Abstract
INTRODUCTION
Age‐related hearing loss is an important risk factor for cognitive decline. However, audiogram thresholds are not good estimators of dementia risk in subjects with normal hearing or mild hearing loss. Here we propose to use distortion product otoacoustic emissions (DPOAEs) as an objective and sensitive tool to estimate the risk of cognitive decline in older adults with normal hearing or mild hearing loss.
METHODS
We assessed neuropsychological, brain magnetic resonance imaging, and auditory analyses on 94 subjects > 64 years of age.
RESULTS
We found that cochlear dysfunction, measured by DPOAEs—and not by conventional audiometry—was associated with Clinical Dementia Rating Sum of Boxes (CDR‐SoB) classification and brain atrophy in the group with mild hearing loss (25 to 40 dB) and normal hearing (<25 dB).
DISCUSSION
Our findings suggest that DPOAEs may be a non‐invasive tool for detecting neurodegeneration and cognitive decline in the older adults, potentially allowing for early intervention.
Keywords: aging, biomarker, brain atrophy, cognitive decline, DPOAE, presbycusis
1. INTRODUCTION
The prevalence of dementia is increasing rapidly, from 50 million in 2015, to 150 million in 2022, impacting global health systems worldwide (Alzheimer's International Report 2020). A key challenge is the identification of reliable biomarkers for early diagnosis that could allow the implementation of prevention strategies for cognitive decline. In the last 5 years, hearing loss (HL) has emerged as one of the most relevant modifiable risk factors for dementia. 1 , 2 , 3 , 4 The World Health Organization defines HL as a an elevation of 25 dB or more in hearing thresholds, which are usually measured using perceptual audiometry, estimating a global prevalence of ≈500 million people (WHO, 2021). Evidence shows that the risk for cognitive decline and all‐cause dementia increases with moderate HL (greater than 40 dB HL) 5 , 6 ; however, the relationship between HL and cognitive decline could start earlier, including mild HL (in the range between 25 and 40 dB), or even subjects with normal hearing thresholds (<25 dB HL). 7 , 8 , 9 It is important to note that individuals with mild HL (25–40 dB HL) or normal hearing thresholds (<25 dB HL) can have additional hearing impairments that are not detected by conventional audiometry, such as cochlear dead regions, cochlear synaptopathy or hidden hearing loss, as well as central auditory processing disorders, which have been associated with cognitive decline in elders. 10 , 11 , 12 , 13 , 14 , 15 , 16
Hearing impairments can be estimated with subjective methods, such as audiometer tests and psychoacoustical tasks, and with objective methods, such as otoacoustic emissions or auditory evoked potentials. 17 Otoacoustic emissions are inaudible low‐level sounds that are emitted by the outer hair cells (OHCs) of the cochlea. 18 Its presence reflects normal cochlear functioning and OHC survival. 19 Recently we measured a subtype of otoacoustic emissions elicited by two tones, known as distortion product otoacoustic emissions (DPOAEs; see Figure 1 and Methods section for more details on DPOAE measurements) and brain structural magnetic resonance imaging (MRI) in a group of older adults with mild hearing loss, evidencing significant associations between the loss of DPOAEs and atrophy of non‐auditory brain regions, including the cingulate cortex, insula, and amygdala. 20 , 21 The structural alterations in the thickness and volume of cortical and the volume of subcortical brain regions were related to cognitive and behavioral impairments in different domains, including face recognition and executive function. 20 , 21 , 22 However, whether these cognitive and behavioral impairments are related to the clinical phenotype of cognitive decline and dementia is unknown.
FIGURE 1.
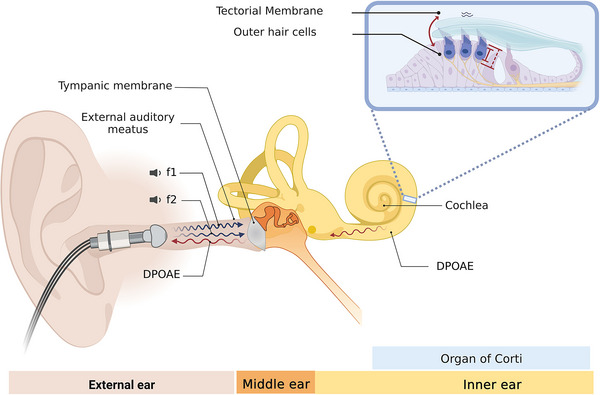
Distortion product otoacoustic emissions (DPOAEs) are sounds emitted by normal cochlear hair cells. This figure describes the biological origin of DPOAEs and the methods used to measure them. A microphone probe with two speakers is inserted and sealed in the external ear canal. The two speakers present two tones of different frequencies (f1 and f2) independently. These two tones are transmitted across the external and middle ear, reaching the cochlear receptor. In the inner ear (cochlear receptor), the two tones generate mechanical distortions at different cochlea basilar membrane positions, including the 2f1‐f2 position, the most widely used DPOAE in clinical and research settings. The presence of DPOAEs depends on the normal functioning of outer hair cells (OHCs). The OHCs possess electromotility, a physiological mechanism in which these cells transduce membrane voltage changes into mechanical vibrations, a physiological process known as the “cochlear amplifier.” As a result of this biological amplification, f1 and f2 tones interact and generate mechanical distortions at different cochlear positions, including the 2f1‐f2 position. These distortions travel back to the external ear, where they can be recorded with a sensitive microphone and measured as a DPOAE at a specific frequency and amplitude in dB sound pressure level (SPL).
RESEARCH IN CONTEXT
Systematic review: we performed a review in PubMed with the terms “presbycusis,” “dementia,” and “otoacoustic emission.” More than 100 articles link presbycusis to the risk of dementia, but studies considering the possible relation between cochlear function measured with otoacoustic emissions and the risk of dementia are scarce.
Interpretation: Hearing impairment is the most important modifiable risk factor for dementia, but the cause of this relation is still not clear. Here we show that cochlear function measured with distortion product otoacoustic emissions (DPOAEs) is associated with cognitive impairment and brain atrophy and that this association is independent of hearing impairment measured with audiometry.
Future directions: We postulate DPOAE as an interesting and innovative biomarker for dementia, since it is a non‐invasive, quick, and objective indicator of neurodegenerative phenomena and cognitive impairment
Here, we hypothesized that the loss of DPOAEs is associated with the clinical phenotype of cognitive decline. To test our hypothesis, we studied the presence of DPOAEs as a proxy of cochlear functioning and OHC survival (as in Belkhiria et al. 20 ), whereas the cognitive clinical profile was assessed independently by blind experienced neurologists determining the Clinical Dementia Rating Sum of Boxes (CDR‐SoB; 23 ). In addition, subjects were evaluated with comprehensive audiological, neuropsychological, and brain MRI evaluations.
2. MATERIALS AND METHODS
2.1. Subjects
A total of 94 older adults from the Auditory and Dementia study (ANDES) cohort 20 were recruited according to the inclusion and exclusion criteria for this study. The patients were recruited from Recoleta's primary health public center in Santiago, Chile. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The inclusion and exclusion criteria were the following: (i) age 65 years or older at the time of recruitment, (ii) no history of neurological or psychiatric illness, (iii) no causes of hearing loss different from presbycusis (e.g., conductive hearing loss), and (iv) no patients using hearing aids. All procedures were approved by the ethics committee of the Clinical Hospital of the University of Chile with permission number OAIC 752/15.
2.2. Clinical assessment
Clinical assessment included: comprehensive cognitive assessment including the Mini‐Mental State Examination (MMSE), 24 functional status as quantified by Pfeffer's Functional Activities Questionnaire, 25 and CDR‐SoB for assessing the severity of cognitive and functional impairments associated with dementia. 23 , 26 Routine neurological and psychiatric examination included daily living questionnaire filled in by a close relative or partner interviewed by a neuropsychologist.
2.3. Audiological evaluations
Evaluations were carried out in the otolaryngology department of the Clinical Hospital of the University of Chile. Air conduction pure tone audiometric hearing thresholds were evaluated at 0.125, 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz for each subject in both ears using a clinical audiometer (AC40, Interacoustics). Bone conduction thresholds were measured at 0.25, 0.5, 1, 2, 3, and 4 kHz to rule out conductive hearing loss. Pure tone average (PTA) at 0.5, 1, 2, and 4 kHz was calculated for each subject in both ears. Subjects were classified according to their hearing level: normal hearing (<25 dB), mild presbycusis (≥25 dB and ≤40 dB) based on the average PTA score of both ears.
2.4. Distortion product otoacoustic emissions
DPOAEs were evaluated as described in. 20 Briefly, DPOAEs (2f1–f2) were measured as a proxy of the cochlear amplifier function, using an ER10C microphone (Etymotic Research), presenting eight pairs of primary tones (f1 and f2, at 65‐ and 55‐dB SPL, f2/f1 ratio of 1.22) in each ear at eight different 2f1–f2 frequencies: 707, 891, 1122, 1414, 1781, 2244, 2828, and 3563 Hz. It is notable that using different pairs of tones (f1 and f2) at different frequencies allows the measurement of the 2f1‐f2 DPOAEs at different cochlear positions that can be used as a proxy of hearing impairments and cochlear hair cell survival in older adults. The amplitude of a DPOAE (dB SPL) should be at least 6 dB above the noise floor. The number of detectable DPOAEs per ear, that is, 0 to 8 was counted and a number “0” denoted the absence of detectable DPOAE in that ear, whereas “8” implied normal cochlear function. We used the total number of detected DPOAEs of both ears (range from 0 to 16).
2.5. Image acquisition
Imaging data were acquired using a MAGNETOM Skyra 3‐Tesla whole‐body MRI Scanner (Siemens Healthcare GmbHR, Erlangen, Germany) using a T1‐Magnetization Prepared ‐ Rapid Gradient Echo (MPRAGE) sequence. Contiguous images across the entire brain were acquired with the following parameters: echo time (TE) = 232 ms, repetition time (TR) = 2300 ms, flip angle = 8, 26 slices, matrix = 256 × 256, voxel size = 0.94 × 0.94 × 0.9 mm3. We also registered T2‐weighted turbo spin echo (TSE) (4500 TR ms, 92 TE ms) and fluid‐attenuated inversion recovery (FLAIR) (8000 TR ms, 94 TE ms, 2500 TI ms) to inspect structural abnormalities. The acquisition duration was 30 minutes, with a total of 440 images for each subject.
2.6. Image preprocessing and analysis
The morphometric analysis was carried out by FreeSurfer, version 6, running under Centos 6. A single Linux workstation was used for the T1‐weighted image analysis of individual subjects as suggested by. 27 The FreeSurfer processes cortical reconstruction 28 through several steps: volume registration with the Talairach atlas, bias field correction, initial volumetric labeling, non‐linear alignment to the Talairach space, and final volume labeling. Briefly, the automatic “recon‐all” function produces representations of the cortical surfaces. It uses both intensity and continuity information from the entire three‐dimensional MR volume in segmentation and deformation procedures. It creates gross brain volume extents for larger‐scale regions (i.e., the total number of voxels per region): total gray and white matter, subcortical gray matter, brain mask volume, and estimated total intracranial volume. The reliability between manual tracing and automatic volume measurements has been validated. The accordance between manual tracings and automatically obtained segmentations was similar to the agreement between manual tracings. 29 All volumes were visually inspected, and if needed, edited by a trained researcher according to standard processes. We selected regions of interest that have been implicated consistently in previous neuroimaging studies relating to audition, cognition, and dementia, such as the hippocampus and the lateral ventricles.
2.7. Statistical analysis
Spearman's rank was used to assess the correlation between variables. For brain volume comparison between high and low DPOAE groups, a Mann‐Whitney U test was used. Receiver‐operator characteristic (ROC) curves and area under the curve (AUC) scores were obtained using scikit‐learn package. 30 For the comparison of pure tone average (PTA) and DPOAE scores, a 1000 bootstrapping approach was used, and a Mann‐Whitney U test for statistical comparison.
3. RESULTS
A total of 94 subjects (65 female) with an average age of 72.7 ± 5 years (mean ± SD), and mean education level of 9.5 ± 5.1 years were obtained from the ANDES cohort. 20 , 21 Data from this cohort include DPOAE measurements, audiogram thresholds (Figure S1), assessed by PTA, a battery of neuropsychological tests for cognitive decline, and structural brain MRI at 3‐Tesla.
The mean PTA of the 94 individuals was 24.3 ± 8 dB HL, including 32 subjects with normal hearing thresholds (<25 dB HL) and 62 with mild hearing loss (≥25 and ≤40 dB HL). None of the individuals used hearing aids at the time of evaluations. Regarding DPOAE, we calculated the total number of detected DPOAE in eight different frequencies in both ears (range between 0 and 16, bigger is better; see Methods section), yielding an average number of 8.4 ± 5 detected DPOAEs per individual.
Because DPOAEs have been used as a proxy of hearing sensitivity, 19 we explored the level of correlation between DPOAE and PTA variables in our data. DPOAE measurements and PTA audiogram thresholds partly converge (Spearman's rank correlation ρ = −0.64, p‐value < 0.0001; Figure 2A), suggesting that they capture similar but not equal auditory characteristics. In relation to cognitive and neurological variables, 70 subjects (74.4%) were classified as cognitively unimpaired (CDR = 0) and 24 subjects (25.3%) were classified as mild cognitive impairment (MCI). The mean MMSE score was 27 ± 3.4 (range 18–30 points), whereas the mean CDR‐SoB was 0.94 ± 2.3 (range 0–4). A summary of demographic data is reported in Table 1.
FIGURE 2.
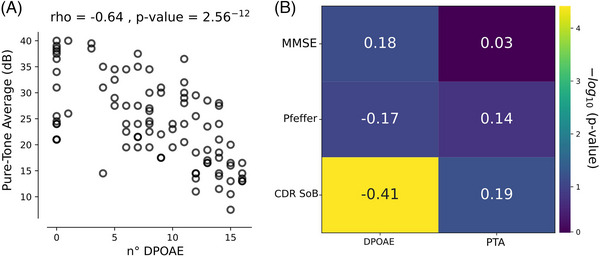
Differential contributions of distortion product otoacoustic emissions (DPOAEs) and pure tone average (PTA) over cognition. (A) PTA correlates negatively with the number of DPOAEs, demonstrating that a larger number of DPOAEs is correlated with better PTA. (B) Correlation matrix with annotated Spearman's rho values in each cell, between audiological measures and Mini‐Mental Status Examination (MMSE), the Pfeffer questionnaire, and the Clinical Dementia Rating Sum of Boxes (CDR‐SoB). Only the correlation between CDR‐SoB and the number of DPOAEs was significant (p < 0.001). Color bar in the y‐axis represents the −log 10 of the p‐value obtained from Spearman's rank correlation.
TABLE 1.
Audiological and neurological profile of the studied subjects (n = 94).
| Characteristic | Low DPOAE (n = 43) | High DPOAE (n = 51) |
|---|---|---|
| Age, y, mean (SD) | 71.5 ± 4.9 | 73.6 ± 4.75 |
|
Sex, n (%) Female |
24 (55.8) | 41 (80.3) |
| Education, y, mean (SD) | 10.2 ± 4.3 | 9.7 ± 3.9 |
| PTA, mean (SD) | 28.3 ± 6.9 | 19.6 ± 6.6 |
| DPOAE, mean (SD) | 4.4 ± 3.3 | 13.1 ± 1.7 |
| Hearing aid use, n (%) | 0 (0.0) | 0 (0.0) |
|
Hearing loss category, n (%) Normal (<25 dB) |
10 (23.2) | 30 (58.8) |
| Mild (25–40 dB) | 33 (76.7) | 21 (41) |
| Pfeffer, mean (SD) | 1.9 ± 4.48 | 1.04 ± 3.98 |
| MMSE, mean (SD) | 26.2 ± 4.2 | 27.9 ± 1.8 |
| Hypertension, n (%) | 29 (67.4) | 29 (56.8) |
| Diabetes, n (%) | 10 (23.2) | 12 (23.5) |
| Cholesterol, mean (SD) | 165.6 ± 59.2 | 168.7 ± 45.6 |
3.1. Less DPOAE is correlated with worse dementia rating
Next, we analyzed the possible relations between the number of DPOAE and PTA with cognitive traits, such as global cognitive performance evaluated through MMSE, functional status quantified using Pfeffer, and the CDR‐SoB, which was obtained by clinical neurological evaluations that were blinded to the DPOAE results, providing a quantitative index of the level of cognitive impairment as a dementia rating. Neither MMSE nor Pfeffer presented a significant correlation with the number of DPOAEs (MMSE Spearman's rank correlation, ρ = 0.18, p‐value = 0.075 and Pfeffer Spearman's rank correlation, ρ = −0.17, p‐value = 0.09) and PTA (MMSE Spearman's rank correlation, ρ = 0.03, p‐value = 0.74 and Pfeffer Spearman's rank correlation, ρ = 0.14, p‐value = 0.16). Interest, CDR‐SoB showed a significant correlation with the number of DPOAEs (Spearman's rank correlation ρ = −0.41, p‐value < 0.00001), even after controlling for relevant covariates such as age and gender (Spearman's rank correlation ρ = −0.37, p‐value = 0.0002) as well as other known relevant factors influencing DPOAEs, such as diabetes, high cholesterol, and hypertension (Spearman's rank correlation ρ = −0.38, p‐value = 0.0002). In contrast, there was a non‐significant correlation between PTA and CDR‐SoB score (Spearman's rank correlation ρ = 0.19, p‐value = 0.06), and after controlling for covariates (Spearman's Rank Correlation ρ = 0.14, p‐value = 0.17). These results suggest that, although the numbers of DPOAE and PTA have a partial statistical convergence (Figure 2A), they correlate differentially with dementia rating, suggesting that DPOAE is a more sensitive hearing assessment for demonstrating the relation between hearing impairments and clinical profile of cognitive decline related to the CDR‐SoB score (Figure 2B).
3.2. Neuroimaging biomarkers correlate with DPOAE
Currently there are well‐validated brain MRI biomarkers that are commonly used for the clinical evaluation of patients with cognitive decline complaints, such as the volumetric changes of the hippocampus and lateral ventricles. To test if cochlear dysfunction is associated with these cognitive decline neuroimaging biomarkers, we first divided subjects by their number of DPOAEs, calculating the median value of the whole population, thus defining low DPOAE and high DPOAE groups. It is striking that we found that the volume of bilateral hippocampus was significantly more atrophied in the low DPOAE group as compared to the high DPOAE group (Figure 3A, left panel, Mann‐Whitney U test p‐value = 0.0015). In addition, we found that the bilateral lateral ventricles were significantly larger in the low DPOAE group (Figure 3B, left panel. Mann‐Whitney U test p‐value = 0.00003). There was a significant correlation between the number of DPOAEs with the volume of both hippocampus (Spearman's rank correlation, ρ = 0.312, p = 0.002, Figure 3A, right panel) and lateral ventricles (Spearman's rank correlation ρ = −0.37, p = 0.0001, Figure 3B, right panel).
FIGURE 3.
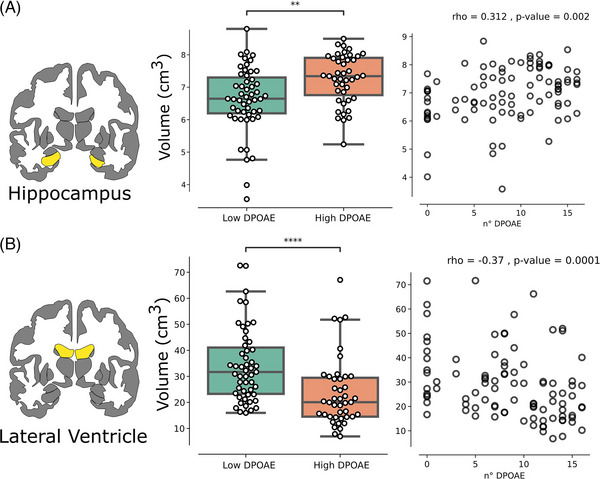
Distortion product otoacoustic emissions (DPOAEs) correlate with neuroimaging biomarkers of cognitive decline. (A) On left, a coronal view of bilateral hippocampal volume. In the middle, total hippocampal volume is more atrophied in the low DPOAE group as compared to the high DPOAE group (Mann‐Whitney U test p‐value = 0.001). On right, a significant correlation between total hippocampal volume and the number of DPOAEs shows a significant positive correlation. (B) On left, a coronal view of the bilateral lateral ventricle volume. In the middle, total ventricular volume is more hypertrophied in the low DPOAE group as compared to the high DPOAE group (p‐value = 0.00003). On right, a significant correlation between total ventricular volume and the number of DPOAEs shows a significant negative correlation. All correlations are Spearman's rank.
3.3. DPOAE predicts cognitive decline
To test if cochlear dysfunction as measured by DPOAEs predicts cognitive decline in our cohort and if it overcomes conventional audiometry as measured by PTA, we evaluated its capacity to discriminate between control (CDR‐SoB <0.5) and risk of cognitive decline (CDR SoB ≥0.5). ROC curve analysis was performed and the AUC was calculated. Five‐fold cross‐validation was performed for PTA (AUC = 0.45 ± 0.26, Figure 4A) showing that its prediction power is near random. In contrast, the number of DPOAE predicted with good discriminability (AUC = 0.81 ± 0.1, Figure 4A). To evaluate if DPOAE consistently predicts better than PTA, we obtained 1000 bootstrapped AUC scores for each term, and statistically compared the scores. Notably, PTA significantly predicts worse than DPOAEs (Mann‐Whitney U test p‐value < 0.0001), and DPOAE shows low AUC variability. These results show that DPOAEs overcomes PTA in terms of discriminability (Figure 4B), and robustly predicts the risk of clinically relevant cognitive decline in a cohort of normal and mild hearing loss older adults.
FIGURE 4.
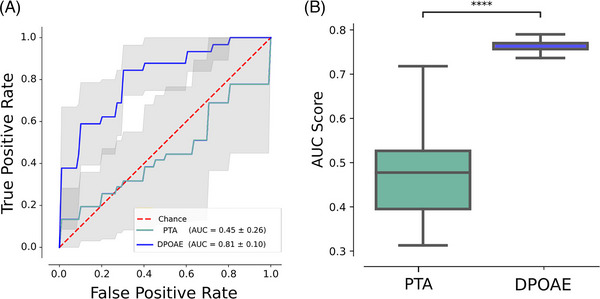
Distortion product otoacoustic emissions (DPOAEs) and not pure tone average (PTA) predicts cognitive impairment in normal and mild hearing loss individuals. (A) Five‐fold cross‐validation using PTA (green, area under the curve [AUC] = 0.45) and DPOAE (blue, AUC = 0.81) to predict cognitive impairment (Clinical Dementia Rating Sum of Boxes [CDR‐SoB] ≥0.5). (B) Boxplot comparing 1000 bootstrap AUC scores, depicting that PTA values significantly predict worse than DPOAEs (Mann‐Whitney U test p‐value < 0.0001).
4. DISCUSSION
We found that in a sample of normal hearing (PTA < 25 dB) and age‐related mild‐hearing loss individuals (PTA ≥25 and ≤40 dB HL), the number of DPOAEs is significantly correlated with the clinical classification of dementia (CDR‐SoB scale) evaluated by expert neurologists who were blinded to the DPOAE results. In the same group of subjects, audiometric hearing thresholds were not correlated with the CDR‐SoB scale. These findings suggest that evaluating cochlear OHC function by means of DPOAE detection is a more sensitive test than audiogram thresholds to estimate clinically relevant cognitive impairment risk in older adults. It is important to note that these results stress that although DPOAEs and PTA are significantly correlated (Figure 2A), they possess relevant differences. For instance, audiometric PTA is obtained by subjective behavioral responses to pure tones, which can be influenced by multiple sources of variability, especially in difficult to test individuals, such as those with cognitive impairment or dementia. 31 In this group of patients, audiological objective measures, such as auditory steady state responses, auditory brainstem responses, and otoacoustic emissions, emerge as important tools that do not need subject cooperation, allowing the reliable assessment of the complete auditory pathway. 13 , 20 , 31 In addition, and concordant with our previous work, 20 , 21 we found significant correlations between the loss of DPOAEs—a measure of cochlear dysfunction—and the volume of different brain structures, such as bilateral hippocampus and lateral ventricle volumes (Figure 3).
4.1. What are the mechanisms that relate DPOAE loss with brain atrophy and risk of cognitive decline?
There are at least three possible mechanisms relating DPOAE loss to the risk of dementia. First, DPOAE is a sensitive and objective measure to detect hearing impairment and is highly correlated with PTA thresholds. Thus DPOAE could be considered as a more sensitive measure than audiometric PTA to estimate hearing impairment (Figure 4). Second, as a proxy of OHC loss, DPOAE loss might reflect the process of cellular aging due to non‐specific neurodegenerative and vascular damage of cells located inside the cranium, and in a speculative statement, DPOAE loss might be a general estimator of neuronal survival in the brain. Finally, DPOAE presence can also be affected by different factors, including chemotherapy, 32 vascular disease, 33 and acoustic trauma. 34 Regarding the latter factor, there is evidence in animal models that acoustic trauma can induce hippocampal atrophy, which is also related to neurodegenerative tau pathology and amyloid beta in the brain. 35 , 36 Therefore, different from PTA thresholds, DPOAE loss might be reflecting a number of biological processes that can contribute in additive ways to cognitive decline in older adults, which could explain the better performance in the AUC score for classifying CDR‐SoB scores in this cohort of normal hearing and mild hearing loss individuals (Figure 4).
It is noteworthy that DPOAE is related to cognitive impairment evaluated by CDR‐SoB, a scale that measures various cognitive and functional domains but does not show a significant relationship with MMSE, which measures memory and cognition, or with Pfeffer, which evaluates functional activities. MMSE has a low sensitivity to predict the presence of initial or mild cognitive impairment and has a better performance in dementia cases.
Our findings may have two practical implications for future research. First, from a pathophysiological point of view, DPOAE loss as a measure of cochlear OHC damage, reinforces the connection between degeneration of auditory structures with broader neurodegenerative phenomena such as those seen in Alzheimer's disease and related dementias. Second, from a clinical point of view, it highlights the potential of DPOAE as a possible biomarker of neurodegenerative phenomena and cognitive impairment. The determination of DPOAEs can constitute an objective, simple, and accessible biomarker to identify older adults who are at risk of cognitive deterioration, considering the AUC over 0.8 in Figure 4. This idea is supported by recent findings in animal models showing a significant relation between auditory functions and tau protein levels in the cerebrospinal fluid. 37
The practical implementation of using DPOAE as a screening tool for identifying older adults at higher risk of developing cognitive decline is supported by the routine use of DPOAE in newborn hearing screening programs worldwide. 38 In this line, newborn screening equipment for DPOAE measurements is available in hospitals in several countries, including universal screening programs where audiologists or other health professionals are already trained in the measurement of DPOAE, and the examination is relatively simple, fast, and available at a reasonable cost. 39 , 40 , 41 Although there are experiences of DPOAE use for the screening of hearing loss in adults and of its good correlation with other audiological measurements, 42 some specific software or hardware adaptations might be necessary for implementing a screening program in older adults. From our work, we propose that DPOAE detection can be used in older adults for estimating the risk of cognitive decline at an early stage, in subjects with normal hearing or mild hearing loss.
CONFLICT OF INTEREST STATEMENT
None of the authors declare any conflict of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All the participants in this study signed an informed consent approved by the local institutional review board before any procedure.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This research work was supported by the National Agency for Research and Development of Chile (ANID), FONDEF ID20I10371, FONDECYT 1221696, FONDECYT 1220607, FONDEQUIP EQM210020, Fondo Basal ANID FB0008 to P.H.D., Centro Nacional de Inteligencia Artificial CENIA, FB210017, BASAL, ANID to R.V., FONDECYT 3230557 to V.M., Proyecto Milenio ICN09_015, and Fundación Guillermo Puelma.
Medel V, Delano PH, Belkhiria C, et al. Cochlear dysfunction as an early biomarker of cognitive decline in normal hearing and mild hearing loss. Alzheimer's Dement. 2024;16:e12467. 10.1002/dad2.12467
Vicente Medel and Paul H. Delano contributed equally to this work and share first authorship.
REFERENCES
- 1. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet North Am Ed. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 2. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet North Am Ed. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brewster KK, Deal JA, Lin FR, Rutherford BR. Considering hearing loss as a modifiable risk factor for dementia. Expert Rev Neurother. 2022;22(9):805‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vergara RC, Zitko P, Slachevsky A, Martin CS, Delgado C. Population attributable fraction of modifiable risk factors for dementia in chile. Alzheimers Dement (Amst). 2022;14(1):e12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the health abc study. J Gerontol A Biol Sci Med Sci. 2017;72(5):703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamblay N, Boggs D, Huidobro B, et al. Prevalence of cognitive impairment and its association with hearing loss among adults over 50 years of age: results from a population‐based survey in santiago, chile. Am J Audiol. 2023;32(1):150‐159. [DOI] [PubMed] [Google Scholar]
- 7. Johnson JCS, Marshall CR, Weil RS, Bamiou DE, Hardy CJD, Warren JD. Hearing and dementia: from ears to brain. Brain. 2021;144(2):391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irace AL, Armstrong NM, Deal JA, et al. Longitudinal associations of subclinical hearing loss with cognitive decline. J Gerontol: Series A. 2022;77(3):623‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chern A, Irace AL, Sharma RK, Zhang Y, Chen Q, Golub JS. The longitudinal association of subclinical hearing loss with cognition in the health, aging and body composition study. Front Aging Neurosci. 2022;13:789515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sardone R, Battista P, Donghia R, et al. Age‐related central auditory processing disorder, mci, and dementia in an older population of southern italy. Otolaryngol Head Neck Surg. 2020;163(2):348‐355. [DOI] [PubMed] [Google Scholar]
- 11. Vinay S, Moore BCJ. Effect of age, test frequency and level on thresholds for the ten (hl) test for people with normal hearing. Int J Audiol. 2020;59(12):915‐920. [DOI] [PubMed] [Google Scholar]
- 12. Bajin MD, Dahm V, Lin VYW. Hidden hearing loss: current concepts. Curr Opin Otolaryngol Head Neck Surg. 2022;30(5):321‐325. [DOI] [PubMed] [Google Scholar]
- 13. Delano PH, Belkhiria C, Vergara RC, et al. Reduced suprathreshold auditory nerve responses are associated with slower processing speed and thinner temporal and parietal cortex in presbycusis. PLoS One. 2020;15(5):e0233224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jayakody DMP, Menegola HK, Yiannos JM, et al. The peripheral hearing and central auditory processing skills of individuals with subjective memory complaints. Front Neurosci. 2020;14:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarawneh HY, Menegola HK, Peou A, Tarawneh H, Jayakody DMP. Central auditory functions of alzheimer's disease and its preclinical stages: a systematic review and meta‐analysis. Cells. 2022;11(6):1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Torrente MC, Vergara R, Moreno‐Gómez FN, Leiva A, et al. Speech perception and dichotic listening are associated with hearing thresholds and cognition, respectively, in unaided presbycusis. Front Aging Neurosci. 2022;14:786330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies RA. Audiometry and other hearing tests. Handb Clin Neurol. 2016;137:157‐176. [DOI] [PubMed] [Google Scholar]
- 18. Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64(5):1386‐1391. [DOI] [PubMed] [Google Scholar]
- 19. Shaffer LA, Withnell RH, Dhar S, Lilly DJ, Goodman SS, Harmon KM. Sources and mechanisms of dpoae generation: implications for the prediction of auditory sensitivity. Ear Hear. 2003;24(5):367‐379. [DOI] [PubMed] [Google Scholar]
- 20. Belkhiria C, Vergara RC, Martín SS, et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Front Aging Neurosci. 2019;11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belkhiria C, Vergara RC, Martin SS, et al. Insula and amygdala atrophy are associated with functional impairment in subjects with presbycusis. Front Aging Neurosci. 2020;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belkhiria C, Vergara RC, Martinez M, Delano PH, Delgado C. Neural links between facial emotion recognition and cognitive impairment in presbycusis. Int J Geriatr Psychiatry. 2021;36(8):1171‐1178. [DOI] [PubMed] [Google Scholar]
- 23. O'Bryant SE, Waring SC, Munro Cullum C, et al, Texas Alzheimer's Research Consortium . Staging dementia using clinical dementia rating scale sum of boxes scores: a texas alzheimer's research consortium study. Arch Neurol. 2008;65(8):1091‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folstein MF. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1992;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 25. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 26. Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140(6):566‐572. [DOI] [PubMed] [Google Scholar]
- 27. Gronenschild EH, Habets P, Jacobs HI, et al. The effects of freesurfer version, workstation type, and macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 2012;7(6):e38234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97(20):11050‐11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341‐355. [DOI] [PubMed] [Google Scholar]
- 30. Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit‐learn: machine learning in Python. J Mach Learn Res. 2011;12:2825‐2830. [Google Scholar]
- 31. Tarawneh HY, Sohrabi HR, Mulders W, Martins RN, Jayakody DMP. Comparison of auditory steady‐state responses with conventional audiometry in older adults. Front Neurol. 2022;1354:924096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jalali MM, Saedi HS, Saadat F. Effect of cisplatin chemotherapy on the inner ear function and serum prestin concentration. Eur Arch Otorhinolaryngol. 2022;279(6):2783‐2789. [DOI] [PubMed] [Google Scholar]
- 33. Cho WK, Kang WS, Lee JB, Park HJ, Chung JW, Ahn JH. Interpreting auditory brainstem evoked responses and distortion product otoacoustic emissions in diabetic patients with normal hearing. Auris Nasus Larynx. 2021;48(2):227‐234. [DOI] [PubMed] [Google Scholar]
- 34. Hamernik RP, Ahroon WA, Jock BM, Bennett JA. Noise‐induced threshold shift dynamics measured with distortion‐product otoacoustic emissions and auditory evoked potentials in chinchillas with inner hair cell deficient cochleas. Hear Res. 1998;118(1‐2):73‐82. [DOI] [PubMed] [Google Scholar]
- 35. Paciello F, Rinaudo M, Longo V, et al. Auditory sensory deprivation induced by noise exposure exacerbates cognitive decline in a mouse model of alzheimer's disease. Elife. 2021;10:e70908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng M, Yan J, Hao W, et al. Worsening hearing was associated with higher β‐amyloid and tau burden in age‐related hearing loss. Sci Rep. 2022;12(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu W, Zhang C, Li JQ, et al. Age‐related hearing loss accelerates cerebrospinal fluid tau levels and brain atrophy: a longitudinal study. Aging (Albany NY). 2019;11(10):3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Janssen T. A review of the effectiveness of otoacoustic emissions for evaluating hearing status after newborn screening. Otol Neurotol. 2013;34(6):1058‐1063. [DOI] [PubMed] [Google Scholar]
- 39. Porter HL, Neely ST, Gorga MP. Using benefit‐cost ratio to select universal newborn hearing screening test criteria. Ear Hear. 2009;30(4):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma Y, Mishra G, Bhatt SH, Nimbalkar S. Neonatal hearing screening programme (nhsp): at a rural based tertiary care centre. Indian J Otolaryngol Head Neck Surg. 2015;67(4):388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bezuidenhout JK, Khoza‐Shangase K, Maayer TD, Strehlau R. Outcomes of newborn hearing screening at an academic secondary level hospital in johannesburg, south africa. S Afr J Commun Disord. 2021;68(1):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramkumar V, Vanaja CS, Hall JW, Selvakumar K, Nagarajan R. Validation of dpoae screening conducted by village health workers in a rural community with real‐time click evoked tele‐auditory brainstem response. Int J Audiol. 2018;57(5):370‐375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


