Abstract
Subepithelial lesions, formerly known as subepithelial tumors, are incidentally discovered protrusions throughout the gastrointestinal tract with normal overlying mucosa. Studies related to the diagnosis and therapy methods are limited due to the low incidence and malignant potential of these lesions. They commonly originating from the second, third, and fourth layers (muscularis mucosa, submucosa, and muscularis propria) of the gastrointestinal wall. They are reported to be more prevalent in the stomach and esophagus than small intestine and colon. Subepithelial lesions in the stomach and duodenum are more prone to malignancy than the lesions in the esophagus. Despite different strategies in the management of subepithelial lesions based on their size and location, there is still not a unique consensus on the issue. In this review, we have attempted to introduce the most practical approach to managing gastrointestinal subepithelial lesions based on current guidelines.
Key Words: Subepithelial lesions, Endoscopic ultrasound, Mucosal incision-assisted biopsy, Endoscopic submucosal resection, Full-thickness resection dissection
Introduction
Subepithelial lesions are usually asymptomatic, so they are usually found unintentionally following extensive usage of screening endoscopies or endoscopies for other indications with an incidence of 0.36-0.76%. (1-3). A limited number of cases are presented with iron deficiency anemia, overt gastrointestinal bleeding, obstruction, and abdominal pain. (4, 5). At the endoscopic view, lesions are covered with normal-appearing mucosa and are protruded into the gastrointestinal tract. Conventional endoscopies suffer from poor accuracy in differentiating various subtypes of subepithelial lesions due to their similar shapes and colors. Chromoendoscopy and narrow-band imaging are not useful since the mucosa of these lesions is normal (6). However, some features are reported to be specific. Suppose that antral lesions with central umbilication and bright yellow protrusions with positive cushion signs are specific for pancreatic rests and lipomas, respectively (7, 8). The Location of SELs out of their color and shape would suggest some of the lesions' characteristics. Gastric and duodenal lesions are harboring more malignant potential than esophageal lesions. The incidence of malignancy in gastric and esophageal lesions is predominantly higher than in small intestinal and large intestinal lesions (4, 5). Size of the lesion is also reported to predict the malignant potential of the lesions. As proof, lesions over 20mm are more susceptible to malignancy than smaller ones (5, 6). Endoscopic ultrasound (EUS) introduction was a significant step in diagnosing subtypes of subepithelial lesions. However, the multiplicity of lesions and limited strength of EUS in the differentiation of most subepithelial lesions are why tissue acquisition is also recommended (9). There is still not a unique consensus on the indications of surveillance, resection, and tissue acquisition of small and large lesions. As mentioned above, the decision on complete resection of the lesions is also controversial because most of these lesions are not malignant. The reported challenges require a review of the current guidelines to design a simple and practical approach to better managing these lesions.
Discussion
Diagnosis: role of endoscopic ultrasound
EUS is the most accepted technique for evaluating SELs in the GI tract due to making a clear distinction among different layers of the GI wall (10, 11). So, it is widely used to demonstrate size, shape, and echogenicity of the lesion and involved layers of the gastrointestinal wall. EUS has also been successful in the differentiation of external pressures from subepithelial lesions (12-14). Some of the ultrasound features are pathognomonic, like lipoma, pancreatic rest, and varices, which are anechoic (15, 16). Leiomyomas and gastrointestinal tumors (GISTs) mainly originate from muscularis propria and other lesions commonly stem from the submucosal layer of the gastrointestinal (GI) tract (10). The hyperechoic feature of lipoma is diagnostic via EUS examination (17, 18). But, the ability of EUS in the differentiation of other subepithelial lesions has been predicted to be less than 37.5% for leiomyoma, 89% for GISTs, and up to 100% for neuroendocrine neoplasms (NENs) (19). Description of some SELs has been seen in Table 1 (6). To have an accurate approach for such lesions, tissue sampling is necessary. The mentioned accuracy will be even less if the lesion is small. Differentiating GISTs and leiomyomas is a serious challenge because both are rooted in the 4th layer of the gastrointestinal wall. Low-grade GISTs are mistaken for leiomyomas; High-grade GISTs, due to their inhomogeneous consistency and hyperechoic parts, are better differentiated from leiomyomas (19, 20). New technologies like artificial intelligence and mini-prob usage during EUS are emerging, but more studies are required to evaluate the superiority of these methods to conventional EUS (21). There are limited studies about the effectiveness of EUS-elastography (22). Presence of hyper-enhanced micro-vascularization in contrast to harmonic enhanced-EUS (CH-EUS) is strongly indicates GIST with a sensitivity and specificity of about 89% and 82%, respectively. Differentiation of high-grade and low-grade GISTs is also feasible with detecting irregular intramural vessels representing high-grade GISTs using CH-EUS. The studies for CH-EUS have not been promising (23, 24).
Table 1.
EUS description of SELs (6)
| Subepithelial lesion | Layer of origin | Ultrasound characteristics | Common location |
|---|---|---|---|
| Duplication cyst | Submucosa | Sharp and anechoic, without Doppler signal | Anywhere in the GI tract |
| Varices | Submucosa | Serpiginous and sharp with anechoic echotexture, without Doppler signal | Anywhere in the GI tract |
| Lymphangiomas | Submucosa | Sharp and anechoic with internal septa, without Doppler signal | Anywhere in the GI tract |
| Granular cell tumors | Muscularis mucosa, Submucosa | Variable border with heterogenous hypoechoic with variable borders | Esophagus |
| Neuroendocrine neoplasm | Muscularis mucosa, Submucosa | Sharp border with various echotextures | Stomach, small bowel, rectum |
| Pancreatic rest | Submucosa, Muscularis propria | Indistinguishable border with heterogenous hypoechoic echotexture, Cysts and ducts in central umbilication | Commonly antrum then, Gastric body and Duodenum |
| Leiomyoma | Muscularis mucosa, Muscularis propria | Sharp border with homogenous hypoechoic echotexture | Esophagus, Stomach, Other parts of GI tract |
| GIST low-risk | Muscularis propria, rarely muscularis mucosa and submucosa | Sharp border with heterogenous hypoechoic echotexture | Stomach, Small intestine, Esophagus, Rectum |
| GIST high-risk | Muscularis propria, rarely muscularis mucosa and submucosa | Irregular border with heterogenous hypoechoic echotexture with cysts or echogenic foci | Stomach, Small intestine, Esophagus, Rectum |
| Schwannoma | Muscularis propria | Sharp border with homogenous hypoechoic echotexture, sometimes with marginal halo | Stomach |
| Lymphoma | Muscularis mucosa, Submucosa, Muscularis propria | Irregular border with hypoechoic echotexture | Stomach, Small intestine |
| Glomus tumor | Submucosa, Muscularis propria | Sharp border with variable echotexture, Hyper vascular with internal echo |
Anywhere in the GI tract |
| Endometriosis | Muscularis propria, Serosa | Irregular border with heterogenous hypoechoic echotexture with extension into the rectovaginal septum | Rectum, Sigmoid colon |
| Lipoma | Submucosa | Sharp border with homogenous hyperechoic echotexture | Anywhere in the GI tract |
| Brunner gland | Muscularis mucosa, Submucosa | Sharp border with homogenous iso-hyperechoic sometimes with ducts | Bulb |
| Metastasis | Any layer | Irregular border with hypoechoic echotexture | Anywhere in the GI tract |
Diagnosis: role of tissue sampling
Ultrasound appearance of some SELs, such as a duplication cyst, lipoma, and ectopic pancreas, is considered diagnostic, so tissue sampling is usually unnecessary. However, a definite diagnosis is not possible for hypoechoic and hetero-echoic lesions originating from the submucosal and muscularis propria. Tissue sampling or removal of such lesions is recommended to diagnose and estimate the malignant potential of lesions definitely. Tissue sampling for these lesions uses forceps biopsy, jumbo-forceps biopsy, EUS tissue acquisition (EUS-TA), ligation-unroof biopsy, and mucosal incision-assisted biopsy (MIAB) (25).
The reason why conventional biopsies using simple forceps or jumbo forceps are not diagnostic for subepithelial lesions is the fact that the overlying mucosa is normal. So other techniques like bite-on-bite biopsies with simple or jumbo-forceps are introduced. The yield of these techniques is low (about 58.9% for jumbo biopsy (23) in comparison with 93% for EUS-FNA technique (26, 27)), and the risk of bleeding particularly for jumbo biopsies is high (estimated to be about 34.9% based on the studies) (28).
Other techniques for tissue sampling include a 6-12mm incision of the top convexity of the lesion, unroofing the lesion, and direct sampling from the lesion called MIAB or single-incision needle knife technique (SINK). The endoscopist should be aware of the risk of bleeding and be ready for prompt hemostatic intervention. Tunneling and sampling in direct view is another recommended procedure for subepithelial lesions (29). The successful tissue sampling has been estimated at 89%, and adverse events containing bleeding have been reported at 5% per the revised guidelines (30, 31). Some studies have introduced ESD-assisted deep biopsy with a better yield and lower adverse events than MIAB (31). Besides the risk of bleeding, tunneling, and MIAB harbor the risk of tissue fibrosis complicating resection of the lesions.
Ligation-unroof biopsy is another recommended technique for resection, histopathologic examination, and even immunohistochemistry (IHC) study of non-pedunculated subepithelial lesions. In this technique, a band/ endo-loop/ cap is wrapped around the lesion after suctioning the lesion. Then, the lesion is unroofed, and a biopsy is taken. Destruction of the lesion is also predictable due to ischemia attributed to the ligation of the lesion. Based on a study, endoscopic submucosal ligation without resection has resulted in the destruction of 95% of leiomyomas (32). Ligation before unroofing would decrease the risk of bleeding and result in more projection of the lesion into the lumen for a better resection. Studies have proved that the accuracy of acquired specimens is comparable with surgical specimens (32).
EUS Tissue acquisition techniques mainly include Fine needle aspiration (FNA) and –biopsy (FNB). Various sizes of FNA and FNB needles (19G, 22G, 25G) have been tried for tissue acquisition with a diagnostic accuracy range of 40–70% based on the lesion size. Clearly, the diagnostic accuracy of smaller lesions is lower than larger lesions. It should be noted that the needle gauge is also a determining factor. Thicker needles arranged for biopsy have better diagnostic accuracy than smaller needles for aspiration. For smaller lesions, however, smaller 25G needles are recommended (33, 34).
The preferred choice of tissue sampling for lesions larger than 20mm is EUS-FNB or mucosal incision-assisted biopsy (MIAB). It is driven by the studies that the results of FNB-EUS and MIAB are comparable for lesions greater than 20mm. The recommended procedure of choice for lesions smaller than 20mm is MIAB, and FNB-EUS lies in the second position because localization of lesions is somehow challenging for EUS (28).
Following tissue sampling, complementary studies are recommended to improve the accuracy of diagnosis. To evaluate cytology of samples, the cytoblock technique has been suggested instead of the traditional smear method (35). There is not a special panel for the usage of IHC and molecular markers, but chromogranin A, synaptophysin, and Ki67 for neuroendocrine neoplasms and mitotic index for GISTs are recommended, although there is a significant difference between surgical and endoscopic samples in case of mitotic index values (36, 37). Studies have shown that proliferation measures underestimate the proliferation indexes more than surgical samples so markers and proliferation indexes are not routinely recommended (38, 39).
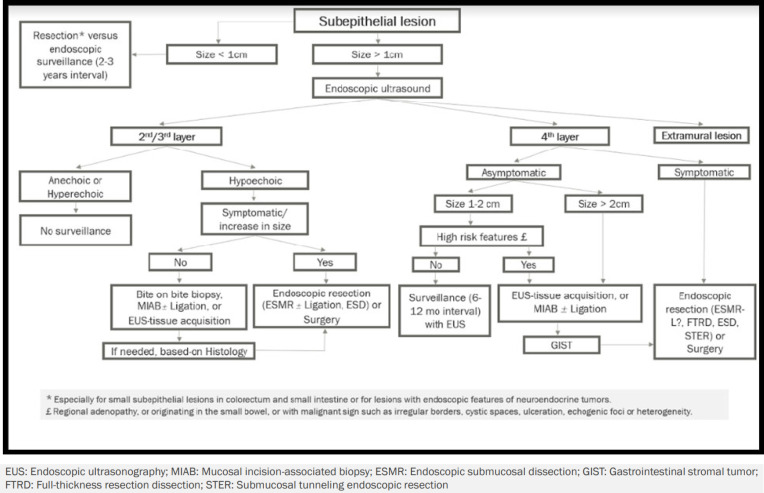
Management: surveillance, tissue acquisition or resection ( algorithm-1 )
Algorithm 1.
Approach to subepithelial lesions
Generally, surveillance without tissue acquisition is confined to a narrow group of patients with subepithelial lesions. Even though some of the lesions are commonly asymptomatic and characteristically benign, failure to diagnose would be stressful. In addition, long-term surveillance would be costly over time. So, trying to have a definite diagnosis via tissue acquisition might be preferred if the lesions were not resected.
Lesions smaller than 10mm without a definite diagnosis
When it comes to the management of subepithelial lesions, a cut-off size of 10mm plays a key role in deciding whether complementary evaluation using EUS is required. Our recommendation for small subepithelial lesions is endoscopically resection without further evaluation (especially for lesions located in large or small intestine) or endoscopic surveillance at two to three-year intervals.
Lesions larger than 10mm without a definite diagnosis
Further evaluation via EUS is recommended for lesions larger than 1cm because the layer of origin and probable diagnosis of the lesion would have a decisive role in the next steps of management. Lesions not originating from the gastrointestinal wall (extramural lesions) are beyond the scope of this study and should be ruled out at the first step. Anechoic or hyperechoic lesions originating from 2nd/3rd layers of the gastrointestinal tract are strongly suggestive of benign characteristics, so they do not require further surveillance.
The presence of symptoms or ongoing growth of lesions larger than 1cm originating from 2nd/3rd layer of the gastrointestinal wall with hypoechoic echotexture definitely necessitates resection. In contrast, asymptomatic lesions larger than 10mm originating from 2nd/3rd layer of the gastrointestinal wall with hypoechoic echotexture are suggested to be precisely evaluated via bite-on-bite biopsy, EUS-tissue acquisition or MIAB (with or without ligation). Further decisions would be made based on the pathology.
Symptomatic lesions rooted from the 4th layer of the gastrointestinal tract are resected endoscopically or surgically. Since manipulation of GISTs is a major source of bleeding, a biopsy is recommended to be avoided, and complete resection of the lesion is suggested to be considered (40, 41). Otherwise, tissue acquisition through EUS or MIAB is recommended for asymptomatic lesions originating from the 4th layer, which are larger than 20mm or measuring about 10-20mm with features of malignant potential like irregular borders, ulceration, cystic spaces, and heterogenous echotexture (42). Asymptomatic lesions measuring about 10-20mm without high-risk stigmata and lesions smaller than 10mm, only require regular endoscopic surveillance at 6-12-month intervals (43).
Lesions larger than 10mm with a definite diagnosis
Documented histology for heterotopic pancreas, leiomyoma, schwannoma, lipoma, granular cell tumor, and glomus tumor in asymptomatic patients obviates the need for resection and follow-up when the risk of complication or malignancy is low. Studies show that small gastric GISTs (smaller than 20mm) do not harbor a risk of malignancy, so surveillance with EUS instead of treatment is an accepted approach. However care should be taken for non-compliant patients who are candidates for long-term follow-up (44, 45). For extra-gastric GISTs, resection is generally suggested independent of their size. Surveillance might only be suggested for patients with comorbidities and patients who do not accept surgery.
Management: endoscopic treatment options
The treatment of goal in subepithelial lesions is complete resection of the lesion with negative borders (6). The best resection technique should be chosen depending on size, involved layer, and location of the lesions. The accepted endoscopic techniques are endoscopic submucosal resection (ESMR), Retract-ligate-unroof-biopsy (RLUB), submucosal tunneling endoscopic resection (STER), endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR) and Submucosal tunneling endoscopic resection (STER). Some revised endoscopic mucosal resections with and without ligation (EMR±L) have also been introduced (46). As explained below, these techniques are applicable for gastric, esophageal, non-ampullary duodenal and colorectal lesions.
ESMR is applicable for lesions with maximum invasion of the submucosa (47). It should be noted that utilization of this method for duodenal lesions and lesions with invasion to muscularis propria might put patients at risk for perforation, tumoral cell seeding, and residual disease. Initial suction and ligation of the lesion with a band or cap (for resection of small lesions) or endo-loop (for larger lesions) could be added to the technique. However, it should be added that resection of lesions larger than 20mm is controversial using the ESMR technique (48).
RLUB is a new technique for larger than 20mm non-pedunculated stromal tumors, especially for patients who are appropriate for surgical intervention. This technique needs simultaneous usage of grasper and loop independently. To achieve this purpose, it is recommended to provide a double-channel endoscope. It retracts the lesion with a 3-branched grasper while implementing the first loop. The subepithelial tumor would be exposed using 2 crosscut incisions. Then, excision of the lesion is performed. Loop-over loop technique might lead to both tumor ischemia and better enucleation of the lesion (49).
Although using ESD is accompanied by a deeper access to the lesion leading to en-bloc resection with clear margins, its usage is confined to lesions smaller than 50mm. In the presence of adherence to the underlying muscularis propria, complete resection often fails due to capsule rupture and the spread of tumor cells (50).
Resection of larger lesions (more than 20mm) and deeper lesions (involvement of muscularis propria) is challenging. EFTR is the technique of choice for these circumstances. But it should be noted that EFTR is also recommended for lesions not larger than 40-50mm. Three different techniques for EFTR are introduced, which are endoscopic full-thickness resection via through-the-scope (TTS) clip, surgical and endoscopic (hybrid) full-thickness resection, and endoscopic full-thickness resection via over-the-scope (OTS) clip (51).
STER has also been introduced as an endoscopic technique for resectioning deep SELs involving the muscularis propria located in the middle/distal parts of the esophagus and gastric cardia where the endoscope is straight. The mucosal incision is performed about 50mm away from the lesion; then the scope is advanced to the submucosal space. Submucosal dissection and en-bloc resection of the lesion were performed when the lesion was seen in the channel using the ESD method. In this technique, the overlying mucosa remains intact. Finally, the defect finally is closed after complete resection of the tumor (51).
Management: surveillance after resection
Complete resection of the lesion
Complete resection of SELs that are not malignant is sufficient, so no surveillance is required after resection of these lesions except for gastric NENs related to atrophic gastritis, in which surveillance is considered at 1-2-year intervals (6). Malignant lesions and their surveillance are outside the area of the present study.
Incomplete resection of the lesion
Having resected SELs, including NENs and GISTs with positive or blurred margins, ESGE recommends re-endoscopic resection of residual tissue in 3–6 months. Surveillance should be followed based on the malignant potential of the lesion if efforts for surgical and endoscopic resection of the lesions failed (6). Surveillance of malignant lesions is beyond the context of this study.
Conclusion
Subepithelial lesions are incidentally discovered protrusions throughout the gastrointestinal tract with normal-appearing mucosa. They commonly originate from the second, third, and fourth layers of the gastrointestinal wall. Studies that are approaching these lesions are controversial. In this review, we have presented the most practical approach to managing gastrointestinal subepithelial lesions based on current guidelines.
Conflict of interests
There is no conflict of interest for authors of this article.
References
- 1.Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: Comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479–89. doi: 10.1111/den.12149. [DOI] [PubMed] [Google Scholar]
- 2.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 3.Lim YJ, Son HJ, Lee JS, Byun YH, Suh HJ, Rhee PL, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010;16:439–44. doi: 10.3748/wjg.v16.i4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon L, Buscaglia JM. Endoscopic approach to subepithelial lesions. Ther Adv Gastroenterol. 2014;7:123–130. doi: 10.1177/1756283X13513538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806–17. doi: 10.3748/wjg.v24.i26.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deprez PH, Moons LMG, OʼToole3 D, Gincul R, Seicean A, Pimentel-Nunes P. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412–429. doi: 10.1055/a-1751-5742. [DOI] [PubMed] [Google Scholar]
- 7.Holman GA, Parasher G. Extra-Pancreatic Pancreatitis: A rare cause of abdominal pain. Dig Dis Sci. 2014;59:1714–1716. doi: 10.1007/s10620-014-3269-1. [DOI] [PubMed] [Google Scholar]
- 8.Law YY, Patel R, Cusick M, Van Eps JL. A case of colonic intussusception and obstruction secondary to giant colonic lipoma. J Surg Case Rep. 2020;2020:rjaa429. doi: 10.1093/jscr/rjaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–62. [PubMed] [Google Scholar]
- 10.Kang JH, Lim JK, Jie H, Hyung W, Chung Y, Choi jy, et al. Role of EUS and MDCT in the diagnosis of gastric submucosal tumors according to the revised pathologic concept of gastrointestinal stromal tumors. Eur Radiol. 2009;19:924–934. doi: 10.1007/s00330-008-1224-2. [DOI] [PubMed] [Google Scholar]
- 11.Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722–727. doi: 10.1016/j.gie.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 12.He G, Wang J, Chen B, Xing X, Wang J, Chen J, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc. 2016;30:4206–4213. doi: 10.1007/s00464-015-4729-1. [DOI] [PubMed] [Google Scholar]
- 13.Bhat YM, Weilert F, Fredrick RT, Shah JN, Hamerski CM, Binmoeller KF, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U S experience over 6 years. Gastrointest Endosc. 2016;83:1164–1172. doi: 10.1016/j.gie.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 14.Sadeghi A, Shahrbaf MA, Asadzadeh Aghdaei H, Esmaeilinejad K, Zali MR. A rare presentation of simple renal cyst: gastrointestinal obstruction. Gastroenterol Hepatol Bed Bench. 2018;11:359–362. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H-T, Xu G-Q, Teng X-D, Chen YP, Chen LH, Li YM. Diagnostic accuracy of endoscopic ultrasonography for rectal neuroendocrine neoplasms. World J Gastroenterol. 2014;20:10470–7. doi: 10.3748/wjg.v20.i30.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q-L, Zhang Y-Q, Chen W-F, Xu MD, Zhong YS, Ma LL, et al. Endoscopic submucosal dissection for foregut neuroendocrine tumors: an initial study. World J Gastroenterol. 2012;18:5799–806. doi: 10.3748/wjg.v18.i40.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kibria R, Butt S, Ali SA, Akram S. An Unusual Case of Giant Gastric Lipoma with Hemorrhage. J Gastrointest Cancer. 2009;40:144–5. doi: 10.1007/s12029-009-9095-6. [DOI] [PubMed] [Google Scholar]
- 18.Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video) Gastrointest Endosc. 2012;75:276–86. doi: 10.1016/j.gie.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Rösch T, Lorenz R, Dancygier H, von Wickert A, Classen M. Endosonographic diagnosis of submucosal upper gastrointestinal tract tumors. Scand J Gastroenterol. 1992;27:1–8. doi: 10.3109/00365529209011157. [DOI] [PubMed] [Google Scholar]
- 20.Kim GH, Park DY, Kim S, Kim DH, Kim DH, Choi CW, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009;15:3376–81. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan S, Zhang R, Fang W, Wang T, Li S, Wang D, et al. Reliability of endoscopic ultrasound using miniprobes and grayscale histogram analysis in diagnosing upper gastrointestinal subepithelial lesions. Gastroenterol Res Pract. 2020;2020:6591341. doi: 10.1155/2020/6591341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji Y, Kusano C, Gotoda T Itokawa F, Fukuzawa M, Sofuni A, et al. Diagnostic potential of endoscopic ultrasonography-elastography for gastric submucosal tumors: A pilot study. Dig Endosc. 2016;28:173–8. doi: 10.1111/den.12569. [DOI] [PubMed] [Google Scholar]
- 23.Tang JY, Tao KG, Zhang LY, Wu KM, Shi J, Zeng X, et al. Value of contrast-enhanced harmonic endoscopic ultrasonography in differentiating between gastrointestinal stromal tumors: a meta-analysis. J Dig Dis. 2019;20:127–34. doi: 10.1111/1751-2980.12710. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–37. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147–52. doi: 10.1016/j.gie.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–19. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750–755. doi: 10.1055/s-2007-1001416. [DOI] [PubMed] [Google Scholar]
- 28.Osoegawa T, Minoda Y, Ihara E, Komori K, Aso A, Goto A, et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: a randomized cross-over study. Dig Endosc. 2019;31:413–21. doi: 10.1111/den.13367. [DOI] [PubMed] [Google Scholar]
- 29.Cho JW. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc. 2016;49:235–240. doi: 10.5946/ce.2015.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanaei O, Fernández-Esparrach G, De La Serna-Higuera C, Carrara S, Kumbhari V, El Zein MH, et al. EUSguided 22-gauge fine needle biopsy versus single-incision with needle knife for the diagnosis of upper gastrointestinal subepithelial lesions: a randomized controlled trial. Endosc Int Open. 2020;8:266–73. doi: 10.1055/a-1075-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhaliwal A, Kolli S, Dhindsa BS, Devani K, Ramai D, Sayles H, et al. Clinical efficacy and safety of mucosal incision-assisted biopsy for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:155–61. doi: 10.20524/aog.2020.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckardt AJ, Jenssen C. Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: optimal or optional? Ann Gastroenterol. 2015;28:160–72. [PMC free article] [PubMed] [Google Scholar]
- 33.Kim GH, Cho YK, Kim EY, Kim HK, Cho JW, Lee TH, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonographyguided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347–54. doi: 10.3109/00365521.2013.867361. [DOI] [PubMed] [Google Scholar]
- 34.Okuwaki K, Masutani H, Kida M, Yamauchi H, Iwai T, Miyata E, et al. Diagnostic efficacy of white core cutoff lengths obtained by EUS-guided fine-needle biopsy using a novel 22G franseen biopsy needle and sample isolation processing by stereomicroscopy for subepithelial lesions. Endosc Ultrasound. 2020;9:187–92. doi: 10.4103/eus.eus_18_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilani SM, Muniraj T, Aslanian H, Cai G. Endoscopic ultrasoundguided fine needle aspiration cytology diagnosis of upper gastrointestinal tract mesenchymal tumors: Impact of rapid onsite evaluation and correlation with histopathologic follow-up. Diagn Cytopathol. 2021;49:203–10. doi: 10.1002/dc.24631. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Kim JY, Oh EH, Yoo C, Park IJ, Yang DH, et al. Chromogranin A expression in rectal neuroendocrine tumors is associated with more aggressive clinical behavior and a poorer prognosis. Am J Surg Pathol. 2020;44:1496–505. doi: 10.1097/PAS.0000000000001526. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Liu Q. Prognostic indicators for gastrointestinal stromal tumors: a review. Transl Oncol. 2020;13:100812. doi: 10.1016/j.tranon.2020.100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: Biochemical markers. Neuroendocrinology. 2017;105:201–11. doi: 10.1159/000472254. [DOI] [PubMed] [Google Scholar]
- 39.Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103:139–43. doi: 10.1159/000443166. [DOI] [PubMed] [Google Scholar]
- 40.Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–30. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29:281–93. doi: 10.21147/j.issn.1000-9604.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akahoshi K, Oya M, Koga T, Koga H, Motomura Y, Kubokawa M, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014;23:405–12. doi: 10.15403/jgld.2014.1121.234.eug. [DOI] [PubMed] [Google Scholar]
- 43.Song JH, Kim SG, Chung S, Kang HY, Yang SY, Kim YS. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy. 2015;47:675–9. doi: 10.1055/s-0034-1391967. [DOI] [PubMed] [Google Scholar]
- 44.Kushnir VM, Keswani RN, Hollander TG, Kohlmeier C, Mullady DK, Azar RR, et al. Compliance with surveillance recommendations for foregut subepithelial tumors is poor: results of a prospective multicenter study. Gastrointest Endosc. 2015;81:1378–84. doi: 10.1016/j.gie.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Dumonceau J-M, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated January 2017. Endoscopy. 2017;49:695–714. doi: 10.1055/s-0043-109021. [DOI] [PubMed] [Google Scholar]
- 46.Asadzadeh Aghdaei H, Sadeghi A, Ghorbanpour A, Nouri GR, Dooghaie Moghadam A, Azizi MR, et al. A new endoscopic submucosal resection -ligation technique for gastric tumors. Gastroenterol Hepatol Bed Bench. 2020;13:149–53. [PMC free article] [PubMed] [Google Scholar]
- 47.Kim GH. Endoscopic resection of subepithelial tumors. Clin Endosc. 2012;45:240–4. doi: 10.5946/ce.2012.45.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SY, Kim KO. Endoscopic treatment of subepithelial tumors. Clin Endosc. 2018;51:19–27. doi: 10.5946/ce.2018.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binmoeller KF, Shah JN, Bhat YM, KaneBS SD. Retract-ligate-unroof-biopsy: a novel approach to the diagnosis and therapy of large nonpedunculated stromal tumors (with video) Gastrointest Endosc. 2013;77:803–8. doi: 10.1016/j.gie.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Ye LP, Mao XL. Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol. 2015;28;21:9503–11. doi: 10.3748/wjg.v21.i32.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Modayil R, Criscitelli T, Stavropoulos SN. Endoscopic resection for subepithelial lesions-pure endoscopic full-thickness resection and submucosal tunneling endoscopic resection. Transl Gastroenterol Hepatol. 2019; 4:39. doi: 10.21037/tgh.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]