Abstract
A conserved 80-kDa minor outer membrane protein, D15, of Haemophilus influenzae has been shown to be a protective antigen in laboratory animals against H. influenzae type a (Hia) or type b (Hib) infection. To localize the protective B-cell epitope(s) within the D15 protein and to further explore the possibility of using synthetic peptides as vaccine antigens, a 20-kDa N-terminal fragment of D15 protein (truncated D15 [tD15]) was expressed as a fusion protein with glutathione S-transferase in Escherichia coli. The tD15 moiety was cleaved from glutathione S-transferase by using thrombin and purified to homogeneity. The purified soluble tD15 appeared to contain immunodominant protective epitope(s) against Hia and Hib, since rabbit antisera directed against tD15 were capable of protecting infant rats from Hia or Hib bacteremia. The ease of purification of soluble tD15, therefore, makes it a better candidate antigen than the full-length recombinant D15 which is produced as inclusion bodies in E. coli. Furthermore, both the purified tD15 fragment and a mixture of tD15-derived peptides spanning amino acid residues 93 to 209 of the mature D15 protein were capable of inhibiting the protection against Hib conferred on infant rats by rabbit anti-tD15 antiserum, indicating that the protective epitopes of D15 may not be conformational. However, the administration of pooled rabbit immune sera raised against the same panel of peptides failed to protect infant rats from Hib infection.
Haemophilus influenzae type b (Hib) is a major cause of bacterial meningitis in children under the age of 5 years (9, 12). The current conjugate vaccines based on the capsular polysaccharide, polyribosyl ribitol phosphate (PRP), selectively protect against Hib-related meningitis (15, 17, 22). However, they do not protect against other invasive typeable strains (such as types a and c) or, more importantly, against nontypeable H. influenzae (NTHI) strains, which are a common cause of neonatal sepsis and otitis media in children (5, 11, 27). To achieve broader protection against various H. influenzae-related diseases, a conserved, cross-protective H. influenzae immunogen is required. One potential antigen is the 80-kDa minor outer membrane protein of H. influenzae, D15, which was detected in all 36 typeable and NTHI isolates tested (29). This protein is expressed on the bacterial surface, and D15-specific antibodies are detected in sera from children who have recovered from H. influenzae meningitis infection (29). In addition, affinity-purified antibodies prepared against native D15 were shown to be protective in the infant rat model of bacteremia (29). In a recent study (18), rabbit antisera raised against a recombinant full-length D15 (rD15) expressed in Escherichia coli were shown to cross-react with H. influenzae serotypes a, b, c, d, e, and f and NTHI. Furthermore, these antisera were also capable of protecting infant rats against bacteremia caused by Hib or H. influenzae type a (Hia). Thus, D15 appears to be an attractive candidate antigen to be included in a vaccine that would provide broader protection against H. influenzae-related diseases.
In recent years, methods for inducing immunity against disease have been constantly improving, and there is presently an interest in using subunits or synthetic peptides as vaccine antigens (1, 20, 26). Well-defined subunit vaccines are more attractive today than whole-cell vaccines. The advantages associated with a synthetic peptide vaccine approach are the elimination of undesirable side effects due to irrelevant epitopes and the possibility of producing vaccines with built-in adjuvanticity. Several bacterial proteins, in particular bacterial toxins, appear to be successful targets for this type of approach (1). Indeed, synthetic fragments of streptococcal M protein (10), diphtheria toxin (2, 3), and cholera toxin (13, 14) were capable of inducing neutralizing antibodies and protective immunity against their representative pathogens. Since the results obtained from earlier studies with D15 were very promising (18), the present study was undertaken to evaluate the protective ability of an amino-terminal D15 fragment (truncated D15 [tD15]; 20 kDa) against bacteremia caused by Hia or Hib and to explore the possibility of using tD15-derived peptides in a synthetic vaccine. tD15 was found to contain protective epitope(s) against both Hia and Hib, since rabbit anti-tD15 antisera protected infant rats against bacteremia induced by Hia or Hib as efficiently as rabbit antisera raised against the entire molecule (18). Furthermore, the protection with anti-tD15 antisera could be blocked by absorption with either purified tD15 protein or a mixture of synthetic peptides derived from the tD15 sequence.
(These data were presented in part at the 94th General Meeting of the American Society for Microbiology, held in Las Vegas, Nev., on 23 to 27 May 1994.)
MATERIALS AND METHODS
Bacterial strains, vectors, and growth conditions.
E. coli BTA282, used for lysogenic growth of recombinant bacteriophage lambda, and the cloning vector lambda gt11 Amp1 have been previously described (4, 28). Hib strain MinnA was kindly provided by R. S. Munson, Jr. (Children’s Hospital Research Foundation, Columbus, Ohio). Hia strain ATCC 9006 was purchased from the American Type Culture Collection. Hia, Hib, and NTHI were grown as described earlier (18).
Construction and expression of a GST-tD15 fusion protein.
Purification of genomic DNA from Hib strain Ca and cloning of the D15 gene were described previously (28). A forward sense primer (5′GGGGAATTCCAAAAGATGTTCGT3′) and a reverse antisense primer (5′CACGAATTCCCTGCAAATC3′) were used to amplify a 600-bp fragment of Hib DNA (Ca isolate) by PCR. This fragment encodes amino acid residues 25 to 220 of the D15 protein (Fig. 1). The PCR product was purified, digested with EcoRI, ligated into the expression vector pGEX-2T, and then transformed into E. coli TG-1. Colonies expressing tD15 linked to the C terminus of glutathione S-transferase (GST) were screened by colony radioimmunoassay with a rabbit affinity-purified anti-D15 antibody (28). Transformed E. coli cells were grown in YT medium to an A578 of 0.3, and isopropyl β-d-thiogalactopyranoside (Sigma) was added to 100 μM for 2 h. Cells from a 2-liter culture were harvested by centrifugation at 10,000 × g for 10 min at 4°C and used to purify the GST-tD15 fusion protein.
FIG. 1.
Amino acid sequence of tD15, based on the nucleotide sequence of the D15 gene obtained by Flack et al. (12). The signal peptide cleavage site, the expected starting position of the GST-tD15 fusion, a cryptic thrombin cleavage site, and the termination of tD15 are all indicated with arrows. The underlined sequences represent the synthetic tD15 peptides (peptides 2 to 9).
Purification of the GST-tD15 fusion protein from E. coli.
Purification of the GST fusion protein was performed as previously described by Smith and Johnson (25), with some modifications. Briefly, the cell pellet from 2 liters of culture was sonicated for 10 min with 20 ml of phosphate-buffered saline (PBS; pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 0.02 mg of soybean trypsin inhibitor ml−1. The homogenate was centrifuged at 10,000 × g for 10 min. The supernatant was saved, and the pellet was subjected to a second extraction. The two supernatants were combined, and Triton X-100 was added to a final concentration of 1%. The combined supernatant was loaded onto a 4-ml glutathione-Sepharose 4B column (Pharmacia) equilibrated in PBS containing 1% Triton X-100. The column was washed with 50 ml of PBS–1% Triton X-100. The GST-tD15 fusion protein was eluted with 50 mM Tris-HCl (pH 8.0) containing 5 mM glutathione. The purity of the protein was assessed by discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) through an SDS–12.5% polyacrylamide gel as described by Laemmli (16), and proteins were visualized by using Rapid Coomassie Blue (Diversified Biotech).
Isolation of tD15 from the GST-tD15 fusion protein.
The purified GST-tD15 fusion protein solution (30 ml) was concentrated in Centriprep 10 concentrators (Amicon) to about 5 ml at 4°C. The solution was dialyzed against 50 mM Tris-HCl (pH 8.5) at 4°C overnight and then treated with 25 U of human thrombin (T-3010; Sigma) per ml of solution at 37°C for 2 h. The reaction was stopped by placing the solution on ice until it was loaded directly onto a glutathione-Sepharose 4B affinity column (2 ml) equilibrated with PBS containing 1% Triton X-100. The tD15 fragment was collected in the run-through fraction. The column was washed with 20 ml of PBS, and GST was eluted with 50 mM Tris-HCl (pH 8.0) containing 5 mM glutathione. The purity of tD15 was determined by SDS-PAGE. Purified GST purchased from Pharmacia was used as a standard. Protein concentrations were determined by the bicinchoninic acid protein assay (Pierce), using bovine serum albumin (BSA; Sigma) as a standard.
Peptide synthesis.
Eight peptides covering the entire tD15 sequence (Fig. 1) were synthesized with an automated ABI 430A peptide synthesizer using optimized tert-butoxycarbonyl chemistry as described previously (7).
Protein sequence analysis.
Chromatographically purified tD15 and GST were separated by SDS-PAGE through 12.5% acrylamide slab gels. The proteins were transferred to Immobilon polyvinylidene difluoride membranes (Millipore) and subjected to Edman degradation in an Applied Biosystems 477A protein sequenator.
CD measurements.
The far-UV circular dichroism (CD) spectrum of the tD15 protein was recorded at room temperature on a Jasco J-720 spectropolarimeter, using a 0.2-cm-light-path cell at a protein concentration of 300 μg ml−1 in PBS containing 0.25% Triton X-100. The spectrum was scanned four times at a rate of 100 nm min−1 from 180 to 260 nm, and the signals were averaged. Mean residue ellipticity data are expressed in degrees square centimeter/decamole. The secondary structure composition of tD15 was estimated by using the program provided by Jasco.
Immunization protocols.
Antisera against tD15 were produced by immunizing two New Zealand White rabbits (Charles River) intramuscularly with 20-μg doses of antigen emulsified in complete Freund’s adjuvant (Difco) on day 1. Animals were boosted on day 28 with another 15-μg dose of protein in incomplete Freund’s adjuvant, and sera were collected on day 42. Anti-Hib strain MinnA and anti-Hia strain ATCC 9006 antisera were generated by using the same protocol except that a heat-inactivated bacterial preparation was used as the immunogen (108 CFU per dose).
To produce antipeptide antisera, two New Zealand White rabbits for each peptide were immunized intramuscularly with 100 μg of antigen emulsified in complete Freund’s adjuvant. Animals were boosted with the same amount of peptide in incomplete Freund’s adjuvant 4 and 6 weeks after priming. Serum samples were collected 2 weeks after the last injection. Equal volumes of serum from the two animals were pooled for infant rat protection studies.
Immunoassays.
Microtiter wells (Nunc-MAXISORP; Nunc, Roskilde, Denmark) were coated with 100 ng of purified tD15 for 16 h at room temperature. The plates were then blocked with 0.1% (wt/vol) BSA in PBS. The sera were serially diluted, added to the wells, and then incubated for 1 h at room temperature. Affinity-purified F(ab′)2 fragments of goat anti-rabbit immunoglobulin G (IgG; Fc specific) conjugated to horseradish peroxidase (Jackson Laboratories) were used as secondary antibodies. The reactions were developed by using tetramethylbenzidine and hydrogen peroxide (ADI), and absorbancies were measured at 450 nm (using 540 nm as a reference wavelength) with a Flow Multiskan MCC microplate reader (ICN). Samples were tested in duplicate. The reactive titer of an antiserum was defined as the reciprocal of the dilution consistently showing a twofold increase in absorbancy over that obtained from the prebleed serum sample.
For peptide-specific enzyme immunoassays (EIAs), microtiter wells were coated with 500 ng of individual peptides. Two pertussis toxin peptides, NAD-S1 (GALATYQSEYLAHRRIPP) and S3-P6 (FVRDGQSVIGACASPYEGRYRDMYDALRRLLY), were included as negative controls. Assays were performed in triplicate, and reactive titers were defined as specified above.
Competitive inhibition assays.
To evaluate the ability of tD15 peptides or tD15 to inhibit the binding of anti-tD15 antibodies to tD15 adsorbed to microtiter wells, two rabbit antisera were adjusted to a concentration corresponding to 50% of the maximum binding to tD15 in EIA and preincubated for 1 h at room temperature with increasing amounts of either D15 peptides or tD15 or with a mixture of 13 peptides derived from H. influenzae outer membrane protein P2 used as a negative control (7). Microtiter wells were coated with 50 ng of tD15 for 16 h at room temperature. Uncoated sites were blocked with 0.1% BSA in PBS. The antigen-antibody mixtures were added to the wells, and the plates were incubated for 1 h at room temperature. Affinity-purified F(ab′)2 fragments of goat anti-rabbit IgG (Fc specific) conjugated to horseradish peroxidase (Jackson Laboratories) were used as a secondary antibody. The remaining steps were as described above for immunoassays. The percent inhibition was calculated from the absorbancy (A) of the antiserum by using the following formula: percent inhibition = [A (antiserum) − A (x)]/A (antiserum), where x is the antiserum mixture containing either tD15 or D15 peptides.
Infant rat protection studies.
The infant rat protection studies were performed as described elsewhere (7). Pregnant Wistar rats were purchased from Charles River. Antilipopolysaccharide and anti-PRP antibodies were removed from rabbit anti-tD15 antisera by adsorption with lipopolysaccharide-Sepharose and PRP-Sepharose prepared as described by Munson et al. (19). In the Hib bacteremia model, groups of 7 to 10 5-day-old infant rats were injected subcutaneously (s.c.) in the dorsal region with 0.1 or 0.2 ml of the antiserum to be tested. The control animals received injections with preimmune sera or PBS only. Twenty hours later, the animals were challenged intraperitoneally (i.p.) with 200 CFU of freshly grown Hib strain MinnA (0.1 ml). Blood samples were collected 20 h postchallenge via cardiac puncture under isoflurane anesthesia and plated on chocolate agar plates. Colonies were counted after 1 day, and the results were statistically analyzed by Fisher’s exact test.
In the Hia bacteremia model, groups of nine five-day-old infant rats were injected s.c. in the dorsal region with 0.1 ml of rabbit anti-tD15 or anti-Hia strain ATCC 9006 antiserum. The animals in the control group were injected with rabbit preimmune serum. Twenty hours later, the animals were challenged i.p. with 105 CFU of freshly grown Hia strain ATCC 9006 (0.1 ml). Blood samples were collected 20 h postchallenge and analyzed as described above.
RESULTS
Purification of tD15.
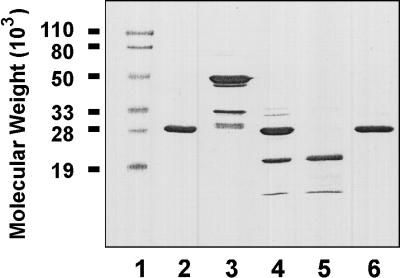
A recombinant D15 fragment of about 20 kDa, encompassing amino acid residues 25 to 220 of the full-length D15 protein according to the D15 gene sequence obtained by Flack et al. (12), was expressed in E. coli as a soluble protein fused to GST (26 kDa) (Fig. 1). The fusion protein (46 kDa) was readily extracted by sonicating the cells in PBS containing protease inhibitors. As shown in Fig. 2, purification of the GST-tD15 fusion protein was achieved by glutathione-Sepharose 4B affinity chromatography (lane 3). Cleavage of the 46-kDa fusion protein with thrombin yielded two major fragments (lane 4), a 26-kDa protein corresponding to purified GST (lane 2) and a 20-kDa protein with the expected size of tD15. The 20-kDa protein was recovered in the run-through fraction (lane 5). Bound GST was eluted with 50 mM Tris-HCl (pH 8.0) containing 5 mM glutathione (lane 6). Approximately 1 mg of purified tD15 was recovered from 1 liter of bacterial culture.
FIG. 2.
SDS-PAGE analysis of tD15 purification, performed on a 12.5% gel. Lane 1, prestained protein molecular weight markers; lane 2, purified GST; lane 3, GST-tD15 fusion protein eluted from glutathione-Sepharose 4B column; lane 4, GST-tD15 fusion protein cleaved by thrombin; lane 5, tD15 recovered in the run-through fraction from the glutathione-Sepharose 4B column; lane 6, GST eluted from the glutathione Sepharose 4B column.
Characterization of tD15.
The identities of the 20- and 26-kDa proteins were determined by protein sequencing. The N-terminal sequence of the 20-kDa protein was found to be NH2-Ser-Leu-Phe-Val-Ser-Gly-Arg-Phe-Asp-Asp-Val-Lys- Ala-His-Gln-Glu-Gly-Asp-Val-Leu-Val-Val-Ser- and to correspond to the sequence starting 38 residues downstream from the predicted N terminus of tD15. This finding indicated that an unexpected thrombin cleavage site was located near the N terminus of D15 (Fig. 1). The N-terminal sequence of the 26-kDa protein (NH2-Met-Ser-Pro-Ile-Leu-Gly-Tyr-Trp-Lys-) confirmed that it was GST (24, 25). The minor, low-molecular-weight protein band in the purified tD15 preparation (Fig. 1, lane 5) was found to be a degradation product of tD15 by immunoblot analysis using a monoclonal anti-tD15 antibody (data not shown). The N-terminal region of D15 is antigenically conserved among Hib and NTHI isolates, since rabbit antiserum raised against purified tD15 recognized an 80-kDa protein in all 11 Hib and 11 NTHI isolates tested by immunoblot analysis (data not shown).
The secondary structure of tD15 was analyzed by CD. The far-UV CD spectrum of tD15 exhibited the characteristics of an α-helical protein as judged by the presence of two ellipticity minima at 208 and 222 nm, respectively (data not shown). The α-helix content for tD15 was established to be 25%.
Protective ability of tD15 against Hib.
The protective ability of tD15 against a live Hib challenge was examined in the infant rat model of bacteremia. As illustrated in Table 1, in the group of 10 infant rats passively immunized with rabbit anti-tD15 antisera, only two animals became bacteremic after challenge, whereas all animals in the control groups (10 immunized with preimmune serum and 9 immunized with PBS) were infected. These results were consistent with those obtained in assays using rabbit anti-rD15 antisera in the same animal model (18).
TABLE 1.
Protective effect of passively transferred rabbit anti-tD15 antiserum against Hib in the infant rat model of bacteremiaa
| Group | Rabbit serum | Anti-tD15 antibody titer | No. bacteremic/no. challenged | Mean CFU/2.5 μl of bloodb |
|---|---|---|---|---|
| 1 | Anti-tD15 | 51,200 | 2/10 | 30 |
| 2 | Preimmune | <200 | 10/10 | 700 |
| 3 | PBS | <200 | 9/9 | 1,250 |
Five-day-old infant rats were passively immunized s.c. with 0.1 ml of rabbit antiserum, immune serum, or PBS. Twenty hours later, the animals were challenged i.p. with freshly grown Hib strain MinnA (200 CFU in 0.1 ml) as described in Materials and Methods. The statistical significance of the difference in results obtained between antisera and preimmune sera (P = 0.0007) was established by Fisher’s exact test.
Duplicate plating.
Protective ability of tD15 against Hia.
The protective ability of tD15 against a live Hia challenge was also tested in a newly developed infant rat model of bacteremia using Hia strain ATCC 9006 (18). As shown in Table 2, none of the nine animals which received rabbit anti-Hia antiserum (group 1) or rabbit anti-tD15 antiserum (group 2) developed bacteremia after live Hia challenge (P = 0.00004). These results demonstrate that the passive transfer of anti-tD15 antibodies is as efficacious as that of anti-D15 antibodies (18) at protecting animals against bacteremia caused by Hia.
TABLE 2.
Protective effect of passively transferred rabbit anti-tD15 antiserum against Hia in the infant rat model of bacteremiaa
| Group | Rabbit serum | Anti-tD15 antibody titer | No. bacteremic/9 challenged | Mean CFU/25 μl of blood | Pb |
|---|---|---|---|---|---|
| 1 | Anti-Hia | 6,400 | 0 | 0 | 0.00004 |
| 2 | Anti-tD15 | 51,200 | 0 | 0 | 0.00004 |
| 3 | Preimmune | <200 | 9 | 190 |
Five-day-old infant rats (nine per group) were passively immunized s.c. with 0.1 ml of indicated rabbit antiserum or preimmune serum. Twenty hours later, the animals were challenged i.p. with freshly grown Hia strain ATCC 9006 (105 CFU, 0.1 ml) as described in Materials and Methods.
Statistical significance of the difference in results obtained between antisera and preimmune sera, established by the Fisher exact test.
Competitive inhibition studies.
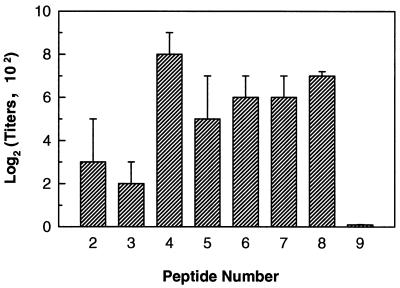
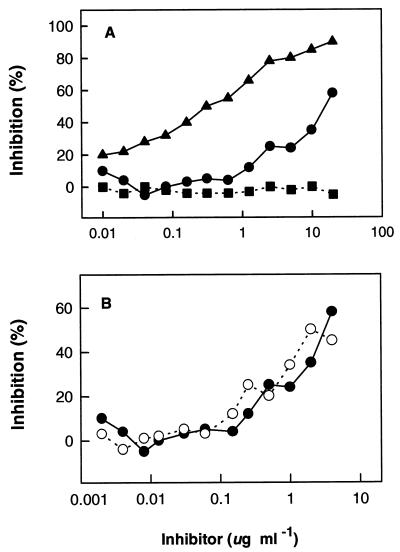
To identify the protective epitope(s) within tD15, eight overlapping peptides were chemically synthesized (Fig. 1). These synthetic peptides were tested for their EIA reactivities with two rabbit antisera raised against purified tD15. Figure 3 shows that the anti-tD15 antisera strongly reacted with peptides 4 to 8 (covering residues 93 to 209). The ability of purified tD15 protein and two mixtures of tD15-derived peptides (peptides 2 to 9 and peptides 4 to 8) to inhibit the binding of rabbit anti-tD15 antibodies to solid-phase tD15 was evaluated in a competitive binding inhibition assay. As shown in Fig. 4, the tD15 protein and both peptide mixtures efficiently blocked the binding of anti-tD15 antibodies to tD15. At a concentration of 20 μg ml−1 (total protein or total peptide), the interaction of anti-D15 antibodies with tD15 was 90 and 60% inhibited by the purified tD15 and peptide mixture (peptides 4 to 8), respectively, whereas no inhibitory effect was observed with an irrelevant peptide mixture at the same concentration (Fig. 4A). When the concentration of each individual peptide was adjusted to the same level (i.e., from 2 ng ml−1 to 4 μg ml−1 for each peptide in the mixture), the replotted inhibition curves for the two peptide mixtures (peptides 2 to 9 and peptides 4 to 8) were almost superimposable (Fig. 4B), indicating that peptides 4 to 8 contain the most immunodominant linear B-cell epitopes of tD15.
FIG. 3.
EIA reactivities of rabbit anti-tD15 antisera with D15 synthetic peptides. Each value represents the mean IgG titer obtained from duplicate determinations on two rabbit sera tested individually. Bars represent the standard deviations of the four measurements.
FIG. 4.
Competitive inhibition of the binding of rabbit anti-tD15 antiserum to tD15-coated microtiter wells by tD15 (▴), D15 peptides 4 to 8 (•) and 2 to 9 (○), or a mixture of irrelevant peptides (13 peptides derived from outer membrane protein P2 sequence) (▪). (A) Rabbit antiserum (2 μl) was adjusted to a concentration corresponding to 50% saturation of plate-bound tD15 and then preincubated for 1 h at room temperature with increasing amounts of either tD15, tD15 peptides 4 to 8, or a mixture of P2 peptides used as a negative control (total protein or total peptide concentration ranged from 10 ng ml−1 to 20 μg ml−1). Each point represents the mean of duplicate determinations for two rabbit immune sera tested individually. (B) Replotting of the data for tD15 peptides 4 to 8 from panel A in comparison with the result obtained with tD15 peptides 2 to 9. The concentration of each peptide in both mixtures (peptides 4 to 8 and 2 to 9) was adjusted to the same level (i.e., from 2 ng ml−1 to 4 μg ml−1).
Characterization of protective epitopes.
Further studies were performed to determine whether the protection of infant rats against Hib observed by passive transfer of rabbit antibodies to tD15 could be suppressed by preincubation of the immune sera with either tD15 protein or a mixture of tD15 peptides. In this study, groups of seven infant rats were passively immunized with an unabsorbed rabbit anti-tD15 antiserum (Table 3, group 1) or with the same antiserum absorbed with either purified tD15 protein (group 2) or a mixture of tD15 peptides 4 to 8 (group 3). As shown in Table 3, the protection provided by rabbit anti-tD15 antiserum alone (group 1) was completely inhibited by preincubating the serum with purified tD15 (group 2). The difference in the number of protected animals between groups 1 and 2 was statistically significant (P = 0.0023). Furthermore, a mixture of five tD15-derived peptides (peptides 4 to 8) representing 59% of the tD15 sequence was also capable of partially inhibiting the protective ability of the anti-tD15 antiserum (group 3, P = 0.0291). Interestingly, the inhibitory effect of the tD15 peptide mixture on the mean bacterial count recovered from infected animals (760 CFU) was significantly lower than that observed with purified tD15 (3810 CFU). This observation is consistent with the fact that the same peptide mixture was less inhibitory (60%) in the in vitro competitive inhibition assay than the tD15 protein (90%) (Fig. 4A).
TABLE 3.
Inhibition of anti-tD15 antibody-induced protection by tD15 and D15-derived peptides in the infant rat model of bacteremiaa
| Group | Inhibitor | No. bacteremic/7 challenged | Mean CFU/2.5 μl of blood | Pb |
|---|---|---|---|---|
| 1 | PBS | 1 | 980 | |
| 2 | tD15 | 7 | 3,810 | 0.0023 |
| 3 | Peptides | 6 | 760 | 0.0291 |
Absorbed immune sera were prepared by mixing 0.5 ml of rabbit anti-tD15 antiserum with either purified tD15 protein (600 μg; group 2) or a mixture of five tD15 peptides (peptides 4 to 8, 250 μg of each peptide; group 3). The final volume of each mixture was adjusted to 1.5 ml with PBS. The mixture was rotated at room temperature for 1 h. Seven-day-old infant rats (seven per group) were injected s.c. with 0.2 ml of the indicated material. After 20 h, animals were challenged i.p. with freshly grown Hib strain MinnA (200 CFU) as described in Materials and Methods.
Statistical significance of the difference in results obtained between group 1 and the other groups, established by Fisher’s exact test.
Nevertheless, these results suggested that certain D15-derived synthetic peptides might be capable of inducing protective antibodies against Hib. Rabbit antisera were therefore raised against the five individual tD15 peptides (peptides 4 to 8). Although anti-tD15 antibodies (titer, 12,800) were detected by EIA in a pool of rabbit antisera directed against each individual peptide, none of the infant rats passively immunized with this antiserum pool was protected against a live Hib challenge (data not shown).
DISCUSSION
Previous studies have indicated that a high-molecular-weight membrane protein, D15, from H. influenzae is a candidate antigen for inclusion in a vaccine against H. influenzae-related diseases (29). A full-length recombinant D15 protein was recently expressed in E. coli and found to protect infant rats against bacteremia caused by Hib or Hia (18). The objectives of the present study were to map the protective epitope(s) of the D15 protein and explore the potential use of a mixture of synthetic peptides as a vaccine against H. influenzae.
Our laboratory had initially performed studies to map the linear B-cell epitopes of D15 by using 36 overlapping synthetic peptides covering the entire protein sequence. It was found that the peptides derived from the N-terminal half of D15 were strongly recognized by the antisera raised against the rD15 protein, whereas the peptides covering the C-terminal half of D15 had very weak, if any, reactivity with anti-rD15 antibodies. These preliminary results indicated that the N-terminus moiety of D15 contains most of its immunodominant linear B-cell epitopes and thus was a potential candidate vaccine. The current study was therefore designed to evaluate the immunoprotective ability of the D15 N-terminal fragment.
A 20-kDa, N-terminal D15 fragment was expressed as a GST-tD15 fusion protein and produced as a soluble protein in E. coli. The tD15 fragment cleaved from the GST fusion protein by thrombin appeared to retain a significant α-helical structure as estimated by CD analysis. This is an important feature since unfolded proteins may lose their immunogenicity and/or antigenicity.
A stretch of 38 amino acid residues was unexpectedly removed from the N terminus of tD15 by thrombin cleavage. Although the thrombin-specific cleavage site is located after an arginine residue (6), an adjacent serine residue is not the preferred amino acid for the enzyme activity. We speculate that the GST-tD15 fusion protein has an altered conformation resulting in the exposure of a cryptic thrombin cleavage site. The purified full-length rD15 (18) was also subjected to thrombin digestion under the same conditions. About 50% of the rD15 was cleaved into two major fragments, with apparent molecular masses of approximately 60 and 20 kDa, respectively. The N-terminal sequence of the 60-kDa fragment was found to be NH2-Ser-Ala-Arg-Ile-Ile-Gly-Asn-Leu-Gly-, identical to the sequence located 266 residues downstream of the N terminus of the mature protein (18). This cleavage site, although different from the cryptic thrombin cleavage within tD15, was also found to be located between an arginine and a serine residue. Analysis of the internal amino acid sequence of the 20-kDa fragment confirmed that it was indeed the N terminus of rD15. The finding that there are two different cryptic thrombin cleavage sites in rD15 and tD15 may be explained by the exposure of an additional cleavage site in the GST-tD15 fusion protein. Nevertheless, the truncated D15 was found to be an immunoprotective antigen since passive transfer of rabbit anti-tD15 antiserum protected 8 of 10 infant rats against Hib bacteremia and 9 of 9 infant rats against Hia bacteremia. This level of protection is similar to that obtained with an antiserum to the full-length rD15 (18), indicating that immunodominant protective B-cell epitopes against Hia and Hib are located within the D15 N-terminal fragment.
Consistent with the results obtained from the in vitro competitive inhibition binding assay, both purified tD15 protein and a mixture of tD15 peptides (peptides 4 to 8) were capable, although with different degrees of effectiveness, of inhibiting the protective ability of rabbit anti-tD15 antisera against Hib in vivo in the infant rat model of bacteremia. This finding suggests that some of the protective epitopes of tD15 are not conformational and are located between residues 93 and 209. However, rabbit antisera raised against peptides 4 to 8 failed to protect infant rats against Hib infection. There are several explanations for this observation. First, to date the theoretical basis for the selection of peptide sequences that are likely to encompass antigenic determinants and might be used to induce protective immunity is still rather crude. We synthesized overlapping peptides based on the hydrophilicity plot of tD15. The antibodies raised against these synthetic peptides might be different in specificity, affinity, or isotype from those elicited by purified tD15, in spite of the fact that rabbit anti-tD15 antisera reacted strongly with these peptides. Second, all of the peptides used in this study were injected into animals as adjuvanted free peptides. Although these peptides induced an immune response, indicating that they contained T-helper epitopes, they might have been more immunogenic had they been coupled to a carrier protein (2, 3, 13). Indeed, the administration of free synthetic diphtheria peptides failed to elicit an antibody response in mice. In contrast, when conjugated to a protein or a synthetic carrier, the same peptides induced not only high antibody titers but also protective antitoxic immunity (3). Whether D15 peptides conjugated to a protein carrier will induce protective antibodies remains to be determined. Third, the approach used in the infant rat study to test the protective ability of antipeptide antisera may have been suboptimal, since the antibodies generated against each individual peptide were diluted in the serum pool.
It was clear to us that the development of a consistently reproducible in vitro bactericidal assay would be of great utility in assessing the functionality of anti-rD15 and anti-tD15 antibodies. Unfortunately, none of the antisera, including those raised against PRP-T (PRP conjugated to tetanus toxoid) or against whole NTHI cells consistently exhibited bactericidal activity in the in vitro assay. Variability in results from in vitro bactericidal assays against H. influenzae has been observed by others and was discussed in detail in our earlier study of rD15 (18). Three main factors, namely, the source of complement, the strain of H. influenzae used, and the types of antibodies (titer, affinity, and source, etc.), largely affect the outcome of bactericidal activity toward this bacterium. The poor reproducibility of our bactericidal assays could be due to the lack of a correct combination of these factors. It was also suggested by other investigators that at least in some cases the opsonic rather than the bactericidal activity of complement is responsible for the clearance of Hib bacteremia (20, 23). This might explain why some antisera raised against PRP-T, whole H. influenzae cells, rD15 (18), or tD15 (as observed in this study) protect infant rats against Hib bacteremia even though they are not bactericidal in vitro. Clearly, this is a question which requires further study and clarification.
In summary, the present study describes the expression and purification of a truncated D15 fragment. Unlike the full-length recombinant D15 which is expressed as inclusion bodies in E. coli, the tD15 appears to be a soluble protein and thus does not require detergent or other denaturing agents for its purification. The expression of tD15 as a fusion protein linked to GST also has the advantage of easy purification. It was found that this 20-kDa N-terminal D15 fragment contains immunodominant B-cell epitopes against Hia and Hib and may represent a good candidate antigen for inclusion in a subunit vaccine to provide broader protection against H. influenzae-caused diseases. The immunoprotective ability of tD15 could not be mimicked with a mixture of synthetic tD15-derived peptides, even though the peptide cocktail was found to be capable of blocking the protection conferred by anti-tD15 antisera in infant rats.
ACKNOWLEDGMENTS
We thank the National Research Council Canada (Ottawa, Ontario, Canada) for providing the monoclonal antibody against tD15. S. Cockle, B. Rovinski, and D. Burt are acknowledged for critical review of the manuscript. We also thank D. Flemming, M. Haer, T. Olivier, D. Persaud, and W. Xu-Li for excellent technical assistance.
REFERENCES
- 1.Arnon R. Synthetic peptides as the basis for future vaccines. Trends Biochem Sci. 1986;11:521–524. [Google Scholar]
- 2.Audibert F, Jolivet M, Chedid L, Alouf J E, Boquet P, Rivaille P, Siffret O. Active antitoxic immunization by a diphtheria toxin synthetic oligopeptide. Nature. 1981;289:593–595. doi: 10.1038/289593a0. [DOI] [PubMed] [Google Scholar]
- 3.Audibert F, Jolivet M, Chedid L, Arnon R, Sela M. Successful immunization with a totally synthetic diphtheria vaccine. Proc Natl Acad Sci USA. 1982;79:5042–5046. doi: 10.1073/pnas.79.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berns C A, Thomas C A. Isolation of high molecular weight DNA from Haemophilus influenzae. J Mol Biol. 1965;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone C D, Klein J O. Otitis media with effusion, atelectasis, and eustachian tube dysfunction. In: Bluestone C D, Stool S E, editors. Pediatric otolaryngology. Philadelphia, Pa: The W. B. Saunders Co.; 1983. pp. 356–402. [Google Scholar]
- 6.Chang J-Y. Thrombin specificity. Eur J Biochem. 1985;151:217–224. doi: 10.1111/j.1432-1033.1985.tb09091.x. [DOI] [PubMed] [Google Scholar]
- 7.Chong P, Yang Y-P, Fahim R, McVerry P, Sia C, Klein M. Immunogenicity of overlapping synthetic peptides covering the entire sequence of Haemophilus influenzae type b outer membrane protein P2. Infect Immun. 1993;61:2653–2661. doi: 10.1128/iai.61.6.2653-2661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong P, Yang Y-P, Persaud D, Haer M, Tripet B, Tam E, Sia C, Klein M. Immunogenicity of synthetic peptides of Haemophilus influenzae type b outer membrane protein P1. Infect Immun. 1995;63:3751–3758. doi: 10.1128/iai.63.10.3751-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochi S L, Fleming D W, Hightower A W, Limpakarnjanarat K, Facklam R R, Smith J D, Sikes R K, Broome C V. Primary invasive Haemophilus influenzae type b disease: a population-based assessment of risk factors. J Pediatr. 1986;108:887–896. doi: 10.1016/s0022-3476(86)80922-2. [DOI] [PubMed] [Google Scholar]
- 10.Dale J B, Beachey E H. Protective antigen determinant of streptococcal M protein shared with sarcolemmal membrane protein of human heart. J Exp Med. 1982;156:1165–1176. doi: 10.1084/jem.156.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans F O, Jr, Sydnor J B, Morre W E C, Moore G R, Manwaring J L, Brill A H, Jakson R T, Hanna S, Skaar J S, Holdeman L V, Fitz-Hugh S, Sande M A, Gwaltney J M., Jr Sinusitis of the maxillary antrum. N Engl J Med. 1975;293:735–739. doi: 10.1056/NEJM197510092931502. [DOI] [PubMed] [Google Scholar]
- 12.Flack F S, Loosmore S, Chong P, Thomas W R. The sequencing of the 80-kDa D15 protective surface antigen of Haemophilus influenzae. Gene. 1995;156:97–99. doi: 10.1016/0378-1119(95)00049-c. [DOI] [PubMed] [Google Scholar]
- 13.Jacob C O, Sela M, Arnon R. Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci USA. 1983;80:7611–7615. doi: 10.1073/pnas.80.24.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob C O, Pines M, Arnon R. Neutralization of heat-labile toxin of E. coli by antibodies to synthetic peptides derived from the B subunit of cholera toxin. EMBO J. 1984;3:2889–2893. doi: 10.1002/j.1460-2075.1984.tb02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kathy H, Eskola J, Peltola H, Stout M G, Samuelson J S, Gordon L K. Immunogenicity in infants of a vaccine composed of Haemophilus influenzae type b capsule polysaccharide mixed with DPT or conjugated to diphtheria toxoid. J Infect Dis. 1987;155:100–106. doi: 10.1093/infdis/155.1.100. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lenoir A A, Granoff P D, Granoff D M. Immunogenicity of Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer membrane protein conjugate vaccine in 2 to 6 month-old infants. Pediatrics. 1987;80:283–287. [PubMed] [Google Scholar]
- 18.Loosmore S M, Yang Y-P, Coleman D C, Shortreed J M, England D M, Klein M H. Outer membrane protein D15 is conserved among Haemophilus influenzae species and may represent a universal protective antigen. Infect Immun. 1997;65:3701–3707. doi: 10.1128/iai.65.9.3701-3707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munson R S, Jr, Shenep J L, Barenkamp S J, Granoff D M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983;72:677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noel G J, Kaza S, Edelson P J. Complement-mediated early clearance of Haemophilus influenzae type b from blood is independent of serum lytic activity. J Infect Dis. 1988;157:85–90. doi: 10.1093/infdis/157.1.85. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M A. Synthetic peptides: prospects for a pili (fimbriae)-based synthetic vaccine. Curr Top Microbiol Immunol. 1990;151:185–204. doi: 10.1007/978-3-642-74703-8_10. [DOI] [PubMed] [Google Scholar]
- 22.Schneerson R, Robbins J B, Parke J C, Jr, Bell C, Schlesslman J J, Sutton A, Wang Z, Schiffman G, Karpas A, Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986;52:519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw S, Smith A L, Anderson P, Smith D H. The paradox of Haemophilus influenzae type b bacteremia in the presence of serum bactericidal activity. J Clin Invest. 1976;58:1019–1029. doi: 10.1172/JCI108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith D B, Davern K M, Board P G, Tiu W U, Garcia E G, Mitchell G F. Mr 26,000 antigen of Schistosoma japonicum recognized by resistant WEHI 129/J mice is a parasite glutathione S-transferase. Proc Natl Acad Sci USA. 1986;83:8703–8707. doi: 10.1073/pnas.83.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith D B, Johnson K S. Single step purification of polypeptide expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 26.Steward M W, Howard C R. Synthetic peptides: a next generation of vaccine? Immunol Today. 1987;8:57–58. doi: 10.1016/0167-5699(87)90239-8. [DOI] [PubMed] [Google Scholar]
- 27.Stool S E, Field M J. The impact of otitis media. Pediatr Infect Dis J. 1989;8:S11–S14. [PubMed] [Google Scholar]
- 28.Thomas W R, Rossi A A. Molecular cloning of DNA coding for outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1986;52:812–817. doi: 10.1128/iai.52.3.812-817.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas W R, Callow M G, Dilworth R J, Audesho A A. Expression in Escherichia coli of a high-molecular-weight protective surface antigen found in nontypeable and type b Haemophilus influenzae. Infect Immun. 1990;58:1090–1913. doi: 10.1128/iai.58.6.1909-1913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]