Abstract
Background
Long waiting times for elective healthcare procedures may cause distress among patients, may have adverse health consequences and may be perceived as inappropriate delivery and planning of health care.
Objectives
To assess the effectiveness of interventions aimed at reducing waiting times for elective care, both diagnostic and therapeutic.
Search methods
We searched the following electronic databases: Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1946‐), EMBASE (1947‐), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), ABI Inform, the Canadian Research Index, the Science, Social Sciences and Humanities Citation Indexes, a series of databases via Proquest: Dissertations & Theses (including UK & Ireland), EconLit, PAIS (Public Affairs International), Political Science Collection, Nursing Collection, Sociological Abstracts, Social Services Abstracts and Worldwide Political Science Abstracts. We sought related reviews by searching the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effectiveness (DARE). We searched trial registries, as well as grey literature sites and reference lists of relevant articles.
Selection criteria
We considered randomised controlled trials (RCTs), controlled before‐after studies (CBAs) and interrupted time series (ITS) designs that met EPOC minimum criteria and evaluated the effectiveness of any intervention aimed at reducing waiting times for any type of elective procedure. We considered studies reporting one or more of the following outcomes: number or proportion of participants whose waiting times were above or below a specific time threshold, or participants' mean or median waiting times. Comparators could include any type of active intervention or standard practice.
Data collection and analysis
Two review authors independently extracted data from, and assessed risk of bias of, each included study, using a standardised form and the EPOC 'Risk of bias' tool. They classified interventions as follows: interventions aimed at (1) rationing and/or prioritising demand, (2) expanding capacity, or (3) restructuring the intake assessment/referral process.
For RCTs when available, we reported preintervention and postintervention values of outcome for intervention and control groups, and we calculated the absolute change from baseline or the effect size with 95% confidence interval (CI). We reanalysed ITS studies that had been inappropriately analysed using segmented time‐series regression, and obtained estimates for regression coefficients corresponding to two standardised effect sizes: change in level and change in slope.
Main results
Eight studies met our inclusion criteria: three RCTs and five ITS studies involving a total of 135 general practices/primary care clinics, seven hospitals and one outpatient clinic. The studies were heterogeneous in terms of types of interventions, elective procedures and clinical conditions; this made meta‐analysis unfeasible.
One ITS study evaluating prioritisation of demand through a system for streamlining elective surgery services reduced the number of semi‐urgent participants waiting longer than the recommended time (< 90 days) by 28 participants/mo, while no effects were found for urgent (< 30 days) versus non‐urgent participants (< 365 days).
Interventions aimed at restructuring the intake assessment/referral process were evaluated in seven studies. Four studies (two RCTs and two ITSs) evaluated open access, or direct booking/referral: One RCT, which showed that open access to laparoscopic sterilisation reduced waiting times, had very high attrition (87%); the other RCT showed that open access to investigative services reduced waiting times (30%) for participants with lower urinary tract syndrome (LUTS) but had no effect on waiting times for participants with microscopic haematuria. In one ITS study, same‐day scheduling for paediatric health clinic appointments reduced waiting times (direct reduction of 25.2 days, and thereafter a decrease of 3.03 days per month), while another ITS study showed no effect of a direct booking system on proportions of participants receiving a colposcopy appointment within the recommended time. One RCT and one ITS showed no effect of distant consultancy (instant photography for dermatological conditions and telemedicine for ear nose throat (ENT) conditions) on waiting times; another ITS study showed no effect of a pooled waiting list on the number of participants waiting for uncomplicated spinal surgery.
Overall quality of the evidence for all outcomes, assessed using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) tool, ranged from low to very low.
We found no studies evaluating interventions to increase capacity or to ration demand.
Authors' conclusions
As only a handful of low‐quality studies are presently available, we cannot draw any firm conclusions about the effectiveness of the evaluated interventions in reducing waiting times. However, interventions involving the provision of more accessible services (open access or direct booking/referral) show some promise.
Keywords: Humans, Elective Surgical Procedures, Elective Surgical Procedures/statistics & numerical data, Interrupted Time Series Analysis, Randomized Controlled Trials as Topic, Time‐to‐Treatment, Time‐to‐Treatment/statistics & numerical data
Plain language summary
Effects of interventions to reduce waiting times for non‐urgent health procedures
Long waiting times for non‐urgent procedures are common in public healthcare systems, where care is provided free of charge and supply is limited by budget constraints. This may cause distress among patients as well as adverse health consequences.
We reviewed the evidence on the effects of interventions in reducing waiting times. We found eight eligible studies (three randomised controlled trials and five interrupted time series studies) involving 135 primary care clinics, seven hospitals and one outpatient clinic. Different interventions, elective procedures and clinical conditions across included studies made pooling of data unfeasible. The quality of the included evidence (to November 2013) ranged from low to very low, as data were obtained from randomised controlled trials that for the most part suffered from serious bias, and from non‐randomised studies without a control group.
The single study that evaluated an intervention aimed at prioritising demand showed that introducing a system for streamlining elective surgery reduced the number of semi‐urgent patients waiting longer than recommended, but did not affect urgent or non‐urgent groups.
Seven studies evaluated interventions aimed at restructuring the intake assessment/referral process. Three of four studies evaluating effects of open access or direct booking/referral showed beneficial effects: One study showed reduced waiting times for open access to sterilisation through keyhole surgery; another showed that open access to investigative services may lead to reduced waiting times for patients with urinary symptoms (but not for patients with microscopic blood in urine); and one study reported that same‐day scheduling reduced waiting times for those seeking child health outpatient services. One study showed no effect of a direct booking system on the proportion of patients reported to have moderate or severe cell changes on the neck of the womb who received an appointment for further investigation within four weeks.
Two studies of distant consultancy (instant photography for skin conditions and telemedicine for ear, nose and throat conditions) showed no effect on waiting times to see a specialist. One study reported that using a pooled waiting list did not change the number of patients waiting for routine back surgery within the recommended time. We found no studies evaluating interventions aimed at increasing capacity or rationing demand.
As only a handful of low‐quality studies are presently available, we cannot draw any firm conclusions about the effectiveness of the evaluated interventions in reducing waiting times. However, interventions involving the provision of more accessible services (open access or direct booking/referral) show some promise.
Summary of findings
Summary of findings for the main comparison. Summary of findings: interventions aimed at rationing and/or prioritising demand.
| Interventions aimed at rationing and/or prioritising demand compared with no intervention | ||||
|
Patient or population: patients scheduled for elective surgery Settings: hospital surgery units Intervention: introduction or suspension of an intervention aimed at prioritising demand Comparison: no intervention | ||||
| Outcomes | Effect measure | Number of studies (hospitals/practices/health professionals/participants) | Quality of the evidence (GRADE) | Comments |
| Introduction of interventions aimed at rationing and/or prioritising demand | ||||
| Number of participants waiting longer than recommended | Number of participants waiting < 90 days: change in slope: ‐27.99 participants/mo (SE 8.58, P value 0.002); change in level: +32.55 participants (SE 54.65, P value 0.55) Number of participants waiting < 30 days: change in slope: ‐1.03 participants/mo (SE 0.51, P value 0.049); change in level: ‐5.40 participants (SE 6.44, P value 0.41) Number of participants waiting < 365 days: change in slope: ‐1.62 participants/mo (SE 2.96, P value 0.59); change in level: +5.50 participants (SE 11.83, P value 0.64) |
1 reanalysed ITS study at 1 public hospital | ⊕⊝⊝⊝ Very lowa |
A single study of very low quality; impossible to draw any conclusions about the effectiveness of the intervention |
| GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. aOne non‐randomised study downgraded for high risk of bias (high risk that the intervention is dependent on other changes and unclear risk of reporting bias). | ||||
Summary of findings 2. Summary of findings: interventions aimed at restructuring referral processes.
| Interventions aimed at restructuring referral processes compared with no intervention | ||||
|
Patient or population: patients needing elective specialist ambulatory visit or surgery Settings: hospitals and primary care practices Intervention: restructuring referral processes Comparison: no intervention | ||||
| Outcomes | Effect measure | Number of studies (hospitals/practices/health professionals/participants) | Quality of the evidence (GRADE) | Comments |
| Direct/open access and direct booking systems | ||||
| Waiting time | Not possible to give a pooled estimate. 1 RCT showed a reduction in waiting time only for participants with lower urinary tract syndrome (ratio of means 0.7, 95% CI 0.5 to 0.9) and not for those with microhaematuria; the other RCT showed a reduction in waiting time for participants randomly assigned to direct laparoscopic sterilisation if compared with standard procedure (108 vs 127 days, P value 0.003) | 2 RCTs (123 general practices and 2 hospitals/1191 participants) |
+ + ⊝⊝ Lowa |
Only 2 RCTs available targeting different elective procedures. Difficult to draw any conclusions about effectiveness or generalisability |
| Proportion of participants waiting below a recommended time threshold | Proportion of participants waiting less than 4 weeks: change in level: ‐14.26%, P value 0.50; change in slope: +6.29% each 3 months, P value 0.62 | 1 reanalysed ITS study (1 community primary care unit and 1 public hospital/2501 women). |
+⊝⊝⊝ Very lowb |
Extremely scarce evidence of very low quality, impossible to draw any conclusions |
| Waiting time | Waiting time (days): change in level: ‐25.20 days, SE 3.83, P value < 0.001; change in slope: ‐3.03 days/mo, SE 0.92, P value 0.005 | 1 reanalysed ITS study (1 outpatient clinic/7594 appointments) |
+⊝⊝⊝ Very lowc |
Scarce evidence of low quality; impossible to draw any conclusions |
| Distant consultancy | ||||
| Waiting time |
Waiting time: mean 55 days (SD = 40, P value >0.05) | 1 RCT (1 hospital, 10 general practices/136 participants) |
+ + ⊝⊝ Lowd |
Scarce evidence of low quality; impossible to draw any conclusions. |
| Waiting time | Waiting time: change in level: ‐0.69 months (SE 0.55, P value 0.23); change in slope: ‐0.21 months each year (SE 0.13, P value 0.15) | 1 reanalysed ITS study (1 ENT clinic/1690 participants) |
+⊝⊝⊝ Very lowe |
Scarce evidence of very low quality; impossible to draw any conclusions |
| Generic waiting list | ||||
| Number of participants waiting less than recommended time threshold or within a recommended time period | Number of participants waiting less than 9 months: change in level: ‐20.59 (SE 22.67, P value 0.37); change in slope: 2.75 participants each month (SE 12.69, P value 0.86) Number of participants waiting between 9 and 18 months: change in level: ‐5.28 (SE 16.20, P value 0.75); change in slope: ‐6.59 participants each month (SE 8.73, P value 0.46) |
1 reanalysed ITS study (1 hospital) |
+⊝⊝⊝ Very lowf |
Scarce evidence of low quality; impossible to draw any conclusions |
| GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. aOne RCT with low risk of bias and one RCT with high risk of bias (due to high risk of attrition and contamination bias) and indirectness (low applicability of the intervention); according to the GRADE rule, the overall quality of evidence for this outcome is that of the trial with the lowest quality (i.e. low). bOne non‐randomised study downgraded for high risk of bias due to high risk that the intervention is dependent on other changes. cOne non‐randomised study downgraded for high risk of bias due to intervention affecting data collection, risk of attrition and reporting bias. dOne RCT with unclear risk of bias (high risk of attrition bias) and imprecision of results. eOne non‐randomised study downgraded for high risk of bias due to high risk of reporting bias. fOne non‐randomised study downgraded for high risk of bias due to unclear risk of intervention not being independent of other changes and having affected data collection, and unclear risk of attrition and reporting bias. | ||||
Background
Description of the condition
Elective health procedures are procedures that are programmed and are not delivered in emergency or urgent situations. Long waiting times for elective health procedures are observed in most health systems and are thought to occur when demand exceeds supply. They tend to occur in health systems that combine public health insurance with zero or low patient cost‐sharing and constraints on capacity (Siciliani 2013); some view them as a structural feature of those systems (Harrison 2000; Hurst 2003; Kreindler 2010; Siciliani 2013). The mere existence of waiting lists is not necessarily a negative phenomenon, as it is sometimes considered a structural and inevitable way of rationing scarce supply (Appleby 2011; Black 2004; Lindsay 1984). Total absence of waiting times would certainly cause dysfunction, as planning of activities would be jeopardised and efficiency would greatly suffer if services were not used at their full capacity.
However, long waiting times can cause distress for patients, in some cases can have adverse health consequences (Kreindler 2010) and are perceived by patients, the public and policy‐makers as lack of appropriate delivery and planning of health care (Kreindler 2010). This explains in part why long waiting times tend to catalyse the tensions between patients' and citizens' expectations on the one side, and health care providers' ability to meet those expectations on the other. The question is therefore how to find a way to keep waiting times at a safe and acceptable level while ensuring quality, equity and wise use of resources (Kreindler 2010).
Extended studies of this phenomenon have not pinpointed specific health system determinants of waiting lists (Hurst 2003; Siciliani 2003; Siciliani 2013). Besides a link between universalistic access to care and long waiting times, researchers have found associations with lower levels of health expenditure and lessened availability of curative beds and of physicians. However some data contradict these associations, and long waiting times are found in countries where health expenditure and availability of services and resources are high. Great variation between countries demonstrates that it is still very difficult to advance tenable inferences on causal relationships, and only descriptive associations can be put forward.
Evidence on how waiting times for elective procedures affect health outcomes appears less conclusive than for urgent procedures (Hirvonen 2007). This could be explained by the fact that patients on a waiting list whose health deteriorates while waiting tend to be shifted to an emergency list and "lost" from the elective procedure waiting lists.
Description of the intervention
Analysis of determinants and implications of waiting times, as well as of the impact of policies targeted at their reduction, has been the object of a number of reviews (Appleby 2005a; Appleby 2011; Harrison 2000; Hurst 2003; Kipping 2002; MacMillan Press 1993; Siciliani 2003; Siciliani 2013; Yates 1987).
Comparative data between countries and health systems experiencing or not experiencing long waiting times for elective procedures do not lend themselves to hypotheses that go beyond the imbalance between supply and demand. However causes of inadequate supply or excessive demand can be numerous and heterogeneous. An inadequate supply can be due to insufficient capacity or inefficient use of existing capacity, which in turn can be affected by several factors such as important changes in technology (Siciliani 2013). Decreasing healthcare demand is quite problematic; probably only effective prevention of illnesses and/or their effective management within primary care would reduce the number of patients who need treatment. Clinical uncertainty and variations in clinical practice have been associated with inappropriate demand.
During the past decade, waiting time guarantees have become a frequent and popular policy tool to address waiting times. Recommended minimum waiting times are established for different types of elective procedures, and hospital incentives or penalties are associated with meeting the set targets. This policy has been successful in England, where publication of waiting times data was coupled with sanctions for poorly performing hospital managers. This strategy appeared to reduce maximum waiting times (Appleby 2005b; Dash 2004; Department of Health 2002; Mayor 2003; Propper 2008), but, as it happened in other countries, it did not seem to affect average waiting times. To meet target waiting time as set by policies, health systems and organisations adopt specific interventions to reach the set target. These can be of three main types: interventions aimed at rationing and/or prioritising demand, at expanding capacity and at improving the organisational management of waiting lists or restructuring the intake assessment/referral process. Examples of interventions aimed at rationing and/or prioritising demand include patients' financial contributions to healthcare services (co‐payment), development and implementation of explicit referral criteria or practice guidelines to increase appropriateness and use of tools that triage patients according to their clinical conditions (clinical priority scores). Interventions aimed at expanding capacity comprise additional funding to increase appointment slots in the public sector, thus subsidising or facilitating access to the private sector. Examples of interventions aimed at restructuring the intake assessment/referral process include queuing strategies, redesign of clinical pathways, open access (patients seen without an appointment), direct booking/referral (specialty visits booked directly by patients), pooled waiting lists (single and pooled waiting lists for different consultants, with patients assigned to the first available appointment) and telemedicine, among others.
All of the above strategies have been used alone or in combination (Hurst 2003) in different settings and countries with heterogeneous results.
How the intervention might work
A lengthy waiting time is thought to result from a misalignment between the demand for procedures as expressed by citizens and the capacity of health systems to supply such procedures in adequate number and time. Therefore interventions implemented to enforce national or regional policies for reduction of waiting times may act on increasing supply or on reducing demand.
Interventions to increase supply involve raising funding/expenditures to buy additional personnel, equipment or time slots for extra numbers of procedures; to provide incentives for extra activity; or to buy extra activity from other providers.
Other interventions include improving efficiency and shortening patients' pathways by eliminating redundancies or obstacles in the process of care (Kreindler 2010).
Interventions to reduce demand include taking actions to discourage inappropriate requests and promote appropriate use of procedures and prioritising patients, while taking into consideration both clinical (e.g. severity of condition, expected benefit, need, urgency) and non‐clinical (e.g. ability to work) parameters (Kreindler 2010).
Why it is important to do this review
Numerous reports (Rachlis 2005; Siciliani 2013; Willcox 2007) and reviews (Kreindler 2010; Miller 2008) have sought to assess and compare the impact of different national policies for regulating and containing length of waiting times, using mainly national administrative data. These reports represent a fundamental contribution to the debate on management of waiting times and waiting lists, but they rarely provide evidence on the effectiveness of interventions. It is therefore important to summarise and evaluate existing evidence to identify what interventions are most effective in reducing waiting times for elective procedures.
Objectives
To assess the effectiveness of interventions aimed at reducing waiting times for elective care, both diagnostic and therapeutic.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs), controlled before‐and‐after studies (CBAs) and interrupted time series (ITS) designs that met the minimum criteria used by the Cochrane Effective Practice and Organisation of Care Group (EPOC) (EPOC 2013). We considered ITS studies as eligible if they had a clearly defined point in time when the intervention occurred, and at least three data collection points before and after the intervention (Ramsay 2003). We included CBAs if they involved at least two (intervention and/or control) sites (EPOC 2013). We included inappropriately analysed ITS studies if they reported data (in graphical or table format) that could be used to reanalyse data while taking into account possible secular trends in the analysis.
Types of participants
We included healthcare providers of any discipline/specialty area and patients referred to any type of elective diagnostic or therapeutic procedure.
Types of interventions
We considered any type of regulatory/administrative, economic, clinical or organisational intervention aimed at reducing waiting times for access to elective diagnostic or therapeutic procedures. We classified interventions according to the following taxonomy.
Interventions aimed at rationing and /or prioritising demand (e.g. co‐payment, explicit referral criteria or practice guidelines, clinical priority scores, waiting time cap strategies).
Interventions aimed at expanding capacity (e.g. additional funding to the public sector, ways of subsidising or facilitating access to the private sector).
Interventions aimed at restructuring the intake assessment/referral process (e.g. different queuing strategies, theatre management strategies, other resource sharing strategies, remuneration schemes, direct access, open access, telemedicine).
We considered as a comparator standard practice (i.e. no intervention) or any kind of active intervention aimed at reducing waiting time.
Types of outcome measures
Commonly reported measures include mean and median waiting times, measured at different points in the patient pathway, which can be the moment at which patients see their general practitioner, the time of referral, the time patients are put on a waiting list, the time at which they undergo the elective procedure or the time they are discharged from hospital. Mean and median waiting times are considered reliable measures, although the mean can be influenced by a small number of patients with long waiting times and tends to be above the median (Siciliani 2013).
We restricted the review to include only studies that provided an objective measure of the impact of the interventions considered, expressed in terms of:
number or proportion of participants whose waiting times were above or below a specified or recommended time threshold; or
participants' mean or median waiting times for elective procedures.
We considered safety outcomes, that is, any health outcomes of participants (e.g. mortality, morbidity, complication rates), as well as costs.
Search methods for identification of studies
M. Fiander, Trials Search Co‐ordinator (TSC) for the EPOC Group, wrote the search strategies, in consultation with the review authors. The TSC searched the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews, as well as the databases listed below for primary studies. Major databases were searched in November 2013; other databases, from which the identification of trials is less likely, were searched in November 2012 (see notations below); exact search dates for each database are included with the search strategies in Appendix 1 (MEDLINE) and Appendix 2 (other).
Neither date nor language limits were applied. Two methodological search filters were used to limit retrieval to appropriate study designs: the Cochrane Highly Sensitive Search Strategy (sensitivity‐ and precision‐maximising version, 2008 revision) (Higgins 2011) to identify randomised trials; and an EPOC methodology filter to identify non‐RCT designs.
Databases
MEDLINE (1946‐2012), In‐Process and other non‐indexed citations, Ovid SP.
EMBASE (1947‐2012), Ovid SP.
Cochrane Central Register of Controlled Trials Evidence‐Based Medicine (EBM) Reviews, Ovid.
Cumulative Index to Nursing and Allied Health Literature (CINAHL), EbscoHost (1980‐2012).
EPOC Register, Reference Manager.
Dissertations and Theses Full Text, UK and Ireland ProQuest.
Health Technology Assessment, Fourth Quarter 2013, EBM Reviews, Ovid.
National Health Service (NHS) Economic Evaluation Database, Fourth Quarter 2013, EBM Reviews, Ovid.
PAIS (Public Affairs International), ProQuest.
Science, Social Sciences and Humanities Citation Indexes, Conference Proceedings, Web of Science (e.g. Web of Knowledge).
ABI Inform (January 2013).
Canadian Research Index (November 2012).
Communication Disorders Database, ProQuest (November 2012).
Political Science Collection, ProQuest (November 2012).
Nursing and Allied Health Source, ProQuest (November 2012).
Sociological Abstracts and Social Services Abstracts, ProQuest (November 2012).
Worldwide Political Science Abstracts, ProQuest (November 2012).
Searching other resources
Trial registries
World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/AdvSearch.aspx).
Grey literature
We undertook a grey literature search that included, but was not limited to, the following sites.
AHRQ (Agency for Healthcare Research and Quality) (http://www.ahrq.gov/) (November 2013).
Centre for Health Services and Policy Research (CHSPR) (http://www.chspr.ubc.ca/pubs/pub‐search) (November 2013).
Centre for Health Economics and Policy Analysis, McMaster University (CHEPA) (http://www.chepa.org/ ) (November 2013).
Health Quality Council (HQC), University of Saskatchewan (November 2013).
Institute for Clinical Evaluative Sciences (ICES) (http://www.ices.on.ca/ ) (November 2013).
Institute of Health Economics (http://www.ihe.ca/publications/library/) (November 2013).
Ontario Health Technology Advisory Committee (OHTAC) (http://www.health.gov.on.ca/english/providers/program/ohtac/tech/recommend/rec_mn.html ) (November 2013).
Organisation for Economic Co‐operation and Development (OECD) (www.oecd.org) (November 2013).
Public Health Agency of Canada (PHAC) (www.phac‐aspc.gc.ca) (November 2013).
World Health Organization (WHO) (http://www.euro.who.int/en/what‐we‐do/data‐and‐evidence/health‐evidence‐network‐hen/publications ) (November 2013).
We also searched lists of references from relevant studies and systematic reviews; and contacted the authors of all eligible studies to ask about other relevant studies.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by the electronic searches to the reference management database Reference Manager (Reference Manager 2010) and removed duplicates. One person (AH) sifted through the search results, discarding obviously irrelevant studies, and produced a long list of possibly eligible studies. Thereafter one of the review authors (GF) assessed these citations. Two review authors (GF, IS) independently obtained and assessed full‐text copies of potentially eligible studies. The review authors were not blinded to study author or location. We resolved disagreements through full‐group discussion.
Data extraction and management
At least two review authors (among LB, SM, LV, IS and JH) independently extracted study data using a modified EPOC data collection checklist (EPOC 2013). We resolved disagreements by discussion and, when necessary, through the involvement of an arbitrator (RG). We contacted study authors to ask for additional data/information. If study authors did not respond the first time, we sent two email reminders.
For inappropriately analysed ITS studies, if data needed for reanalyses were reported in tables or graphs in the original paper, we contacted the study authors to request original data to ensure minimum approximation. If data were not available, or if we received no response from the study authors, we used the software xyExtract to extract data from graphs (Wagner 2002).
To evaluate the impact of interventions on outcomes, we classified all included studies, according to the above described taxonomy, into three different intervention categories: rationing and/or prioritising demand; expanding capacity; and restructuring the intake assessment/referral process.
Assessment of risk of bias in included studies
At least two review authors (among SM, LV, LB, IS and JH) independently assessed the risk of bias of each included study using the criteria suggested by EPOC (EPOC 2013) and Davey (Davey 2013). We assessed RCTs for generation of allocation sequence, concealment of allocation, similar baseline outcome measurements, similar baseline characteristics, incomplete outcome data, blinding of participants, blinding of outcome assessors, protection against contamination, selective outcome reporting and other risks of bias. Criteria for assessing ITS design included independence of the intervention from other changes, appropriate analysis of data, prespecified shape of the intervention, intervention unlikely to affect data collection, knowledge of the allocated intervention during the study adequately prevented, incomplete outcome data, selective outcome reporting and other risks of bias.
We scored each study for risk of bias as follows: 'low' if all key domains were scored as 'low risk'; 'unclear' if one or two key domains were scored as 'unclear risk'; and 'high' if more than two key domains were scored 'unclear risk' or 'high risk' (adapted from Davey 2013).
Measures of treatment effect
We calculated the effects of interventions by study design.
For RCTs, when available, we reported preintervention and postintervention values for outcomes of intervention and control groups, and we calculated the absolute change from baseline with 95% confidence interval (CI), or the effect (e.g. mean difference, ratio of means) with 95% CI.
We reanalysed ITS studies that were inappropriately analysed as simple before‐and‐after studies using segmented time‐series regression techniques to estimate the effect of the intervention, taking into account the time trend and autocorrelation among observations. Adjustment for autocorrelation involved estimating the autocorrelation parameter and including it in the segmented regression model if necessary (Wagner 2002). We obtained estimates for regression coefficients corresponding to two standardised effect sizes for each study: a change in step or level, and a change in slope before and after the intervention. A change in step or level was defined as the difference between the predicted level al the first intervention time point and the level predicted by the preintervention time trend. A change in slope was defined as the difference between postintervention and preintervention slopes (Ramsay 2003).
A change in level and/or slope with a negative value may indicate:
an effect in terms of a reduction in waiting time (i.e. a beneficial intervention effect);
an effect in terms of a reduction in the number/proportion of participants waiting longer than the recommended time (i.e. a beneficial intervention effect); or
an effect in terms of a reduction in the number/proportion of participants waiting within the recommended time (i.e. a non‐beneficial intervention effect).
A change in level and/or slope with a positive value may indicate:
an effect in terms of an increase in the number or proportion of participants treated within the recommended time (i.e. a beneficial intervention effect); or
an effect in terms of an increase in the number or proportion of participants waiting longer than the recommended time (i.e. a non‐beneficial intervention effect).
We used STATA 12 (Stata 2011) for all analyses.
Unit of analysis issues
Included cluster trials were analysed appropriately, hence there was no need for reanalysis.
Dealing with missing data
For eligible studies, when data on outcomes of interest were missing or were incompletely reported, we contacted study authors to ask for additional information. Authors of only one study (Hofstetter 2010) were able to provide data for the ITS reanalysis. Authors of two RCTs (Leggett 2004; McKessock 2001) were unable to provide data on the outcomes of interest for this review.
Assessment of heterogeneity
We identified too few studies to explore heterogeneity. We descriptively reported heterogeneity of studies by assessing differences in populations of interest, types/categories of interventions, outcomes, study design and measures of effect.
Data synthesis
We summarised separately and qualitatively described the results of RCTs and reanalysed ITS studies.
Although initially planned, we decided against meta‐analysis because of the significant heterogeneity of eligible studies in terms of targeted elective procedures, participant and healthcare provider populations and characteristics and components of the intervention and setting. We performed no subgroup or sensitivity analyses.
'Summary of findings' tables
We assessed the quality of evidence for primary outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (Guyatt 2008; Higgins 2011) and reported this information in Table 1 and Table 2. We rated the quality of the body of evidence for each outcome as 'high,' 'moderate,' 'low' or 'very low.'
Results
Description of studies
Results of the search
After duplicates were removed, electronic searches yielded 3040 citations. Of these, 2939 were judged not relevant and were excluded. Of the remaining 101 citations, 93 did not meet our inclusion criteria and were excluded. Eight publications (Hofstetter 2010; Leach 2004; Leggett 2004; Lowthian 2011; Lukman 2004; Mallard 2004; McKessock 2001; Thomas 2003) were found eligible for inclusion in this review. See PRISMA study flow diagram (Figure 1).
1.
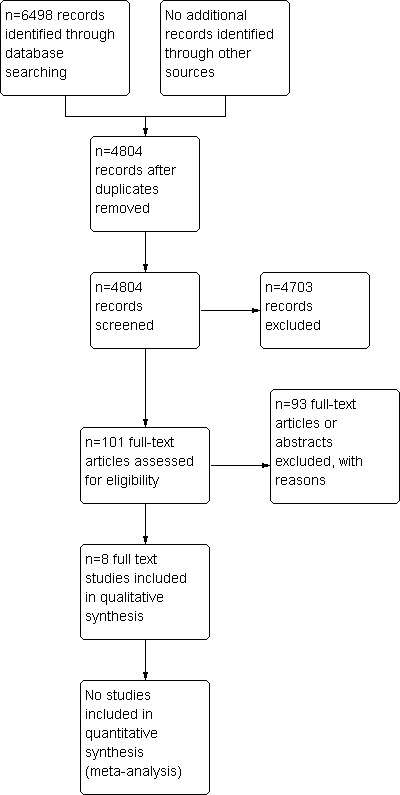
Study flow diagram.
The additional search run up to November 2013, resulting in 662 citations. Of these, 468 were judged not relevant and excluded. Of the remaining 194, 189 did not meet our inclusion criteria and were thus excluded. Four studies from this new search are listed in the Characteristics of studies awaiting classification table, and one protocol is listed in the Characteristics of ongoing studies table. The six publications are awaiting final classification.
Included studies
The eight included studies are described in the Characteristics of included studies and are summarised, according to the taxonomy of interventions, in Table 3.
1. Summary of characteristics of included studies.
| Reference | Study design | Setting and participants | Intervention (duration) | Control | Outcomes | Risk of bias |
| Interventions aimed at rationing and/or prioritising demand | ||||||
|
Lowthian 2011 |
Reanalysed ITS study | 1 hospital, patients waiting for elective surgery | Redesigning and streamlining of perioperative services (3 years) | Routine practice | Number of participants waiting longer than recommended wait time | High |
| Interventions aimed at restructuring referral processes | ||||||
| Direct/open access and direct booking systems | ||||||
| McKessock 2001 | Cluster‐RCT | 1 hospital and 57 general practices, 232 patients referred for elective laparoscopic sterilisation | Direct booking laparoscopic service (10 months) |
Routine referral from GP to clinic | Waiting time | High |
| Thomas 2003 | Cluster‐RCT | 1 hospital and 66 general practices, 959 patients with lower urinary tract symptoms (LUTS) or microscopic haematuria (MH) | Direct booking investigation service (10 months) |
Routine practice, consisting of initial outpatient appointment plus 1 further appointment for routine day case investigation | Waiting time | Low |
| Lukman 2004 | Reanalysed ITS study | 1 hospital and 1 community primary care, 2501 patients with cervical cytology abnormality and needing a colposcopy | Direct booking colposcopy service (24 months) |
Referral and appointment made by GP | Proportion of participants obtaining an appointment within the recommended time threshold | High |
| Mallard 2004 | Reanalysed ITS study | 1 clinic, 7594 appointments for outpatients attending a public health clinic | Same‐day scheduling (12 months) | Routine practice, consisting of complex appointment guidelines and next place available schedule | Waiting time | High |
| Distant consultancy | ||||||
| Hofstetter 2010 | Reanalysed ITS study | 1 hospital and 1 community primary care, 1690 patients needing ENT specialty care in a rural area | Telemedicine consultations (6 years) |
Face‐to‐face visit in main city hospital | Waiting time | High |
| Leggett 2004 | RCT | 1 hospital and 10 general practitioners, 136 patients requiring dermatology referral | Instant photography (unknown duration) | Face‐to‐face first appointment with dermatology consultant | Waiting time | Unclear |
| Single generic waiting list | ||||||
| Leach 2004 | Reanalysed ITS study | 1 hospital, patients requiring routine spinal surgery | Generic waiting list (14 months) |
Each consultant managing own waiting list | Number of participants waiting less than or longer than the prespecified time threshold | High |
Study designs
Among the eight included studies were two cluster‐RCTs (McKessock 2001; Thomas 2003), one RCT (Leggett 2004) and five reanalysed ITS studies (Hofstetter 2010; Leach 2004; Lowthian 2011; Lukman 2004; Mallard 2004).
Geographical location of study
Five studies were conducted in the UK (Leach 2004; Leggett 2004; Lukman 2004; McKessock 2001; Thomas 2003); two in the USA (Hofstetter 2010; Mallard 2004); and one in Australia (Lowthian 2011).
Settings and participants
The review involved a total of seven hospitals, one outpatient clinic and 135 general practices (GPs)/primary care clinics. Two studies (Leach 2004; Lowthian 2011) involved only hospitals. One study involved one outpatient clinic (7594 appointments) (Mallard 2004). Five studies were carried out in general practices/primary care and hospitals: one hospital and one community primary care clinic (1690 participants) (Hofstetter 2010); one hospital and 10 GPs (136 participants) (Leggett 2004); one hospital and one community primary care clinic (2501 female participants) (Lukman 2004); one hospital and 57 general practices (232 female participants) (McKessock 2001); and one hospital and 66 general practices (959 participants) (Thomas 2003).
Targeted elective procedures
The eight included studies reported interventions that targeted elective procedures for different clinical conditions: referrals for ENT (Hofstetter 2010), uncomplicated spinal surgery (Leach 2004). dermatology (Leggett 2004), elective surgery (Lowthian 2011), colposcopy for abnormal cervical cytology (Lukman 2004), any paediatric clinical conditions treated in an outpatient clinic (Mallard 2004), laparoscopic sterilisation (McKessock 2001) and urological investigations (Thomas 2003).
Types of interventions and comparators
Following our classification, study interventions were grouped according to whether they were aimed at rationing/and or prioritising demand; expanding capacity; or restructuring the intake assessment/referral process.
Interventions aimed at rationing and/or prioritising demand
One study (Lowthian 2011) evaluated the effects of introducing a system for streamlining elective surgery patients according to urgency, and compared this system with routine practice. The introduction of the intervention coincided with the construction of a dedicated elective surgery and procedural facility. The intervention lasted three years.
No studies evaluating interventions aimed at rationing demand were found.
Interventions aimed at expanding capacity
No studies evaluating interventions aimed at expanding capacity were found, although an increase in capacity was introduced as a co‐intervention in one study (Lowthian 2011).
Interventions aimed at restructuring the intake assessment/referral process
Seven studies evaluated interventions aimed at restructuring the referral process (Hofstetter 2010; Leach 2004; Leggett 2004; Lukman 2004; Mallard 2004; McKessock 2001; Thomas 2003). Median duration of intervention was 11.8 months, ranging from 7 months (Mallard 2004) to five years (Hofstetter 2010); for one study (Leggett 2004) the duration of the intervention was not specified.
Three studies (Lukman 2004; McKessock 2001; Thomas 2003) explored direct booking/referral. McKessock et al (McKessock 2001) evaluated the impact of direct access to laparoscopic sterilisation in general practices against routine referral from GP to clinic. Thomas et al (Thomas 2003) evaluated a direct booking urological investigation service for patients referred by their GPs for lower urinary tract symptoms (LUTS) or microscopic haematuria (MH) and compared it versus current practice, consisting of an initial outpatient appointment plus one further appointment for routine day case investigation. Lukman et al (Lukman 2004) evaluated a direct booking system in a colposcopy clinic for women with abnormal cervical cytology versus referral and appointment made by GP: A "fail safe" pathway to retrieve patients failing to respond to the new referral system was set in place.
Distance consultancy interventions were evaluated in two studies (Hofstetter 2010; Leggett 2004). Hofstetter 2010 assessed the introduction of telehealth for participants needing ear nose throat (ENT) specialty care in a rural area and compared the intervention versus ENT face‐to‐face visit in the main city hospital. In Leggett 2004, instant photography was introduced to diagnose and manage dermatology conditions in general practices located near a major teaching hospital, and was compared versus face‐to‐face index appointment with a dermatology consultant.
In one study (Leach 2004), the effects of introducing a generic waiting list and pooling all initial outpatient appointments and dates for routine spinal surgery were compared against current practice, consisting of each consultant managing his or her own waiting list. A second intervention was to integrate the MRI booking system with outpatient review appointments. However, it was unclear when this intervention was introduced.
One study (Mallard 2004) evaluated open access/same‐day scheduling for paediatric outpatients attending a public health clinic and compared this approach with standard routine based on complex appointment guidelines and next place available schedule.
Outcomes
One study that evaluated an intervention aimed at prioritising of demand measured the absolute number of participants waiting longer than a recommended waiting time (Lowthian 2011), that is, the number of urgent elective participants waiting longer than 30 days, semi‐urgent participants waiting longer than 90 days and non‐urgent participants waiting longer than 365 days.
Among the seven studies assessing interventions aimed at improving the organisational management of waiting lists or restructuring the intake assessment/referral processes, five studies measured the effects on waiting time (Hofstetter 2010; Leggett 2004; Mallard 2004; McKessock 2001; Thomas 2003); one study measured the proportion of participants obtaining an appointment within the recommended four weeks waiting time (Lukman 2004) and one study the absolute number of participants waiting less than nine months, between nine and 18 months or longer than 18 months (Leach 2004).
Of the three included RCTs, one (Thomas 2003) reported estimated effect with the 95% CI, while the other two (Leggett 2004; McKessock 2001) reported estimated effect and P value.
No safety outcomes were reported in the included studies. Two RCTs (McKessock 2001; Thomas 2003) measured direct and indirect costs (NHS and participant time and travel costs, respectively).
Excluded studies
Reasons for exclusion of the 93 citations were as follows: ineligible study design (n = 59); inappropriately analysed ITS study with no graphically reported data, and/or lacking baseline data or inappropriately analysed ITS study with graphically reported data but with insufficient number of data points before and/or after the intervention (n = 23); CBA studies with only one intervention and/or control site and without graphically presented data (n = 5), ineligible intervention (n = 4) and one study could not be located. See Characteristics of excluded studies for details. We listed two relevant study protocols (Augestad 2008; Wahlberg 2013;) under ongoing studies (see Characteristics of ongoing studies).
Risk of bias in included studies
See 'Risk of bias' tables in Characteristics of included studies, Figure 2 and Figure 3 for RCTs, and in Figure 4 and Figure 5 for reanalysed ITS studies.
2.
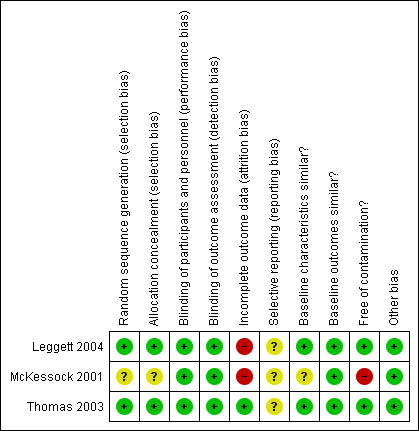
Risk of bias graph for RCTs: review authors' judgements about each risk of bias item for each included study.
3.
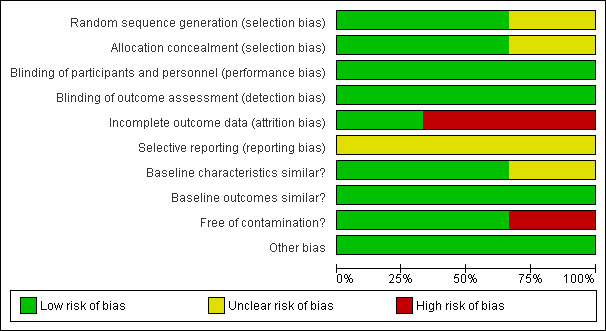
Risk of bias graph for RCTs: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias graph for reanalysed ITS studies: review authors' judgements about each risk of bias item for each included study.
5.
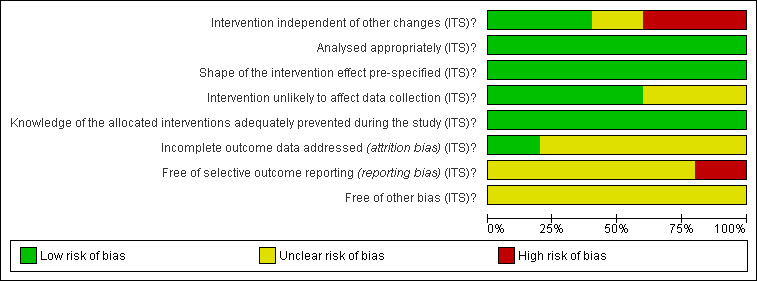
Risk of bias graph for reanalysed ITS studies: review authors' judgements about each risk of bias item presented as percentages across all included studies.
RCTs
See 'Risk of bias' in Characteristics of included studies table, Figure 2 and Figure 3.
One of the three included trials (Thomas 2003) had an overall low risk of bias, and two trials (Leggett 2004; McKessock 2001) were at high risk of bias. In these trials high attrition and contamination were the main sources of bias: in Leggett 2004, 36.6% of intervention participants could not be diagnosed, and among those who received a diagnosis, 38.0% still needed to see a dermatologist face‐to‐face for management; in McKessock 2001, only 10 out of 75 participants allocated to the intervention group actually received the intervention.The McKessock 2001 trial was also at high risk of contamination, as a large proportion of participants assigned to the intervention group were treated according to standard referral practice. The other two trials were at low risk of contamination. In two trials (Leggett 2004; Thomas 2003), both the sequence generation process and allocation concealment were adequate and at low risk of bias, and in one trial (McKessock 2001), risk of bias for sequence generation and allocation concealment was unclear. Baseline characteristics were similar in two trials (Leggett 2004; Thomas 2003) and unclear in one trial, as baseline characteristics were not provided (McKessock 2001). All trials had an unclear risk of selective outcome reporting, as no trial protocols were available. All trials were at low risk of bias for baseline outcome measures (as no baseline measure of outcomes can be provided for the outcome of interest), blinding (due to objective outcomes) and other risk of bias.
Reanalysed ITS studies
See 'Risk of bias' in Characteristics of included studies table, Figure 4 and Figure 5.
All five ITS studies (Hofstetter 2010; Leach 2004; Lowthian 2011; Lukman 2004; Mallard 2004) were at overall high risk of bias.
Intervention independent of other changes
Risk of bias was low in two studies (Hofstetter 2010; Mallard 2004): In one, the number of available appointment slots was stable during the study period (Hofstetter 2010); in another (Mallard 2004), extra clinics were organised at the beginning of the intervention period, but as these data were not included in the analysis, risk of bias must be considered low. In one study (Leach 2004), risk of bias was unclear, as it was unclear whether a second intervention was implemented at the same time as the main intervention, or later during the intervention period, which may have affected the results. In two studies, risk of bias was high (Lowthian 2011; Lukman 2004): In one (Lowthian 2011), three different interventions were implemented over time, of which the main intervention (streamlining of services) was one; this complicates interpretation of results; in the other (Lukman 2004), extra clinics (of unclear duration) were introduced after the start of the main intervention to meet extra demand, and a second intervention (introduction of colposcopy nurse) was put in place during the intervention period.
Appropriate analysis and shape of intervention effect prespecified
All included ITS studies were reanalysed by the review authors; therefore risk of bias is low for these items.
Intervention unlikely to affect data collection
Data were retrospectively collected in three studies (Hofstetter 2010; Lowthian 2011; Lukman 2004); thus risk of bias for this item is low; two studies had unclear risk of bias, as no information was provided on how data had been collected (Leach 2004; Mallard 2004).
Knowledge of allocated interventions adequately prevented during the study
All outcomes of interest for this review are objective and are unlikely to be affected by non‐blinding; therefore risk of bias is low.
Incomplete outcome data
Low risk of bias due to incomplete outcome data is reported for one study (Lowthian 2011), and unclear risk of attrition bias is reported for four studies (Hofstetter 2010; Leach 2004; Lukman 2004; Mallard 2004), as no information on number of participants who withdrew or were lost to follow‐up was provided.
Selective outcome reporting
Usually ITS studies do not have a study protocol with prespecified outcomes, and it is sometimes difficult to judge whether all important outcomes have been reported; therefore most of the included ITS studies had an unclear risk of bias for this item (Leach 2004; Lowthian 2011; Lukman 2004; Mallard 2004). Another study (Hofstetter 2010) was at high risk of bias because of different waiting time outcomes measured in preintervention and postintervention periods: For preintervention, waiting time was measured by referral to a face‐to‐face specialist appointment, which, in many cases, involved treatment given to the participant, but in the postintervention period, waiting time was measured with referral until the consultant looked at participant data sent by store and forward telehealth, but not when the participant actually received feedback/treatment.
Other bias
All five ITS studies had unclear risk of bias (Hofstetter 2010; Leach 2004; Lowthian 2011; Lukman 2004; Mallard 2004). In Hofstetter 2010, it was unclear whether all participants who received a telehealth specialist consultation could be diagnosed and subsequently treated, or if some had to see a specialist face‐to‐face to be diagnosed. Telemedicine techniques and equipment most likely improved over the study years, which may have had implications for whether consultations were successful. In Leach 2004, it was unclear whether preintervention and/or postintervention data related to waiting times for all 10 surgeons, or for only the seven who agreed to the intervention. In Lowthian 2011, it was unclear whether study authors each month added up the number of people who were currently waiting too long and were still waiting. If so, participants may appear on the graph for several consecutive months, that is, from the time they exceed the recommended time to the time they have surgery, and if so, this may have affected the results of the analysis. In Lukman 2004, the source of data for analysis (Figure 2 in Lukman 2004) seems to include data for all types of referrals (inadequate, abnormal or other), but the study aims to assess the impact of direct booking on waiting time from abnormal smear report to colposcopy clinic (direct booking available only for abnormal smears, not for the other referrals), but it is unclear how this may have affected the results. In Mallard 2004, the definition of 'waiting time' was unclear, and it was unclear whether the analysis included also waiting time for prescheduled appointments, for which shorter waiting time presumably was not desired.
Effects of interventions
See Table 1 and Table 2 for the main comparisons.
Effects of interventions aimed at rationing and/or prioritising demand
See Table 4.
2. Interventions aimed at rationing and/or prioritising demand: reanalysed ITS studies.
| Study | Outcome | Postintervention period | Secular trend (SE, P) | Change in level (SE, P) | Change in slope (SE, P) |
| Lowthian 2011 | Number of participants waiting longer than recommended time threshold ("urgent" participants waiting less than 30 days) every month | 3 years | +0.25 (SE 0.41, P value 0.55) | ‐5.40 (SE 6.44, P value 0.41) | ‐1.03 (SE 0.51, P value 0.049) |
| Number of participants waiting longer than recommended time threshold ("semi‐urgent" patients waiting less than 90 days) every month |
+13.72 (SE 6.23, P value 0.032) | +32.55 (SE 54.65, P value 0.55) | ‐27.99 (SE 8.58, P value 0.002) | ||
| Number of participants waiting longer than recommended time threshold ("non‐urgent" participants waiting less than 365 days) every month |
‐0.15 (SE 1.85, P value 0.94) | +5.50 (SE 11.83, P value 0.64) | ‐1.62 (SE 2.96, P value 0.59) |
One ITS study (Lowthian 2011) evaluated the effects of interventions aimed at prioritising demand. Results of this study show that streamlining of elective surgery services had an effect on the waiting time of ‘semi‐urgent’ patients only, with 28 (SE 8.58, P value 0.002) fewer participants per month waiting longer than recommended (< 90 days). No effects on waiting times were found for 'urgent' or 'non‐urgent' participant groups (with recommended waiting times of less than 30 days and 365 days, respectively).
No effectiveness data for interventions aimed at rationing demand were included in this review.
Effects of interventions aimed at expanding capacity
No effectiveness data for interventions aimed at increasing capacity were included in this review.
Effects of interventions aimed at improving the organisational management of waiting lists or restructuring the intake assessment/referral process
3. Interventions aimed at restructuring referral processes: RCTs.
| Study | Outcome | Preintervention value | Postintervention value | Effect |
| Direct/open access and direct booking systems | ||||
| McKessock 2001 | Mean waiting time from referral to operation (days) | Intervention: NA Control: NA |
Intervention: 108 days Control: 127 days |
Statistically significant difference (P value 0.003) |
| Thomas 2003 | Median waiting time from referral to initial hospital appointment (IQR, days) | LUTS Intervention: 106 (70‐170) Control: 130 (77‐175) MH Intervention: 65 (41‐107) Control: 65 (48‐96) |
LUTS Intervention (IQR): 36 (24‐64) Control: 75 (39‐99) MH Intervention: 41 (31‐58) Control: 47 (34‐62) |
LUTS Ratio of means (95% CI): 0.7 (0.5 to 0.9) MH Ratio of means (95% CI): 1.0 (0.7 to 1.2) |
| Distant consultancy | ||||
| Leggett 2004 | Mean waiting time for appointments (days) | NA | NA | "Median waiting time in study and control groups were similar (mean 55 days; SD=40)" |
NA: not available.
IQR: interquartile range.
LUTS: lower urinary tract syndrome.
MH: microscopic haematuria
4. Interventions aimed at restructuring referral processes: reanalysed ITS studies.
| Study | Outcome | Postintervention period | Secular trend (SE, P) | Change in level (SE, P) | Change in slope (SE, P) |
| Direct/open access and direct booking systems | |||||
| Lukman 2004 | Proportion of participants waiting less than recommended time threshold (percentage of participants with moderate/severe lesions waiting less than 4 weeks) every 3 months | 24 months | +0.86% (SE 3.78 P value 0.83) | ‐14.26% (SE 19.83, P value 0.50) | +6.29% (SE 12.26, P value 0.62) |
| Mallard 2004 | Waiting time (days) per month | 12 months | +1.40 (SE 0.8, P value 0.13) | ‐25.20 (SE 3.83, P value < 0.001) | ‐3.03 (SE 0.92, P value 0.005) |
| Distant consultancy | |||||
| Hofstetter 2010 | Waiting time (months) per year | 6 years | ‐0.04 (SE 0.06, P value 0.51) |
‐0.69 (SE 0.55, P value 0.23) | ‐0.21 (SE 0.13, P value 0.15) |
| Single generic waiting list | |||||
| Leach 2004 | Number of participants waiting less than recommended time threshold (less than 9 months) per month |
14 months | +9.44 (SE 10.93, P value 0.40) | ‐20.59 (SE 22.67, P value 0.37) | +2.75 (SE 12.69, P value 0.86) |
| Number of participants waiting within a recommended time threshold (between 9 and 18 months) per month |
‐3.30 (SE 7.78, P value 0.68) | ‐5.28 (SE 16.20, P value 0.75) | ‐6.59 (SE 8.73, P value 0.46) | ||
Seven studies evaluated the effects of improving organisational management and restructuring of referral processes: three RCTs (Leggett 2004; McKessock 2001; Thomas 2003) and four ITS studies (Hofstetter 2010;Leach 2004; Lukman 2004; Mallard 2004).
Effects of direct/open access and direct booking systems
Four studies (two ITS: Lukman 2004; Mallard 2004; and two RCTs: McKessock 2001; Thomas 2003) evaluated the effects of direct/open access or direct booking systems.
Both trials showed beneficial effects of direct/open access interventions on waiting times: One of the trials (McKessock 2001) enrolling 232 participants showed a reduction in mean waiting time for those randomly assigned to direct access to laparoscopic sterilisation as compared with control participants (108 vs 127 days, P value 0.003), and the other (Thomas 2003) showed that introducing an open access urological investigation service reduced waiting times for participants with lower urinary tract syndrome by 30% (ratio of means 0.7, 95% CI 0.5 to 0.9), although no significant difference was found for those with microscopic haematuria (total n = 959). McKessock 2001 suffered from high attrition, as only 10 out of 75 participants allocated to the intervention group actually received the intervention.
Both trials evaluated costs. In McKessock 2001, evaluation of total costs to patients and total NHS costs showed no differences between intervention and control groups. Thomas 2003 reported no differences in costs between intervention and control groups.
One ITS study (Mallard 2004) showed that open access resulted in a direct reduction in mean waiting times for paediatric patients (total n = 7594) at health clinic appointments (step change: ‐25.20 days, SE 3.83, P value < 0.001; slope change: ‐3.03 days/mo; SE 0.92, P value 0.005).
The other ITS study (Lukman 2004) showed no effect of introducing a direct booking system on the proportion of participants (n = 2501) ‐ with moderate or severe cellular abnormalities of the uterine cervix ‐ who received a colposcopy appointment within the recommended four weeks of waiting time (step change: ‐14.26%; SE 19.83, P value 0.50; slope change: 6.29; SE 12.26, P value 0.62).
Effects of distant consultancy
Two studies ‐ one trial (Leggett 2004) and one ITS study (Hofstetter 2010) ‐ evaluated the effects of distance consultancy on waiting times.
Both studies showed no effect of distance consultancy on waiting times: Leggett 2004 showed no effect of using instant photography to diagnose and manage dermatology referrals on the waiting time of dermatology patients (n = 136) (mean 55 days, SD = 40, P value > 0.05), and the ITS study (Hofstetter 2010) showed no effect of introducing telemedicine to manage rural ENT patients (n = 1690) on waiting times (step change: ‐0.69 months; SE 0.55, P value 0.23; slope change: ‐0.21 months each year; SE 0.13, P value 0.15).
Leggett 2004 suffered from high attrition: 36.6% of intervention participants did not receive the intervention, and among those who did, 38.0% still needed to see a dermatologist face‐to‐face.
Effects of introducing generic waiting lists (pooling of patients)
One ITS study (Leach 2004) showed no effect of introducing a generic waiting list for non‐complex spinal surgery on the number of participants waiting less than nine months (step change: ‐20.59 participants; SE 22.67, P value 0.37) and on the number of participants waiting between nine months and 18 months (step change: ‐5.28 participants; SE 16.20, P value 0.75).
Discussion
The aim of this review was to identify interventions that are effective in reducing waiting time for elective procedures.
Summary of main results
See Table 1 and Table 2 for main results.
The review included eight studies evaluating the effects of interventions aimed at reducing waiting times for elective procedures: three RCTs (Leggett 2004; McKessock 2001; Thomas 2003) and five reanalysed ITS studies (Hofstetter 2010; Leach 2004; Lowthian 2011; Lukman 2004; Mallard 2004). One study evaluated interventions aimed at prioritising demand (Lowthian 2011), and seven studies evaluated interventions aimed at restructuring the intake assessment/referral process (Hofstetter 2010; Leach 2004; Leggett 2004; Lukman 2004; Mallard 2004; McKessock 2001; Thomas 2003). The included studies were heterogeneous in terms of types of interventions, target conditions and elective procedures, study design and outcome measures, thus hindering meta‐analysis.
The Lowthian 2011 study, which evaluated a system using explicit referral guidelines for streamlining patients according to the urgency of their condition, showed a reduced number of semi‐urgent patients waiting longer than the recommended time, but unchanged numbers of urgent and non‐urgent elective patients waiting too long. However, no information was provided on how the number of patients not waiting too long was affected. Of concern in interpreting the results of this study are the discrepancies found between the numbers reported in text and in figures, which suggest that a participant may have been counted more than once. Another problem is that we cannot say how the results are affected by co‐interventions introduced during the intervention period.
Among the seven studies that evaluated interventions aimed at restructuring the intake assessment/referral process, three studies showed decreased waiting time (Mallard 2004; McKessock 2001; Thomas 2003), and four studies reported no effect (Hofstetter 2010; Leach 2004; Leggett 2004; Lukman 2004). However, important caveats were related to all of these studies; their results should therefore be interpreted with caution.
In McKessock 2001, only 14/75 (18.7%) women referred from intervention practices for laparoscopic sterilisation were eligible for direct referral according to the inclusion criteria, and of these, only 10/14 women actually received the intervention. In the discussion, study authors highlighted that participants seemed to prefer the current referral system and suggest the need to conduct preliminary studies before implementing new services based on assumptions of acceptance of revised clinical pathways. In one study (Mallard 2004), it was unclear exactly what the definition of waiting time was, and if the waiting time reported also included prescheduled appointments (i.e. appointments for which shorter waiting time presumably was not desired). In another study (Lukman 2004), extra clinics were introduced after the start of the main intervention to meet extra demand, and a dedicated colposcopy nurse was introduced halfway through the intervention period, which complicates the interpretation of results.
Distant consultancy resulted in no effect on improvement of mean waiting time (Hofstetter 2010; Leggett 2004). However, in Hofstetter 2010, outcomes measured in the preintervention and postintervention periods differed: For control participants, time from referral to specialist appointment and presumably also to treatment was measured, while for intervention participants, time from referral to examination/consultation was measured. It remains unclear when participants who received telehealth consultations received treatment. Also, study authors provided no information on the number of unsuccessful telehealth appointments for which a face‐to‐face appointment was required. In Leggett 2004, a large proportion of intervention participants could not be diagnosed through the use of instant photography, which indicates that this approach may not be suitable for some dermatological conditions. An intervention aimed at restructuring means of queuing using a generic waiting list showed no effect of the intervention on the number of participants waiting less than the recommended time threshold (Leach 2004). In this study, seven out of 10 consultants participated, but it was not clear whether preintervention and/or postintervention data related to waiting times for all 10 surgeons, or for only the seven who agreed to the intervention, which may have affected results of reanalysis of this study.
We found no studies evaluating interventions directly aimed at rationing demand or increasing capacity.
On the basis of available evidence, it is difficult to draw any firm conclusions about the effectiveness of interventions to reduce waiting time.
Overall completeness and applicability of evidence
Despite media and journal coverage given to waiting time policies implemented nationwide in different health systems, we could not find and include studies with usable empirical data measuring their impact. This was disappointing, as it implies failure of researchers to adequately evaluate policy initiatives to improve waiting times.
Most included studies were conducted in the UK (5/8) or in the USA (2/8) ‐ both high‐income countries but with different healthcare systems. No studies were conducted in low‐ and middle‐income countries. All interventions targeted elective therapeutic or test‐and‐treat procedures.
Most of the evaluated interventions were aimed at improving the organisational management of waiting lists or restructuring the intake assessment/referral process. These studies however, did not cover all possible interventions, for example, resource sharing strategies or remuneration schemes. Only one study involved interventions aimed at prioritising demand, but no study evaluated interventions including co‐payments, practice guidelines or clinical priority scores. No study evaluated the effects of interventions aimed at expanding capacity (e.g. providing additional funding to the public sector, subsidising or facilitating access to the private sector).
None of the included studies reported on adverse effects of the interventions (e.g. morbidity, mortality), and only two studies reported on costs.
Quality of the evidence
More than half of the evidence included in this review was derived from non‐randomised low‐quality time series studies with no control groups, involving only one or two intervention sites, which we reanalysed to remove the risk of bias due to secular trends in uncontrolled data.
The overall quality of the evidence for all outcomes ranged from low to very low, which is why no robust conclusions regarding the effectiveness of the evaluated interventions can be drawn. The quality of evidence for the effectiveness of interventions aimed at rationing and/or prioritising demand was low, as only one reanalysed ITS study (Lowthian 2011), conducted at a single site, was included in this review. Even though this study showed a beneficial effect of streamlining services for semi‐urgent patients, the intervention was not independent of other changes, which made it difficult to isolate the effect of the main intervention. This type of intervention needs further investigation, during controlled conditions, to determine its effectiveness in reducing waiting times for elective surgery.
The quality of the evidence on the effectiveness of direct booking/open access or same‐day scheduling was low because of high risk of bias in most studies (3/4). Bias was mainly due to high attrition/contamination (McKessock 2001) and other changes concurrent with the main intervention (Lukman 2004; Mallard 2004). One of the four studies was at low risk of bias (Thomas 2003). These interventions, all of which involve the provision of more accessible services, show some promise, as three of the four studies show a beneficial intervention effect in terms of reduced waiting times.
Data on the effectiveness of distant consultancy on waiting time were limited to two studies, which evaluated two different types of distance consultancy for two different conditions: one providing specialist consultations for ENT patients through telemedicine (Hofstetter 2010), and the other using teledermatology for specialist consultations (Leggett 2004). Both studies were at high risk of bias ‐ the first study because of selective reporting bias, as it appeared to measure and report different things in the preintervention and postintervention periods and did not provide information on the numbers of successful or failed teleconsultations; the latter had high risk of attrition bias, with only 25.4% of intervention participants who received a 'photo‐diagnosis' not needing to be seen by a dermatologist, while 38.0% needed to be seen face‐to‐face for further management, and for 36.6%, photo‐diagnosis was not possible. Neither study showed a significant intervention effect.
Finally, evidence on the effect of introducing a generic waiting list in spinal surgery on the number of patients waiting less than a recommended time threshold or within a recommended time period was limited to only one observational study with high risk of bias (Leach 2004).
Potential biases in the review process
The search strategy used in this review was carefully developed by an experienced information technologist, and a comprehensive search, involving a large number of databases, was performed. One review author sifted all references identified by the electronic searches, excluding papers that clearly were not eligible, and two review authors independently assessed all potentially eligible titles and abstracts against the eligibility criteria to ensure that no important references were missed. We also searched reference lists of included studies and contacted study authors about other published or unpublished studies. In addition, we searched trials registers for ongoing trials, along with a number of sources of grey literature. Despite all this, we cannot exclude the possibility that important references may have been missed.
Few studies were identified for inclusion in this review, and none claimed negative results that could be suggestive of publication bias. Unfortunately, because too few studies were identified for inclusion in this review, we could not assess publication bias.
Agreements and disagreements with other studies or reviews
The impact of different national policies for regulating and containing length of waiting times has been evaluated by reports (Rachlis 2005; Siciliani 2013; Willcox 2007), overviews and reviews (Kreindler 2010; Miller 2008). However these documents do not provide data on the effects of specific interventions used to enforce or implement national and regional policies on waiting times for elective procedures.
Authors' conclusions
Implications for practice.
Decision‐makers should be aware that for interventions aimed at prioritising demand (e.g. co‐payment, explicit referral criteria or practice guidelines, clinical priority scores), evidence is incomplete, and for those aimed at rationing demand or expanding capacity (e.g. providing additional funding to the public sector, subsidising or facilitating access to the private sector), evidence is lacking. Thus, implementation of such interventions should be monitored for both effectiveness and possible drawbacks.
Implications for research.
Despite the importance of long waiting times as a relevant healthcare problem, only scarce evidence of low quality is presently available.
RCTs and cluster‐RCTs are ideally the best study designs to be applied to fill in this knowledge gap. Large and robust experimental studies might be difficult and expensive to set up, and represent unfamiliar ground in policy‐making. However robust and useful evidence on the effectiveness of interventions aimed at reducing waiting time could be obtained with good quality interrupted time series studies, which are both feasible and practical.
Some points must be taken into consideration before one can plan and embark on a study addressing the effectiveness of any intervention to reduce waiting times for elective procedures.
Greater attention should be paid to the quality of study designs, and cluster‐RCTs should be carefully controlled for contamination bias across interventions among the included clusters.
Researchers designing ITS studies should adhere to the quality criteria described by the EPOC Group (EPOC 2013), for example, allow a sufficient number of data points before as well as after the intervention to enable reliable statistical inference, and use formal tests for trend, taking into account any secular trends.
A reliable primary outcome should be chosen: It is still uncertain which could be the most appropriate outcome measure ‐ among the many available ‐ that could best depict the 'long waiting time phenomenon'; however the proportion of patients waiting above a recommended time threshold appears to be suitable in terms of practical relevance, effective communication of results and statistical reliability. As interventions tend to act on supply or on demand, process outcomes ‐ such as increase in supply or decrease in demand ‐ should also be monitored to evaluate tenure of the causal mechanism between variation on supply/demand and waiting time; if the number of participants waiting too long is provided, the number of participants not waiting too long should also be reported.
Future research
Research evaluating interventions aimed at rationing services and/or prioritising demands or interventions aimed at expanding capacity is lacking and therefore needed.
Future researchers should make greater efforts to collect and analyse data on undesired consequences of interventions, as well as on economic outcomes in different health settings.
Interventions showing some promise (e.g. direct booking, open access, same‐day scheduling) but also streamlining of services needs further evaluation.
Interventions involving advanced technology (i.e. distance consultancy) (telemedicine or photo specialist consultations) may need reevaluation in the light of rapidly evolving new and better technology.
Acknowledgements
We thank Michelle Fiander for having developed the search strategy for this review and for running the electronic search.
We thank Monica Taljaard, Tomas Pantoja, Sam Sheps and Luigi Siciliani for their very useful comments on the review, and Julia Worswick for her assistance throughout the review process.
This review was funded by the Italian Ministry of Health Research Fund (grant number: GR ‐ 2010 ‐ 2317133) and by the NIHR Cochrane EPOC Programme Grant.
Appendices
Appendix 1. MEDLINE search strategy
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) < 1946 to November 2012>. 1 Waiting lists/ (7789) 2 ((wait or waiting) adj2 (time or times or list or lists)).ti. (2254) 3 ((wait or waiting) adj2 (time or times or list or lists) adj4 (reduce? or reduction or eliminat$ or lower or fewer or intervention or policy or policies or reform$ or effectiveness or impact or improv$ or organi?ational$ or quality)).ab. (1729) 4 (eliminat$ patient? wait$ or improv$ patient? wait$ or lower? patient? wait$ or lowering patient? wait$ or reduc$ patient? wait$).ti,ab. (62) 5 or/1‐4 [Waiting Lists/Waiting Times‐MeSH] (9487) 6 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (4186) 7 ((wait$ list? or waitlist?) adj2 (control? or controlled or group? or intervention or trial or study)).ti,ab. (1891) 8 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (4186) 9 Watchful Waiting/ (612) 10 5 not (or/6‐9) [Waiting Lists/Time] (8961) 11 exp transplantation/ (391733) 12 (transplant? or transplantation?).ti,ab,hw. (488983) 13 Emergency Service, Hospital/ or Trauma centres/ or exp Emergency Medical Services/ or exp Emergency Treatment/ or Emergencies/ or Emergency Medicine/ (194007) 14 (emergency or emergencies).ti,hw. (130333) 15 (cell or cells or cellular or molecular$ or animal? or lab or labs or laborator$).ti. (1638971) 16 patient? delay$.ti,ab. (1129) 17 or/11‐16 [terms to exclude] (2251755) 18 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. (819631) 19 exp animals/ not humans.sh. (3807583) 20 18 not 19 [Cochrane RCT Filter 6.4.d Sens/Precision Maximizing] (757438) 21 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or DESIGN$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or GP or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab. (137446) 22 (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab. [added 2.4] (8029) 23 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. (670660) 24 demonstration project?.ti,ab. (1793) 25 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab. (56179) 26 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. (511) 27 trial.ti. or ((study adj3 aim?) or "our study").ab. (533289) 28 (before adj10 (after or during)).ti,ab. (327726) 29 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab,hw. [ML] (92136) 30 ("time series" adj2 interrupt$).ti,ab,hw. [ML] (791) 31 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab. (7481) 32 pilot.ti. (34197) 33 Pilot projects/ [ML] (74430) 34 (clinical trial or controlled clinical trial or multicenter study).pt. [ML] (594981) 35 (multicentre or multicenter or multi‐centre or multi‐center).ti. (25360) 36 random$.ti,ab. or controlled.ti. (669876) 37 (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt. [ML] (363890) 38 "comment on".cm. or review.ti,pt. or randomized controlled trial.pt. [ML] (2695157) 39 (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti. (1288069) 40 exp animals/ not humans.sh. [ML] (3807583) 41 (or/21‐37) not (or/38,39‐40) [EPOC Methods Filter ML 2.4] (1920409) 42 (10 not 17) and 20 [WL RCT] (216) 43 ((10 not 17) and 41) not 42 [WL EPOC] (1773) 44 ((wait or waiting) adj2 (time or times or list or lists) adj4 (reduce? or reduction or eliminat$ or lower or fewer or intervention or policy or policies or reform$ or effectiveness or impact or improv$ or organi?ational$ or quality)).ti. (211) 45 (eliminat$ patient? wait$ or improv$ patient? wait$ or lower? patient? wait$ or lowering patient? wait$ or reduc$ patient? wait$).ti,ab. (62) 46 (or/44‐45) not (or/6‐9,17,42‐43) [Keyword Results] (99) 47 review.pt. or ((systematic adj3 review?) or metaanalys$ or meta‐analys$ or literature review).ti. (1779206) 48 (10 not 17) and 47 (187) 49 42 not 48 [RCT Results] (200) 50 43 not 48 [EPOC EPOC Filter Results] (1773) 51 42 not 49 [Reviews Set 1] (16) 52 46 and 47 [KW Reviews Set 2] (4) 53 or/51‐52 [Reviews‐‐Results to export] (20) 54 46 not 53 [Keyword Results to export] (95)
An update of the above reported search was performed in November 2013.
Appendix 2. Additional search strategies
ABI Inform, ProQuest
January 31, 2013
all(waiting list or waiting lists or wait lists or waiting list) NOT (all(organ or organs or transplant or transplantation or emergency room* or emergency department* or trauma ) or ti(therapy))
Canadian Research Index
Jan 5, 2012 (((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review)))
Communication Disorders Database, ProQuest
Jan 5, 2012 (49 results) (((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review))) =49
Dissertations, UK & Ireland; and Dissertations & Theses, ProQuest
November 28, 2013 (35 results)
November 16, 2012 (192 results)
(((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review))) NOT (((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) AND (randomi?ed or randomly or controlled or trial or study or pilot or effectiveness or organi?ational or improv* or experimental or pre‐intervention or post‐intervention or workshop* or quasiexperiment* or quasi‐experiment* or (time n/10 period?) or (time n/10 series)) )) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review)))
ECON‐LIT (ProQuest)
January 31, 2013 (129 results)
November 16, 2012 (158 results)
(ti("wait* list*") OR ab("wait* list*")) AND (health or healthcare or primary care or referral* or physician* or doctor* or non‐urgent or non‐emerg* or elective). No limits
EMBASE Classic + EMBASE < 1947 to 2012 November 08>
1 ((wait or waiting) adj2 (time or times or list or lists)).ti. (2773) 2 wait target?.ti,ab. (17) 3 ((wait or waiting) adj2 (time or times or list or lists) adj4 (reduce? or reduction or eliminat$ or lower or fewer or intervention or policy or policies or reform$ or effectiveness or impact or improv$ or organi?ational$ or quality)).ab. (2347) 4 (eliminat$ patient? wait$ or improv$ patient? wait$ or lower? patient? wait$ or lowering patient? wait$ or reduc$ patient? wait$).ti,ab. (95) 5 or/1‐4 [Wait Lists/Time‐‐EMTREE not available for this concept] (4802) 6 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (5692) 7 ((wait$ list? or waitlist?) adj2 (control? or controlled or group? or intervention or trial or study)).ti,ab. (2423) 8 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (5692) 9 watchful waiting/ (1031) 10 exp organ transplantation/ or emergency/ or emergency health service/ or emergency ward/ or rescue personnel/ (392259) 11 (transplant? or transplantation?).ti,ab,hw. (598772) 12 (emergency or emergencies).ti,hw. (208162) 13 donor?.ti,ab,hw. (289744) 14 (cell or cells or cellular or molecular$ or animal? or lab or labs or laborator$).ti. (1982518) 15 patient? delay$.ti,ab. (1493) 16 or/6‐15 [terms to exclude] (2840055) 17 5 not 16 (2796) 18 controlled clinical trial/ or controlled study/ or randomized controlled trial/ [EM] (3980271) 19 (book or conference paper or editorial or letter or review).pt. not randomized controlled trial/ [Per BMJ Clinical Evidence filter] (3836581) 20 (random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not randomized controlled trial/ [Per BMJ Clinical Evidence filter] (47405) 21 (animal$ not human$).sh,hw. (3771897) 22 18 not (or/19‐21) [Trial filter per BMJ CLinical Evidence] (2612246) 23 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or DESIGN$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or GP or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab. (175866) 24 (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab. [added 2.4] (10294) 25 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. (1448525) 26 demonstration project?.ti,ab. (2224) 27 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab. (80635) 28 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. (689) 29 trial.ti. or ((study adj3 aim?) or "our study").ab. (727350) 30 (before adj10 (after or during)).ti,ab. (439645) 31 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab. (9936) 32 pilot.ti. or (pilot adj (project? or study or trial)).ab. (78137) 33 (multicentre or multicenter or multi‐centre or multi‐center).ti. (34541) 34 random$.ti,ab. or controlled.ti. (842407) 35 (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. (555613) 36 *experimental design/ or *pilot study/ or quasi experimental study/ (5386) 37 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab. (120056) 38 ("time series" adj2 interrupt$).ti,ab. (913) 39 or/23‐38 (3656448) 40 (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti. (1594402) 41 (animal$ not human$).sh,hw. (3771897) 42 review.ti. (286471) 43 39 not (or/40‐42) [EPOC Filter 2.4a EMBASE] (3203340) 44 (17 and 22) not (or/40‐42) [RCT Results] (346) 45 (17 and 43) not 44 [EPOC Filter Results] (1576)
Cochrane Central Register of Controlled Trials, EBM Reviews, Ovid <October 2012> 1 Waiting lists/ (204) 2 ((wait or waiting) adj2 (time or times or list or lists)).ti,kw. (97) 3 ((wait or waiting) adj2 (time or times or list or lists) adj4 (reduce? or reduction or eliminat$ or lower or fewer or intervention or policy or policies or reform$ or effectiveness or impact or improv$ or organi?ational$ or quality)).ab. (446) 4 (eliminat$ patient? wait$ or improv$ patient? wait$ or lower? patient? wait$ or lowering patient? wait$ or reduc$ patient? wait$).ti,ab. (4) 5 or/1‐4 [Waiting Lists/Waiting Times‐MeSH] (658) 6 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (327) 7 ((wait$ list? or waitlist?) adj2 (control? or controlled or group? or intervention or trial or study)).ti,ab. (1487) 8 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (327) 9 Watchful Waiting/ (31) 10 exp transplantation/ (8073) 11 (transplant? or transplantation?).ti,ab,hw. (14302) 12 Emergency Service, Hospital/ or Trauma centres/ or exp Emergency Medical Services/ or exp Emergency Treatment/ or Emergencies/ or Emergency Medicine/ (5206) 13 (emergency or emergencies).ti,hw. (3487) 14 (cell or cells or cellular or molecular$ or animal? or lab or labs or laborator$).ti. (17817) 15 patient? delay$.ti,ab. (300) 16 donor?.ti,ab,hw. (3773) 17 or/6‐16 [terms to exclude] (40068) 18 5 not 17 (168) 19 18 not placebo?.ti,ab,hw. (161)
ACP Journal Club <1991 to October 2012>, Cochrane Database of Systematic Reviews <2005 to October 2012>, Database of Abstracts of Reviews of Effects <4th Quarter 2012>, Health Technology Assessment <4th Quarter 2012>, EBM Reviews, Ovid 1 Waiting lists/ (22) 2 ((wait or waiting) adj2 (time or times or list or lists)).ti,kw,hw. (41) 3 ((wait or waiting) adj2 (time or times or list or lists) adj4 (reduce? or reduction or eliminat$ or lower or fewer or intervention or policy or policies or reform$ or effectiveness or impact or improv$ or organi?ational$ or quality)).ab. (18) 4 (eliminat$ patient? wait$ or improv$ patient? wait$ or lower? patient? wait$ or lowering patient? wait$ or reduc$ patient? wait$).ti,ab. (0) 5 or/1‐4 [Waiting Lists/Waiting Times‐MeSH] (58) 6 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (21) 7 ((wait$ list? or waitlist?) adj2 (control? or controlled or group? or intervention or trial or study)).ti,ab. (44) 8 (waiting room? or waiting area? or watchful wait$ or "wait and see" or "wait until").ti,ab. (21) 9 Watchful Waiting/ (0) 10 exp transplantation/ (257) 11 (transplant? or transplantation?).ti,ab,hw,kw. (963) 12 Emergency Service, Hospital/ or Trauma centres/ or exp Emergency Medical Services/ or exp Emergency Treatment/ or Emergencies/ or Emergency Medicine/ (99) 13 (emergency or emergencies).ti,hw,kw. (411) 14 (cell or cells or cellular or molecular$ or animal? or lab or labs or laborator$).ti. (987) 15 patient? delay$.ti,ab. (4) 16 donor?.ti,ab,hw. (136) 17 or/6‐16 [terms to exclude] (2297) 18 5 not 17 (40) 19 18 not placebo?.ti,ab,hw,kw. (38)
Nursing, ProQuest :
Nov 15, 2012 (231 results) ((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* NEAR/7 group*) OR (wait* list* NEAR/7 control*) OR (wait* list* NEAR/7 cohort?) OR (wait* list* NEAR/7 random*))) AND (randomi?ed OR randomly OR controlled OR trial OR study OR pilot OR effectiveness OR organi?ational OR improv* OR experimental OR pre‐intervention OR post‐intervention OR workshop* OR quasiexperiment* OR quasi‐experiment* OR (time NEAR/10 period?) OR (time NEAR/10 series))) NOT (transplant* OR organ donat*)) NOT ti(emergency)) NOT ti(review)
PAIS, ProQuest
November 15, 2012 (71 results) & Feb 7, 2013 (9 results)
(((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective OR hospital OR hospitals OR hospitalized OR hospitalised OR inpatient* OR outpatient* OR out‐patient* OR surgery)) NOT (emergency OR emergencies OR transplant* OR "organ* donor*" OR donation* OR "wait* list control*")) AND ((quality NEAR/2 improv*) OR (quality NEAR/2 manag*) OR policy OR policies OR organisation* OR organization* OR reform OR reforms OR government* OR qualitativ* OR incentiv* OR theory OR theories)
PILOTS (Published International Literature on Traumatic Stress)
January 5, 2012: Search identified 23 citations, none relevant.
((ti("wait* list*") OR ab("wait* list*")) AND (health or healthcare or primary care or referral* or physician* or doctor* or non‐urgent or non‐emerg* or elective)) NOT (("wait* list*" p/5 group*) OR ("wait* list*" p/5 control*) OR ("wait* list*" p/5 random*))
Political Science, ProQuest
Nov 16, 2012 (106 results)
Social Services Abstracts, ProQuest
November 16, 2012 January 6, 2012
Strategy 1:
(((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review))) NOT (((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) AND (randomi?ed or randomly or controlled or trial or study or pilot or effectiveness or organi?ational or improv* or experimental or pre‐intervention or post‐intervention or workshop* or quasiexperiment* or quasi‐experiment* or (time n/10 period?) or (time n/10 series)) )) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review)))
Strategy 2: (((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) AND (randomi?ed or randomly or controlled or trial or study or pilot or effectiveness or organi?ational or improv* or experimental or pre‐intervention or post‐intervention or workshop* or quasiexperiment* or quasi‐experiment* or (time n/10 period?) or (time n/10 series)) )) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review)))
Sociological Abstracts, ProQuest
November 16, 2012 (((((((ti("wait* list*") OR ti("wait* time*") OR ab("wait* list*") OR ab("wait* time*")) AND (health OR healthcare OR primary care OR referral* OR physician* OR doctor* OR non‐urgent OR non‐emerg* OR elective)) NOT ((wait* list* n/7 group*) or (wait* list* n/7 control*) or (wait* list* n/7 cohort?) or (wait* list* n/7 random*))) AND (randomi?ed or randomly or controlled or trial or study or pilot or effectiveness or organi?ational or improv* or experimental or pre‐intervention or post‐intervention or workshop* or quasiexperiment* or quasi‐experiment* or (time n/10 period?) or (time n/10 series)) )) NOT ((transplant* or organ donat*) OR ti(emergency) OR ti(review))) = 115
Web of Knowledge Science & Social Sciences Citation Indexes
November 28, 2013 & November 13, 2012
| Web of Knowledge Science & Social Sciences Citation Indexes Search Strategy; Databases=SCI‐EXPANDED, SSCI Timespan=All Years | ||
| Search Date | November 13, 2012 to November 28, 2013 | |
| Set | Results | Search query |
| 183 | #25 OR #28 [2013 results] | |
| 29 | 733 | #25 OR #28 [2012 results] |
| 28 | 688 | (#9 not #16) and #17 |
| Refined by: [excluding] Web of Science Categories=( TRANSPORTATION SCIENCE TECHNOLOGY OR ENGINEERING MULTIDISCIPLINARY OR FOOD SCIENCE TECHNOLOGY OR OPERATIONS RESEARCH MANAGEMENT SCIENCE OR COMPUTER SCIENCE HARDWARE ARCHITECTURE OR STATISTICS PROBABILITY OR COMPUTER SCIENCE SOFTWARE ENGINEERING OR MATHEMATICAL COMPUTATIONAL BIOLOGY OR ENGINEERING INDUSTRIAL OR PHYSICS FLUIDS PLASMAS OR PERIPHERAL VASCULAR DISEASE OR PHYSICS ATOMIC MOLECULAR CHEMICAL OR ENGINEERING CIVIL OR PLANNING DEVELOPMENT OR PHYSICS CONDENSED MATTER OR SOCIAL SCIENCES MATHEMATICAL METHODS OR TRANSPORTATION OR TRANSPLANTATION OR ENGINEERING MANUFACTURING OR BUSINESS FINANCE OR ENGINEERING ELECTRICAL ELECTRONIC OR AUTOMATION CONTROL SYSTEMS OR ERGONOMICS OR PHYSICS MATHEMATICAL OR HOSPITALITY LEISURE SPORT TOURISM OR COMPUTER SCIENCE INFORMATION SYSTEMS OR INSTRUMENTS INSTRUMENTATION OR NUTRITION DIETETICS OR COMPUTER SCIENCE CYBERNETICS OR PHYSICS MULTIDISCIPLINARY OR BIOTECHNOLOGY APPLIED MICROBIOLOGY OR CELL BIOLOGY OR MATERIALS SCIENCE MULTIDISCIPLINARY OR COMPUTER SCIENCE ARTIFICIAL INTELLIGENCE OR MATHEMATICS OR ENGINEERING CHEMICAL OR COMPUTER SCIENCE THEORY METHODS OR MATHEMATICS INTERDISCIPLINARY APPLICATIONS OR SPORT SCIENCES OR TELECOMMUNICATIONS OR MATHEMATICS APPLIED OR ZOOLOGY ) | ||
| 27 | 988 | (#9 not #16) and #17 |
| 26 | 356 | ((#9 NOT #16) AND #17) NOT #25 |
| 25 | 632 | #23 or #24 |
| 24 | 632 | ((#9 NOT #16) AND #17) AND TS=(health or healthcare or medicine or hospital* or surgery or surgical or inpatient* or outpatient* or "out‐patient*" or family doctor* or family practitioner* or family physician* or family practice or general practitioner* or general practice or primary care or treatment or therapy or physio* or cancer or oncolog* or pediatrician* or specialist* or orthopedic* or orthopaedic* or hip or hips or joints) |
| 23 | 397 | ((#9 NOT #16) AND #17) AND TI=(health or healthcare or medicine or hospital* or surgery or surgical or inpatient* or outpatient* or "out‐patient*" or family doctor* or family practitioner* or family physician* or family practice or general practitioner* or general practice or primary care or treatment or therapy or physio* or cancer or oncolog* or pediatrician* or specialist* or orthopedic* or orthopaedic* or hip or hips or joints) |
| 22 | 39 | (#20 OR #21) AND Document Types=(Review) |
| 21 | 1 | ((#9 not #16) AND #19) AND Document Types=(Review) |
| 20 | 39 | ((#9 not #16)) AND Document Types=(Review) |
| 19 | 45613 | TI=((systematic NEAR/3 review?) or metaanalys* or meta‐analys* or "literature review") |
| 18 | 988 | (#9 not #16) and #17 |
| 17 | 8525837 | (TI=(random* OR trial OR study OR pilot OR piloted or piloting or comparative OR tool OR tools OR innovat* OR organisation* OR organization* OR impact OR influence OR changing OR quality OR implement* or intervention*)) OR TS=(random* OR controlled OR "control group" or "control groups" OR pilot OR innovat* OR organisational* OR organizational* OR impact OR "quality improv*" or quality manag* OR implement* or team or teams or team‐based or multifaceted or multi‐faceted or complex intervention* or cooperat* or "patient focus*" or "physician led" or "nurse led" or "pharmacist led" or "nurse practitioner" or "skill mix") |
| 16 | 2845710 | #15 OR #14 OR #13 OR #12 OR #11 OR #10 |
| 15 | 26 | TI=("patient? delay*") |
| 14 | 2376784 | TI=(cell or cells or cellular or molecular* or animal? or lab or labs or laborator*) |
| 13 | 52661 | TI=(emergency or emergencies) |
| 12 | 127493 | TS=("hospital emergency service" or "trauma centres" or "emergency medical services" or "emergency treatment" or emergencies or "emergency medicine" OR "emergency room*") |
| 11 | 309957 | TI=(transplant* or transplantation or organ or organs or cadaver*) OR TS=((organ near/3 donor*) or (organ* near/3 donat*) or (cadaver* near/3 organ*)) |
| 10 | 308791 | TS=(transplantation) |
| 9 | 4348 | #5 not (#6 or #7 or #8) |
| 8 | 1299 | TS=("watchful waiting") |
| 7 | 135 | TI=(("wait* list*" or waitlist*) NEAR/2 (control or controlled or group* or intervention or trial or study)) OR TS=(("wait* list?" or waitlist?) NEAR/2 (control or controlled or group* or intervention or trial or study)) |
| 6 | 3716 | TI=("waiting room*" or "waiting area*" or "watchful wait*" or "wait and see" or "wait until") OR TS=("waiting room*" or "waiting area*" or "watchful wait*" or "wait and see" or "wait until") |
| 5 | 4485 | #4 OR #3 OR #2 OR #1 |
| 4 | 45 | TI=("eliminat* patient* wait*" or "improv* patient* wait*" or "lower* patient* wait*" or "lowering patient* wait*" or "reduc* patient* wait*") OR TS=("eliminat* patient* wait*" or "improv* patient* wait*" or "lower* patient* wait*" or "lowering patient* wait*" or "reduc* patient* wait*") |
| 3 | 226 | Title=((wait or waiting) NEAR/2 (time or times or list or lists) NEAR/4 (reduce? or reduction or eliminat* or lower or fewer or intervention or policy or policies or reform* or effectiveness or impact or improv* or organi?ational* or quality)) |
| 2 | 3537 | TI=((wait or waiting) NEAR/2 (time or times or list or lists)) |
| 1 | 1399 | Topic=("waiting lists") |
Worldwide Political Science Abstracts, ProQuest
Nov 16, 2012 (11 results) (ti("wait* list*") OR ab("wait* list*")) AND (health or healthcare or primary care or referral* or physician* or doctor* or non‐urgent or non‐emerg* or elective) [No limits]
CINAHL EbscoHost
Run November 12, 2012
| # | Query | Results |
| 1 | (MH "Quasi‐Experimental Studies") | 5,248 |
| 2 | TI ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) or AB ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) | 131,711 |
| 3 | TI ( pre‐test* or pretest* or posttest* or post‐test* ) or AB ( pre‐test* or pretest* or posttest* or "post test* ) OR TI ( preimplement*" or pre‐implement* ) or AB ( pre‐implement* or preimplement* ) | 6,201 |
| 4 | MH Experimental Studies or Community Trials or Community Trials or Pretest‐Posttest Design + or Quasi‐Experimental Studies + Pilot Studies or Policy Studies + Multicenter Studies | 30,735 |
| 5 | TI ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) or AB ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) | 9,402 |
| 6 | MH "Multiple Time Series" or MH "Time Series" | 1,201 |
| 7 | TI pre w7 post or AB pre w7 post | 8,019 |
| 8 | TI ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) or AB ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) | 10,891 |
| 9 | TI ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) or AB ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) | 44,180 |
| 10 | AB ( before* n10 during or before n10 after ) or AU ( before* n10 during or before n10 after ) | 29,013 |
| 11 | TI time series | 218 |
| 12 | AB time series | 1,570 |
| 13 | AB "before‐and‐after" | 15,296 |
| 14 | (MH "Pilot Studies") | 26,572 |
| 15 | TI pilot | 10,293 |
| 16 | TI ( collaborativ* or collaboration* or tailored or personalised or personalized ) or AB ( collaborativ* or collaboration* or tailored or personalised or personalized ) | 33,761 |
| 17 | (intervention n6 clinician*) or (intervention n6 community) or (intervention n6 complex) or (intervention n6 design*) or (intervention n6 doctor*) or (intervention n6 educational) or (intervention n6 family doctor*) or (intervention n6 family physician*) or (intervention n6 family practitioner*) or (intervention n6 financial) or (intervention n6 GP) or (intervention n6 general practice*) Or (intervention n6 hospital*) or (intervention n6 impact*) Or (intervention n6 improv*) or (intervention n6 individualize*) Or (intervention n6 individualise*) or (intervention n6 individualizing) or (intervention n6 individualising) or (intervention n6 interdisciplin*) or (intervention n6 multicomponent) or (intervention n6 multi‐component) or (intervention n6 multidisciplin*) or (intervention n6 multi‐disciplin*) or (intervention n6 multifacet*) or (intervention n6 multi‐facet*) or (intervention n6 multimodal*) or (intervention n6 multi‐modal*) or (intervention n6 personalize*) or(intervention n6 personalise*) or (intervention n6 personalizing) or (intervention n6 personalising) or (intervention n6 pharmaci*) or (intervention n6 pharmacist*) or (intervention n6 pharmacy) or (intervention n6 physician*) or (intervention n6 practitioner*) Or (intervention n6 prescrib*) or (intervention n6 prescription*) or (intervention n6 primary care) or (intervention n6 professional*) or (intervention* n6 provider*) or (intervention* n6 regulatory) or (intervention n6 regulatory) or (intervention n6 tailor*) or (intervention n6 target*) or (intervention n6 team*) or (intervention n6 usual care) | 36,648 |
| 18 | TI ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) or AB ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) | 1,191 |
| 19 | TI ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) or AB ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) | 283 |
| 20 | TI ( trial or (study n3 aim) or "our study" ) or AB ( (study n3 aim) or "our study" ) | 73,438 |
| 21 | TI random* OR controlled | 30,013 |
| 22 | TI ( multicentre or multicenter or multi‐centre or multi‐center ) or AB random* or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) | 90,896 |
| 23 | TI ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) or AB ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) | 41,234 |
| 24 | TI ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) or AB ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) | 1,347 |
| 25 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 | 380,833 |
| 26 | (MM "Clinical Trials+") | 7,527 |
| 27 | TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) | 6,281 |
| 28 | TI random* or AB random* | 97,366 |
| 29 | TI controlled or AB controlled | 56,119 |
| 30 | TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) | 1 |
| 31 | #26 or #27 or #28 or #29 or #30 | 131,204 |
| 32 | (MH "Waiting Lists") | 2,403 |
| 33 | TI wait list or wait lists or wait time or wait times | 232 |
| 34 | TI wait target* OR AB wait target* | 8 |
| 35 | AB (reduc* or eliminat* or decreas* or short*) n3 (wait or waiting) | 606 |
| 36 | S32 OR S33 OR S34 OR S35 | 2,934 |
| 37 | (MH "Transplantation+") | 20,147 |
| 38 | (MH "Transplant Donors") | 2,795 |
| 39 | (MH "Waiting Rooms") | 308 |
| 40 | TI ( (waiting room* or waiting area or waiting areas ) ) OR AB ( (waiting room* or waiting area or waiting areas ) ) | 621 |
| 41 | (MH "Emergencies") | 3,961 |
| 42 | (MH "Emergency Medical Services+") OR (MH "Emergency Service") | 45,999 |
| 43 | (MH "Emergency Nursing") OR (MH "Emergency Nurses Association") OR (MH "Emergency Nurse Practitioners") OR (MH "Emergency Care") | 23,948 |
| 44 | TI ( emergency or emergencies ) OR AB ( emergency or emergencies ) | 42,206 |
| 45 | TI ( transplant* or donor or donors or (organ* n3 donat*) ) OR AB ( transplant* or donor or donors or (organ n3 donat*) ) | 21,682 |
| 46 | S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 | 115,127 |
| 47 | S36 NOT S46 | 1,798 |
| 48 | S31 and S47 | 105 |
| 49 | ( S25 and S47 ) NOT s48 | 359 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hofstetter 2010.
| Methods |
Study design: re‐analysed ITS study Aim: to determine the relationship between use of telemedicine consultations and changes in patient waiting times, access to care and travel‐related costs Timing: 1992‐2007; before intervention period: from 1992 to December 2001; after intervention period: from January 2002 to 2007 Data collection: retrospective data collected from routine records |
|
| Participants |
Providers: 2 units, 1 audiologist at Norton Sound Health Corporation in Nome and 1 consulting ear nose throat (ENT) specialist at Alaska Native Medical Centre Participants: All 1690 new patients referred to ENT from 1992 to 2007 (people not previously seen by ENT but for whom the opinion or care of the ENT specialist is requested); unknown number of participants in preintervention and postintervention periods Participant baseline characteristics: Age: no information Gender: no information Ethnicity: no information Clinical problem: audiology patients with a medical need (treatment or surgery) who required an ENT specialist consultation Setting: rural area of Alaska Country: USA (state of Alaska) |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
Duration of intervention: 5 years (2002‐2007) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS)? | Low risk | Number of available slots stable during the study period; no change in supply of in‐person appointments |
| Analysed appropriately (ITS)? | Low risk | Data reanalysed by review authors |
| Shape of the intervention effect pre‐specified (ITS)? | Low risk | Data reanalysed by review authors |
| Intervention unlikely to affect data collection (ITS)? | Low risk | Data retrospectively collected from routine records; therefore low risk of bias |
| Knowledge of the allocated interventions adequately prevented during the study (ITS)? | Low risk | Outcomes objective in nature; thus unlikely to be affected by possible unblinded assessment |
| Incomplete outcome data addressed (attrition bias) (ITS)? | Unclear risk | No information provided; not clear whether a truncation had occurred at the end of 2007 |
| Free of selective outcome reporting (reporting bias) (ITS)? | High risk | Study protocol not available ‐ so outcomes reported in the paper cannot be checked against any prespecified outcomes. However, it appears that different details were measured during the preintervention and postintervention periods. Before intervention, the waiting time for the participant to see the consultant face‐to‐face, and presumably get treatment, was measured. After intervention, the waiting time until the consultant sees the store and forward participant data sent by telemedicine was measured. But this is not when the participant gets treatment because no information is available on when the consultant diagnosis and treatment are given to the participant |
| Free of other bias (ITS)? | Unclear risk | No information about whether or not all telehealth consultations were successful, that is, whether all participants who received a specialist consultation through telemedicine could be diagnosed, or if some had to see a specialist face‐to‐face. Telemedicine techniques and equipment may have improved over the study years, thus improving the accuracy of diagnoses made by the consultant |
Leach 2004.
| Methods |
Study design: re‐analysed ITS study Aim: to evaluate effects of implementation of 2 systems for managing generic outpatient waiting list on meeting national targets (3 months for routine outpatient appointment; 6 months for inpatient treatment) Timing: from June 2001 to November 2002; before intervention period: from June 2001 to mid September 2001; after intervention period: from mid September 2001 to November 2002 Data collection: data collected before and after the intervention; not further described |
|
| Participants |
Providers: consultants and secretariat for integration of MRI appointment; number of providers not reported Participants: outpatients referred to neurosurgical services of Hope Hospital site in Salford for elective non‐complex spinal surgery (clear‐cut signs or symptoms of cervical or lumbar neural compromise, and no obvious underlying disease that might require spinal fixation, e.g. rheumatoid arthritis). Total number of participants not provided Participant baseline characteristics: Age: no information Gender: no information Ethnicity: no information Type of spinal surgery: no information Clinical problem: non‐complex (elective) spinal surgery Setting: all neurosurgical services within Greater Manchester Country: UK |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
The managed generic outpatient waiting list begins with a consultant screening all new GP‐referred spinal cases to assess their suitability for inclusion in a pooled waiting list. Participants are then allocated to the next available appointment, irrespective of who the consultant might be. The managed generic surgical waiting list works through a similar process. When consultants list a patient for elective non‐complex spinal surgery, they indicate whether the patient should remain under their care or be entered onto a pooled waiting list. Pooled patients are then allocated dates for surgery sequentially vs
Duration of intervention: approximately 15 months (mid September 2001‐November 2002) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS)? | Unclear risk | Quote: "To reduce waiting times, we employed two strategies. First, managed generic waiting lists were introduced for both initial outpatient appointments and dates for surgery. Subsequently the computerized MRI booking system was integrated with outpatient review appointments" The 2 interventions were implemented at different times; it is unclear whether there would be an impact on the waiting times on the surgical list. No information was given on other possible concurrent interventions |
| Analysed appropriately (ITS)? | Low risk | Reanalysed as ITS by review authors |
| Shape of the intervention effect pre‐specified (ITS)? | Low risk | Data reanalysed by review authors |
| Intervention unlikely to affect data collection (ITS)? | Unclear risk | No information given |
| Knowledge of the allocated interventions adequately prevented during the study (ITS)? | Low risk | Outcomes objective in nature; thus unlikely to be affected by a possible unblinded assessment |
| Incomplete outcome data addressed (attrition bias) (ITS)? | Unclear risk | No information given |
| Free of selective outcome reporting (reporting bias) (ITS)? | Unclear risk | Study protocol not available ‐ so outcomes reported in the paper cannot be checked against any prespecified outcomes |
| Free of other bias (ITS)? | Unclear risk | Only 7 out of 10 consultants participated. Not clear whether preintervention and/or postintervention data relate to waiting times for all 10 surgeons, or only for the 7 who agreed to the intervention. In addition, a problem was observed with discrepancies between numbers reported in the figures ‐ with a greater number of participants undergoing surgery than was reported among those referred by the GP |
Leggett 2004.
| Methods |
Study design: RCT Aim: to establish whether instant photography allows a correct dermatological diagnosis and reduces the number of patients needing an outpatient appointment with a dermatologist Unit of allocation: participants Unit of analysis: participants Unit of analysis issue: no (participants were the unit of both allocation and analysis) Stratification: not done Timing: not reported Data collection: prospective recording by investigators |
|
| Participants |
Providers: 10 GPs participating from 5 practices (but 20 agreed to participate) Patients: 136 patients referred to a GP for a dermatological problem Clinical problem: dermatology referrals Setting: general practices and a teaching hospital Country: UK |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
1 camera was placed in each practice, and GPs were trained for 15 minutes in its use Duration of intervention: not reported |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | This is a feasibility study conducted to assess possible adverse effects; waiting time monitored to assess whether participants initially assessed via photography but for whom diagnosis was not possible suffered from longer waiting times for an appointment than participants not assessed via photography | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The GP took photograph(s) of the skin condition and sent them with a referral letter to the dermatologist in a numbered, sealed envelope. The numbers previously were allocated randomly to study and control groups using a computer program" |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocations were only revealed at hospital where photographs were removed from control group letters and appointments were made as usual" |
| Blinding of participants and personnel (performance bias) Waiting time and number of visits to GPs before and after operation | Low risk | Blinding was not possible; however, waiting time is an objective outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified whether investigators were blinded, but given the objective nature of the assessed outcomes, whether the investigators were blinded was not likely to affect study results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 18 of 71 (25.4%) intervention participants received a photo‐diagnosis and did not need to be seen by a dermatologist; 27 participants (38.0%) needed to be seen face‐to‐face for further management; for 26 intervention participants (36..6%), photo‐diagnosis was not possible |
| Selective reporting (reporting bias) | Unclear risk | Impossible to check against protocol (protocol not published) |
| Baseline characteristics similar? | Low risk | Quote: "Study and control groups were similar in age (range: 5 months–94 years; mean 38.5 years, SD 23.2), gender [55 (40%) male; 81 (60%) female], numbers of patients not attending appointments and range of diagnoses (Table 1)" |
| Baseline outcomes similar? | Low risk | Not possible to provide baseline data for the outcome of interest |
| Free of contamination? | Low risk | Participants were the unit of randomisation, but despite this, It is unlikely that control group participants received the intervention |
| Other bias | Low risk | No obvious other risk of bias was identified |
Lowthian 2011.
| Methods |
Study design: re‐analysed ITS study Aim: to show whether streamlining perioperative services reduces hospital‐initiated postponement (HIP), decreases numbers of patients waiting for elective surgery beyond nationally recommended waiting periods or increases hospital surgical treatment capacity Timing: before intervention period: February 2005 to January 2007; intervention introduction: February 2007; after intervention period: February 2007 to February 2010 Data collection: retrospective data collected from an administrative database: de‐identified patient data drawn from a computerised patient management system tracking people from admission to discharge |
|
| Participants |
Providers: 1 public hospital Participants: patients requiring elective surgery; total number of participants not given Participant baseline characteristics: Age: no information Gender: no information Ethnicity: no information Clinical problem: elective surgery Setting: tertiary hospital Country: Australia |
|
| Interventions |
Type of intervention: intervention aimed at prioritising demand (and expanding capacity)
Duration of intervention: 3 years (February 2007‐February 2010) |
|
| Outcomes |
|
|
| Notes | During the intervention period, 3 different interventions were implemented over time, of which streamlining of services was one, which complicates reanalysis and interpretation of results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS)? | High risk | Initial redesign process started in May 2006, i.e. during the period used as the control period in the reanalysis. Additional short‐stay beds were made available from mid 2008; also, from April 2008 to January 2010, elective surgery throughout the Alfred Centre was reduced during further building works, which may have impacted outcomes |
| Analysed appropriately (ITS)? | Low risk | Quote: "Comparing data from February 2010 with February 2005, there was a 45% decrease in the numbers of Category 2 patients (semi urgent) waiting longer for surgery than the recommended time of < 90 days" Comment: reanalysed as ITS |
| Shape of the intervention effect pre‐specified (ITS)? | Low risk | Data reanalysed by review authors |
| Intervention unlikely to affect data collection (ITS)? | Low risk | Retrospective data collection Quote: "Data comprising aggregated monthly figures and patient information were extracted and de‐identified by the Clinical Performance Unit from the computerised patient‐management system (HOMER), which tracks patients from admission to discharge. [...] Data from 12 months before (February 2005 – February 2006) and after (February 2009 – February 2010) the process redesign were analysed" |
| Knowledge of the allocated interventions adequately prevented during the study (ITS)? | Low risk | Outcomes objective; thus unlikely to be affected by possible unblinded assessment |
| Incomplete outcome data addressed (attrition bias) (ITS)? | Low risk | Quote: "Data comprising aggregated monthly figures and patient information were extracted and de‐identified by the Clinical Performance Unit from the computerised patient‐management system (HOMER), which tracks patients from admission to discharge. Aggregated monthly data included: summaries of all elective surgery procedures performed; [...]; numbers of elective surgery patients waiting longer than nationally recommended maximum waiting times (including patients ready and not ready for care)" Comment: Study authors looked at all operations ‐ so data should be complete |
| Free of selective outcome reporting (reporting bias) (ITS)? | Unclear risk | Study protocol not available ‐ so outcomes reported in the paper cannot be checked against any prespecified outcomes |
| Free of other bias (ITS)? | Unclear risk | Discrepancies noted between figures ‐ Figure 2 plots the number of participants waiting too long for elective surgery. Figure 6 plots the number of elective surgery admissions. According to these figures, the number of admissions is lower than the number of participants who had to wait too long, which does not make sense, It is unclear whether study authors each month added up the number of people who were currently waiting too long and were still waiting. So a person may appear on the graph for several consecutive months, that is, from the time they went over the recommended time to the time they underwent surgery. We contacted the study authors for clarification but received no response |
Lukman 2004.
| Methods |
Study design: reanalysed ITS study Aim: to review results for the 2 years following introduction of direct booking for colposcopy Timing: before intervention period: December 2000 to August 2001; intervention period: September 2001; after intervention period: September 2001 to August 2003 Data collection: Participant information is collected and stored using extensively used regional database; data from this are used to produce the returns required nationally |
|
| Participants |
Provider: laboratory Participants: women with abnormal cervical cytology needing colposcopy; 2501 women with abnormal cytology referred through direct booking Participant baseline characteristics: Age: no information Gender: 100% female Ethnicity: no information Clinical problem: moderate or severe abnormal cervical cytology Setting: public health setting, in Portsmouth and South East Hampshire Country: UK |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
Duration of intervention: 24 months |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS)? | High risk | Quote: "We organized extra clinics to meet the extra demand" Also, a second intervention (introduction of a dedicated colposcopy nurse) was put in place during the intervention period, which may have affected the results |
| Analysed appropriately (ITS)? | Low risk | Reanalysed as ITS |
| Shape of the intervention effect pre‐specified (ITS)? | Low risk | Data reanalysed by review authors |
| Intervention unlikely to affect data collection (ITS)? | Low risk | Quote: "Patient information is collected and stored using an approved database used extensively within the region" |
| Knowledge of the allocated interventions adequately prevented during the study (ITS)? | Low risk | Outcomes objective; thus unlikely to be affected by possible unblinded assessment |
| Incomplete outcome data addressed (attrition bias) (ITS)? | Unclear risk | No information given |
| Free of selective outcome reporting (reporting bias) (ITS)? | Unclear risk | Study protocol not available ‐ so outcomes reported in the paper cannot be checked against any prespecified outcomes |
| Free of other bias (ITS)? | Unclear risk | Figure 2 (showing the percentage of participants meeting guidelines on standards of waiting time for patients with abnormal cytology and source of data for analysis) seems to include data for all types of referrals (inadequate smears, abnormal cytology or other referrals), but the study aims to assess the impact of direct booking on waiting time from abnormal cytology smear referral to colposcopy clinic |
Mallard 2004.
| Methods |
Study design: reanalysed ITS study Aim: to test the following propositions in support of same‐day scheduling, using actual data from a public health clinic:
Timing: preintervention period: January 2001 to June 2001; intervention period: July 2001 to August 2001; postintervention period: September 2001 to February 2002 Data collection: not reported |
|
| Participants |
Providers: paediatricians, nurses; number of providers not given Participants: outpatients calling for routine visits at a public paediatric health clinic; preintervention period: 4063 appointments and 78 new patients/mo; postintervention period: 3531 appointments and 95 new patients/mo Baseline characteristics of participants: Age: no information Gender: no information Ethnicity: no information Clinical problem: all conditions treated at the outpatient public paediatric health clinic (primary health care) Setting: outpatient paediatric clinic, urban Country: USA (state of Alabama) |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
Duration of intervention: 8 months |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS)? | Low risk | Quote: "Since the first two months following the initiation of the project were already booked according to the prior guideline, the clinicians doubled up and saw both the existing appointments and the same‐day scheduled patients" Comment: Nevertheless, as analysis of data excluded the period of extra activity, risk of bias is presumed to be low |
| Analysed appropriately (ITS)? | Low risk | Reanalysed as ITS |
| Shape of the intervention effect pre‐specified (ITS)? | Low risk | Data reanalysed by review authors |
| Intervention unlikely to affect data collection (ITS)? | Unclear risk | No information given on data collection |
| Knowledge of the allocated interventions adequately prevented during the study (ITS)? | Low risk | Outcomes objective; thus unlikely to be affected by a possible unblinded assessment |
| Incomplete outcome data addressed (attrition bias) (ITS)? | Unclear risk | No information given |
| Free of selective outcome reporting (reporting bias) (ITS)? | Unclear risk | Study protocol not available ‐ so outcomes reported in the paper cannot be checked against any prespecified outcomes |
| Free of other bias (ITS)? | Unclear risk | Unclear definition on how waiting time was calculated; unclear also how waiting time for prescheduled appointments contributed, i.e. appointments for which shorter wait time presumably was not desired |
McKessock 2001.
| Methods |
Study design: cluster‐RCT Aim: to establish and evaluate a new referral service for women referred to laparoscopic sterilisation, and to report on some methodological issues Unit of allocation: GP practices Unit of analysis: participants Unit of analysis issue: yes, as practices were randomly assigned and participants analysed; unclear also if effects of clustering were taken into account in the analysis Stratification: practices randomly assigned from prestratified lists according to size, location, etc Timing: 1 June 1996 to 31 March 1997 Data collection: prospective data collection through specific questionnaires and hospital records |
|
| Participants |
Providers: gynaecologists, 230 general practitioners from 57 general practices, and nurses Participants randomly assigned: n = 232; intervention: 75, control: 157 referred for laparoscopic sterilisation Participants withdrawn or lost to follow‐up: intervention: n = 65, control: n = 57 (35 participants later crossed over from control to intervention group again) Baseline characteristics of participants: Age: no information Gender: 100% female Ethnicity: no information Clinical problem: referral for laparoscopic sterilisation Setting: general practices and hospital in the Grampian region, Aberdeen Royal Infirmary Country: Scotland, UK |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
GPs randomly assigned to direct referral (intervention) were supplied with a referral pack, which included:
A newsletter kept practices up‐to‐date with the study's progress and encouraged continuing participation; a "theatre list" of two consultants was dedicated to direct referral sterilisation (separate waiting list) Duration of intervention: 10 months |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | This study was terminated before the expected date after discussions with the funding body, as lower than expected recruitment rates made timely completion impossible. Findings of this trial were reported despite the small numbers, as important lessons were learnt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was by referring practice to encourage consistency of referral behaviour and to avoid administrative complications within practices. Participating practices were randomised into intervention and control groups stratified by list size, fund holding status and rural or urban location. The practices in the eight blocks were placed into different coloured envelopes (one colour for each block). A coin was flipped to decide to commence with intervention or control allocation. From the first block of small list practices an envelope was selected and at the same time an envelope from a large list block was selected. These practices were randomised to the same arm of the trial and this process continued, allocating control or intervention alternately until all practices in those two groups were allocated into one of the two arms of the trial: one receiving and implementing the guidelines (intervention arm) and one maintaining the status quo (control arm). This process was carried out for each of the eight blocks" Comment: Method of randomisation is obscure: unclear whether envelopes were opaque and numbered; also unclear whether the envelopes were shuffled |
| Allocation concealment (selection bias) | Unclear risk | See quote above. Comment: Actual method of allocation is obscure. Unclear whether envelopes were opaque and numbered; also unclear whether the envelopes were shuffled |
| Blinding of participants and personnel (performance bias) Waiting time and number of visits to GPs before and after operation | Low risk | No masking possible but unlikely to affect results (objective nature of the outcomes of interest of the review) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear whether assessors were blinded, but results unlikely to be affected (objective nature of the outcomes of interest of the review) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the control arm, 57/157 (36.3%) participants withdrew from the trial; among participants randomly assigned to the intervention group, only 10 of 75 received the intervention |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; not possible to check whether all prespecified outcomes have been evaluated |
| Baseline characteristics similar? | Unclear risk | Baseline data only partially reported Quote: "There were no significant differences found in patients' characteristics between control and intervention groups" See Table 1. Referral criteria ‐ suitability for direct referral Comment: This was according to study authors; however, they reported mainly on different clinical criteria, and age was the only non‐clinical characteristic reported |
| Baseline outcomes similar? | Low risk | Not possible to provide baseline outcome data for the outcome of interest |
| Free of contamination? | High risk | A large proportion (65/75, 86.7%) of participants assigned to the experimental/intervention group were treated by the standard referral procedure (not eligible for or refused direct referral) |
| Other bias | Low risk | No obvious other risk of bias identified |
Thomas 2003.
| Methods |
Study design: cluster‐RCT (2 × 2 balanced incomplete block design) Aim: to establish whether guideline‐based open access reduces outpatient waiting times, provides a management decision earlier, completes hospital care sooner and reduces hospital management cost Unit of allocation: primary general practices (Grampian, Scotland) Unit of analysis: participants Unit of analysis issue: adjustment for preintervention data and clustering of participants by practice Stratification: by location and fund holding status Timing: preintervention: February to July 1995 (intervention introduced in August 1995); post‐intervention: August 1995 to May 1996 Data collection: Data were collected from hospital medical records 12 months after referral to determine waiting time to initial appointment and dates of management decision and discharge from hospital care; routine data on waiting times for all new referrals to urology were obtained from GUHT |
|
| Participants |
Providers: 66 general practices from Grampian region, Scotland, UK, referring for LUTS and MH Participants randomly assigned: n = 959 participants with suspected LUTS or MH: intervention: n = 479; control: n = 480 Participants withdrawn or lost to follow‐up: intervention: no retrievable information; control: no retrievable information Baseline characteristics of participants: Age: 60 years old Gender: 25% female Ethnicity: no retrievable information Clinical condition: lower urinary tract symptoms (LUTS) and microscopic haematuria (MH) Setting: general practices and Grampian Uniiversity Hospital NHS Trust (GUHT) Country: Scotland, UK |
|
| Interventions |
Type of intervention: intervention aimed at restructuring the referral process
Duration of intervention: 10 months (August 1995‐May 1996) |
|
| Outcomes |
|
|
| Notes | Satisfaction of GPs also investigated for possible side effects (e.g. increased GP workload). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[General practices] were randomised by a statistician independent of the research team using computer‐generated numbers (stratified by location and fund holding status)" |
| Allocation concealment (selection bias) | Low risk | Quote: "[General practices] were randomised by a statistician independent of the research team using computer‐generated numbers (stratified by location and fund holding status)" |
| Blinding of participants and personnel (performance bias) Waiting time and number of visits to GPs before and after operation | Low risk | Unfeasible but not likely to affect study results as the outcome of interest (waiting time) is objective |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | During data collection and entry, researchers were blind to the intervention status of the general practices. The outcome of interest (waiting time) is objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In total, 10/76 practices (7 from LUTS intervention and 3 from MH intervention) were excluded from analysis, as possibly picked up guideline‐based open access referrals only. Intention‐to‐treat analysis declared as carried out |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available; not possible to check whether all prespecified outcomes have been evaluated |
| Baseline characteristics similar? | Low risk | Baseline characteristics similar (information provided in supplementary Table 2) |
| Baseline outcomes similar? | Low risk | Baseline outcome measures for outcome of interest similar (information provided in supplementary Table 2) |
| Free of contamination? | Low risk | As this was a cluster‐RCT, the risk of the control group receiving the intervention must be considered low |
| Other bias | Low risk | Low risk of unit of analysis error; quote: "All outcome measures except the number of referrals, costs and waiting time for all urology referrals were analysed using the patient as the unit of analysis and multilevel modelling using MLWiN version 1.01 to account for the clustering of patients within practices" Comment: Waiting times for LUTS and MH were calculated while adjusting for the cluster effect Quote: "However, control patients also experienced a reduction in waiting time. This was partly because of the increase in the available number of new out‐patient slots as intervention group patients referred to the guideline‐based open access service bypassed the initial outpatient appointment. This dilutes the effect of the intervention. Thus the effects found in this study are likely to be underestimates of the true effect of the intervention" Comment: The described phenomenon could have reduced the effect of the intervention&& |
GOPD: Gynaecology Outpatient Department.
GP: general practitioner.
GUHT: Grampian University Hospital NHS Trust.
HIP: hospital‐initiated postponement.
ITS: interrupted time series.
LUTS: lower urinary tract symptoms.
MH: microscopic haematuria.
MRI: Magnetic Resonance Imaging.
NHS: National Health Service.
RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlburg 2005 | Ineligible study design |
| Arnaout 2012 | Uncontrolled before‐and‐after (BA) study. No graphs. Conference abstract only |
| Bassi 2004 | Controlled before‐and‐after study (CBA) with only 1 intervention and 1 control site. No graphs |
| Bellan 2004 | Case study. Inadequate outcome measure |
| Bergin 2009 | Ineligible study design |
| Bibi 2007 | Uncontrolled BA study. No graphs |
| Blick 2010 | Uncontrolled BA study. No graphs |
| Boisjoly 2010 | Ineligible study design |
| Borg 1991 | Ineligible study design |
| Borugian 2001 | Not about reducing waiting lists, but about reducing within‐clinic waiting times between diagnosis and treatment |
| Brook 2010 | Not about reducing waiting lists, but about reducing within‐clinic waiting times between diagnosis and treatment |
| Bungard 2009 | Uncontrolled BA study. No graphs |
| Carrington 1991 | Ineligible study design |
| Chandler 2005 | Ineligible study design. Poster abstract |
| Ciardullo 2003 | Uncontrolled BA study, with no baseline data |
| Clark 1999 | Uncontrolled BA study, with no baseline data |
| Clemente 2006 | CBA study with only 1 intervention and 1 control site. No graphs |
| Cootauco 2007 | Ineligible study design |
| Dennis 1994 | Ineligible study design (simplified time series) |
| Dewson 2001 | Ineligible study design |
| Droulers 1995 | Ineligible study design |
| Elkhuizen 2007 | Uncontrolled BA study, with only 1 data point before the intervention. Also already short waiting times (14 days) |
| Fitzgerald 2006 | Uncontrolled BA. No graphs |
| Fortune 2012 | Ineligible study design |
| Garfield 1976 | 1‐site CBA. No graphs |
| Graves 2006 | Ineligible study design |
| Gustafson 2013 | Comparison of 3 different implementation strategies for the same intervention |
| Haggarty 2012 | CBA with 1 intervention and 4 control sites. No graphs |
| Hanning 1996 | Uncontrolled BA study with fewer than 3 data points before the intervention |
| Hanning 2007 | Testing the suspension of an intervention instead of testing its introduction |
| Harding 2012 | Ineligible study design |
| HMT 2012 | Ineleigible study design. Descriptive |
| Hobday 2003 | Ineligible study design |
| Jibawi 2005 | Ineigible study design. Abstract |
| Jones 2000 | Ineligible study design |
| Keller 1997 | Uncontrolled BA study with no baseline data |
| Kendall 2009 | Ineligible study design |
| Kew 2001 | Inappropriately analysed interrupted time series (ITS) study. No graphs |
| Khawaja 2000 | Ineligible study design |
| Kielar 2010 | Ineligible study design. Descriptive study |
| Kirkwood 2006 | Ineligible study design |
| Kumari 2001 | Ineligible study design |
| Lal 2011 | Inappropriately analysed ITS study. No graph |
| Lim 2012 | CBA with 1 intervention and 1 control site and no baseline |
| Lizan‐Garcia 2001 | Inappropriately analysed ITS study. No graphs. Main aim with intervention was to assess its acceptability by patients |
| Magnusson 2010 | Inappropriately analysed ITS study with no graph. Conference abstract |
| Marden 2001 | Ineligible study design |
| Marquez 1994 | Ineligible study design. Intervention sites volunteered to receive the intervention |
| Martin 2005 | Inappropriately analysed ITS study. No graphs |
| Maruthachalam 2005 | Ineligible study design |
| May 2008 | Ineligible study design |
| McLeod 2003 | Ineligible study design |
| Menzies 2001 | Ineligible study design |
| Mitchell 2002 | Ineligible study design |
| Montoro 2002 | Ineligible study design. Non‐randomised study comparing medical specialities (intervention group) vs surgical specialities (control group) |
| Newey 2006 | Ineligible study design |
| Newman 2011 | Ineligible study design |
| Nichols 2011 | Ineligible study design. Abstract only |
| Ogunbamise 2005 | Inappropriately analysed ITS study. No graphs |
| Old 2001 | Ineligible study design |
| Perez 2005 | Ineligible study design |
| Phillips 2001 | Inappropriately analysed ITS study. No graphs |
| Pomerantz 2008 | Inappropriately analysed ITS study. No graphs |
| Poot 2011 | Ineligible study design |
| Proenca 2003 | Inappropriately analysed ITS study. No graphs |
| Rayner 2008 | Ineligible study design |
| Reece 2001 | Ineligible study design. Abstract only |
| Reid 2009 | Ineligible study design |
| Rochester 2008 | Ineligible study design |
| Ross 2010 | Ineligible study design. Abstract only |
| Salam 2006 | Inappropriately analysed ITS study. No graphs |
| Sanderson 2003 | Ineligible study design. Descriptive |
| Scheurmier 2001 | Ineligible study design |
| Seagger 2011 | Ineligible study design. Non‐randomised, 1‐site, 2‐group comparison |
| Shetty 2004 | Ineligible study design |
| Siofradh 2000 | Neither abstract nor full text available, impossible to contact study author |
| Smigorowsky 2007 | Inappropriately analysed ITS study. No graphs |
| Snow 2009 | Ineligible study design |
| Sri‐Ram 2006 | Ineligible study design. Non‐randomised, 1‐site, 2‐group comparison |
| Sulaiman 2004 | CBA study with 1 intervention site only. No graphs |
| Tavakol 2011 | Ineligible study design |
| Tebe 2012 | Ineligible study design. Descriptive |
| Tinkler 2004 | Ineligible study design |
| Toustrup 2011 | Inappropriately analysed ITS study. No graphs |
| Unknown 1950 | Ineligible study design |
| Unknown 1975 | Ineligible study design |
| Unknown 1995 | Ineligible study design |
| West 2001 | Ineligible study design |
| White 2010 | Ineligible study design. Abstract only |
| Wijesekara 2011 | Ineligible study design. Case study. Abstract only |
| Wong 2000 | Ineligible study design |
| Wynn‐Williams 1950 | Ineligible study design |
Characteristics of studies awaiting assessment [ordered by study ID]
Heptulla 2013.
| Methods | Time series analysis |
| Participants | Children needing a consultation for endocrine problems |
| Interventions |
|
| Outcomes |
|
| Notes |
Salisbury 2013.
| Methods | Ransomised controlled trial |
| Participants | Adults (aged ≥ 18 years) who were referred by general practitioners, or who referred themselves, for physiotherapy for a musculoskeletal problem |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Notes |
Sobolev 2012.
| Methods | Cohort study |
| Participants | Patients needing elective coronary artery bypass grafting (CABG) |
| Interventions |
|
| Outcomes |
|
| Notes | Could be considered inappropriate interrupted time series (ITS) study ‐ study authors to be contacted for additional data |
Stewart 2013.
| Methods | Controlled before‐and‐after study |
| Participants | Community‐based alcohol and other drug (AOD) services providing treatment programmes for detoxification, inpatient, residential and outpatient treatment services |
| Interventions |
|
| Outcomes |
|
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Augestad 2008.
| Trial name or title | 1‐Stop trial |
| Methods | Randomised controlled trial |
| Participants | Patients diagnosed by general practitioners for an inguinal hernia, gallstone disease or pilonidal sinus requiring surgical treatment |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | October 2010 |
| Contact information | Knut Magne Augestad (knut.magne.augestad@telemed.no), Norwegian Centre for Telemedicine, Norway, and Departement of Gastrointestinal Surgery, University Hospital of North Norway, Norway |
| Notes | Trial registration number in ClinicalTrials.gov: NCT00692497 |
Wahlberg 2013.
| Trial name or title | Practical health co‐operation ‐ the impact of a referral template on quality of care and health care co‐operation: study protocol for a cluster randomized controlled trial |
| Methods | Cluster‐randomised controlled trial |
| Participants | Unit of randomisation: 14 general practitioner offices (in an area primarily served by the same University Hospital) Unit of analysis: patients referred from general practices to hospital care for dyspepsia or suspected colonic malignancy or chest pain or chronic obstructive pulmonary disease or suspected chronic obstructive pulmonary disease |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | September 2011. |
| Contact information | Henrik Wåhlberg (henrik.wahlberg2@unn.no; henrik.wahlberg@uit.no), Department of Community Medicine, Faculty of Health Sciences, University of Tromsø, Tromsø, and University Hospital of North Norway Harstad, St. Olavsgate 70, 9480, Harstad, Norway |
| Notes | Trial registration number in ClinicalTrials.gov: NCT01470963 |
Differences between protocol and review
The original protocol included "number of patients in a waiting list" among the outcomes. Considerations of the clear inadequacy of this measure of outcome (Siciliani 2008; Siciliani 2013) led the review authors to exclude it from the inclusion criteria of the review.
Except for one (RG), all authors of this review are different from those of the protocol.
Contributions of authors
RG wrote the protocol for the original review. LB led this review. GF and AH (Alison Hirst) assessed studies for inclusion. LB, LV, SM, AN, GF, IS and JH participated in data extraction and contributed to data analysis. AN and JH carried out the statistical analyses. LB, LV, AN and SM drafted the review, and all review authors commented on this.
Sources of support
Internal sources
No sources of support supplied
External sources
Research grant by the Italian Ministry of Health (grant number: GR ‐ 2010 ‐ 2317133), Italy.
Declarations of interest
LB is editor for the Cochrane Effective Practice and Organisation of Care Review Group.
New
References
References to studies included in this review
Hofstetter 2010 {published data only}
- Hofstetter PJ, Kokesh J, Ferguson AS, Hood LJ. The impact of telehealth on wait time for ENT specialty care. Telemedicine Journal & E‐Health 2010;16(5):551‐6. [DOI] [PubMed] [Google Scholar]
Leach 2004 {published data only}
- Leach P, Rutherford SA, King AT, Leggate JR. Generic waiting lists for routine spinal surgery. Journal of the Royal Society of Medicine 2004;97(3):119‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leggett 2004 {published data only}
- Leggett P, Gilliland AE, Cupples ME, McGlade K, Corbett R, Stevenson M, et al. A randomized controlled trial using instant photography to diagnose and manage dermatology referrals. Family Practice 2004;21(1):54‐6. [DOI] [PubMed] [Google Scholar]
Lowthian 2011 {published data only}
- Lowthian JA, Curtis AJ, Comitti BL, Cameron PA, Keogh MJ, Johnson WR, et al. Streamlining elective surgery care in a public hospital: the Alfred experience. Medical Journal of Australia 2011;194(9):448‐51. [DOI] [PubMed] [Google Scholar]
Lukman 2004 {published data only}
- Lukman H, Bevan JR, Greenwood E. Direct booking colposcopy clinic ‐ The Portsmouth experience. Cytopathology 2004;15(4):217‐20. [DOI] [PubMed] [Google Scholar]
Mallard 2004 {published data only}
- Mallard SD, Leakeas T, Duncan WJ, Fleenor ME, Sinsky RJ. Same‐day scheduling in a public health clinic: a pilot study. Journal of Public Health Management & Practice 2004;10(2):148‐55. [DOI] [PubMed] [Google Scholar]
McKessock 2001 {published data only}
- McKessock L, Smith BH, Scott A, Graham W, Terry PB, Templeton A, Fitzmaurice AE. A randomized controlled trial of direct access for laparoscopic sterilization. Family Practice 2001;18(1):1‐8. [DOI] [PubMed] [Google Scholar]
Thomas 2003 {published data only}
- Thomas RE, Grimshaw JM, Mollison J, McClinton S, McIntosh E, Deans H, Repper J. Cluster randomized trial of a guideline‐based open access urological investigation service. Family Practice 2003;20(6):646‐54. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahlburg 2005 {published data only}
- Ahlburg P. Operation for lumbar disc prolapse on an outpatient basis. Ambulatory Surgery.Conference. I.A.A.S.8th International Congress on Ambulatory Surgery Brisbane, QLD Australia.Conference Start: 20090703 Conference End: 20090706 2009;15(3):22. [Google Scholar]
- Ahlburg P, Duel P, Spangsberg NLH, Albeck MJ. Operation for lumbar disc prolapse on an outpatient basis. Ugeskrift for Laeger 2005;167(17):1852‐5. [PubMed] [Google Scholar]
Arnaout 2012 {published data only}
- Arnaout A, Seely J, Robertson S, Smylie S, Knight K, Lad S, et al. An innovation in breast cancer care in Ottawa, Canada: the evaluation and validation of a rapid diagnostic and support clinic for women assessment for breast cancer. European Journal of Cancer.Conference: European Breast Cancer Conference March 2012, EBCC8 Vienna Austria 2012;48:S59. [Google Scholar]
Bassi 2004 {published data only}
- Bassi RS, Haidar SG, Gupta AK, Sinha AK, Deshmukh SC. Can single‐stop carpal tunnel syndrome assessment clinics reduce waiting time to surgery?. Clinical Governance 2004;9(4):222‐4. [Google Scholar]
Bellan 2004 {published data only}
- Bellan L. The impact of allocation of additional resources on the waiting time for cataract surgery. Healthcare Quarterly (Toronto, Ont.) 2004;7(4):54‐6. [DOI] [PubMed] [Google Scholar]
Bergin 2009 {published data only}
- Bergin Shan. Getting a foot in the door: can expanding the role of podiatry assistant improve access to public podiatry services?. Australian Journal of Primary Health 2009;15(1):45‐9. [Google Scholar]
Bibi 2007 {published data only}
- Bibi Y, Cohen AD, Goldfarb D, Rubinshtein E, Vardy DA. Intervention program to reduce waiting time of a dermatological visit: managed overbooking and service centralization as effective management tools. International Journal of Dermatology 2007;46(8):830‐4. [DOI] [PubMed] [Google Scholar]
Blick 2010 {published data only}
- Blick C, Bailey D, Haldar N, Bdesha A, Kelleher J, Muneer A. The impact of the two‐week wait rule on the diagnosis and management of bladder cancer in a single UK institution. Annals of the Royal College of Surgeons of England 2010;92(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boisjoly 2010 {published data only}
- Boisjoly H, Freeman EE, Djafari F, Aubin MJ, Couture S, Bruen RP, et al. Reducing wait time for cataract surgery: comparison of 2 historical cohorts of patients in Montreal. Canadian Journal of Ophthalmology 2010;45(2):135‐9. [DOI] [PubMed] [Google Scholar]
Borg 1991 {published data only}
- Borg S. New organization shortens the waiting list for methadone therapy. Lakartidningen 1991;88(18):1673. [PubMed] [Google Scholar]
Borugian 2001 {published data only}
- Borugian MJ, Kan L, Chu CCY, Ceballos K, Gelmon KA, Gordon PB, et al. Facilitated "Fast Track" referral reduces time from abnormal screening mammogram to diagnosis. Canadian Journal of Public Health 2001;99(4):252‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 2010 {published data only}
- Brook MG, Baveja T, Smondulak L, Shukla S. The effect of electronic patient records (EPR) on the time taken to treat patients with genital Chlamydia infection. Sexually Transmitted Infections 2010;86(5):384‐7. [DOI] [PubMed] [Google Scholar]
Bungard 2009 {published data only}
- Bungard TJ, Smigorowsky MJ, Lalonde LD, Hogan T, Doliszny KM, Gebreyesus G, et al. Cardiac EASE (Ensuring Access and Speedy Evaluation) ‐ the impact of a single‐point‐of‐entry multidisciplinary outpatient cardiology consultation program on wait times in Canada. Canadian Journal of Cardiology 2009;25(12):697‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrington 1991 {published data only}
- Carrington S. A waiting list initiative for varicose vein surgery in Bristol. British Journal of Theatre Nursing 1991;1(5):23‐5. [PubMed] [Google Scholar]
Chandler 2005 {published data only}
- Chandler L, Callister MEJ, Williams SE, Meghjee SPL. A protocol driven nurse led clinic leads to cost savings and shorter waiting times for routine respiratory referrals. Thorax 2005;60:II96. [Google Scholar]
Ciardullo 2003 {published data only}
- Ciardullo AV, Daghio MM, Brunetti M, Luca ML, Magrini N. Cooperative effort between local health services, doctors and the elderly population to reduce bone densitometry waiting lists. Aging‐Clinical & Experimental Research 2003;15(4):352‐3. [DOI] [PubMed] [Google Scholar]
Clark 1999 {published data only}
- Clark K, Voase R, Fletcher IR, Thomson PJ. Improving patient throughput for oral day case surgery. The efficacy of a nurse‐led pre‐admission clinic. Ambulatory Surgery 1999;7(2):101‐6. [Google Scholar]
Clemente 2006 {published data only}
- Clemente C, McGrath R, Stevenson C, Barnes J. Evaluation of a waiting list initiative in a child and adolescent mental health service. Child and Adolescent Mental Health 2006;11(2):98‐103. [DOI] [PubMed] [Google Scholar]
Cootauco 2007 {published data only}
- Cootauco M, Groth J, Rayner CFJ. Nurse led rapid access clinic reduces waiting times and ensures timely and effective diagnosis and management of tuberculosis. Thorax 2007;62:A83. [Google Scholar]
Dennis 1994 {published data only}
- Dennis ML, Ingram PW, Burks ME, Rachal JV. Effectiveness of streamlined admissions to methadone treatment: a simplified time‐series analysis. Journal of Psychoactive Drugs 1994;26(2):207‐16. [DOI] [PubMed] [Google Scholar]
Dewson 2001 {published data only}
- Dewson SA, Field RJ. Applying the triage process to manage a podiatry waiting list for rheumatology patients. Rheumatology 2001;45:I13. [Google Scholar]
Droulers 1995 {published data only}
- Droulers AM, O'Connor HT. Two methods for waiting list management in a hospital based obesity programme. InternationalJournal of Obesity 1995;19(3):217. [Google Scholar]
Elkhuizen 2007 {published data only}
- Elkhuizen SG, Burger MP, Jonkers RE, Limburg M, Klazinga N, Bakker PJ. Using business process redesign to reduce wait times at a university hospital in the Netherlands. Joint Commission Journal on Quality & Patient Safety 2007;33(6):332‐41. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2006 {published data only}
- Fitzgerald M. Operator assistance with process improvement. NIATx helps one agency reduce waiting times from 21 days to 24 hours. Behavioral Healthcare 2006;26(5):20, 22, 2006. [PubMed] [Google Scholar]
Fortune 2012 {published data only}
- Fortune GM, Magee JA, Brien C. Development of a waiting list initiative and implementation of an early intervention clinic for non‐epileptic attack disorder. Epilepsia 2012;53:69. [Google Scholar]
Garfield 1976 {published data only}
- Garfield SR, Collen MF, Feldman R. Evaluation of an ambulatory medical care delivery system. New England Journal of Medicine 1976;294(8):426‐31. [DOI] [PubMed] [Google Scholar]
Graves 2006 {published data only}
- Graves J, Fleischman MH, Goldstein GD. Derm Access: a new triage system to rapidly identify suspicious skin lesions. Dermatologic Surgery 2006;32(12):1486‐90. [DOI] [PubMed] [Google Scholar]
Gustafson 2013 {published data only}
- Gustafson DH, Quanbeck AR, Robinson JM, Ford JH 2nd, Pulvermacher A, French MT, et al. Which elements of improvement collaboratives are most effective? A cluster‐randomized trial. Addiction 2013;108(6):1145‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanbeck AR, Gustafson DH, Ford JH 2nd, Pulvermacher A, French MT, McConnell KJ, et al. Disseminating quality improvement: study protocol for a large cluster‐randomized trial. Implementation Science 2011;6(44):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haggarty 2012 {published data only}
- Haggarty JM, Jarva JA, Cernovsky Z, Karioja K, Martin L. Wait time impact of co‐located primary care mental health services: the effect of adding collaborative care in northern Ontario. Canadian Journal of Psychiatry ‐ Revue Canadienne de Psychiatrie 2012;57(1):29‐33. [DOI] [PubMed] [Google Scholar]
Hanning 1996 {published data only}
- Hanning M. Maximum waiting‐time guarantee ‐ an attempt to reduce waiting lists in Sweden. Health Policy 1996;36(1):17‐35. [DOI] [PubMed] [Google Scholar]
Hanning 2007 {published data only}
- Hanning M, Lundstrom M. Waiting for cataract surgery ‐ effects of a maximum waiting‐time guarantee. Journal of Health Services Research and Policy 2007;12(1):5‐10. [DOI] [PubMed] [Google Scholar]
Harding 2012 {published data only}
- Harding KE, Taylor NF, Leggat SG, Stafford M. Effect of triage on waiting time for community rehabilitation: a prospective cohort study. Archives of Physical Medicine and Rehabilitation 2012;93(3):441‐5. [DOI] [PubMed] [Google Scholar]
HMT 2012 {published data only}
- HMT. Solution helps shorten referral wait times. University health system uses referral‐automation and decision‐support software to reduce wait times for specialty care. Health Management Technology 2012;33(3):12‐3. [PubMed] [Google Scholar]
Hobday 2003 {published data only}
- Hobday A, Dickson C. Information surgeries: a waiting list management initiative. Clinical Child Psychology and Psychiatry 2003;6(1):41‐2. [Google Scholar]
Jibawi 2005 {published data only}
- Jibawi A, Jackson T, Scally J, Baker J, Gurnani L, Arnett L, et al. Redesigning breast service to reduce waiting times, improve patient satisfaction and enhance the quality of care: a successful innovative approach. British Journal of Surgery 2005;92:16‐7. [Google Scholar]
Jones 2000 {published data only}
- Jones E, Lucey C, Wadland L. Triage: a waiting list initiative in a child mental health service. Psychiatric Bulletin 2000;24(2):57‐9. [Google Scholar]
Keller 1997 {published data only}
- Keller GA. Management for quality: continuous quality improvement to increase access to outpatient mental health services. Psychiatric Services 1997;48(6):821‐5. [DOI] [PubMed] [Google Scholar]
Kendall 2009 {published data only}
- Kendall N. Improving access to oral surgery services in primary care. Primary Dental Care 2009;16(4):137‐42. [DOI] [PubMed] [Google Scholar]
Kew 2001 {published data only}
- Kew FM, Appleby D, Whittaker V, Cruickshank DJ, Knott S. Providing a quality service: direct referral from the cytology laboratory to the colposcopy clinic. Journal of Medical Screening 2001;12(1):3‐6. [DOI] [PubMed] [Google Scholar]
Khawaja 2000 {published data only}
- Khawaja AR, Allan SM. Has the breast cancer 'two week wait' guarantee for assessment made any difference?. European Journal of Surgical Oncology 2000;26(6):536‐9. [DOI] [PubMed] [Google Scholar]
Kielar 2010 {published data only}
- Kielar AZ, El‐Maraghi RH, Schweitzer ME. Improving equitable access to imaging under universal‐access medicine: the Ontario wait time information program and its impact on hospital policy and process. Journal of the American College of Radiology 2010;7(8):573‐81. [DOI] [PubMed] [Google Scholar]
Kirkwood 2006 {published data only}
- Kirkwood BJ, Pesudovs K, Latimer P, Coster DJ. The efficacy of a nurse‐led preoperative cataract assessment and postoperative care clinic. Medical Journal of Australia 2006;184(6):278‐81. [DOI] [PubMed] [Google Scholar]
Kumari 2001 {published data only}
- Kumari M, Nikolopoulos I, Huf S, Corry D, Thakur K. Does the "two week wait" target improve the waiting times for specialist review and also waiting time between first seen by colorectal cancer specialist and diagnosis of colorectal cancer?. European Journal of Cancer 2001;47:S411. [Google Scholar]
Lal 2011 {published data only}
- Lal A, Phillips S, Russell C, Woolhouse I. The novel use of fast track CT to select patients for lung cancer clinics: effect on clinic efficiency, waiting times, and patient satisfaction. Postgraduate Medical Journal 2011;87(1026):264‐8. [DOI] [PubMed] [Google Scholar]
Lim 2012 {published data only}
- Lim D, Oakley AM, Rademaker M. Better, sooner, more convenient: a successful teledermoscopy service. Australasian Journal of Dermatology 2012;53(1):22‐5. [DOI] [PubMed] [Google Scholar]
Lizan‐Garcia 2001 {published data only}
- Lizan‐Garcia M, Planchuelo JL, Lizan‐Garcia ML, Garbizu‐Esteban I. Evaluation of a program to reduce surgical waiting‐lists according to change in the surgical center. Revista de Calidad Asistencial 2001;16(3):195‐8. [Google Scholar]
Magnusson 2010 {published data only}
- Magnusson C, Collins D. RA diagnosis clinic: an experiment in prioritizing appointments for suspected RA. Journal of Rheumatology Conference: 65th Annual Meeting of the Canadian Rheumatology Association, CRA Quebec City, QC Canada. 2010; Vol. 37, 6 Suppl 2, issue var.pagings:1337.
Marden 2001 {published data only}
- Marden PF, Newton A, Williamson M, Robertson D. Colonoscopy waiting lists: revalidation by general practitioners is cost effective and reduces unnecessary endoscopy commissioning. Gut 2001;56:A16‐7. [Google Scholar]
Marquez 1994 {published data only}
- Marquez S, Portella E. Evaluation of a program for reducing the surgical waiting list on the basis of on the spot payment. Medicina Clinica 1994;103(5):169‐73. [PubMed] [Google Scholar]
Martin 2005 {published data only}
- Martin J, Bowden P, Stephens R, Andrews J, Bishop M. Managing waiting time for radiotherapy: a single machine unit experience. Australasian Radiology 2005;49(6):480‐4. [DOI] [PubMed] [Google Scholar]
Maruthachalam 2005 {published data only}
- Maruthachalam K, Stoker E, Chaudhri S, Noblett S, Horgan AF. Evolution of the two‐week rule pathway ‐ direct access colonoscopy vs outpatient appointments: one year's experience and patient satisfaction survey. Colorectal Disease 2005;7(5):480‐5. [DOI] [PubMed] [Google Scholar]
May 2008 {published data only}
- May C, Giles L, Gupta G. Prospective observational comparative study assessing the role of store and forward teledermatology triage in skin cancer. Clinical and Experimental Dermatology 2008;33(6):736‐9. [DOI] [PubMed] [Google Scholar]
McLeod 2003 {published data only}
- McLeod H, Ham C, Kipping R. Booking patients for hospital admissions: evaluation of a pilot programme for day cases. British Medical Journal 2003;327(7424):1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzies 2001 {published data only}
- Menzies RD, Young RA. Referrals from a primary care‐based sports medicine department to an orthopaedic department: a retrospective cohort study. British Journal of Sports Medicine 2001;45(13):1064‐7. [DOI] [PubMed] [Google Scholar]
Mitchell 2002 {published data only}
- Mitchell J, Hussaini H, McGovern D, Farrow R, Maskell G, Dalton H. The "jaundice hotline" for the rapid assessment of patients with jaundice. British Medical Journal 2002;325(7357):213‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montoro 2002 {published data only}
- Montoro JGC, Medina Ferrer E, Custardoy Olavarrieta J, Pineda Cuenca M, Orozco Beltran D, Quirce Andres F. Impact of an intervention on the waiting‐list for medical specialists in a health area. Atencion Primaria 2002;30(9):549‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newey 2006 {published data only}
- Newey M, Clarke M, Green T, Kershaw C, Pathak P. Nurse‐led management of carpal tunnel syndrome: an audit of outcomes and impact on waiting times. Annals of the Royal College of Surgeons of England 2006;88(4):399‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 2011 {published data only}
- Newman RD, Nightingale J. Improving patient access to videofluoroscopy services: role of the practitioner‐led clinic. Radiography 2011;17(4):280‐3. [Google Scholar]
Nichols 2011 {published data only}
- Nichols N, Oldford D. NP access clinic ‐ a collaborative approach to ensuring timely access for low risk outpatient cardiology referrals. Canadian Journal of Cardiology Conference: 64th Annual Meeting of the Canadian Cardiovascular Society, CCS 2011 Vancouver, BC Canada 2011;27(6 Suppl 1):S349. [Google Scholar]
Ogunbamise 2005 {published data only}
- Ogunbamise A, Reardon M, Mohoboob M, Lelliott P. Impact of a fast‐track assessment clinic on waiting times and non‐attendance rates for new referrals to a community mental health centre. Psychiatric Bulletin. 2005;29(11):413‐5. [Google Scholar]
Old 2001 {published data only}
- Old O, Rogers T. Restructuring services to reduce waiting times and prevent complications: neonatal inguinal hernias at Bristol Children's Hospital. Clinical Governance 2001;17(1):39‐44. [Google Scholar]
Perez 2005 {published data only}
- Perez FPF, Perez CF, Ledo MJS, Garcia ER, Casas IJ, Morante DF, et al. Impact of a priority scale in a waiting list for bariatric surgery. Obesity Surgery 2005;15(7):929. [Google Scholar]
Phillips 2001 {published data only}
- Phillips SA, Ross PD, Chalmers K, MacDougall G. Can we improve dysphagia referrals?. Journal of Laryngology & Otology 2001;121(6):584‐7. [DOI] [PubMed] [Google Scholar]
Pomerantz 2008 {published data only}
- Pomerantz A, Cole BH, Watts BV, Weeks WB. Improving efficiency and access to mental health care: combining integrated care and advanced access. General Hospital Psychiatry 2008;30(6):546‐51. [DOI] [PubMed] [Google Scholar]
Poot 2011 {published data only}
- Poot B. A nurse‐led cough clinic improves patient wait times. Respirology Conference: TSANZ and ANZSRS Annual Scientific Meetings Perth, WA Australia. 2011; Vol. 16:9.
Proenca 2003 {published data only}
- Proenca G, Ferreira D, Freitas A, Madeira F, Soares AO, Ferreira R. Special program to reduce cardiology consultation waiting lists: report on an innovatory experience. Revista Portuguesa de Cardiologia 2003;22(11):1335‐42. [PubMed] [Google Scholar]
Rayner 2008 {published data only}
- Rayner WJ, Neal JJ. Pre‐assessment triage of orthodontic referrals at an East Yorkshire Hospital. British Dental Journal 2008;204(9):493‐5. [DOI] [PubMed] [Google Scholar]
Reece 2001 {published data only}
- Reece RJ, Hesselden A. Clinical specialist physiotherapy assessment of patients referred to an out‐patient rheumatology service is effective in managing waiting lists. Annals of the Rheumatic Diseases 2001;62:340. [Google Scholar]
Reid 2009 {published data only}
- Reid MJ, David LA, Nicholl JE. A one‐stop carpal tunnel clinic. Annals of the Royal College of Surgeons of England 2009;91(4):301‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rochester 2008 {published data only}
- Rochester M, Scurrell S, Parry JR. Prospective evaluation of a novel one‐stop testicular clinic. Annals of the Royal College of Surgeons of England 2008;90(7):565‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2010 {published data only}
- Ross C. Reduction in osteoporosis physiotherapy waiting times through service redesign. Osteoporosis International.Conference: Osteoporosis Conference December 2010 Liverpool United Kingdom. 2010; Vol. 21:S508.
Salam 2006 {published data only}
- Salam MA, Matai V, Salhab M, Hilger AW. The facial skin lesions "see and treat" clinic: a prospective study. European Archives of Oto‐Rhino‐Laryngology 2006;263(8):764‐6. [DOI] [PubMed] [Google Scholar]
Sanderson 2003 {published data only}
- Sanderson D, Limber C, Eldred C, Harrison K. Patient access. To the ENT degree. Health Service Journal 2003;113(5844):26‐7. [PubMed] [Google Scholar]
Scheurmier 2001 {published data only}
- Scheurmier N, Breen AC. A pilot study of the purchase of manipulation services for acute low back pain in the United Kingdom. Journal of Manipulative & Physiological Therapeutics 2001;21(1):14‐8. [PubMed] [Google Scholar]
Seagger 2011 {published data only}
- Seagger R, Bunker T, Hamer P. Surgeon‐operated ultrasonography in a one‐stop shoulder clinic. Annals of the Royal College of Surgeons of England 2011;93(7):528‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shetty 2004 {published data only}
- Shetty A, Manimaran N, Reece‐Smith H. Direct access day‐case hernia surgery: a logical option for reduction in waiting time. Ambulatory Surgery 2004;11(1‐2):41‐3. [Google Scholar]
Siofradh 2000 {published data only}
- Siofradh MK. Waiting list interventions at clinical psychology departments: an evaluation of a multiple‐group, CBT based, "anxiety management programme" for adult outpatients. ProQuest Dissertations and Theses ‐ UK & Ireland. University College Dublin, 2000. [Google Scholar]
Smigorowsky 2007 {published data only}
- Smigorowsky MJ. The effect of a multidisciplinary outpatient clinic on wait times for cardiology consult. ProQuest Dissertations and Theses 2007:87‐n/a.
Snow 2009 {published data only}
- Snow BW, Cartwright PC, Everitt S, Ekins M, Maudsley W, Aloi S. A method to improve patient access in urological practice. Journal of Urology 2009;182(2):663‐7. [DOI] [PubMed] [Google Scholar]
Sri‐Ram 2006 {published data only}
- Sri‐Ram K, Irvine T, Ingham Clark CL. A direct booking hernia service ‐ a shorter wait and a satisfied patient. Ambulatory Surgery 2006;12(3):113‐7. [Google Scholar]
Sulaiman 2004 {published data only}
- Sulaiman S, Chong KW, Gaudoin M. One‐stop postmenopausal bleeding clinics reduce patient waiting times and theatre costs. Scottish Medical Journal 2004;49(4):152‐4. [DOI] [PubMed] [Google Scholar]
Tavakol 2011 {published data only}
- Tavakol P, Labruto F, Bergstrand L, Blomqvist L. Effects of outsourcing magnetic resonance examinations from a public university hospital to a private agent. Acta Radiologica 2011;52(1):81‐5. [DOI] [PubMed] [Google Scholar]
Tebe 2012 {published data only}
- Tebe C, Adam P, Alomar S, Espallargues M. Impact of a prioritisation system for patients on waiting list for hip and knee arthroplasties and cataract surgery (Structured abstract). Health Technology Assessment Database.2012 Issue 4, J. John Wiley and Sons, Ltd.Chichester, UK.Division: ST., 2012, issue 4.
Tinkler 2004 {published data only}
- Tinkler K. Setting up, piloting, implementing and reviewing a GP direct access service for the diagnosis of lower limb deep vein thrombosis. PQDT ‐ UK & Ireland. Ethos‐ Electronic Thesis Online Service , 2004.
Toustrup 2011 {published data only}
- Toustrup K, Lambertsen K, Birke‐Sorensen H, Ulhoi B, Sorensen L, Grau C. Reduction in waiting time for diagnosis and treatment of head and neck cancer ‐ a fast track study. Acta Oncologica 2011;50(5):636‐41. [DOI] [PubMed] [Google Scholar]
Unknown 1950 {published data only}
- Unknown 1950. Experiment in Cambridgeshire; reducing hospital waiting lists. Nursing Times 1950;46(17):448. [PubMed] [Google Scholar]
Unknown 1975 {published data only}
- Unknown 197. Day surgery cuts wait time, saves $550,000 in 4 years. Employee Benefit Plan Review 1975;30(6):16, 1975. [PubMed] [Google Scholar]
Unknown 1995 {published data only}
- Unknown. Henry Ford Health's 'Saturn' system slashes waiting times. Healthcare Systems Strategy Report 1995;12(11):1‐2. [PubMed] [Google Scholar]
West 2001 {published data only}
- West B, Bennett JA, Deegan PC, Merry P, Watson L, Jones NS, et al. Reducing waiting times for sleep apnoea hypopnoea syndrome and snoring using a questionnaire and home oximetry: results of a second audit cycle. Journal of Laryngology and Otology 2001;115(8):645‐7. [DOI] [PubMed] [Google Scholar]
White 2010 {published data only}
- White S, Magrane T, Hamer A, Fisher N. Echocardiographer technician led valve follow‐up clinic (valve clinic). Heart Lung and Circulation.Conference: New Zealand Annual Scientific Meeting of the Cardiac Society of Australia and New Zealand Rotorua New Zealand 2010;19:S34. [Google Scholar]
Wijesekara 2011 {published data only}
- Wijesekara G. One stop access clinic ‐ a model for the future. Journal of Vascular Access Conference: Inaugural Meeting of the Vascular Access Society of Britain and Ireland September 2010 Manchester United Kingdom Conference. Manchester, UK, 2011; Vol. 12:106.
Wong 2000 {published data only}
- Wong BCY, Chi Kuen C, Ka Wah W, Wai Man W, Man Fung Y, Kam Chuen L, et al. Evaluation of a new referral system for the management of dyspepsia in Hong Kong: role of open‐access upper endoscopy. Journal of Gastroenterology and Hepatology 2000;15(11):1251‐6. [PubMed] [Google Scholar]
Wynn‐Williams 1950 {published data only}
- Wynn‐Williams N, Shaw JB, Mashiter WE. A scheme which has successfully reduced a sanatorium waiting‐list. Lancet 1950;1(6597):221‐4. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Heptulla 2013 {published data only}
- Heptulla RA, Choi SJ, Belamarich PF. A quality improvement intervention to increase access to pediatric subspecialty practice. Pediatrics 2013;131(2):e585‐90. [DOI] [PubMed] [Google Scholar]
Salisbury 2013 {published data only}
- Salisbury C, Foster NE, Hopper C, Bishop A, Hollinghurst S, Coast J, et al. A pragmatic randomised controlled trial of the effectiveness and cost‐effectiveness of 'PhysioDirect' telephone assessment and advice services for physiotherapy. Health Technology Assessment.(Winchester, England) 2013;17(2):1‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury C, Montgomery AA, Hollinghurst S, Hopper C, Bishop A, Franchini A, et al. Effectiveness of PhysioDirect telephone assessment and advice services for patients with musculoskeletal problems: pragmatic randomised controlled trial. BMJ 2013;346:f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobolev 2012 {published data only}
- Sobolev BG, Fradet G, Kuramoto L, Sobolyeva R, Rogula B, Levy AR. Evaluation of supply‐side initiatives to improve access to coronary bypass surgery. BMC Health Services Research 2012;12:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2013 {published data only}
- Stewart MT, Horgan CM, Garnick DW, Ritter G, McLellan AT. Performance contracting and quality improvement in outpatient treatment: effects on waiting time and length of stay. Journal of Substance Abuse Treatment 2013;44(1):27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Augestad 2008 {published data only}
- Augestad KM, Revhaug A, Vonen B, Johnsen R, Lindsetmo RO. The one‐stop trial: does electronic referral and booking by the general practitioner (GPs) to outpatient day case surgery reduce waiting time and costs? A randomized controlled trial protocol. BC Surgery 2008;8(14):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahlberg 2013 {published data only}
- Wahlberg H, Valle PC, Malm S, Broderstad AR. Practical health co‐operation ‐ the impact of a referral template on quality of care and health care co‐operation: study protocol for a cluster randomized controlled trial. Trials [Electronic Resource] 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Appleby 2005a
- Appleby J, Boyle S, Devlin N, Harley M, Harrison A, Locock L. Sustaining reduction in waiting times. Identifying successful strategies. King's Fund 2005.
Appleby 2005b
- Appleby J. Cutting NHS waiting times. Identifying strategies for sustainable reductions. King's Fund 2005.
Appleby 2011
- Appleby J. What's happening to waiting times?. British Medical Journal (Clinical research ed) 2011;342:d1235. [DOI] [PubMed] [Google Scholar]
Black 2004
- Black N. Surgical waiting lists are inevitable: time to focus on work undertaken. Journal of the Royal Society of Medicine 2004;97:159‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dash 2004
- Dash P. New providers in UK health care. British Medical Journal (Clinical Research ed) 2004;328(7435):340‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davey 2013
- Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD003543.pub3] [DOI] [PubMed] [Google Scholar]
Department of Health 2002
- Department of Health. Growing capacity. Independent Sector Diagnosis and Treatment Centres. Department of Health Publications. London (UK) 2002.
EPOC 2013
- Effective Practice and Organisation of Care Group. EPOC Website. http://epoc.cochrane.org/epoc‐author‐resources (last accessed 10 February 2014).
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, et al. What is ’quality of evidence’ and why is it important to clinicians?. British Medical Journal 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2000
- Harrison A, New B. Access to Elective Care. What Should Really Be Done About Waiting Lists. 2nd Edition. London: Kings Fund Publishing, 2000. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochranehandbook.org. [Google Scholar]
Hirvonen 2007
- Hirvonen J, Blom M, Tuominen U, Seitsalo S, Lehto M, Paavolainen P, et al. Evaluating waiting time effect on health outcomes at admission: a prospective randomized study on patients with osteoarthritis of the knee joint. Journal of Evaluation in Clinical Practice 2007;13(5):728‐33. [DOI] [PubMed] [Google Scholar]
Hurst 2003
Kipping 2002
- Kipping R, Robert G, McLeod H, Clark J. A review of priority scoring and slot systems for elective surgery. Birmingham (UK), University of Birmingham, School of Public Policy, Health Services Management Centre, 2002.
Kreindler 2010
- Kreindler S. Policy strategies to reduce waits for elective care: a synthesis of international evidence. British Medical Bulletin 2010;95(1):7‐32. [DOI] [PubMed] [Google Scholar]
Lindsay 1984
- Lindsay CM, Feigenbaum B. Rationing by waiting lists. American Economic Review 1984;74(3):404‐17. [PubMed] [Google Scholar]
MacMillan Press 1993
- Rationing and rationality in the National Health Service. The persistence of waiting lists. The MacMillan Press 1993.
Mayor 2003
- Mayor S. Privately run surgery centres for NHS patients proposed. British Medical Journal 2003;327(640):6. [Google Scholar]
Miller 2008
- Miller AR, Armstrong RW, Mâsse LC, Klassen AF, Shen J, O'Donnell ME. Waiting for child developmental and rehabilitation services: an overview of issues and needs. Developmental Medicine and Child Neurology 2008;50(11):815‐21. [DOI] [PubMed] [Google Scholar]
Propper 2008
- Propper C, Sutton M, Whitnall C, Windmeijer F. Did targets and terror reduce waiting times in Englandfor hospital care?. B E Journal of Economic Analysis & Policy 2008;8(2):Article 5. [Google Scholar]
Rachlis 2005
- Rachlis MM. Solutions to health care wait lists. Canadian Centre for Policy Alternatives 2005;9:1‐38. [Google Scholar]
Ramsay 2003
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment of Health Care 2003;19:613‐23. [DOI] [PubMed] [Google Scholar]
Reference Manager 2010 [Computer program]
- Thomson Reuters. Reference Manager. Version 12. London: Thomson ISI ResearchSoft, 2010.
Siciliani 2003
- Siciliani L, Hurst J. Explaining waiting times variations for elective surgery across OECD countries. OECD. Working Papers 2003.
Siciliani 2008
- Siciliani L. A note on the dynamic interaction between waiting times and waiting lists. Health Economics 2008;17:639‐47. [DOI] [PubMed] [Google Scholar]
Siciliani 2013
- Siciliani L, Borowitz M, Moran V (eds). Waiting time polizie in the health sector: what works?. OECD Health Policy Studies, OECD Publishing; http://dx.doi.org/10.1787/9789264179080‐en (last accessed 16 July 2013) 2013.
Stata 2011 [Computer program]
- StataCorp. Stata Statistical Software. Version 12. College Station, TX: StataCorp LP, 2011.
Wagner 2002
- Wagner AK, Soumerai SB, Zhang F, Ross D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of Clinical Pharmacy and Therapeutics 2002;27:299‐309. [DOI] [PubMed] [Google Scholar]
Willcox 2007
- Willcox S, Seddon M, Dunn S, Edwards RT, Pearse J, Tu JV. Measuring and reducing waiting times: a cross‐national comparison of strategies. Health Affairs (Millwood) 2007;26(4):1078‐87. [DOI] [PubMed] [Google Scholar]
Yates 1987
- Yates J. Why Are We Waiting? An Analysis of Hospital Waiting Lists. Why Are We Waiting? An Analysis of Hospital Waiting Lists. Oxford, UK: Oxford University Press, 1987. [Google Scholar]


