Abstract
Acral melanoma (AM) is the most common histologic subtype of melanoma in dark-skinned patients and is associated with a worse prognosis and a high mortality rate, largely due to the inconspicuous nature of early-stage lesions, which can lead to late diagnosis. Because of the overlapping clinical and histopathological features of AM with other forms of cutaneous melanomas, early detection of AM requires a multidisciplinary approach that integrates various diagnostic modalities, including clinical examination, dermoscopy, histopathology, molecular testing, radiological imaging, and blood tests. While surgery is the preferred method of treatment for AM, other therapeutic options may be employed based on the stage and underlying etiology of the disease. Immune checkpoint inhibitors, molecular targeted therapy, radiotherapy, chemotherapy, and oncolytic virotherapy represent promising advanced treatment options for AM. In this review, we provide an overview of the latest advancements in diagnostic and therapeutic methods for AM, highlighting the importance of early detection and the prompt, individualized management of this challenging disease.
Keywords: Acral melanoma, Acral lentiginous melanoma, Acral nevus, Cutaneous malignant melanoma, Dermoscopy, Oncolytic virotherapy
Abstract
肢端黑色素瘤(AM)是深色皮肤患者中最常见的黑色素瘤组织学亚型,常伴有更差的预后和高死亡率,主要原因为早期病变的不典型特征导致诊断延误。由于AM的临床和组织病理学特征与其他形式的皮肤黑色素瘤相近,其早期检测需要综合各种诊断模式的多学科方法,包括临床检查、皮肤镜检查、组织病理学检查、分子检测、放射学成像和血液检测。虽然手术是AM的首选治疗方法,但是根据疾病的分期和潜在病因,也可以采用其他治疗方案。免疫检查点抑制剂、分子靶向治疗、放疗、化疗和溶瘤病毒治疗在AM治疗上具有应用前景。在本文中,我们概述了AM诊断和治疗方法的最新进展,强调了早期发现和及时、个性化管理在这一具有挑战性疾病诊疗上的重要性。
Keywords: 肢端黑色素瘤, 肢端雀斑样黑色素瘤, 肢端痣, 皮肤恶性黑色素瘤, 皮肤镜检查, 溶瘤病毒治疗
1. Introduction
Acral melanoma (AM), a distinct histopathological subgroup of cutaneous malignant melanomas (CMMs), represents 1%–3% of CMM cases and affects acral areas such as the soles, palms, and nails (Bradford et al., 2009; Wu et al., 2011; Huang et al., 2020). Despite the incidence of AM being similar across racial and ethnic groups, it disproportionately affects individuals with dark skin (Bradford et al., 2009). AM carries a poorer prognosis compared to other subtypes of melanoma, possibly due to delayed diagnosis related to its unique appearance (Cascinelli et al., 1994; Ridgeway et al., 1995; Chang et al., 1998; Gumaste et al., 2014). Compared to CMM, it is less linked to sun exposure and typically appears later and more severely, as well as being more prevalent in older patients (mean age of 63 years versus 59 years for CMM) (Hayward et al., 2017; Liang et al., 2017; Huang et al., 2020). There is inadequate evidence to show a causal link between stress, genetics, carcinogen exposure, viral infection and AM.
Clinical findings differ between AM and non-AM CMMs, with clinical research indicating that AM has a worse survival rate. In a recent Surveillance, Epidemiology, and End Results (SEER) analysis, five-year disease-specific survival rate for AM was found to be 81% compared to 93% for non-AM CMM (Huang et al., 2020). Despite controlling for the melanoma stage, studies indicate that AM has poorer survival outcomes than non-AM due to inherent molecular/biological differences (Curtin et al., 2005; Bello et al., 2013). Late diagnosis has been suggested as a possible factor but other research shows that, even when tumor diameter and stage are also adjusted for, AM still has a worse prognosis (Cascinelli et al., 1994; Ridgeway et al., 1995; Weyers et al., 1999; Bradford et al., 2009; Bello et al., 2013; Wada et al., 2017; Teramoto et al., 2018; Huang et al., 2020). Prognosis is influenced by age, race, and socioeconomic status (Zemelman et al., 2014; York et al., 2016).
Population-based studies analyzing data from SEER, epidemiology, surveillance, and the American National Cancer Database (NCDB) have investigated AM’s survival rate. For instance, the NCDB was used by Bian et al. (2021) to compare survival rates of AM and non-AM patients; they analyzed prognostic and therapeutic factors and stage-specific therapy, as well as AM survival. The authors investigated cutaneous melanoma patients aged 18–90 years with malignant tumor characteristics in American Joint Committee on Cancer (AJCC) cancer stages I–IV between 2004 and 2015, gathering data on critical circumstances, sex, age, diagnosis date, histopathology, and the Charlson-Deyo comorbidity index (for predicting long-term survival). The results reflected that AM had a less favorable overall prognosis in stages I, III, and IV compared to non-AM. Moreover, the difference in overall survival (OS) rates between AM and non-AM (stages I, II, and IV) was ≤4%, suggesting that conventional treatment is typically required for early cases but is ineffective for later ones. Huang et al. (2020) found that AM patients in stage III may not be given established standard therapy or receive heterogeneous care. This may amplify the disease’s biological differences compared to non-AM and could potentially lead to significant implications for patient outcomes.
The serine/threonine-protein kinase B-raf (BRAF) mutation rate in AM is significantly lower than that in non-AM (Maldonado et al., 2003; Curtin et al., 2005; Beadling et al., 2008; Viros et al., 2008; Greaves et al., 2013),which may lead to a decrease in the effectiveness of targeted therapy. Furthermore, AM displays notable genetic differences, such as localized amplifications and deletions, along with neuroblastoma RAS viral oncogene homologue (NRAS) mutations and mast/stem cell growth factor receptor Kit (KIT) mutations/amplifications (Curtin et al., 2006; Woodman and Davies, 2010; Lee et al., 2011; Omholt et al., 2011; Ascierto et al., 2013; Yeh et al., 2019). Moreover, AM may be less vulnerable to immune checkpoint inhibitors (ICIs) because of a lower tumor mutational burden and tumor-infiltrating lymphocytes (TILs) in the absence of the ultraviolet (UV) mutational signal observed in non-AM patients (Castaneda et al., 2017; Hayward et al., 2017; Kaunitz et al., 2017; Liang et al., 2017).
There are several diagnostic methods used to identify AM; these methods include a visual examination of the skin, a biopsy of the suspicious area, histopathology, and imaging tests such as X-rays, computed tomography (CT) scans, and magnetic resonance imaging (MRI). In some cases, a sentinel lymph node (SLN) biopsy (SLNB) may be performed to determine whether cancer has spread to nearby lymph nodes. Dermoscopy, a non-invasive technique that uses a handheld device to magnify the skin, can also be used to identify suspicious lesions and improve diagnostic accuracy. Molecular testing may be used to analyze the genetic mutations that drive the growth of cancer cells and help guide treatment decisions (Abbasi et al., 2004; Phan et al., 2010; Fernandez-Flores and Cassarino, 2017). Consequently, clinical information, including dermoscopic findings, is occasionally important for diagnosing AM and determining the most accurate treatment method. The standard treatment for AM is surgery, which involves removing the cancerous tissue and some of the surrounding healthy tissues. However, additional treatment may be needed, such as radiation therapy, chemotherapy, or immunotherapy. The use of targeted therapy, which involves drugs that specifically target the genetic mutations that drive the growth of cancer cells, has shown promising results in some cases (Yeh et al., 2019).
This paper provides an in-depth review of various diagnostic methods and treatment modalities, together with the latest research developments in AM, to underscore the importance of early detection for the management of this malignancy.
2. Methods
In this study, we aimed to identify relevant scientific articles related to acral malignant melanoma. To accomplish this, we searched electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar, using the search terms: acral melanoma; acral malignant melanoma; acral lentiginous melanoma; lentiginous melanoma; and malignant melanoma. Articles were included if they were directly relevant to acral malignant melanoma and covered aspects such as clinical manifestations, epidemiology, risk factors, diagnosis, treatment options, or outcomes. Non-English articles and those unrelated to our research objectives were excluded. After a thorough evaluation, a total of 144 articles met our inclusion criteria and were included in the review, ensuring a comprehensive and reliable basis for our study.
3. Diagnosis
3.1. Clinical findings
The close observation of the AM lesion at the initial growth stage shows an irregular fringe with a mottled pigmentation appearance or asymmetric tan macule. During early research on melanomas, Saida (1989) analyzed 144 pigmented lesions of the soles, and suggested a clinical guideline to detect the plantar malignant melanoma at an early stage. The guideline states that elderly patients (>50 years) are more affected by plantar malignant melanoma, and resection and histological examination should be considered when the pigmented lesion’s diameter is more than 7 mm. Other clinical guidelines, such as “Asymmetry, Border, Color, Diameter, and Evolution” (ABCDE) and the acronym CUBED (colored lesion, uncertain diagnosis, bleeding lesion, enlargement of a lesion, and delay in healing), are supposed to assist physicians in evaluating questionable skin lesions—particularly of the acral sites—and in making decisions about the need for further observation and diagnosis (Arrington et al., 1977; Bristow and de Berker, 2010). However, these guidelines have not included a systematic investigation of AM and lack specific morphological standards.
3.2. Dermoscopic features
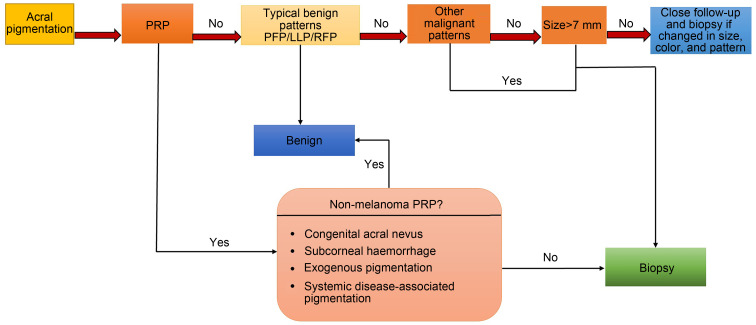
Dermoscopy is a vital tool for the visual assessment of pigmented skin lesions, particularly for the identification of AM, which presents in two distinct dermoscopic patterns: the parallel ridge pattern (PRP) and irregular diffuse pigmentation (IDP). In contrast, acral melanocytic nevi can be identified through three dermoscopic patterns, namely the parallel furrow pattern (PFP), lattice-like pattern (LLP), and regular fibrillar pattern (RFP). Benign acral lesions are characterized by the presence of at least one of these patterns. When comparing acral nevi to AM, the former are typically smaller with less asymmetric borders (Saida et al., 2011; Chuah et al., 2015; Darmawan et al., 2019). During the early stages of AM, the PRP typically manifests as a band-like pigmentation on the skin ridges, with the presence of asymmetrical, light brown lesions. As the disease progresses, the lesions tend to darken, eventually resulting in the formation of a black lesion during the later stages of the disease (Kim et al., 2014). Studies have shown that atypical melanosis of the foot (AMF) presents a PRP similar to that of AM. As a result, distinguishing between the two based on dermoscopic features can be challenging. However, recent research utilizing biopsies has indicated that AMF lesions are likely an early stage in the formation of AM in situ (Kilinc Karaarslan et al., 2007; Oh et al., 2011; Menis et al., 2015). Although PRP commonly presents with dermoscopic features in AM, it can also be manifested in benign lesions like subcorneal hemorrhage, drug-induced acral pigmentation, Peutz-Jeghers syndrome, and Laugier-Hunziker syndrome. Distinguishing between these conditions and AM can be achieved by considering the patient’s clinical history and evaluating the cutaneous characteristics (Tanioka, 2011). Mun et al. (2018) identified distinctive patterns that aid in differentiating in situ AM from invasive AM. Both exhibit asymmetry and PRP patterns; however, in situ AM demonstrates irregular dots, globules, and blotches, while invasive AM presents irregular blotches, polychromia, ulcers, blue-white veils, and atypical vascular patterns. Dermoscopy has significantly advanced the evaluation of pigmented lesions on acral sites by providing several dermoscopic patterns that aid in differentiation beyond what can be detected by the naked eye. Dermoscopy has played a crucial role in the early detection and management of pigmented lesions, especially AM, and in avoiding unnecessary biopsies of benign lesions. To facilitate the early diagnosis and management of AM, Saida and Koga (2007) proposed a three-step algorithm. The first step involves examining for PRP. If PRP is present, direct excision and histopathological examination are performed. If PRP is absent, the second step involves measuring the lesion size. Lesions with a diameter smaller than 7 mm require careful observation, while lesions with a larger diameter require the third step, which involves examining the dermoscopic patterns. Clinical follow-up is recommended for lesions with typical benign patterns, such as PFP, LLP, and RFP. However, biopsy and histopathological examination are preferred for lesions with atypical patterns. The scientists who proposed the three-step algorithm for the diagnosis and management of AM on acral sites reviewed and updated the algorithm after four years. In the updated algorithm, it was determined that close follow-up of acral nevi with benign dermoscopic patterns is unnecessary (Saida et al., 2011). In a 2018 retrospective study, Costello et al. (2018) found that the three-step algorithm was highly accurate in diagnosing and managing AM, but was unable to evaluate small multi-component and invasive melanomas. As a result, the multi-component pattern was included among the high-risk dermoscopic features. Costello et al. (2018) also suggested that improving the ability to recognize the RFP as a benign appearance could lower the number of necessary biopsies for diagnosis. In 2015, the BRAAFF (irregular blotch, parallel ridge pattern, asymmetry of structures, asymmetry of colours, parallel furrow pattern, fibrillar pattern) algorithm emerged as a new approach to AM diagnosis and management, with a sensitivity of 93.1% and a specificity of 86.7% (Lallas et al., 2015). The BRAAFF algorithm was based on the absence of PRP in one-third of AM cases and considered additional dermoscopic features for assessing AMs. BRAAFF is a six-variable algorithm for diagnosing AM, consisting of four positive variables (including PRP, asymmetry of color and structure, and irregular blotches) and two negative variables (including furrow and fibrillar patterns). An AM diagnosis is made when the score is one or higher. Lesions with a PRP appearance should be excised, while lesions with a symmetric PEP or fibrillar pattern may not require excision. However, lesions that exhibit either asymmetry of color or structure, as well as those with irregular blotches, should be excised. The presence of PFP and RFP, which are often seen in nevi, should not exclude the diagnosis of AM as they may also be present in AM, albeit at a low percentage (Lallas et al., 2015). The three-step algorithm proposed by Saida and Koga (2007) for early AM diagnosis is less effective with 6 mm AM lesions. PRP appearance is a crucial factor in the initial evaluation of melanocytic lesions on hairless skin. Lesions with PRP indicate a malignant lesion and a biopsy is necessary for confirmation. Lesions without PRP require further examination using IDP to determine their nature. If the lesion exhibits sophisticated features, such as PRP, IDP, atypical vascular patterns, or blue-white veil, histopathology examination is required. Biopsy is recommended for lesions of >7 mm in diameter without malignant features, while lesions of ≤7 mm should be monitored for changes in color, size, and pattern (Saida and Koga, 2007; Costello et al., 2018; Han et al., 2020) (Fig. 1).
Fig. 1. Diagnosis and management of acral melanoma (AM) based on dermoscopic features. PRP: parallel ridge pattern; PFP: parallel furrow pattern; LLP: lattice-like pattern; RFP: regular fibrillar pattern. Reprinted from Han et al. (2020) licensed under a Creative Commons Attribution 4.0 International License, with some modifications.
3.3. Histopathological examination
The gold standard for analyzing melanocytic lesions is an excisional biopsy, which is not typically preferred for AM due to the large lesion size and wound closure difficulties. Multiple punch biopsies are preferred to provide a primary diagnosis of AM, taken from the highly pigmented area (Scolyer et al., 2006; Park and Cho, 2010). The diagnosis of melanoma in situ can be made with large hyperchromatic melanocytic nuclei compared to keratinocyte nuclei. The malignant lesion is characterized by thick elongated dendrites and angulated vertically arranged nuclei. AM may have thick elongated dendrites near the epidermis at superficial layers, with their processes around the basal keratinocytes in a web shape (Bravo Puccio and Chian, 2011). It is important to note that the presence of pagetoid melanocytes in the sole is not sufficient to confirm the diagnosis of AM in situ, as it can also be present in benign acral nevi (LeBoit, 2000). In benign acral nevi, there is an increase in melanocytic nests proliferation, which differs from the single unit diffuse proliferation in AM. The cells in AM tend to aggregate and form nests of varying sizes, poor consistency, and circumscription (Fernandez-Flores and Cassarino, 2017). In addition, an obvious sign of early-stage AM can be seen histologically, where the crist profunda intermedia contains solitary melanocytes with preferential proliferation (Ishihara et al., 2006). Histologically, the presence of solitary melanocytes with preferential proliferation in the crist profunda intermedia is a hallmark of early-stage AM. Stratum corneum should be closely evaluated for pigment separation, as it is a key feature of AM diagnosis, whereas benign nevus pigmentation is found in the vertical columns (Fernandez-Flores and Cassarino, 2017). Dermal inflammation is a distinguishing feature of AM as it is not present in benign nevi (Ishihara et al., 2006). AM diagnosis can be aided by the use of melanocyte markers such as homatropine methylbromide 45, S-100, and melan-A. Solitary melanocytes found in the crista profunda intermedia on homatropine-methylbromide 45 staining also suggest an AM diagnosis (Boyd and Rapini, 1994; Ishihara et al., 2006).
3.4. Biomarker tests
Biomarker tests or molecular tests search for a specific gene, protein, or other molecules in the tissue sample to confirm the diagnosis of AM. Upregulation of cyclin D1 (CCND1) was reported to be a melanoma oncogene, more specifically in AM, which has more upregulated CCND1 than other types of CM. The early radial growth phase of AM can be detected through upregulated CCND1 in proliferating melanocytes, which is visible on fluorescence in situ hybridization (FISH) (Signoretti et al., 1999). The diagnostic accuracy of early in situ AM increased with the FISH assay because it detects various amplificated genes such as aurora kinase A (AURKA), Ras-responsive element-binding protein 1 (RREB1), telomerase reverse transcriptase gene (TERT), chromosome 6 centromere (CEP6), and v-myb avian myeloblastosis viral oncogene homolog (MYB), which are found in early in situ AM at a high percentage compared with other melanomas (Diaz et al., 2014; Su et al., 2017; Ogata et al., 2018). In addition, when the acral lesion has a PRP pattern but without histological findings, then the application of the FISH assay assists with early AM diagnosis (Diaz et al., 2014; Ogata et al., 2018). According to genomic studies, AMs and mucosal melanoma have a significantly lower mutation burden controlled by wider structural variants compared with CMs (Tod et al., 2020; Yang et al., 2023). BRAF, NRAS, neurofibromin 1 gene (NF1), and KIT proto-oncogene mutations distinguish AMs from other melanomas, as they are remarkably higher in AMs (de Lima Vazquez et al., 2016; Hayward et al., 2017; Ravaioli et al., 2019).
3.5. Radiological imaging
The identification of AM using non-invasive imaging techniques could be essential for providing further detail for the purpose of AM diagnosis and surgical planning, as well as for minimizing the diagnostic challenge associated with the classic diagnostic criteria. The diagnostic effectiveness of MRI was reported in a study by Kong et al. (2020), in which a presurgical MRI of a small lesion on the right heel showed an abnormal mass with internal hyperintensity on T1-weighted imaging (T1WI) and hypointensity on T2-weighted imaging (T2WI). The previous findings have mainly been related to melanoma lesions. On the other hand, non-melanoma lesions appear with a hypointense nodule on T1WI and a hyperintense nodule on T2WI (Kong et al., 2020).
The pre-operative CT and positron emission tomography (PET)/CT efficacy in AM patients has been evaluated to detect nodal or distant metastasis. Pre-operative CT and PET/CT in patients without palpable adenopathy (lymphadenopathy) have limited detection efficacy due to low metastasis rate and low staging accuracy; thus, they are not appropriate (Swetter et al., 2019; Garbe et al., 2020). Generally, radiological imaging has high false-positive results in the detection of nodal disease or distant metastases associated with early AM. In contrast, AM patients with lymphadenopathy were detected with a high metastasis rate by the CT and PET/CT; therefore, it is necessary to perform pre-operative CT or PET/CT to determine a better treatment strategy and assess the metastatic lesion (Ide et al., 2021).
3.6. Non-invasive imaging methods
There are several non-invasive imaging methods that are commonly used to diagnose and monitor AM. Digital dermatoscopy uses a specialized camera to capture high-resolution images of the skin. Digital dermatoscopy can be used to identify the early signs of AM, such as pigmented lesions, and can also be used to monitor the progress of the disease (Kraus and Haenssle, 2013; Cabrera and Recule, 2018). Reflectance confocal microscopy (RCM) uses laser technology to produce high-resolution images of the skin’s surface and subsurface structures. RCM can be used to diagnose AM by identifying the characteristic patterns of pigmentation and architecture that are associated with the disease (Cinotti et al., 2016). Optical coherence tomography (OCT) applies light waves to produce high-resolution images of the skin’s surface and subsurface structures. OCT can be used to diagnose AM by identifying the characteristic patterns of pigmentation and architecture that are associated with the disease (Rajabi-Estarabadi et al., 2019). Ultrasound imaging uses high-frequency sound waves to produce images of the skin’s surface and subsurface structures. Ultrasound imaging can be used to diagnose AM by identifying characteristic patterns of pigmentation and architecture, as well as to monitor the progress of the disease (Kwon et al., 2019).
3.7. Blood tests
Blood tests are capable of indicating the nature of the AM, especially for late-stage AM. Lactate dehydrogenase (LDH) is a converting enzyme that is present in most cells. High blood LDH level refers to tissue damage and the advanced cancer stage. When the AM patient has a high level of LDH, this means that AM has metastasized or spread to the surrounding organs (Palmer et al., 2011).
4. Treatment/management methods of acral melanoma
4.1. Surgical method
Primary AM treatment typically involves broad local excision, similar to other types of melanoma. In the past, aggressive melanoma was treated with substantial local excision using 3–5 cm horizontal boundaries. However, numerous studies have shown that narrow-margin and broad-margin excisions result in no discernible differences in survival rate or prognosis. For 1–4 mm thick melanomas, previous studies found no statistically significant difference between 2 and 4 cm margins (Khayat et al., 2003; Felton et al., 2016).
A randomized study conducted by Khayat et al. (2003) found no significant differences in 10-year survival rates or recurrence when investigating melanoma with a thickness of ≤2 mm in relation to 2–5 cm margins. However, Kunishige et al. (2012) revealed that 5 mm margins for in situ melanoma excision were insufficient, eliminating only around 86% of the lesion, and suggested standard excision margins of approximately 9 mm. Therefore, 5–10 mm margins are currently recommended for in situ melanoma (Bichakjian et al., 2011). After surgical removal, various methods are considered for restoring the functional and aesthetic features, such as primary closure, grafting of the skin, subsequent intended repair, and local and free flaps (Nakamura and Fujisawa, 2018). If primary closure is not possible, full-thickness skin grafting is often employed for restoration and negative pressure closure (NPC) is performed for skin graft stabilization (Llanos et al., 2006; Oh et al., 2013).
Following extensive excision of heel AM, local flaps such as a distally-based sural flap and a medial plantar flap can provide excellent aesthetic and functional outcomes. However, these flaps may negatively impact lymphatic circulation to localized lymph nodes, potentially increasing the risk of tumor growth (Nakamura et al., 2018).
Micrographic surgery, also known as Mohs surgery, is a specialized surgical procedure used to treat specific types of skin cancer, including AM. Compared to traditional surgical techniques, Mohs surgery offers several advantages, such as precise removal of cancerous tissue layer by layer while minimizing the removal of healthy tissue, resulting in higher cure rates, better cosmetic outcomes, and potentially reduced need for additional treatments such as radiation therapy or chemotherapy. However, Mohs surgery is a complex and time-consuming procedure that requires specialized training and equipment, and not all cases of AM are suitable for this surgery. It is crucial to work with an experienced healthcare team to ensure the best possible outcome and explore alternative treatments if needed (Loosemore et al., 2013; Seo et al., 2021).
4.2. Molecular targeted therapies
Most treatments for metastatic melanoma are currently designed to target transcription factors associated with specific driver mutations (BRAF, NRAS, phosphatase and tensin homolog (PTEN)) or to enhance the immune system response of tumors. However, the available treatment options have mainly been developed and tested on patients with superficial spreading melanoma/nodular melanoma (SSM/NM) (Dobson et al., 2014; Miller et al., 2014). Although there are genetic and multifactorial differences between AM and SSM/NM, it is difficult to generalize outcomes from current medical studies of melanoma therapies such as vemurafenib and ipilimumab. Nonetheless, novel medications are being developed and used to treat advanced AM, including targeting KIT (often mutated in AM) as a therapeutic strategy. Although imatinib or sunitinib has been utilized to treat advanced AM, their effectiveness in this group is still being studied (Zebary et al., 2013; Miller et al., 2014).
Malignant melanoma often exhibits prevalent mutations, such as the BRAF gene mutation, a serine-threonine kinase mutation occurring in 40% to 60% of patients (Manzano et al., 2016). This mutation activates the rapidly accelerated fibrosarcoma (Raf), extracellular signal-regulated kinase (ERK), and mitogen-activated protein kinase (MAPK) kinase (MEK) components of the MAPK pathway, leading to ERK phosphorylation and cellular proliferation, which serves as a protective mechanism and plays a critical role in cancer biology. The majority of cases involve a valine substitution at the 600 codon (V600E), with lysine (V600K) or arginine (V600R) mutation occurring rarely. According to Chapman et al. (2017), a randomized phase 3 study (BRIM-3) using the BRAF inhibitor vemurafenib demonstrated a significantly better response rate and increased progression-free survival (PFS) and OS in comparison to dacarbazine (DTIC) for patients with advanced BRAF mutant melanoma. Furthermore, the MEK inhibitor, which suppresses downstream BRAF, also extended the OS rate in these patients compared to DTIC (Flaherty et al., 2012). As MEK stimulation is a recognized drug resistance mechanism for BRAF suppression in BRAF mutant melanoma, clinical trials have combined BRAF and MEK inhibitors. The combination of trametinib (MEK inhibitor) and dabrafenib has been shown to have better efficacy and higher survival rates, and prolonged PFS compared to dabrafenib monotherapy. Therefore, the combined treatment with BRAF and MEK inhibitors is now recommended for patients with advanced BRAF-mutant melanoma (Coit et al., 2016a). The incidence of BRAF mutations in melanoma is known to be closely linked with significant solar exposure. However, such mutations are found to be relatively infrequent in AM in comparison to other types of melanoma (Zebary et al., 2013). Previous studies have reported BRAF mutation prevalence in AM to be around 15% to 20%, whereas it is much higher, ranging from 50% to 65%, in SSM (Curtin et al., 2005; Beadling et al., 2008; Greaves et al., 2013; Bastian, 2014; Kim et al., 2015; Yamazaki et al., 2015). On the other hand, KIT mutations are found to be more prevalent in AM, ranging from 10% to 20%, as compared to other melanomas (Curtin et al., 2005; Omholt et al., 2011). Promising outcomes have been observed in several phase II studies involving KIT inhibitors in KIT-mutant melanoma cases (Woodman and Davies, 2010). Besides KIT, NRAS mutations have also been reported in AM, and recent clinical trials of the potent MEK inhibitor, MEK162, in patients with NRAS-mutant melanoma have shown the modest efficacy (Lee et al., 2011; Ascierto et al., 2013). Consequently, given the low incidence of BRAF mutants in AM, a small group of AM patients may benefit from innovative drugs targeting these molecules in the future.
4.3. Immunological treatment
Immunological treatment, also known as immunotherapy, has emerged as a promising approach to treating AM. By enhancing the body’s immune response, this therapy targets cancer cells, leading to better outcomes for patients. With continued research, immunotherapy may become a standard treatment option for AM. Moreover, programmed cell death-1 (PD-1) is an immunoglobulin superfamily protein mainly expressed on the T lymphocytes’ surfaces, with the capability to attach to programmed cell death-ligand 1 (PD-L1) and PD-L2 and significantly suppress cluster of differentiation 28 (CD28) co-stimulation and T-cell receptor (TCR) signaling (Sharpe et al., 2007; Arasanz et al., 2017). Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is generally expressed on both types of T cells (conventional and regulatory). It could interact with CD28 for B7 and block the signaling of TCRs (Linsley et al., 1996). Thus, PD-1 and CTLA-4 are essential determinants for inhibiting T-cell stimulation. The most recent discovery of immune checkpoint drugs that target such molecules has substantially improved metastatic melanoma treatment. The survival rate of metastatic melanoma patients has increased due to anti-CTLA-4 and anti-PD-1 therapies (Robert et al., 2011, 2015; Hamid et al., 2017). Coit et al. (2016b) recommended ICIs as the first-line treatment for BRAF metaststic melanoma. On the other hand, Kaunitz et al. (2017) observed that AM is less responsive to ICIs than SSM or lentigo malignant melanoma (LMM). Furthermore, Nakamura and Fujisawa (2018) discovered that the highest performance response rates for individuals with SSM and AM achieved by anti-PD-1 antibodies were 80% and 25%, respectively. In addition, the response of TILs to immunotherapies was significant. The number of TILs in AM was reduced markedly compared with that in non-AM patients (Castaneda et al., 2017). Hence, further investigation is needed to improve AM response to immune checkpoint-blocked treatment through the combinations of ICIs and other immunostimulants.
Fan et al. (2015) showed that topical imiquimod is the potential for LMM treatment with positive surgical margins. Several studies have established the efficacy of imiquimod in different AM categories (non-subungual and subungual); however, the imiquimod-induced inflammatory response appears to be considerably challenging in causing an ulcerated acral lesion due to the dense corneum and epidermal layers, which inhibits component absorption (Savarese et al., 2015; Ocampo-Garza et al., 2017). Thus, imiquimod may be a potential therapy option for individuals unable to undergo surgery due to patient desire, comorbidities, or functional impairment.
Furthermore, type I interferons (IFNs) exhibit diverse biological functions, including antiangiogenic, immunoregulatory, antiproliferative, differentiation-inducing, and antiapoptotic activities (Kirkwood et al., 2002). Regarding immunoregulation, the dendritic cell reactivity to tumor antigens may improve with type I IFNs, which can also increase cross-linking antigens; therefore, an antitumor immunity enhancement occurs (Kirkwood et al., 2002; Wang et al., 2007). IFNs have been routinely utilized as adjuvant therapy for individuals whose primary tumor has been removed. Prior randomized controlled studies showed the effectiveness of IFN doses (at high-dose or pegylated) as adjuvant treatment (Rafique et al., 2015), but unfortunately, the advantage of IFN adjuvant therapy was slight. Recently, Long et al. (2017a) and Eggermont et al. (2018) reported that, when IFNs were used as adjuvant therapy, the BRAF and ICIs greatly extended the survival rate; therefore, studies of IFN coupled with anti-CTLA-4 or anti-PD-1 antibodies for the treatment of metastatic melanoma are ongoing (Rafique et al., 2015).
4.4. Radiation therapy
Radiotherapy is not the primary treatment for melanocytes due to low radio-sensitivity. However, it may be used for adjuvant therapy, palliation, and recurring cases (Seegenschmiedt et al., 1999). SLNs, which drain from the initial tumor, are crucial in determining prognosis. Thus, SLNB is recommended for intermediate-thickness melanoma patients (Coit et al., 2019). SLNB is recommended by the National Comprehensive Cancer Network (Coit et al., 2019) for first melanomas with an AJCC stage of T1b or above. Because of the high probability of loco-regional recurrence, SLNB may be particularly significant for AM. Swetter et al. (2021) recommend supplementing SLNB with ultrasound monitoring of the node pelvis, rather than extensive lymph node dissection, for cases with a higher risk of progression.
Moreover, no increase in melanoma-specific survival was observed in two separate trials comparing the benefits of complete lymph node dissection following positive SLNB assessment against clinical evaluation with nodal ultrasound surveillance. Multiple studies have shown that AM patients with positive SLNs had shorter OS and disease-free survival (DFS) (Ito et al., 2015). Recently, Marek et al. (2016) found that, in superficial melanoma, AM had the greatest prevalence of positive SLNs amongst all histologic categories and was an independent predictor of the positivity of SLN. Nowadays, the use of elective lymph node dissection (ELND) has suggested that an SLNB reveals metastatic disease (Coit et al., 2016a). Conversely, Faries et al. (2017) conducted a randomized study (the second Multicenter Selective Lymphadenectomy Trial (MSLT-II)) that compared positive SLN patients with versus without rapid ELND, indicating that quick ELND did not increase disease-specific survival. Moreover, Nakamura et al. (2018) indicated that ELND might enhance tumor development via a deficient adaptive immune response. Hence, in the coming years, the existing recommendation for immediate ELND following a positive SLNB may be modified.
4.5. Chemotherapy
Chemotherapies have been utilized to fight cancer due to their cytotoxic activity on tumor cells. For decades, chemotherapy with DTIC has been regarded as the mainstay treatment for individuals with malignant melanoma (Nakamura and Fujisawa, 2018). Nevertheless, it is recognized that responses to DTIC are minimal, and prior trials have not shown a survival advantage with DTIC. However, current research has demonstrated that several chemotherapy medications have immunosuppressive properties. According to Hervieu et al. (2013), DTIC had no immediate effect on immune cells and it eventually caused the overexpression of natural killer group 2 member D (NKG2D) ligands on cancerous cells, which resulted in natural killer (NK) upregulation and IFN release in mice and humans. NK cell-derived IFN then promoted the expression of the main histocompatibility complex class I molecules on malignant cells, making them vulnerable to cytotoxic CD8+ T lymphocytes. Furthermore, until 2011, chemotherapy with DTIC was regarded as the standard therapy for individuals with inoperable or malignant melanoma (Manzano et al., 2016).
Over the past decade, the management of stage IV melanoma has seen significant advancements with the introduction of immunotherapy and targeted therapy. Prior to this, phase III trials using chemotherapy achieved objective response rates (ORRs) ranging from 5% to 15% but did not have a noticeable impact on OS, resulting in an average OS of 8–10 months with approved therapies (Flaherty et al., 2012; Hauschild et al., 2012; Manzano et al., 2016). A study by Häfliger et al. (2018) demonstrated that primary chemotherapy for stage IV AM had an ORR of 44%, which was higher than the previously reported results for CM, yet the OS remained within the literature limits (8–9 months) (Flaherty et al., 2012; Hauschild et al., 2012; Long et al., 2017b). The use of ICIs, such as ipilimumab, in combination with DTIC, has shown improved OS in phase III randomized trials (Coit et al., 2016a; Lee et al., 2016; Russo et al., 2017). This is achieved by increasing anti-cancer T-cell immunity through the suppression of cytotoxic T-lymphocyte-associated antigen 4, enhancing the immunostimulatory properties towards melanoma. In addition to having a superior ORR compared to chemotherapy, immunotherapy and targeted therapy have revolutionized the management of metastatic melanoma.
4.6. Oncolytic virotherapy
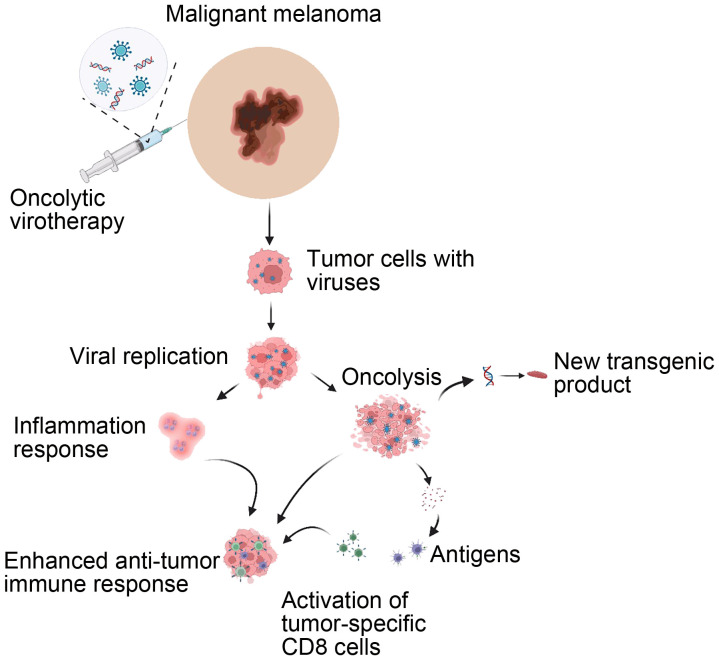
Oncolytic virotherapy is a virus-related therapy, in which a genetically modified virus is injected into the tumor. It is part of intralesional immune therapies that include proinflammatory cytokines, innate immune agonists, and vaccines, providing an alternative treatment for metastasized cancers, especially inoperable or malignant melanoma, where traditional treatment methods, such as surgical resection, chemotherapy, and immune checkpoint therapy, have unsatisfactory outcomes with high recurrence rates (Beasley et al., 2009; Perone et al., 2018) (Fig. 2). The selective replication of the oncolytic viruses within the cancer cells and the ability to enhance anticancer immunity in the body, along with high cytotoxicity, enable them to induce apoptosis and destroy the tumor cells (Ito et al., 2006). The association between viruses and cancer treatment has been investigated since the early 20th century, when many clinical experiments were performed to test the effectiveness of different viruses, including the herpes simplex virus, reovirus, adenovirus, and vaccinia, as anticancer agents (Kuruppu and Tanabe, 2005; Alemany, 2013). The anticancer effects of viruses were reported in cervical cancer, Hodgkin’s lymphoma, and Burkitt’s lymphoma after the patients had a viral infection or immunization (Kuruppu and Tanabe, 2005). In 2004, the enteric cytopathic human orphan virus type 7 (ECHO-7) was the first oncolytic virus approved in Latvia and was used to treat skin melanoma (Lichty et al., 2014; Doniņa et al., 2015), but in 2019 the state agency of medicine in Latvia withdrew it and suspended its registration (De Facto and Eng.LSM.lv, 2019). Since then, many other viruses have been genetically modified to target specific cancer cells. The China’s State Food and Drug Administration (SFDA) New Drug Application (NDA) approved a modified adenovirus called H101 (Oncorine) in 2005 for head-and-neck cancer treatment (Frew et al., 2008). Ten years later, the US and the European Union approved the first oncolytic virus known as talimogene laherparepvec (T-VEC), which was a genetic modification of the herpes virus involving genetic deletion of infected cell protein 34.5 (ICP34.5) and ICP47, a neurovirulence gene and antigen presentation inhibiter, respectively (Pol et al., 2016), and the addition of the Granulocyte-macrophage colony-stimulating factor (GMCSF) gene for antitumor immune response (Kaufman et al., 2015). T-VEC is used to treat inoperable melanoma by being injected directly into the lesion to produce an anticancer immune response and invade the cancer cells, and then start replicating; as a result, the cancer cells will be swollen and burst (Fukuhara et al., 2016). Another clinical trial investigated the efficacy of T-VEC in 50 patients with unresectable metastatic melanoma. It found that the virus increased survival rate and had systemic efficacy and robustness (Senzer et al., 2009). Furthermore, Andtbacka et al. (2015)’s clinical trial was the main reason for the approval of T-VEC for treating inoperable stages III and IV melanoma, where the durable response rate and overall response rate of T-VEC were greater than those of GM-CSF. T-VEC efficacy is higher when it is combined with systemic therapy, such as ipilimumab or pembrolizumab, in treating advanced melanoma (Ribas et al., 2017; Chesney et al., 2018). There are many other oncolytic viruses under investigation for providing better treatment for advanced melanoma. Some of these viruses are ONCOS-102 (modified adenovirus), coxsackievirus A21, poliovirus (PVSRIPO), and vesicular stomatitis virus (VSV) (Koski et al., 2010; Wollmann et al., 2013; Kaufman et al., 2015; Farrow et al., 2020). In the future, oncolytic virotherapy will contribute greatly to treating unresectable AM or other lesions due to extensive animal and clinical research.
Fig. 2. Oncolytic virotherapy mechanism in treating advanced malignant melanoma. Reprinted from Robinson et al. (2022) licensed under a Creative Commons Attribution 4.0 International License, with some modifications.
5. Modern development of acral melanoma research
Recent studies have been attempting to find the most suitable treatment methods for AM; some of these studies will be discussed in this section. Bian et al. (2021) described a study on a significant multi-institutional cohort of 4796 AM patients during 2004–2015 using NCDB to define the demographic and therapeutic aspects of AM. The findings showed that AM patients had a lower 5-year survival rate when categorized by phases than non-AM patients, especially for stage III patients (47.5% vs. 56.7%, P<0.001). Older age, male sex, comorbidity load, positive lymph nodes, higher tumor thickness, ulceration, and positive metastasis were all independently related to a lower rate of 5-year survival in AM patients. Furthermore, stage III AM patients were found to have a higher survival rate under a multimodal treatment (surgery along with radiation and/or systemic therapies) in contrast with lower stages. The aforementioned findings highlight the need for further research into the potential of therapy intensification in the AM patient population. Yang et al. (2011) tested the therapeutic efficacy of various therapies for malignant melanoma of the finger. The most common therapies were surgery, chemotherapy (one pre-operative chemotherapy cycle and four to six postoperative chemotherapy cycles), and immunotherapy (using IFNα2b and four cases had interleukin-2 (IL-2)). They concluded that the most effective treatment was a combination of surgery, chemotherapy, and immunotherapy, and that the survival rates for 1-, 3-, and 5-year follow-ups were 86.4%, 42.1%, and 31.0%, respectively. Moreover, Kwon et al. (2019) aimed to identify the most prevalent primary metastatic location of AM and the use of pre-operative ultrasonography for SLN mapping. The experiments were carried out on 98 Korean AM cases. The results showed that the most prevalent site of origin was the sole (33.7%) and loco-regional recurrence was the most frequent site of primary metastasis (64.7%). Moreover, pre-operative SLN ultrasonography’s sensitivity, specificity, and prediction values (negative and positive) were 29.1%, 94.6%, 63.0%, and 80.0%, respectively. The limited sensitivity of pre-operative ultrasound observations of SLN underscores the necessity of pathological confirmation of SLN in AM patients, especially at earlier stages of the disease (Sanki et al., 2009). Therefore, the authors hypothesized that pre-operative ultrasound for SLN mapping in AM is ineffective.
It has been indicated that the Wnt/β-catenin signalling pathway plays a central role in melanoma development (Espada et al., 2009). The essential components of the Wnt signalling system are β-catenin and lymphoid enhancer-binding factor-1 (LEF-1) (Takahashi et al., 2008). Under physiological settings, β-catenin gene mutations and other molecular alterations improve the cytoplasmic stabilization of β-catenin (Friedlander and Hodi, 2010; Gartner et al., 2012). According to earlier research, heparanase-1 (HPA-1) is also a risk factor for the emergence of skin cancer (Liu et al., 2012). In addition, HPA-1 is implicated in the dissemination and invasion of cancer cells and is increased in metastatic tumors (Orgaz and Sanz-Moreno, 2013). Also, 14.5% and 16.0% of the AMs were evaluated for NRAS and BRAF mutations, respectively. Consequently, Xu et al. (2015) examined AM cases without mutant variants of BRAF and NRAS and analyzed the expression of LEF-1, β-catenin, and HPA-1 in peritumoral tissue, pigmented nevus, and malignant melanoma, as well as their roles in the prognosis. The results reflected that AM patients had positive expression of LEF-1, β-catenin, and HPA-1, with corresponding percentages of 62%, 72%, and 64%, respectively. Moreover, LEF-1, β-catenin, and HPA-1 expression levels were not associated with age, gender, or diseased body parts (P>0.05) but were strongly correlated with tumor node metastasis (TNM) phase and metastasis. In patients with harmful mutations in BRAF exons 11 and 15 and NRAS exons 1 and 2, the expression of LEF-1, β-catenin, and HPA-1 was evaluated and compared between malignant melanoma, benign nevus, and peritumoral tissues, and the results identified LEF-1, β-catenin, and HPA-1 as potential therapies for metastatic AM.
The recent progress in immune checkpoint regulation and exceptional clinical activity in AM opens the way to new combinations that may bypass the tolerogenic tumor mechanisms that are recognized to limit the powerful antitumor effect of IFN-α. Rafique et al. (2015) reported that IFN has considerable immunomodulatory and anticancer clinical efficacy, contributing to its antiproliferative properties in metastatic melanoma and its complementary effectiveness in more than 20 studies of phase III. Despite the potential anticancer benefits of immunotherapy treatment, metastatic cancer has been found to induce immunological suppression, thereby reducing the efficacy of this treatment approach. However, it has become more feasible to overcome tumor-induced immune suppression in metastatic cancers through techniques that utilize the immunomodulatory and therapeutic potential benefits of IFN. In the case of malignant lesions such as AM, modifying the consequences of immune checkpoints may allow therapeutic antibodies to control or destroy malignancies by reducing the restrictions that inhibit the response to novel antigens. These approaches have the potential to improve the effectiveness of adjuvant therapy in patients with significant tumor loads. Antibodies that interrupt suppressive mechanisms, including those recognized for clinical use as an anticancer drug that targeted PD-1, CTLA-4, and PD-L1, are appropriate partners to the cytokine/IFN response system, to form different effects such as synergistic or complementary effects. In addition, specific checkpoint-blocking antibodies showed a significant effect in treating various types of malignancies, as a monotherapy or combined with other immunotherapeutic agents. Furthermore, the combination of anti-CTLA-4 immunotherapy with high-dose IFN and tremelimumab has exhibited long-lasting anticancer activity that is attracting interest concerning experimental studies of international cooperating associations. Another area of focus is the evaluation of the effectiveness of combining ipilimumab or pembrolizumab with IFN for treating metastatic melanoma.
6. Conclusions
Clinical information, including dermoscopic findings, histological examination, radiological imaging, biomarkers, and blood tests, is important for diagnosing AM and determining the most accurate treatment method. Many algorithms have been established for diagnosing AM, which have considered PRP the unique dermoscopic pattern of AM and the first step that the physician should be aware of. Although surgical intervention is the first line of treatment, other advanced methods are available such as targeted molecular therapies and ICIs; these are the first line of treatment for BRAF wild-type metastatic melanoma.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LS21H060001), the Alibaba Youth Studio Project (No. ZJU-032), and the Zhejiang Province Medical and Health Science and Technology Program (Nos. 2022KY1455 and 2022RC136). The funding bodies had no role in the design of the study, in collection, analysis, and interpretation of data, or in drafting the manuscript. We thank Fatima ALALIWI and Ma LING for their invaluable support and encouragement.
Author contributions
Hui LU, Ahmad ALHASKAWI, Sohaib Hasan Abdullah EZZI, Mohamed Hasan Abdulla Hasan ABDULLA, Jingtian LAI, and Yanzhao DONG designed the study. Chengjun YAO, Vishnu Goutham KOTA, Zewei WANG, and Mohamed Hasan Abdulla Hasan ABDULLA performed literature collection. Sohaib Hasan Abdullah EZZI, Ahmad ALHASKAWI, Hui LU, and Yanzhao DONG wrote the original draft. Zewei WANG, Jingtian LAI, Haiying ZHOU, Chengjun YAO, and Vishnu Goutham KOTA edited the draft. Hui LU, Ahmad ALHASKAWI, Sohaib Hasan Abdullah EZZI, Haiying ZHOU, Yanzhao DONG, and Zewei WANG analyzed the results. Hui LU, Ahmad ALHASKAWI, Haiying ZHOU, and Sohaib Hasan Abdullah EZZI contributed to manuscript supervision. All authors have read and approved the final manuscript.
Compliance with ethics guidelines
Ahmad ALHASKAWI, Sohaib Hasan Abdullah EZZI, Yanzhao DONG, Haiying ZHOU, Zewei WANG, Jingtian LAI, Chengjun YAO, Vishnu Goutham KOTA, Mohamed Hasan Abdulla Hasan ABDULLA, and Hui LU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Abbasi NR, Shaw HM, Rigel DS, et al. , 2004. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA, 292(22): 2771-2776. 10.1001/jama.292.22.2771 [DOI] [PubMed] [Google Scholar]
- Alemany R, 2013. Viruses in cancer treatment. Clin Transl Oncol, 15(3): 182-188. 10.1007/s12094-012-0951-7 [DOI] [PubMed] [Google Scholar]
- Andtbacka RHI, Kaufman HL, Collichio F, et al. , 2015. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol, 33(25): 2780-2788. 10.1200/jco.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- Arasanz H, Gato-Cañas M, Zuazo M, et al. , 2017. PD1 signal transduction pathways in T cells. Oncotarget, 8(31): 51936-51945. 10.18632/oncotarget.17232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington JH III, Reed RJ, Ichinose H, et al. , 1977. Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma. Am J Surg Pathol, 1(2): 131-143. [PubMed] [Google Scholar]
- Ascierto PA, Schadendorf D, Berking C, et al. , 2013. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol, 14(3): 249-256. 10.1016/s1470-2045(13)70024-x [DOI] [PubMed] [Google Scholar]
- Bastian BC, 2014. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol: Mech Dis, 9: 239-271. 10.1146/annurev-pathol-012513-104658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Jacobson-Dunlop E, Hodi FS, et al. , 2008. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res, 14(21): 6821-6828. 10.1158/1078-0432.Ccr-08-0575 [DOI] [PubMed] [Google Scholar]
- Beasley GM, Caudle A, Petersen RP, et al. , 2009. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg, 208(5): 706-715. 10.1016/j.jamcollsurg.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Bello DM, Chou JF, Panageas KS, et al. , 2013. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol, 20(11): 3618-3625. 10.1245/s10434-013-3089-0 [DOI] [PubMed] [Google Scholar]
- Bian SX, Hwang L, Hwang J, et al. , 2021. Acral lentiginous melanoma—population, treatment, and survival using the NCDB from 2004 to 2015. Pigment Cell Melanoma Res, 34(6): 1049-1061. 10.1111/pcmr.12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichakjian CK, Halpern AC, Johnson TM, et al. , 2011. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol, 65(5): 1032-1047. 10.1016/j.jaad.2011.04.031 [DOI] [PubMed] [Google Scholar]
- Boyd AS, Rapini RP, 1994. Acral melanocytic neoplasms: a histologic analysis of 158 lesions. J Am Acad Dermatol, 31(5 Pt 1): 740-745. 10.1016/s0190-9622(94)70235-7 [DOI] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, McMaster ML, et al. , 2009. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986‒2005. Arch Dermatol, 145(4): 427-434. 10.1001/archdermatol.2008.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Puccio F, Chian C, 2011. Acral junctional nevus versus acral lentiginous melanoma in situ: a differential diagnosis that should be based on clinicopathologic correlation. Arch Pathol Lab Med, 135(7): 847-852. 10.5858/2010-0323-rar.1 [DOI] [PubMed] [Google Scholar]
- Bristow IR, de Berker DAR, 2010. Development of a practical guide for the early recognition for malignant melanoma of the foot and nail unit. J Foot Ankle Res, 3: 22. 10.1186/1757-1146-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera R, Recule F, 2018. Unusual clinical presentations of malignant melanoma: a review of clinical and histologic features with special emphasis on dermatoscopic findings. Am J Clin Dermatol, 19(S1): 15-23. 10.1007/s40257-018-0373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascinelli N, Zurrida S, Galimberti V, et al. , 1994. Acral lentiginous melanoma. A histological type without prognostic significance. J Dermatol Surg Oncol, 20(12): 817-822. 10.1111/j.1524-4725.1994.tb03711.x [DOI] [PubMed] [Google Scholar]
- Castaneda CA, Torres-Cabala C, Castillo M, et al. , 2017. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin Transl Oncol, 19(12): 1478-1488. 10.1007/s12094-017-1685-3 [DOI] [PubMed] [Google Scholar]
- Chang AE, Karnell LH, Menck HR, 1998. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84, 836 cases from the past decade. Cancer, 83(8): 1664-1678. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Robert C, Larkin J, et al. , 2017. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol, 28(10): 2581-2587. 10.1093/annonc/mdx339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Puzanov I, Collichio F, et al. , 2018. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol, 36(17): 1658-1667. 10.1200/jco.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah SY, Tsilika K, Chiaverini C, et al. , 2015. Dermoscopic features of congenital acral melanocytic naevi in children: a prospective comparative and follow-up study. Br J Dermatol, 172(1): 88-93. 10.1111/bjd.13187 [DOI] [PubMed] [Google Scholar]
- Cinotti E, Debarbieux S, Perrot JL, et al. , 2016. Reflectance confocal microscopy features of acral lentiginous melanoma: a comparative study with acral nevi. J Eur Acad Dermatol Venereol, 30(7): 1125-1128. 10.1111/jdv.13399 [DOI] [PubMed] [Google Scholar]
- Coit DG, Thompson JA, Algazi A, et al. , 2016a. Melanoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw, 14(4): 450-473. 10.6004/jnccn.2016.0051 [DOI] [PubMed] [Google Scholar]
- Coit DG, Thompson JA, Algazi A, et al. , 2016b. NCCN guidelines insights: melanoma, version 3.2016. J Natl Compr Canc Netw, 14(8): 945-958. 10.6004/jnccn.2016.0101 [DOI] [PubMed] [Google Scholar]
- Coit DG, Thompson JA, Albertini MR, et al. , 2019. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw, 17(4): 367-402. 10.6004/jnccn.2019.0018 [DOI] [PubMed] [Google Scholar]
- Costello CM, Ghanavatian S, Temkit M, et al. , 2018. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol, 32(9): 1450-1455. 10.1111/jdv.14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, et al. , 2005. Distinct sets of genetic alterations in melanoma. N Engl J Med, 353(20): 2135-2147. 10.1056/NEJMoa050092 [DOI] [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, et al. , 2006. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol, 24(26): 4340-4346. 10.1200/jco.2006.06.2984 [DOI] [PubMed] [Google Scholar]
- Darmawan CC, Jo G, Montenegro SE, et al. , 2019. Early detection of acral melanoma: a review of clinical, dermoscopic, histopathologic, and molecular characteristics. J Am Acad Dermatol, 81(3): 805-812. 10.1016/j.jaad.2019.01.081 [DOI] [PubMed] [Google Scholar]
- De Facto, Eng. LSM. lv, 2019. Rigvir cancer treatment at center of fresh controversy. https://eng.lsm.lv [Google Scholar]
- de Lima Vazquez V, Vicente AL, Carloni A, et al. , 2016. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res, 26(2): 93-99. 10.1097/cmr.0000000000000222 [DOI] [PubMed] [Google Scholar]
- Diaz A, Puig-Butillé JA, Valera A, et al. , 2014. TERT and AURKA gene copy number gains enhance the detection of acral lentiginous melanomas by fluorescence in situ hybridization. J Mol Diagn, 16(2): 198-206. 10.1016/j.jmoldx.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Dobson R, Burgess MI, Valle JW, et al. , 2014. Serial surveillance of carcinoid heart disease: factors associated with echocardiographic progression and mortality. Br J Cancer, 111(9): 1703-1709. 10.1038/bjc.2014.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniņa S, Strēle I, Proboka G, et al. , 2015. Adapted ECHO-7 virus rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res, 25(5): 421-426. 10.1097/cmr.0000000000000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont AMM, Blank CU, Mandala M, et al. , 2018. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med, 378(19): 1789-1801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- Espada J, Calvo MB, Díaz-Prado S, et al. , 2009. Wnt signalling and cancer stem cells. Clin Transl Oncol, 11(7): 411-427. 10.1007/s12094-009-0380-4 [DOI] [PubMed] [Google Scholar]
- Fan Q, Cohen S, John B, et al. , 2015. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J, 15(4): 443-447. [PMC free article] [PubMed] [Google Scholar]
- Faries MB, Thompson JF, Cochran AJ, et al. , 2017. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med, 376(23): 2211-2222. 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow NE, Leddy M, Landa K, et al. , 2020. Injectable therapies for regional melanoma. Surg Oncol Clin N Am, 29(3): 433-444. 10.1016/j.soc.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton S, Taylor RS, Srivastava D, 2016. Excision margins for melanoma in situ on the head and neck. Dermatol Surg, 42(3): 327-334. 10.1097/dss.0000000000000648 [DOI] [PubMed] [Google Scholar]
- Fernandez-Flores A, Cassarino DS, 2017. Histopathological diagnosis of acral lentiginous melanoma in early stages. Ann Diagn Pathol, 26: 64-69. 10.1016/j.anndiagpath.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Robert C, Hersey P, et al. , 2012. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med, 367(2): 107-114. 10.1056/NEJMoa1203421 [DOI] [PubMed] [Google Scholar]
- Frew SE, Sammut SM, Shore AF, et al. , 2008. Chinese health biotech and the billion-patient market. Nat Biotechnol, 26(1): 37-53. 10.1038/nbt0108-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander P, Hodi FS, 2010. Advances in targeted therapy for melanoma. Clin Adv Hematol Oncol, 8(9): 619-627. [PubMed] [Google Scholar]
- Fukuhara H, Ino Y, Todo T, 2016. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci, 107(10): 1373-1379. 10.1111/cas.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe C, Amaral T, Peris K, et al. , 2020. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics ‒ Update 2019. Eur J Cancer, 126: 141-158. 10.1016/j.ejca.2019.11.014 [DOI] [PubMed] [Google Scholar]
- Gartner JJ, Davis S, Wei XM, et al. , 2012. Comparative exome sequencing of metastatic lesions provides insights into the mutational progression of melanoma. BMC Genomics, 13: 505. 10.1186/1471-2164-13-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves WO, Verma S, Patel KP, et al. , 2013. Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn, 15(2): 220-226. 10.1016/j.jmoldx.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaste PV, Fleming NH, Silva I, et al. , 2014. Analysis of recurrence patterns in acral versus nonacral melanoma: should histologic subtype influence treatment guidelines? J Natl Compr Canc Netw, 12(12): 1706-1712. 10.6004/jnccn.2014.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfliger EM, Ramelyte E, Mangana J, et al. , 2018. Metastatic acral lentiginous melanoma in a tertiary referral center in Switzerland: a systematic analysis. Melanoma Res, 28(5): 442-450. 10.1097/cmr.0000000000000465 [DOI] [PubMed] [Google Scholar]
- Hamid O, Puzanov I, Dummer R, et al. , 2017. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer, 86: 37-45. 10.1016/j.ejca.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Han B, Hur K, Ohn J, et al. , 2020. Acral lentiginous melanoma in situ: dermoscopic features and management strategy. Sci Rep, 10: 20503. 10.1038/s41598-020-77425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, et al. , 2012. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet, 380(9839): 358-365. 10.1016/s0140-6736(12)60868-x [DOI] [PubMed] [Google Scholar]
- Hayward NK, Wilmott JS, Waddell N, et al. , 2017. Whole-genome landscapes of major melanoma subtypes. Nature, 545(7653): 175-180. 10.1038/nature22071 [DOI] [PubMed] [Google Scholar]
- Hervieu A, Rébé C, Végran F, et al. , 2013. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol, 133(2): 499-508. 10.1038/jid.2012.273 [DOI] [PubMed] [Google Scholar]
- Huang K, Fan J, Misra S, 2020. Acral lentiginous melanoma: incidence and survival in the United States, 2006‒2015, an analysis of the SEER registry. J Surg Res, 251: 329-339. 10.1016/j.jss.2020.02.010 [DOI] [PubMed] [Google Scholar]
- Ide T, Ito T, Wada-Ohno M, et al. , 2021. Preoperative screening CT and PET/CT scanning for acral melanoma: is it necessary? J Clin Med, 10(4): 811. 10.3390/jcm10040811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara Y, Saida T, Miyazaki A, et al. , 2006. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol, 28(1): 21-27. 10.1097/01.dad.0000187931.05030.a0 [DOI] [PubMed] [Google Scholar]
- Ito H, Aoki H, Kühnel F, et al. , 2006. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst, 98(9): 625-636. 10.1093/jnci/djj161 [DOI] [PubMed] [Google Scholar]
- Ito T, Wada M, Nagae K, et al. , 2015. Acral lentiginous melanoma: who benefits from sentinel lymph node biopsy? J Am Acad Dermatol, 72(1): 71-77. 10.1016/j.jaad.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Kohlhapp FJ, Zloza A, 2015. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov, 14(9): 642-662. 10.1038/nrd4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunitz GJ, Cottrell TR, Lilo M, et al. , 2017. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest, 97(9): 1063-1071. 10.1038/labinvest.2017.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat D, Rixe O, Martin G, et al. , 2003. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer, 97(8): 1941-1946. 10.1002/cncr.11272 [DOI] [PubMed] [Google Scholar]
- Kilinc Karaarslan I, Akalin T, Unal I, et al. , 2007. Atypical melanosis of the foot showing a dermoscopic feature of the parallel ridge pattern. J Dermatol, 34(1): 56-59. 10.1111/j.1346-8138.2007.00217.x [DOI] [PubMed] [Google Scholar]
- Kim JY, Hwang EJ, Choi M, et al. , 2014. Recurrent acral lentiginous melanoma in situ suggesting the field cell theory. Ann Dermatol, 26(6): 779-781. 10.5021/ad.2014.26.6.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kim SN, Hahn HJ, et al. , 2015. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol, 72(6): 1036-1046.e2. 10.1016/j.jaad.2015.02.1113 [DOI] [PubMed] [Google Scholar]
- Kirkwood JM, Richards T, Zarour HM, et al. , 2002. Immunomodulatory effects of high-dose and low-dose interferon α2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer, 95(5): 1101-1112. 10.1002/cncr.10775 [DOI] [PubMed] [Google Scholar]
- Kong LQ, Tan KP, Tan HW, et al. , 2020. Use of magnetic resonance imaging as a noninvasive technique to identify acral lentiginous melanoma. J Am Acad Dermatol, 83(2): e121-e123. 10.1016/j.jaad.2019.09.025 [DOI] [PubMed] [Google Scholar]
- Koski A, Kangasniemi L, Escutenaire S, et al. , 2010. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther, 18(10): 1874-1884. 10.1038/mt.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus SL, Haenssle HA, 2013. Early detection of cutaneous melanoma by sequential digital dermatoscopy (SDD). J Dtsch Dermatol Ges, 11(6): 509-512. 10.1111/ddg.12072 [DOI] [PubMed] [Google Scholar]
- Kunishige JH, Brodland DG, Zitelli JA, 2012. Surgical margins for melanoma in situ. J Am Acad Dermatol, 66(3): 438-444. 10.1016/j.jaad.2011.06.019 [DOI] [PubMed] [Google Scholar]
- Kuruppu D, Tanabe KK, 2005. Viral oncolysis by herpes simplex virus and other viruses. Cancer Biol Ther, 4(5): 524-531. 10.4161/cbt.4.5.1820 [DOI] [PubMed] [Google Scholar]
- Kwon MR, Choi SH, Jang KT, et al. , 2019. Acral malignant melanoma; emphasis on the primary metastasis and the usefulness of preoperative ultrasound for sentinel lymph node metastasis. Sci Rep, 9: 15894. 10.1038/s41598-019-52180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallas A, Kyrgidis A, Koga H, et al. , 2015. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol, 173(4): 1041-1049. 10.1111/bjd.14045 [DOI] [PubMed] [Google Scholar]
- LeBoit PE, 2000. A diagnosis for maniacs. Am J Dermatopathol, 22(6): 556-558. 10.1097/00000372-200012000-00012 [DOI] [PubMed] [Google Scholar]
- Lee JH, Choi JW, Kim YS, 2011. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol, 164(4): 776-784. 10.1111/j.1365-2133.2010.10185.x [DOI] [PubMed] [Google Scholar]
- Lee KT, Kim EJ, Lee DY, et al. , 2016. Surgical excision margin for primary acral melanoma. J Surg Oncol, 114(8): 933-939. 10.1002/jso.24442 [DOI] [PubMed] [Google Scholar]
- Liang WS, Hendricks W, Kiefer J, et al. , 2017. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res, 27(4): 524-532. 10.1101/gr.213348.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty BD, Breitbach CJ, Stojdl DF, et al. , 2014. Going viral with cancer immunotherapy. Nat Rev Cancer, 14(8): 559-567. 10.1038/nrc3770 [DOI] [PubMed] [Google Scholar]
- Linsley PS, Bradshaw J, Greene J, et al. , 1996. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity, 4(6): 535-543. 10.1016/s1074-7613(00)80480-x [DOI] [PubMed] [Google Scholar]
- Liu XY, Fang H, Chen HC, et al. , 2012. An artificial miRNA against HPSE suppresses melanoma invasion properties, correlating with a down-regulation of chemokines and MAPK phosphorylation. PLoS ONE, 7(6): e38659. 10.1371/journal.pone.0038659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos S, Danilla S, Barraza C, et al. , 2006. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg, 244(5): 700-705. 10.1097/01.sla.0000217745.56657.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Hauschild A, Santinami M, et al. , 2017a. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med, 377(19): 1813-1823. 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- Long GV, Flaherty KT, Stroyakovskiy D, et al. , 2017b. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol, 28(7): 1631-1639. 10.1093/annonc/mdx176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosemore MP, Morales-Burgos A, Goldberg LH, 2013. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg, 39(1 Pt 1): 136-138. 10.1111/j.1524-4725.2012.02569.x [DOI] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, et al. , 2003. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst, 95(24): 1878-1890. 10.1093/jnci/djg123 [DOI] [PubMed] [Google Scholar]
- Manzano JL, Layos L, Bugés C, et al. , 2016. Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med, 4(12): 237. 10.21037/atm.2016.06.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek AJ, Ming ME, Bartlett EK, et al. , 2016. Acral lentiginous histologic subtype and sentinel lymph node positivity in thin melanoma. JAMA Dermatol, 152(7): 836-837. 10.1001/jamadermatol.2016.0875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menis D, Maroñas-Jiménez L, Rodríguez-Peralto J, et al. , 2015. Two Spanish cases of atypical melanosis of the foot, an early stage of acral lentiginous melanoma in situ . Br J Dermatol, 172(5): 1436-1438. 10.1111/bjd.13485 [DOI] [PubMed] [Google Scholar]
- Miller DM, Flaherty KT, Tsao H, 2014. Current status and future directions of molecularly targeted therapies and immunotherapies for melanoma. Semin Cutan Med Surg, 33(2): 60-67. 10.12788/j.sder.0081 [DOI] [PubMed] [Google Scholar]
- Mun JH, Jo G, Darmawan CC, et al. , 2018. Association between Breslow thickness and dermoscopic findings in acral melanoma. J Am Acad Dermatol, 79(5): 831-835. 10.1016/j.jaad.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Fujisawa Y, 2018. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol, 19(8): 42. 10.1007/s11864-018-0560-y [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Fujisawa Y, Okiyama N, et al. , 2018. Surgical damage to the lymphatic system promotes tumor growth via impaired adaptive immune response. J Dermatol Sci, 90(1): 46-51. 10.1016/j.jdermsci.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Ocampo-Garza J, di Chiacchio NG, Haneke E, et al. , 2017. Subungual melanoma in situ treated with imiquimod 5% cream after conservative surgery recurrence. J Drugs Dermatol, 16(3): 268-270. [PubMed] [Google Scholar]
- Ogata D, Arai E, Goto Y, et al. , 2018. Pilot study on the correlation between dermoscopic patterns and fluorescence in situ hybridization findings using whole-slide digital imaging for acral volar melanocytic lesions. J Dermatol, 45(7): 830-836. 10.1111/1346-8138.14324 [DOI] [PubMed] [Google Scholar]
- Oh BH, Lee SH, Nam KA, et al. , 2013. Comparison of negative pressure wound therapy and secondary intention healing after excision of acral lentiginous melanoma on the foot. Br J Dermatol, 168(2): 333-338. 10.1111/bjd.12099 [DOI] [PubMed] [Google Scholar]
- Oh TS, Bae EJ, Ro KW, et al. , 2011. Acral lentiginous melanoma developing during long-standing atypical melanosis: usefulness of dermoscopy for detection of early acral melanoma. Ann Dermatol, 23(3): 400-404. 10.5021/ad.2011.23.3.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omholt K, Grafström E, Kanter-Lewensohn L, et al. , 2011. KIT pathway alterations in mucosal melanomas of the vulva and other sites. Clin Cancer Res, 17(12): 3933-3942. 10.1158/1078-0432.Ccr-10-2917 [DOI] [PubMed] [Google Scholar]
- Orgaz JL, Sanz-Moreno V, 2013. Emerging molecular targets in melanoma invasion and metastasis. Pigment Cell Melanoma Res, 26(1): 39-57. 10.1111/pcmr.12041 [DOI] [PubMed] [Google Scholar]
- Palmer SR, Erickson LA, Ichetovkin I, et al. , 2011. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin Proc, 86(10): 981-990. 10.4065/mcp.2011.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Cho KH, 2010. Acral lentiginous melanoma in situ: a diagnostic and management challenge. Cancers, 2(2): 642-652. 10.3390/cancers2020642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone JA, Farrow N, Tyler DS, et al. , 2018. Contemporary approaches to in-transit melanoma. J Oncol Pract, 14(5): 292-300. 10.1200/jop.18.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Dalle S, Touzet S, et al. , 2010. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol, 162(4): 765-771. 10.1111/j.1365-2133.2009.09594.x [DOI] [PubMed] [Google Scholar]
- Pol J, Kroemer G, Galluzzi L, 2016. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology, 5(1): e1115641. 10.1080/2162402x.2015.1115641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique I, Kirkwood JM, Tarhini AA, 2015. Immune checkpoint blockade and interferon-α in melanoma. Semin Oncol, 42(3): 436-447. 10.1053/j.seminoncol.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi-Estarabadi A, Bittar JM, Zheng CW, et al. , 2019. Optical coherence tomography imaging of melanoma skin cancer. Lasers Med Sci, 34(2): 411-420. 10.1007/s10103-018-2696-1 [DOI] [PubMed] [Google Scholar]
- Ravaioli GM, Dika E, Lambertini M, et al. , 2019. Acral melanoma: correlating the clinical presentation to the mutational status. G Ital Dermatol Venereol, 154(5): 567-572. 10.23736/s0392-0488.18.05791-7 [DOI] [PubMed] [Google Scholar]
- Ribas A, Dummer R, Puzanov I, et al. , 2017. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell, 170(6): 1109-1119.e10. 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway CA, Hieken TJ, Ronan SG, et al. , 1995. Acral lentiginous melanoma. Arch Surg, 130(1): 88-92. 10.1001/archsurg.1995.01430010090019 [DOI] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, et al. , 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med, 364(26): 2517-2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, et al. , 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med, 372(4): 320-330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Robinson C, Xu MM, Nair SK, et al. , 2022. Oncolytic viruses in melanoma. Front Biosci, 27(2): 63. 10.31083/j.fbl2702063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Zorzetto L, Frigo AC, et al. , 2017. A comparative study of the cutaneous side effects between BRAF monotherapy and BRAF/MEK inhibitor combination therapy in patients with advanced melanoma: a single-centre experience. Eur J Dermatol, 27(5): 482-486. 10.1684/ejd.2017.3069 [DOI] [PubMed] [Google Scholar]
- Saida T, 1989. Malignant melanoma in situ on the sole of the foot. Its clinical and histopathologic characteristics. Am J Dermatopathol, 11(2): 124-130. 10.1097/00000372-198911020-00003 [DOI] [PubMed] [Google Scholar]
- Saida T, Koga H, 2007. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol, 143(11): 1423-1426. 10.1001/archderm.143.11.1423 [DOI] [PubMed] [Google Scholar]
- Saida T, Koga H, Uhara H, 2011. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol, 38(1): 25-34. 10.1111/j.1346-8138.2010.01174.x [DOI] [PubMed] [Google Scholar]
- Sanki A, Uren RF, Moncrieff M, et al. , 2009. Targeted high-resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. J Clin Oncol, 27(33): 5614-5619. 10.1200/jco.2008.21.4882 [DOI] [PubMed] [Google Scholar]
- Savarese I, Papi F, D'Errico A, et al. , 2015. Acral lentiginous melanoma treated with topical imiquimod cream: possible cooperation between drug and tumour cells. Clin Exp Dermatol, 40(1): 27-30. 10.1111/ced.12469 [DOI] [PubMed] [Google Scholar]
- Scolyer RA, Thompson JF, McCarthy SW, et al. , 2006. Letters to the editor. Australas J Dermatol, 47(1): 71-73. 10.1111/j.1440-0960.2006.00230.x [DOI] [PubMed] [Google Scholar]
- Seegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, et al. , 1999. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. Int J Radiat Oncol Biol Phys, 44(3): 607-618. 10.1016/s0360-3016(99)00066-8 [DOI] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, et al. , 2009. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol, 27(34): 5763-5771. 10.1200/jco.2009.24.3675 [DOI] [PubMed] [Google Scholar]
- Seo J, Oh Y, Kim SK, et al. , 2021. Slow Mohs micrographic surgery for acral melanoma treatment in Korean patients. Dermatol Surg, 47(2): e42-e46. 10.1097/DSS.0000000000002827 [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, et al. , 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol, 8(3): 239-245. 10.1038/ni1443 [DOI] [PubMed] [Google Scholar]
- Signoretti S, Annessi G, Puddu P, et al. , 1999. Melanocytic nevi of palms and soles: a histological study according to the plane of section. Am J Surg Pathol, 23(3): 283-287. 10.1097/00000478-199903000-00006 [DOI] [PubMed] [Google Scholar]
- Su J, Yu WJ, Liu JY, et al. , 2017. Fluorescence in situ hybridisation as an ancillary tool in the diagnosis of acral melanoma: a review of 44 cases. Pathology, 49(7): 740-749. 10.1016/j.pathol.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Swetter SM, Tsao H, Bichakjian CK, et al. , 2019. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol, 80(1): 208-250. 10.1016/j.jaad.2018.08.055 [DOI] [PubMed] [Google Scholar]
- Swetter SM, Thompson JA, Albertini MR, et al. , 2021. NCCN Guidelines® insights: melanoma: cutaneous, version 2.2021. J Natl Compr Canc Netw, 19(4): 364-376. 10.6004/jnccn.2021.0018 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Suehara T, et al. , 2008. Gene silencing of β-catenin in melanoma cells retards their growth but promotes the formation of pulmonary metastasis in mice. Int J Cancer, 123(10): 2315-2320. 10.1002/ijc.23812 [DOI] [PubMed] [Google Scholar]
- Tanioka M, 2011. Benign acral lesions showing parallel ridge pattern on dermoscopy. J Dermatol, 38(1): 41-44. 10.1111/j.1346-8138.2010.01128.x [DOI] [PubMed] [Google Scholar]
- Teramoto Y, Keim U, Gesierich A, et al. , 2018. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol, 178(2): 443-451. 10.1111/bjd.15803 [DOI] [PubMed] [Google Scholar]
- Tod BM, Schneider JW, Bowcock AM, et al. , 2020. The tumor genetics of acral melanoma: what should a dermatologist know? JAAD Int, 1(2): 135-147. 10.1016/j.jdin.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viros A, Fridlyand J, Bauer J, et al. , 2008. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med, 5(6): e120. 10.1371/journal.pmed.0050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Ito T, Tsuji G, et al. , 2017. Acral lentiginous melanoma versus other melanoma: a single-center analysis in Japan. J Dermatol, 44(8): 932-938. 10.1111/1346-8138.13834 [DOI] [PubMed] [Google Scholar]
- Wang WJ, Edington HD, Rao UNM, et al. , 2007. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNαb. Clin Cancer Res, 13(5): 1523-1531. 10.1158/1078-0432.Ccr-06-1387 [DOI] [PubMed] [Google Scholar]
- Weyers W, Euler M, Diaz-Cascajo C, et al. , 1999. Classification of cutaneous malignant melanoma: a reassessment of histopathologic criteria for the distinction of different types. Cancer, 86(2): 288-299. [DOI] [PubMed] [Google Scholar]
- Wollmann G, Davis JN, Bosenberg MW, et al. , 2013. Vesicular stomatitis virus variants selectively infect and kill human melanomas but not normal melanocytes. J Virol, 87(12): 6644-6659. 10.1128/jvi.03311-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Davies MA, 2010. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol, 80(5): 568-574. 10.1016/j.bcp.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XC, Eide MJ, King J, et al. , 2011. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999‒2006. J Am Acad Dermatol, 65(5 Suppl 1): S26.e1-S37.e13. 10.1016/j.jaad.2011.05.034 [DOI] [PubMed] [Google Scholar]
- Xu SX, Yang ZZ, Zhang JY, et al. , 2015. Increased levels of β-catenin, LEF-1, and HPA-1 correlate with poor prognosis for acral melanoma with negative BRAF and NRAS mutation in BRAF exons 11 and 15 and NRAS exons 1 and 2. DNA Cell Biol, 34(1): 69-77. 10.1089/dna.2014.2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki N, Tanaka R, Tsutsumida A, et al. , 2015. BRAF V600 mutations and pathological features in Japanese melanoma patients. Melanoma Res, 25(1): 9-14. 10.1097/cmr.0000000000000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Yu S, Ke CK, et al. , 2023. The genomic landscape of melanoma and its therapeutic implications. Genes (Basel), 14(5): 1021. 10.3390/genes14051021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Xie L, Huang YC, et al. , 2011. Clinical features of malignant melanoma of the finger and therapeutic efficacies of different treatments. Oncol Lett, 2(5): 811-815. 10.3892/ol.2011.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh I, Jorgenson E, Shen L, et al. , 2019. Targeted genomic profiling of acral melanoma. J Natl Cancer Inst, 111(10): 1068-1077. 10.1093/jnci/djz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York K, Dlova NC, Wright CY, et al. , 2016. Primary cutaneous malignancies in the Northern Cape Province of South Africa: a retrospective histopathological review. S Afr Med J, 107(1): 83-88. 10.7196/SAMJ.2016.v107.i1.10924 [DOI] [PubMed] [Google Scholar]
- Zebary A, Omholt K, Vassilaki I, et al. , 2013. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci, 72(3): 284-289. 10.1016/j.jdermsci.2013.07.013 [DOI] [PubMed] [Google Scholar]
- Zemelman VB, Valenzuela CY, Sazunic I, et al. , 2014. Malignant melanoma in Chile: different site distribution between private and state patients. Biol Res, 47: 34. 10.1186/0717-6287-47-34 [DOI] [PMC free article] [PubMed] [Google Scholar]