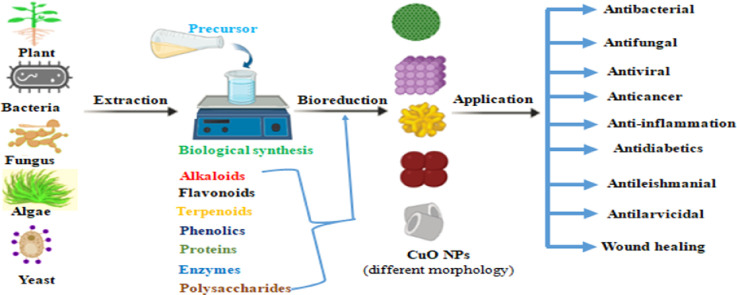
Highlights
-
•
This review provides a comprehensive assessment of the biofabrication of CuO NPs, focusing on green and cost-effective approaches for antimicrobial and anticancer applications.
-
•
Highlights the current advancements and insights in the green synthesis of CuO NPs, with a particular emphasis on the utilization of plants, microbes, and natural biomolecules.
-
•
Various methodologies employed for the production of CuO NPs are discussed, shedding light on the different techniques and processes involved.
-
•
Antimicrobial and santicancer applications of biosynthesized CuO NPs, elucidating their mechanism of action and exploring their potential in combating microbial infections and cancer cells.
Keywords: Copper oxide nanoparticles; Biological synthesis; Antimicrobial activity; Anticancer activity; Green synthesis; Nanotechnology, Microbial infection
Abstract
Nanotechnology has made remarkable advancements in recent years, revolutionizing various scientific fields, industries, and research institutions through the utilization of metal and metal oxide nanoparticles. Among these nanoparticles, copper oxide nanoparticles (CuO NPs) have garnered significant attention due to their versatile properties and wide-range applications, particularly, as effective antimicrobial and anticancer agents. CuO NPs can be synthesized using different methods, including physical, chemical, and biological approaches. However, conventional chemical and physical approaches are expensive, resource-intensive, and involve the use of hazardous chemicals, which can pose risks to human health and the environment. In contrast, biological synthesis provides a sustainable and cost-effective alternative by eliminating chemical pollutants and allowing for the production of CuO NPs of tailored sizes and shapes. This comprehensive review focused on the green synthesis of CuO NPs using various biological resources, such as plants, microorganisms, and other biological derivatives. Current knowledge and recent trends in green synthesis methods for CuO NPs are discussed, with a specific emphasis on their biomedical applications, particularly in combating cancer and microbial infections. This review highlights the significant potential of CuO NPs in addressing these diseases. By capitalizing on the advantages of biological synthesis, such as environmental safety and the ability to customize nanoparticle characteristics, CuO NPs have emerged as promising therapeutic agents for a wide range of conditions. This review presents compelling findings, demonstrating the remarkable achievements of biologically synthesized CuO NPs as novel therapeutic agents. Their unique properties and mechanisms enable effective combating against cancer cells and various harmful microbial infections. CuO NPs exhibit potent anticancer activity through diverse mechanisms, including induction of apoptosis, inhibition of angiogenesis, and modulation of signaling pathways. Additionally, their antimicrobial activity manifests through various mechanisms, such as disrupting microbial membranes, generating reactive oxygen species, and interfering with microbial enzymes. This review offers valuable insights into the substantial potential of biologically synthesized CuO NPs as an innovative approach for future therapeutic interventions against cancer and microbial infections.
Graphical abstract
1. Introduction
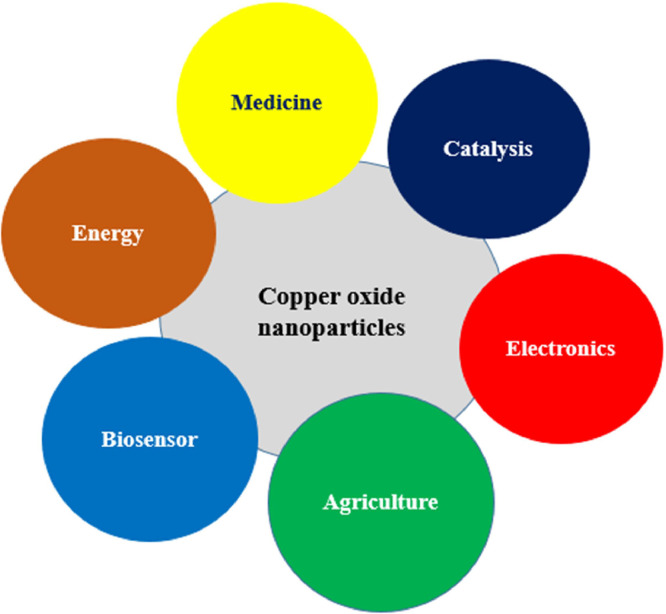
Nanotechnology has emerged as a rapidly advancing field of research that focuses on the design, fabrication, surface analysis, and application of materials at the nanoscale, typically ranging from 1 to 100 nm [1]. These materials, known as nanoparticles (NPs), possess unique physicochemical and biological properties due to their remarkably small size and large surface-area-to-volume ratio. As a result, they exhibit significant variations in properties compared to their bulk counterparts [2,3]. The exceptional and intriguing properties of nanoparticles have spurred extensive multidisciplinary research, offering numerous benefits and opportunities in various fields such as agriculture, food, pharmaceuticals, cosmetics, catalysis, biosensors, electronics, energy, medicine, and the environment [4], [5], [6]. In the medical field, nanoparticles have been applied in disease diagnosis, treatment, drug delivery, and the development of novel drugs [7,8]. Among the various types of nanoparticles used, metal and metal oxide nanoparticles are considered highly efficient due to their exceptional attributes, including high stability, biocompatibility, and remarkable antimicrobial and anticancer properties [9], [10], [11], [12].
Among metal oxide nanoparticles, copper oxide nanoparticles (CuO NPs) have garnered significant interest recently due to their distinct optical, electrical, magnetic, biological, and catalytic properties [13], [14], [15]. These fascinating characteristics have prompted extensive exploration of CuO NPs in diverse fields, including energy, electronics, cosmetics, biosensors, storage devices, supercapacitors, catalysis, food and agriculture, and healthcare (Fig. 1) [16,17]. Moreover, their antimicrobial and anticancer properties have positioned them as promising therapeutic agents [18], [19], [20].
Fig. 1.
General applications of CuO NPs.
With the increasing demand for environmentally friendly and sustainable synthesis methods, biogenic synthesis (green synthesis) has become prominent in the fabrication of CuO NPs. This approach utilizes biological entities such as plants and microorganisms to produce CuO NPs, offering advantages in terms of availability, simplicity, cost-effectiveness, and environmental compatibility [8,21,22]. Green synthesis avoids the use of harmful chemicals by utilizing natural reducing, capping, and stabilizing agents present in biological sources, thereby preventing nanoparticle agglomeration [23], [24], [25].
Recent studies have emphasized the potential applications of CuO NPs synthesized through green methods, including antimicrobial [26,27], anticancer agent [28], [29], [30], antioxidant [19], drug delivery vehicle [31], [32], [33], anti-inflammatory agent [34], antidiabetic agent [35], and antitumor agents [36], [37], [38], [39]. These nanoparticles demonstrate outstanding antimicrobial activity, making them valuable for wound dressings and antiseptics [40]. They also exhibit fungicidal properties against various fungal strains [41,42]. Furthermore, CuO NPs serve as effective nonenzymatic biosensors for clinically relevant analytes and show promise as nanocarriers and antitumor agents in cancer treatment [43], [44], [45], [46], [47]. However, addressing the toxicity concerns associated with CuO NPs is crucial since their excessive production of reactive oxygen species (ROS) can induce oxidative stress, potentially endangering the well-being of normal cells and vital organs [48]. Therefore, a comprehensive evaluation of the toxicity profile is essential before CuO NPs can be applied in biomedical applications. Considering these factors, the primary objective of this review is to extensively investigate bioinspired synthesis approaches for CuO NPs. This review describes the synthesis, biomedical applications, and toxicological analysis of these nanoparticles. Furthermore, the specific mechanisms that underlie the antimicrobial and anticancer potential of CuO NPs will be elucidated, highlighting their importance in developing powerful antimicrobial formulations and advancing strategies for cancer treatment. By concentrating on these aspects, this review aims to make a valuable contribution to the expanding body of knowledge in the field and promote further research utilizing CuO NPs for combating microbial diseases, cancer, and other biomedical applications.
2. Biogenic synthesis of copper oxide nanoparticles
The synthesis of CuO NPs using biological methods involves the utilization of plant and microorganism extracts, including bacteria and fungi. This eco-friendly approach is renowned for its simplicity and cost-effectiveness, in contrast to conventional methods that rely on expensive and hazardous chemicals. Biological synthesis eliminates the need for chemical reducing and capping agents. Instead, plant extracts contain phytochemicals such as alkaloids, phenols, flavonoids, and terpenoids, while microorganisms possess enzymes and proteins. These natural components fulfill the dual role of reducing and capping, resulting in the production of nontoxic, biocompatible, and highly stable nanoparticles [1,[49], [50], [51]]. However, the use of bacteria-based green synthesis has drawbacks, requiring a sterile culture environment, specialized growth media, and meticulous monitoring throughout the entire process. Additionally, the cost of media for bacterial growth can be prohibitive for large-scale commercial production [52]. Similarly, green synthesis methods based on algae also have limitations, as they tend to be slow and time-consuming [53]. Overall, the biological synthesis of copper oxide nanoparticles represents a promising and sustainable alternative, harnessing the power of nature to create nanoparticles with desirable properties. By exploring and addressing the challenges associated with various biological approaches, researchers can optimize and enhance the efficiency of this eco-friendly nanoparticle synthesis method.
2.1. Plant-based approaches for the synthesis of CuO NPs
The eco-friendly synthesis of CuO NPs using plant extracts, microorganisms, and other biological derivatives has been reported. Plants as production aggregates for CuO NPs have garnered considerable interest due to their safety, simplicity, and ability to serve as rich resources for stabilizing and reducing agents [13,54]. Natural extracts from plant components offer a rapid, low-cost, and environmentally benign alternative that does not require expensive equipment, resulting in highly pure and concentrated products free of impurities [55]. The metabolites and phytochemicals present in plant extracts, such as phenolic compounds, alkaloids, terpenoids, saponins, tannins, amino acids, proteins, enzymes, and vitamins, play crucial roles in reducing copper ions in salt solution, leading to the formation of corresponding CuO NPs [19,56]. Table 1 presents the biological synthesis of CuO NPs utilizing diverse plant components, such as flowers, leaves, fruits, and stems, which are briefly discussed in this review. Previous studies have successfully demonstrated the preparation of CuO NPs through a simple approach involving mixing copper salt with plant extract. This mixture undergoes a reaction within minutes to hours under normal laboratory conditions. The presence of phytochemicals aids in reducing the copper salt, leading to the formation of nanoparticles. The synthesis of CuO NPs was initially confirmed by a noticeable change in color, followed by characterization via various spectroscopic and microscopic techniques [57,58].
Table 1.
Plant-mediated synthesis of CuO NPs according to their size, morphology, and brief experimental conditions.
| Plants used | Precursor(s) | Synthesis conditions | CuO NPs | Applications | References | ||
|---|---|---|---|---|---|---|---|
| Size (nm) | Morphology | ||||||
| A. spectabilis | CuSO4 | Reaction: 27 °C, 2 h | 50 | Spherical | Antinociceptive and anti-inflammatory effects | [72] | |
| A. millefolium | CuSO4·5H2O | Reaction: room temp., 24 h | 28 | Semispherical | Catalytic, antimicrobial and photocatalytic activity | [41] | |
| A. indicum | Cu(NO3)2·3H2O | Reaction: 2–5 min,4 h Heating: 400 5 °C, 2–3 min |
16.78 | Spherical | Antioxidant, antibacterial and photocatalytic activity | [73] | |
| A. altissmia | Cu(Ac)2 | Reaction: 27 °C, 4 h Drying: 400 °C, 4 h |
5 – 20 | Spherical | Antibacterial activity | [74] | |
| A. sativum | Cu(NO3)2 | Reaction: 70 °C, 2–3 h; Drying:80 °C, overnight Calcination: 400 °C, 3–4 h |
20 – 40 | Spherical and oval | Antimicrobial, antioxidant, and anti-larvicidal activity | [34] | |
| A. vera | Cu(NO3)2·3H2O | Reaction: 100–120 °C, 24 h | 20 | Monoclinic | Antibacterial activity | [75] | |
| B. tomentosa | CuSO4 | Reaction: Room temp.,4 days | 22 – 40 | Spherical | Antibacterial activity | [76] | |
| C. papaya | Cu(NO3)2·3H2O | Reaction: 70–80 °C, until color change; Calcination: 450 °C, 2 h |
85–140 | Spherical | Photocatalytic activity | [77] | |
| C. quadrangularis | Cu(Ac)2 | Reaction:60 °C; 2 h; Calcination: 300 °C, 2 h |
28–32 | Spherical | Antifungal activity | [42] | |
| C. sebestena | Cu(NO3)2·3H2O | Reaction:80 °C; 4 h; Calcination: 400 °C, 2 h |
20–35 | Spherical | Catalytic and antibacterial activity | [78] | |
| D. gangeticum | CuCl2 | Reaction: 80 °C; Until color change | 28 | Spherical | Antioxidant and antimicrobial activity | [79] | |
| E. globulus | CuSO4 | Reaction: 30–140 °C, 2–6 h, pH 8; Irradiation:140 °C, 10 min; Drying: 80 °C, 4 h |
12–68 | Spherical, cubical and oval | Anticancer and antifungal activity | [80] | |
| F. japonica | CuSO4·5H2O | Reaction: 90 °C, 1 h, pH 10 | 5–10 | Spherical | Catalytic activity | [58] | |
| M. champaca | Cu(Ac)2 | Reaction: 37 °C, 24 h Drying: 60 °C, overnight |
20–40 | Spherical | Antioxidant activity | [81] | |
| M. charantia | CuSO4·5H2O | Reaction: 60 °C, pH 11, until color change Drying: 80 °C, 8 h; Calcination: 500 °C, 3 h |
61.48 | Rods | Antimicrobial activity | [18] | |
| M. koenigii | CuSO4 | Reaction: Room temp., 1 h, pH 11 | 8.4 | Spherical | Catalytic activity | [82] | |
| O. europea | CuSO4·5H2O | Reaction: 100 °C, 24 h | 20–50 | Spherical | Antioxidant and anticancer activity | [67] | |
| P. vulgaris | CuSO4·5H2O | Reaction: 120 °C, 7–8 h Calcination: 400 °C, 2 h |
26.6 | Spherical | Anticancer activity | [83] | |
| P. guajava | Cu(Ac)2.H2O | Reaction: 60 °C, 4 h, pH 7.5; Calcination: 400 °C, 4 h |
2–6 | Spherical | Photocatalytic activity | [84] | |
| P. marsupium | CuSO4·5H2O | Reaction: 50 °C, 15 min | 20–25 | Spherical | Antibacterial activity | [85] | |
| P. hexapetalun | CuSO4·5H2O | Reaction: 60 °C, 2 h; Drying: 80 °C, 2 h; Calcination: 400 °C, 4 h |
10–50 | Spherical | Antibacterial and anticancer potential | [86] | |
| P. pyrifolia | Cu(NO3)2·6H2O | Reaction: 80 °C, until color change; Calcination: 400 °C, 3 h |
24 | Spherical | Photocatalytic activity | [87] | |
| R. serpentine | Cu(NO3)2·3H2O | Reaction: room temp., 10 min Calcination: 400 10 °C, 5 min |
10–20 | Monoclinic | Photocatalytic and antibacterial activity | [69] | |
| R. palmatum | CuCl2 | Reaction: 70 °C, 10 min; Drying: 70 °C, 24 h; Calcination: 350 °C, 2 h |
30 | Spherical | photocatalytic activity | [88] | |
| R. glaucus Benth. | Cu(NO3)2·3H2O | Reaction: 75–80 °C, 6 h | 45 | Spherical | Antioxidant activity | [68] | |
| S. officinarum | Cu(NO3)2 | Reaction: 80 °C, 2 h, pH 10 Drying: 80 °C, 8 h; Calcination: 500 °C, 3 h |
29.5–60.5 | Spherical, square, cube, rectangular | Antibacterial activity | [89] | |
| S. acuta | CuSO4·5H2O | Reaction: 100 °C, 5–7 h | 50 | Rod | Photocatalytic and antimicrobial activity | [90] | |
| S. torvum | CuSO4 | Reaction: 37 °C, 6 h | 32 | Spherical | Biological activity | [91] | |
| S. lavandulifolia | CuCl2·2H2O | Reaction: 50 °C, pH 10 | <80 | Spherical | Antifungal activity | [92] | |
| S. alternifolium | CuSO4·5H2O | Reaction: 60–80 °C, 2 h, pH 9 | 3–85 | Spherical | Photocatalytic activity | [93] | |
| T. aestivum | CuSO4·5H2O | Reaction: 70 °C, 30 min, pH 7 Drying: 90 °C, 2 h; |
20.5–23.5 | Spherical | Catalytic activity | [94] | |
| T. indica | Cu(Ac)2·4H2O | Reaction: 80 °C, until color change; Calcination: 400 °C, 2 h |
12 | Spherical | Antioxidant and anticancer activity | [19] | |
| T. cordifolia | Cu(NO3)2·3H2O | Combustion: 400 10 °C, 5 min | 6–8 | Spherical | Antioxidant, antibacterial and photocatalytic activity | [95] | |
| T. terrestris | Cu(NO3)2 | Reaction: 90 °C, 2 h; Drying: 80 °C, 2 h |
5–22 | Spherical | Antimicrobial and anticancer activity | [56] | |
| T. procumbens | CuSO4 | Reaction: 80 °C, 4 h; Calcination: 400 °C, 5 h |
16 | Spherical | Larvicidal activity | [96] | |
The leaf extract of Aleo barbadenesis Miller was observed to enable simple, rapid, and environmentally benign synthesis of CuO NPs [59]. Microscopic analysis revealed the formation of spherical CuO NPs with sizes ranging from 15 to 30 nm. Tabernaemontan divaricata leaf extract was used as a reducing and capping agent in the biosynthesis of spherical CuO NPs, demonstrating potential antibacterial effects [60]. Acalypha indica leaf extract has also been used as a sustainable and facile method for the fabrication of CuO NPs, the average size of which ranges from 26 to 30 nm [61]. Microscopic analysis confirmed the formation of spherical CuO nanoparticles. These biogenic CuO NPs exhibited robust antimicrobial activity against C. albicans, E. coli, and P. fluorescens. Additionally, the green synthesis of CuO NPs using Abutilon indicum leaf extract has been demonstrated, highlighting its considerable potential in anticancer and antibacterial applications [62].
CuO NPs were synthesized using Calotropis gigantean leaf extract [25]. Flavonoids, terpenoids, steroids, and alkaloids present in plant extracts play vital roles in the reduction and stabilization of CuO NPs. Banana peel extract was used to prepare CuO NPs with an average size of 60 nm [63]. These nanoparticles were evaluated for their photocatalytic degradation of Congo red dye under solar irradiation. The green-synthesized CuO NPs exhibited excellent photocatalytic performance, demonstrating their potential application in industrial wastewater treatment. CuO NPs were synthesized using Acanthospermum hispidum leaf extract as a reducing and capping agent [64]. Phytochemicals present in plant extracts, such as coumarins, saponins, phenols, tannins, flavonoids, sterols, and essential oils, played vital roles in the bioreduction and stabilization of CuO NPs. These biogenic CuO NPs show potential as effective agents for antimicrobial, antimalarial, and antimycobacterial applications in biomedicine. Camellia sinensis leaf extract was used for the green synthesis of CuO NPs [13]. The resulting CuO NPs, ranging from 25 to 32 nm in length, were evaluated for antibacterial activity against S. aureus, B. subtilis, K. pneumoniae, and E. coli. The highest antibacterial activity was observed against S. aureus. Additionally, these nanoparticles showed remarkable cytotoxic effects against the MCF-7 human breast cancer cell line.
CuO NPs were successfully prepared by a rapid, cost-effective, simple, and green approach by employing leaf extracts of Acanthospermum hispidum and Eupatorium odoratum [65]. Research findings indicate that these green-synthesized CuO NPs exhibit remarkable bactericidal activity against S. aureus, B. cereus, and E. coli. Moreover, the green synthesis of CuO NPs using extracts from Matricaria chamomilla, Andean blackberry, and Olea europaea has also been reported [66–68]. The synthesized nanoparticles showed antioxidant activity that varied with concentration. Moreover, CuO nanoparticles synthesized from Rauvolfia serpentia leaf extract exhibited remarkable bactericidal effects against both gram-positive and gram-negative bacterial strains, indicating their potential for use in commercial antibacterial preparations [69]. Furthermore, the dried peel extract of Zea mays was identified as a natural source for preparing CuO NPs and demonstrated substantial antimicrobial and photocatalytic activities [70]. CuO NPs synthesized through a green chemistry approach displayed exceptional catalytic activity in the synthesis of N-monosubstituted urea and the reduction of 4-nitrophenol [71].
2.2. Microbe-mediated green synthesis of CuO NPs
In recent years, microorganisms have emerged as significant nanofactories and have attracted considerable attention due to their reliability, eco-friendliness, and cost-effectiveness. They offer a viable alternative to reduce the use of toxic chemicals and the high energy requirements associated with physical and chemical synthesis [97]. Microbes have the ability to accumulate and detoxify heavy metals while simultaneously reducing metal salts into metal or metal oxide nanoparticles through a range of enzymes. Bacteria, fungi, and yeast have been utilized for intra- or extracellular biosynthesis of metal and metal oxide nanoparticles [98], [99], [100], [101]. The extracellular synthesis method, in particular, has advantages over the intracellular approach because it eliminates several synthesis steps, such as sonication for cell wall degradation, multiple centrifugations, and washing steps for nanoparticle purification, increasing the practicality of the approach [101]. Microbes play a crucial role in nanoparticle synthesis by providing enzymes and proteins, reducing cofactors, and organic materials as reducing agents. Additionally, the proteins secreted by microbes act as natural capping agents, preventing nanoparticle aggregation and ensuring long-term stability, thereby providing additional benefits [102].
Among the various available nanoparticles, CuO NPs synthesized using microbes have shown promise due to their well-defined shapes and sizes (Table 2). Microbial-assisted green synthesis of CuO NPs has proven to be a reliable, low-cost, and environmentally safe alternative to physicochemical approaches. Nonetheless, this approach has several drawbacks, including time-consuming microbial screening, the need for careful monitoring of the culture broth, and difficulty in controlling nanoparticle shape and size [55]. Biogenic CuO NPs synthesized through microbe-mediated processes exhibit remarkable characteristics such as high fluorescence, water dispensability, high stability, and reduced aggregation [103]. For instance, Shewanella indica extract was used to synthesize polydispersed CuO NPs with an average size of 400 nm [104]. The formation of CuO NPs was confirmed by UV–Vis analysis, which showed a broad absorption band at 399 nm. FTIR analysis indicated that the presence of hydroxyl, carboxyl, and amide groups contributing to the reduction and stabilization of CuO NPs. A simple and cost-effective approach yielded spherical CuO NPs ranging from 40 to 110 nm [103]. FTIR analysis further demonstrated the presence of amine, carboxylic, hydroxyl, amide I, and amide II functional groups in the bacterial membrane proteins, revealing their role in the reduction of copper salts to nanoparticles. The synthesized nanoparticles showed excellent antibacterial activity against S. aureus and P. aeruginosa. Furthermore, these materials significantly inhibited cell proliferation in human gastric and colorectal cancer cell lines (AGS and HT-29), demonstrating their potential as antimicrobial and anticancer agents for biomedical and industrial applications. Polydispersed CuO NPs with average sizes ranging from 10 to 30 nm were synthesized using Serratia sp [100]. Specific intracellular membrane-bound proteins are involved in the bioreduction and subsequent stabilization of CuO NPs. A highly efficient microbial system, Escherichia coli, was employed for the extracellular synthesis of quasi-spherical CuO NPs with an average particle size of 10–40 nm [105].
Table 2.
Microbes-mediated synthesis of CuO NPs.
| Microbes | Precursor(s) | Synthesis conditions | CuO NPs | Applications | References | |
|---|---|---|---|---|---|---|
| Size (nm) | Shape | |||||
| Bacillus sp. | CuSO4·5H2O | Reaction: 37 °C, 48–96 h | 2–41 | Spherical | Antibacterial activity | [118] |
| E. coli | CuSO4 | Reaction: 28 °C, 42 h | 10–40 | Quasi – spherical | – | [105] |
| Gluconacetobacterhanseniiendnote | Cu(NO3)2, NH4OH | Reaction: 1 h, Heating: 150 °C, 3–48 h |
25–35 | – | Antimicrobial activity | [119] |
| Halomonas elongate | CuSO4 | Reaction: 28 °C, 10 min | 57–79 | Rectangular | Antibacterial effect | [120] |
| Lactobacillus casei | CuSO4 | Reaction: 37 °C, pH 6, 48 h; Drying: 40 °C, 2 h |
40–110 | Spherical | Antibacterial and anticancer activity | [103] |
| Morganellamorganii | CuSO4·5H2O | Reaction: 37 °C, 24 h | 7 | Spherical | Antibacterial activity | [121] |
| CuSO4·5H2O | Reaction: 37 °C, 24 h, pH 7 | 3–10 | Polydispersed | – | [122] | |
| Morganellapsychrotolerans | Cu(NO3)2 | Reaction: 30 °C, pH 7; Drying: 100 °C, 2 h |
10 | Quasi – spherical | – | [123] |
| Proteus mirabilis | Cu(NO3)2 | Reaction: 30 °C, pH 7; Drying: 100 °C, 2 h |
8–15 | spherical | Antimicrobial activity | [123] |
| Pseudomonas stutzeri | CuSO4 | Reaction: 37 °C, 48 h | 10–30 | Polydispersed | – | [100] |
| CuSO4·5H2O | Irradiation: 30 kGy | 29.8 | Spherical | Antimicrobial activity | [124] | |
| Serratia sp. | CuSO4·5H2O | Reaction: 30–32 °C, 1 h | 1.72–13.49 | Spherical | Antimicrobial activity | [125] |
| Streptomyces cyaneus | CuSO4·5H2O | Reaction: 30 °C, 48 h; Drying: Room temp., overnight |
15.75 3.95 | Spherical | Anticancer activity | [126] |
| Streptomyces sp. | CuSO4·5H2O | Reaction: 30 °C, 1 h, pH 8 | 1.72–13.49 | Spherical | Antimicrobial activity | [125] |
| Streptomyces zaomyceticus | CuSO4·5H2O | Reaction: 30 °C, 6 h, pH 9 | 78 | Spherical | Antimicrobial, anticancer and anti-larvicidal activity | [117] |
| Streptomyces pseudogriseolus | CuSO4·5H2O | Reaction: 35 °C, 6 h, pH 7 | 80 | Spherical | Antimicrobial, anticancer and anti-larvicidal activity | [117] |
| Aspergillus oryzae | CuSO4·5H2O | Reaction: 25 °C, 7.0 | 9.7 | Spherical | Antifungal activity | [124] |
| Aspergillus fumigates | Cu(Ac)2·2H2O | Reaction: 30 °C, 48 h; Drying: 80 °C, 48 h |
10.5–59.7 | Spherical | Antimicrobial activity | [15] |
| Penicillium chrysogenum | CuCl2 | Reaction: 25 °C, pH 9, 5 days | 5–20 | Spherical | – | [108] |
| Rhodotorulamucilaginosa | Cu(NO3)2·3H2O | Reaction: 70–80 °C, 3 h; Drying: 80 °C, 2 h |
10–190 | Spherical | Anticancer activity | [127] |
| Trichodermaasperellum | Cu(NO3)2·3H2O | Reaction: 40 °C, overnight; Heating: 75–80 °C, 2 h Drying: 80 °C, 2 h |
110 | Spherical | Anticancer activity | [127] |
Fungal species offer significant advantages for the extracellular synthesis of metal and metal oxide nanoparticles, due to their economic viability, convenient downstream processing, and scalability [106]. Compared to bacteria, fungal strains exhibit superior tolerance and metal bioaccumulation properties [107]. The white-rot fungus Stereu hirsuta was utilized for the eco-friendly synthesis of CuO NPs [108]. TEM analysis revealed predominantly spherical nanoparticles ranging in size from 5 to 20 nm. FTIR analysis indicated the involvement of extracellular proteins and polysaccharides from the fungus in the reduction and stabilization of the nanoparticles. In another study, Penicillium chrysogenum was used for the synthesis of CuO NPs [109]. TEM analysis showed spherical nanoparticles with an average size of 9.7 nm. Proteins were proposed to be involved in the reduction and stabilization of nanoparticles. The biosynthesized CuO NPs exhibited significant antifungal activity against Aspergillus niger (ZOI 26.5 mm), Alternaria solani (ZOI 28.0 mm), and Fusarium oxysporum (ZOI 37 mm). These nanoparticles also showed substantial bactericidal activity against Ralstonia solanacearum and Erwinia amylovora with ZOIs of 22.0 and 19 mm, respectively.
Alga, known for its abundant secondary metabolites and enzymes, are widely utilized for both intra- and extracellular synthesis of nanoparticles [110,111]. Researchers have reported the use of an extract from the microalgae Anabaena cylindrical for the environmentally friendly synthesis of rod-like CuO NPs, which are 50–60 nm in size [112]. Algae harbor a diverse array of compounds, including flavones, terpenoids, and polysaccharides, which have proven to be efficient agents for reducing and capping CuO NPs during synthesis. Excitingly, these nanoparticles produced through biogenic methods have shown great potential in combating E. coli infection, and thus display notable antibacterial activity. Similarly, the utilization of aqueous extracts of Sargassum polycytum has yielded CuO NPs with remarkable antimicrobial and anticancer activities, opening up new possibilities for medical applications [113]. Another study successfully produced spherical CuO NPs with sizes ranging from 5 to 45 nm using an extract of Bifurcaria bifurcata, a type of brown algae [114]. The visual color change from dark blue to dark brown in the reaction mixture containing metal salts and algal extracts confirmed the formation of CuO NPs. The antibacterial potential of CuO NPs synthesized from Bifurcaria bifurcata extract highlights their promising biomedical applications. Table 2 provides an overview of commonly used microorganisms for the synthesis of CuO NPs.
2.3. Actinomycetes-mediated synthesis of CuO NPs
Actinomycetes are exploited for synthesizing nanoparticles because they can produce secondary metabolites such as enzymes and proteins. These compounds serve as capping and stabilizing agents, enabling the production of nanoparticles with diverse shapes and sizes [22,115]. For instance, Nabila and Kannabiran [116] were the first to report the synthesis of highly stable spherical CuO NPs with an average size of 61.7 nm using actinomycetes. These nanoparticles demonstrated promising bactericidal potential against various human pathogenic bacteria. Another study described the eco-friendly synthesis of CuO NPs using two endophytic actinomycetes, Streptomyces pseudogriseolus (Acv-11) and Streptomyces zaomyceticus (Oc-5), isolated from Oxalis corniculata leaves [117]. The nanoparticles synthesized from Acv-11 and Oc-5 were spherical with average sizes of 80 nm and 78 nm, respectively. Furthermore, these biosynthesized CuO NPs displayed interesting antibacterial, antifungal, larvicidal, and antioxidant properties, as revealed by the results of the present study.
2.4. CuO NPs synthesized using biomolecules and other biological products
In addition to plant- and microbial-mediated synthesis, researchers have developed simple and eco-friendly methods for synthesizing CuO NPs using various biological derivatives. These biological products play vital roles in reducing and stabilizing nanoparticles. For instance, ovalbumin, a protein found in egg white, has been used in the green synthesis of Momordica-like CuO nanorods with a length of less than 100 nm and a width of 30 to 50 nm [128]. CuO/pectin nanocomposites, prepared by utilizing copper acetate as a precursor and pectin as a reducing and stabilizing agent, have demonstrated dose-dependent antioxidant and anticancer activities [129]. Starch, a natural polymer, has also been employed as both a capping and reducing agent in the green synthesis of CuO NPs [130]. The resulting nanoparticles exhibited a spherical morphology with an average size of 54 nm. The antibacterial activities of the strains were subsequently assessed against B. cereus, E. coli, P. aeruginosa, Enterococcus, S. epidermidis, and Shigella sonnei. Notably, the nanoparticles displayed strong antimicrobial and anticancer activities, particularly against B. cereus, suggesting their potential application in antibacterial formulations targeting this specific bacterium.
Ascorbic acid was utilized in the synthesis of a chitosan/CuO nanocomposite, which formed a cubic shape with an average size of 17 nm [131]. This nanocomposite exhibited significant antimicrobial activity against B. subtilis, P. aeruginosa, E. coli, and P. notatum. It also displayed potent antibiofilm activity, reducing B. subtilis biofilms by 69 % at 100 μg/mL and P. aeruginosa biofilms by 63 % at 100 μg/mL. In another study, CuO NPs synthesized from sinapic acid were shown to have dose- and time-dependent cytotoxic effects on breast cancer cell lines [132]. An inexpensive and environmentally benign approach involving sugarcane juice was utilized for the synthesis of CuO NPs [89]. TEM analysis confirmed the formation of spherical CuO NPs with an average size of 29.5 nm. The sugar molecules and other organic acids present in the sugarcane juice served as surface modifiers, stabilizers, and capping agents. Additionally, the prepared CuO NPs exhibited strong bactericidal activity against E. coli, S. aureus, P. aeruginosa, and B. subtilis. Similarly, almond gum was utilized to synthesize spherical CuO NPs ranging in size from 16 to 25 nm [133]. These biologically synthesized nanoparticles exhibited excellent antimicrobial effects, suggesting their potential for use in the treatment of urinary tract infections.
3. Applications of CuO NPs
3.1. Antimicrobial potential of CuO NPs
In underdeveloped countries with economic burdens, infectious diseases caused by microorganisms pose serious public health concerns and result in substantial financial burdens. These diseases often result from the transmission of pathogens through contaminated water, food, or soil, and involve various bacteria, viruses, and parasites. While antibiotics have been widely used, the emergence of antibiotic resistance has limited their effectiveness in combating microbial infections. Thus, there is an urgent need for alternative antimicrobial treatments [134]. Nanotechnology-based therapies have gained prominence in the diagnosis, treatment, and development of new drugs [135]. Among the various kinds of green nanoparticles, CuO NPs have shown marked effects on different human pathogenic microorganisms [136]. Recently, environmentally friendly CuO NPs have garnered considerable attention as antimicrobial agents due to their unique morphology, large surface area-to-volume ratio, and biocompatibility for treating a wide range of human pathogens [20,60,75,137]. For example, CuO NPs prepared from the leaf extract of Phyllanthus amarus exhibited significant antimicrobial efficacy against various bacterial strains such as B. subtilis, E. coli, P. aeruginosa, and S. aureus [138]. Similarly, CuO NPs biofabricated using leaf extracts of Pterolobium hexapetalum exhibited promising antibacterial activity against E. coli, B. subtilis, and S. aureus, with zones of inhibition of 14 mm, 15 mm and 15 mm, respectively [86].
Actinomycete-produced CuO NPs exhibited antimicrobial activity against disease-causing gram-positive bacteria (B. subtilis, B. diminuta, and S. aureus), gram-negative bacteria (P. aeruginosa and E. coli), and fungi (A. brasiliensis and C. albicans) [117]. The synthesized nanoparticles exhibit potent inhibitory effects, due to their decreased size and high surface area-to-volume ratio. This characteristic facilitates interactions with microbial cell membranes, leading to cellular death. The effectiveness of CuO NPs against microorganisms depends on factors such as size, morphology, dosage, and the type of extracts used for synthesis. The antimicrobial efficacy of CuO nanoparticles increases with decreasing particle size [139]. CuO NPs prepared with Acanthospermum hispidum extract exhibited dose-dependent antibacterial activity [140]. Researchers investigated the antimicrobial efficacy of CuO and ZnO nanoparticles synthesized from an extract derived from Penicillium chrysogenum [17]. Fungal extracellular enzymes and proteins are involved in the bioreduction and capping of manufactured nanoparticles. The results demonstrated zones of inhibition at a concentration of 5 mg/mL for CuO NPs against S. Typhimurium, E. coli, P. aeruginosa, B. subtilis, and S. aureus, at 11.66 ± 0.33, 11.93 ± 0.52, 13.6 ± 0.4, 16.26 ± 0.63, and 22 ± 0.57 mm, respectively. Similarly, for the same concentration of ZnO NPs, the inhibition zones against E. coli, S. Typhimuriu, P.aeruginosa, B.subtilis, and S.aureus were 11.06 0.34, 11.13 0.41, 12.43 0.23, 13.5 ± 0.26, and 16.33 0.88 mm, respectively. CuO NPs exhibited a superior inhibitory effect against bacterial pathogens compared to that of ZnO NPs. The researchers proposed that the antibacterial activity of CuO NPs and ZnO NPs could be attributed to electrostatic interactions between the NPs and bacterial proteins, resulting in protein inactivation, membrane impermeability, and thereby inhibition of bacterial growth.
CuO NPs synthesized using green methods have shown excellent bactericidal activity against S. aureus and E. coli when applied to clothes [141]. These findings suggested that CuO NPs-coated cotton fabrics could be beneficial for wound dressings, bed pads, and bandages in hospitals. In a study, an eco-friendly and biocompatible Allium sativum extract was employed to synthesize CuO NPs, which were subsequently evaluated for their antimicrobial activity against bacterial strains (B. subtilis, E. coli, K. pneumoniae, P. aeruginosa, S. pyogenes, and S. aureus) and fungal strains (A. flavus, A. fumigatus, A. niger, and C. albicans) [34]. The results suggested that these biomediated CuO NPs displayed efficient antimicrobial efficacy, making them suitable for biomedical applications. Furthermore, the antifungal activity of CuO NPs was investigated against Colletotrichum gloeosporioides, a pathogen responsible for anthracnose disease in crops [142]. The study reported growth inhibition rates of 74.2 % and 89 % at concentrations of 500 mg/L and 1000 mg/L, respectively, indicating a concentration-dependent response of CuO NPs against fungal pathogens. Similarly, tea-mediated CuO NPs exhibited significant antifungal activity against Fusarium solani, with more than 90 % inhibition of mycelial growth observed at a concentration of 50 ppm [143]. These findings highlight the potential of biogenic CuO NPs for treating multidrug-resistant microorganisms and suggest that they may be a promising solution for combating pathogenic infections. Numerous other studies have also reported the remarkable antimicrobial activity of CuO NPs synthesized using green methods, as summarized in Table 3.
Table 3.
Antimicrobial potential of green-synthesized CuO NPs.
| Bacterial species | CuO NPs | Method | ZOI (mm) /inhibition (%) | References | ||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Shape | Concentration/ amount | ||||||
| B. cereus | 5–22 | Spherical | MIC: 21 µg/mL | Broth microdilution | – | [56] | ||
| B. subtilis | 10–50 | Spherical | 50 µg/mL | Disc diffusion | 150.29 | [86] | ||
| 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 0.23 | [34] | |||
| 29.5–60.5 | Spherical, square, cube, plate, and rectangular | 100 µg | Agar well diffusion | 9 | [89] | |||
| E. coli | 5–10 | Spherical | 500 µg/50 µL | Agar well diffusion | 7.330.33 | [144] | ||
| 5–20 | Spherical | 100 µg/mL | Disc diffusion | 18 | [74] | |||
| 5–22 | Spherical | MIC:16 µg/mL | Broth microdilution | – | [56] | |||
| 20–30 | Spherical | 25 µg/mL | 151 | [61] | ||||
| 10–50 | Spherical | 50 µg/mL | Disc diffusion | 140.22 | [86] | |||
| 20 | Spherical | 1000 µg/mL | 13 | [145] | ||||
| 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 3.900.27 | [34] | |||
| 28–30 | Spherical | 50 µL | Agar well diffusion | 170.25 | [146] | |||
| 29.5–60.5 | Spherical, square, cube, plate, and rectangular | 100 µg | Agar well diffusion | 5 | [89] | |||
| 16–25 | Spherical | – | Agar well diffusion | 14 | [133] | |||
| 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 3.500.24 | [34] | |||
| K. aerogenes | 5–10 | Spherical | 500 µg/50 µL | Agar well diffusion | 12.000.00 | [144] | ||
| 16–25 | Spherical | – | Agar well diffusion | 18 | [133] | |||
| 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 3.750.26 | [34] | |||
| 5–22 | Spherical | MIC:17.5 µg/mL | Broth microdilution method | – | [56] | |||
| 5–10 | Spherical | 500 µg/50 µL | Agar well diffusion | 2.670.33 | [144] | |||
| 16–25 | Spherical | – | Agar well diffusion | 19 | [133] | |||
| 5–10 | Spherical | 500 µg/50 µL | Agar well diffusion | 3.330.33 | [144] | |||
| 5–20 | Spherical | 80 µg/mL | Disc diffusion | 20 | [74] | |||
| 5–22 | Spherical | MIC:19.5 µg/mL | Broth microdilution | – | [56] | |||
| 10–50 | Spherical | 50 µg/mL | Disc diffusion | 150.47 | [34] | |||
| 20 | Spherical | 1000 µg/mL | 15 | [145] | ||||
| 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 2.800.19 | [34] | |||
| 29.5–60.5 | Spherical, square, cube, and rectangular | 100 µg | Agar well diffusion | 9 | [89] | |||
| 16–25 | Spherical | – | Agar well diffusion | 17 | [133] | |||
| 20 | Spherical | 100 µg/mL | Broth microdilution | 13 | [145] | |||
| 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 3.050.21 | [34] | |||
| Fungal species | CuO NPs | Method | ZOI (mm) /inhibition (%) | References | ||||
| Size (nm) | Shape | Concentration/ amount | ||||||
| A. flavus | 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 2.350.16 | [34] | ||
| 28 | Semispherical | 50 µg/mL | Disc diffusion | 20.31.1 | [41] | |||
| 5–24 | Spherical, oval-shaped, cubical | 50 µg/mL | Well diffusion | 16.90.42 | [80] | |||
| A. fumigates | 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 2.700.18 | [34] | ||
| A. niger | 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 2.700.18 | [34] | ||
| C. albicans | 20–40 | Spherical and oval-shaped | 50 µg/mL | Disc diffusion | 2.950.20 | [34] | ||
| 16–25 | Spherical | – | Agar well diffusion | 25 | [133] | |||
| G. albicans | 28 | Semispherical | 50 µg/mL | Disc diffusion | 19.50.6 | [41] | ||
| G. globrata | 28 | Semispherical | 50 µg/mL | Disc diffusion | 18.61.2 | [41] | ||
| M. canis | 28 | Semispherical | 50 µg/mL | Disc diffusion | 21.61.5 | [41] | ||
3.1.1. Antimicrobial mechanism of CuO NPs
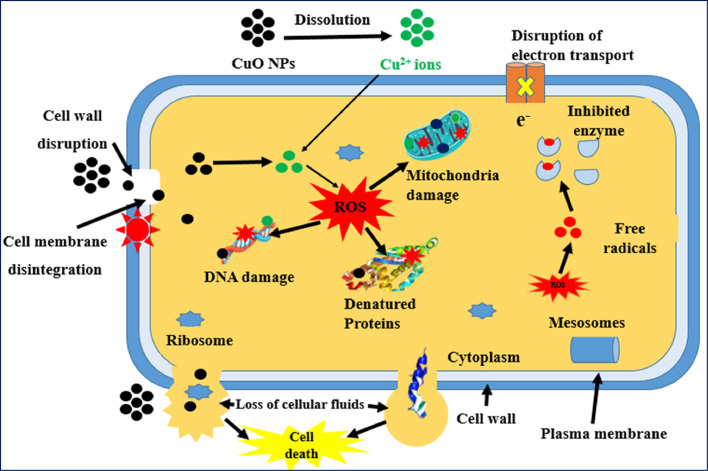
The exact mechanism by which CuO NPs exert their effects on various microbial strains has yet to be fully comprehended. Nevertheless, several suggested mechanisms provide insights into the antimicrobial effects of CuO NPs (as shown in Fig. 2): 1) Oxidative stress: CuO NPs generate a large amount of reactive oxygen species (ROS) on their surface, leading to oxidative stress and severe damage to microbial cells. This process is considered the primary mechanism contributing to the antimicrobial potential of CuO NPs [34,56,147,148]. Biogenic CuO NPs produce ROS, such as hydroxyl radicals, hydrogen peroxide, and superoxide, which can disrupt microbial cell walls, disrupt cell membrane integrity, and provoke cell death by damaging proteins, DNA helices, and mitochondria [117,149,150]. In addition, CuO NPs can inhibit the respiratory enzymes of microbial cells, leading to their death [15]. 2) Accumulation on cell surfaces: CuO NPs can accumulate on the surface of microbial cells, potentially causing direct damage to the cell wall [151,152]. This accumulation can induce morphological, structural, and physiological changes in microbial cell membranes, including alterations in membrane potential, deactivation of surface proteins responsible for material transport, and disruption of cellular homeostasis, ultimately leading to cell death [56,117]. 3) Release of copper ions: CuO NPs release copper (II) ions that attach to the microbial cell membrane through electrostatic interactions. These positively charged Cu2+ ions attach to the carboxyl and sulfhydryl groups of cell membrane proteins, causing protein denaturation, membrane impermeability, and cell death [144]. Cu2+ ions released from internalized CuO NPs can also bind to DNA molecules, disrupting their helical structure through intra- and internuclear cross-linking [23,144]. Moreover, copper ions inside the microbial cytoplasm produce ROS, which subsequently leads to mitochondrial and DNA damage [23]. The antimicrobial characteristics of CuO NPs can be attributed to their small size and large surface area, which enable them to interact effectively with microbial cell membranes [86,103]. These interactions may result in the deformation of the bacterial cell membrane (depicted in Fig. 3), leading to the leakage of cytoplasmic contents and eventual cell death [56]. CuO NPs are capable of penetrating the bacterial cell envelope, reaching the cytoplasm, and disrupting cellular compartments, ultimately causing abnormal metabolism [153]. In summary, the antimicrobial effects of CuO NPs involve oxidative stress, accumulation on cell surfaces, and the release of copper ions, which collectively contribute to the disruption and death of microbial cells.
Fig. 2.
Antimicrobial mechanism of CuO NPs.
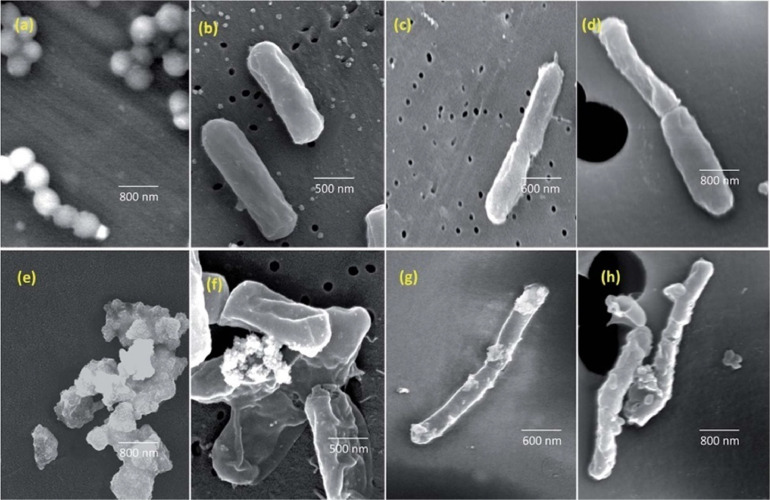
Fig. 3.
FE-SEM analysis revealing the impact of CuO NPs on bacterial morphology. (a-d) Preexposure images of S. aureus, B. cereus, E. coli, and P. aeruginosa, respectively. (e-h) Images illustrating the disruptive effects of CuO NPs on the cell membranes of the corresponding bacteria) [56].
Similarly, bioinspired synthesis of CuO NPs has potent fungicidal effects by generating abundant free radicals and reactive oxygen species. This process induces the distortion of fungal hyphae and impedes the proliferation of conidia and conidiophores [15]. The remarkable antifungal activity of environmentally friendly CuO NPs can be attributed to their ability to disrupt the cellular membrane structure and inhibit cell division through strong interactions with the respiratory chain [41]. Devipriya and Roopan [42] made intriguing observations that CuO NPs synthesized using eco-friendly techniques displayed remarkable antifungal efficacy against Aspergillus niger and Aspergillus flavus, surpassing the effectiveness of the standard commercial drug carbendazim. The heightened fungicidal properties of these nanoparticles stemmed from their deleterious interaction with the cell wall, which subsequently resulted in the release of intracellular components and compromised the integrity of the membrane. These studies underscore the importance of direct contact between nanoparticles and the surfaces of microbial cells, accompanied by the liberation of Cu2+ ions and the formation of ROS. This intricate interplay ultimately leads to the cell wall damage, culminating in the triggering of cell death.
3.2. Antioxidant potential of CuO NPs
Cells that are crucial for essential functions such as immunity and respiration can naturally produce free radicals. However, excessive production of free radicals can result in interactions with other biomolecules such as proteins, nucleic acids, lipids, and carbohydrates, causing various adverse effects on human health, including atherosclerosis, ageing, cardiovascular disease, inflammation, and even cancer [154]. Additionally, when food compounds are exposed to air, they can react with oxygen and generate free radicals, posing a health risk to humans [155].
In recent years, nanoparticles have gained recognition as valuable assets because of their antimicrobial activity and ability to target free radicals [49,156]. Researchers have shown considerable interest in exploring the antioxidant capacity, reducing power, and scavenging abilities of nanomaterials in terms of free radicals. For example, the in vitro antioxidant activity of CuO NPs has been extensively investigated using various methods such as DPPH, ABTS, nitric oxide scavenging, and reducing power assays [157]. The findings of this study revealed that the ability of CuO NPs to neutralize DPPH, ABTs, and nitric oxide free radicals significantly increases with increasing concentrations of CuO NPs, ranging from 10 to 100 μg/mL. At a concentration of 100 μg/mL, CuO NPs exhibited dose-dependent scavenging activities against DPPH (60.74 %), ABTS (70.88 %), and nitric oxide (65.46 %). Furthermore, CuO NPs demonstrated dose-dependent reducing effects, reaching a maximum activity of 71.44 % at a concentration of 100 μg/mL. In vitro studies assessing the antioxidant activity of CuO NPs synthesized from O. europaea extracts have demonstrated the significant potential of these NPs for scavenging DPPH radicals [67]. The antioxidant capacity of CuO NPs prepared from Andean blackberry fruits (ABFs) and leaves (ABLs) was evaluated using DPPH assay. CuO NPs at a concentration of 1 mM had dose-dependent scavenging activity, with ABF-mediated CuO NPs demonstrating greater antioxidant potential (89.02 %) than ABL-mediated CuO NPs (75.92 %). The enhanced antioxidant capacity of ABF-mediated CuO NPs can be attributed to the greater presence of antioxidant phytochemicals which act as capping agents for CuO NPs, in the ABF extract. The significant scavenging activity of CuO NPs against DPPH radicals is likely attributed to the electrostatic attraction between negatively charged bioactive compounds (COO−, O−) and neutrallly or positively charged nanoparticles [68].
CuO NPs synthesized from T. cordifolia extract exhibited strong DPPH scavenging activity, with an IC50 value of 566 μg/mL [95]. In another study, the antioxidant potential of Magnolia champaca-mediated CuO NPs was investigated using DPPH and ABTS assays, revealing dose-dependent inhibition with maximum values of 76.30 % and 88.53 %, respectively, at a concentration of 500 μg/mL [81]. Similarly, the antioxidant potential of CuO NPs/pectin nanocomposites increases with increasing concentrations of nanoparticles [129]. The CuO NPs/pectin nanocomposite displayed a maximum DPPH scavenging activity of 91.25 % at a concentration of 1000 μg/mL. CuO NPs synthesized from D. gangeticum exhibited significant effectiveness against oxidative stress and showed lower toxicity than their precursor materials [79]. In another study [19], the antioxidant capacity of CuO NPs prepared via green and chemical methods was compared using ABTS, DPPH, and hydrogen peroxide radical scavenging assays. The green synthesized CuO NPs exhibited considerable radical scavenging activity compared to those synthesized by chemical methods. This difference may be due to the presence of phytochemicals in the plant extracts used for the synthesis of CuO NPs. CuO NPs synthesized from T. indica and H. rosa also exhibited greater scavenging activity than did other synthesized green nanoparticles, potentially due to the presence of additional secondary metabolites such as flavonoids, glycosides, saponins, tannins, phenolic compounds, carbohydrates, proteins, and amino acids present in the plant extracts used for synthesis. These findings strongly suggest the use of green synthesized CuO NPs as useful antioxidants to support overall health against various degenerative diseases associated with oxidative stress. However, further in vitro and in vivo evaluation of the antioxidant properties of CuO NPs is essential before considering their use in human applications.
3.3. Anticancer activity of CuO NPs
Cancer, a disease marked by the aberrant proliferation of cells, is a major cause of death worldwide, especially in less developed countries [158]. Although conventional treatments such as radiotherapy, chemotherapy, hormone therapy, and surgery have been employed, they are associated with high costs and often lead to significant side effects. Furthermore, the emergence of drug resistance presents a challenge for successful cancer treatment [159]. Consequently, alternative therapies are urgently needed to address this cancer issue. In recent years, researchers have turned their focus to nanoparticle-based approaches for cancer diagnosis and treatment due to their efficacy, simplicity, and minimal side effects [30,160]. Numerous studies have explored the anticancer potential of green-synthesized CuO nanoparticles against different cancer types, such as breast, cervical, colon, skin, gastric, lung, and ovarian cancers, as depicted in Table 4. For example, the impact of CuO NPs synthesized using Ficus religiosa extract on A549 lung cancer cells was investigated [161]. The results revealed that the nanoparticles had dose-dependent anticancer effects on A549 cells. The efficacy of these NPs is influenced by various factors, including size, shape, cell type, and the specific plant utilized in the synthesis process.
Table 4.
Anticancer potential of biosynthesized CuO NPs.
| Types of cells/cell lines | CuO NPs | Method | Toxicity (IC50) (µg/mL) |
References | ||
|---|---|---|---|---|---|---|
| Size (nm) | Shape | |||||
| Breast cancer | ||||||
| AMJ-13 | 20–50 | Spherical | MTT assay | 1.47 | [67] | |
| MCF-7 | 5–24 | Spherical, oval-shaped, cubical |
MTT assay |
>100 | [80] | |
| 12 | Spherical | 19.77–27.44 (depends on plant extract) |
[19] | |||
| 20 | Spherical | 85.58 | [145] | |||
| 20 | Spherical | 24.5 | [163] | |||
| 30–40 | Spherical, cubical | 35 | [164] | |||
| 36 | Spherical | 21.56 | [165] | |||
| >200 | Spherical | 21.5 | [163] | |||
| MDA-MB-231 | 10–50 | Spherical | MTT assay | 30 | [86] | |
| 20 | Spherical | 11 | [163] | |||
| >200 | Spherical | 7.5 | [163] | |||
| 108.83 | Hexagonal, oval | 21.03 1.85 | [31] | |||
| Cervical cancer | ||||||
| HeLa | 12 | Spherical | MTT assay | 26.73–20.32 (depends on the source of plant extract) |
[19] | |
| 36 | Spherical | 24.74 | [165] | |||
| 26.6 | Spherical | SRB assay | 0.5 | [83] | ||
| 10 −15 | Quasispherical | MTT assay | 740 | [129] | ||
| HNCF-PI 52 | 10 −15 | Quasispherical | MTT assay | 838 | [129] | |
| CCI-PI 19 | 10 −15 | Quasispherical | MTT assay | 900 | [129] | |
| Epithelioma | ||||||
| Hep-2 | 12 | Spherical | MTT assay | 21.66–29.58 (depends on the source of plant extract) |
[19] | |
| Colon cancer | ||||||
| HCT-116 | – | Agglomerated cluster | MTT assay | 40 | [28] | |
| Gastric cancer | ||||||
| AGS | 5–22 | Spherical | MTT assay | 25–50 | [56] | |
| Lung cancer | ||||||
| A549 | 12 | Spherical |
MTT assay |
18.11–37.19 (depends on the source of plant extract) |
[19] | |
| 20 | Spherical | 81.57 | [145] | |||
| 33.47 | Spherical, irregular | 25 | [166] | |||
| 577 | Spherical | 200 | [161] | |||
| 577 | Spherical | 200 | [167] | |||
| Ovarian cancer | ||||||
| SKOV-3 | 20–50 | Spherical | MTT assay | 2.27 | [67] | |
CuO NPs fabricated from different plant extracts via a green chemistry approach were evaluated for their anticancer effects on four human tumor cell lines: lung (A549), epithelioma (Hep-2), cervical (HeLa), and breast (MCF-7) [19]. The cytotoxicity of the CuO NPs varied among the cell lines. Notably, CuO NPs prepared from the leaf extract of Tamarindus indica exhibited greater anticancer activity against all the tested cell lines than nanoparticles synthesized from other plant extracts. This enhanced anticancer potential can be attributed to the antioxidant properties of the phytochemicals present in the Tamarindus indica extract. Furthermore, CuO NPs synthesized using the bean extract of Phaseolus vulgaris showed strong cytotoxic effects on HeLa cell lines [83]. These nanoparticles dose-dependently induced the intracellular generation of ROS and significantly suppressed cervical cancer colony formation. Likewise, CuO NPs synthesized from Tirbulus terrestis extract induced apoptosis and toxicity in AGS cancer cells while remaining relatively safe for normal mammalian cells [56]. Similar results were observed in SKOV-3 and AMJ-13 cancer cells using biologically synthesized CuO NPs [67]. However, the cytotoxic effect on human dermal fibroblasts (HuFB) was observed only at high concentrations. These findings revealed the potential use of bioinspired CuO NPs in pharmaceutical applications.
The biomedical applications of CuO NPs prepared from four different plant extracts were compared to those of chemically synthesized CuO NPs [19]. CuO NPs biosynthesized through a cost-effective and green approach exhibited greater toxicity against various human cancer cell lines than chemically synthesized nanoparticles. Among the plant extracts employed, the copper oxide nanoparticles synthesized with Tamarindus indica demonstrated greater toxicity against human lung, cervical, and breast cancer cells than did the chemically synthesized CuO NPs. Despite their potent cytotoxicity in cancer cells, biologically prepared CuO NPs have demonstrated reduced toxicity in healthy animals. For instance, in a zebrafish model, both green-synthesized and commercially available CuO NPs decreased viability in a dose-and time-dependent manner [162]. However, the median lethal dose (LC50) of the biosynthesized CuO NPs (17510 mg/L) was greater than that of commercially available CuO NPs (4510 mg/L). These findings highlight the potential of using green-synthesized CuO NPs for cancer therapy without causing significant toxicity to normal cells.
3.3.1. Anticancer mechanism of CuO NPs
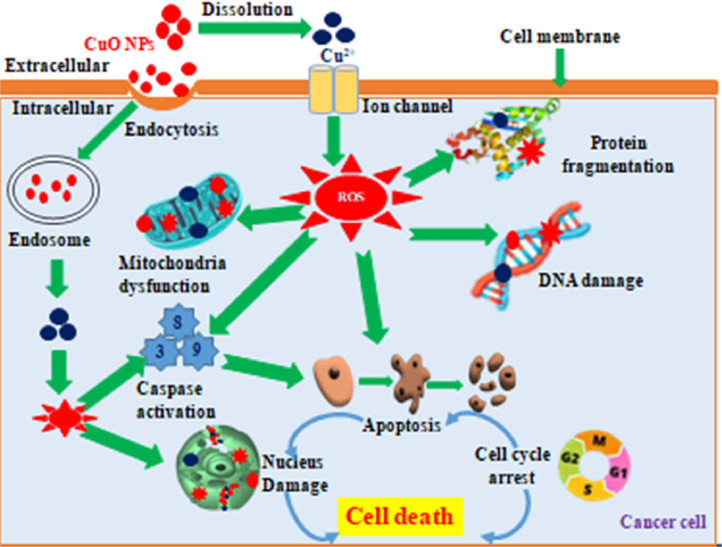
The specific mechanism by which CuO NPs act against cancer cells is still under investigation. The primary mechanism underlying the anticancer properties of CuO NPs synthesized through green methods involves reactive oxygen species (ROS)-dependent apoptosis and caspase-mediated apoptosis [80,83,167] (Fig. 4). When CuO NPs interact with cancer plasma membranes, they initiate nanoparticle invagination via endocytosis, enabling entry into the intracellular environment. Once inside, the nanoparticles release ROS, which can potentially harm mitochondria, enzymes, DNA, and the nucleus. They also reduce the activity of key nonprotein free radical scavengers. Treatment with biogenic CuO NPs leads to significant morphological changes in cancer cells, such as shrinkage, cell clumping, cytoplasmic blebbing, loss of membrane integrity, chromatin condensation, and organelle damage [83,161,166]. Copper (II) ions internalized through endocytosis disrupt the redox properties of the mitochondrial respiratory chain, causing oxidative stress and ultimately triggering cell death [165]. CuO NPs also elevate intracellular ROS levels and upregulate nitric oxide (NO) in certain cancer cell types [80,83,86,161,165].
Fig. 4.
Proposed mechanism for the anticancer activity of CuO NPs.
On the other hand, CuO NPs synthesized from Matricaria chamomilla flower extract directly interact with plasmids, thereby causing DNA cleavage and apoptosis [66]. Intracellular ROS generation induced by CuO NPs leads to DNA damage and upregulation of tumor suppressor genes (p21 and p53), which are responsible for cell cycle arrest and apoptosis [167]. CuO NPs prepared from Eucalyptus globulus and Beta Vulgaris extracts induce cell cycle arrest at the G2/M phase in breast cancer cells (MCF-7) and A549 cancer cells, respectively [80,166]. In MCF-7 cells, the collective complications of ROS generation, mitochondrial damage, cell cycle dysfunction, and apoptosis likely upregulate the expression of tumor suppressor genes (p53) [80]. CuO NPs synthesized from F. religiosa extract suppress the total amount of histone deacetylases (HDACs) and downregulate the mRNA and protein expression levels of p53 and p21 and the protein levels of oncogenes (MMP-2, and MMP-9) potentially protecting against angiogenesis and inflammation [167].
Apoptosis, a programmed cell death mechanism, is initiated and carried out by a sequence of caspase proteins through intrinsic (mitochondria-mediated) and extrinsic (death receptor-mediated) pathways [168], [169], [170]. The intrinsic pathway involves the activation of caspase-9, followed by the activation of caspase-3. The presence of the proapoptotic protein Bak/Bax on mitochondria and the antiapoptotic protein Bcl-2 leads to the permeabilization of the membrane and the release of cytochrome c. In addition to the Apaf-1 adaptor and pro-caspase-9, cytochrome c forms an apoptosome [171]. Treatment with CuO NPs has been demonstrated to enhance the expression of caspases 3, 8, and 9, cytochrome c, and Bax in HeLa and MCF-7 cells, indicating activation of the intrinsic pathway [165]. Similarly, biogenic CuO NPs stimulate caspase-9, suggesting the induction of the apoptosis pathway in A549 cells [167]. Furthermore, cancer cells treated with CuO NPs exhibited decreased Bcl2 expression and increased Bax and cytochrome c expression. An increase in the Bax/Bcl2 ratio, coupled with the activation of p53, prompts mitochondria to release cytochrome c and initiate the apoptosis caspase cascade pathway [80,165,167].
The extrinsic pathway is activated by death-receptor-mediated caspase-8 and then by caspase-3 activation. Both activated caspase-9 and caspase-8 trigger apoptosis through caspase-3 and caspase-7 [170]. The expression of caspase-8 was also concomitantly increased in cells treated with CuO NPs, indicating that CuO NPs may induce extrinsic apoptosis [80,165,167]. Overall, CuO NPs synthesized using green methods have demonstrated anticancer potential through multiple routes, including ROS generation, cell cycle arrest, and apoptosis. However, the mode of action may vary depending on the synthesis route, the source of CuO NPs, and the type of cell line used.
3.4. Other potential biomedical applications of CuO NPs
In addition to the aforementioned applications, CuO NPs have various applications, including antimalarial, antilarvicidal, antiviral, anti-inflammatory, antidiabetic, and antirheumatic effects. Although limited reports are available on these applications, we will briefly discuss them here. CuO NPs synthesized from Acanthospermum hispidum L. extract exhibited excellent antimalarial activity against Plasmodium falciparum, outperforming standard drugs such as chloroquine and quinine [64]. CuO NPs also showed antimycobacterial activity against the mycobacterium tuberculosis H37RV. CuO NPs synthesized from Tridax procumbens showed excellent antilarval activity against Aedes aegypti mosquitoes in a dose-dependent manner. The IC50 value for this activity was 4.21 mg/L [96]. Similarly, CuO NPs synthesized using Streptomyces zaomyceticus Oc-5 and Streptomyces pseudogriseolus Acv-11, which were isolated from healthy leaves of O. corniculata, exhibited larvicidal effects on Musca domestica and Culex pipiens mosquitoes [117]. In another study, CuO NPs synthesized from Allium sativum exhibited excellent antilarval activity against Anopheles subpictus mosquito larvae [34]. The researchers proposed that the larvicidal effects of the nanoparticles could be attributed to their accumulation in the airways of mosquito larvae, resulting in severe damage to the alimentary canal and tissue rupture. Additionally, CuO NPs synthesized with Rubia cordifolia bark extract showed significant larvicidal activity against Aedesaegypti, Anopheles stephensi, and Culex quinquefasciatus mosquito larvae, with LC50 values of 26.88, 16.93 and 26.60 ppm, respectively [157].
CuO NPs synthesized using Syzygium alternifolium demonstrated effective antiviral effects on Newcastle disease virus (NDV) [172]. After the integration of CuO NPs with electrospun nanofibers combined with PVP, researchers discovered 70 % of H1N1 viruses after hours of exposure [173]. Additionally, CuO NPs, along with other antimicrobial nanomaterials, have been shown to reduce the viability of severe acute respiratory syndrome coronavirus 2 [174]. These findings underscore the strong antiviral potential of CuO NPs. In various mouse models, biogenic CuO NPs notably alleviated pain and inflammation induced by different stimuli, effectively inhibiting nociceptive responses and reducing inflammation [72]. Moreover, CuO NPs synthesized with A. sativum demonstrated even greater anti-inflammatory activity than did standard diclofenac sodium [34]. However, commercial CuO NPs and their ions have been found to activate the NLRP3 inflammasome, a regulator of proinflammatory cytokine release [175]. Nonetheless, the bioactive molecules involved in the biosynthesis of CuO NPs, which act as capping and stabilizing agents, have the potential to suppress the inflammatory response.
CuO NPs synthesized using Bacopa monnieri leaf extracts showcased impressive properties in managing diabetes by significantly reducing glucose levels in diabetic mice [35]. Compared to their chemically prepared counterparts, Ag/CuO nanocomposites synthesized from Murraya koenigii and Zingiber officinale exhibited promising potential as antidiabetic agents [176]. The superior antidiabetic effects of the green-synthesized Ag/CuO composite may be attributed to the presence of phytochemicals in the plant extracts.
Ficus religiosa-mediated CuO NPs exhibited remarkable wound-healing activity, promoting efficient wound closure and tissue restoration [40]. Wounds treated with CuO NPs achieved 93 % closure, surpassing the 80 % closure observed in control wounds. The accelerated wound closure and reduced wound size can be attributed to the inhibition of pathogenic bacteria at the wound site and the restoration of tissue integrity, thereby facilitating the healing process. Furthermore, CuO NPs fabricated using extracts derived from Cassia auriculata extract hold potential as antirheumatic agents for the treatment of rheumatoid arthritis [177]. These studies highlight the promising potential of green-synthesized CuO NPs for reducing inflammation, alleviating pain, and facilitating the process of wound healing.
3.5. Assessment of the toxicity of CuO NPs
The therapeutic effects of bioderived CuO NPs are diverse, but evaluating their potential toxicity on normal human cells and vital organs is important to avoid unwanted side effects. Several studies have demonstrated the relative safety of biogenic CuO NPs for use with normal human cell lines (Table 5). The IC50 values, which indicate the concentration required to inhibit cell growth by 50 %, for biosynthesized CuO NPs on normal human cell lines are greater than those for cancer cells. For example, the IC50 value for HEK293 cells is 410 µg/mL, and for normal human dermal fibroblast NHDF/L929 cells, it is greater than 100 µg/mL [145,178]. Although the IC50 value of CuO NPs in human dermal fibroblasts (HuFb) is 54.34 µg/mL, these nanoparticles have an IC50 value of less than 2.5 µg/mL in ovarian and breast cancer cell lines [67]. These findings suggest that green-synthesized CuO NPs have lower toxicity in normal cells and exhibit cytotoxic effects on cancer cells at lower concentrations, indicating their safety. In a separate study [67], oral administration of biomediated green-synthesized CuO NPs to male Swiss albino mice affected the digestive system. This process resulted in decreased spleen and thymus weights, and a concentration-dependent increase in the weight of the liver and kidney. However, no significant toxicity was observed up to a dose of 400 mg/kg in this study, but it was lethal at 800 mg/kg. Furthermore, the NPs induced significant atrophy in the lymphoid organs, indicating damage to the immune system. Another study conducted on zebrafish embryos showed that biogenic CuO NPs tended to accumulate on the skin surface and in the chorion, leading to abnormalities such as yolk sacs and pericardial edema [81]. Thus, further studies are necessary to understand the toxicity of biosynthesized CuO NPs in different animal models.
4. Conclusions and future perspectives
In this review, we explored the field of biogenic synthesis of CuO NPs and their biomedical applications. Our findings demonstrate that the biological synthesis of NPs has garnered significant attention due to their rapid, eco-friendly, cost-effective, nontoxic, and straightforward nature. Unlike physicochemical methods, biogenic synthesis negates the requirement for toxic chemicals, high temperatures, pressure, and energy. Various biological sources, including plants, bacteria, yeasts, fungi, actinomycetes, and other biological derivates, have successfully provided natural reducing and stabilizing agents for the fabrication of CuO NPs in a wide range of biomedical and pharmaceutical applications.
Notably, the biosynthesized CuO NPs have shown substantial antimicrobial potential against diverse microbial species. The mechanism of action against these pathogens involves the overproduction of ROS and the deactivation of scavenging enzymes. ROS disrupt the plasma membrane, cellular compartments, and biomolecules, leading to impaired cellular functions and eventual cell death. Moreover, the biosynthesized CuO NPs have shown promising outcomes against multidrug-resistant microbes, indicating their potential as effective antimicrobial agents against challenging pathogens in the future. However, further studies are necessary to fully comprehend the underlying mechanisms and assess any potential adverse effects. Furthermore, green-synthesized CuO NPs have demonstrated excellent anticancer and antioxidant potential in vitro models. However, their toxicity and optimal dosage still require careful consideration. Future research should focus on addressing these aspects to ensure their safe and effective use. Additionally, there is a need to investigate the synthesis mechanism of CuO NPs and explore strategies for minimizing their toxicity, facilitating large-scale production for potential biomedical applications.
Overall, this review highlights the significance of the use of biogenic CuO NPs in various therapeutic approaches, including antimicrobial, anticancer, antioxidant, anti-inflammatory, and wound-healing applications. Further research is needed to unravel the complete underlying mechanisms and address potential toxicity concerns. By gaining a comprehensive understanding of the rapidly evolving synthesis methodologies discussed herein, future studies can build upon this foundation to further explore the potential of CuO NPs in biomedical applications. In conclusion, our review provides valuable insights into the fabrication of CuO NPs and their diverse biomedical applications. This finding sets the stage for future investigations, aiming to unravel the full potential of CuO NPs and their safe integration into biomedical practices.
CRediT authorship contribution statement
Yemane Tadesse Gebreslassie: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Fisseha Guesh Gebremeskel: Conceptualization, Writing – review & editing.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Funding
The authors received no external funding.
Data availability
No data was used for the research described in the article.
References
- 1.Ahmed S., Chaudhry S.A., Ikram S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: a prospect towards green chemistry. J. Photochem. Photobiol. B: Biol. 2017;166:272–284. doi: 10.1016/j.jphotobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Thakkar K., Mhatre S., Parikh R. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):252–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Mohanpuria P., Rana N., Yadav S. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 2008;10:507–517. [Google Scholar]
- 4.Kubik T., Bogunia-Kubik K., Sugisaka M. Nanotechnology on duty in medical applications. Curr. Pharm. Biotechnol. 2005;6(1):17–33. doi: 10.2174/1389201053167248. [DOI] [PubMed] [Google Scholar]
- 5.Frewer L., et al. Consumer attitudes towards nanotechnologies applied to food production. Trends Food Sci. Technol. 2014;40(2):211–225. [Google Scholar]
- 6.Gebreslassie Y.T., Gebretnsae H.G. Green and cost-effective synthesis of tin oxide nanoparticles: a review on the synthesis methodologies, mechanism of formation, and their potential applications. Nanoscale Res. Lett. 2021;16(1):97. doi: 10.1186/s11671-021-03555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith D., Simon J., Baker J.J.J. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subbaiya R., et al. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017;11(8):965–972. doi: 10.1049/iet-nbt.2016.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espitia P.J.P., et al. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioproc. Tech. 2012;5:1447–1464. [Google Scholar]
- 10.Singh P., et al. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018;19(7):1979. doi: 10.3390/ijms19071979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed S., et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asghar M., et al. Comparative analysis of synthesis, characterization, antimicrobial, antioxidant, and enzyme inhibition potential of roses petal based synthesized copper oxide nanoparticles. Mater. Chem. Phys. 2022;278 [Google Scholar]
- 13.Jeronsia J.E., et al. Camellia sinensis leaf extract mediated synthesis of copper oxide nanostructures for potential biomedical applications. Mater. Today: Proc. 2019;8:214–222. [Google Scholar]
- 14.Dastjerdi R., Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf. B: Biointerfaces. 2010;79(1):5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed A.A., et al. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 2021;199:2788–2799. doi: 10.1007/s12011-020-02369-4. [DOI] [PubMed] [Google Scholar]
- 16.Katwal R., et al. Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J. Ind. Eng. Chem. 2015;31:173–184. [Google Scholar]
- 17.Aaga G.F., Anshebo S.T. Green synthesis of highly efficient and stable copper oxide nanoparticles using an aqueous seed extract of Moringa stenopetala for sunlight-assisted catalytic degradation of Congo red and alizarin red s. Heliyon. 2023;9(5) doi: 10.1016/j.heliyon.2023.e16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qamar H., et al. Green synthesis, characterization and antimicrobial activity of copper oxide nanomaterial derived from Momordica charantia. Int. J. Nanomed. 2020:2541–2553. doi: 10.2147/IJN.S240232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehana D., et al. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017;89:1067–1077. doi: 10.1016/j.biopha.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 20.Singh D., et al. Bacteria assisted green synthesis of copper oxide nanoparticles and their potential applications as antimicrobial agents and plant growth stimulants. Front. Chem. 2023;11 doi: 10.3389/fchem.2023.1154128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akintelu S., Folorunso A. Characterization and antimicrobial investigation of synthesized silver nanoparticles from Annona muricata leaf extracts. J. Nanotechnol. Nanomed. Nanobiotechnol. 2019;6:1–5. [Google Scholar]
- 22.Subbaiya R., Selvam M. Synthesis and characterization of silver nanoparticles from Streptomyces olivaceus sp-1392 and its anticancerous activity against non-small cell lung carcinoma cell line (NCI-H460) Curr. Nanosci. 2014;10(2):243–249. [Google Scholar]
- 23.Letchumanan D., et al. Plant-based biosynthesis of copper/copper oxide nanoparticles: an update on their applications in biomedicine, mechanisms, and toxicity. Biomolecules. 2021;11(4):564. doi: 10.3390/biom11040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayanan K.B., Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid. Interface Sci. 2010;156(1–2):1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Sharma J.K., et al. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compd. 2015;632:321–325. [Google Scholar]
- 26.Almasi H., Jafarzadeh P., Mehryar L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: characterization, antimicrobial and release properties. Carbohydr. Polym. 2018;186:273–281. doi: 10.1016/j.carbpol.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 27.Rajesh K., et al. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: physical, optical and antimicrobial properties. Optik (Stuttg.) 2018;154:593–600. [Google Scholar]
- 28.Gnanavel V., Palanichamy V., Roopan S.M. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116) J. Photochem. Photobiol. B: Biol. 2017;171:133–138. doi: 10.1016/j.jphotobiol.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Saravanakumar K., et al. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B: Biol. 2019;190:103–109. doi: 10.1016/j.jphotobiol.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Sarfraz M.H., et al. Comparative analysis of phyto-fabricated chitosan, copper oxide, and chitosan-based CuO nanoparticles: antibacterial potential against Acinetobacter baumannii isolates and anticancer activity against HepG2 cell lines. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1188743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariadoss A.V.A., et al. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2020;164:2073–2084. doi: 10.1016/j.ijbiomac.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadhassan Z., et al. Preparation of copper oxide nanoparticles coated with bovine serum albumin for delivery of methotrexate. J. Drug Deliv. Sci. Technol. 2022;67 [Google Scholar]
- 33.Assadi Z., Emtiazi G., Zarrabi A. Hyperbranched polyglycerol coated on copper oxide nanoparticles as a novel core-shell nano-carrier hydrophilic drug delivery model. J. Mol. Liq. 2018;250:375–380. [Google Scholar]
- 34.Velsankar K., et al. Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J. Environ. Chem. Eng. 2020;8 [Google Scholar]
- 35.Faisal S., et al. In vivo analgesic, anti-inflammatory, and anti-diabetic screening of Bacopa monnieri-synthesized copper oxide nanoparticles. ACS Omega. 2022;7(5):4071–4082. doi: 10.1021/acsomega.1c05410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maor I., et al. Laser-induced thermal response and controlled release of copper oxide nanoparticles from multifunctional polymeric nanocarriers. Sci. Technol. Adv. Mater. 2021;22(1):218–233. doi: 10.1080/14686996.2021.1883406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dey A., et al. Immunostimulatory effect of chitosan conjugated green copper oxide nanoparticles in tumor immunotherapy. Cytokine. 2020;127 doi: 10.1016/j.cyto.2019.154958. [DOI] [PubMed] [Google Scholar]
- 38.Benguigui M., et al. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019;9(1):12613. doi: 10.1038/s41598-019-48959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., et al. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis. 2013;4(8):e783. doi: 10.1038/cddis.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sankar R., et al. Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J. Mater. Sci. Mater. Med. 2015;26(7):214. doi: 10.1007/s10856-015-5543-y. [DOI] [PubMed] [Google Scholar]
- 41.Rabiee N., et al. Biosynthesis of copper oxide nanoparticles with potential biomedical applications. Int. J. Nanomed. 2020:3983–3999. doi: 10.2147/IJN.S255398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devipriya D., Roopan S.M. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mater. Sci. Eng.: C. 2017;80:38–44. doi: 10.1016/j.msec.2017.05.130. [DOI] [PubMed] [Google Scholar]
- 43.Xu D., et al. Design and fabrication of Ag-CuO nanoparticles on reduced graphene oxide for nonenzymatic detection of glucose. Sens. Actuat. B: Chem. 2018;265:435–442. [Google Scholar]
- 44.Reddy S., Swamy B., Jayadevappa H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta. 2012;61:78–86. [Google Scholar]
- 45.Uzunoglu A., Stanciu L. Novel CeO 2–CuO-decorated enzymatic lactate biosensors operating in low oxygen environments. Anal. Chim. Acta. 2016;909:121–128. doi: 10.1016/j.aca.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 46.Chen M., et al. An ultrasensitive electrochemical DNA biosensor based on a copper oxide nanowires/single-walled carbon nanotubes nanocomposite. Appl. Surf. Sci. 2016;364:703–709. [Google Scholar]
- 47.Naz S., et al. Nanomaterials as nanocarriers: a critical assessment why these are multi-chore vanquisher in breast cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018;46(5):899–916. doi: 10.1080/21691401.2017.1375937. [DOI] [PubMed] [Google Scholar]
- 48.Chang Y.-N., et al. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials (Basel) 2012;5(12):2850–2871. [Google Scholar]
- 49.Abhimanyu P., Arvind M., Kishor N. Biosynthesis of CuO nanoparticles using plant extract as a precursor: characterization, antibacterial, and antioxidant activity. Nano Biomed. Eng. 2023 [Google Scholar]
- 50.Nzilu D.M., et al. Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic. Sci. Rep. 2023;13(1):14030. doi: 10.1038/s41598-023-41119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed S., et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal H., Kumar S.V., Rajeshkumar S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour.-Eff. Technol. 2017;3(4):406–413. [Google Scholar]
- 53.Javed, R., et al., Phytonanotechnology: a greener approach for biomedical applications, in Biogenic Nanoparticles for Cancer Theranostics2021, Elsevier. p. 43–86.
- 54.Priya M., et al. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci. Rep. 2023;13(1):18838. doi: 10.1038/s41598-023-46002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinlaan M., et al. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 56.Gopinath V., et al. In vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticles. RSC Adv. 2016;6(112):110986–110995. [Google Scholar]
- 57.Narasaiah P., Mandal B.K., Sarada N. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. Biosynthesis of copper oxide nanoparticles from Drypetes sepiaria leaf extract and their catalytic activity to dye degradation. [Google Scholar]
- 58.Singh S., et al. Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem. Eng. J. 2017;313:283–292. [Google Scholar]
- 59.Gunalan S., Sivaraj R., Venckatesh R. Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: optical properties. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2012;97:1140–1144. doi: 10.1016/j.saa.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 60.Sivaraj R., et al. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014;133:178–181. doi: 10.1016/j.saa.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 61.Sivaraj R., et al. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014;129:255–258. doi: 10.1016/j.saa.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 62.Sathiyavimal S., et al. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaves extract and their evaluation of antibacterial, anticancer in human A549 lung and MDA-MB-231 breast cancer cells. Food Chem. Toxicol. 2022;168 doi: 10.1016/j.fct.2022.113330. [DOI] [PubMed] [Google Scholar]
- 63.Aminuzzaman M., Kei L.M., Liang W.H. AIP conference proceedings. AIP Publishing LLC; 2017. Green synthesis of copper oxide (CuO) nanoparticles using banana peel extract and their photocatalytic activities. [Google Scholar]
- 64.Pansambal S., et al. Phytosynthesis and biological activities of fluorescent CuO nanoparticles using Acanthospermum hispidum L. extract. J. Nanostruct. 2017;7(3):165–174. [Google Scholar]
- 65.Gowri M., Latha N., Rajan M. Copper oxide nanoparticles synthesized using eupatorium odoratum, Acanthospermum hispidum leaf extracts, and its antibacterial effects against pathogens: a comparative study. BioNanoSci. 2019;9:545–552. [Google Scholar]
- 66.Duman F., Ocsoy I., Kup F. Chamomile flower extract-directed CuO nanoparticle formation for its antioxidant and DNA cleavage properties. Mater. Sci. Eng. C. 2016;60:333–338. doi: 10.1016/j.msec.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 67.Sulaiman G., Tawfeeq A., Jaaffer M. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: an in vivo and in vitro study. Biotechnol. Prog. 2018;34(1):218–230. doi: 10.1002/btpr.2568. [DOI] [PubMed] [Google Scholar]
- 68.Kumar B., et al. Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf. J. Saudi Chem. Soc. 2017;21:S475–S480. [Google Scholar]
- 69.Lingaraju K., et al. Rauvolfia serpentina-mediated green synthesis of CuO nanoparticles and its multidisciplinary studies. Acta Metall. Sin. (Engl. Lett.) 2015;28(9):1134–1140. [Google Scholar]
- 70.Nwanya A., et al. Industrial textile effluent treatment and antibacterial effectiveness of Zea mays L. Dry husk mediated bio-synthesized copper oxide nanoparticles. J. Hazard. Mater. 2019;375:281–289. doi: 10.1016/j.jhazmat.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Nasrollahzadeh M., Maham M., Sajadi S. Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N-monosubstituted ureas and reduction of 4-nitrophenol. J. Colloid Interface Sci. 2015;455:245–253. doi: 10.1016/j.jcis.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 72.Liu H., et al. Biosynthesis of copperoxide nanoparticles using Abies spectabilis plant extract and analyzing its antinociceptive and anti-inflammatory potency in various mice models. Arab. J. Chem. 2020;13:6995–7006. [Google Scholar]
- 73.Faheem I., et al. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop. J. Phar. Res. 2016;16(4):743–753. [Google Scholar]
- 74.Awwad A., Amer M. Biosynthesis of copper oxide nanoparticles using Ailanthus altissima leaf extract and antibacterial activity. Chem. Int. 2020;6:210–217. [Google Scholar]
- 75.Kumar V., et al. Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogen. Bio. Nano Sci. 2015;5:135–139. [Google Scholar]
- 76.Sharmila G., et al. Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: characterization and its antibacterial application. J. Mol. Struct. 2018;1165:288–292. [Google Scholar]
- 77.Phang Y., et al. Green Synthesis and Characterization of CuO Nanoparticles Derived from Papaya Peel Extract for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME) Sustainability. 2021;13(796):1–15. [Google Scholar]
- 78.Prakash S., et al. Green synthesis of copper oxide nanoparticles and its effective applications in Biginelli reaction, BTB photodegradation and antibacterial activity. Adv. Powder Technol. 2018;29(12):3315–3326. [Google Scholar]
- 79.Rohit G., Shakila B., Gino A. Synthesis of Copper oxide nanoparticles using Desmodium gangeticum aqueous root extract. Int. J. Pharm. Pharm. Sci. 2014;7(1):60–65. [Google Scholar]
- 80.Ali K., et al. Bio-functionalized CuO nanoparticles induced apoptotic activities in human breast carcinoma cells and toxicity against Aspergillus flavus: an In Vitro approach. Process. Biochem. 2020;91:387–397. [Google Scholar]
- 81.Santhosh Kumar J., Shanmugam V. Green synthesis of copper oxide nanoparticles from magnolia champaca floral extract and its antioxidant & toxicity assay using Danio Rerio. Int. J. Recent Technol. Eng. 2020;8:5444–5449. [Google Scholar]
- 82.Nordin N., Shamsuddin M. Biosynthesis of copper(II) oxide nanoparticles using Murayya koeniggi aqueous leaf extract and its catalytic activity in 4-nitrophenol reduction. Malays. J. Fundam. Appl. Sci. 2019;15(2):218–224. [Google Scholar]
- 83.Nagajyothi P., et al. Green synthesis: invitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017;10(2):215–225. [Google Scholar]
- 84.Singh J., et al. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 2019;177 doi: 10.1016/j.envres.2019.108569. [DOI] [PubMed] [Google Scholar]
- 85.Rajgovind S., et al. Pterocarpus marsupium derived phytosynthesis of copper oxide nanoparticles and their antimicrobial activities. J. Micro. Biochem. Technol. 2015;7:140–144. [Google Scholar]
- 86.Nagaraj E., et al. Exploration of Bio-synthesized Copper Oxide Nanoparticles Using Pterolobium hexapetalum Leaf Extract by Photocatalytic Activity and Biological Evaluations. J. Cluster Sci. 2019;30:1157–1168. [Google Scholar]
- 87.Sundaramurthy N., Parthiban C. Biosynthesis of copper oxide nanoparticles using Pyrus pyrifolia leaf extract and evolve the catalytic activity. Int. Res. J. Eng. Technol. 2015;2:332–338. [Google Scholar]
- 88.Bordbar M., Sharifi-Zarchi Z., Khodadadi B. Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: high catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue. J. Solgel. Sci. Technol. 2017;81(3):724–733. [Google Scholar]
- 89.Mary A., Ansari A., Subramanian R. Sugarcane juice mediated synthesis of copper oxide nanoparticles, characterization and their antibacterial activity. J. King Saud Univ. Sci. 2019;31(4):1103–1114. [Google Scholar]
- 90.Sathiyavimal S., et al. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B Biol. 2018;188:126–134. doi: 10.1016/j.jphotobiol.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 91.Ezealisiji K., Siwe-Noundou K. Copper oxide nano-hydrogel composite and their toxicology studies: a green chemistry approach. J. Mater. Sci. Nanotechnol. 2019;7(1):1–5. [Google Scholar]
- 92.Khatami M., et al. Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnol. 2017;11:709–713. [Google Scholar]
- 93.Yugandhar P., et al. Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci. 2017;7(7):417–427. [Google Scholar]
- 94.Buazar F., et al. Biofabrication of highly pure copper oxide 559 nanoparticles using wheat seed extract and their catalytic activity: a mechanistic approach. Green Process. Synth. 2019;8(1):691–702. [Google Scholar]
- 95.Udayabhanu P., et al. Tinospora cordifolia, mediated facile green synthesis of cupric oxide nanoparticles and their photo catalytic, antioxidant and antibacterial properties. Mater. Sci. Semicond. Process. 2015;33:81–88. [Google Scholar]
- 96.Selvan S., et al. Green synthesis of copper oxide nanoparticles and mosquito larvicidal activity against dengue, zika and chikungunya causing vector Aedes aegypti. IET Nanobiotechnol. 2018;12(8):1042–1046. doi: 10.1049/iet-nbt.2018.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh P., et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 98.Li X., et al. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011;2011 [Google Scholar]
- 99.Alshami H.G.A., Al-Tamimi W.H., Hateet R.R. The antioxidant potential of copper oxide nanoparticles synthesized from a new bacterial strain. Biodivers. J. Biol. Divers. 2023;(5):24. [Google Scholar]
- 100.Saif Hasan S., et al. Bacterial synthesis of copper/copper oxide nanoparticles. J. Nanosci. Nanotechnol. 2008;8(6):3191–3196. doi: 10.1166/jnn.2008.095. [DOI] [PubMed] [Google Scholar]
- 101.Yusof H.M., Mohamad R., Zaidan U.H. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 2019;10(1):57. doi: 10.1186/s40104-019-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitching M., Ramani M., Marsili E. Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb. Biotechnol. 2015;6:904–917. doi: 10.1111/1751-7915.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kouhkan M., et al. Biosynthesis of copper oxide nanoparticles using Lactobacillus casei subsp. casei and its anticancer and antibacterial activities. Curr. Nanosci. 2020;16(1):101–111. [Google Scholar]
- 104.Zarasvand K., Rai V. Inhibition of a sulfate reducing bacterium, Desulfovibrio marinisediminis GSR3, by biosynthesized copper oxide nanoparticles. 3 Biotech. 2016;6(1):84. doi: 10.1007/s13205-016-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh A., et al. Biological synthesis of copper oxide nano particles using Escherichia coli. Curr. Nanosci. 2010;6(4):365–369. [Google Scholar]
- 106.Azizi S., et al. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014;116:275–277. doi: 10.3390/ma6125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pati R., et al. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed. Nanotechnol., Biol., Med. 2014;10:1195–1208. doi: 10.1016/j.nano.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 108.Cuevas R., et al. Extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from chilean forests. J. Nanomater. 2015;2015:1–8. [Google Scholar]
- 109.El-Batal A., et al. Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J. Clust. Sci. 2020;32(1):79–90. [Google Scholar]
- 110.Siddiqi K., Husen A. Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res. Lett. 2016 Dec 1;11(1):363. doi: 10.1186/s11671-016-1580-9. 363., 2016. 11(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Papaspyrides C., Pavlidou S., Vouyiouka S. Development of advanced textile materials: natural fibre composites, antimicrobial, and flame-retardant fabrics. J. Mat. Design. Appl. 2009;223:91–102. [Google Scholar]
- 112.Bhattacharya P., et al. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J. Environ. Chem. Eng. 2019;7(1) [Google Scholar]
- 113.Vishnu S., et al. Potentiating effect of ecofriendly synthesis of copper oxide nanoparticles using brown alga: antimicrobial and anticancer activities. Bull. Mater. Sci. 2016;39:361–364. [Google Scholar]
- 114.Abboud Y., et al. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata) Appl. Nanosci. 2014;4(5):571–576. [Google Scholar]
- 115.Fouda A., et al. Springer Nature; Switzerland: 2019. Interaction Between Plants and Bacterial Endophytes Under Salinity stress. Endophytes and Secondary Metabolites. [Google Scholar]
- 116.Nabila M., Kannabiran K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018;15:56–62. [Google Scholar]
- 117.Hassan S., et al. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. JBIC J. Biol. Inorg. Chem. 2019;24(3):377–393. doi: 10.1007/s00775-019-01654-5. [DOI] [PubMed] [Google Scholar]
- 118.Taran M., Rad M., Alavi M. Antibacterial Activity of Copper Oxide (CuO) Nanoparticles Biosynthesized by Bacillus sp. FU4: optimization of Experiment Design. Pharma. Sci. 2018;23:198–223. [Google Scholar]
- 119.Araújo I., et al. Hydrothermal synthesis of bacterial cellulose–copper oxide nanocomposites and evaluation of their antimicrobial activity. Carbohydr. Polym. 2018;179:341–349. doi: 10.1016/j.carbpol.2017.09.081. [DOI] [PubMed] [Google Scholar]
- 120.Rad M., Taran M., Alavi M. Effect of incubation time, CuSO4 and glucose concentrations on biosynthesis of copper oxide (CuO) nanoparticles with rectangular shape and antibacterial activity: taguchi method approach. Nano. Biomed. Eng. 2018;10:25–33. [Google Scholar]
- 121.Ghasemi N., Jamali-Sheini F., Zekavati R. CuO and Ag/CuO nanoparticles: biosynthesis and antibacterial properties. Mater. Lett., 2017;196:78–82. [Google Scholar]
- 122.Ramanathan R., Bhargava S., Bansal V. Chemica 2011: Engineering a Better World: Sydney Hilton Hotel, NSW, Australia, 2011. 2011. Biological synthesis of copper/copper oxide nanoparticles; pp. 18–21. [Google Scholar]
- 123.Eltarahony M., Zaki S., Abd-El-Haleem D. Concurrent synthesis of zero -and one-dimensional, spherical,rod-, needle-, and wire-shaped CuO nanoparticles by Proteus mirabilis 10B. J. Nanomater. 2018;2018:1–15. [Google Scholar]
- 124.El-Batal A., et al. Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J. Clust. Sci. 2017;28(3):1083–1112. [Google Scholar]
- 125.Bukhari S., et al. Biosynthesis of copper oxide nanoparticles using Streptomyces MHM38 and its biological applications. J. Nanomater. 2021;2021:1–6. [Google Scholar]
- 126.Mousa A., et al. Biosynthetic new composite material containing CuO nanoparticles produced by Aspergillus terreus for 47Sc separation of cancer theranostics application from irradiated Ca target. Appl. Radiat. Isotopes. 2020;166 doi: 10.1016/j.apradiso.2020.109389. [DOI] [PubMed] [Google Scholar]
- 127.Saravanakumar K., et al. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B. Biol. 2019;190:103–109. doi: 10.1016/j.jphotobiol.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 128.Zou Y.-L., et al. Hydrothermal synthesis of momordica-like CuO nanostructures using egg white and their characterisation. Chem. Papers. 2012;66:278–283. [Google Scholar]
- 129.Liang X., et al. Pectin mediated green synthesis of CuO nanoparticles: evaluation of its cytotoxicity, antioxidant and anti-human cervical cancer properties. J. Exp. Nanosci. 2022;17(1):315–325. [Google Scholar]
- 130.Alishah H., et al. Green synthesis of starch-mediated CuO nanoparticles: preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. Rendiconti. Lincei. 2017;28:65–71. [Google Scholar]
- 131.Logpriya S., et al. Preparation and characterization of ascorbic acid-mediated chitosan–copper oxide nanocomposite for anti-microbial, sporicidal and biofilm-inhibitory activity. J. Nanostruct. Chem. 2018;8:301–309. [Google Scholar]
- 132.Raj Preeth D., et al. Green synthesis of copper oxide nanoparticles using sinapic acid: an underpinning step towards antiangiogenic therapy for breast cancer. JBIC J. Biol. Inorg. Chem. 2019;24:633–645. doi: 10.1007/s00775-019-01676-z. [DOI] [PubMed] [Google Scholar]
- 133.Nithiyavathi R., et al. Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J. Infect. Public Health. 2021;14(12):1893–1902. doi: 10.1016/j.jiph.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 134.Engler A.C., et al. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today. 2012;7(3):201–222. [Google Scholar]
- 135.Nadeem M., et al. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem. Lett. Rev. 2017;10(4):216–227. [Google Scholar]
- 136.Applerot G., et al. Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small. 2012;8(21):3326–3337. doi: 10.1002/smll.201200772. [DOI] [PubMed] [Google Scholar]
- 137.Awwad A., Albiss B., Salem N. Antibacterial activity of synthesized copper oxide nanoparticles using Malva sylvestris leaf extract. SMU Med. J. 2015;2(1):91–101. [Google Scholar]
- 138.Acharyulu N., et al. Green synthesis of CuO nanoparticles using Phyllanthus amarus leaf extract and their antibacterial activity against multidrug resistance bacteria. Int. J. Eng. Res. Tech. 2014;3(4):638–641. [Google Scholar]
- 139.Azam A., et al. Size-dependent antimicrobial properties of CuO nanoparticles against gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012;7:3527–3535. doi: 10.2147/IJN.S29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pansambal S., et al. Phytosynthesis and biological activities of fluorescent CuO nanoparticles using Acanthospermum hispidum L. extract. J. Nanostruct. 2017;7:165–174. [Google Scholar]
- 141.Perelshtein I., et al. CuO–cotton nanocomposite: formation, morphology, and antibacterial activity. Surf. Coat. Technol. 2009;204:54–57. [Google Scholar]
- 142.Oussou-Azo A., et al. Antifungal potential of nanostructured crystalline copper and its oxide forms. Nanomaterials (Basel) 2020;10(5):1003. doi: 10.3390/nano10051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khatami M., et al. Copper oxide nanoparticles greener synthesis using tea and its antifungal efficiency on Fusarium solani. Geomicrobiol. J. 2019;36(9):777–781. [Google Scholar]
- 144.Raja Naika H., et al. Green synthesis of CuO nanoparticles using Gloriosa superba L extract and their antibacterial activity. J. Taibah. Univ. Sci. 2015;9(1):7–12. [Google Scholar]
- 145.Rajamma R., et al. Antibacterial and anticancer activity of biosynthesised CuO nanoparticles. IET Nanobiotechnol. 2020;14(9):833–838. doi: 10.1049/iet-nbt.2020.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sharma B.K., Shah D.V., Roy D.R. Green synthesis of CuO nanoparticles using Azadirachta indica and its antibacterial activity for medicinal applications. Mater. Res. Exp. 2018;5(9) [Google Scholar]
- 147.Das D., et al. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces. 2013;101:430–433. doi: 10.1016/j.colsurfb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 148.Chang Y., et al. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials (Basel) 2012;5(12):2850–2871. [Google Scholar]
- 149.Meghana S., et al. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015;15:12293–12299. [Google Scholar]
- 150.Sukumar S., Rudrasenan A., Padmanabhan Nambiar D. Green-synthesized rice-shaped copper oxide nanoparticles using caesalpinia bonducella seed extract and their applications. ACS Omega. 2020;5:1040–1051. doi: 10.1021/acsomega.9b02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blecher K., Nasir A., Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence. 2011;2(5):395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- 152.Huh A.J., Kwon Y.J. Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release. 2011;156(2):128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 153.Jadhav M.S., et al. Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. N. J. Chem. 2018;42(1):204–213. [Google Scholar]
- 154.Javed B., Ikram M., Farooq F. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: a mechanistic overview. Appl. Microbiol. Biotechnol. 2021;105:2261–2275. doi: 10.1007/s00253-021-11171-8. [DOI] [PubMed] [Google Scholar]
- 155.Yu Z., Wang W., Kong F. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019;129:889–894. doi: 10.1016/j.ijbiomac.2019.02.084. [DOI] [PubMed] [Google Scholar]
- 156.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomedicine. 2017;12:1227. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vinothkanna A., et al. Biosynthesis of copper oxide nanoparticles using Rubia cordifolia bark extract: characterization, antibacterial, antioxidant, larvicidal and photocatalytic activities. Environ. Sci. Pollu. Res. 2022:1–12. doi: 10.1007/s11356-022-18996-4. [DOI] [PubMed] [Google Scholar]
- 158.Kim G.J., Nie S. Targeted cancer nanotherapy. Mater. Today. 2005;8(8):28–33. [Google Scholar]
- 159.Housman G., et al. Drug resistance in cancer: an overview. Cancers (Basel) 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Z., Kiessling F., Gätjens J. Advanced nanomaterials in multimodal imaging: design, functionalization, and biomedical applications. J. Nanomater. 2010;2010 [Google Scholar]
- 161.Sankar R., et al. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:234–239. doi: 10.1016/j.msec.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 162.Kumari P., et al. Mechanistic insight to ROS and apoptosis regulated cytotoxicity inferred by green synthesized CuO nanoparticles from Calotropis gigantea to embryonic zebrafish. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-017-16581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Preeth D.R., et al. Green synthesis of copper oxide nanoparticles using sinapic acid: an underpinning step towards antiangiogenic therapy for breast cancer. J. Biol. Inorgan. Chem. 2019;24:633–645. doi: 10.1007/s00775-019-01676-z. [DOI] [PubMed] [Google Scholar]
- 164.Sukumar K., et al. Eco-friendly cost-effective approach for synthesis of copper oxide nanoparticles for enhanced photocatalytic performance. Optik (Stuttg) 2019:202. [Google Scholar]
- 165.Dey A., et al. Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J. Saudi Chem. Soc. 2019;23(2):222–238. [Google Scholar]
- 166.Chandrasekaran R., Yadav S.A., Sivaperumal S. Phytosynthesis and characterization of copper oxide nanoparticles using the aqueous extract of beta vulgaris L and evaluation of their antibacterial and anticancer activities. J. Cluster Sci. 2020;31:221–230. [Google Scholar]
- 167.Kalaiarasi A., et al. Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol. Lett. 2018;40:249–256. doi: 10.1007/s10529-017-2463-6. [DOI] [PubMed] [Google Scholar]
- 168.Ola M.S., Nawaz M., Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 169.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 170.Pfeffer C., Singh A. Apoptosis: a target for anticancer therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Berens H.M., Tyler K.L. The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J. Virol. 2011;85(8):3858–3871. doi: 10.1128/JVI.01958-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yugandhar P., et al. Cost effective, green synthesis of copper oxide nanoparticles using fruit extract of Syzygium alternifolium (Wt.) Walp, characterization and evaluation of antiviral activity. J. Clust. Sci. 2018;29(4):743–755. [Google Scholar]
- 173.Cui W., et al. Electrospun nanofibers embedded with copper oxide nanoparticles to improve antiviral function. J. Nanosci. Nanotechnol. 2021;21(8):4174–4178. doi: 10.1166/jnn.2021.19379. [DOI] [PubMed] [Google Scholar]
- 174.Merkl P., et al. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials. 2021;11(5):1312. doi: 10.3390/nano11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tao X., et al. A tandem activation of NLRP3 inflammasome induced by copper oxide nanoparticles and dissolved copper ion in J774A. 1 macrophage. J. Hazard. Mater. 2021;411 doi: 10.1016/j.jhazmat.2021.125134. [DOI] [PubMed] [Google Scholar]
- 176.Arumai Selvan D., et al. Antidiabetic activity of phytosynthesized Ag/CuO nanocomposites using Murraya koenigii and Zingiber officinale extracts. J. Drug Deliv. Sci. Technol. 2022;67 [Google Scholar]
- 177.Shi L., et al. Green synthesis of CuO nanoparticles using Cassia auriculata leaf extract and in vitro evaluation of their biocompatibility with rheumatoid arthritis macrophages (RAW 264.7) Trop. J. Pharm. Res. 2017;16(1):185–192. [Google Scholar]
- 178.Kumari P., et al. Molecular insight to in vitro biocompatibility of phytofabricated copper oxide nanoparticles with human embryonic kidney cells. Nanomedicine. 2018;13(19):2415–2433. doi: 10.2217/nnm-2018-0175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.