Abstract
A seed lectin from Manilkara zapota (MZSL) was purified using ammonium sulphate precipitation and affinity chromatography. Hemagglutination activity, neutral sugar content and physicochemical properties of the lectin were determined and toxicity was checked by brine shrimp toxicity assay. Antimicrobial, antioxidant as well as in vitro anticancer activities of MZSL were also evaluated. Our findings showed the molecular weight of MZSL to be 33.0 ± 1 kDa. Minimum hemagglutination concentration of the lectin was 15.625 μg/ml. With a neutral sugar content of 6.32 %, the lectin was fully active at a temperature range of 30–50 °C and pH 7.0–8.0 and it was mildly toxic with an LC50 value of 107.93 μg/ml. The lectin demonstrated bacteriostatic activity against gram-positive bacteria in contrast to gram-negative bacteria at a concentration of 31.25 μg/ml, agglutinated Staphylococcus aureus and Shigella dysenteriae and exerted fungistatic activity against Aspergillus niger. MZSL dose-dependently reduced the formation of biofilm by E. coli. DPPH assay confirmed its antioxidant activity with an IC50 value of 96.42 μg/ml. MZSL showed 21.64 % growth inhibition against Ehrlich ascites carcinoma (EAC) cells at 80 μg/ml whereas its antiproliferative potential against MCF-7 and A-549 cancer cell lines became evident with IC50 values of 70.66 μg/ml and 107.64 μg/ml, respectively.
Keywords: Seed lectin, Manilkara zapota, Bacteriostatic, Fungistatic, Antibiofilm, Anticancer
Abbreviations
- kDa
Kilodalton
- TBS
Tris-buffered saline
- EDTA
Ethylenediamine tetraacetic acid
- Ca2+ Calcium
- Mn2+
Manganese
- Mg2+
Magnesium
- LD50
Lethal dose, 50 %
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- IC50
Half-maximal inhibitory concentration
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- LC50
Lethal dose, 50 %
1. Introduction
Lectins are naturally occurring proteins that recognize and bind specifically to glycans attached on the surfaces of proteins, lipids and RNA [1]. These biomolecular recognition proteins interact with glycans through their carbohydrate recognition domains and this capability of binding to specific saccharide moieties is often used in a variety of biological applications [[2], [3], [4], [5], [6], [7], [8]]. Lectins are reported to be involved in cell-cell communications, cell signaling, host-pathogen interactions, immune responses and determining the cellular fate [9,10]. Lectin-based biosensors and lectin microarray techniques recognize the alteration of glycan structures on tumor cell surface supporting the application of lectins as biomarkers to detect certain cancer diseases and improve their therapeutics [11,12].
Seeds are the richest source of plant lectins though these lectins are found in leaves, barks, roots, tubers and fruits [13]. Ricin, the first lectin was isolated from Castor bean (Ricinus communis) seeds in 1888. Till then, a good number of seed lectins had been found with unique glycan specificities and diverse physiological roles in immunity and inflammation [[14], [15], [16]]. Those lectins also exhibited significant antiviral, anticancer and antimicrobial effects through the interaction with various cancer cells and microbes including viruses, fungi and pathogenic bacteria [[17], [18], [19], [20], [21], [22], [23]].
Sapotaceae family of plants belongs to the order Ericale and includes 65 genera and 800 species in total. Manilkara zapota (also known as sapodilla, naseberry, or sapota) is also a member of this family of flowering plants [24]. There are 32 species in the genus Manilkara, the majority of which are commercially used for fruits, timber, and latex [25]. In a previous study, a 33-kDa lectin was isolated from the fleshy part of Manilkara zapota fruits which showed affinity to lactose and maltose sugars [26]. Two lectin-like proteins from seeds of Pouteria torta (Pouterin) and Labramia bojeri (Labramin), belonging to the sapotaceae family, have been reported to possess antifungal, anticancer and insecticidal activities [[27], [28], [29]]. Another galactose-binding lectin, CCL from the leaf extract of Chrysophyllum caimito (Star apple, family sapotaceae) inhibited the growth of E. coli [30]. Lectins were also found in fruits of Synsepalum dulcificum, another member of the sapotaceae family [31].
All these evidences encouraged us to isolate the lectins found in seeds of Manilkara zapota and investigate their potential as antimicrobial and anticancer agents. This study also focused on the characterization, toxicity level and antioxidant property of Manilkara zapota seed lectin.
2. Materials and methods
2.1. Materials
Lactose-agarose gel was bought from J-Oil Mills Inc., Tokyo, Japan. A standard protein marker mix was purchased from Takara Bio Inc, Japan. We purchased fetal calf serum, DMEM, RPMI-1640 media and Hoechst-33342 dye from Sigma Chemicals Co., USA. Streptomycin and penicillin were bought from CarlRoth Co., Germany. All other reagents were bought from Merck, Germany, Wako Pure Chemical Co., Japan and Sigma Chemicals Co., USA and were of the highest purity grades.
2.2. Purification of manilkara zapota seed lectin (MZSL)
Manilkara Zapota fruits were collected from the local market of Rajshahi, Bangladesh. After cleaning and washing, the seeds were crushed into powders with a homogenizer. TBS buffer (10 mM Tris (hydroxymethyl) aminomethane-HCl with 150 mM NaCl, pH 8.2) was added and the homogenate was filtered through a clean muslin cloth. The obtained supernatant was centrifuged at 4 °C at 10,000 rpm for 15 min. The crude protein sample was subjected to 70 % ammonium sulphate saturation. After collecting the precipitate by centrifugation, it was dialyzed sequentially against distilled water and TBS and was applied on a pre-equilibrated lactosyl-agarose affinity column in a Poly-Prep chromatography column (J-Oil Mills Inc., Tokyo, Japan). The column was washed well with TBS buffer and 50 mM galactose-containing TBS buffer was then applied to elute the lectin. Protein fractions were collected by a fraction collector (FRC-10 A, Shimadzu Corporation, Tokyo, Japan). Purity of MZSL was checked using 16 % (w/v) polyacrylamide gel by sodium dodecyl sulphate poly acrylamide gel electrophoresis (SDS PAGE) [20,26].
2.3. Hemagglutination activity assay
Blood from Swiss albino mice was collected in phosphate buffer saline (PBS) and centrifuged at 3000 rpm for 5 min to obtain 2 % red blood cells. To perform the hemagglutination test, hemagglutination buffer (50 μl) was added to the titer plate wells. Equal volume of MZSL was applied to the first well and diluted serially. Red blood cells from different sources were taken in phosphate buffer saline and 50 μl of 2 % red blood cells was taken in each well. The plate was shaken for 5 min and incubated for 1 h at 34 °C [32].
2.4. Estimation of sugar content in MZSL
d-glucose was used as a standard to determine the sugar content of MZSL according to the phenol-sulphuric acid method [33].
2.5. Determination of temperature and pH stability of the lectin
1 mg/ml of MZSL (pH 8.0) was kept for 30 min at different temperatures (30–100 °C). After cooling the samples to room temperature, hemagglutination activities were checked according to the assay described in section 2.3. To check the pH stability, 0.5 ml of MZSL (1 mg/ml) was taken in 100 mM of glycine-NaOH buffer (pH 9.0–10.0), Tris-HCl buffer (pH 8.2), PBS (pH 7.0) and sodium acetate buffer (pH 4.0–6.0). After incubating the lectin in those buffers at 30 °C for 6 h, hemagglutination activity of MZSL samples were determined for each buffer. Three replicates were used in each experiment.
2.6. Effect of divalent metal ions on MZSL
The metal chelator EDTA (100 mM) was mixed with MZSL for 2 h at 20 °C. The lectin was dialyzed in 20 mM TBS (pH 7.8) at 4 °C for 10 h. Twenty five mM of salt solutions of Ca2+, Mn2+ and Mg2+were prepared and hemagglutination activity of MZSL was then observed in the presence and absence of those solutions. The activity was compared to that of control. Three replicates were used for each experiment.
2.7. Brine shrimp lethality assay
Artificial sea water (1 L) was prepared adding 38 g of NaCl in distilled water. Sodium tetraborate salt was added to adjust its pH to 7.0. Cysts of brine shrimp were hatched in that water at a temperature of 25–28 °C under constant aeration and illumination. The cysts were incubated for 48 h (1 gm/liter of artificial seawater). MZSL was added to vials containing artificial sea water with final concentrations of 20, 40, 80, and 160 μg/ml.Ten Artemia nauplii were then added to each vial. Sea water was added to each vial to adjust their volume to 4 ml. A test tube with 10 nauplii and no added lectin was taken as the negative control. After 24 h, the death percentages and LD50 values for each concentration were assessed by using Probit analysis [34].
2.8. Antimicrobial activity assay
2.8.1. Determination of bacterial growth inhibition
Staphylococcus aureus (ATCC 25923), Shigella boydii (ATCC 231903), Shigella dysenteriae (ATCC 238135) and Escherichia coli (ATCC 27853) were grown in liquid nutrient medium at 37 °C for 24 h and the concentration of bacterial solution was fixed by adjusting its optical density to 1.0 at 640 nm. Manilkara zapota seed lectin was added to a microtiter plate through serial dilution and 100 μl of bacterial suspension was added to a total volume of 200 μl in each well. Four wells were kept as control wells containing only bacteria and nutrient media. After shaking the microtiter plate, absorbance values were recorded by a microtiter plate reader.
2.8.2. Fungistatic activity assay by disc diffusion method
Mycelia of a fungal strain, Aspergillus niger, was dissolved in distilled water and spread out on petri dishes containing solidified potato dextrose agar. Sterile paper discs were placed on the top of the surface. Manilkara zapota seed lectin at various concentrations (50 and 100 μg/disc) and standard antifungal agent (1 % Clioquinol + 0.02 % Flumetasone Pivalate) were soaked out by the discs. The petri dishes were then incubated at 30 °C until the growth of mycelia. Antifungal activity was observed by the formation of transparent rings around the discs.
2.8.3. Bacterial agglutination and antibiofilm activity (against Escherichia coli) by MZSL
Shigella dysenteriae (ATCC 238135) and Staphylococcus aureus (ATCC 25923) were grown following the procedure described in section 2.8.1. MZSL was serially diluted in the wells of a microtiter plate, 100 μl of bacterial suspension was added to each well and the plate was shaken using a titer plate shaker to observe under a microscope. Four wells were kept as control wells containing only bacteria and nutrient media.
Antibiofilm activity of MZSL against Escherichia coli (ATCC 27853) was investigated according to a previous study [20].
2.9. Antioxidant activity of MZSL by DPPH scavenging assay
The DPPH scavenging assay was carried out as described by Kedare and Singh, 2011 [35] with minor modifications. Different concentrations of MZSL and vitamin C (5, 10, 20, 30, 40 and 50 μg/ml) were taken and brought up to 1 ml by adding distilled water. The sample and standard were then mixed thoroughly with 3 ml of newly produced DPPH solution (0.1 mM DPPH solution: 0.00197 gm DPPH was dissolved in 50 ml methanol). The mixture was left in darkness for around 30 min and its absorbance was measured at 517 nm. DPPH solution and methanol were used as control and blank solutions.
The result was expressed as % of inhibition, S.
In this DPPH assay, IC50 (μg/mL) values were calculated from the graphs of percentage scavenging inhibition versus concentration of the sample.
2.10. Antiproliferative activity of MZSL against cancer cells
2.10.1. In vivo culture of ehrlich ascites carcinoma cells in swiss albino mice and study of antiproliferative activity of MZSL by MTT assay
Swiss albino mice (male, 3–4 weeks old, weighing 20–27 g) were purchased from the Department of Pharmacy, Jahangirnagar University, Savar, Bangladesh to culture Ehrlich ascites carcinoma (EAC) cells in vivo. EAC cells were then collected in sterile isotonic saline following a standard protocol [20]. A fixed number of viable cells (3 × 106 cells/ml) were transferred into each mouse. After six days, the mice were sacrificed and intraperitoneal tumor cells were harvested with normal saline.
The number of EAC cells was adjusted (3 × 106 cells in 200 μl) and seeded to the wells of a 96-well flat bottom culture plate. Different concentrations (0–400 μg/ml) of MZSL were also added and kept in a CO2 incubator for 24 h at 37 °C. Three wells were used as ‘control’ wells containing only EAC cells. The aliquot from each well was carefully removed to add 180 μl of PBS and 20 μl of MTT reagent. After incubating it further for 8 h at 37 °C, it was again drained out. Acidic isopropanol (200 μl) was added and kept in the incubator at 37 °C for another hour. Absorbance values of these wells were recorded at 570 nm with a titer plate reader. The proliferation inhibition ratio of EAC cells was determined from the equation:
A and B denoted OD of the homogenates of untreated (control) and lectin-treated EAC cells at 570 nm, respectively.
2.10.2. Determination of antiproliferative activity assay (in vitro) of MZSL against different human cancer lines
DMEM media with 10 % FBS was taken in cell culture flasks to culture MCF-7 and A-549 cells at 37 °C in a CO2 incubator. Penicillin, neomycin and streptomycin were used as antibiotics. Subcultures were carried out at 80−90 % confluence. A-549 (2 × 104 cells) and MCF-7 (1 × 104 cells) were seeded in each well containing 150 μL of DMEM media and incubated for 24 h at 37 °C. MZSL was serially diluted in 50 μl of DMEM media and added to each well of the plate to final concentrations of 5–80 μg/ml and incubated again for 48 h. And finally, the MTT assay was performed according to section 2.10.1.
2.11. Statistical analysis
Results of triplicate experiments have been presented with standard errors (SE). Data are shown as mean ± SE (n = 3). Asterisks (*P < 0.05 and **P < 0.01) denote statistically significant differences compared with controls by one-way ANOVA with Dunnett's t-test using SPSS software version 22 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Isolation of Manilkara zapota seed lectin
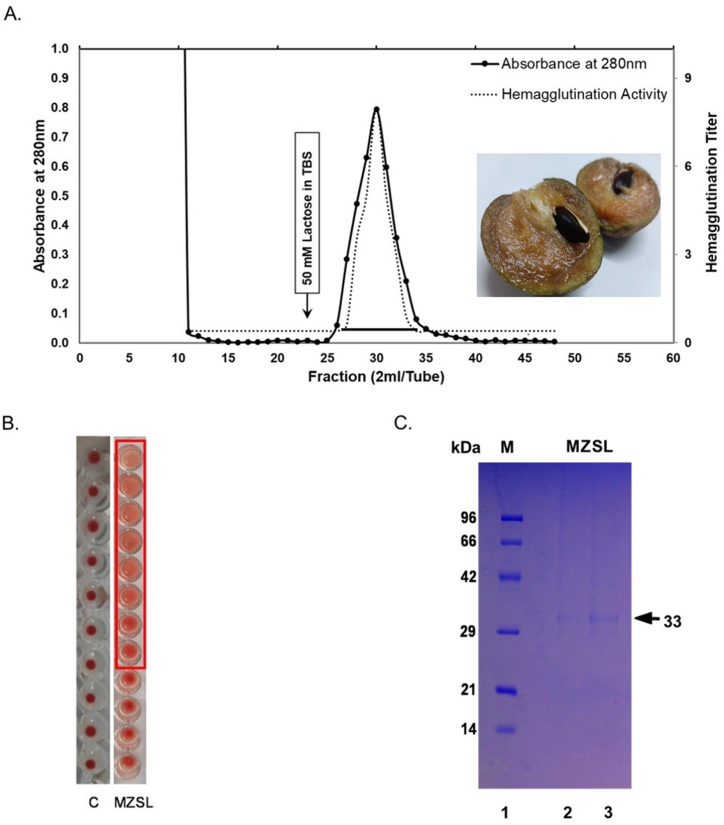
The lectin from Manilkara zapota seeds was purified following the method described above (Fig. 1A). The protein was termed as Manilkara zapota seed lectin (MZSL) and was eluted. The elution profile was monitored at 280 nm and after its elution from the affinity column, the lectin was dialyzed for 48 h against water and TBS to remove free sugars. Purification profile of MZSL is shown in Table 1. Concentration of the purified lectin was determined considering bovine serum albumin as standard [36].
Fig. 1.
(A) Crude protein sample (100 ml) was subjected to 100 % ammonium sulphate precipitation. Fractions with hemagglutination activity were collected and applied to a lactose-agarose column. After washing with TBS, fractions (2 ml/tube) were eluted (flow rate: 1 ml/min). (B) Hemagglutination activity of MZSL (C) The eluted fraction (MZSL) showed strong hemagglutination activity and SDS-PAGE of the lectin was performed in 15 % (w/v) polyacrylamide gel. Lane 1 (M): Marker proteins, Lane 2 and 3: Purified MZSL. Uncropped and unprocessed image of the polyacrylamide gel can be found as the supplementary material (Supplementary File).
Table 1.
Purification of Manilkara zapota seed Lectin.
| Purification step | Total protein (mg) | Total activity (HU) | Specific activity (HU/mg) | Purification Fold |
% of Yield (Per step) |
|---|---|---|---|---|---|
| Crude extract | 30,000 | 7,20,000 | 24 | 1 | 100 |
| Ammonium sulphate precipitation | 600 | 96,000 | 160 | 6.66 | 13.33 |
| Affinity chromatography | 19.5 | 8192 | 420 | 17.5 | 1.13 |
The given data is calculated for 100 g seeds. Mice RBCs were used for the hemagglutination assay.
3.2. Hemagglutination activity of MZSL
To confirm the presence of lectins, hemagglutination activity was performed. Five wells of serially diluted solutions of MZSL in the microtiter plate showed agglutination activity with mice erythrocytes (Fig. 1B). The minimum agglutination concentration was determined to be 15.625 μg/ml.
3.3. Molecular weight of MZSL
Depending on the movement of marker proteins like Phosphorylase B (96 kDa), Bovine serum albumin (66 kDa), Ovalbumin (42 kDa), Carbonic anhydrase (29 kDa), Trypsin inhibitor (21 kDa) and Lysozyme (14 kDa), molecular weight of MZSL was determined. A single band was detected as MZSL and the calculated molecular weight of MZSL was 33.0 ± 1 kDa (Fig. 1C).
3.4. Sugar content in MZSL
MZSL was glycoprotein in nature as the phenol-sulphuric acid test gave orange-yellow colour and the neutral sugar content of this lectin was determined to be 6.32 %.
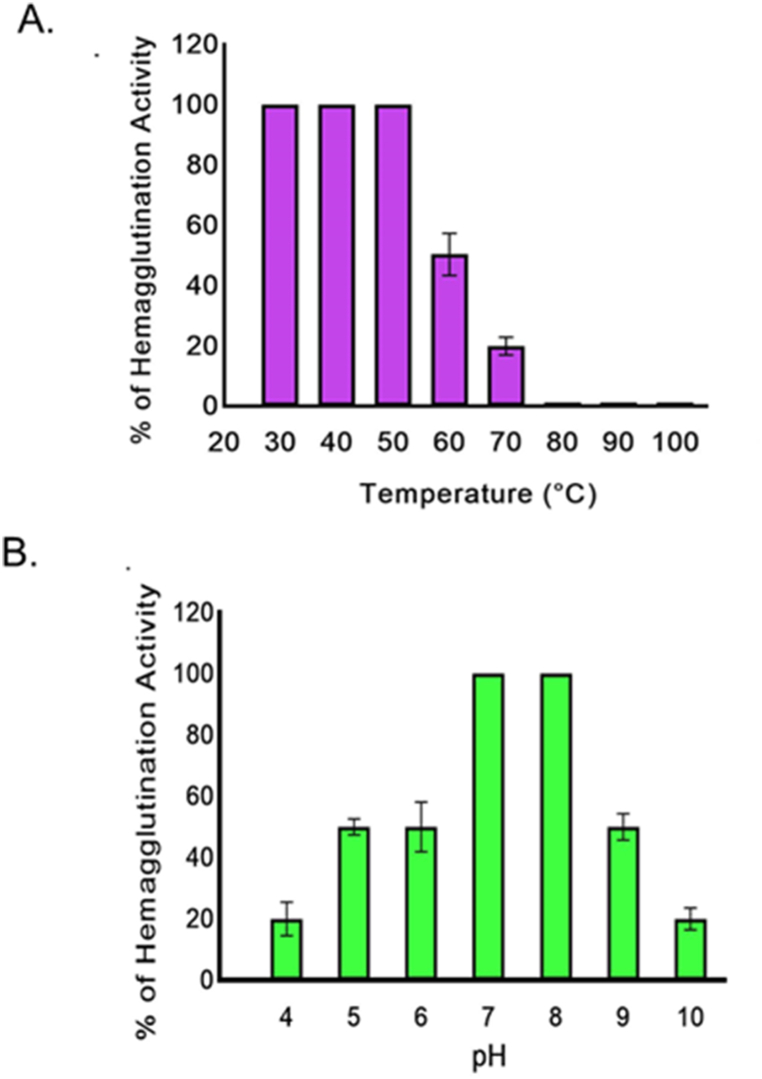
3.5. Temperature and pH stability of MZSL
Hemagglutination activity of MZSL did not reduce at the temperature range of 30–50 °C. The activity suddenly decreased to 50 % and 20 % at 60 and 70 °C, respectively and was lost completely at higher temperatures (80–100 °C) (Fig. 2A). The lectin was fully active at pH 7.0–8.0, decreased to 50 % at pH 5.0, 6.0 and 9.0 whereas only 20 % activity was observed at pH 4.0 and 10.0 (Fig. 2B).
Fig. 2.
Consequences of increasing (A) temperature and (B) pH on the hemagglutination activity of MZSL. Error bars: Results of triplicate experiments have been presented with standard errors (SE). Data are presented as mean ± SE (n = 3).
3.6. Effect of divalent metal ions on the activity of MZSL
MZSL lost its activity completely when treated with 100 mM EDTA. After the addition of 25 mM of salt solutions of Ca2+, Mn2+ and Mg2+, hemagglutination activity of the lectin was almost recovered. Surprisingly, after the reconstitution of Ca2+, the hemagglutination titer surpassed the value we got for the native protein (Table 2).
Table 2.
Hemagglutination activity of MZSL after demetallization and addition of metal ions.
| Native Lectin | Chelated Lectin | Chelated lectins after reconstitution with metal ions | ||
|---|---|---|---|---|
| Hemagglutination Titer | Ca2+ | Mg2+ | Mn2+ | |
| 2−13 ± 0 | 0 | 2−16± 0 | 2−12± 0 | 2−10± 0 |
Values are expressed in mean ± SEM. Three independent tests were performed for each experiment.
3.7. Toxicity of MZSL against Artemia salina nauplii
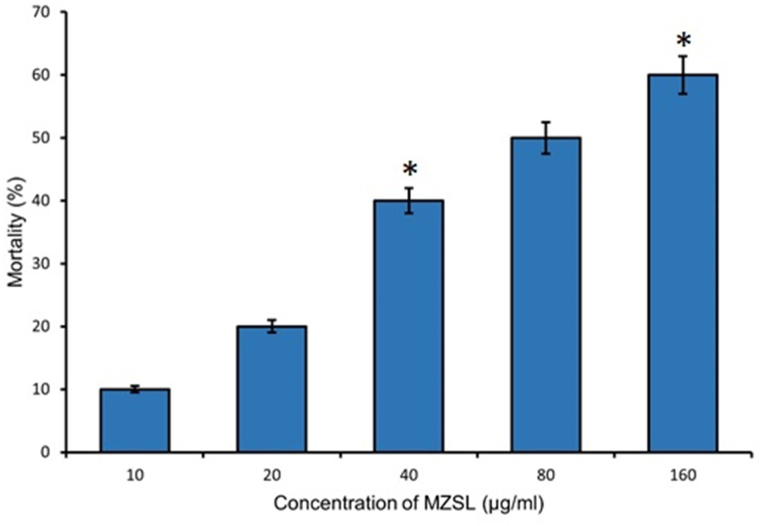
Toxicity of MZSL against Artemia salina nauplii was determined. Number of dead shrimp nauplii raised with increasing concentrations of the lectin and the maximum mortality of nauplii occurred at a concentration of 160 μg/ml. The estimated LC50 value was 107.93 μg/ml by Probit analysis (Fig. 3).
Fig. 3.
Mortality percentage of Artemia salina nauplii treated with various concentrations of MZSL after an exposure for 24 h. Error bars: Results of triplicate experiments have been presented with standard errors (SE). Data are presented as mean ± SE (n = 3). Asterisks (*P < 0.05) denote statistically significant differences compared to controls.
3.8. Determination of bacteriostatic and fungistatic activity of MZSL
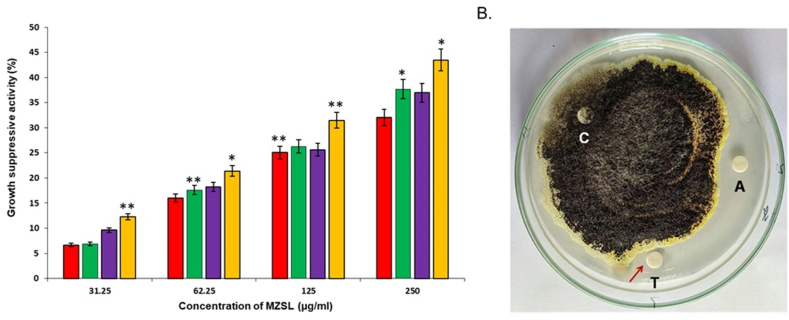
MZSL suppressed the growth of Shigella boydii (ATCC 231903), Shigella dysenteriae (ATCC 238135), Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 27853) in a dose-dependent manner. The lectin has 43.48 % growth suppressive effect against Staphylococcus aureus, 37 % against Escherichia coli, 36.95 % against Shigella dysenteriae but only 32.03 % against Shigella boydii at a concentration of 250 μg/ml (Fig. 4A). MZSL also suppressed the growth of Aspergillus niger when applied at a dose of 100 μg/disc (Fig. 4B).
Fig. 4.
Antimicrobial activity of MZSL. A. Inhibition of bacterial growth by MZSL. Red, green, purple and yellow columns indicate the growth reduction of Staphylococcus aureus, Escherichia coli, Shigella dysenteriae and Shigella boydii, respectively. B. Fungistatic activity of MZSL against Aspergillus niger. A and T represent the discs soaked with 100 μg of MZSL and Antifungal agent (1 % Clioquinol + 0.02 % Flumetasone Pivalate), respectively. C is the control disc with no MZSL. Error bars: Results of triplicate experiments have been presented with standard errors (SE). Data are presented as mean ± SE (n = 3). Asterisks (*P < 0.05, **p < 0.01) denote statistically significant differences compared to controls.
3.9. Bacterial agglutination and antibiofilm activity against Escherichia coli by MZSL
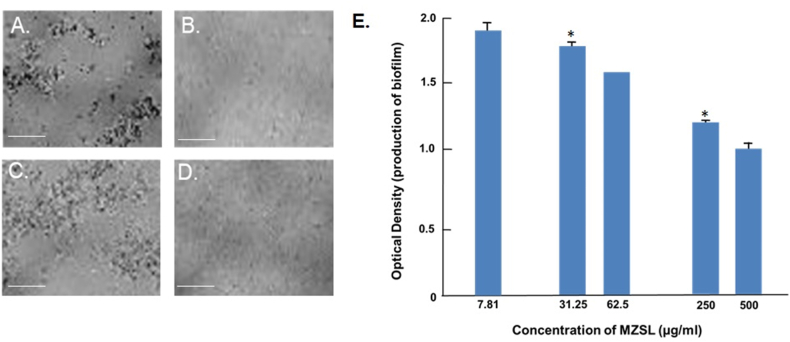
MZSL agglutinated two pathogenic bacteria (Staphylococcus aureus and Shigella dysenteriae) at a concentration of 500 μg/ml (Fig. 5A and 5C), whereas no agglutination was observed without MZSL (Fig. 5B and 5D). The lectin dose-dependently inhibited the growth of biofilm produced by Escherichia coli (Fig. 5E).
Fig. 5.
Agglutination and antibiofilm activity MZSL. A and B. Agglutination of Staphylococcus aureus by MZSL at a concentration of 500 and 0 μg/ml, respectively. C and D. Agglutination of Shigella dysenteriae by MZSL at a concentration of 500 and 0 μg/ml, respectively. Scale bar: 50 μm. E. Antibiofilm activity of MZSL against Escherichia coli. Error bars: Results of triplicate experiments have been presented with standard errors (SE). Data are presented as mean ± SE (n = 3). Asterisks (*P < 0.05) denote statistically significant differences compared to controls.
3.10. Determination of antioxidant activity by DPPH scavenging assay
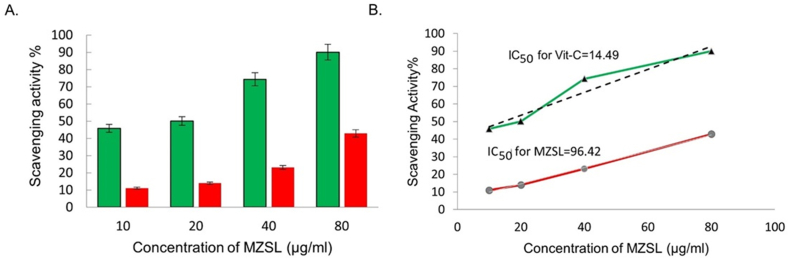
The absorption maxima of the free radical DPPH with an odd electron are 517 nm. Antioxidants react with DPPH in the presence of hydrogen donors and become reduced to DPPHH, resulting a drop in DPPH absorbance. The effect of various MZSL concentrations, as well as ascorbic acid as a control, on DPPH radical antioxidant activity is depicted in Fig. 6A. For a concentration of 80 μg/ml of MZSL, the activity was found to be 42.83 % while Vitamin C activity was found to be 87.57 %. The IC50 values for vitamin C and MZSL were 14.49 μg/ml and 96.42 μg/ml, respectively which revealed MZSL has mild antioxidant activity comparing to the standard (Fig. 6B).
Fig. 6.
DPPH scavenging activity of MZSL. A. Green and red colored columns showed the concentration-dependent scavenging activity of vitamin C and MZSL. B. IC50 values of vitamin C and MZSL. Error bars: Results of triplicate experiments have been presented with standard errors (SE). Data are presented as mean ± SE (n = 3).
3.11. In vitro antiproliferative activity of MZSLs against EAC cells by MTT assay
From the MTT assay it became evident that EAC cell death was induced by the dose dependent effect of MZSL. Growth inhibition of EAC cells were concentration dependent whereas the highest growth inhibition was 21.64 % at a concentration of 80 μg/ml. In addition, 11.98 %, 11.04 % and 6.94 % growth inhibition were observed at a concentration of 40, 20 and 10 μg/ml, respectively. The lowest growth inhibition was found to be 5.30 % at a concentration of 5 μg/ml (Fig. 7A).
Fig. 7.
Antiproliferative activity of MZSL. A. Growth inhibition of EAC cells after treating with MZSL for 24 h. B. Effect of MZSL on the growth of MCF-7 (red bar) and A-549 (green bar) cell lines. Error bars: Results of triplicate experiments have been presented with standard errors (SE). Data are presented as mean ± SE (n = 3). Asterisks (*P < 0.05, **p < 0.01) denote statistically significant differences compared to controls.
3.12. Antiproliferative activity (in vitro) of MZSL against human cancer cell lines (MCF-7 and A-549) by MTT assay
MZSL showed promising antiproliferative activity against breast cancer cell line (MCF-7). In both cases, MZSL induced the death of MCF-7 cell line in a dose-dependent manner as shown in Fig. 7B. MZSL showed about 51.2 %, 41.25 %, 34.41 %, 24.65 % and 18.83 % of growth inhibition when incubated at 80, 40, 20, 10 and 5 μg/ml concentrations for 72 h, respectively. In this case, the calculated IC50 value was 70.66 μg/ml. The lectin showed moderate antiproliferative activity against lung cancer cell line (A-549) with IC50 value of 107.64 μg/ml and inhibited 40.3 %, 31.65 %, 26.22 %, 22.87 % and 14.73 % of growth against this cell line when incubated at 80, 40, 20, 10 and 5 μg/ml of concentrations for 72 h, respectively (Fig. 7B).
4. Discussion
MZSL was isolated from Manilkara zapota seeds by a lactosyl-agarose affinity chromatographic column with an apparent molecular weight of 33.0 ± 1 kDa. Another lactose-binding lectin from the fleshy part of Manilkara zapota fruits with the same molecular weight has been reported as well [26]. We came across with galactose-binding lectins in seeds and leaves of Pouteria torta and Chrysophyllum cainitol, two other members of the Sapotaceae family, with molecular weights of 14 and 33 kDa, respectively [27,30]. But labramin (19 kDa), another seed lectin from Labramia bojeri of the same family showed specificity to glycoproteins and N-acetylglucosamine [13]. Using ion-exchange and gel filtration chromatography, a lectin named AGL was extracted from Amaranthus gangeticus seeds with a molecular weight of 15 kDa [20]. A lectin from Trichosanthes dioica seeds (57 ± 2 kDa) was successfully isolated with galactose-sepharose-4B affinity column [21] whereas the 17 kDa lectin from Moringa oleifera seeds was purified using a chitin column [17]. Therefore, several distinct seed lectins with varied molecular weights and glycan specificity had been purified following different isolation procedures.
When treated with 2 % mouse erythrocytes, the minimum agglutination concentration of MZSL was found to be 15.625 μg/ml. Pouterin agglutinated human and rabbit erythrocytes at a concentration of 3.6 μg/ml [27]. In case of the lectin from Manilkara Zapota fruits, the minimum hemagglutination concentration for human erythrocytes was 10 μg/ml [26]. Below and above the temperature range of 30–50 °C, hemagglutination activity of MZSL decreased significantly. Similarly, Pouterin, CCL (Chrysophyllum cainitol lectin) and Vitellaria paradoxa seed lectin were found to be heat stable up to 60–70 °C and lost their activity at higher temperatures [27,30,37]. Manilkara fruit lectin was comparatively less stable, losing the activity completely at or above 40 °C. MZSL, CCL and Vitellaria paradoxa seed lectin were fully active at a narrower range of pH (6.0–9.0) comparing to that of Pouterin (5.0–10.0) and Manilkara fruit lectin (9.0–13.0) [26,27,30,37]. Both MZSL and Manilkara fruit lectins showed enhanced activity in the presence of divalent metal ions whereas CCL's activity did not depend on metal ions [26,30]. Demetallization of lectins brought conformational changes, causing alteration in the glycan-binding activity of these proteins [38]. Reconstitution of divalent metal ions allowed MZSL not only to recover that activity (Mg2+ and Mn2+) but also to exceed (Ca2+) that of the native lectin.
Varying percentages of neutral sugar content were observed in seed lectins isolated from plants included in the Sapotaceae family. The percentage was low in MZSL (6.32 %) if compared to that of Pouterin (22 %) whereas no sugar was detected in Vitellaria paradoxa seed lectin [27,37]. Though from a different family, a lactose specific seed lectin from Erythrina senegalensis showed its glycoprotein nature with a neutral sugar content of 6.5 % [39]. Other physicochemical properties like temperature and pH stability and effect of metal cations on biological activity of this seed lectin were also similar to MZSL. Until now, not many lectins have been evaluated in the Artemia lethality test which is a suitable preliminary toxicity assay. According to this assay, LC50 values of five seed lectins from different species of Canavalia genus have been determined to be 54.38–376.48 μg/ml [40]. Other seed lectins from Moringa oleifera, Trichosanthes dioica and Momordica charantia were found to be more cytotoxic, with LC50 values of 131, 84 and 49.7 μg/ml [17,21,41]. Therefore, in terms of toxicity, MZSL was mildly toxic against Artemia with an LC50 value of 107.93 μg/ml.
Bacteriostatic activity of MZSL demonstrated that it may act as a self-defense chemical compound against pathogenic bacteria. Lectins are reported to exert their bacteriostatic effect through the interaction with glycans present on bacterial outer surface, probably blocking the biosynthesis of cell wall components, proteins and nucleic acids [11]. The gram-positive S. aureus (ATCC 25923) was comparatively more sensitive to MZSL than three other gram-negative bacteria: E. coli (ATCC 27853), S. dysenteriae (ATCC 238135) and S. boydii (ATCC 231903). Another galactose binding seed lectin from Vatairea macrocarpa also showed bactericidal activity against S. aureus [42]. The antibacterial activity of Eugenia uniflora seed lectin (EuniSL) against gram-positive and gram-negative bacteria was very much in line with the present study [43]. The lectin from Moringa oleifera seeds reduced the growth of S. aureus leaving E. coli unaffected [44]. Interestingly, antibacterial activity of CCL, a galactose-specific lectin, was just opposite. It inhibited the growth of E. coli but had no effect on S. aureus [30].
Most of the antifungal lectins bind to chitin or N-acetyl-d-glucosamine, but a few of those were specific to other sugars, especially to glucose, mannose and galactose [11]. MZSL mildly suppressed the growth of Aspergillus niger though Pouterin strongly inhibited the growth of Saccharomyces cerevisae, Fusarium oxysporum and Colletotrichum musae [27]. Another galactose-binding seed lectin from Bauhinia ungulate also exerted potent antifungal activity against A. niger [22]. MZSL also agglutinated Staphylococcus aureus and Shigella dysenteriae to different degrees and inhibited the formation of biofilm by E. coli. Capability of producing biofilm helps bacteria to survive in adverse conditions. Lectins are known to alter the biosynthesis and polymerization of biofilm matrix and inhibition of signaling pathways [45]. Antibiofilm activity of seed lectins have been observed previously from Canavalia marítima, Moringa oleifera and Amaranthus gangeticus against biofilms produced by Streptococcus mutans, Bacillus sp., Serratia marcescens and E. coli [20,46,47].
Seeds are rich sources of bioactive proteins with antioxidant activity. But this activity of lectins is not well reported. MZSL exerted mild DPPH scavenging activity in a dose dependent manner and IC50 values for MZSL and vitamin C were 96.42 μg/mL and 14.49 μg/mL, respectively. Another seed lectin from Chickpea (Cicer arietinum) demonstrated promising antioxidant activity with an IC50 values of 0.88 and 0.52 μg/mL for vitamin C [48]. Lectins from Mucuna pruriens seeds and Pleurotus flabellatus mushroom also exhibited antioxidant properties [49,50].
Ehrlich ascites carcinoma (EAC) cells are well known for their propensity to multiply rapidly due to the lack of H2 histocompatibility antigens [51]. In this study, MZSL inhibited the growth of EAC cells up to 21.64 % at a concentration of 80 μg/ml. At higher concentrations (200 and 120 μg/ml), seed lectins from Moringa oleifera and Pisum sativum exerted 71.08 % and 84 % of growth inhibition of these cells, possibly following apoptotic pathways [17,52]. A previous work reported significant anticancer activity of the stem bark of M. zapota extract against EAC cells [53].
When applied against human cancer cell lines, MCF-7 and A-549, antiproliferative activity of MZSL was observed with IC50 values of 70.66 and 107.64 μg/ml, respectively. A seed lectin from Cicer arietinum showed potent anticancer activity against MCF-7 with an IC50 value of 53.54 μg/ml [49]. Much higher activity against A-549 cells was detected by another lectin from Entada rheedii seeds (IC50 = 28 μg/ml) which was purified in combination of ammonium sulphate precipitation and lactose affinity chromatography, similar to MZSL [16]. Two very recent studies with seed and leaf extracts of Manilkara zapota also indicated to their activity against different human cancer cell lines via multiple signaling pathways [54,55].
5. Conclusion
From Manilkara zapota seed, a novel lectin (MZSL) with 33.0 ± 1 kDa was purified and characterized. The main limitation of our study included (i) not performing the biological activity assays in the presence of galactose (inhibitory sugar of MZSL) and (ii) not determining its amino acid sequence and/or three-dimensional structure. A previous study on another seed lectin from Cucurbitaceae family (Snake Gourd seed lectin) showed its crystal structure and interaction with LacNac, Lac, Gal and Me-α-Gal, like MZSL [56]. This seed lectin possessed bacteriostatic, fungistatic, antibiofilm and antioxidant activities whereas moderate antiproliferative activity of MZSL against Ehrlich ascites carcinoma cells, MCF-7 and A-549 cell lines was also observed. If this lectin can identify subtle variations in glycan structures on microbial and cancer cells, it can be a useful tool in biomedical research and therapy, though further investigations are required to explore its chemopreventive properties in more details.
Funding
This study was partly supported by the research grant from Rajshahi University Research Project (Grant No. 297/5/52/R.U/Science-31/2021–2022), University of Rajshahi, Bangladesh.
Declaration of Interest’s statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Ethics approval and consent to participate
Ethical clearance of the experiments using Swiss albino mice was provided by the Institutional Animal, Medical Ethics, Bio-safety and Bio-security Committee (IAMEBBC) for Experimentations on Animals, Human, Microbes and Living Natural Sources (Memo No. 249 (35)/320/IAMEBBC/IBSc), Institute of Biological Sciences (IBSc), University of Rajshahi, Bangladesh.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Munna Kumar Podder: Writing – original draft, Methodology, Investigation, Formal analysis. Md. Mikail Hossain: Investigation, Formal analysis. Syed Rashel Kabir: Validation, Data curation. A. K. M. Asaduzzaman: Validation, Software, Methodology. Imtiaj Hasan: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24592.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
figs1.
References
- 1.Sharon N., Lis H. The Molecular Immunology of Complex Carbohydrates-2. Springer; 2001. The structural basis for carbohydrate recognition by lectins; pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 2.Bhutia S.K., Panda P.K., Sinha N., Praharaj P.P., Bhol C.S., Panigrahi D.P., Mahapatra K.K., Saha S., Patra S., Mishra S.R., Behara B.P., Patil S., Maiti T.K. Plant lectins in cancer therapeutics: targeting apoptosis and autophagy-dependent cell death. Pharmacol. Res. 2019;144:8–18. doi: 10.1016/j.phrs.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Fu L., Zhou C., Yao S., Yu J., Liu B., Bao J. Plant lectins: targeting programmed celldeath pathways as antitumor agents. Int. J. Biochem. Cell Biol. 2011;43:1442–1449. doi: 10.1016/j.biocel.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Gong T., Wang X., Yang Y., Yan Y., Yu C., Zhou R., Jiang W. Plant lectins activate the NLRP3 inflammasome to promote inflammatory disorders. J. Immunol. 2017;198:2082–2092. doi: 10.4049/jimmunol.1600145. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Q.-L., Zhang S., Tian M., Zhang S.‐Y., Xie T., Chen D., Chen Y., He J., Liu J., Ouyang L., jiang X. Plant lectins, from ancient sugar‐binding proteins to emerging anti‐cancer drugs in apoptosis and autophagy. Cell Prolif. 2015;48:17–28. doi: 10.1111/cpr.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lis H., Sharon N. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 7.Pusztai A., Bardocz S., Ewen S.W.B. Uses of plant lectins in bioscience and biomedicine. Front. Biosci. 2008;13:1130–1140. doi: 10.2741/2750. [DOI] [PubMed] [Google Scholar]
- 8.Rillahan C.D., Paulson J.C. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira Figueiroa E., Albuquerque da Cunha C.R., Albuquerque P., de Paula R.A., Aranda-Souza M.A., Alves M.S., Zagmignan A., Carneiro-da-Cunha M.G., Nascimento da Silva L.C., dos Santos Correia M.T. Lectin-carbohydrate interactions: implications for the development of new anticancer agents. Curr. Med. Chem. 2017;24:3667–3680. doi: 10.2174/0929867324666170523110400. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Takahashi M., Gu J., Miyoshi E., Matsumoto A., Kitazume S., Taniguchi N. Functional roles of N‐glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008;99:1304–1310. doi: 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konozy E.H.E., Osman M.E.M. Plant lectin: a promising future anti-tumor drug. Biochimie. 2022;202:136–145. doi: 10.1016/j.biochi.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro A.C., Ferreira R., Freitas R. Plant lectins: bioactivities and bioapplications. Stud. Nat. Prod. Chem. 2018;58:1–42. doi: 10.1016/B978-0-444-64056-7.00001-5. [DOI] [Google Scholar]
- 13.Macedo M.L.R., Oliveira C.F.R., Oliveira C.T. Insecticidal activity of plant lectins and potential application in crop protection. Molecules. 2015;20:2014–2033. doi: 10.3390/molecules20022014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida A.C., da Silva Osterne V.J., Santiago M.Q., Pinto-Junior V.R., Silva-Filho J.C., Lossio C.F., Nascimento F.L.F., Almeida R.P.H., Teixeira C.S., Leal R.B. Structural analysis of Centrolobium tomentosum seed lectin with inflammatory activity. Arch. Biochem. Biophys. 2016;596:73–83. doi: 10.1016/j.abb.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Kabir S. Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J. Immunol. Methods. 1998;212:193–211. doi: 10.1016/s0022-1759(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 16.Naik S., Kumar S. Biochemical Characterization of lactose binding Entadin lectin from Entada rheedii seeds with cytotoxic activity against cancer cell lines. ACS Omega. 2020;5:16430–16439. doi: 10.1021/acsomega.0c00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asaduzzaman A.K.M., Hasan I., Chakrabortty A., Zaman S., Islam S.S., Ahmed F.R.S., Kabir K.M.A., Nurujjaman M., Uddin M.B., Alam M.T., Shaha R.K., Kabir S.R. Moringa oleifera seed lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of Bak and NF-κB gene expression. Int. J. Biol. Macromol. 2018;107:1936–1944. doi: 10.1016/j.ijbiomac.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 18.Brustein V.P., Souza-Araujo F.V., Vaz A.F.M., Araujo R.V.S., Paiva P.M.G., Coelho L., Carneiro-Leao A.M.A., Teixeira J.A., Carneiro-da-Cunha M.G., Correia M.T.S. A novel antimicrobial lectin from Eugenia malaccensis that stimulates cutaneous healing in mice model. Inflammopharmacology. 2012;20:315–322. doi: 10.1007/s10787-011-0113-5. [DOI] [PubMed] [Google Scholar]
- 19.de Souza Carvalho A., da Silva M.V., Gomes F.S., Paiva P.M.G., Malafaia C.B., da Silva T.D., de Melo Vaz A.F., da Silva A.G., de Souza Arruda I.R., Napoleao T.H., das Carneiro-da-Cunha M., dos Santos Correia M.T. Purification, characterization and antibacterial potential of a lectin isolated from Apuleia leiocarpa seeds. Int. J. Biol. Macromol. 2015;75:402–408. doi: 10.1016/j.ijbiomac.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Hasan I., Rahman S.N., Islam M.M., Ghosh S.K., Mamun M.R., Uddin M.B., Shaha R.K., Kabir S.R. A N-acetyl-D-galactosamine-binding lectin from Amaranthus gangeticus seeds inhibits biofilm formation and Ehrlich ascites carcinoma cell growth in vivo in mice. Int. J. Biol. Macromol. 2021;181:928–936. doi: 10.1016/j.ijbiomac.2021.04.052. [DOI] [PubMed] [Google Scholar]
- 21.Islam S.S., Karim M.R., Asaduzzaman A.K.M., Alam A.H.M.K., Mahmud Z.H., Kabir S.R. Trichosanthes dioica seed lectin inhibits Ehrlich ascites carcinoma cells growth in vivo in mice by inducing G0/G1 cell cycle arrest. J. Food Biochem. 2021;45 doi: 10.1111/jfbc.13714. [DOI] [PubMed] [Google Scholar]
- 22.Silva H.C., Pinto L. da S., Teixeira E.H., Nascimento K.S., Cavada B.S., Silva A.L.C. BUL: a novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process Biochem. 2014;49:203–209. doi: 10.1016/j.procbio.2013.10.020. [DOI] [Google Scholar]
- 23.Wang W., Li Q., Wu J., Hu Y., Wu G., Yu C., Xu K., Liu X., Wang Q., Huang W. Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg. Microb. Infect. 2021;10:1519–1529. doi: 10.1080/22221751.2021.1957720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milind P., Preeti M. Chickoo: a wonderful gift from nature. Int. J. Res. Ayurveda Pharm. 2015;6:544–550. [Google Scholar]
- 25.Bangar S.P., Sharma N., Kaur H., Kaur M., Sandhu K.S., Maqsood S., Ozogul F. A review of Sapodilla (Manilkara Zapota) in human nutrition, health, and industrial applications. Trends Food Sci. Technol. 2022;127:319–334. doi: 10.1016/j.tifs.2022.05.016. [DOI] [Google Scholar]
- 26.Chaki S., Shah T., Dutta R. Isolation and characterization of lectin from Carica papaya and Manilkara zapota. Int. J. Pharm. Biol. Sci. 2015;5:31–39. [Google Scholar]
- 27.Boleti A.P., Freire M.D.G.M., Coelho M.B., Da Silva W., Baldasso P.A., Gomes V.M., Marangoni S., Novello J.C., Macedo M.L.R. Insecticidal and antifungal activity of a protein from Pouteria torta seeds with lectin-like properties. J. Agric. Food Chem. 2007;55:2653–2658. doi: 10.1021/jf0636317. [DOI] [PubMed] [Google Scholar]
- 28.Boleti A.P., Ventura C.A. de A., Justo G.Z., Silva R.A., de Sousa A.C.T., Ferreira C.V., Yano T., Macedo M.L.R. Pouterin, a novel potential cytotoxic lectin-like protein with apoptosis-inducing activity in tumorigenic mammalian cells. Toxicon. 2008;51:1321–1330. doi: 10.1016/j.toxicon.2008.03.007. 32. [DOI] [PubMed] [Google Scholar]
- 29.Teodoro Martinez D.S., das Freire Gracas Machado M., Mazzafe P., Araujo-Junior R.T., Delmond Bueno R., Rodrigues Macedo M.L. Insecticidal effect of labramin, a lectin–like protein isolated from seeds of the beach apricot tree, Labramia bojeri, on the Mediterranean flour moth, Ephestia kuehniella. J. Insect Sci. 2012;12:62. doi: 10.1673/031.012.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madayi D., Sreekala S., Mohan M., Deepthi V.C., Anusha T.S., Elyas K.K. Partial purification and characterization of a galactose specific lectin from Chrysophyllum cainitol, a plant that shows hypoglycemic activity. Int. J. Pharmaceut. Sci. Rev. Res. 2016;41:263–268. [Google Scholar]
- 31.Menendez-Rey A., Gonzalez-Martos R., Ye P., Quiroz-Troncoso J., Alegría-Aravena N., Sanchez-Diez M., Maestu-Unturbe C., Bensadon-Naeder L., Ramirez-Castillejo C. Quantification of lectins in Synsepalum dulcificum and comparison with reference foods. Food Chem. 2021;352 doi: 10.1016/j.foodchem.2021.129341. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson H.M., Trust T.J. Hemagglutination properties and adherence ability of Aeromonas hydrophila. Infect. Immun. 1980;27:938–946. doi: 10.1128/iai.27.3.938-946.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Zhang B., Ibrahim S.A., Gao S.S., Yang H., Huang W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016;145:71–77. doi: 10.1016/j.carbpol.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Finney D.J. third ed. vol. 60. Cambridge University Press; 1971. p. 1432. (Probit Analysis). J Pharm Sci. [DOI] [Google Scholar]
- 35.Kedare S.B., Singh R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein estimation with bovine serum albumin as standard. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 37.Nafiu M.I., Abdullahi R.I., Lawal N., Bayero U., Kontagora G.F. Biochemical charactarization of lectin isolated from the seeds of shear butter tree (Vitellaria paradoxa) Int. J. Sci. Glob. Sustain. 2021;7:11. [Google Scholar]
- 38.Valadez-Vega C., Lugo-Magana O., Betanzos-Cabrera G., Villagomez-Ibarra J.R. Partial characterization of lectins purified from the surco and vara (furrow and rod) varieties of black Phaseolus vulgaris. Molecules. 2022;27:8436. doi: 10.3390/molecules27238436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuku A., Odekanyin O.O., Okonji R.E. Physicochemical properties of a lactose specific lectin from the seeds of Erythrina senegalensis DC. IFE J. Sci. 2012;14:143–153. [Google Scholar]
- 40.Arruda F.V.S., Melo A.A., Vasconcelos M.A., Carneiro R.F., Barroso-Neto I.L., Silva S.R., Pereira-Junior F.N., Nagano C.S., Nascimento K.S., Teixeira E.H., Saker-Sampaio S., Cavada B.S., Sampaio A.H. Toxicity and binding profile of lectins from the Genus canavalia on brine shrimp. BioMed Res. Int. 2013 doi: 10.1155/2013/154542. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabir S.R., Nabi M., Nurujjaman M., Reza M., Alam A.H.M., Zaman R.U., Khalid-Bin-Ferdaus K.M., Amin R., Khan M.M.H., Hossain M.A., Uddin M.S., Mahmud Z.H. Momordica charantia seed lectin: toxicity, bacterial agglutination and antitumor properties. Appl. Biochem. Biotechnol. 2015;175:2616–2628. doi: 10.1007/s12010-014-1449-2. [DOI] [PubMed] [Google Scholar]
- 42.Santos V.F., Costa M.S., Campina F.F., Rodrigues R.R., Santos A.L.E., Pereira F.M., Batista K.L.R., Silva R.C., Pereira R.O., Rocha B.A.M., Coutinho H.D.M., Teixeira C.S. The Galactose-Binding lectin isolated from Vatairea macrocarpa seeds enhances the effect of antibiotics against Staphylococcus aureus–resistant strain, Probiotics Antimicrob. Proteins. 2020;12:82–90. doi: 10.1007/s12602-019-9526-z. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira M.D.L., Andrade C.A.S., Santos‐Magalhaes N.S., Coelho L., Teixeira J.A., Carneiro‐da‐Cunha M.G., Correia M.T.S. Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol. 2008;46:371–376. doi: 10.1111/j.1472-765X.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira R.S., Napoleao T.H., Santos A.F.S., Sa R.A., Carneiro‐da‐Cunha M.G., Morais M.M.C., Silva‐Lucca R.A., Oliva M.L.V., Coelho L.C.B.B., Paiva P.M.G. Coagulant and antibacterial activities of the water‐soluble seed lectin from Moringa oleifera. Lett. Appl. Microbiol. 2011;53:186–192. doi: 10.1111/j.1472-765X.2011.03089.x. [DOI] [PubMed] [Google Scholar]
- 45.Souza G.M., de Oliveira Vieira K.C., Naldi L.V., Pereira V.C., Winkelstroter L.K. Nanotechnology for Advances in Medical Microbiology. Springer; 2021. Green synthesized nanoparticles as a promising strategy for controlling microbial biofilm; pp. 1–28. [DOI] [Google Scholar]
- 46.Cavalcante T.T.A., Carneiro V.A., Neves C.C., de Queiroz Martins M.G., Arruda F.V.S., de Vasconcelos M.A., dos Santos H.S., da Silva Cunha R.M., Cavada B.S., Teixeira E.H. A ConA-like lectin isolated from Canavalia maritima seeds alters the expression of genes related to virulence and biofilm formation in Streptococcus mutans. Adv. Biosci. Biotechnol. 2013;4:1073–7078. doi: 10.4236/abb.2013.412143. [DOI] [Google Scholar]
- 47.Moura M.C., Trentin D.S., Napoleao T.H., Primon‐Barros M., Xavier A.S., Carneiro N.P., Paiva P.M.G., Macedo A.J., Coelho L.C.B.B. Multi‐effect of the water‐soluble Moringa oleifera lectin against Serratia marcescens and Bacillus sp.: antibacterial, antibiofilm and anti‐adhesive properties. J. Appl. Microbiol. 2017;123:861–874. doi: 10.1111/jam.13556. [DOI] [PubMed] [Google Scholar]
- 48.Gautam A.K., Gupta N., Narvekar D.T., Bhadkariya R., Bhagyawant S.S. Characterization of chickpea (Cicer arietinum L.) lectin for biological activity. Physiol. Mol. Biol. Plants. 2018;24:389–397. doi: 10.1007/s12298-018-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murugesan A., Mohanraj K.G., Wungpam Shimray K., Khan M.Z.I., Seppan P. Therapeutic potential of Mucuna pruriens (Linn.) on high-fat diet-induced testicular and sperm damage in rats. Avicenna J. Phytomedicine. 2022;12:489–502. doi: 10.22038/AJP.2022.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto I.R., Chaves H.V., Vasconcelos A.S., de Sousa F.C.F., Santi-Gadelha T., de Lacerda J.T.J.G., Ribeiro K.A., Freitas R.S., Maciel L.M., Viana A.F.S.C., de Almeida Gadelha C.A., Filho G.C., de Paulo Teixeira Pinto V., Pereira K.M.A., Silva A.A.R.E., Bezerra M.M. Antiulcer and antioxidant activity of a lectin from Mucuna pruriens seeds on ethanol-induced gastropathy: involvement of alpha-2 adrenoceptors and prostaglandins. Curr. Pharmaceut. Des. 2019;25:1430–1439. doi: 10.2174/1381612825666190524081433. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Watkins J.F. Evidence against the presence of H2 histocompatibility antigens in Ehrlich ascites tumour cells. Nature. 1970;225:734–735. doi: 10.1038/225734a0. [DOI] [PubMed] [Google Scholar]
- 52.Kabir S.R., Nabi M.M., Haque A., Zaman R.U., Mahmud Z.H., Reza M.A. Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine. 2013;20:1288–1296. doi: 10.1038/225734a0. [DOI] [PubMed] [Google Scholar]
- 53.Osman M.A., Rashid M.M., Aziz M.A., Habib M.R. Inhibition of Ehrlich ascites carcinoma by Manilkara zapota L. stem bark in Swiss albino mice. Asian Pac. J. Trop. Biomed. 2011;1:448–451. doi: 10.1016/S2221-1691(11)60098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saradha S., Debbie J.V., Meera M., Ruckmani A., Arunkumar R. Anti-cancer activity of Manilkara zapota seed and skin extracts on hela cell lines. J. Posit. Sch. Psychol. 2022;6:10422–10427. [Google Scholar]
- 55.Tan B.L., Norhaizan M.E., Chan L.C. ROS-mediated mitochondrial pathway is required for Manilkara zapota (L.) P. Royen leaf methanol extract inducing apoptosis in the modulation of caspase activation and EGFR/NF-κB activities of HeLa human cervical cancer cells, Evidence-Based Complement. Alternative Med. 2018 doi: 10.1155/2018/6578648. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandran T., Sivaji N., Surolia A., Vijayan M. Ligand binding and retention in snake gourd seed lectin (SGSL), a crystallographic, thermodynamic and molecular dynamics study. Glycobiology. 2018;28:968–977. doi: 10.1093/glycob/cwy072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.