Abstract
Background
The absolute and relative benefits of adjuvant bisphosphonates on disease-free survival and overall survival in patients receiving contemporary systemic therapy for early breast cancer is uncertain.
Methods
Data from randomized trials of adjuvant bisphosphonates that recruited patients exclusively after 2000 and reported disease free survival and overall survival was utilized. Five-year disease-free survival and overall survival in bisphosphonates and control group along with associated hazard ratios were extracted. Absolute data were weighted by sample size and hazard ratios were pooled using inverse variance and random effects modelling. Meta-regression comprising linear regression weighted by sample size (mixed effects) was performed to explore association between disease and treatment related factors and absolute differences in benefit from bisphosphonates.
Results
Eleven trials comprising 24023 patients were included in the analysis. For disease free survival, pooled hazard ratio was 0.89 (0.81–0.97, p = 0.008) with a 1.5 % weighted mean difference favoring bisphosphonates over control. There was no significant overall survival benefit (0.92, 0.82–1.03, p = 0.16). Among patients receiving anthracycline and taxane based chemotherapy, there were no differences in either disease free survival (0.95, 0.80–1.12) or overall survival (1.04, 0.81–1.32). Meta-regression showed lower benefits in higher risk patients (node-positive, larger tumor size, estrogen receptor-, grade 3 or those receiving chemotherapy). Overall, 1 % (95 % CI 0.75–1.15) of patients experienced osteonecrosis of jaw related to zoledronic acid.
Conclusions
Compared to the Early Breast Cancer Trialist's Collaborative Group meta-analysis, benefit from adjuvant bisphosphonates is lower in recent trials especially in higher risk patients receiving contemporary chemotherapy. The balance between benefits and risks of adjuvant bisphosphonates should be considered in individual patients.
Keywords: Adjuvant bisphosphonates, Disease-free survival, Overall survival, Chemotherapy, Meta-analysis, Osteonecrosis of jaw
Highlights
-
•
Benefits of adjuvant bisphosphonates are less certain with contemporary therapy.
-
•
Meta-Analysis of contemporary trials showed a small DFS (HR 0.89, 0.81–97) benefit.
-
•
There was no OS benefit (HR 0.92, 0.82–1.03) with adjuvant bisphosphonates.
-
•
Both absolute and relative benefits were smaller than reported in the EBCTCG analysis.
-
•
1 % of patients receiving zoledronic acid developed osteonecrosis.
Abbreviations
- EBCTCG
Early Breast Cancer Trialists Collaborative Group
- HR
Hazard ratio
- ASCO
American Society of Clinical Oncology
- ESMO
European Society of Medical Oncology
- DFS
Disease free survival
- OS
Overall survival
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ONJ
osteonecrosis jaw
1. Introduction
There is increased risk of skeletal complications in patients with early breast cancer including bone loss, fractures and bone metastasis. Moreover, risk of systemic recurrence outside bone and death from breast cancer remains elevated long after completion of definitive therapy especially in hormone receptor positive breast cancer [1] Bisphosphonates are bone modifying agents with a strong pre-clinical rationale for efficacy in prevention of metastatic recurrence [2] Based on the pre-clinical rationale, multiple randomized trials have tested various bisphosphonates as adjuvant therapy in early breast cancer. Data from these trials was variable and therefore, a synthesis by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) in which individual patient data were pooled showed a modest reduction in bone recurrence [hazard ratio (HR) 0.83, p = 0.04] and breast cancer-specific mortality (HR 0.91, p = 0.04). This benefit was limited to post-menopausal women [3] There was no effect on local recurrence or recurrence outside bone. Inclusion of older trials with bone density as primary end point, heterogenous definition of menopause, variable chemotherapy exposure especially modern agents such as taxanes, and non-standardized control arms were some limitations of the studies included in the meta-analysis [[4], [5], [6], [7], [8]].
In the modern era, breast cancer events are substantially lower than those observed over a decade ago. This likely reflects improvements in screening, surgery, more effective chemotherapy (e.g. anthracycline and taxanes) and endocrine therapy (e.g. aromatase inhibitors) [9].
International guidelines including American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) recommend a discussion of adjuvant bisphosphonates in post-menopausal women irrespective of hormone receptor status with individualised decisions based on risk of recurrence [10,11]. However, concerns have been raised about the uncertain absolute benefit of these treatments, the practicalities of giving repeat infusions and toxicities, leading to variable uptake [12,13]. At the 2019 St Galen Consensus conference, only 42.6 % of the expert panel reported routine use of adjuvant bisphosphonates in their practise despite previous strong endorsement for their use. Routine use is even lower across Ontario [14]. In addition, the results of a recent phase III trial in post-menopausal patients was negative raising questions about efficacy in the current era [15].
Given the variable uptake and the uncertain benefit in the setting of contemporary adjuvant therapy, we performed a meta-analysis of randomized trials of adjuvant bisphosphonates in which contemporary systemic therapy was used. The primary outcome was the impact of bisphosphonates on disease-free survival (DFS) and overall survival (OS). We also planned to explore toxicity as a key secondary endpoint.
2. Methods
2.1. Literature review and study identification
The review and meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16] (Supplementary Table 1-checklist). The studies used to formulate the ASCO guidelines on use of adjuvant bisphosphonates formed the data source for this review [10,17]. AM and FT collected data from each study independently and discrepancies were resolved by discussion with a third reviewer (EA). All data were extracted from primary publications and their associated online appendices.
2.2. Data extraction
The following data were collected from each study: number of patients, year of publication, details of the bisphosphonate used (name of agent, duration and frequency of administration), trial summary data including median age, proportion of pre and post-menopausal patients, proportion of patients with node-positive disease, hormone receptor negative, grade 3 and large tumor size (defined as T3 or T4 disease at baseline). Details regarding chemotherapy including type when available were also recorded. Finally, we collected data on 5-year DFS and OS and absolute number of DFS and OS events in bisphosphonate and control arm. When these estimates were not available as absolute numbers in publications, these were derived from Kaplan Meir survival curves. For analysis of toxicity, absolute number of patients having elevated creatinine, hypocalcemia and osteonecrosis jaw (ONJ) (when available) were collected. The primary outcome measures were 5-year DFS and OS in bisphosphonate and control arm. The most mature data was extracted for publications where 5-year outcomes were not available.
2.3. Data synthesis and statistical analysis
Two authors (AM and FT) reviewed all references of these guidelines independently and identified randomized trials of adjuvant bisphosphonates that accrued patients exclusively beyond 2000 (to reflect contemporary chemotherapy use). For multiple publications, those with most mature follow-up data were selected. Data on 5-year DFS and OS in bisphosphonates and control group arm along with HR (when reported) were extracted along with relevant clinical parameters. Trials where only neoadjuvant bisphosphonates was used or those utilising denosumab were excluded. Descriptive statistics were used to report individual trial characteristics. Confidence intervals of individual study estimates were calculated using confidence interval of one proportion. Heterogeneity was assessed using the I2 statistic. With the study eligibility criteria limited to high quality randomized trials, a formal risk of bias assessment was not performed as based on available quality scales, differentiation of studies would be based almost exclusively on blinding. This was not felt to be a valid criterion for quality assessment. With the method of administration of most bisphosphonates being IV and heterogenous use of placebo control in trials, there would have been inadvertent unblinding had blinding been utilized. Individual studies were weighted by sample size and pooled mean 5-year DFS and OS were calculated. HR for DFS and OS were pooled in a meta-analysis using generic inverse variance and random effects modelling. Trial level absolute differences in DFS and OS between bisphosphonate and control arms were calculated. Meta-regression comprising linear regression weighted by sample size (mixed effects) was performed to explore association between disease and treatment related factors and absolute differences in benefit from bisphosphonates as well as HR [18]. Due to limited power from a small number of included studies, first we performed only univariable analyses as multivariable models could not be fitted adequately. Second, rather than using statistical significance, we explored associations quantitatively using methods described by Burnand et al. [19] Analyses were performed using SPSS version 28 (IBM Corp, Armonk NY) and Review manager v5.4.
3. Results
Eleven trials comprising 24,023 patients were included in the analysis (Table 1A, Table 1BA and 1B). Among these, the SWOG S0307 compared three different bisphosphonates in the adjuvant setting (zoledronic acid, clodronate, ibandronate) and SUCCESS A trial compared different duration of adjuvant zoledronic acid (5 years vs 2 years) [20,21] (Table 1A). Therefore, these trials were included only for calculation of pooled absolute DFS and OS estimates but not hazard ratios. All patients in 4 trials (N = 6248) received anthracycline and taxanes (Table 1A). Six trials reported renal side effects but only two reported on hypocalcemia. CI of pooled estimates could not be estimated for the SUCCESS A and NSABP-34 studies as the total number of events at five years were not reported (Table 1B).
Table 1A.
Clinical characteristics of patients in included studies.
| Study details | Final year of recruitment | No of patients (N) | Bisphosphonate used | Median Age (years) | Node+ (%) | >T3 (%) | Hormone receptor negative (%) | Grade 3 (%) | Receiving chemotherapy (%) | Receiving anthracycline, taxane (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| ZO-FAST [[22], [23], [24]] | 2004 | 1065 | ZA | 57 | 56.8 | NA | 0 | NA | 53.2 % | NA |
| NSABP-B34 [25] | 2005 | 3323 | CL | NA | 25 | 5 | 22 | 35 | 64 | 59,16 |
| AZURE [5,26] | 2006 | 3306 | ZA | 51.4 | 98 | 17.4 | 21 | NA | 100 | 93,23 |
| ABCSG-12 [4,27,28] | 2006 | 1803 | ZA | 45 | 30.4 | NA | 16.1 | 20.9 | 5.4 | NA |
| University of Washington [29,30] | 2006 | 119 | ZA | 50 | 59.6 | NA | 35.2 | 52.1 | 100 | 100,100 |
| SUCCESS-A [20] | 2007 | 3421 | ZA | 53 | 69.7 | 6 | 27.7 | 46.1 | 100 | 100,100 |
| GAIN [31] | 2008 | 2015 | IB | 49 | 100 | 12 | 23 | 46.4 | 100 | 100,100 |
| NATAN [32] | 2009 | 693 | ZA | NA | 72.9 | 15.4 | 20.7 | 31.7 | 100 | 100,100 |
| SWOG S0307 [21] | 2010 | 6097 | ZA,IB, CL | 53 | 50.4 | NA | 21.4 | NA | 79.6 | NA |
| TEAM IIB [15] | 2014 | 1116 | IB | 62 | 50 | 5.8 | NA | 26 | 53.8 | 53.8,37.8 |
| HOBOE [33] | 2015 | 1065 | ZA | 45 | 45.26 | 3.6 | 0 | 33.5 | 62.6 | NA |
NA-not available, ZA-zoledronic acid, IB-ibandronate, C-clodronate, ONJ-osteonecrosis of jaw.
Table 1B.
Outcomes of interest in included studies.
| Study details | 5y DFS bisphosphonates (%) | 5y DFS Control (%) | DFS HR | 5y OS bisphosphonates (%) | 5y OS control (%) | OS HR | ONJ (%) |
|---|---|---|---|---|---|---|---|
| ZO-FAST [[22], [23], [24]] | 92.1 (89.48–94.25) | 88.4 (85.34–90.96) | 0.66 (0.44–0.97) | 95.1 (92.92–96.78) | 93.25 (90.77–95.22) | 0.69 (0.42–1.14) | 1.3 (0.78–3.19) |
| NSABP-B34 [25] | 88 (86.1–90.2) | 87 (85.1–90.2) | 0.91 (0.78–1.07) | 95 (93.1–97.54) | 94 (91.64–96.72) | 0.83 (0.67–1.05) | 0.06 (0–0.34) |
| AZURE [5,26] | 76.9 (75.5–79.55) | 77.1 (75.58–79.63) | 0.94 (0.82–1.06) | 85.4 (83.77–87.19) | 83.1 (81.69–85.3) | 0.93 (0.81–1.08) | 1.7 (1–2.4) |
| ABCSG-12 [4,27,28] | 91.5 (89.54–93.29) | 87.8 (85.5–89.8) | 0.7 (0.51–0.91) | 96.6 (95.28–97.74) | 95.24 (93.64–96.53) | 0.66 (0.41–1.07) | 0 |
| University of Washington [29,30] | 71.6 (58.56–82.55) | 71.2 (57.92–92.24) | 0.98 (0.44–2.15) | 86.6 (75.41–94.06) | 84.75 (73.01–92.78) | 0.98 (0.34–2.8) | 1.7 (0.04–8.94) |
| SUCCESS-A [20] | 85* | NA | NA | 92* | NA | NA | 0.5 (0.27–0.76) |
| GAIN [31] | 80.5 (78.56–82.26) | 81.3(78.8–83.77) | 0.94 (0.77–1.16) | 91* | 92* | 1.04 (0.76–1.42) | 0.1(0.01–0.4) |
| NATAN [32] | 75 (70.04–79.66) | 75.1 (70.2–79.58) | 0.96 (0.71–1.3) | 84 (82.8–90.38) | 87 (81.6–89.21) | 1.19 (0.79–1.79) | 1.5 (0.5–3.5) |
| SWOG S0307 [21] | 87.8 (86.9–88.7) | NA | NA | 92.6 (91.4–93.6) | NA | NA | 0.8 (0.62–1.1) |
| TEAM IIB [15] | 89 (86–91) | 86 (83–88) | 0.97 (0.76–1.24) | 93 (91–95) | 92 (89–94) | 1.1 (0.82–1.98) | 2.1 (1.27–4.24) |
| HOBOE [33] | 91 (87.5–93.8) | 85.1 (82.2–87.7) | 0.6 (0.4–0.87) | 97.7 (95.6–99.02) | 96.1 (94.2–97.3) | 0.55 (0.25–1.23) | 1.1 (0.3–2.86) |
HR-hazard ratio, ONJ-osteonecrosis of jaw, DFS- disease free survival, OS-overall survival, NA-not available.
*confidence intervals of these estimates could not be calculated due to unavailability of number of events.
3.1. Disease free survival
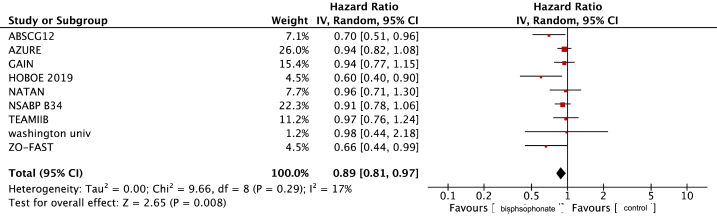
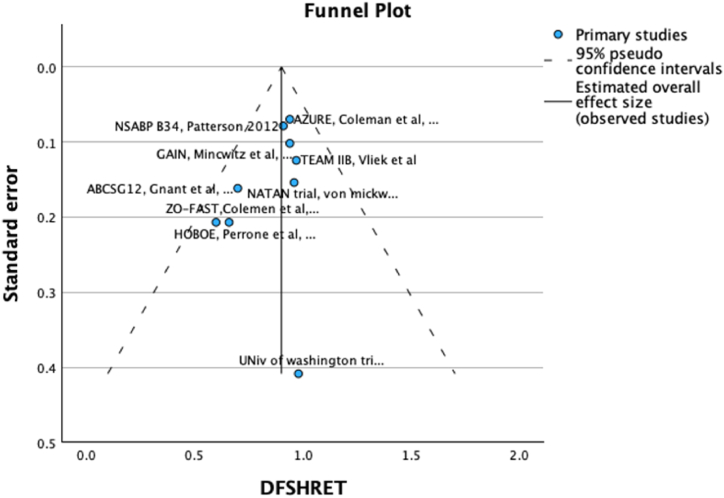
The weighted mean 5-year-DFS for patients receiving bisphosphonates was 84.8 % and was 82.1 % for those in the control arm. The pooled HR for DFS was 0.89 (95 % CI 0.81–0.97, p = 0.008, Fig. 1) with a weighted mean absolute difference of 1.5 %. No publication bias was detected on visual examination of funnel plot (Fig. 2)
Fig. 1.
Forest Plot for Disease free Survival Hazard ratio.
Fig. 2.
Funnel plot for Disease free survival.
In trials where all patients received anthracyclines and taxanes, there was no DFS benefit (HR 0.95, 95 % CI 0.80–1.12, see Supplementary Fig. 1) and a higher number of DFS events at five years in the bisphosphonates group (absolute difference −0.57). Negative quantitative significance for absolute DFS was observed for post -menopausal patients, node positivity, greater tumour size, hormone receptor negative and chemotherapy exposure, see Table 2A). Trends in DFS HR were consistent with absolute DFS results (Table 2A).
Table 2A.
Meta regression analysis for Disease free survival.
| Delta DFS |
DFS hazard ratio |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Beta coefficient | Studies (N) | p value | SE | 95 % CI | Beta coefficient | N studies | p value | SE | 95 % CI |
| Final year of accrual | 0.41 | 9 | 0.27 | 84.98 | −200.54 to 201.36 | −0.12 | 9 | 0.75 | 5.64 | −13.46 to 13.22 |
| Duration of bisphosphonates | 0.19 | 9 | 0.63 | 91.74 | −216.74 to 217.12 | −0.25 | 9 | 0.51 | 5.50 | −13.26 to 12.76 |
| Median age | −0.085 | 7 | 0.86 | 107.5 | −276.42 to 276.25 | 0.41 | 7 | 0.36 | 5.87 | −14.68 to 15.50 |
| Post-menopausal | −0.29 | 9 | 0.45 | 89.46 | −211.83 to 211.25 | 0.48 | 7 | 0.19 | 4.98 | −12.32 to 13.28 |
| pNode+ | −0.62 | 9 | 0.07 | 59.67 | −144.72 to 140.48 | 0.42 | 9 | 0.25 | 4.63 | −10.53 to 11.37 |
| >=pT3 | −0.68 | 5 | 0.14 | 75.72 | −241.65 to 240.29 | 0.46 | 6 | 0.36 | 4.71 | −12.62 to 13.54 |
| HR negative | −0.72 | 7 | 0.06 | 56.65 | −146.34 to 144.90 | 0.82 | 7 | 0.023 | 2.97 | −6.81 to 8.45 |
| Grade 2 | 0.21 | 6 | 0.68 | 94.33 | −261.69 to 262.11 | −0.11 | 5 | 0.84 | 5.16 | −16.53 to 16.31 |
| Grade 3 | −0.71 | 7 | 0.07 | 67.98 | −175.46 to 174.04 | 0.48 | 7 | 0.27 | 4.99 | −12.35 to 13.31 |
| Received NACT/ACT | −0.76 | 9 | 0.02 | 60.24 | −143.20 to 141.68 | 0.66 | 9 | 0.054 | 4.28 | −9.46 to 10.78 |
| Taxane% | −0.47 | 6 | 0.34 | 50.84 | −141.62 to 140.68 | 0.48 | 6 | 0.34 | 0.91 | −2.05 to 3.01 |
| Anthracycline% | −0.88 | 6 | 0.02 | 26.99 | −75.82 to 74.06 | 0.39 | 6 | 0.45 | 0.95 | −2.25 to 3.03 |
SE−standard error, CI- confidence interval, HR = hormone receptor, NACT = neoadjuvant chemotherapy. ACT = Adjuvant chemotherapy, DFS = disease free survival.
3.2. Qverall survival
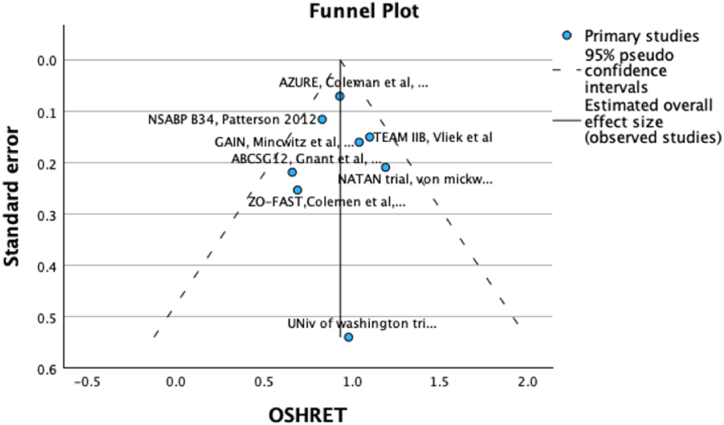
The weighted mean 5-year OS in bisphosphonates and control groups was 92.1 % and 90.9 % respectively with no statistically significant benefit (HR 0.92, 95 % CI 0.83–1.03, p = 0.16, see Fig. 3) and an absolute difference of <1 %. No publication bias was detected on visual inspection of funnel plot (Fig. 4). As with DFS, in contemporary chemotherapy studies, there was no difference in OS (HR 1.04, 95 % CI 0.81–1.32) with more OS events in the bisphosphonates group (absolute difference −1.37 %, Supplementary Fig. 2). Meta regression results for absolute and relative OS effect were consistent with DFS results (Table 2)
Fig. 3.
Forest Plot for Overall Survival Hazard ratio.
Fig. 4.
Funnel plot for Overall Survival.
Table 2b.
Meta-regression analysis for overall survival.
| Delta OS |
OS hazard ratio |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Beta coefficient | N studies | p value | SE | 95 % CI | Beta coefficient | N studies | p value | SE | 95 % CI |
| Final year of accrual | −0.19 | 9 | 0.62 | 60.99 | −144.41 to 144.003 | 0.71 | 8 | 0.05 | 5.12 | −11.82 to 13.24 |
| Duration of bisphosphonates | 0.46 | 9 | 0.21 | 55.27 | −130.23 to 131.15 | −0.06 | 8 | 0.88 | 7.25 | −17.80 to 17.68 |
| Median age | 0.13 | 7 | 0.78 | 55.32 | −142.07 to 142.33 | 0.40 | 6 | 0.43 | 7.12 | −19.37 to 20.17 |
| Post-menopausal | −0.18 | 9 | 0.64 | 61.13 | −144.73 to 144.37 | 0.38 | 8 | 0.35 | 6.72 | −16.06 to 16.82 |
| pNode+ | −0.13 | 9 | 0.74 | 72.42 | −171.38 to 171.12 | 0.57 | 9 | 0.14 | 5.34 | −12.06 to 13.20 |
| >=pT3 | 0.05 | 6 | 0.93 | 59.36 | −164.76 to 164.86 | 0.32 | 5 | 0.6 | 6.27 | −19.63 to 20.27 |
| HR negative | −0.27 | 7 | 0.56 | 70.23 | −180.80 to 180.26 | 0.58 | 7 | 0.17 | 5.93 | −14.66 to 15.82 |
| Grade 2 | −0.51 | 6 | 0.31 | 53.66 | −149.49 to 148.47 | 0.98 | 5 | 0.003 | 1.25 | −3.00 to 4.96 |
| Grade 3 | −0.49 | 7 | 0.26 | 51.42 | −132.67 to 131.69 | 0.5 | 6 | 0.31 | 7.04 | −19.05 to 20.05 |
| Received NACT/ACT | −0.24 | 9 | 0.54 | 60.37 | −142.99 to 142.51 | 0.73 | 8 | 0.04 | 5.00 | −11.50 to 12.96 |
| Taxane% | −0.83 | 6 | 0.037 | 42.98 | −120.16 to 118.50 | 0.76 | 6 | 0.08 | 3.73 | −9.60 to 11.12 |
| Anthracycline% | −0.27 | 6 | 0.60 | 76.01 | −211.31 to 210.77 | 0.46 | 6 | 0.36 | 5.10 | −13.70 to 14.62 |
SE−standard error, CI- confidence interval, HR = hormone receptor, NACT = neoadjuvant chemotherapy. ACT = Adjuvant chemotherapy, DFS = disease free survival.
3.2.1. Toxicity
Pooled incidence of ONJ was 0.78 % (95 % CI 0.6–0.87). This was higher in an analysis of zoledronic acid studies (1 %, 95 % CI 0.75–1.15). Pooled incidence of any grade renal toxicity was 0.27 % (95 % CI 0.13–0.31); this was 0.15 % (95 % CI 0.07–0.24) in a sensitivity analysis excluding data from GAIN study which reported this toxicity as combined renal and urinary [31]. A total of 7 events (0.5 %) across two trials of hypocalcaemia were reported.
4. Discussion
The EBCTCG meta-analysis, which is the primary evidence base for the recommendation of adjuvant bisphosphonates for postmenopausal women included many older trials with non-cancer outcomes as primary endpoints and did not provide detailed information on other systemic therapies [3]. In this updated meta-analysis of more contemporary studies (some published after the EBCTCG meta-analysis), we found a modest absolute benefit in DFS, which was attenuated in patients with high risk disease or those who received chemotherapy. The OS benefit was not statistically significant.
The EBCTCG meta-analysis evaluated several end points including recurrence within and outside bone, local recurrence as well as mortality related and unrelated to breast cancer. DFS is a composite endpoint which includes both local and contralateral events (both new primaries and true recurrences), distant disease, mortality from any cause and often unrelated secondary invasive cancers. In the EBCTCG meta-analysis, the majority of benefit with bisphosphonates was seen in terms of decrease in bone recurrence [3]. There was no effect on local recurrence or non-bone distant recurrence. Furthermore, with competing risks being an important event in EBC, emphasis on breast cancer specific mortality may have overestimated effects [34]. This supports concerns about the impact of adjuvant bisphosphonates in the modern era.
Recent work has shown a decrease in distant DFS events by 20–30 % in more contemporary studies [9] likely due to earlier diagnosis and improvements in systemic therapy. In such a setting even if relative effects of adjuvant bisphosphates remain unchanged, this will result in smaller absolute benefits. In our study, which included more contemporary studies, a small relative benefit in DFS was observed, although as expected, this translated to small absolute benefit.
OS remains the gold standard endpoint in adjuvant breast cancer trials especially because surrogacy between DFS and OS has not been established for most EBC subtypes except HER2 positive disease [35,36]. The previous EBCTCG meta-analysis did not report a statistically significant difference in all-cause mortality overall, a finding which was confirmed in the current study [3]. In fact, higher number of deaths was observed in patients who received adjuvant bisphosphonates after receiving anthracycline and taxane based chemotherapy. Therefore, failure to translate the small DFS benefit observed into an OS benefit is of concern.
The EBCTCG meta-analysis reported highest absolute benefits in patients who were node-positive and ER-negative; a subgroup that derive the maximum benefit from chemotherapy as well. We observed consistent diminishing benefit in patients with clinical high risk disease including ER-negative disease. It is plausible that effective chemotherapy modifies the prognosis of these high risk patients with very little (if any) added benefit of bisphosphonates. This observation is in contrast to both ESMO and ASCO recommendations who recommend bisphosphonates in high risk (ESMO) or all (ASCO) post-menopausal patients irrespective of risk factors such as hormone receptor status [10,11].
Data from individual trials suggest the potential value of absence of MAF gene amplification for predicting benefit from adjuvant bisphosphonates [37,38]. The role of adjuvant bisphosphonates based on genomic risk evaluated using a multi-gene assay has not been defined and is an area of ongoing research. Recent studies have identified composite scores including clinical and genetic risk factors for better risk stratification of patients with hormone receptor positive and HER-2 negative breast cancer and considering these while assessing benefits of adjuvant bisphosphonates might provide further insight into who may derive greater benefit from these therapies [39,40].
ONJ was observed in ∼1 % of patients in trials using zoledronic acid. Although ONJ is seen much more frequently in metastatic disease where treatment intervals are typically shorter (2–3% at 3 years) [41], 1 % risk of ONJ is significant especially given the modest benefits on DFS. The risk of renal dysfunction and hypocalcaemia was low but small number of studies reporting these side effects limited the power of the analysis.
Our study has limitations. Included studies used different bisphosphonates (including clodronate, ibandronate, pamidronate and zoledronic acid) and for different durations leading to heterogeneity. However, given the lack of heterogeneity observed in the EBCTCG analysis and the equivalence of these drugs in the SWOG S0307 trial [21] we felt that pooling of these studies was consistent with prior practice-changing methodology. Also, due to the small number of included studies, meta-regression was underpowered. Therefore, data were interpreted quantitatively rather than based on statistical significance. Finally, our analyses were based on reported summary statistics rather than individual patient data. This will increase uncertainty and will not allow for direct comparison with the EBCTCG analysis [3].
5. Conclusions
In a cohort of randomized trials with contemporary systemic therapy, the absolute DFS benefit of adjuvant bisphosphonates is lower than shown in EBCTCG meta-analysis and seems limited to patients with lower risk disease. Patients treated with anthracycline and taxane based chemotherapy did not derive a DFS or OS benefit. About 1 % of patients developed ONJ related to treatment with zoledronic acid. These findings should be taken into consideration when counselling patients about the role of adjuvant bisphosphonates.
Data availability statement
| Question | Response |
|---|---|
|
Data Availability Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication. Has data associated with your study been deposited into a publicly available repository? |
No |
| Please select why. Please note that this statement will be available alongside your article upon publication. as follow-up to "Data Availability Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication. Has data associated with your study been deposited into a publicly available repository? " |
The data used for analysis is derived from published randomised trials and is available in the public domain. Specific variables collected related to this manuscript can be made available at reasonable request from the corresponding author |
CRediT authorship contribution statement
Abhenil Mittal: Writing - review & editing, Writing - original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Faris Tamimi: Writing - review & editing, Investigation, Formal analysis. Consolacion Molto: Writing - review & editing, Investigation, Formal analysis, Data curation. Massimo Di Iorio: Writing - review & editing, Formal analysis. Eitan Amir: Writing - review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24793.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P., et al. 20-Year risks of breast-cancer recurrence after Stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017 Nov 9;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santini D., Stumbo L., Spoto C., D'Onofrio L., Pantano F., Iuliani M., et al. Bisphosphonates as anticancer agents in early breast cancer: preclinical and clinical evidence. Breast Cancer Res. 2015 Sep 2;17(1):121. doi: 10.1186/s13058-015-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015 Oct 3;386(10001):1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 4.Gnant M., Mlineritsch B., Schippinger W., Luschin-Ebengreuth G., Pöstlberger S., Menzel C., et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 2009 Feb 12;360(7):679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 5.Coleman R.E., Marshall H., Cameron D., Dodwell D., Burkinshaw R., Keane M., et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011 Oct 13;365(15):1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 6.Powles T., Paterson S., Kanis J.A., McCloskey E., Ashley S., Tidy A., et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J. Clin. Oncol. 2002 Aug 1;20(15):3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 7.Paterson A.H.G., Anderson S.J., Lembersky B.C., Fehrenbacher L., Falkson C.I., King K.M., et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012 Jul;13(7):734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diel I.J., Solomayer E.F., Costa S.D., Gollan C., Goerner R., Wallwiener D., et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 1998 Aug 6;339(6):357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 9.Wilson B.E., Desnoyers A., Al-Showbaki L., Nadler M.B., Amir E. A retrospective analysis of changes in distant and breast cancer related disease-free survival events in adjuvant breast cancer trials over time. Sci. Rep. 2022 Apr 15;12(1):6352. doi: 10.1038/s41598-022-09949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisen A., Somerfield M.R., Accordino M.K., Blanchette P.S., Clemons M.J., Dhesy-Thind S., et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: ASCO-OH (CCO) guideline update. J. Clin. Orthod. 2022 Mar;40(7):787–800. doi: 10.1200/JCO.21.02647. [DOI] [PubMed] [Google Scholar]
- 11.Coleman R., Hadji P., Body J.J., Santini D., Chow E., Terpos E., et al. Bone health in cancer: ESMO clinical practice guidelines. Ann. Oncol. 2020 Dec 1;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 12.McGee S., Alzahrani M., Vandermeer L., Cole K., Larocque G., Awan A., et al. Adjuvant bisphosphonate use in patients with early stage breast cancer: a physician survey. Breast Cancer Res. Treat. 2021;187(2):477–486. doi: 10.1007/s10549-021-06147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter I., Theodoulou E., Holen I., Harper-Wynne C., Baron-Hay S., Wilson C., et al. Adoption of adjuvant bisphosphonates for early breast cancer into standard clinical practice: challenges and lessons learnt from comparison of the UK and Australian experience. J Bone Oncol. 2021 Nov 1;31 doi: 10.1016/j.jbo.2021.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balic M., Thomssen C., Würstlein R., Gnant M., Harbeck N. St. Gallen/vienna 2019: a brief summary of the Consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019 Apr;14(2):103–110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vliek S.B., Noordhoek I., Meershoek-Klein Kranenbarg E., van Rossum A.G.J., Dezentje V.O., Jager A., et al. Daily oral ibandronate with adjuvant endocrine therapy in postmenopausal women with estrogen receptor-positive breast cancer (BOOG 2006-04): randomized phase III TEAM-IIB trial. J. Clin. Oncol. 2022 Sep 1;40(25):2934–2945. doi: 10.1200/JCO.21.00311. [DOI] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhesy-Thind S., Fletcher G.G., Blanchette P.S., Clemons M.J., Dillmon M.S., Frank E.S., et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a cancer care Ontario and American society of clinical Oncology clinical practice guideline. J. Clin. Orthod. 2017 Jun 20;35(18):2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 18.Stanley T.D., Doucouliagos H. Neither fixed nor random: weighted least squares meta-regression. Res. Synth. Methods. 2017 Mar;8(1):19–42. doi: 10.1002/jrsm.1211. [DOI] [PubMed] [Google Scholar]
- 19.Burnand B., Kernan W.N., Feinstein A.R. Indexes and boundaries for “quantitative significance” in statistical decisions. J. Clin. Epidemiol. 1990;43(12):1273–1284. doi: 10.1016/0895-4356(90)90093-5. [DOI] [PubMed] [Google Scholar]
- 20.Friedl T.W.P., Fehm T., Müller V., Lichtenegger W., Blohmer J., Lorenz R., et al. Prognosis of patients with early breast cancer receiving 5 Years vs 2 Years of adjuvant bisphosphonate treatment: a phase 3 randomized clinical trial. JAMA Oncol. 2021 Aug 1;7(8):1149–1157. doi: 10.1001/jamaoncol.2021.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gralow J.R., Barlow W.E., Paterson A.H.G., M’iao J.L., Lew D.L., Stopeck A.T., et al. Phase III randomized trial of bisphosphonates as adjuvant therapy in breast cancer: S0307. J. Natl. Cancer Inst. 2020 Jul 1;112(7):698–707. doi: 10.1093/jnci/djz215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bundred N.J., Campbell I.D., Davidson N., DeBoer R.H., Eidtmann H., Monnier A., et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008 Mar 1;112(5):1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 23.Eidtmann H., de Boer R., Bundred N., Llombart-Cussac A., Davidson N., Neven P., et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann. Oncol. 2010 Nov;21(11):2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 24.Coleman R., de Boer R., Eidtmann H., Llombart A., Davidson N., Neven P., et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann. Oncol. 2013 Feb;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 25.Paterson A.H.G., Anderson S.J., Lembersky B.C., Fehrenbacher L., Falkson C.I., King K.M., et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012 Jul;13(7):734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman R., Cameron D., Dodwell D., Bell R., Wilson C., Rathbone E., et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014 Aug;15(9):997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 27.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Heck D., Menzel C., et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011 Jul;12(7):631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 28.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Knauer M., Moik M., et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 2015 Feb;26(2):313–320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 29.Aft R., Naughton M., Trinkaus K., Watson M., Ylagan L., Chavez-MacGregor M., et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010 May;11(5):421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aft R.L., Naughton M., Trinkaus K., Weilbaecher K. Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breast cancer. Br. J. Cancer. 2012 Jun 26;107(1):7–11. doi: 10.1038/bjc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Minckwitz G., Möbus V., Schneeweiss A., Huober J., Thomssen C., Untch M., et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J. Clin. Oncol. 2013 Oct 1;31(28):3531–3539. doi: 10.1200/JCO.2012.47.2167. [DOI] [PubMed] [Google Scholar]
- 32.von Minckwitz G., Rezai M., Tesch H., Huober J., Gerber B., Zahm D.M., et al. Zoledronate for patients with invasive residual disease after anthracyclines-taxane-based chemotherapy for early breast cancer - the Phase III NeoAdjuvant Trial Add-oN (NaTaN) study (GBG 36/ABCSG 29) Eur. J. Cancer. 2016 Sep;64:12–21. doi: 10.1016/j.ejca.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Perrone F., De Laurentiis M., De Placido S., Orditura M., Cinieri S., Riccardi F., et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur. J. Cancer. 2019 Sep;118:178–186. doi: 10.1016/j.ejca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Ethier J.L., Anderson G.M., Austin P.C., Clemons M., Parulekar W., Shepherd L., et al. Influence of the competing risk of death on estimates of disease recurrence in trials of adjuvant endocrine therapy for early-stage breast cancer: a secondary analysis of MA.27, MA.17 and MA.17R. Eur. J. Cancer. 2021 May;149:117–127. doi: 10.1016/j.ejca.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Gyawali B., Hey S.P., Kesselheim A.S. Evaluating the evidence behind the surrogate measures included in the FDA's table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine. 2020 Apr;21 doi: 10.1016/j.eclinm.2020.100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saad E.D., Squifflet P., Burzykowski T., Quinaux E., Delaloge S., Mavroudis D., et al. Disease-free survival as a surrogate for overall survival in patients with HER2-positive, early breast cancer in trials of adjuvant trastuzumab for up to 1 year: a systematic review and meta-analysis. Lancet Oncol. 2019 Mar;20(3):361–370. doi: 10.1016/S1470-2045(18)30750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman R., Hall A., Albanell J., Hanby A., Bell R., Cameron D., et al. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol. 2017 Nov;18(11):1543–1552. doi: 10.1016/S1470-2045(17)30603-4. [DOI] [PubMed] [Google Scholar]
- 38.Paterson A.H.G., Lucas P.C., Anderson S.J., Mamounas E.P., Brufsky A., Baez-Diaz L., et al. MAF amplification and adjuvant clodronate outcomes in early-stage breast cancer in NSABP B-34 and potential impact on clinical practice. JNCI Cancer Spectr. 2021 Aug 1;5(4):pkab054. doi: 10.1093/jncics/pkab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garutti M., Griguolo G., Botticelli A., Buzzatti G., De Angelis C., Gerratana L., et al. Definition of high-risk early hormone-positive HER2-negative breast cancer: a Consensus review. Cancers. 2022 Apr 9;14(8):1898. doi: 10.3390/cancers14081898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puglisi F., Gerratana L., Lambertini M., Ceppi M., Boni L., Montemurro F., et al. Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer. npj Breast Cancer. 2021 Jun 28;7(1):1–9. doi: 10.1038/s41523-021-00286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Poznak C.H., Unger J.M., Darke A.K., Moinpour C., Bagramian R.A., Schubert M.M., et al. Association of osteonecrosis of the jaw with zoledronic acid treatment for bone metastases in patients with cancer. JAMA Oncol. 2021 Feb 1;7(2):246–254. doi: 10.1001/jamaoncol.2020.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
| Question | Response |
|---|---|
|
Data Availability Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication. Has data associated with your study been deposited into a publicly available repository? |
No |
| Please select why. Please note that this statement will be available alongside your article upon publication. as follow-up to "Data Availability Sharing research data helps other researchers evaluate your findings, build on your work and to increase trust in your article. We encourage all our authors to make as much of their data publicly available as reasonably possible. Please note that your response to the following questions regarding the public data availability and the reasons for potentially not making data available will be available alongside your article upon publication. Has data associated with your study been deposited into a publicly available repository? " |
The data used for analysis is derived from published randomised trials and is available in the public domain. Specific variables collected related to this manuscript can be made available at reasonable request from the corresponding author |