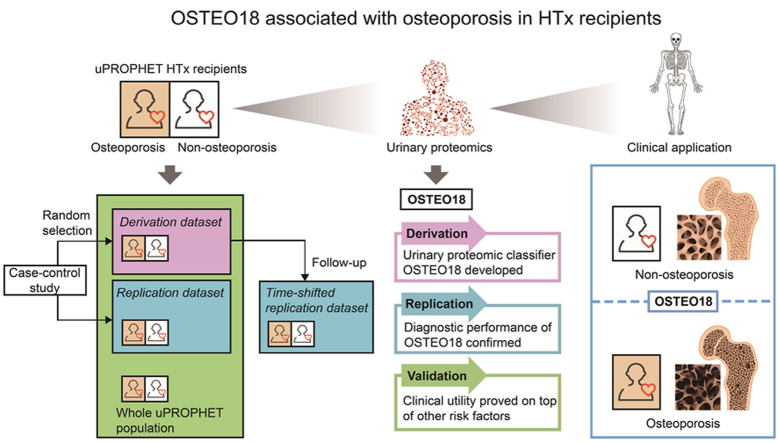
Abstract
Background
Immunosuppressive treatment in heart transplant (HTx) recipient causes osteoporosis. The urinary proteomic profile (UPP) includes peptide fragments derived from the bone extracellular matrix. Study aims were to develop and validate a multidimensional UPP biomarker for osteoporosis in HTx patients from single sequenced urinary peptides identifying the parent proteins.
Methods
A single-center HTx cohort was analyzed. Urine samples were measured by capillary electrophoresis coupled with mass spectrometry. Cases with osteoporosis and matching controls were randomly selected from all available 389 patients. In derivation case-control dataset, 1576 sequenced peptides detectable in ≥30 % of patients. Applying statistical analysis on these, an 18-peptide multidimensional osteoporosis UPP biomarker (OSTEO18) was generated by support vector modeling. The 2 replication datasets included 118 and 94 patients. For further validation, the whole cohort was analyzed. Statistical methods included logistic regression and receiver operating characteristic curve (ROC) analysis.
Results
In derivation dataset, the AUC, sensitivity and specificity of OSTEO18 were 0.83 (95 % CI: 0.76–0.90), 74.3 % and 87.1 %, respectively. In replication datasets, results were confirmatory. In the whole cohort (154 osteoporotic patients [39.6 %]), the ORs for osteoporosis increased (p < 0.0001) across OSTEO18 quartiles from 0.39 (95 % CI: 0.25–0.61) to 3.14 (2.08–4.75). With full adjustment for known osteoporosis risk factors, OSTEO18 improved AUC from 0.708 to 0.786 (p = 0.0003) for OSTEO18 categorized (optimized threshold: 0.095) and to 0.784 (p = 0.0004) for OSTEO18 as continuously distributed classifier.
Conclusion
OSTEO18 is a clinically meaningful novel biomarker indicative of osteoporosis in HTx recipients and is being certified as in-vitro diagnostic.
Keywords: Osteoporosis, Heart transplantation, Bone, Urinary proteomics
Graphical abstract
1. Introduction
Osteoporosis is a common age-related disease caused by imbalance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption, resulting in a decline of bone mineral density (BMD) and an enhanced risk of osteoporotic fractures [1,2]. In recipients of a heart transplant (HTx), immunosuppressants and glucocorticosteroids are routinely prescribed to prevent allograft rejection, thereby increasing the risk of post-transplant osteoporosis due to trabecular bone loss, reduced bone formation and increased bone destruction [3].
Urine contains more than 20,000 peptides, mainly collagen fragments, which are generated in nephron or pass from circulation to tubular fluid through the glomerular barrier [4,5]. Single sequenced urinary peptides allow identifying the parental proteins [6], thereby providing bodywide information on the molecular mechanisms underlying pathophysiological processes. Collagen type I is the most abundant constituent of the organic extracellular matrix in bones [7]. Accelerated biological aging, as captured by urinary proteomic profiling (UPP), is associated with osteoporosis and osteoporotic fractures in the general population [8]. Disease-specific multidimensional urinary classifiers have diagnostic and prognostic value in HTx patients at risk of graft failure [9] or renal dysfunction [10]. However, no previous study investigated whether there is a specific UPP signature indicative of osteoporosis in HTx patients. Using the database of the urinary PROteomics in Predicting HEart Transplantation outcomes study (uPROPHET) [11], the objective of this study was therefore to develop and validate a multidimensional UPP marker from single sequenced urinary peptides with different levels in HTx recipients with and without osteoporosis.
2. Material and methods
2.1. Study population and study endpoint
uPROPHET (registration number, NCT03152422) is a single-center study with as objective to identify and validate UPP signatures for clinical use in HTx patients [11]. uPROPHET complies with the Declaration of Helsinki for research in humans [12]. The Ethics Committee of the University Hospitals Leuven [numbers B322201421186 (S56384) and B322201421045 (S56472)] and the European Research Council Executive Agency approved the protocol. HTx recipients provided written informed consents. Recruitment of HTx patients took place at the University Hospital Gasthuisberg in Leuven in collaboration with the transplantation team. All HTx recipients in regular follow-up at the University Hospitals Leuven were invited to provide a 5-mL mid-morning urine sample for UPP analysis.
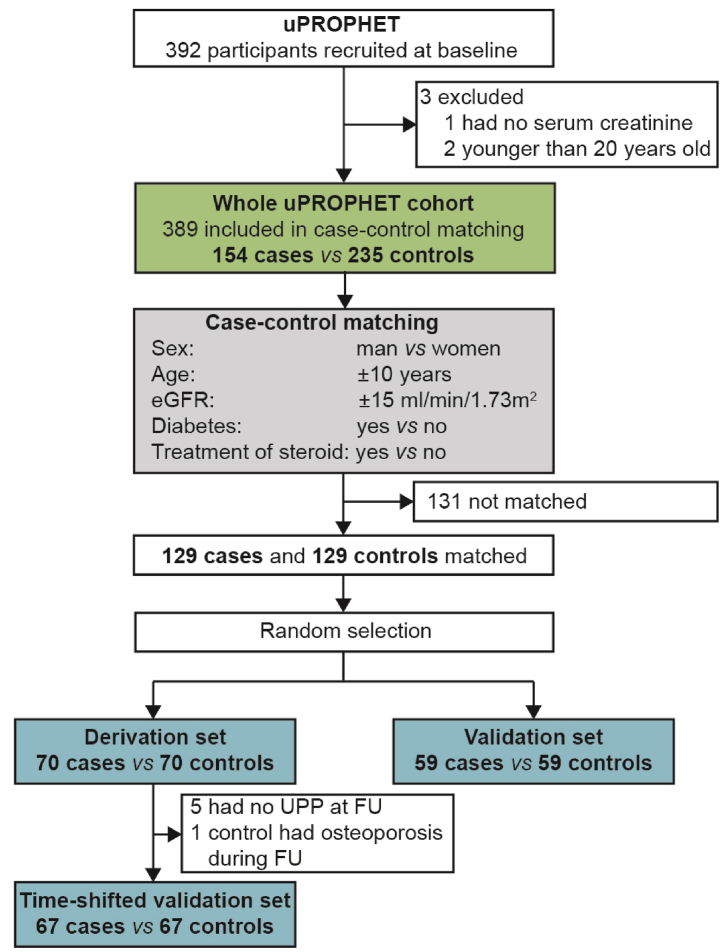
Of 392 HTx patients enrolled in uPROPHET, 3 were excluded because they had no serum creatinine measurement at baseline (n = 1) or because they were younger than 20 years, an age group for which BMD reference values are unavailable (n = 2). Therefore, 389 uPROPHET participants were statistically analyzed (Fig. 1). The osteoporotic endpoint included: (i) a T-score of ≤ -2.5 in the hip or lumbar BMD measurements (Data Supplement p 2) as determined by dual energy X-ray absorptiometry (XRA) [13,14] (ii) a history of osteoporotic fracture or the necessity of spine stabilizing surgery; and (iii) clinical, biochemical, hormonal or radiographic signs to start anti-osteoporotic treatment with calcium supplements, vitamin D and/or bisphosphonates. Measurements monitored at the HTx day hospital include loss of body height, complaints compatible with spinal nerve root compression, the development of thoracal kyphosis, lumbar lordosis or scoliosis, serum parathyroid hormone [15] and osteocalcin [16], alkaline phosphatase and bone-specific alkaline phosphatase [17], sex hormone levels (follicle stimulating hormone, estrogens progestogens and testosterone), 25-hydroxy-vitamin D (calcidiol) or 1,25-dihydroxy-vitamin D (calcitriol) [18], 24-h urinary calcium excretion balanced against estimates of dietary calcium intake, and radiographic evidence of vertebral compression fractures [13,14,19].
Fig. 1.
Flow chart. HTx indicates heart transplantation and FU follow up.
To generate a multidimensional urinary classifier indicative of osteoporosis, analyses started with a case-control study, in which 129 patients with incident osteoporosis were matched with 129 controls for sex, age (±10 years), eGFR (±15 mL/min/1.73 m2) and intake of methylprednisolone (Fig. 1). Cases and controls were randomly subdivided into a derivation dataset (70 cases vs 70 controls) and a replication dataset (59 cases vs 59 controls). Of the patients in the derivation dataset, 67 cases and 67 controls underwent a second UPP assessment after a median interval of 0.75 years (range: 0.25–6.26 years). These patients constituted the time-shifted replication dataset. For final validation, the urinary classifier was tested in the whole cohort, which included 154 patients with osteoporosis (Fig. 1).
2.2. Assessment of clinical variables
A detailed description of the construction and contents of uPROPHET database is available in the published protocol [11] and the Data Supplement (page 2). All potentially relevant clinical information, including anthropometrics, medical antecedents, biochemical measurements and use of immunosuppressive, antihypertensive, lipid-lowering and antidiabetic drugs, was retrieved from the computerized information system of the University Hospitals Leuven. The urine samples was collected within 6 months of the study osteoporotic outcome. The treatments of calcium supplement, vitamin D supplement, bisphosphonates and immunosuppression drugs were administered at the time of urine sampling.
2.3. Urinary proteomic profiling
The methods for urine sample preparation, capillary electrophoresis coupled with mass spectrometry (CE-MS), peptide sequencing, calibration, and quality control of the mass spectrometric data have been published [6,20,21] and are described in the Data Supplement (page 3–5). The 1576 sequenced urinary peptides with a detectable signal in ≥30 % of study participants (Fig. S1), a common approach in UPP analysis [22,23], were selected to generate the classifier differentiating cases from controls.
2.4. Statistical analysis
For database management and statistical analysis, we used the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). Means were compared using the large-sample z-test or ANOVA and proportions by Fisher's exact test. The 95 % confidence intervals (CIs) of rates were computed as R ± 1.96 × √ (R × (100 – R)/T), where R is the rate and T is the total number of patients. Derivation, replication and validation of the osteoporosis classifier were done according to predefined steps (Fig. S1). First, the 1576 selected urinary peptides were compared between cases and controls in the derivation dataset, using the Mann-Whitney U test (Fig. S2). The significance of the between-group differences was adjusted for multiple comparisons by the Benjamini-Hochberg approach [24] Next, searching a balance between computational complexity and the minimum number of peptides to construct a UPP classifier, the urinary peptides in the top tail of significance distribution were combined to generate the classifier [25]. The 30 most significant peptides with higher urinary levels in cases and the 30 most significant peptides with higher levels in controls (Fig. S2) were selected and combined using support vector modeling (SVM) with 94 support vectors and the radial basis kernel function. Models were optimized using a take-one-out procedure until no further optimization was possible by reducing the numbers of support vectors. The so-obtained classifier, OSTEO18 (Table S1), included 18 of the original 60 urinary peptides. Reference signals of 29 abundant peptides were used as internal standards for calibration using local linear regression [20], therefore OSTEO18 scores are the dimensionless ratio with the calibration signal in the denominator.
The risk of osteoporosis in the derivation and replication case-control studies and in the whole uPROHPET cohort (Fig. S1) was related to the OSTEO18 level by logistic regression models, first unadjusted, next partially adjusted for sex, age, the glomerular filtration rate estimated from serum creatinine (eGFR) [26], diabetes (yes vs no) and treatment with methylprednisolone (yes vs no), and finally additionally adjusted for the time interval since HTx, ionized calcium, treatment with thiazides (yes vs no) [27], tacrolimus [28] (yes vs no) and cyclosporine (yes vs no) [29]. Ionized calcium was calculated from serum total calcium and serum albumin, using the following formula: ionized calcium (mmol/l) = serum total calcium (mmol/l) + 0.02 × (40 - serum albumin (g/l)) [30]. These covariables were selected because their clinical relevance to osteoporosis in HTx patients [[27], [28], [29],[31], [32], [33]]. In these models, the OSTEO18 score was introduced as categorized variable approximating the quartiles of the OSTEO18 distribution (<-1, -1-0, 0–1 and ≥1) and as a continuously distributed variable. For the analysis of OSTEO18 as categorized variable the deviation from mean coding was applied, a method which does not require the definition of a reference group, provides 95 % confidence intervals for all categories and expresses the risk relative to the average risk in the whole study population [34]. The performance of the OSTEO18 was further assessed by the receiver operating curve (ROC) and the area under curve (AUC) approach. The OSTEO18 threshold optimized by the Youden index was 0.095. The 95 % CI of the AUC was calculated by the DeLong method [35]. Significance was a 2-sided p-value of ≤0.05.
3. Results
3.1. Case-control studies
Table 1 lists the baseline characteristics of the patients included in the case-control studies. In the derivation dataset, 80.0 % of the participants were men (n = 112) and 34.3 % were on treatment with methylprednisolone (n = 48), 67.7 % tacrolimus (n = 94) and 29.3 % cyclosporine (n = 41). In the derivation (70 cases vs 70 controls), replication (59 vs 59) and time-shifted replication (67 vs 67) datasets, cases and controls (Table 1) were well matched. Table S2 shows that patients included in the 3 case-control studies had largely similar characteristics with some differences in the treatment with immunosuppressant drugs associated with the time interval since HTx and over-time changing treatment policies.
Table 1.
Patient characteristics in the derivation, replication and time-shifted case-control studies (starts).
| Characteristic | Derivation dataset |
Replication dataset |
Time-shifted replication dataset |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 70) | Cases (n = 70) | p-value | Controls (n = 59) | Cases (n = 59) | p-value | Controls (n = 67) | Cases (n = 67) | p-value | |
| Time after HTx (years) | 9.91 (4.53, 14.3) | 11.1 (5.28, 18.3) | 0.17 | 3.28 (0.41, 9.29) | 5.75 (0.76, 12.9) | 0.20 | 11.3 (5.26, 15.0) | 12.0 (5.74, 19.8) | 0.18 |
| Age (years) | 61.3 (12.1) | 60.2 (13.5) | 0.61 | 56.59 (12.6) | 59.44 (13.0) | 0.23 | 62.2 (12.4) | 60.9 (13.2) | 0.58 |
| BMI (kg/m2) | 25.4 (3.36) | 25.3 (4.26) | 0.99 | 24.81 (4.28) | 24.83 (4.53) | 0.98 | 27.0 (11.5) | 25.5 (4.53) | 0.31 |
| SBP (mm Hg) | 145.6 (19.1) | 143.5 (22.3) | 0.55 | 140.0 (19.9) | 143.1 (21.3) | 0.43 | 143.8 (19.3) | 143.0 (21.4) | 0.83 |
| DBP (mm Hg) | 86.2 (12.6) | 86.0 (13.1) | 0.94 | 87.2 (11.2) | 88.0 (10.6) | 0.71 | 85.0 (11.1) | 85.0 (13.3) | 0.99 |
| Serum creatinine (μmol/L) | 136.5 (45.1) | 130.2 (40.6) | 0.38 | 124.75 (41.6) | 129.81 (42.0) | 0.51 | 140.3 (48.0) | 133.8 (40.4) | 0.40 |
| eGFR (ml/min/1.73 m [2]) | 52.3 (21.1) | 54.1 (19.6) | 0.59 | 58.99 (21.4) | 56.00 (22.2) | 0.46 | 51.2 (21.8) | 52.2 (19.6) | 0.77 |
| Serum albumin (g/L) | 44.1 (2.97) | 43.9 (3.48) | 0.71 | 43.3 (3.78) | 44.1 (2.90) | 0.17 | 44.7 (2.77) | 43.9 (3.24) | 0.14 |
| Total serum calcium (mmol/L) | 2.39 (0.09) | 2.40 (0.12) | 0.58 | 2.40 (0.13) | 2.40 (0.12) | 0.78 | 2.40 (0.10) | 2.39 (0.13) | 0.65 |
| Ionized calcium (mmol/L) | 2.47 (0.14) | 2.48 (0.16) | 0.82 | 2.46 (0.19) | 2.48 (0.16) | 0.46 | 2.49 (0.14) | 2.47 (0.17) | 0.37 |
| Sex | |||||||||
| Men, n (%) | 57 (81.4 %) | 55 (78.6 %) | 0.67 | 46 (78.0 %) | 48 (81.4 %) | 0.65 | 56 (83.6 %) | 53 (79.1 %) | 0.51 |
| Women, n (%) | 13 (18.6 %) | 15 (21.4 %) | 13 (22.0 %) | 11 (18.6 %) | 12 (16.7 %) | 14 (20.9 %) | |||
| indication for HTx | |||||||||
| Ischemic CMP, n (%) | 33 (47.1 %) | 25 (35.7 %) | 0.17 | 19 (32.2 %) | 29 (49.2 %) | 0.061 | 31 (46.3 %) | 24 (35.8 %) | 0.22 |
| Dilated CMP, n (%) | 27 (38.6 %) | 27 (38.6 %) | … | 29 (49.2 %) | 21 (35.6 %) | 0.14 | 26 (38.8 %) | 26 (38.8 %) | … |
| Other, n (%) | 10 (14.3 %) | 18 (25.7 %) | 0.091 | 8 (13.6 %) | 6 (10.2 %) | 0.57 | 10 (14.9 %) | 17 (25.4 %) | 0.13 |
| Past smoker, n (%) | 47 (67.1 %) | 43 (61.4 %) | 0.48 | 37 (62.7 %) | 36 (62.1 %) | 0.94 | 44 (65.7 %) | 43 (64.2 %) | 0.86 |
| Hypertension, n (%) | 66 (94.3 %) | 65 (92.9 %) | 0.73 | 50 (84.7 %) | 55 (93.2 %) | 0.14 | 65 (97.0 %) | 60 (89.6 %) | 0.084 |
| Diabetes, n (%) | 15 (21.4 %) | 10 (14.3 %) | 0.27 | 6 (10.2 %) | 11 (18.6 %) | 0.19 | 15 (22.4 %) | 9 (13.4 %) | 0.18 |
| Osteoporotic fracture, n (%) | … | 7 (10.0 %) | … | … | 4 (6.8 %) | … | … | 6 (9.0 %) | … |
| BMD T-score ≤ -2.5, n (%) | … | 23 (32.9 %) | … | … | 20 (33.9 %) | … | … | 22 (32.8 %) | … |
| Calcium supplements, n (%) | … | 53 (75.7 %) | … | … | 47 (79.7 %) | … | … | 51 (76.1 %) | … |
| Vitamin D, n (%) | … | 52 (74.3 %) | … | … | 47 (79.7 %) | … | … | 50 (74.6 %) | … |
| Bisphosphonates, n (%) | … | 14 (20.0 %) | … | … | 5 (8.5 %) | … | … | 13 (19.4 %) | … |
| Thiazides, n (%) | 15 (21.4 %) | 5 (7.1 %) | 0.016 | 4 (11.8 %) | 3 (9.1 %) | 0.72 | 11 (16.4 %) | 4 (6.0 %) | 0.055 |
| Immunosuppression | |||||||||
| Tacrolimus, n (%) | 53 (75.7 %) | 41 (58.6 %) | 0.031 | 36 (62.1 %) | 27 (47.4 %) | 0.15 | 49 (73.1 %) | 37 (55.2 %) | 0.031 |
| Cyclosporine, n (%) | 14 (20.0 %) | 27 (38.6 %) | 0.016 | 5 (8.5 %) | 12 (20.3 %) | 0.067 | 14 (20.9 %) | 26 (38.8 %) | 0.024 |
| Everolimus, n (%) | 4 (5.7 %) | 3 (4.3 %) | 0.70 | 4 (9.3 %) | 3 (6.0 %) | 0.56 | 0 (0 %) | 1 (1.5 %) | 0.32 |
| Mycophenolic acid, n (%) | 55 (78.6 %) | 51 (72.9 %) | 0.43 | 32 (57.1 %) | 33 (61.1 %) | 0.42 | 49 (73.1 %) | 48 (71.6 %) | 0.85 |
| Azathioprine, n (%) | 6 (8.6 %) | 6 (8.6 %) | … | 3 (7.0 %) | 1 (2.1 %) | 0.26 | 0 (0 %) | 0 (0 %) | … |
| Methylprednisolone, n (%) | 25 (35.7 %) | 23 (32.9 %) | 0.72 | 12 (20.3 %) | 13 (22.0 %) | 0.82 | 0 (0 %) | 0 (0 %) | … |
Values are mean (SD), median (IQR) or number of patients (%). Abbreviations: HTx, heart transplantation; BMI, body mass index; SBP/DBP, systolic/diastolic blood pressure; eGFR, estimated glomerular filtration rate; CMP, cardiomyopathy; BMD, bone mineral density. To convert serum creatinine from μmol/L to mg/dL, multiply by 0.0113. To convert serum albumin from g/L to g/dL, multiply by 0.1. To convert serum total and ionized calcium from mmol/L to mg/dL, multiply by 4. p-values refer to the difference between cases and controls in the derivation, replication and time-shifted case-control studies. An ellipsis indicates that the variable was not measured or that the p-value was not calculated.
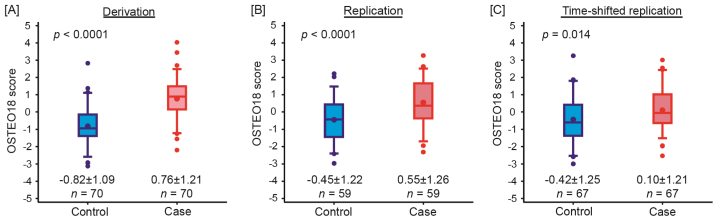
The OSTEO18 scores were significantly and consistently higher in patients with osteoporosis compared with controls in the derivation (p < 0.0001, Fig. 2A), replication (p < 0.0001, Fig. 2B) and time-shifted (p = 0.014, Fig. 2C) case-control studies. In the derivation dataset, the AUC was 0.83 (95 % CI: 0.76–0.90; p < 0.0001) and sensitivity and specificity were 74.3 % and 87.1 %, respectively (Table S3). Analysis of the replication and time-shifted replication datasets confirmed the discriminatory performance of OSTEO18 (Table S3). In the replication case-control study, the AUC, sensitivity and specificity were 0.71 (95 % CI: 0.62–0.80; p < 0.0001), 69.5 % and 62.7 %, and in the time-shifted replication dataset 0.62 (0.53–0.72; p < 0.011), 73.1 % and 53.7 %, respectively.
Fig. 2.
Distribution of the multidimensional classifier OSTEO18 in cases (red) and controls (blue) in the derivation (A), replication (B) and time-shifted (C) case-control studies. OSTEO18 was developed by support vector modelling and optimized by take-one-out cross validation procedure. The central line, the upper and lower lines, and the upper and lower whiskers represent the median, interquartile range, and the 10th to 90th percentile interval, respectively. The arithmetic means and extreme measurements are represented by circles inside the box and outside the whiskers, respectively. Arithmetic means and standard deviations are presented along the horizontal axis. n denotes the number of patients in each group. p values denote the significance in the OSTEO18 score between cases and controls in each dataset. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Performance of OSTEO18 in all uPROPHET patients
Table 2 and Table S4 list the characteristics of all 389 uPROPHET patients as well as the characteristics of 154 patients (39.6 %) with osteoporosis and 235 patients (60.4 %) without any sign of osteoporosis. Of 389 patients, 78 (20.1 %) were examined within the first year after HTx. Of 154 patients with osteoporosis (Table S4), only 8 (5.19 %) were diagnosed before HTx. As shown in Fig. S3, the distribution of OSTEO18 was close to normally distributed (p = 0.034).
Table 2.
Characteristics of 389 uPROPHET patients by OSTEO18 categories (starts).
| Characteristic | OSTEO18 score |
p-value | |||
|---|---|---|---|---|---|
| < −1 | -1–0 | 0–1 | ≥1 | ||
| Number in group, n (%) | 93 (23.9) | 107 (27.5) | 97 (24.9) | 92 (23.7) | |
| Time after HTx (years) | 4.33 (1.26, 13.2) | 6.26 (1.52, 11.2) | 7.53 (1.74, 15.6) | 10.2 (3.87, 16.1) | 0.0024 |
| Age (years) | 54.0 (13.5) | 55.3 (15.4) | 57.1 (14.2) | 62.5 (12.2) | <0.0001 |
| BMI (kg/m2) | 25.7 (4.12) | 25.3 (4.50) | 24.4 (4.24) | 25.8 (4.04) | 0.78 |
| SBP (mm Hg) | 142.3 (18.8) | 141.2 (20.7) | 141.7 (21.8) | 144.3 (21.6) | 0.50 |
| DBP (mm Hg) | 86.4 (11.2) | 85.0 (12.1) | 84.4 (11.5) | 86.6 (10.9) | >0.99 |
| Serum creatinine (μmol/L) | 118.4 (35.9) | 121.0 (45.8) | 126.7 (45.0) | 135.0 (47.6) | 0.0062 |
| eGFR (ml/min/1.73 m [2]) | 63.7 (24.2) | 63.6 (26.2) | 58.3 (25.3) | 51.6 (20.1) | 0.0002 |
| Serum albumin (g/L) | 43.9 (3.06) | 44.2 (3.36) | 44.0 (3.03) | 43.0 (3.47) | 0.084 |
| Total serum calcium (mmol/L) | 2.38 (0.11) | 2.38 (0.12) | 2.40 (0.11) | 2.40 (0.12) | 0.16 |
| Ionized serum calcium (mmol/L) | 2.46 (0.15) | 2.46 (0.17) | 2.48 (0.14) | 2.46 (0.17) | 0.76 |
| Sex | |||||
| Men, n (%) | 73 (78.5) | 81 (75.7) | 70 (72.2) | 70 (76.1) | 0.58 |
| Women, n (%) | 20 (21.5) | 26 (24.3) | 27 (27.8) | 22 (23.9) | |
| Indication for HTx | |||||
| Ischemic cardiomyopathy, n (%) | 34 (36.6) | 38 (35.5) | 34 (35.1) | 44 (47.8) | 0.14 |
| Dilated cardiomyopathy, n (%) | 38 (40.9) | 45 (42.1) | 40 (41.2) | 34 (37.0) | 0.58 |
| Other, n (%) | 21 (22.6) | 23 (21.5) | 23 (23.7) | 14 (15.2) | 0.30 |
| Past smoker, n (%) | 58 (63.0) | 61 (57.0) | 61 (63.5) | 59 (64.8) | 0.58 |
| Hypertension, n (%) | 79 (84.9) | 95 (88.8) | 88 (90.7) | 86 (93.5) | 0.056 |
| Diabetes, n (%) | 22 (23.7) | 23 (21.5) | 22 (22.7) | 23 (25.0) | 0.78 |
| Osteoporotic fracture, n (%) | 1 (1.1) | 2 (1.9) | 2 (2.1) | 7 (7.6) | 0.021 |
| BMD T-score ≤ -2.5, n (%) | 10 (10.8) | 11 (10.3) | 17 (17.5) | 18 (19.6) | 0.037 |
| Anti-osteoporotic treatment, n (%) | 11 (11.8) | 21 (19.6) | 39 (40.2) | 52 (56.5) | <0.0001 |
| Thiazides, n (%) | 10 (10.8) | 6 (5.6) | 14 (14.4) | 12 (13.0) | 0.25 |
| Immunosuppression | |||||
| Tacrolimus, n (%) | 77 (82.8) | 86 (80.4) | 66 (68.0) | 50 (54.3) | <0.0001 |
| Cyclosporine, n (%) | 12 (12.9) | 10 (9.3) | 22 (22.7) | 32 (34.8) | <0.0001 |
| Everolimus, n (%) | 5 (5.4) | 8 (7.5) | 4 (4.1) | 4 (4.3) | 0.52 |
| Mycophenolic acid, n (%) | 65 (69.9) | 82 (76.6) | 70 (72.2) | 64 (69.6) | 0.77 |
| Azathioprine, n (%) | 9 (9.7) | 4 (3.7) | 9 (9.3) | 4 (4.3) | 0.40 |
| Methylprednisolone, n (%) e | 28 (30.1) | 36 (33.6) | 33 (34.0) | 37 (40.2) | 0.17 |
Values are mean (SD), median (IQR) or number of patients (%). Abbreviations: HTx, heart transplantation; BMI, body mass index; SBP/DBP, systolic/diastolic blood pressure; eGFR, estimated glomerular filtration rate; BMD, bone mineral density. Anti-osteoporotic treatment refers to calcium supplementation, vitamin D supplements and/or bisphosphonates. To convert serum creatinine from μmol/L to mg/dL, multiply by 0.0113. To convert serum albumin from g/L to g/dL, multiply by 0.1. To convert serum total and ionized calcium from mmol/L to mg/dL, multiply by 4. p-values refer to the difference between cases and controls in the derivation, replication and time-shifted replication datasets.
In exploratory analyses without any adjustment, the 389 uPROPHET patients were stratified by their OSTEO18 score: low (<-1), medium-low (−1 to 0), medium-high (0–1) and high (≥1). Across increasing OSREO18 categories (Table 2), age (low vs high: 54.0 vs 62.5 years; trend p-value: <0.0001), the time interval since HTx (4.33 vs 10.2 years; p = 0.0024), serum creatinine (118.4 vs 135.0 μmol/L; p = 0.0062) and the use of cyclosporine (12.9 % vs 34.8 %; p < 0.0001) increased, whereas eGFR (63.7 vs 51.6 mL/min/1.73 m2; p = 0.002) and tacrolimus treatment (82.8 % vs 54.3 %; p = 0.0001) decreased. The components of the osteoporotic endpoint were positively associated with higher OSTEO18 strata (Table 2). The osteoporosis rates expressed in cases per 100 patients were 17/93 (18.3 % [95 % CI: 10.4–26.1 %]) in the low OSTEO18 category, 27/107 (25.2 % [17.0–33.5]) and 48/97 (49.5 % [39.5–59.4]) in the medium-low and medium-high categories, and 62/92 (67.4 % [57.8–77.0 %]) in the high category (trend p-value: 0.019).
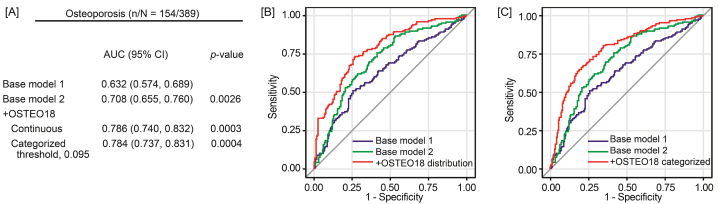
Using unadjusted, partially adjusted and fully adjusted logistic regression, there was a significantly positive association between the risk of osteoporosis and the OSTEO18 level, irrespective of adjustment (Table 3) and irrespective of whether OSTEO18 was analyzed as a categorical or continuously distributed variable with an optimized threshold of 0.095 (Table 4). From the low to high OSTEO18 category (Table 3), the ORs increased from 0.39 (95 % CI: 0.25–0.61) to 3.14 (2.08–4.75), resulting in a trend p-value of <0.0001. With full adjustments applied (model 2), OSTEO18 significantly improved the AUC from 0.708 to 0.786 (p = 0.0003) for the continuously distributed classifier (Fig. 3A and B) and to 0.784 (p = 0.0004) for the categorized classifier (Fig. 3A and C). To place the discriminatory performance into context, Table S5 lists the ORs associated with single risk factors for osteoporosis for which the model 2 (Fig. 3A, B and 3C) was adjusted. The AUC of OSTEO18 was 0.74 (95%CI: 0.69–0.79), which achieved good discriminative performance compared with other single risk factors (Table S5).
Table 3.
Association of osteoporosis with OSTEO18 in 389 uPROPHET patients.
| Variable | n/N (%) | Unadjusted |
Adjusted |
Fully adjusted |
|||
|---|---|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | ||
| OSTEO18 score (categorized) | |||||||
| <-1 | 17/93 (18.3) | 0.36 (0.23, 0.55) | <0.0001 | 0.37 (0.24, 0.58) | <0.0001 | 0.39 (0.25, 0.61) | <0.0001 |
| -1–0 | 27/107 (25.2) | 0.54 (0.37, 0.79) | 0.0016 | 0.54 (0.36, 0.79) | 0.0017 | 0.53 (0.35, 0.79) | 0.0017 |
| 0–1 | 48/97 (49.5) | 1.57 (1.09, 2.24) | 0.015 | 1.57 (1.09, 2.27) | 0.016 | 1.57 (1.07, 2.29) | 0.020 |
| ≥1 | 62/92 (67.4) | 3.31 (2.26, 4.84) | <0.0001 | 3.18 (2.14, 4.73) | <0.0001 | 3.14 (2.08, 4.75) | <0.0001 |
| p for linear trend | <0.0001 | <0.0001 | <0.0001 | ||||
| OSTEO18 score (continuous, +1) | 154/389 (39.6) | 2.07 (1.70, 2.50) | <0.0001 | 2.02 (1.67, 2.46) | <0.0001 | 2.03 (1.65, 2.49) | <0.0001 |
Abbreviations: OR, odds ratio; CI, confidence interval. n/N indicates the number of participants with osteoporosis, the number of participants at risk in each category, and the corresponding percentages. In logistic regression, OSTEO18 was introduced as categorical and as continuously distributed variable. The categorized OSTEO18 score (<-1, -1-0, 0–1 and ≥1) was modeled by the deviation from mean coding, so that ORs express the relative risk compared with the average risk in the whole study population. Adjusted logistic models accounted for sex, age, eGFR, diabetes (yes vs no) and treatment with methylprednisolone (yes vs no). Fully adjusted logistic models additionally accounted for the time interval since HTx, ionized calcium, treatment with thiazides (yes vs no), tacrolimus (yes vs no) and cyclosporine (yes vs no).
Table 4.
Discriminative performance of OSTEO18 in 389 uPROPHET patients.
| Performance | Estimate (95%CI) |
|---|---|
| n/N | 154/389 |
| Continuously distributed OSTEO18 | |
| AUC | 0.74 (0.69, 0.79) |
| Categorized OSTEO18 | |
| Youden cut-off threshold | 0.095 |
| Sensitivity, % | 71.4 (63.6, 78.4) |
| Specificity, % | 69.8 (63.5, 75.6) |
| PLR | 2.36 (1.90, 2.94) |
| NLR | 0.41 (0.31, 0.53) |
| Accuracy | 70.4 (65.6, 74.9) |
Abbreviations: CI, confidence interval; AUC, area under curve; PLR, positive likelihood ratio (true positive rate/false positive rate); NLR, negative likelihood ratio (false negative rate/true negative rate). n/N indicates the number of participants with osteoporosis and the number of participants at risk. Accuracy is the overall probability that a patient is correctly classified. All estimates in this table were unadjusted for other osteoporosis risk factors.
Fig. 3.
Performance of the OSTEO18 classifier on top of other osteoporosis risk factors in 389 uPROPHET patients. n/N indicates the number of patients with osteoporosis and the number of participants at risk (panel A). Base model 1 includes sex, age, the estimated glomerular filtration rate, diabetes (yes vs no) and treatment with methylprednisolone (yes vs no). Base model 2 additionally accounted for the time interval since HTx, ionized calcium, treatment with thiazides (yes vs no), tacrolimus (yes vs no) and cyclosporine (yes vs no). In subsequent steps, OSTEO18 was added to the previous model as a continuously distributed variable (panel B) or as a categorized variable (panel C) based on the 0.095 optimized threshold. At each step, p-values are for the comparison with the preceding model. AUC is the area under curve given with 95 % confidence interval.
4. Discussion
The key finding of the current study was the development, replication and validation of a novel 18-peptide multidimensional urinary biomarker indicative of osteoporosis in HTx patients. On top of relevant osteoporosis risk factors, OSTEO18 improved the AUC as categorized variable with optimized threshold of 0.095 as well as continuously distributed biomarker. Collagens type I, III, and V are the most abundant constituents of the organic extracellular matrix in bones [36,37]. The main function of collagens is mechanical support and to act as a scaffold for bone cells. Type-I collagen accounts for 90 % of total collagen in bone tissue [36]. Only breakdown products of mature collagen appear in urine [38]. In the first step of type-I collagen degradation small fragments are released by collagenases, while larger fragments are further degraded by gelatinases with the resulting smaller fragments being further cleaved by gelatinases [38]. The OSTEO18 urinary biomarker includes 8 fragments (44.4 %) derived from type-I collagen and 2 (11.1 %) from type-III collagen and plausibly reflects the turnover of the extracellular bone matrix. Furthermore, calcium is absorbed in the mammalian small intestine by two general mechanisms: a transcellular active transport process, located largely in the duodenum and upper jejunum; and a paracellular, passive process that functions throughout the length of the intestine [39]. The polymeric immunoglobulin receptor (PIGR) has the dual role of transporting locally produced dimeric IgA across mucosal epithelia, and serving as the precursor of secretory component and plays a key role in mucosal immunity [40]. The presence of a PIGR-derived urinary peptide in OSTEO18 might be explained by dysregulation of its role by immunosuppression.
In addition to aging, menopause and renal dysfunction compromising calcium homeostasis and the activation of calcidiol to active calcitriol, the root causes of osteoporosis in HTx patients are the use of glucocorticosteroids and immunosuppressive drugs, such as cyclosporin and tacrolimus, to prevent allograft rejection. Glucocorticosteroids reduce bone formation mainly at the trabecular core of the vertebrae [41]. The calcineurin blockers cyclosporine and tacrolimus stimulate the secretion of parathyroid hormone [3] and are nephrotoxic [42,43]. Cyclosporine induces tubular renal cell apoptosis and increases the calcium levels of renal tubular cells [29]. Furthermore, cyclosporine stimulates both osteoblasts and osteoclasts. However, the osteoclastic activity dominates and induces elevated bone turnover and delayed repair of renal osteodystrophy [3,42]. In experimental rat studies [28], tacrolimus decreased bone mineral density in the tibia and femur without influence on bone formation. Keeping in mind that over half of the peptide fragments included in OSTEO18 are derived from type-I and type-III collagens, the predominant collagens in bone tissue, the novel biomarker might be applied to monitor the osteotoxic effects of glucocorticosteroids, cyclosporine and tacrolimus.
Osteoporosis is a silent disease until it is complicated by fractures—fractures that occur following minimal trauma or, in some cases, with no trauma. Therefore, osteoporosis represents a major public health issue [44], in terms of the quality of life of the patients and social health cost. Several risk factors and circulating biomarkers reflecting osteoporosis in HTx patients can be monitored, although on their own they have an AUC not exceeding 60 % (Table S5), whereas a 1-unit increment in OSTEO18 was associated with a doubling of the osteoporosis risk with an AUC of 0.74 (95 % CI: 0.69–0.79). More importantly, on top of sex, age, the time interval since HTx, eGFR, diabetes, and treatment with methylprednisolone, tacrolimus, cyclosporin and thiazide diuretics, OSTEO18 maintained its discriminatory performance, irrespective of whether the optimized 0.095 threshold was applied or OSTEO18 was analyzed as continuously distributed variable. BMD testing by dual energy XRA is consensually the gold standard in the diagnosis and management of osteoporosis, because the so-determined BMD at the lumbar spine and hip closely correlates with bone strength and is an excellent predictor of future fracture risk [13,14]. Nevertheless, XRA also has limitation due to the confounding effects on the BMD measurement of surrounding soft tissue, bone artefacts caused by osteoarthritis, degenerated discs, aortic calcification and vertebral compression fractures [14]. Other limitations in the administration of XRA scans are imposed by the applicable health insurance. In Belgium (nomenclatuurart17ter_20230401_01.pdf available at www.riziv.be), a dual energy XRA (code: N72) is reimbursed at a rate of €40.25. In the context of HTx, it should be noted that the examination is only reimbursed if the patient has a low-impact vertebral fracture or has been on therapy with glucocorticosteroids for ≥3 months at a daily dose equivalent to 7.5 mg prednisolone. However, in 2012, legislation changed making dual energy XRA only reimbursable at 5-year instead of annual intervals. These rules do not support efficient prevention of osteoporotic complications in HTx patients, because a vertebral fracture already indicates the presence of the disease and because the nomenclature disregards the osteotoxic side-effects of cyclosporine and tacrolimus. Given reimbursement rules, which differ from country to country, the low diagnostic and prognostic value of single osteoporosis risk factors and some pitfalls potentially associated with dual energy XRA, OSTEO18 might be added testing armamentarium provided that cost-effectiveness can be demonstrated, as discussed in the next paragraph.
Cost-effectiveness balances health-care costs against non-monetary units, such as quality-adjusted life-years (QALYs) [45]. The QALY-based value proposition is well established in the UK, Sweden, Belgium, the Netherlands, Luxembourg, and some eastern European countries, but health-care insurers in Germany and France prefer assessing changes in clinical outcomes instead [45]. Bone loss progresses rapidly after HTx. Osteoporosis causes a high economic and social burden, predominantly due to severe fractures. In the United States, among 885,676 Medicare beneficiaries, the per-patient annual health insurance costs from January 2010 until September 2014 were substantially higher in patients (mean age: 80.5 years; 94 % female) with an osteoporosis-related fracture than in the matched nonfracture population ($47163.25 vs $16034.61) [46]. In 12 Belgian hospitals, the in-patient costs Belgium associated with total hip replacement, which is the feasible treatment for severe fracture, averaged €8013 [47]. In addition, the estimated total costs were €465 million for anti-osteoporotic medication, preventive supplement and the treatment of osteoporosis-related fracture, according the Achmea Health Database (108,013 individuals) in 2010 in the Nederland [48]. Along similar lines, in France, the annual per-person osteoporosis-related costs amounted to €18,040 per person, of which €17,905 was directly related to the fracture and €135 to the management of osteoporosis [49]. The timely institution of anti-osteoporotic is therefore of paramount clinical importance to avoid bone fractures and associated debilitating complications and risky and costly orthopedic surgery [2]. OSTEO18 is being certified as an in-vitro diagnostic and is therefore ready to enter the clinical management of HTx patients. However, the aforementioned costs have to be balanced against those of an OSTEO18 test (€850), which requires further clinical validation in HTx patients.
4.1. Strong points and limitations
Strong points of current study are the derivation and replication of the multidimensional urinary classifier OSTEO18 in the case-control studies, as well as the validation in the whole uPROPHET cohort. The UPP is unbiased in the sense that it does not depend on predefined markers, but is only determined by peptides passing the glomerular barrier or generated along the nephron, and because it is platform independent [6]. Notwithstanding the consistency of the findings, the current study must also be interpreted within the context of its limitations. First, the current study had a cross-sectional design, albeit that the time-shifted case-control study added some longitudinal dimension in the replication of the results from the discovery dataset. Second, cases and controls in the discovery and two replication datasets were well matched for the use of methylprednisolone, but it was impossible to achieve complete matching for immunosuppressive drugs, because treatment strategies changed over time. Third, there was a preponderance of short amino-acid chains among the sequenced peptides, which may be due to the reduced success of sequencing longer-chain peptides. Unknown posttranslational modifications are the most likely cause for the failure to identify a peptide sequence with confidence. Peptide abundance also impacts on the quality of the MS/MS spectra. However, while only 17 % of the detected urinary peptides were sequenced, those currently sequenced represented 63 % of the total peptide mass [50]. Fourth, the current results were generated in a single-center study of HTx recipients, with specific immunosuppressant treatments and vulnerability inducing higher risks of osteoporosis [3] and other aging-related diseases [51]. The OSTEO18 findings cannot be extrapolated to other patients, such as for instance postmenopausal women. Finally, because our proof-of-concept study is retrospective, osteoporotic biomarkers other than bone specific alkaline phosphatase were not measured.
5. Conclusion
OSTEO18 is a clinically meaningful novel biomarker indicative of osteoporosis in HTx recipients. Given that OSTEO18 is being certified as in-vitro diagnostic, the test is ready to enter the clinical management of HTx patients. However, to be endorsed by HTx transplantation guidelines, OSTEO18 required further evidence from prospective studies and demonstration of its cost-effectiveness.
Funding
uPROPHET was supported by the European Research Council (Advanced Researcher Grant-2011-294713-EPLORE and Proof-of-Concept Grant-713601-uPROPHET awarded to JAS). OMRON Healthcare, Co., Ltd., Kyoto, Japan provided a non-binding grant to the Non-Profit Research Association Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium. Dries S. Martens holds a postdoctoral grant by the Research Foundations Flanders (FWO grants 12 × 9623 N).
Data sharing statement
Pseudonymize-anonymized UPROPHET data can be made available but only after the request to access data has been approved by the Ethics Review Board of the University Hospitals of Leuven. Requests have to be directed to the corresponding author. Proposals will be reviewed by the authors with scientific merit as sole evaluation criterion. Data can be shared via a secure online platform after signing a data access and confidentiality agreement. All data will be made available for up to 3 years after publication of the current article.
CRediT authorship contribution statement
Yu-Ling Yu: Writing – original draft, Visualization, Formal analysis, Conceptualization. Qi-Fang Huang: Writing – review & editing, Methodology, Investigation, Data curation. De-Wei An: Writing – review & editing, Methodology. Julia Raad: Resources, Methodology, Formal analysis. Dries S. Martens: Writing – review & editing. Agnieszka Latosinska: Writing – review & editing, Methodology, Investigation, Formal analysis. Katarzyna Stolarz-Skrzypek: Writing – review & editing, Supervision. Johan Van Cleemput: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Conceptualization. Ying-Qing Feng: Writing – review & editing. Harald Mischak: Writing – review & editing, Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Karel Allegaert: Writing – review & editing, Supervision. Peter Verhamme: Writing – review & editing, Supervision. Stefan Janssens: Writing – review & editing, Supervision, Methodology, Investigation. Tim S. Nawrot: Writing – review & editing, Supervision, Methodology, Investigation. Jan A. Staessen: Writing – original draft, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: uPROPHET was supported by the European Research Council (Advanced Researcher Grant-2011-294713-EPLORE and Proof-of-Concept Grant-713601-uPROPHET awarded to JAS). OMRON Healthcare, Co., Ltd., Kyoto, Japan provided a non-binding grant to the Non-Profit Research Association Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium. Dries S. Martens holds a postdoctoral grant by the Research Foundations Flanders (FWO grants 12X9623N).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24867.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Committee on Clinical Practice Guideline-Gynecology. Osteoporosis prevention, screening, and disgnosis: ACOG clinical practice guideline No. 1. Obstet. Gynecol. 2012;138:494–506. doi: 10.1097/AOG.0000000000004514. [DOI] [PubMed] [Google Scholar]
- 2.Löfdahl E., Rådegran G. Osteoporosis following heart transplantation and immunosuppressive therapy. Transplant. Rev. 2017;31:232–239. doi: 10.1016/j.trre.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Kovvuru K., Kanduri S.R., Vaitla P., et al. Risk factors and management of osteoporosis post-transplant. Medicina (Kaunas) 2020;56:302. doi: 10.3390/medicina56060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mischak H., Kolch W., Aivaliotis M., et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl. 2010;4:464–478. doi: 10.1002/prca.200900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavrogeorgis E., Mischak H., Latosinska A., et al. Reproducibility evaluation of urinary peptide detection using CE-MS. Molecules. 2021;26:7260. doi: 10.3390/molecules26237260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein J., Papadopoulos T., Mischak H., et al. Comparison of CE-MS/MS and LC-MS/MS sequencing demonstrates significant complementarity in natural peptide identification in human urine. Electrophoresis. 2014;35:1060–1064. doi: 10.1002/elps.201300327. [DOI] [PubMed] [Google Scholar]
- 7.Saito M., Marumo K. Effects of collagen crosslinking on bone material properties in health and disease. Calcif. Tissue Int. 2015;97:242–261. doi: 10.1007/s00223-015-9985-5. [DOI] [PubMed] [Google Scholar]
- 8.Martens D.S., Thijs L., Latosinska A., et al. Urinary peptidomics to address age-related disabilities: a prospective population study with replication in patients. Lancet Healthy Longevity. 2021;2:e690–e703. doi: 10.1016/S2666-7568(21)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q.F., Trenson S., Zhang Z.Y., et al. Biomarkers to assess right heart pressures in recipients of a heart transplant: a proof-of-concept study. Transplant Direct. 2018;4:e346. doi: 10.1097/TXD.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Q.F., Zhang Z.Y., Van Keer J., et al. Urinary peptidomic biomarkers of renal function in heart transplant recipients. Nephrol. Dial. Transplant. 2019;34:1336–1343. doi: 10.1093/ndt/gfy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Q.F., Trenson S., Zhang Z.Y., et al. Urinary proteomics in predicting heart transplantation outcomes (uPROPHET) - Rationale and database description. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Cosman F., de Beur S.J., LeBoff M.S., et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2014;25:2539. doi: 10.1007/s00198-014-2794-2. 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorentzon M., Cummings S.R. Osteoporosis: the evolution of a diagnosis. J. Intern. Med. 2015;277:650–661. doi: 10.1111/joim.12369. [DOI] [PubMed] [Google Scholar]
- 15.Braverman E.R., Chen T.J.H., Chen A.L.C., et al. AGe-related increases in parathyroid hormaone may be antecedent to both osteoporosis and dementia. BMC Endocr. Disord. 2023;9:21. doi: 10.1186/1472-6823-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S., Kumar D., Lal A.K. Diagnostic biomarker for primary osteoporosis in women. J. Clin. Diagn. Res. 2015;9:RC04–RC07. doi: 10.7860/JCDR/2015/14857.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata K., Tokuhiro H., Nakatsuka K., et al. Measurement of bone-specific alkaline phosphatase by an immunoselective enzyme assay method. Ann. Clin. Biochem. 1996;33:127–131. doi: 10.1177/000456329603300205. [DOI] [PubMed] [Google Scholar]
- 18.Richart T., Li Y., Staessen J.A. Renal versus extrarenal activation of vitamin D in relation to atherosclerosis, arterial stiffening, and hypertension. Am. J. Hypertens. 2007;20:1007–1015. doi: 10.1016/j.amjhyper.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Bouquegneau A., Salam S., Delanaye P., et al. Bone disease after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2016;11:1282–1296. doi: 10.2215/CJN.11371015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jantos-Siwy J., Schiffer E., Brand K., et al. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J. Proteome Res. 2009;8:268–281. doi: 10.1021/pr800401m. [DOI] [PubMed] [Google Scholar]
- 21.Mischak H., Vlahou A., Ioannidis J.P.A. Technical aspects and inter-laboratory variability in native peptide profiling : the CE-MS experience. Clin. Biochem. 2013;46:432–443. doi: 10.1016/j.clinbiochem.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z.Y., Ravassa S., Nkuipou-Kenfack E., et al. Novel urinary peptidomic classifier predicts incident heart failure. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An D.W., Yu Y.L., Martens D.S., et al. Statistical approaches applicable in managing OMICS data: urinary proteomics as exemplary case. Mass Spec Rev. 2023;42:1–18. doi: 10.1002/mas.21849. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57:289–300. [Google Scholar]
- 25.Wendt R., Thijs L., Kalbitz S., et al. A specific urinary peptidomic profile predicts outcome in SARS-CoV-2-infected patients. eClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng L., Zhang K., Zhang Z. Effectiveness of thiazides on serum and urinary calcium levels and bone mineral density in patients with osteoporosis: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:3929–3935. doi: 10.2147/DDDT.S179568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda J., Izumo N., Furukawa M., et al. Effects of the calcineurin inhibitors cyclosporine and tacrolimus on bone metabolism in rats. Biomed Res. 2018;39:131–139. doi: 10.2220/biomedres.39.131. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook P.N. Cyclosporine and bone mass. Clin. Exp. Rheumatol. 2000;18:S93–S96. [Google Scholar]
- 30.Karbhari D.S., Karbhari N.S., Patel S. Significance of measurement of corrected calcium inpatients with normoalbuminemia. Int J Sci Public Health. 2017;6(6):1069–1071. [Google Scholar]
- 31.Pouresmaeili F., Kamalidehghan B., Kamareher M., et al. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14:2029–2049. doi: 10.2147/TCRM.S138000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anastasilakis A.D., Tsourdi E., Makras P., et al. Bone disease following solid organ transplatation: a narrative review and recommendations for management from the European Calcified Tissue Society. Bone. 2019;127:401–418. doi: 10.1016/j.bone.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Hsu C.-Y., Chen L.-R., Chen K.-H. Osteoporosis in patients with chronic kidney disease: a systemic review. Int. J. Mol. Sci. 2020;21:6846. doi: 10.3390/ijms21186846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosmer D.W., Jr., Lemeshow S. John Wiley & Sons; New York: 1989. Polytomous Independent Variable. Applied Logistic Regression; pp. 47–56. [Google Scholar]
- 35.DeLong E.R., DeLong D.M., Clarke-Peterson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 36.Lin X., Patil S., Gao Y.G., et al. The bone extracellular matrix in bone formation and regeneration. Front Phrmacol. 2020;11:757. doi: 10.3389/fphar.2020.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licini C., Vitale-Brovarone C., Mattiololi-Belmonte M. Collagen and non-collagenous proteins molecular crosstalk in the pathophysiology of osteoporosis. Cytokine Growth Factor Rev. 2019;49:59–69. doi: 10.1016/j.cytogfr.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Lόpez B., González A., Ravassa S., et al. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. JACC (J. Am. Coll. Cardiol.) 2015;65:2449–2456. doi: 10.1016/j.jacc.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Bronner F. Mechanisms of intestinal calcium absorption. J. Cell. Biochem. 2003;88:387–393. doi: 10.1002/jcb.10330. [DOI] [PubMed] [Google Scholar]
- 40.Johansen F.E., Kaetzel C.S. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4:598–602. doi: 10.1038/mi.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid I.R., Billington E.O. Drug therapy for osteoporosis in older adults. Lancet. 2022;399:1080–1092. doi: 10.1016/S0140-6736(21)02646-5. [DOI] [PubMed] [Google Scholar]
- 42.Sikma M.A., van Maarseveen E.M., van de Graaf E.A., et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am. J. Transplant. 2015;15:2301–2313. doi: 10.1111/ajt.13309. [DOI] [PubMed] [Google Scholar]
- 43.Wu Q., Wang X., Nepovimova E., et al. Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophay, and signaling. Food Chem. Toxicol. 2018;118:889–907. doi: 10.1016/j.fct.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 44.Bliuc D., Nguyen N.D., Milch V.E., et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. J. Am. Med. Assoc. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 45.Johannesson M. The relationship between cost-effectiveness analysis and cost-benefit analysis. Soc. Sci. Med. 1995;41:483–489. doi: 10.1016/0277-9536(94)00353-u. [DOI] [PubMed] [Google Scholar]
- 46.Williams S.A., Daigle S.G., Weiss R., et al. Economic burden of osteoporosis-related fractures in the US medicasre population. Ann. Pharmacother. 2021;55:821–829. doi: 10.1177/1060028020970518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dehanne F., Gourdin M., Devleesschauwer B., et al. Cost-DALY comparison of hip replacement care in 12 Belgian hospitals. BMJ Open Quality. 2021;10 doi: 10.1136/bmjoq-2020-001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunnewind T., Dvortsin E.P., Smeets H.M., et al. Economic consequences and potentially preventable costs related to osteoporosis in The Netherlands. Value Health. 2017;20:762–768. doi: 10.1016/j.jval.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Thomas T., Tubach F., Bizouard G., et al. The economic burden of severe osteoporotic fractures in the French healthcare database: the FRACTOS study. J. Bus. Manag. Res. 2022;37:1811–1822. doi: 10.1002/jbmr.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latosinska A., Siwy J., Mischak H., et al. Peptidomics and proteomics based on CE-MS as a robust tool in clinical application: the past, the present, and the future. Electrophoresis. 2019;40:2294–2308. doi: 10.1002/elps.201900091. [DOI] [PubMed] [Google Scholar]
- 51.Kobashigawa J., Shah P., Joseph S., et al. Frailty in heart transplatation: report from the heart workgroup of a consensus conference on frailty. Am. J. Transplant. 2021;21:636–644. doi: 10.1111/ajt.16207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.