Abstract
BACKGROUND
Lack of mobilization and prolonged stay in the intensive care unit (ICU) are major factors resulting in the development of ICU-acquired muscle weakness (ICUAW). ICUAW is a type of skeletal muscle dysfunction and a common complication of patients after cardiac surgery, and may be a risk factor for prolonged duration of mechanical ventilation, associated with a higher risk of readmission and higher mortality. Early mobilization in the ICU after cardiac surgery has been found to be low with a significant trend to increase over ICU stay and is also associated with a reduced duration of mechanical ventilation and ICU length of stay. Neuromuscular electrical stimulation (NMES) is an alternative modality of exercise in patients with muscle weakness. A major advantage of NMES is that it can be applied even in sedated patients in the ICU, a fact that might enhance early mobilization in these patients.
AIM
To evaluate safety, feasibility and effectiveness of NMES on functional capacity and muscle strength in patients before and after cardiac surgery.
METHODS
We performed a search on Pubmed, Physiotherapy Evidence Database (PEDro), Embase and CINAHL databases, selecting papers published between December 2012 and April 2023 and identified published randomized controlled trials (RCTs) that included implementation of NMES in patients before after cardiac surgery. RCTs were assessed for methodological rigor and risk of bias via the PEDro. The primary outcomes were safety and functional capacity and the secondary outcomes were muscle strength and function.
RESULTS
Ten studies were included in our systematic review, resulting in 703 participants. Almost half of them performed NMES and the other half were included in the control group, treated with usual care. Nine studies investigated patients after cardiac surgery and 1 study before cardiac surgery. Functional capacity was assessed in 8 studies via 6MWT or other indices, and improved only in 1 study before and in 1 after cardiac surgery. Nine studies explored the effects of NMES on muscle strength and function and, most of them, found increase of muscle strength and improvement in muscle function after NMES. NMES was safe in all studies without any significant complication.
CONCLUSION
NMES is safe, feasible and has beneficial effects on muscle strength and function in patients after cardiac surgery, but has no significant effect on functional capacity.
Keywords: Neuromuscular electrical stimulation, Cardiac surgery, coronary artery bypass grafting, Heart valve replacement, Peak VO2, Safety
Core Tip: Data regarding the effects of neuromuscular electrical stimulation (NMES) in cardiac surgery patients still remains limited. We investigated the safety and the effectiveness of NMES on functional capacity and muscle strength and function in patients before and after cardiac surgery. We observed that NMES has beneficial effects on muscle strength and function, but its effect on functional capacity is not clear. Moreover, NMES is safe and feasible for cardiac surgery patients without any major adverse events.
INTRODUCTION
Polyneuromyopathy, defined as a disorder of both the muscle and the peripheral nerve or lower motor neuron, is a common complication in the majority of patients during their stay in the intensive care unit (ICU) before and after cardiac surgery[1,2]. It is characterized by muscle weakness, increased loss of muscle mass, as well as degeneration of the deep ligaments and tendons[3,4]. During the last decade, the term that was proposed and used to characterize this neuromuscular weakness was intensive care unit acquired weakness (ICUAW)[5]. Lack of mobilization and prolonged stay in the ICU are major factors resulting in the development of muscle weakness[6]. Weaning from mechanical ventilation is quite difficult and muscle strength is limited for several months in patients with ICUAW, with tremendous effects on their quality of life and their mortality rates[7]. Muscle mass is associated with increased muscle strength and therefore, it is a prognostic marker for the clinical outcomes in patients with polyneuromyopathy[3].
Most therapeutical strategies of polyneuromyopathy are focused on prevention of muscle atrophy and degeneration of muscle proteins. Early mobilization has been associated with increased muscle strength and functional capacity[8,9]. Active exercise is not feasible for most patients due to difficulty in producing sufficient muscle contractions and hemodynamic instability[10]. Recent research has shown that neuromuscular electrical stimulation (NMES) is an alternative modality of exercise in patients with muscle weakness due to chronic obstructive pulmonary disease and chronic heart failure[11,12]. NMES causes muscle contraction without patient’s effort, so it can be implemented even in intubated patients under mechanical ventilation. It has beneficial effects as its daily application prevents the progression of neuromyopathy and results in shorter length of stay in the ICU[13]. It also increases muscle strength, exercise endurance and maximum oxygen uptake (peak VO2), reduces protein catabolism and sympathetic nervous system activity in patients with chronic heart failure[11,12,14] and has positive influence on the peripheral microcirculation of skeletal muscles[11,15], which is directly related to the endothelial function[16,17]. All these effects on clinical outcomes may lead to improvement of patients' quality of life.
NMES could be an alternative method of activating skeletal muscles and improving muscle function in patients who cannot exercise after cardiac surgery and present high risk of ICUAW. It may also prevent the progression of ICU-related muscular dystrophy and post-intensive care syndrome. We hypothesized that NMES is safe and feasible in patients before and after cardiac surgery, leading in improvement in functional capacity and prevention or reduction of neuromyopathy. The aim of this systematic review was to evaluate safety and effectiveness of NMES on functional capacity and mobility in patients before or after undergoing cardiac surgery.
MATERIALS AND METHODS
Search strategy
Authors searched literature for suitable articles that included in-hospital implementation of NMES in patients before and after cardiac surgery. The search was conducted between April of 2023 and May of 2023 in 4 science databases; Pubmed, Physiotherapy Evidence Database (PEDro), Embase and CINAHL. Terms which were used included (“cardiac surgery” OR “left ventricular assist device” OR “LVAD” OR “ECMO” OR “extracorporeal membrane oxygenation” OR “coronary artery bypass grafting” OR “CABG” OR “heart valve replacement”) AND (“electrotherapy” OR “electrical stimulation” OR “electrical muscle stimulation” OR “electromyostimulation” OR “electrostimulation” OR “neuromuscular stimulation” OR “Functional Electrical Stimulation” OR “FES” OR “Neuromuscular Electrical Stimulation” OR “NMES”). Studies were selected according to the PRISMA and the PRISMA checklist. Duplicates were removed from the initial number of studies and the rest were initially screened using only the title and the abstract and then, the full text of the articles. Two independent reviewers reviewed all these articles for eligibility. The final evaluation of the process was performed by a third independent reviewer.
Study selection criteria
Inclusion criteria were: (1) Studies available as full texts in English; (2) published randomized controlled trials (RCTs) in peer-reviewed journals; (3) study groups including patients before and after cardiac surgery such as CABG, valve replacement, LVAD, cardiac transplantation, etc.; (4) patients aged ≥ 18 years, (5) NMES protocols of at least 1 session compared to usual care or sham NMES of the control group, and (6) outcome measures focused on safety, functional capacity assessed by 6MWT or other indices and muscle strength (ambulation ability, MRC values, etc.).
Exclusion criteria were: (1) Non RCTs, reviews, guidelines, commentaries, case reports, editorials or conference abstracts; (2) additional interventions in study groups except for NMES; (3) studies including patients with hemodynamic instability of high risk; (4) studies including patients with other types of surgeries, (5) studies including patients aged < 18 years; and (6) studies including NMES and other exercise modalities that were unable to be quantified.
Quality assessment
Two independent reviewers used PEDro in order to assess all the included RCTs for methodological rigor and risk of bias, using similar methods with a recently published study[18]. PEDro is an 11-point scale for assessing RCTs for internal validity and control of bias. Maximum score is 10 as the first question does not contribute to total score. A study with a score of 6-10 is considered of excellent quality, a study with 4-5 of fair quality, and a score of 3 or less gives a poor-quality study. If the 2 reviewers did not agree for their quality score, then an independent third reviewer made the final decision.
Outcome measures
The primary outcome measures were functional capacity, assessed by 6MWT or other indices, and safety of NMES. The secondary outcome measure was muscle strength and mass assessed by several indices such as the 1 repetition maximum test (1RM test), the sit-and-stand test (SST), perimeter of the thighs, grip strength, knee extensors strength, cross-sectional area of the quadriceps femoris, etc. All outcomes were evaluated at baseline and after NMES intervention.
RESULTS
Screening of the articles
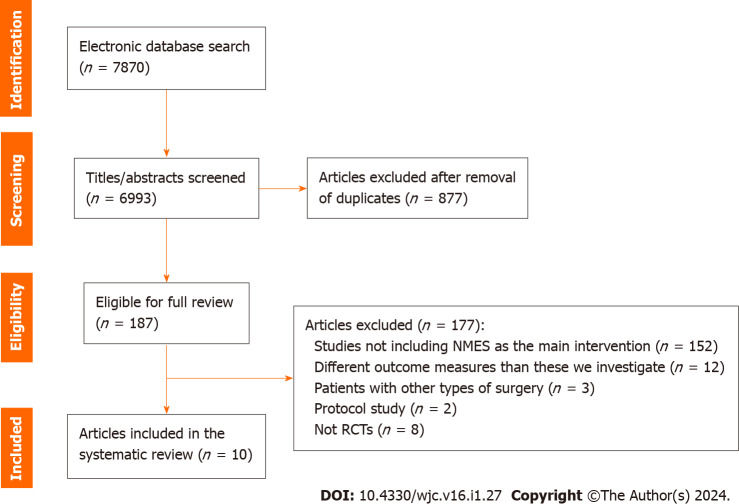
From the initial 7870 studies derived from Pubmed, Embase, Physiotherapy Evidence Database (PEDro) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) database, 877 duplicates were removed and 6993 studies remained for title and abstract screening. Among these, 187 studies were eligible for full text review. After the full text review of these 187 studies, 152 were excluded because they did not include NMES, 12 presented different endpoints, 3 included other surgeries, 2 articles were RCT protocols without results, while 8 studies were clinical trials without randomization. As a result, we finally found 10 RCTs eligible for our systematic review[19-28] (Figure 1).
Figure 1.
PRISMA flowchart regarding the screening results of the systematic review. RCT: Randomized controlled trial; NMES: Neuromuscular electrical stimulation.
Quality assessment
Scores from the PEDro scale, which was used as a quality assessment tool, ranged from 3 to 9 for these studies (Table 1). A single study scored 3 points, being assessed as poor-quality study. Seven studies out of 10 scored 6-8 points being assessed as good-quality studies, while 2 out of 10 studies scored 9 points and were of excellent quality. Blindness of therapists and participants, concealed allocation and adequate follow-up had the lowest scores.
Table 1.
Quality assessment of the included studies using the physiotherapy evidence database
|
|
Fischer et al[19]
|
Schardong et al[20]
|
Kitamura et al[21]
|
Fontes Cerqueira et al[22]
|
Fontes Cerqueira et al[23]
|
Sumin et al[24]
|
Rengo et al[25]
|
Cerqueira et al[26]
|
Takino et al[27]
|
Sumin et al[28]
|
| Eligibility criteria1 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Random allocation | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Concealed allocation | √ | √ | √ | √ | √ | |||||
| Baseline comparability | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Blinded subjects | √ | √ | √ | √ | ||||||
| Blinded therapists | ||||||||||
| Blinded assessors | √ | √ | √ | √ | √ | √ | ||||
| Adequate follow-up | √ | √ | √ | √ | √ | |||||
| Intention-to-treat analysis | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Between-group comparisons | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Point estimates and variability | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Total score | 8/10 | 9/10 | 7/10 | 6/10 | 7/10 | 6/10 | 3/10 | 7/10 | 9/10 | 6/10 |
Eligibility criteria item does not contribute to total score.
Characteristics of participants
The 10 RCTs resulted in 703 patients before and after cardiac surgery separated almost equally between the interventional and the control group. The majority of them were males (475 vs 228 females). Patients were from 42 to 74 years with a mean BMI ranging from 19.3 to 29.1 kg/m2. Cardiac surgeries included a variety of cases such as aortic valve replacement, CABG, heart transplantation, mitral/aortic/tricuspid valve replacement or reconstruction and Bentall surgery. Studies were conducted in 5 countries; Brazil[20,22,23,26], Japan[21,27], the United States[25], Austria[19] and Russia[24,28]. Table 2 demonstrates baseline characteristics of patients.
Table 2.
The main baseline characteristics among patients after cardiac surgery in each study included in the systematic review
|
Ref.
|
Groups
|
Males/Females (N)
|
Age (yr)
|
Weight (kg)
|
Height (cm)
|
BMI (kg/m2)
|
Type of surgery
|
| Fischer et al[19] | NMES (n = 27); CG (n = 27) | 18/9; 20/7 | 63.3 ± 15.5; 69.7 ± 13.1 | NA | NA | 27.6 ± 3.7; 27.7 ± 4.6 | Aortic valve replacement; CABG; Heart transplantation; Other cardiothoracic surgery; Mitral valve replacement; Mitral valve reconstruction; Tricuspid valve reconstruction; Bentall surgery |
| Schardong et al[20] | FES (n = 10); CG (n = 10) | 7/3; 7/3 | 60 ± 7.3; 63.5 ± 5 | NA | NA | 27.3 ± 3.1; 29.1 ± 6.2 | CABG; Heart valve surgery |
| Kitamura et al[21] | NMES (n = 60); CG (n = 59) | 39/21; 37/22 | 67 (55-74); 70 (61-77) | NA | NA | 25.5 (20.4-24.8); 22.3 (20.4-24.9) | CABG; Valvular surgery; Thoracic Aorta |
| Fontes Cerqueira et al[22] | NMES (n = 26); CG (n = 33) | 18/8; 23/10 | 41.8 ± 13.17; 42.21 ± 14.36 | 66.12 ± 13.29; 61.85 ± 12.69 | 160 ± 6; 165 ± 8 | 25. ± 4.72; 21.96 ± 4.2 | Mitral valve replacement; Aortic valve replacement; Mitral valve reconstruction; Aortic valve reconstruction; Mitral valve replacement + Aortic valve reconstruction |
| Fontes Cerqueira et al[23] | NMES (n = 15); CG (n = 15) | 9/6; 5/10 | 49.87 ± 14.37; 50.93 ± 14.56 | NA | NA | NA | CABG; Valve replacement; CABG + Valve replacement |
| Sumin et al[24] | NMES (n = 18); CG (n = 19) | 12/6; 13/6 | 61.5 (52-70); 64 (60-68) | NA | NA | 28.4 [25.2-30.9]; 28.4 [25.8-32.5] | CABG; Aortic valve replacement; Mitral valve replacement; CABG + valve replacement; Multivalve operations; Bentall surgery; Aortic dissection; Heart transplantation |
| Rengo et al[25] | NMES (n = 18); CG (n = 19) | 16/2; 17/2 | 66.5 ± 1.6; 66.2 ± 1.4 | 89.4 ± 2.7; 90.9 ± 3.8 | 173 ± 1; 176 ± 3 | 29.7 ± 0.8; 29.0 ± 0.8 | CABG; CABG + Valve replacement |
| Cerqueira et al[26] | NMES (n = 23); CG (n = 22) | 12/11; 15/7 | 47.8 ± 13.9; 46.4 ± 13.5 | 72.3 ± 14.8; 68.5 ± 13.6 | 163.1 ± 10.4; 165.2 ± 7.2 | 27.2 ± 4.9; 25.1 ± 4.5 | CABG; Aortic valve replacement; Mitral valve replacement; Mitral valve replacement; + Aortic valve reconstruction |
| Takino et al[27] | NMES (n = 90); CG (n = 90) | 61/29; 63/27 | 74 ± 5; 74 ± 5 | NA | NA | 19.8 (18.0-21.8); 19.3 (18.2-20.8) | CABG; Valvular surgery; Thoracic aorta; Other surgery; Combined surgery |
| Sumin et al[28] | NMES n = 62); CG (n = 60) | 44/18; 39/21 | 62.0 [57.5-66.6]; 63.5 [59.0-69.0] | NA | NA | 27.4 [25.4-31.5]; 28.7 [25.9-33.3] | Prehabilitation (before cardiac surgery) |
Values are presented as a mean ± SD or median (interquartile range). CABG: Coronary artery bypass grafting; CG: Control group; FES: Functional electrical stimulation; NMES: Neuromuscular electrical stimulation group; NA: Not available.
NMES protocols
Table 3 demonstrates details regarding NMES protocols, as well as populations, intervention, comparison, outcomes and study designs (PICOS). NMES was performed in the intervention group in all studies with differences however, in intensity and sessions duration among studies. In 3 studies, stimulator electrodes were applied to the control group but no electricity was delivered[19,20,23] while in the rest 7 studies the control group received only usual care after the surgery[21,22,24-28]. Most studies included at least 5 sessions of NMES except for one that included a single session of NMES[23]. Sessions were performed from 2 to 5 times weekly with a duration from 30 min to 90 min.
Table 3.
Population, intervention, comparison, outcomes, and study design of each study included in the systematic review
| Ref. |
Interventions by group
|
Frequency
|
Session duration
|
Intervention
Duration |
Outcomes
|
Main results
|
Adverse events
|
| Fischer et al[19] | NMES: biphasic rectangular pulses at 66 Hz, pulse duration 0.4 ms, duty cycle 3.5 s on and 4.5 s off to quadriceps muscle bilaterally. CG: stimulator electrodes were applied but no electricity was delivered | 2 times/d for 7 d/wk | 30 min | From POD 1 until ICU exit or POD 14 | Muscle layer thickness, Muscle strength; Functional capacity | No significant effect on MLT. ↑ 4.5 times in recovering muscle strength to NMES group during ICU stay. Positive correlation between change in MLT and cumulative fluid balance (r = 0.43, P = 0.01) the first 3 PODs. No significant effect on functional ability | 5 patients in the NMES group mentioned a feeling of discomfort |
| Schardong et al[20] | 1FES: symmetric biphasic rectangular pulses at 15 Hz, pulse duration 0.5 ms, duty cycle 5 s on and 10 s off to vastus medialis and lateralis muscle bilaterally. CG: Stimulator electrodes were applied but no electricity was delivered | 2 times/wk | 40 min | 8 wk | Functional capacity; Muscle strength; Muscle endurance; Muscle mass | ↑ Distance to 6MWT in the FES group by 11.0% (49.6 m, 95%CI: 15.9-83.3) and in the CG by 10.4% (41.5 m, 95%CI: 7.8-75.2) with no significant between-groups. ↑ muscle strength (7.2 kg, 95%CI: 0.2-14.2). ↑ Muscle endurance (2.2 repetitions, 95%CI: 1.0-3.4) | No complications |
| Kitamura et al[21] | NMES: Symmetric biphasic square pulses, duty cycle 0.4 s on and 0.6 s off, 10 pulse trains (10 s) with 30 s intervals to quadriceps femoris and triceps surae muscle bilaterally. Usual postoperative rehabilitation program. CG: Usual postoperative rehabilitation program | 1 time/d | 30 min | 3 d before surgery and from POD 1 to POD 5 (8 sessions) | The mean concentration of 3-MH/Cre; Physical function; Walking speed; Grip strength | No significant difference in the mean 3-MH/Cre from POD 1 to POD 6 between groups (225.3 [204.0-248.3] μmol/g vs 227.3 [206.3-259.9] μmol/g, P = 0.531). No significant difference in the KEIS on POD 7 between groups (0.44 ± 0.13 kgf/kg vs 0.41 ± 0.12 kgf/kg, P = 0.149. No significant difference in walking speed between groups (1.04 ± 0.24 m/s vs 0.99 ± 0.23 m/s, P = 0.294). No significant difference in grip strength between groups (29.1 ± 10.5 kg vs 26.9 ± 8.7 kg, P = 0.213) | 1 patient mentioned muscle soreness |
| Cerqueira et al[22] | NMES: Stimulation at 50 Hz, duration 400 ms duty cycle 3 s on and 9 s off, to quadriceps and gastrocnemius muscle bilaterally. Regular physiotherapy care. CG: Usual physiotherapy care twice a day | 2 times/d | 60 min | from POD 1 to POD 5 | Ambulation ability; Muscle strength; Functional independence; Quality of life | No significant difference in distance walked (95%CI: -64.87 to 65.97) and walking speed (95%CI: -0.55 to 0.57) between groups. No significant difference in muscle strength in the upper- limb, lower limb, and total MRC values, functional independence, and quality of life between groups | 2 patients reported hypotension, and 1 patient complained of pain |
| Cerqueira et al[23] | NMES: Stimulation at 50 Hz, duration 200 ms duty cycle 3 s on and 9 s off, to quadriceps and gastrocnemius muscle bilaterally. Regular physiotherapy care. CG: stimulator electrodes were applied but no electricity was delivered | Once during the first 48 h of ICU stay | 60 min | 60 min | Hemodynamic responses; Respiratory responses | No difference in heart rate, systolic blood pressure, diastolic blood pressure, mean blood pressure respiratory rate, and oxygen saturation between groups | No complications |
| Sumin et al[24] | NMES: biphasic rectangular pulses at 45 Hz, duty cycle 12 s on and 5 s off to quadriceps muscle bilaterally. CG: Usual postoperative rehabilitation program | 1 time/d | 90 min | from POD 3 to exit the hospital (12 sessions or more) | Knee extensors strength; Handgrip strength; Knee flexor strength CSA of quadriceps femoris |
↑ Knee extensors strength in the NMES group [28.1 (23.8; 36.2) kg on the right and 27.45 (22.3; 33.1) kg on the left] vs CG [22.3 (20.1; 27.1) and 22.5 (20.1; 25.9) kg, respectively; P < 0.001]. No difference in handgrip strength, knee flexor strength, quadriceps CSA, and 6MWT at discharge between groups | Non mentioned |
| Rengo et al[25] | 2NMES: biphasic rectangular pulses at 25 Hz, pulse duration 400 ms, duty cycle 10 s on and 30 s off to quadriceps muscle bilaterally. CG: no intervention | 1 times/d for 5 d/wk | 45 min | 4 wk | Physical function Mental and physical health |
From discharge to 4-wk post-discharge: No significant interaction effect for total SPPB score (P = 0.11; ηp2 = 0.073; CG: 2.89 ± 0.50 vs NMES: 4.11 ± 0.54 units). Time effects for 6MWT distance (P < 0.01; ηp2 = 0.207; CG: 194 ± 18 vs NMES: 267 ± 16 m) and 6MWT power output (P = 0.01; ηp2 = 0.168; CG: 0.4 ± 0.1 vs NMES: 0.6 ± 0.1 W; P = 0.01) | No complications |
| Cerqueira et al[26] | NMES: Stimulation at 50 Hz, duration 400 ms duty cycle 3 s on and 9 s off, to rectus femoris and gastrocnemius muscle bilaterally. Regular physiotherapy care twice a day. CG: Usual physiotherapy care twice a day | 2 times/d | 60 min | From POD 1 to POD 5 | Distance walked; Gait speed; Lactate levels Muscle strength Electromyographic activity of the rectus femoris; Functional Independence Measure | No significant difference in the distance walked (P = 0.650) between NMES group (239.06 ± 88.55) and CG (254.43 ± 116.67) as well as gait speed (P = 0.363), lactate levels (P = 0.302), knee extensor strength (P = 0.117), handgrip strength (P = 0.882), global muscle strength (P = 0.104), electromyographic activity (P = 0.179) and Functional Independence Measure (P = 0.059) | No complications |
| Takino et al[27] | NMES: Biphasic symmetric square pulses at 20 and 200 Hz, duty cycle 0.4 s on and 0.6 s off to vastus lateralis, vastus medialis and triceps surae muscle bilaterally. Standard post- surgical rehabilitation. CG: Standard post- surgical rehabilitation. | 1 time/d | 60 min | from POD 1 to POD 7 | % change in isometric knee strength; % change in usual and maximum walking speed; % change in grip strength | ↓ %ΔIKES in the NMES than CG [NMES: Mean -2%, 95% confidence interval (CI) -6 to 1; CG: -13%, 95% CI -17 to -9, P < 0.001]. ↓ %ΔMWS (P = 0.04). ↓ %ΔUWS and %ΔGS in the NMES compare to CG but not statistically significant | Non mentioned |
| Sumin et al[28] | NMES: rectangular pulses at 45 Hz, duty cycle 12 s on and 5 s off to quadriceps muscle bilaterally. Standard preoperative rehabilitation program; CG: Standard preoperative rehabilitation program | 1 time/d | 90 min | from the 2nd day of hospital stay until the day before surgery (7–10 sessions) | Exercise capacity; Muscle strength | ↑ in KES, KFS, and 6MWT distance (all P < 0.001) in the NMES group compared to the CG. Slight ↑ in HS to the NMES group and slight ↓ to the CG but not statistically significant (P = 0.054 on the right hand and P = 0.062 on the left) | No complications |
Patients were discharged from hospital and submitted to phase I of cardiac rehabilitation.
Home-based intervention after hospital discharge.
CG: Control group; CSA: Cross-sectional area; FES: Functional electrical stimulation; ICU: Intensive care unit; KEIS: Knee extensor isometric strength; MLT: Muscle layer thickness; NMES: Neuromuscular electrical stimulation POD: Post operative day; SPPB: Short physical performance battery; 3-MH/Cre: 3-methylhistidine concentration corrected for urinary creatinine content; 6MWT: Six minutes walking test; KFS: Knee flexor strength; KES: Knee extensor strength; NA: Not available.
Effectiveness of NMES on functional capacity
Effects of NMES on functional capacity were assessed by several indices in 9 studies out of 10. Only a single study did not investigate functional capacity[23]. Functional capacity seemed to improve only in 2 recent studies out of 9 examined; in the first case in patients before cardiac surgery who received NMES as prehabilitation[28] and in the second case in older individuals with diabetes mellitus with postsurgical muscle weakness[27].
Specifically, Fischer et al[19] did not found significant differences in the average mobility level, functional independence measure (FIM) score, the Timed Up and Go Test as well as the mental component score (MCS-12) and physical component score (PCS-12) of the SF-12 between the intervention and the control group from preoperative day to ICU or hospital discharge. Similarly, Schardong et al[20] assessed 6MWT and, although they found an increase in the distance to 6MWT by 11.0% (49.6 m, 95%CI: 15.9-83.3) in the FES group and by 10.4% (41.5 m, 95%CI: 7.8-75.2) in the control group, no significant difference between groups was observed. Kitamura et al[21] also assessed functional capacity via walking speed and found no significant difference between groups after the surgery (NMES: 1.04 ± 0.24 m/s vs Control group: 0.99 ± 0.23 m/s, P = 0.294).
Fontes Cerqueira et al[22] found no influence of NMES on functional capacity as there was no statistically significant difference in distance walked (0.10 m, 95%CI: -64.87 to 65.97) and walking speed (0.01m/s, 95%CI: -0.55 to 0.57) between intervention and control group in cardiac valve surgery patients in the immediate postoperative period. Four years later, Fontes Cerqueira et al[23] examined the effect of NMES on functional capacity of patients in the immediate postoperative period of cardiac surgery again and found similar conclusions; no significant difference in the distance walked (P = 0.650) between NMES group (239.06 ± 88.55) and control group (254.43 ± 116.67) as well as gait speed (P = 0.363) and FIM score (P = 0.059).
In another study of Rengo et al[25], physical function measures improved from discharge to 4 wk post-surgery (P < 0.001) in the total sample and, NMES group showed greater improvements in 6MWT distance and power output compared with controls (P < 0.01). However, no differences between NMES and control groups were found in total Short Physical Performance Battery score or 6MWT measured pre-surgery (range of P values: 0.19-0.61), or at post-surgery discharge (range of P values: 0.21-0.56).
Sumin et al[24] did not find any significant difference in the 6MWT at discharge between NMES and control group (P = 0.166) in early rehabilitation of patients with postoperative complications after cardiovascular surgery. In the contrary, some years later, the same investigators found a statistically significant increase in the 6MWT within NMES group [from 300.0 m (261.0-371.0) to 331.0 m (280.0-375.0); P < 0.01] compared to the control group [from 304.5 m (253.0-380.0) to 285.5 m (246.0-342.0); P < 0.01], as well as between groups (P < 0.001) in patients before cardiac surgery as a kind of prehabilitation[28].
Finally, Takino et al[27] managed to show a statistically significant improvement in the percent change in maximum walking speed from preoperative to postoperative day 7 [treatment effect: 6.2 (0.3 to 12.1); P = 0.04], but the percent change in usual walking speed from preoperative to postoperative day 7 remained unchanged [treatment effect: 3.6 (-0.7 to 7.9); P = 0.10] between groups.
Safety of NMES
Regarding safety, there was no study that demonstrated severe complications during NMES sessions. Adverse events included only minor events and concerned only a very small number of patients. Specifically, 5 patients in the NMES group mentioned a feeling of discomfort in the study of Fischer et al[19], 1 patient mentioned muscle soreness in the study of Kitamura et al[21], and 2 patients reported hypotension and 1 patient complained of pain in the study of Fontes Cerqueira et al[22]. In the rest of the studies, no complications were mentioned.
Effectiveness of NMES on muscle function, strength and endurance
All of the above studies investigated the effectiveness of NMES on muscle mass and/or strength except for 2 studies[23,25].
Fischer A et al[19] assessed muscle layer thickness of the quadriceps muscle of both thighs using two-dimensional B-mode ultrasound and muscle strength via the Medical Research Council (MRC) scale and found that at hospital discharge, NMES patients regained preoperative levels of muscle strength [NMES compared to controls: 0.09 points (0.03 to 0.14); P = 0.002], but not of MLT [NMES compared to controls: 0.02 cm (−0.01 to 0.06); P = 0.21]. As a result, NMES had no significant effect on MLT although patients in the NMES group regained muscle strength 4.5 times faster than patients in the control group. Moreover, there was no difference in grip strength between groups [NMES compared to controls: 0.89 kgf (−5.16 to 6.94); P = 0.77]. In the study of Schardong et al[20], there were significant between-group differences for quadriceps muscle strength (7.2 kg, 95%CI: 0.2-14.2) assessed by the 1RM test and muscle endurance (2.2 repetitions, 95%CI: 1.0-3.4) assessed by the SST test, in favor of the NMES group. Muscle mass above the patella did not differ between the 2 groups (P > 0.05).
Sumin et al[24] examined muscle strength in 37 patients with postoperative complications after cardiovascular surgery. They showed that knee extensors strength at discharge was significantly higher in the NMES group [28.1 kg (23.8; 36.2) on the right and 27.45 kg (22.3; 33.1) on the left] than in the control group [22.3 kg (20.1; 27.1) and 22.5 kg (20.1; 25.9), respectively; P < 0.001) while there was no difference in the handgrip strength, knee flexor strength and quadriceps cross-sectional area between groups (P > 0.05). In the other modality of exercise, prehabilitation, Sumin et al[28] demonstrated statistically significant increase in right and left knee extensors and knee flexors strength in the NMES group compared to the controls (P < 0.001), but handgrip strength was similar between the 2 groups (P = 0.054 on the right hand and P = 0.062 on the left hand).
Finally, Takino et al[27] showed that isometric knee extension strength from preoperative to postoperative day 7 was significantly lower in the NMES than the SHAM group [NMES: mean -2%, 95%CI: -6 to 1 vs sham: Mean -13%, 95%CI: -17 to -9; P < 0.001], indicating the benefits of NMES in postsurgical muscle weakness and functional decline in older persons with diabetes mellitus after cardiac surgery. However, the percent change in grip strength from preoperative to postoperative day 7 did not differ statistically significant between the 2 groups.
There were studies that did not show statistically significant differences on muscle strength and muscle function after NMES. Specifically, Kitamura et al[21] assessed muscle function via knee extensor isometric strength (KEIS) and the mean concentration of 3-methylhistidine concentration corrected for urinary creatinine (Cre) content (3-MH/Cre), which is an objective measure of muscle proteolysis[29]. Authors concluded that there was no significant difference in the mean 3-MH/Cre from post-operative day 1 to post-operative day 6 [225.3 μmol/g (204.0-248.3) vs 227.3 μmol/g (206.3-259.9); P = 0.531), in the KEIS on post-operative day 7 (0.44 ± 0.13 kgf/kg vs 0.41 ± 0.12 kgf/kg; P = 0.149) and in grip strength (29.1 ± 10.5 kg vs 26.9 ± 8.7 kg; P = 0.213) between groups. Fontes Cerqueira et al[22] came in agreement with the findings of Kitamura et al[21] in their study, as no significant difference in muscle strength in the upper- limb (P = 0.54), lower limb(P = 0.67), and total MRC values (P = 0.57) were observed between NMES and control group in post-cardiac surgery patients. Muscle strength was assessed by measuring the peak strength and representative maximum voluntary contraction through manual testing, ranging from 0 (no muscular contraction) to 5 (active movement against complete resistance) for 6 lower and upper limbs movements. In the other RCT they performed some years later[26], the same investigators confirmed their previous results as they also did not find differences in knee extensor strength (P = 0.117), handgrip strength (P = 0.882), global muscle strength (P = 0.104) and electromyographic activity (P = 0.179) between NMES and controls.
DISCUSSION
Safety, feasibility and effectiveness of NMES on functional capacity and muscle strength in patients before undergoing or immediately after cardiac surgery, and comparison between NMES and SHAM or usual care, were assessed in this article. Through our systematic review, we demonstrated that NMES is safe and feasible for patients before and after cardiac surgery and seems to be beneficial in muscle strength in order to prevent ICUAW. However, it did not seem to be beneficial on functional capacity after cardiac surgery, but, mainly before cardiac surgery as a type of prehabilitation.
ICUAW is a type of skeletal muscle dysfunction and a common complication of patients after cardiac surgery, and has been associated with a poor 2-year survival of critically ill patients[30]. It may be a risk factor for prolonged duration of mechanical ventilation[31], associated with a higher risk of readmission[32] and higher mortality[33]. The incidence of ICUAW ranges from 25% to 31% worldwide[34,35]. Patients with ICUAW may have critical illness polyneuropathy and critical illness myopathy, followed by muscle atrophy[36]. A previous study from our Institution showed that skeletal quadriceps muscle mass tends to decrease in ICU patients after cardiac surgery and seems to be associated with prolonged duration of mechanical ventilation and ICU length of stay[37]. Muscle atrophy may occur due to reduced synthesis and increased degradation of muscle proteins. Muscle mass and volume decrease, shrinkage of the muscle fiber cross-section area, and transformation of the type of muscle fibers from I to II are some of the pathophysiological mechanisms of muscle atrophy, also correlated with age[38]. Moreover, muscle atrophy and dysfunction is a result of increased reactive oxygen species due to long-term muscle inactivity[39]. The ubiquitin–proteasome system, calpain, caspase 3, and the autophagy–lysosome system are the major proteolytic systems causing massive loss of myosin and myoglobin-related proteins and leading to muscle atrophy[36,40]. Structural remodeling of the neuromuscular junction is also an important cause of aging-related muscle atrophy[41].
Early mobilization in the ICU after cardiac surgery has been found to be low with a significant trend to increase over ICU stay and is also associated with a reduced duration of mechanical ventilation and ICU length of stay[9,42-44]. In Greek ICUs, only 19% of ICU physiotherapists practice early mobilization in critical ill patients[45]. Similarly, low mobilization rates are also referred in ICUs in Australia, New Zealand and Scotland[46,47]. NMES is safe and feasible as an alternative form of exercise with beneficial effects on preserving muscle mass and strength[9,12], local and systemic microcirculation[15,16] in critically ill patients and may also reduce the duration of mechanical ventilation and ICU stay[11,14]. A major advantage of NMES is that it can be applied even in sedated patients in the ICU, a fact that might enhance early mobilization in these patients. Most RCTs included in our systematic review showed that early implementation of NMES increases muscle strength and endurance of the upper and lower limbs, and improves muscle function in patients after cardiac surgery[19,20,24,27]. However, none of these studies demonstrated significant increase of the muscle mass and handgrip strength remained also unchanged. A possible explanation of these findings may be the small number of sessions performed by patients due to their short length of stay in the ICU. Moreover, functional capacity did not improve after NMES in most studies except for one[28]. This may happen due to the fact that NMES is applied locally in the upper or lower extremities for a short time and thus, its effect is not satisfying on functional capacity. Our findings come in agreement with the findings of a recent meta-analysis by Zhang et al[48] who found no effects of NMES on 6MWT (MD = 44.08; P = 0.22) and walking speed (MD = 0.05; P = 0.24) in 400 cardiac surgery patients.
There are many factors influencing length of stay in ICU after adult cardiac surgery including age, gender, increased BMI, smoking and other cardiovascular and non-cardiovascular risk factors[49]. Preoperative functional capacity and exercise tolerance are among them[49]. NMES, as a form of prehabilitation, could be a crucial approach in order to prevent muscle atrophy and polyneuromyopathy. A recently published RCT showed that 62 patients who underwent 7–10 sessions of NMES prior to cardiac surgery, significantly increased knee extensor strength, knee flexor strength, and 6MWT distance compared to 60 controls who carried out only breathing exercises and an educational program (P < 0.001), indicating improvement on functional capacity and muscle strength[28]. These findings could be quite promising and guide clinicians to target prehabilitation as a significant part of the therapeutic strategy of ICUAW. Unfortunately, data regarding the use of NMES as a form of prehabilitation is still limited.
Potential pathophysiological mechanisms regarding the effects of NMES on functional capacity and muscle function have been proposed over the years. NMES activates muscle fibers by bypassing motor neurons. A positive correlation between the intensity, the electrical filed and the number of recruited type I and II muscle fibers has been found[50]. Moreover, it seems that higher benefits are derived by higher current intensity[51]. NMES should be applied specifically to the muscles of the lower limbs of frail patients with the maximal tolerable intensities, high frequencies (> 30 Hz and rather 50–80 Hz), optimal width pulses, short contractions interspersed with long recovery times[51]. NMES both stimulates anabolic pathways and negatively modulates muscle catabolism, which increases protein synthesis and reduces protein degradation and activates satellite cells in aged individuals[52-54]. As a result, NMES induces an increase in the size of type II muscle fibers[53]. Finally, there is a hypothesis that peripheral application of NMES can evoke a wide range of activities in the central nervous system, which can lead to a series of neural adjustments and adaptations[55].
Clinical perspectives
Patients after cardiac surgery may present impaired functional capacity and muscle function, reduced muscle strength, exercise intolerance and poor prognosis due to complications including ICUAW and polyneuromyopathy. The present systematic review evaluated the beneficial effects of NMES on functional capacity, muscle strength and muscle function. The most significant fact is that NMES is safe and feasible for these patients, without severe complications or major adverse effects even in high-risk patients. Moreover, it was proven to efficient, too. NMES should be initiated in patients as a form of prehabilitation before a major cardiac surgery and be continued immediately after the surgery until hospital discharge. A multidisciplinary team approach is necessary for its implementation. Preventing ICUAW and polyneuromyopathy via NMES could result in better prognosis, reduced length of stay in the ICU, less complications and improved exercise tolerance and mobility of cardiac surgery patients. Other additional benefits of NMES could be better quality of life and improved hemodynamic and respiratory responses.
Limitations
More RCTs regarding the effects of NMES after cardiac surgery are required. Especially as a form of prehabilitation before cardiac surgery, NMES has been investigated only in one single study[28]. Another significant limitation is that the different samples from the included RCTs may present heterogeneity due to different mean age, type of surgery and functional capacity at baseline. Moreover, the number of NMES sessions was low in most studies and, as a result, the effectiveness of NMES on functional capacity may not have been shown in these studies. Finally, the lack of adjustment for multiple comparisons and possible confounders in the analysis makes it difficult for researchers to conclude whether these results are generalizable for the whole population of these patients. However, all these limitations are related mostly with each RCT separately, and not directly with our systematic review. The reason we preferred a systematic review over a meta-analysis was due to the fact that access to data of all the included RCTs was not feasible.
CONCLUSION
NMES is safe and feasible for patients before and after cardiac surgery and seems to be beneficial in muscle strength in order to prevent ICUAW in these patients. NMES did not present beneficial effects on functional capacity and muscle mass after cardiac surgery, possibly due to the low number of sessions that patients performed. However, NMES before cardiac surgery, as a form of prehabilitation, showed promising results on functional capacity and muscle strength and function. This form of rehabilitation could be a valuable strategy of preventing ICUAW after cardiac surgery. In order to discover all beneficial effects of NMES, fully understand its pathophysiological mechanisms in muscle function and functional capacity, and define the appropriate dose including duration, frequency and intensity, bigger number of multicenter RCTs with higher number of patients are required.
ARTICLE HIGHLIGHTS
Research background
Lack of mobilization and prolonged stay in the intensive care unit (ICU) are major factors resulting in the development of ICU-acquired muscle weakness (ICUAW). Early mobilization in the ICU after cardiac surgery is associated with a reduced duration of mechanical ventilation and ICU length of stay.
Research motivation
Neuromuscular electrical stimulation (NMES) is an alternative modality of exercise in patients with muscle weakness. A major advantage of NMES is that it can be applied even in sedated patients in the ICU, a fact that might enhance early mobilization in these patients.
Research objectives
To evaluate safety, feasibility and effectiveness of NMES on functional capacity and muscle strength in patients before and after cardiac surgery.
Research methods
We performed a search on Pubmed, PEDro, Embase and CINAHL databases, selecting papers published between December 2012 and April 2023 and identified published randomized controlled trials (RCTs) that included implementation of NMES in patients before after cardiac surgery. RCTs were assessed for methodological rigor and risk of bias via the Physiotherapy Evidence Database. The primary outcomes were safety and functional capacity and the secondary outcomes were muscle strength and function.
Research results
Ten studies were included in our systematic review, resulting in 703 participants. Almost half of them performed NMES and the other half were included in the control group, treated with usual care. Nine studies investigated patients after cardiac surgery and 1 study before cardiac surgery. Functional capacity was assessed in 8 studies via 6MWT or other indices, and improved only in 1 study before and in 1 after cardiac surgery. Nine studies explored the effects of NMES on muscle strength and function and, most of them, found increase of muscle strength and improvement in muscle function after NMES. NMES was safe in all studies without any significant complication.
Research conclusions
NMES is safe, feasible and has beneficial effects on muscle strength and function in patients after cardiac surgery, but has no significant effect on functional capacity.
Research perspectives
The present systematic review evaluated the beneficial effects of NMES on functional capacity, muscle strength and muscle function. NMES should be initiated in patients as a form of prehabilitation before a major cardiac surgery and be continued immediately after the surgery until hospital discharge. A multidisciplinary team approach is necessary for its implementation. Preventing ICUAW and polyneuromyopathy via NMES could result in better prognosis, reduced length of stay in the ICU, less complications and improved exercise tolerance and mobility of cardiac surgery patients.
Footnotes
Conflict-of-interest statement: All the authors received no financial support for the research, authorship, and/or publication of this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 29, 2023
First decision: December 12, 2023
Article in press: January 3, 2024
Specialty type: Rehabilitation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Gendy HA, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
Contributor Information
Christos Kourek, Medical School of Athens, National and Kapodistrian University of Athens, Athens 15772, Greece.
Marios Kanellopoulos, Clinical Ergospirometry, Exercise and Rehabilitation Laboratory, Evangelismos Hospital, Athens 10676, Greece.
Vasiliki Raidou, Clinical Ergospirometry, Exercise and Rehabilitation Laboratory, Evangelismos Hospital, Athens 10676, Greece.
Michalis Antonopoulos, Intensive Care Unit, Onassis Cardiac Surgery Center, Kallithea 17674, Greece.
Eleftherios Karatzanos, Clinical Ergospirometry, Exercise and Rehabilitation Laboratory, Evangelismos Hospital, Athens 10676, Greece.
Irini Patsaki, Department of Physiotherapy, University of West Attica, Athens 12243, Greece.
Stavros Dimopoulos, Clinical Ergospirometry, Exercise and Rehabilitation Laboratory, Evangelismos Hospital, Athens 10676, Greece; Intensive Care Unit, Onassis Cardiac Surgery Center, Kallithea 17674, Greece. stdimop@gmail.com.
References
- 1.Intiso D, Centra AM, Bartolo M, Gatta MT, Gravina M, Di Rienzo F. Recovery and long term functional outcome in people with critical illness polyneuropathy and myopathy: a scoping review. BMC Neurol. 2022;22:50. doi: 10.1186/s12883-022-02570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Confer J, Wolcott J, Hayes R. Critical illness polyneuromyopathy. Am J Health Syst Pharm. 2012;69:1199–1205. doi: 10.2146/ajhp110343. [DOI] [PubMed] [Google Scholar]
- 3.Nanas S, Kritikos K, Angelopoulos E, Siafaka A, Tsikriki S, Poriazi M, Kanaloupiti D, Kontogeorgi M, Pratikaki M, Zervakis D, Routsi C, Roussos C. Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand. 2008;118:175–181. doi: 10.1111/j.1600-0404.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32:140–163. doi: 10.1002/mus.20304. [DOI] [PubMed] [Google Scholar]
- 5.Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidiras G, Patsaki I, Karatzanos E, Dakoutrou M, Kouvarakos A, Mitsiou G, Routsi C, Stranjalis G, Nanas S, Gerovasili V. Long term follow-up of quality of life and functional ability in patients with ICU acquired Weakness - A post hoc analysis. J Crit Care. 2019;53:223–230. doi: 10.1016/j.jcrc.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Kourek C, Nanas S, Kotanidou A, Raidou V, Dimopoulou M, Adamopoulos S, Karabinis A, Dimopoulos S. Modalities of Exercise Training in Patients with Extracorporeal Membrane Oxygenation Support. J Cardiovasc Dev Dis. 2022;9 doi: 10.3390/jcdd9020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Hu W, Cai Z, Liu J, Wu J, Deng Y, Yu K, Chen X, Zhu L, Ma J, Qin Y. Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS One. 2019;14:e0223185. doi: 10.1371/journal.pone.0223185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel HJ, Needham DM, Morris PE, Gropper MA. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013;41:S69–S80. doi: 10.1097/CCM.0b013e3182a240d5. [DOI] [PubMed] [Google Scholar]
- 11.Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13:R161. doi: 10.1186/cc8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karatzanos E, Gerovasili V, Zervakis D, Tripodaki ES, Apostolou K, Vasileiadis I, Papadopoulos E, Mitsiou G, Tsimpouki D, Routsi C, Nanas S. Electrical muscle stimulation: an effective form of exercise and early mobilization to preserve muscle strength in critically ill patients. Crit Care Res Pract. 2012;2012:432752. doi: 10.1155/2012/432752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos DR, Bueno TBC, Anjos JSGG, Zoppi D, Dantas BG, Gosselink R, Guirro RRJ, Borges MC. Early Neuromuscular Electrical Stimulation in Addition to Early Mobilization Improves Functional Status and Decreases Hospitalization Days of Critically Ill Patients. Crit Care Med. 2022;50:1116–1126. doi: 10.1097/CCM.0000000000005557. [DOI] [PubMed] [Google Scholar]
- 14.Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, Tripodaki E, Markaki V, Zervakis D, Nanas S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14:R74. doi: 10.1186/cc8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelopoulos E, Karatzanos E, Dimopoulos S, Mitsiou G, Stefanou C, Patsaki I, Kotanidou A, Routsi C, Petrikkos G, Nanas S. Acute microcirculatory effects of medium frequency versus high frequency neuromuscular electrical stimulation in critically ill patients - a pilot study. Ann Intensive Care. 2013;3:39. doi: 10.1186/2110-5820-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanou C, Karatzanos E, Mitsiou G, Psarra K, Angelopoulos E, Dimopoulos S, Gerovasili V, Boviatsis E, Routsi C, Nanas S. Neuromuscular electrical stimulation acutely mobilizes endothelial progenitor cells in critically ill patients with sepsis. Ann Intensive Care. 2016;6:21. doi: 10.1186/s13613-016-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Ierssel SH, Jorens PG, Van Craenenbroeck EM, Conraads VM. The endothelium, a protagonist in the pathophysiology of critical illness: focus on cellular markers. Biomed Res Int. 2014;2014:985813. doi: 10.1155/2014/985813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahmati M, Malakoutinia F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Physiotherapy. 2021;113:12–28. doi: 10.1016/j.physio.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Spiegl M, Altmann K, Winkler A, Salamon A, Themessl-Huber M, Mouhieddine M, Strasser EM, Schiferer A, Paternostro-Sluga T, Hiesmayr M. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care. 2016;20:30. doi: 10.1186/s13054-016-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schardong J, Kuinchtner GC, Sbruzzi G, Plentz RDM, Silva AMVD. Functional electrical stimulation improves muscle strength and endurance in patients after cardiac surgery: a randomized controlled trial. Braz J Phys Ther. 2017;21:268–273. doi: 10.1016/j.bjpt.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura H, Yamada S, Adachi T, Shibata K, Tamaki M, Okawa Y, Usui A. Effect of Perioperative Neuromuscular Electrical Stimulation in Patients Undergoing Cardiovascular Surgery: A Pilot Randomized Controlled Trial. Semin Thorac Cardiovasc Surg. 2019;31:361–367. doi: 10.1053/j.semtcvs.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Fontes Cerqueira TC, Cerqueira Neto ML, Cacau LAP, Oliveira GU, Silva Júnior WMD, Carvalho VO, Mendonça JT, Santana Filho VJ. Ambulation capacity and functional outcome in patients undergoing neuromuscular electrical stimulation after cardiac valve surgery: A randomised clinical trial. Medicine (Baltimore) 2018;97:e13012. doi: 10.1097/MD.0000000000013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontes Cerqueira TC, Cerqueira Neto ML, Carvalho AJG, Oliveira GU, Araújo Filho AA, Carvalho VO, Cacau LAP, Silva Júnior WMD, Mendonça JT, Santana Filho VJ. Neuromuscular Electrical Stimulation on Hemodynamic and Respiratory Response in Patients Submitted to Cardiac Surgery: Pilot Randomized Clinical Trial. Int J Cardiovasc Sci. 2019;32:483–489. [Google Scholar]
- 24.Sumin AN, Oleinik PA, Bezdenezhnykh AV, Ivanova AV. Neuromuscular electrical stimulation in early rehabilitation of patients with postoperative complications after cardiovascular surgery: A randomized controlled trial. Medicine (Baltimore) 2020;99:e22769. doi: 10.1097/MD.0000000000022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rengo JL, Savage PD, Hirashima F, Leavitt BJ, Ades PA, Toth MJ. Improvement in Physical Function After Coronary Artery Bypass Graft Surgery Using a Novel Rehabilitation Intervention: A RANDOMIZED CONTROLLED TRIAL. J Cardiopulm Rehabil Prev. 2021;41:413–418. doi: 10.1097/HCR.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerqueira TCF, de Cerqueira Neto ML, Cacau LAP, de Araújo Filho AA, Oliveira GU, da Silva Júnior WM, Carvalho VO, de Mendonça JT, de Santana Filho VJ. Effect of neuromuscular electrical stimulation on functional exercise capacity in patients undergoing cardiac surgery: A randomized clinical trial. Clin Rehabil. 2022;36:789–800. doi: 10.1177/02692155211070945. [DOI] [PubMed] [Google Scholar]
- 27.Takino K, Kameshima M, Asai C, Kawamura I, Tomita S, Sato H, Hirakawa A, Yamada S. Neuromuscular electrical stimulation after cardiovascular surgery mitigates muscle weakness in older individuals with diabetes. Ann Phys Rehabil Med. 2023;66:101659. doi: 10.1016/j.rehab.2022.101659. [DOI] [PubMed] [Google Scholar]
- 28.Sumin AN, Oleinik PA, Bezdenezhnykh AV, Bezdenezhnykh NA. Prehabilitation in Cardiovascular Surgery: The Effect of Neuromuscular Electrical Stimulation (Randomized Clinical Trial) Int J Environ Res Public Health. 2023;20 doi: 10.3390/ijerph20032678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iida Y, Yamazaki T, Arima H, Kawabe T, Yamada S. Predictors of surgery-induced muscle proteolysis in patients undergoing cardiac surgery. J Cardiol. 2016;68:536–541. doi: 10.1016/j.jjcc.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Saccheri C, Morawiec E, Delemazure J, Mayaux J, Dubé BP, Similowski T, Demoule A, Dres M. ICU-acquired weakness, diaphragm dysfunction and long-term outcomes of critically ill patients. Ann Intensive Care. 2020;10:1. doi: 10.1186/s13613-019-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 32.Adler D, Dupuis-Lozeron E, Richard JC, Janssens JP, Brochard L. Does inspiratory muscle dysfunction predict readmission after intensive care unit discharge? Am J Respir Crit Care Med. 2014;190:347–350. doi: 10.1164/rccm.201404-0655LE. [DOI] [PubMed] [Google Scholar]
- 33.Medrinal C, Prieur G, Frenoy É, Robledo Quesada A, Poncet A, Bonnevie T, Gravier FE, Lamia B, Contal O. Respiratory weakness after mechanical ventilation is associated with one-year mortality - a prospective study. Crit Care. 2016;20:231. doi: 10.1186/s13054-016-1418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, Casaer MP, Meersseman P, Debaveye Y, Van Cromphaut S, Wouters PJ, Gosselink R, Van den Berghe G. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190:410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 35.Kasotakis G, Schmidt U, Perry D, Grosse-Sundrup M, Benjamin J, Ryan C, Tully S, Hirschberg R, Waak K, Velmahos G, Bittner EA, Zafonte R, Cobb JP, Eikermann M. The surgical intensive care unit optimal mobility score predicts mortality and length of stay. Crit Care Med. 2012;40:1122–1128. doi: 10.1097/CCM.0b013e3182376e6d. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Xu C, Ma X, Zhang X, Xie P. Intensive Care Unit-Acquired Weakness: A Review of Recent Progress With a Look Toward the Future. Front Med (Lausanne) 2020;7:559789. doi: 10.3389/fmed.2020.559789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimopoulos S, Raidou V, Elaiopoulos D, Chatzivasiloglou F, Markantonaki D, Lyberopoulou E, Vasileiadis I, Marathias K, Nanas S, Karabinis A. Sonographic muscle mass assessment in patients after cardiac surgery. World J Cardiol. 2020;12:351–361. doi: 10.4330/wjc.v12.i7.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallinson JE, Murton AJ. Mechanisms responsible for disuse muscle atrophy: potential role of protein provision and exercise as countermeasures. Nutrition. 2013;29:22–28. doi: 10.1016/j.nut.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalamgi RC, Larsson L. Mechanical Signaling in the Pathophysiology of Critical Illness Myopathy. Front Physiol. 2016;7:23. doi: 10.3389/fphys.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudolf R, Deschenes MR, Sandri M. Neuromuscular junction degeneration in muscle wasting. Curr Opin Clin Nutr Metab Care. 2016;19:177–181. doi: 10.1097/MCO.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raidou V, Dimopoulos S, Chatzivasiloglou F, Kourek C, Tsagari V, Pitsolis T, Papadopoulos K, Kriaras I, Tasouli A, Nanas S, Karabinis A. Early mobilization is associated with decreased mechanical ventilation and ICU length of stay following cardiac surgery. Health Res J. 2021;7:184–193. [Google Scholar]
- 43.Moradian ST, Najafloo M, Mahmoudi H, Ghiasi MS. Early mobilization reduces the atelectasis and pleural effusion in patients undergoing coronary artery bypass graft surgery: A randomized clinical trial. J Vasc Nurs. 2017;35:141–145. doi: 10.1016/j.jvn.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Anekwe DE, Biswas S, Bussières A, Spahija J. Early rehabilitation reduces the likelihood of developing intensive care unit-acquired weakness: a systematic review and meta-analysis. Physiotherapy. 2020;107:1–10. doi: 10.1016/j.physio.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Christakou A, Seitaridi A, Koutsioumba E, Papaioannou S, Spinou A, Anaouni E et al Current physiotherapy practice in Greek intensive care units: a national study. Eur J Physiother 2019; 21: 210-216. [Google Scholar]
- 46.TEAM Study Investigators. Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, Denehy L, Harrold M, Higgins A, Presneill J, Saxena M, Skinner E, Young P, Webb S. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrold ME, Salisbury LG, Webb SA, Allison GT Australia and Scotland ICU Physiotherapy Collaboration. Early mobilisation in intensive care units in Australia and Scotland: a prospective, observational cohort study examining mobilisation practises and barriers. Crit Care. 2015;19:336. doi: 10.1186/s13054-015-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Peng Y, Zhong F, Li S, Huang X, Huang Q, Chen L, Lin Y. Effects of neuromuscular electrical stimulation on functional capacity and quality of life among patients after cardiac surgery: A systematic review and meta-analysis. J Cardiol. 2022;79:291–298. doi: 10.1016/j.jjcc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 49.Almashrafi A, Elmontsri M, Aylin P. Systematic review of factors influencing length of stay in ICU after adult cardiac surgery. BMC Health Serv Res. 2016;16:318. doi: 10.1186/s12913-016-1591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc Sport Sci Rev. 2007;35:102–109. doi: 10.1097/jes.0b013e3180a0321b. [DOI] [PubMed] [Google Scholar]
- 51.Paillard T. Muscle plasticity of aged subjects in response to electrical stimulation training and inversion and/or limitation of the sarcopenic process. Ageing Res Rev. 2018;46:1–13. doi: 10.1016/j.arr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Barber L, Scicchitano BM, Musaro A. Molecular and Cellular Mechanisms of Muscle Aging and Sarcopenia and Effects of Electrical Stimulation in Seniors. Eur J Transl Myol. 2015;25:231–236. doi: 10.4081/ejtm.2015.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mancinelli R, Toniolo L, Di Filippo ES, Doria C, Marrone M, Maroni CR, Verratti V, Bondi D, Maccatrozzo L, Pietrangelo T, Fulle S. Neuromuscular Electrical Stimulation Induces Skeletal Muscle Fiber Remodeling and Specific Gene Expression Profile in Healthy Elderly. Front Physiol. 2019;10:1459. doi: 10.3389/fphys.2019.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paillard T. Neuromuscular or Sensory Electrical Stimulation for Reconditioning Motor Output and Postural Balance in Older Subjects? Front Physiol. 2021;12:779249. doi: 10.3389/fphys.2021.779249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110:223–234. doi: 10.1007/s00421-010-1502-y. [DOI] [PubMed] [Google Scholar]