Key Points
Question
What are the characteristics of colorectal cancer screening programs in Latin America?
Findings
This systematic review and meta-analysis included 17 studies conducted in upper middle-income and high-income countries in Latin America; the pooled participation rate in fecal immunochemical test (FIT)-based programs was 85.8%. For a positive FIT, detection rates were 39.0% for any adenoma, 13.3% for advanced adenomas, and 4.9% for colorectal cancer; these yields for neoplasia were comparable with those observed in high-income settings.
Meaning
These findings suggest that establishing population-level structured screening programs in Latin America, at least in upper middle-income countries, would be effective at reducing colorectal cancer–related disease burden, as they have been in higher-income regions.
This systematic review and meta-analysis characterizes and quantifies colorectal cancer screening program coverage and performance in Latin American countries, with attention to screening test used, program structure, and geographic region.
Abstract
Importance
Colorectal cancer (CRC) is a leading cause of cancer-related mortality globally, with increasing incidence and mortality in Latin America. CRC screening programs can reduce disease burden, but information on screening programs in Latin America is limited.
Objective
To describe characteristics (eg, type of program, uptake, neoplastic yield) of CRC screening programs in Latin America.
Data Sources
PubMed, Ovid MEDLINE, EMBASE, Cochrane, PsycINFO, Web of Science Core Collection, LILACS, and SciELO were searched from inception to February 2023. Relevant references from bibliographies, conference proceedings, and gray literature were considered. The search strategy included English, Spanish, and Portuguese terms.
Study Selection
Included were studies of CRC screening programs in Latin America using fecal immunochemical test (FIT) or colonoscopy as the primary screening method. Four reviewers independently assessed study eligibility based on titles, with review of abstracts and full texts as needed.
Data Extraction and Synthesis
Guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were followed for data abstraction and quality assessment. Descriptive information was extracted, and data were pooled using a random-effects model.
Main outcomes and Measures
Program performance indicators included rates of participation and FIT positivity, adenoma detection rate (ADR), advanced adenoma detection rate (AADR), CRC detection rate, and colonoscopy quality indicators.
Results
There were 17 studies included from upper middle-income and high-income countries in Latin America with a total of 123 929 participants. Thirteen studies used FIT as the initial screening method, whereas 4 used screening colonoscopy. The participation rate in FIT-based programs was 85.8% (95% CI, 78.5%-91.4%). FIT positivity rates were 15.2% (95% CI, 9.6%-21.8%) for the 50-ng/mL threshold and 9.7% (95% CI, 6.8%-13.0%) for the 100-ng/mL threshold. For FIT-based studies, the pooled ADR was 39.0% (95% CI, 29.3%-49.2%) and CRC detection rate was 4.9% (95% CI, 2.6%-7.9%); for screening colonoscopy–based studies, the pooled ADR was 19.9% (95% CI, 15.5%-24.8%) and CRC detection rate was 0.4% (95% CI, 0.1%-0.8%).
Conclusions and Relevance
This systematic review and meta-analysis suggests that CRC screening in upper middle-income countries in Latin America is feasible, detecting rates of neoplasia comparable with those of high-income regions. Population-based screening programs should be developed or enhanced in these settings. There is a knowledge gap regarding feasibility and yield of screening programs in lower middle-income countries.
Introduction
Colorectal cancer (CRC) is the second-leading cause of cancer-related mortality globally, disproportionately affecting high-income countries.1,2,3 CRC incidence and mortality are increasing in low middle-income countries (LMICs) and high middle-income countries (HMICs). This includes Latin America,2,3,4,5,6,7 predominately comprised of LMICs and HMICs in economic transition. CRC is the second-most common malignant neoplasm among Latinos in the US.8 This upward trend in the Americas is likely due to both westernization of lifestyle and increased life expectancy.2,9,10,11 CRC incidence is predicted to increase by 60% in the Americas by 2030.6,12
CRC screening has been shown to reduce disease incidence and mortality.13,14,15 Globally there are multiple national and professional guidelines recommending screening using a variety of methods starting at different ages,6,13,16,17,18 and they generally consist of either 1-step primary colonoscopy or a less-invasive 2-step method of noninvasive testing (with fecal occult blood test or multitarget stool DNA) with colonoscopy for those with a positive noninvasive test. The staged method has the advantages of convenience, ease of scalability, and lower cost, and it may be the preferred method for new CRC screening programs in resource-limited settings.19
Studies of CRC screening programs in Latin America are sparce due to the lack of resources for sustainable nationally scaled programs. The first CRC screening program to be established in Latin America was in Uruguay in 2005.20 Since then, Brazil, Mexico, and Chile have developed national screening guidelines and focused programs.21
Although CRC incidence is increasing,2,22,23,24,25 the true incidence of CRC in much of Latin America is still unknown because of the lack of reliable cancer registries.26,27 The incidence of precancerous polyps is even less known. To characterize and quantify screening program coverage and performance in Latin American countries, we performed a systematic review and meta-analysis of studies of CRC screening in these countries, with attention to screening test used, program structure, and geographic region.
Methods
The protocol for this systematic review and meta-analysis was registered in PROSPERO (CRD42022322437) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.28 We systematically searched PubMed, Ovid MEDLINE, EMBASE, and Cochrane from inception to February 2023. Keywords and MeSH terms used are shown in eTable 1 in Supplement 1. To identify studies on CRC screening programs in Latin America, a comprehensive search strategy was developed and applied to 5 other databases: PsycINFO (EBSCO Information Services), Web of Science Core Collection, LILACS (Latin American and Caribbean Health Sciences Literature/Literatura Latino-Americana e do Caribe em Ciências da Saúde), and SciELO (Scientific Electronic Library Online).
We conducted 8 separate searches in Google Scholar to discover additional relevant gray literature. When searching Latin American databases and Google Scholar, we included the terms translated into Spanish and Portuguese. Results from all searches are in eTable 1 in Supplement 1. The searches were developed and executed by a medical librarian (M.R.). The results from all databases were aggregated in Endnote version 20 (Clarivate) and deduplicated using the Covidence tool.
Study Selection (Inclusion and Exclusion Criteria)
We sought to include CRC screening programs that used either fecal immunochemical test (FIT) or open-access colonoscopy as the principal test. We excluded studies evaluating follow-up colonoscopies (after a prior negative screen), programs using several different screening tests (such as computed tomography, magnetic resonance imaging, and fecal DNA tests), and studies that only looked at population rather than individual patient outcomes. If 2 publications described the same study population or overlapping research protocols, we included the study with the larger sample size.
Data Extraction
Four independent reviewers (E.M.S., R.B., M.G., D.A.N.) reviewed titles and abstracts, followed by full texts to determine study eligibility. Two independent reviewers extracted descriptive information on author, year of publication, year of conduction of the study, country, World Bank country classification at time of study, study type and design, sample size, funding source, recruitment strategy, type of screening program, and FIT cutoff. We attempted to contact corresponding authors to obtain missing information from their studies. Discrepancies were resolved by consensus and adjudicated (M.K.D., E.M.S., D.R.M., T.I.).
Sampling methods were categorized as voluntary response, convenience sampling, and population-based studies. Voluntary response, also known as self-selection sampling, occurs when individuals or participants respond to a public invitation to enroll in a study. Convenience sampling involves recruiting individuals who are easily accessible and available for the research study, such as a certain clinic panel. Population-based sampling, also known as probability sampling or random sampling, aims to select a sample that represents the entire population accurately.
Study Quality Assessment
We assessed the risk of bias (ROB) of included studies using the Newcastle-Ottawa Scale version for cross-sectional studies to assess 9 items across 3 domains: (1) selection of the study groups, (2) comparability of groups, and (3) exposure or outcome according to the study design. We assigned 1 point for each item (2 points for a comparability item), for a maximum total score of 9 points.29 Scores of 8 to 9 were defined as low ROB, scores of 5 to 7 as medium ROB, and scores of 1 to 4 as high ROB. ROB was assessed independently by 2 reviewers (E.M.S., R.B.), with discrepancies resolved through discussion with a third reviewer (M.K.D.).
Data Synthesis
Studies were classified by screening modality to be either FIT-based (patients underwent colonoscopy only if their FIT test was positive) or colonoscopy-based (all patients offered colonoscopy, with or without paired FIT). The outcomes were the following: adenoma detection rate (ADR: percentage of colonoscopies with either nonadvanced adenomas, advanced adenomas, or CRC), advanced adenoma detection rate (AADR: percentage of colonoscopies with either advanced adenoma or CRC), CRC detection rate, and colonoscopy quality indicators (cecal intubation rate, mean withdrawal time, and bowel preparation score);30 for FIT-based programs, we also assessed the study uptake rate (number of invited individuals who returned the FIT), FIT positivity rate (proportion of individuals who tested positive), and rate of follow-up colonoscopy for positive FITs.
Statistical Analysis
A random-effects model was used to pool the rates with proportions and 95% CIs for the studies with data available.31,32 Freeman-Tukey double arcsine transformation was used to stabilize the variances,33 and heterogeneity was assessed by the inconsistency index (I2). Funnel plots were used to evaluate small-study effects (mainly publication bias), along with the Egger test for statistical significance of funnel plot asymmetry (2-sided P < .05 statistically significant). Metaregression random-effects models and subgroup analyses according to a variety of study features (eg, sampling strategy), screening test (eg, FIT hemoglobin cutoff), and patient population (eg, familial risk) factors were used to investigate statistical heterogeneity of the main pooled estimates. All analyses were performed using the metan packages in Stata 18 (StataCorp).
Results
A total of 5661 articles were identified from the combined databases, with 4267 citations remaining after deduplication. We excluded 3950 based on content of titles and abstracts. We assessed 44 full-text articles for eligibility, and we included 17 in the review with a total of 123 929 participants (eFigure 1 in Supplement 1). Reasons for excluding 27 studies at the full text stage are listed in eTable 2 in Supplement 1.
Study Characteristics and Quality Assessment
All included studies were published after 2006, with study data collection occurring from 1999 to 2019. All studies were conducted in HMICs or high-income countries in Latin America, defined by World Bank classification at the start date of the specific study. Eleven (65%) of 17 were conducted in South America (Argentina [n = 2]; Brazil [n = 4]; Chile [n = 3]; Uruguay [n = 2]),20,34,35,36,37,38,39,40,41,42,43 and the remaining 6 studies were conducted in Mexico and Central America (Mexico [n = 4]; Costa Rica [n = 1]; Panama [n = 1]).44,45,46,47,48,49 The articles were published in English (n = 10),20,34,37,40,42,43,44,47,48,49 Spanish (n = 6),35,36,38,39,45,46 and Portuguese (n = 1).41 Funding sources were governmental (n = 5), governmental with other agencies (n = 2), private (n = 3), international (n = 2), and not reported (n = 5). Four studies were colonoscopy-based without prior FIT.34,36,44,49 Of the 13 FIT-based studies, several included a cohort of high-risk study participants (familial/genetic risk or symptomatic)38,40,41,42 who underwent colonoscopy; for our analysis, however, we only included the subset with a positive FIT.20,40 Most FIT studies reported hemoglobin cutoffs of 50 ng/mL (5 studies) or 100 ng/mL (6 studies), although 2 did not report the cutoff.41,46 Descriptive characteristics of included studies are presented in Table 1.
Table 1. Descriptive Characteristics of Included Studies.
| Study (country) | Project period | Language | Income level (WBI) | Sample size | Type of screening program | Funding | Risk of biasa | FIT brand | FIT cutoff value, ng/mL |
|---|---|---|---|---|---|---|---|---|---|
| Fenocchi et al,20 2006 (Uruguay) | 1999-2004 | English | UMICs | 11734 | FIT | International (JICA) | Low | OC-Hemodia | 100 |
| Rettally,49 2008 (Panama) | 2004-2007 | English | HICs | 306 | Colonoscopy | NR | Medium | NA | NA |
| Silva et al,36 2011 (Chile) | 2003-2008 | Spanish | UMICs | 1158 | Colonoscopy | Private | Medium | NA | NA |
| Lopez-Kostner et al,38 2012 (Chile) | 2007-2009 | Spanish | UMICs | 6348 | FIT | Governmental | Low | OC-sensor Micro, Eiken | 100 |
| Fenocchi et al,40 2015 (Uruguay) | 2015 | English | UMICs | 902 | FIT | NR | Low | OC-Eiken | 100 |
| Garcia-Osogobio et al,44 2015 (Mexico) | 2009-2010 | English | UMICs | 123 | Colonoscopy | Private | Low | NA | NA |
| Braga et al,41 2017 (Brazil) | 2014-2015 | Portuguese | UMICs | 438 | FIT | NR | Medium | NR | NA |
| Teixeira et al,37 2017 (Brazil) | 2015-2016 | English | UMICs | 1039 | FIT | Private | Medium | OC-Light | 50b |
| Alfaro-Seguro et al,46 2020 (Costa Rica) | 2017-2019 | Spanish | UMICs | 53003 | FIT | Governmental | Low | NR | NA |
| Galvez-Rios et al,45 2020 (Mexico) | 2015-2016 | Spanish | UMICs | 900 | FIT | Governmental | Medium | OC FIT-CHEK | 100 |
| Manzano-Robleda et al,47 2020 (Mexico) | 2017-2019 | English | UMICs | 810 | FIT | Governmental/nonprofit | Low | OC FIT-CHEK | 50c |
| Okada et al,42 2016 (Chile) | 2012-2014 | English | UMICs | 26444 | FIT | Governmental | Low | NR | 100 |
| Remes-Troche et al, 48 2020 (Mexico) | 2015-2016 | English | UMICs | 473 | FIT | International (US NCI) | Medium | OC FIT-CHEK | 100 |
| Averbach et al,34 2021 (Brazil)d | 2014-2017 | English | UMICs | 2022 | Colonoscopy | Governmental/private | Low | Bioptix | NA |
| Fernandez et al,39 2021 (Argentina) | 2019-2020 | Spanish | UMICs | 730 | FIT | Governmental | Medium | SD. BIOLINE FOB | 50b |
| Guimaraes et al,43 2021 (Brazil) | 2015-2017 | English | UMICs | 6737 | FIT | NR | Low | Hemosure | 50b |
Abbreviations: FIT, fecal immunochemical test; HICs, high-income countries; JICA, Japan International Cooperation Agency; NA, not available; UMICs, upper- and middle-income countries; US NCI, United States National Cancer Institute; WBI, World Bank Index.
Per dual review using the Newcastle-Ottawa Scale for comparative studies (Wells et al,29 2014).
Values converted from ug/g to ng/mL.
The 50 ng/mL cutoff was used for comparability with the other studies; results were also provided for cutoffs of 20 ng/mL and 100 ng/mL.
Averbach et al administered colonoscopies and FIT to all individuals.
All but 1 study had an eligibility age of 50 years or older.44 Most studies excluded individuals at elevated risk, such as those with a personal or family history of colorectal neoplasm or inflammatory bowel disease. Where studies included a group of diagnostic or nonaverage risk screening procedures, we included only the average-risk screening population in the analyses whenever possible,49 although some studies still had some contamination of familial risk36,42,48,49 or symptomatic participants,41 which led to lower comparability ratings in risk-of-bias assessment. All studies were judged to have a low or medium risk of bias according to Newcastle-Ottawa (eTable 3 in Supplement 1). Recruitment strategies included media advertisement only (n = 5), personal invitation (n = 7), combination of personal and media (n = 3), and unspecified (n = 2). Strategies for sampling and recruiting are presented in eTable 4 in Supplement 1.
FIT-Based Programs: Participation
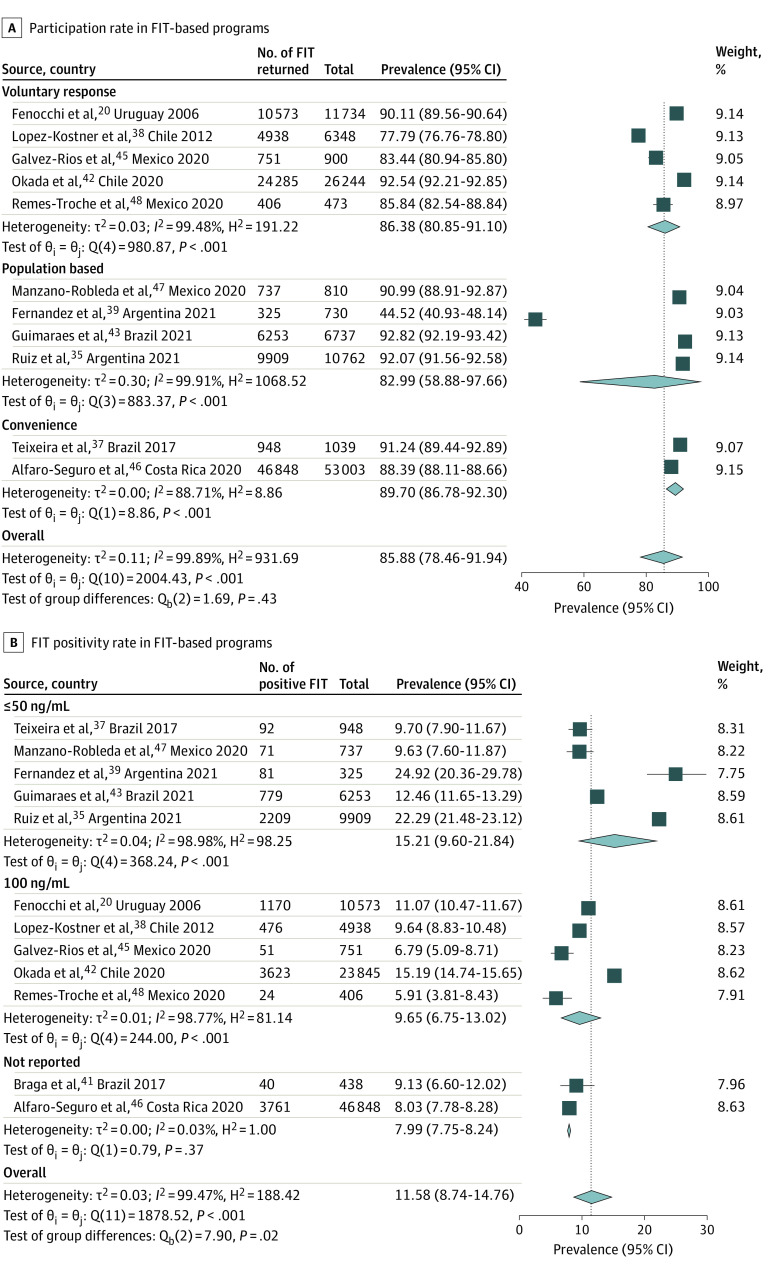
A summary of pooled estimates of clinical outcomes and performance indicators is presented in Table 2. FIT studies reported participation rates ranging from 44.5% to 92.8% among 118 780 individuals invited, with an overall pooled participation rate of 85.8% (95% CI, 78.5%-91.4%; I2 = 99.9%). Uptake was not associated with sampling strategy (voluntary response: 86.4% [95% CI, 80.9%-91.1%]; convenience: 89.7% [95% CI, 86.8%-92.3%]; population-based: 83.0% [95% CI, 58.9%-97.7%]; P = .43) (Figure 1A) or recruitment strategy (media recruitment: 87.9% [95% CI, 84.2%-91.1%]; individual recruitment: 88.9% [95% CI, 83.5%-93.4%]; P = .72) (eFigure 3 in Supplement 1). The pooled participation rate was 85.0% (95% CI, 72.5%-94.2%) in South America and was 87.4% (95% CI, 84.0%-90.4%) in Mexico and Central America (P = .68) (eFigure 2 in Supplement 1). Studies with larger sample sizes reported higher participation rates (sample size <1000: 78.0% [95% CI, 56.2%-93.8%]; sample size 1000-10 000: 88.0% [95% CI, 77.9%-95.3%]; sample size >10 000: 90.2% [95% CI, 88.0%-92.2%]), although this was not a statistically significant difference (P = .33) (eFigure 4 in Supplement 1).
Table 2. Pooled Estimates of Performance Indicators for FIT and Colonoscopy-Based Programs.
| Variable | Studies, No. | Population, No. | Positive test, No. | Range, % | Point estimate, % (95% CI)a |
|---|---|---|---|---|---|
| Participation rate | |||||
| Overall | 12 | 118 780 | 105 971 | 44.5-92.8 | 85.8 (78.5-91.4) |
| Voluntary | 5 | 45 699 | 40 513 | 77.8-98.2 | 86.4 (80.9-91.1) |
| Population-based | 4 | 19 039 | 17 224 | 44.5-92.82 | 83 (58.9-97.7) |
| Convenience | 3 | 54 042 | 48 234 | 88.4-91.24 | 89.7 (86.8-92.3) |
| FIT positivity rate | |||||
| Overall | 12 | 105 971 | 12 377 | 5.9-24.9 | 11.6 (8.7-14.8) |
| 50 ng/dL | 5 | 18 172 | 3232 | 9.6-24.9 | 15.2 (9.6-21.8) |
| 100 ng/dL | 5 | 40 513 | 5344 | 5.9-15.2 | 9.7 (6.8-13.0) |
| Not reported | 2 | 47 286 | 3801 | 8.0-9.1 | 8 (7.8-8.2) |
| Detection rates per positive FITb | |||||
| ADR | 12 | 12 377 | 3377 | 5.5-55.07 | 28.5 (19.1-38.8) |
| AADR | 8 | 7788 | 282 | 1.1-28.2 | 8.3 (3.5-14.9) |
| CRC detection rate | 12 | 12 377 | 410 | 0.0-12.0 | 3.5 (1.6-6.0) |
| Any adenoma (ADR) | |||||
| FIT-positivec | 12 | 9373 | 3377 | 13.9-65.0 | 39 (29.3-49.2) |
| Screening colonoscopy | 4 | 3494 | 699 | 16.6-26.1 | 19.9 (15.5-24.8) |
| Advance Adenoma (AADR) | |||||
| FIT-positivec | 10 | 7240 | 648 | 2.2-34.5 | 13.3 (7.4-20.5) |
| Screening colonoscopy | 2 | 2258 | 62 | 2.6-3.9 | 2.9 (1.8-4.3) |
| CRC detection rate | |||||
| FIT-positivec | 12 | 9373 | 420 | 0.0-15.1 | 5.4 (3.1-8.2) |
| Screening colonoscopy | 4 | 3494 | 15 | 0.0-1.3 | 0.4 (0.1-0.8) |
| Colonoscopy quality indicators | |||||
| Cecal intubation rate | 12 | 12 203 | 11 515 | 86.8-100.0 | 95.7 (92.7-97.9) |
| Withdrawal time, mean, min | 4 | 4825 | 9.9 | 7.3-17.0 | 8.4 (4.3-12.4) |
| Bowel preparation (adequate) | 4 | 6459 | 6109 | 83.5-99.6 | 94.6 (86.6-99.5) |
Abbreviations: AADR, advanced adenoma detection rate; ADR, adenoma detection rate (any adenoma); CRC, colorectal cancer; FIT, fecal immunochemical test.
Random-effects meta-analysis of proportions.
In FIT-based programs.
Rates per colonoscopy after positive FIT, from FIT-based programs only.
Figure 1. Forest Plots of the Outcomes of Colorectal Cancer Screening Programs in Latin America.
FIT indicates fecal immunochemical test.
FIT-Based Programs: Positivity Rate and Outcomes
Twelve FIT studies reported FIT positivity rates, which ranged from 5.9% to 24.9%, with a pooled FIT positivity rate of 11.6% (95% CI, 8.7%-14.8%; I2 = 99.5%). This rate was significantly associated with FIT threshold, with 15.2% (95% CI, 9.6%-21.8%) positivity for thresholds of 50 ng/dL or less and 9.7% (95% CI, 6.8%-13.0%) for 100 ng/dL (P = .02) (Figure 1B). Approximately three-fourths (79.6% [95% CI, 65.4%-90.8%]) of individuals with a positive FIT underwent diagnostic colonoscopy (eFigure 5 in Supplement 1). There was minimal description of interventions to navigate FIT-positive participants to colonoscopy beyond efforts of study personnel, although the 2 underperforming outlier studies were more pragmatic35,39 pilot studies that acknowledged limitations of the follow-up colonoscopy process during the study period.
The pooled ADR per positive FIT test was 28.5% (95% CI, 19.1%-38.8%); pooled AADR per positive FIT test was 8.3% (95% CI, 3.5%-14.9%); and pooled CRC detection rate per positive FIT test was 3.5% (95% CI, 1.6%-6.0%) (eFigures 5-7 in Supplement 1). These outcomes provide the neoplasia yield of the entire FIT-based program, accounting for the variation in colonoscopy follow-up. There was no difference in ADR, AADR, or CRC detection rate by FIT cutoff. ADR was 29.4% (95% CI, 9.1%-55.4%) for 50 ng/dL and 29.9% (95% CI, 24.2%-36.0%) for 100 ng/dL (P = .97) (eFigure 6 in Supplement 1); AADR was 9.2% (95% CI, 2.1.8%-20.7%) for 50 ng/dL and 11.9% (95% CI, 7.8%-16.5%) for 100 ng/dL (P = .02) (eFigure 7 in Supplement 1); and CRC detection rate was 4.7% (95% CI, 1.3%-9.8%) for 50 ng/dL and 2.9% (95% CI, 0.5%-6.8%) for 100 ng/dL (P = .86) (eFigure 8 in Supplement 1).
Yield of Colonoscopy
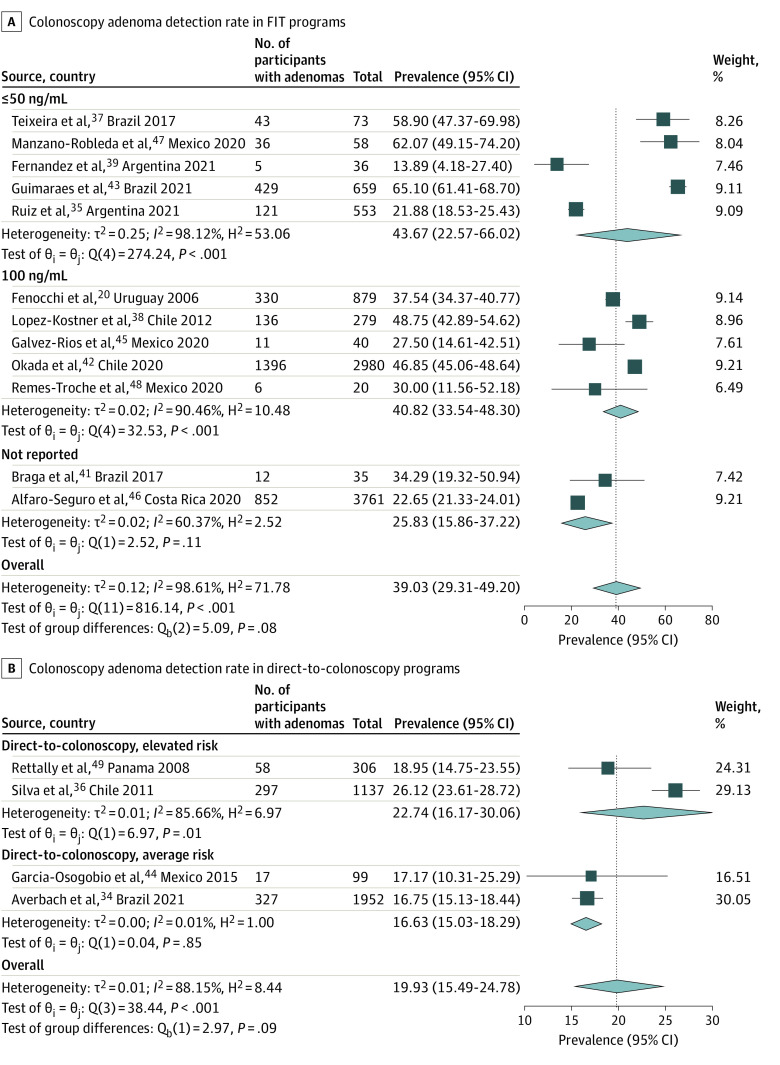
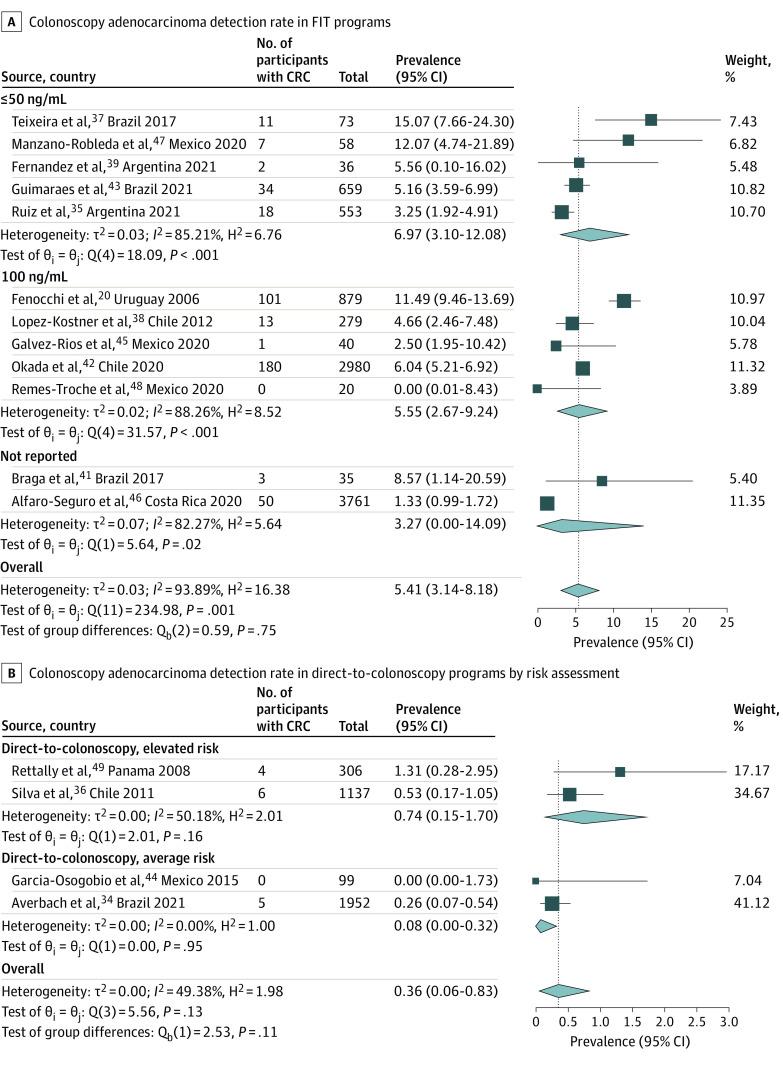
A total of 18 037 colonoscopies were performed among all studies. ADR per procedure ranged from 13.9% to 65.1%, higher following a positive FIT (39.0% [95% CI, 29.3%-49.2%]) (Figure 2A) than for primary screening colonoscopy (19.9% [95% CI, 15.5%-24.8%]) (Figure 2B). AADR data were available in 12 studies (10 FIT programs and 2 screening colonoscopy programs), with a pooled rate of 13.3% (95% CI, 7.4%-20.6%) for FIT (eFigure 9 in Supplement 1) and 2.9% (95% CI, 1.8%-4.3%) for primary screening colonoscopy (eFigure 10 in Supplement 1). The CRC detection rate ranged from 0% to 15.1%, with a pooled rate of 3.5% (95% CI, 1.6%-6.0%) (eFigure 11 in Supplement 1). CRC detection was higher in FIT-based programs (5.4% [95% CI, 3.1%-8.2%]) (Figure 3A) compared with primary screening colonoscopy (0.4% [95% CI, 0.1%-0.8%]) (Figure 3B), a statistically significant difference (P < .001) (eFigure 11 in Supplement 1).
Figure 2. Colonoscopy Adenoma Detection Rate in FIT and Direct-to-Colonoscopy Programs.
FIT indicates fecal immunochemical test.
Figure 3. Colonoscopy Adenocarcinoma Detection Rate in FIT and Direct-to-Colonoscopy Programs.
CRC indicates colorectal cancer; FIT, fecal immunochemical test.
Colonoscopy Quality
A total of 12 studies reported at least 1 additional quality indicator for colonoscopy beyond ADR. Cecal intubation rate was reported in 12 studies, with a pooled rate of 95.7% (95% CI, 92.7%-98.0%) (eFigure 12 in Supplement 1). Mean withdrawal time from the cecum was reported in 4 studies, with a mean time of 8.4 minutes (95% CI, 4.3-12.4 minutes) (eFigure 13 in Supplement 1) and rate of adequate bowel preparation in 4 studies with a pooled estimate of 94.6% (95% CI, 86.6%-99.5%) (eFigure 14 in Supplement 1). Overall, only 5 studies reported the type of colonoscopy equipment utilized, which were comparable (Olympus 180 or Fujifilm 530, each with high-definition endoscopes), except for Fenocchi et al,20 which used Olympus 140-series.20 One study used endoscopic accessories,47 resulting in one of the highest ADRs. Another study with high ADR used indigo carmine chromoendoscopy in all procedures, with a correspondingly longer withdrawal time.43
Heterogeneity and Subgroup Analyses
Assessments for heterogeneity (Galbraith plots) and publication bias (Funnel plot of small study effects with Egger P value) can be found in eFigures 14, 15, 16, 17, and 18 in Supplement 1. No significant small study effects were detected, but statistical heterogeneity was high in all analyses. We explored this heterogeneity in the FIT-based studies for the principal outcomes of neoplasia yield (detection rates of adenomas, advanced adenomas, and CRC) with metaregression, which was a single variable regression given the limited number of studies (eTable 5 in Supplement 1). Among significant modifiers, populations with more female individuals demonstrated higher ADRs and AADRs; greater mean age was associated with higher rates of CRC. Exclusion of studies using visualization enhancements for polyp detection decreased the overall ADR from 39.0% (95% CI, 29.3%-49.2%) to 34.2% (95% CI, 25.5%-43.3%) (eFigure 19 in Supplement 1), but also realigned the expected direction of ADR as higher at higher FIT cutoff (40.8% [95% CI, 33.5%-48.3%] at 100 ng/mL vs 30.4% [95% CI, 8.3%-58.7%] at 50 ng/mL; eFigure 20 in Supplement 1). Stratification by familial risk was not associated with detection of CRC, however, at least among FIT positives (eFigure 21 in Supplement 1).
To explore the effect of such outliers on summary estimates, in addition to the Galbraith and forest plots, we performed leave-one-out meta-analyses for neoplasia outcomes per colonoscopy in FIT-based studies (eFigures 22-24 in Supplement 1), although no single study affected overall ADR more than 2.5%, AADR more than 2.0%, or CRC detection rate more than 0.7%. Overall, while these subgroup analyses helped to explain much of the clinical heterogeneity, the I2 metric remained high in all main analyses.
Discussion
To our knowledge, this is the first systematic review to quantitatively evaluate the nascent CRC screening programs in Latin America. This review found that colon cancer screening, either with a FIT-based or screening colonoscopy–based strategy, was successfully implemented in a variety of upper middle-income countries throughout Latin America, with high yields of neoplasia. The detection rates of adenomas (19.9% for screening colonoscopy and 39.0% after positive FIT), advanced adenomas (2.9% for screening colonoscopy and 13.3% after positive FIT), and CRC (0.4% for screening colonoscopy and 5.4% after positive FIT) were comparable to those in mature programs in North American and European countries, where ADR averages 23% to 42% and CRC detection 0.5% in direct colonoscopy,50,51,52,53,54 and ADR is 48% and CRC detection is 5.1% among individuals with a positive FIT.55 Other program metrics are also in the same general range (FIT positivity: 5.9%-24.9% vs 4.3%-10.1%) or slightly superior (participation rate: 44.5%-92.8% vs 48.2%-64.7%) to those of higher-income regions.7,25,56 Population-based screening programs may represent a cost-efficient opportunity to address the growing burden of CRC in Latin America, with the caveat that this review did not identify studies from LMICs, nor did it identify screening programs that were unsuccessful.
Limited resources, infrastructure, and lack of public awareness have posed challenges for population-based CRC screening programs in Latin America.6 Despite these obstacles, our review of upper-middle-income countries found high uptake (85.8%) of screening measures, with FIT-based studies reporting greater than 75% colonoscopy completion rates after positive FIT. Data on quality metrics, such as cecal intubation rates (95.7%) and bowel preparation (94.6%), was limited, however, it aligns with global standards, suggesting the feasibility of CRC screening in Latin American upper-middle-income countries.30,57,58,59,60
The rising incidence and mortality of CRC in Latin American countries underscores the need for screening programs at the population level. Although our study found that both primary colonoscopy and FIT-based programs were associated with higher detection rates, given the resource constraints in these countries, a stool-based strategy is likely most feasible and cost-effective.61,62,63,64,65,66,67 A stool-based strategy allows for country-specific FIT cutoffs tailored to CRC prevalence and colonoscopy capacity.68,69 Stool test distribution is more easily integrated into existing public health infrastructure, such as population-based H. pylori, cervical cancer, or diabetes screening.
Regardless of a country’s initial screening strategy, colonoscopy resources are a crucial and potentially rate-limiting factor. Developing these resources may be guided through international collaboration, as previously modeled in Latin America with Japanese support of the Chilean CRC national program.38,42 Established in 2012, this collaboration between a private entity, the government, and an academic institution demonstrated promising results,42 with significant improvement in ADR and cancer detection rate after local Chilean physicians received colonoscopy skills–improvement training.42 In addition to overseas collaborations, however, our study suggests that enough Latin American centers have developed sufficient expertise in CRC screening that intracontinental collaborations could fill some of this need for mentorship and professional development for the more nascent programs. Furthermore, regional networks among Latin American countries, with potential advocacy from the Pan-American Health Organization (PAHO), to share screening outcomes will strengthen knowledge of both disease epidemiology and optimal screening practices. Additionally, national health ministries’ commitment to program funding will be important for sustainability, even if research or nongovernmental funding is used initially.70
Limitations
This systematic review and meta-analysis had limitations. The main limitation of this review is statistical heterogeneity among the included studies. This is due to a variety of causes, both clinical and statistical. In the pooled analysis of participation rate for FIT-based studies, all studies except 1 (Fernandez et al39) had participation rates from 78% to 93%, and 8 of 10 studies fell between 83% and 93%. This variation may not be considered clinically important, but even with exclusion of the most extreme outlier (Fernandez et al39)—a small study with shortcomings in project implementation—I2 remains high at 99.5%. There are other study factors, such as recruitment and sampling methods, that likely contribute to this variation. However, the majority of this heterogeneity is purely statistical, due to the number of large studies with low within-study variance, which artificially inflates the I2 in the presence of relatively small between-study variances.
Clinical heterogeneity is evident in FIT positivity and ADR rates. Two Argentine pilot programs, being new, involved nonresearch staff, potentially leading to errors in FIT interpretation and inclusion of symptomatic patients. While ADR outliers could be linked to equipment differences, we could not identify other factors that may have contributed to heterogeneity. Stratification by (familial) risk or FIT positivity threshold surprisingly did not significantly affect neoplasia yields, possibly due to unreported factors or true population differences.20,37 Incomplete reporting of FIT cutoff by studies and the ecological nature of comparisons limit the ability to explore sources of heterogeneity.41,46 Caution is advised in interpreting summary estimates, serving as the best available estimates for future study planning based on a limited and emerging literature.
Teixeira et al’s study37 reported high neoplasia detection despite normal FIT positivity in an average-risk population. Possible explanations include unreported endoscopic accessory use or high-detecting endoscopists. Another outlier study from Uruguay suggested a higher-risk population with disproportionate AADR and CRC detection due to lower image resolution colonoscopes.20 The main negative outlier for ADR also showed low FIT positivity and participation rates, with ongoing protocol development and potential errors in FIT interpretation and participant recruitment acknowledged by the authors.39
Other limitations of this review highlight future challenges in stemming the impending wave of CRC in Latin America. This review highlights challenges in addressing the growing CRC burden in Latin America. Two countries (Costa Rica, Panama) lacked reported CRC screening programs in the 2016 PAHO report, and we identified studies from 7 countries, none of which were LMICs. While no publication bias was found in our funnel plots, there could be unpublished CRC screening efforts due to implementation failures, constituting infeasibility. The LMIC “double cancer burden” is well-documented,26,71 with increasing rates of CRC in Latin American lower- and upper-middle-income countries, emphasizing the need for research on CRC prevention in the region.3,71
Conclusions
This systematic review and meta-analysis found both the substantial burden of colorectal neoplasia and the feasibility of organized screening programs in Latin America. It also highlights the need for more data on CRC burden or screening feasibility in the Latin American LMICs. CRC incidence is rising in Latin America as fast as anywhere in the world, warranting effective preventive measures, particularly with cost-effective, FIT-based screening programs. CRC screening should become a greater research and public health priority in Latin America.
eTable 1. Search Strategy
eFigure 1. Flow Diagram of Study Inclusion
eTable 2. Excluded Studies and Reasons for Exclusion
eTable 3. Risk of Bias Using the New Castle Ottawa Scale for Comparative Studies
eTable 4. Strategies for Sampling and Recruitment of Included Studies
eTable 5. Univariate Meta Regression
eFigure 2. Participation Rate (FIT Given/FIT Returned) by Region
eFigure 3. Participation Rate (FIT Given/FIT Returned) by Recruitment Strategy
eFigure 4. Participation Rate (FIT Given/FIT Returned) by Sample Size
eFigure 5. Colonoscopy Follow-Up (Colonoscopies Performed After a Positive FIT Test)
eFigure 6. Outcome ADR (Adenoma Detection Rate) per Positive FIT Test in FIT Based Programs
eFigure 7. Outcome AADR (Advanced Adenomas Detection Rate) per Positive FIT Test in FIT Based Programs
eFigure 8. Outcome CRCDR (Colorectal Cancer Detection Rate) per Positive FIT Test in FIT Based Programs
eFigure 9. Yield of Colonoscopy: AADR (Advanced Adenomas Detection Rate). FIT Programs
eFigure 10. Yield of Colonoscopy: AADR (Advanced Adenomas Detection Rate). Direct-to-Colonoscopy Programs
eFigure 11. Yield of Colonoscopy: CRC-DR (Adenocarcinoma Detection Rate) by Type of Program
eFigure 12. Colonoscopy Quality Indicators: Cecal Intubation Rate
eFigure 13. Colonoscopy Quality Indicators: Mean Withdrawal Time From Cecum (Mean, Minutes)
eFigure 14. Colonoscopy Quality Indicators: Adequate Bowel Preparation
eFigure 15. Heterogeneity and Publication Bias (Small Study Effects) for FIT Based Studies
eFigure 16. Heterogeneity and Publication Bias (Small Study Effects) for Outcomes per Positive FIT Test in FIT Based Programs
eFigure 17. Heterogeneity and Publication Bias (Small Study Effects) for Outcomes in Direct-to-Colonoscopy Programs
eFigure 18. Heterogeneity and Publication Bias (Small Study Effects) for Colonoscopy Quality Indicators
eFigure 19. ADR (Adenomas Detection Rate) FIT Programs by Risk: Leave-One-Out Analysis
eFigure 20. ADR (Adenomas Detection Rate) FIT Programs by FIT Cut-Off: Leave-One-Out Analysis
eFigure 21. CRC (Adenocarcinoma Detection Rate) FIT Programs: Leave-One-Out Analysis
eFigure 22. ADR (Adenomas Detection Rate) FIT Programs: Leave-One-Out Analysis
eFigure 23. AADR (Advanced Adenomas Detection Rate) FIT Programs: Leave-One-Out Analysis
eFigure 24. CRC (Adenocarcinoma Detection Rate) FIT Programs: Leave-One-Out Analysis
Data Sharing Statement
References
- 1.Rabeneck L, Chiu HM, Senore C. International perspective on the burden of colorectal cancer and public health effects. Gastroenterology. 2020;158(2):447-452. doi: 10.1053/j.gastro.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Sierra MS, Soerjomataram I, Antoni S, et al. Cancer patterns and trends in Central and South America. Cancer Epidemiol. 2016;44(suppl 1):S23-S42. doi: 10.1016/j.canep.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691. doi: 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 4.Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer Epidemiol. 2016;44(suppl 1):S62-S73. doi: 10.1016/j.canep.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Demb J, Gupta S. Racial and ethnic disparities in colorectal cancer screening pose persistent challenges to health equity. Clin Gastroenterol Hepatol. 2020;18(8):1691-1693. doi: 10.1016/j.cgh.2019.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan American Health Organization . Colorectal cancer screening in the Americas: situation and challenges. Accessed April 2023. https://www3.paho.org/hq/dmdocuments/2016/Colorectal-Cancer-Screening-Landscape-English.pdf
- 7.Sharma R, Abbasi-Kangevari M, Abd-Rabu R, et al. ; GBD 2019 Colorectal Cancer Collaborators . Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7(7):627-647. doi: 10.1016/S2468-1253(22)00044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KD, Ortiz AP, Pinheiro PS, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. 2021;71(6):466-487. doi: 10.3322/caac.21695 [DOI] [PubMed] [Google Scholar]
- 9.Bray F, Soerjomataram I. The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer: Disease Control Priorities. Vol 3. 3rd ed. The International Bank for Reconstruction and Development / The World Bank; 2015. [PubMed] [Google Scholar]
- 10.Pilleron S, Soerjomataram I, Soto-Perez-de-Celis E, et al. Aging and the cancer burden in Latin America and the Caribbean: Time to act. J Geriatr Oncol. 2019;10(5):799-804. doi: 10.1016/j.jgo.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 11.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713-732. doi: 10.1038/s41575-019-0189-8 [DOI] [PubMed] [Google Scholar]
- 12.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 13.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 14.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371. doi: 10.1056/NEJM199305133281901 [DOI] [PubMed] [Google Scholar]
- 15.Senore C, Riggi E, Armaroli P, et al. ; SCORE Working Group . Long-Term Follow-up of the Italian Flexible Sigmoidoscopy Screening Trial. Ann Intern Med. 2022;175(1):36-45. doi: 10.7326/M21-0977 [DOI] [PubMed] [Google Scholar]
- 16.Sung JJY, Chiu HM, Lieberman D, et al. Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut. 2022;71(11):2152-2166. doi: 10.1136/gutjnl-2022-327377 [DOI] [PubMed] [Google Scholar]
- 17.Davies J, Chew C, Bromham N, Hoskin P; National Institute for Health and Care Excellence (NICE) . NICE 2020 guideline for the management of colorectal cancer. Lancet Oncol. 2022;23(6):e247. doi: 10.1016/S1470-2045(22)00256-X [DOI] [PubMed] [Google Scholar]
- 18.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250-281. doi: 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 19.Dan YY, Chuah BY, Koh DC, Yeoh KG. Screening based on risk for colorectal cancer is the most cost-effective approach. Clin Gastroenterol Hepatol. Published online March 12, 2012;10(3):266-71 e1-6. doi: 10.1016/j.cgh.2011.11.011 [DOI] [PubMed]
- 20.Fenocchi E, Martínez L, Tolve J, et al. Screening for colorectal cancer in Uruguay with an immunochemical faecal occult blood test. Eur J Cancer Prev. 2006;15(5):384-390. doi: 10.1097/00008469-200610000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Sanguinetti JM, Lotero Polesel JC, Piscoya A, Sáenz Fuenzalida R. Colorectal cancer screening: a South American perspective. Rev Gastroenterol Peru. 2020;40(3):238-245. [PubMed] [Google Scholar]
- 22.Dominguez RL, Crockett SD, Lund JL, et al. Gastric cancer incidence estimation in a resource-limited nation: use of endoscopy registry methodology. Cancer Causes Control. 2013;24(2):233-239. doi: 10.1007/s10552-012-0109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sierra MS, Forman D. Cancer in Central and South America: methodology. Cancer Epidemiol. 2016;44(suppl 1):S11-S22. doi: 10.1016/j.canep.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 24.Strasser-Weippl K, Chavarri-Guerra Y, Villarreal-Garza C, et al. Progress and remaining challenges for cancer control in Latin America and the Caribbean. Lancet Oncol. 2015;16(14):1405-1438. doi: 10.1016/S1470-2045(15)00218-1 [DOI] [PubMed] [Google Scholar]
- 25.Sierra MS, Forman D. Burden of colorectal cancer in Central and South America. Cancer Epidemiol. 2016;44(suppl 1):S74-S81. doi: 10.1016/j.canep.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 26.Piñeros M, Frech S, Frazier L, et al. Advancing Reliable Data for Cancer Control in the Central America Four Region. J Glob Oncol. 2018;4:1-11. doi: 10.1200/JGO.2016.008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piñeros M, Abriata MG, de Vries E, et al. Progress, challenges and ways forward supporting cancer surveillance in Latin America. Int J Cancer. 2021;149(1):12-20. doi: 10.1002/ijc.33407 [DOI] [PubMed] [Google Scholar]
- 28.Montalvan-Sanchez EEBR, Ramirez-Rojas M. What are the uptake and yield of CRC screening programs in Latin America. NIHR . Accessed December 27, 2023. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022322437
- 29.Wells GA, Wells G, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed December 27, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 30.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90. doi: 10.1038/ajg.2014.385 [DOI] [PubMed] [Google Scholar]
- 31.Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol. 2008;61(1):41-51. doi: 10.1016/j.jclinepi.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32(4):138. doi: 10.1080/00031305.1978.10479283 [DOI] [Google Scholar]
- 34.Averbach P, Ferrari AP, Toscano CM, Borges JL, Averbach M. Implementation and results of a gastrointestinal cancer screening program in an Amazon rainforest village: a descriptive study. Endosc Int Open. 2021;9(6):E770-E776. doi: 10.1055/a-1386-2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz EF, Hasdeu S. Rastreo de cáncer colorrectal: análisis de resultados en la provincia del Neuquén, Argentina, 2015-2019. Rev Argent Salud Publica. 2021;13:11-20. [Google Scholar]
- 36.Silva MdeL, Santander R, Gobelet J, et al. Plan de tamizaje de cáncer colorrectal (“Mes del Colon”) en la Clínica Alemana de Santiago de Chile. Acta Gastroenterol Latinoam. 2011;41(1):10-16. [PubMed] [Google Scholar]
- 37.Teixeira CR, Bonotto ML, Lima JP, Figueiredo LF, Conrado L, Frasca C. Clinical impact of the immunochemical fecal occult blood test for colorectal cancer screening in Brazil. Ann Gastroenterol. 2017;30(4):442-445. doi: 10.20524/aog.2017.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Köstner F, Kronber U, Zárate AJ, et al. Programa de detección de neoplasias colorrectales en población mayor de 50 años. Rev Med Chil. 2012;140(3):281-286. doi: 10.4067/S0034-98872012000300001 [DOI] [PubMed] [Google Scholar]
- 39.Fernández NE, Enrique CI. Prueba piloto para la implementación del Programa Nacional de Prevención y Detección Temprana de Cáncer Colorrectal en Entre Ríos. Rev Argent Salud Publica. 2021;13:131-140. [Google Scholar]
- 40.Fenocchi E, Gaggero P, Rondán M, et al. Usefulness of the fecal immunochemical test in the detection of advanced adenomas in subjects at average risk for colorectal cancer. Endoscopia. 2015;27(2):64-68. doi: 10.1016/j.endomx.2015.07.002 [DOI] [Google Scholar]
- 41.Braga DC, Bortolini SM, Quadros NJ, et al. Rastreamento do câncer colorretal através da pesquisa de sangue oculto fecal-um estudo de base populacional. GED Gastroenterol Endosc Dig. 2017;36(2):60-64.
- 42.Okada T, Tanaka K, Kawachi H, et al. International collaboration between Japan and Chile to improve detection rates in colorectal cancer screening. Cancer. 2016;122(1):71-77. doi: 10.1002/cncr.29715 [DOI] [PubMed] [Google Scholar]
- 43.Guimarães DP, Mantuan LA, de Oliveira MA, et al. The performance of colorectal cancer screening in Brazil: the first two years of the implementation program in barretos cancer hospital. Cancer Prev Res (Phila). 2021;14(2):241-252. doi: 10.1158/1940-6207.CAPR-20-0179 [DOI] [PubMed] [Google Scholar]
- 44.García-Osogobio S, Téllez-Ávila FI, Méndez N, Uribe-Esquivel M. Results of the first program of colorectal cancer screening in Mexico. Endoscopia. 2015;27(2):59-63. doi: 10.1016/j.endomx.2015.06.001 [DOI] [Google Scholar]
- 45.Gálvez-Ríos S, Sobrino-Cossío S, Siu A, et al. Results of the fecal immunochemical test in a colorectal cancer screening program in Mexico. Cir Cir. 2020;88(5):635-642. [DOI] [PubMed] [Google Scholar]
- 46.Alfaro Segura K. Primer programa organizado de tamizaje de cáncer colorrectal en Costa Rica: resultados de primera ronda, provincia de Cartago. Published 2020. Accessed January 5, 2024. https://www.kerwa.ucr.ac.cr/handle/10669/81411
- 47.Manzano-Robleda MDC, Espinosa-Tamez P, Potter MB, et al. Fecal immunologic test results and diagnostic colonoscopy in a Mexican population at average risk for colorectal cancer. Cancer Prev Res (Phila). 2020;13(11):959-966. doi: 10.1158/1940-6207.CAPR-20-0076 [DOI] [PubMed] [Google Scholar]
- 48.Remes-Troche JM, Hinojosa-Garza G, Espinosa-Tamez P, et al. Faecal immunochemical test-based colorectal cancer screening in Mexico: an initial experience. Fam Pract. 2020;37(3):321-324. doi: 10.1093/fampra/cmz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rettally C. Colorectal cancer screening in Latin America: the Panama experience. Am J Gastroenterol. 2008;103(3):806-807. doi: 10.1111/j.1572-0241.2007.01612_10.x [DOI] [PubMed] [Google Scholar]
- 50.Shaukat A, Holub J, Pike IM, et al. Benchmarking adenoma detection rates for colonoscopy: results from a US-based registry. Am J Gastroenterol. 2021;116(9):1946-1949. doi: 10.14309/ajg.0000000000001358 [DOI] [PubMed] [Google Scholar]
- 51.Klair JS, Ashat M, Johnson D, et al. Serrated polyp detection rate and advanced adenoma detection rate from a US multicenter cohort. Endoscopy. 2020;52(1):61-67. doi: 10.1055/a-1031-5672 [DOI] [PubMed] [Google Scholar]
- 52.Penz D, Ferlitsch A, Waldmann E, et al. Impact of adenoma detection rate on detection of advanced adenomas and endoscopic adverse events in a study of over 200,000 screening colonoscopies. Gastrointest Endosc. 2020;91(1):135-141. doi: 10.1016/j.gie.2019.08.038 [DOI] [PubMed] [Google Scholar]
- 53.Quintero E, Castells A, Bujanda L, et al. ; COLONPREV Study Investigators . Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697-706. doi: 10.1056/NEJMoa1108895 [DOI] [PubMed] [Google Scholar]
- 54.Bretthauer M, Løberg M, Wieszczy P, et al. ; NordICC Study Group . Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi: 10.1056/NEJMoa2208375 [DOI] [PubMed] [Google Scholar]
- 55.Mohan BP, Khan SR, Daugherty E, et al. Pooled rates of adenoma detection by colonoscopy in asymptomatic average-risk individuals with positive fecal immunochemical test: a systematic review and meta-analysis. Gastrointest Endosc. 2022;96(2):208-222.e14. doi: 10.1016/j.gie.2022.04.004 [DOI] [PubMed] [Google Scholar]
- 56.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152(5):1217-1237.e3. doi: 10.1053/j.gastro.2016.08.053 [DOI] [PubMed] [Google Scholar]
- 57.Spada C, Koulaouzidis A, Hassan C, et al. Colonoscopy quality across Europe: a report of the European Colonoscopy Quality Investigation (ECQI) Group. Endosc Int Open. 2021;9(10):E1456-E1462. doi: 10.1055/a-1486-6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lieberman DA, Faigel DO, Logan JR, et al. Assessment of the quality of colonoscopy reports: results from a multicenter consortium. Gastrointest Endosc. 2009;69(3 Pt 2):645-653. doi: 10.1016/j.gie.2008.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikematsu H, Sakamoto T, Togashi K, et al. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc. 2017;86(2):386-394. doi: 10.1016/j.gie.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 60.Xin L, Gao Y, Cheng Z, et al. Utilization and quality assessment of digestive endoscopy in China: results from 5-year consecutive nationwide surveys. Chin Med J (Engl). 2022;135(16):2003-2010. doi: 10.1097/CM9.0000000000002366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Areia M, Fuccio L, Hassan C, Dekker E, Dias-Pereira A, Dinis-Ribeiro M. Cost-utility analysis of colonoscopy or faecal immunochemical test for population-based organised colorectal cancer screening. United European Gastroenterol J. 2019;7(1):105-113. doi: 10.1177/2050640618803196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopes G, Stern MC, Temin S, et al. Early detection for colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol. 2019;5:1-22. doi: 10.1200/JGO.18.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zauber AG. Cost-effectiveness of colonoscopy. Gastrointest Endosc Clin N Am. 2010;20(4):751-770. doi: 10.1016/j.giec.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winawer S, Classen M, Lambert R. World Gastroenterology Organisation/International Digestive Cancer Alliance guidelines: Colorectal cancer screening. 2007. Accessed December 27, 2023. https://www.worldgastroenterology.org/guidelines/colorectal-cancer-screening/colorectal-cancer-screening-english
- 65.Sharaf RN, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol. 2013;108(1):120-132. doi: 10.1038/ajg.2012.380 [DOI] [PubMed] [Google Scholar]
- 66.Zhong GC, Sun WP, Wan L, Hu JJ, Hao FB. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91(3):684-697.e15. doi: 10.1016/j.gie.2019.11.035 [DOI] [PubMed] [Google Scholar]
- 67.Pinzon Florez CE, Rosselli D, Gamboa Garay OA. Análisis de Costo-Efectividad de las Estrategias de Tamización de Cáncer Colorrectal en Colombia. Value Health Reg Issues. 2012;1(2):190-200. doi: 10.1016/j.vhri.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 68.Kooyker AI, Toes-Zoutendijk E, Opstal-van Winden AWJ, et al. The second round of the Dutch colorectal cancer screening program: impact of an increased fecal immunochemical test cut-off level on yield of screening. Int J Cancer. 2020;147(4):1098-1106. doi: 10.1002/ijc.32839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen MM, Ferm L, Kleif J, et al. Triage may improve selection to colonoscopy and reduce the number of unnecessary colonoscopies. Cancers (Basel). 2020;12(9):2610. doi: 10.3390/cancers12092610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schliemann D, Ramanathan K, Matovu N, et al. The implementation of colorectal cancer screening interventions in low-and middle-income countries: a scoping review. BMC Cancer. 2021;21(1):1125. doi: 10.1186/s12885-021-08809-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norwood DA, Montalvan-Sanchez EE, Corral JE, et al. Western Honduras Copán population-based cancer registry: initial estimates and a model for rural Central America. JCO Glob Oncol. 2021;7:1694-1702. doi: 10.1200/GO.21.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy
eFigure 1. Flow Diagram of Study Inclusion
eTable 2. Excluded Studies and Reasons for Exclusion
eTable 3. Risk of Bias Using the New Castle Ottawa Scale for Comparative Studies
eTable 4. Strategies for Sampling and Recruitment of Included Studies
eTable 5. Univariate Meta Regression
eFigure 2. Participation Rate (FIT Given/FIT Returned) by Region
eFigure 3. Participation Rate (FIT Given/FIT Returned) by Recruitment Strategy
eFigure 4. Participation Rate (FIT Given/FIT Returned) by Sample Size
eFigure 5. Colonoscopy Follow-Up (Colonoscopies Performed After a Positive FIT Test)
eFigure 6. Outcome ADR (Adenoma Detection Rate) per Positive FIT Test in FIT Based Programs
eFigure 7. Outcome AADR (Advanced Adenomas Detection Rate) per Positive FIT Test in FIT Based Programs
eFigure 8. Outcome CRCDR (Colorectal Cancer Detection Rate) per Positive FIT Test in FIT Based Programs
eFigure 9. Yield of Colonoscopy: AADR (Advanced Adenomas Detection Rate). FIT Programs
eFigure 10. Yield of Colonoscopy: AADR (Advanced Adenomas Detection Rate). Direct-to-Colonoscopy Programs
eFigure 11. Yield of Colonoscopy: CRC-DR (Adenocarcinoma Detection Rate) by Type of Program
eFigure 12. Colonoscopy Quality Indicators: Cecal Intubation Rate
eFigure 13. Colonoscopy Quality Indicators: Mean Withdrawal Time From Cecum (Mean, Minutes)
eFigure 14. Colonoscopy Quality Indicators: Adequate Bowel Preparation
eFigure 15. Heterogeneity and Publication Bias (Small Study Effects) for FIT Based Studies
eFigure 16. Heterogeneity and Publication Bias (Small Study Effects) for Outcomes per Positive FIT Test in FIT Based Programs
eFigure 17. Heterogeneity and Publication Bias (Small Study Effects) for Outcomes in Direct-to-Colonoscopy Programs
eFigure 18. Heterogeneity and Publication Bias (Small Study Effects) for Colonoscopy Quality Indicators
eFigure 19. ADR (Adenomas Detection Rate) FIT Programs by Risk: Leave-One-Out Analysis
eFigure 20. ADR (Adenomas Detection Rate) FIT Programs by FIT Cut-Off: Leave-One-Out Analysis
eFigure 21. CRC (Adenocarcinoma Detection Rate) FIT Programs: Leave-One-Out Analysis
eFigure 22. ADR (Adenomas Detection Rate) FIT Programs: Leave-One-Out Analysis
eFigure 23. AADR (Advanced Adenomas Detection Rate) FIT Programs: Leave-One-Out Analysis
eFigure 24. CRC (Adenocarcinoma Detection Rate) FIT Programs: Leave-One-Out Analysis
Data Sharing Statement