Graphical abstract
Keywords: Chronic stroke, Aphasia, Recovery, Lesion expansion, Longitudinal
Highlights
-
•
Progressive lesion necrosis is present in 80% of chronic stroke survivors.
-
•
Extent of lesion expansion is associated with longitudinal behavioral changes in persons with chronic aphasia.
-
•
Semi-automated lesion demarcation method, LINDA, can successfully capture changes in lesion boundaries over time.
Abstract
Background
Volumetric investigations of cortical damage resulting from stroke indicate that lesion size and shape continue to change even in the chronic stage of recovery. However, the potential clinical relevance of continued lesion growth has yet to be examined. In the present study, we investigated the prevalence of lesion expansion and the relationship between expansion and changes in aphasia severity in a large sample of individuals in the chronic stage of aphasia recovery.
Methods
Retrospective structural MRI scans from 104 S survivors with at least 2 observations (k = 301 observations; mean time between scans = 31 months) were included. Lesion demarcation was performed using an automated lesion segmentation software and lesion volumes at each timepoint were subsequently calculated. A linear mixed effects model was conducted to investigate the effect of days between scan on lesion expansion.
Finally, we investigated the association between lesion expansion and changes on the Western Aphasia Battery (WAB) in a group of participants assessed and scanned at 2 timepoints (N = 54) using a GLM.
Results
Most participants (81 %) showed evidence of lesion expansion. The mixed effects model revealed lesion volumes significantly increase, on average, by 0.02 cc each day (7.3 cc per year) following a scan (p < 0.0001).
Change on language performance was significantly associated with change in lesion volume (p = 0.025) and age at stroke (p = 0.031). The results suggest that with every 10 cc increase in lesion size, language performance decreases by 0.9 points, and for every 10-year increase in age at stroke, language performance decreases by 1.9 points.
Conclusions
The present study confirms and extends prior reports that lesion expansion occurs well into the chronic stage of stroke. For the first time, we present evidence that expansion is predictive of longitudinal changes in language performance in individuals with aphasia. Future research should focus on the potential mechanisms that may lead to necrosis in areas surrounding the chronic stroke lesion.
1. Introduction
With great advances in treatment of acute stroke in the hospital setting, the rate of stroke-related deaths has declined, leaving an ever-increasing number of stroke survivors with long-standing chronic deficits. Arguably the most debilitating of these deficits is aphasia, which refers to impairments in speech production, comprehension, or a combination of the two. The conventional understanding of chronic left hemisphere (LH) stroke-induced aphasia suggests that language recovery typically plateaus after entering the chronic stage (>6mos post-stroke onset). However, recent evidence from our group and others has shown that about 30 % of stroke survivors demonstrate marked declines over time (Johnson et al., 2019, Holland et al., 2017, Hope et al., 2017), even after therapeutic intervention involving intensive speech-language therapy (Kristinsson et al., 2021, Breitenstein et al., 2017). Though considerable work has aimed to investigate participant factors associated with the extent of aphasia recovery, many seminal works are from small groups and case studies (Aftonomos et al., 1999, Robey, 1998) or primarily investigate the effect of aphasia treatment related to included participant factors. Research investigating the impact of detailed participant factors on aphasia recovery has suggested that much of the variance in individual recovery is dependent on both lesion- and non-lesion-related factors (Plowman et al., 2012, Hope et al., 2013).
Participant factors such as age at stroke, initial severity, presence of diabetes, and rate of exercise have been reported to be associated with both long-term recovery (Johnson et al., 2019, Johnson et al., 2022, Basilakos et al., 2019) and treated recovery (Harnish et al., 2018). Lesion-related factors, however, have been consistently reported to explain a considerable amount of variance in individual performance (Plowman et al., 2012, Forkel et al., 2014, Marebwa et al., 2017, Thye and Mirman, 2018). In fact, lesion profiles (dependent on size and location) have been shown to explain more than 25 % of the variance in long-term recovery (Johnson et al., 2022).
There is longstanding evidence to support that larger lesions result in more severe deficits (Goldenberg and Spatt, 1994), however, in persons with aphasia, focal lesions can still cause significant language impairments. More recently, significant work has established the importance of spared white matter connections to minimize aphasia severity (Johnson et al., 2022, Kümmerer et al., 2013, Lee et al., 2021, Rosso et al., 2015). Further, in acute stroke, brain plasticity is enhanced in areas immediately surrounding the lesion (perilesional cortex) (Nudo, 1999, Stroemer et al., 1995). Similar studies have demonstrated that functional changes in perilesional cortex impact aphasia recovery even in the chronic stage (Fernandez et al., 2004, Wierenga et al., 2006, Fridriksson et al., 2010). In a follow-up study, Fridriksson et al., (2012) found that increased activation in the perilesional cortex is associated with treatment-related improvement in a language task (Fridriksson et al., 2012).
The pathophysiology and neuronal recovery patterns during the acute stage have been well-defined, leaving such efforts in the chronic stage unexplored. Some investigations in subacute and early chronic stroke recovery have shown evidence of necrotic tissue and edema remaining in perilesional regions which has been shown to lead to neuronal decay. In a study by Seghier and colleagues (2014), 56 patients were observed to increase in lesion volume by 1.59 cc per year (Seghier et al., 2014). Further, there was a greater degree of lesion expansion for individuals whose time intervals between repeated visits was larger after controlling for baseline lesion volume, age at stroke, years post-onset, and total intracranial volume.
Therefore, given the evidence emphasizing the importance of in tact perilesional cortex to rehabilitation and performance on speech-language tasks, gradual lesion expansion (GLE) may explain chronic behavioral declines in a sample of participants with chronic aphasia. Additionally, and most relevant to individuals with aphasia and servicing clinicians, an individual’s rate of perilesional degeneration could also explain the inherent unreliability in treatment response to post-stroke therapy.
Therefore, the aims of the present project were two-fold. Using our large, retrospective longitudinal dataset of high-resolution structural MR images, we investigated patterns of GLE in the chronic stage of aphasia recovery. Specifically, we measured 1) longitudinal lesion volumes to investigate the presence of lesion expansion in a population with chronic aphasia and 2) the relative importance of lesion expansion in a statistical model to predict longitudinal changes in aphasia severity.
2. Materials and methods
2.1. Participants
The present study utilized retrospectively collected longitudinal data of individuals in the chronic stage of aphasia recovery (≥6 months post-stroke at initial scan and testing timepoint) drawn from the open-source Aphasia Recovery Cohort (ARC) dataset (https://openneuro.org/datasets/ds004512/versions/2.0.0). Upon initial scan, participants (or their caretaker) completed a detailed case history survey that inquired about demographic and health data. A total of 104 participants (33 female) with at least two imaging sessions (with average time between = 952 days between) met inclusion criteria available resulting in a total of 303 imaging observations across all participants. Two participant scans were removed from the analysis due to presence of significant imaging artifacts that hindered automated lesion detection methods (described below). Therefore, a total of 301 scans for 104 participants were included in the present study.
All studies from which the utilized data originated from were conducted in accordance with the Declaration of Helsinki. The need for informed consent for the present study was waived due to the retrospective nature of the study.
2.2. Scanning protocol
Magnetic resonance imaging (MRI) scans at each time point were collected for an independent study or at baseline for a treatment study at the Center for the Study of Aphasia Recovery (C-STAR) laboratory at the University of South Carolina or the Medical University of South Carolina. For the purposes of the present study, only structural MRI images (T1s) were examined. Lesions were automatically identified and delineated using freely available lesion-segmentation software (described below), and lesion volumes were calculated based on the output of this software. High-resolution T1 images were acquired using a Siemens Trio/Prisma 3 T scanner equipped with a 20-element head-neck coil with the following parameters: an MP-RAGE sequence with 1 mm isotropic voxels, a 256 × 256 matrix size, a 9-degree flip angle, and a 92-slice sequence with repetition time = 2250 ms, inversion time = 925 ms, and echo time = 4.11 ms.
2.3. Lesion segmentation protocol
Lesion segmentation was conducted using Lesion Identification with Neighborhood Data Analysis (LINDA), an automated lesion segmentation software (Pustina et al., 2016), with default settings, resulting in a single probabilistic lesion mask for each participant at each time point (i.e., 301 total masks). Subsequently, each probabilistic lesion mask was reviewed by authors AT and/or LJ to ensure successful lesion demarcation. Upon review, it was noted that scans with considerable artifacts (caused by excessive motion or lesions that interfere with the lateral ventricles) resulted in poor automated lesion demarcation. Therefore, all scans and respective LINDA demarcations were reviewed by trained lesion raters (authors LJ and AT) to ensure successful lesion identification. Two scans were removed from the dataset due to scan quality being affected by excessive motion. Following automatic lesion segmentation, the final prediction mask output from LINDA (Prediction3) in native space was used to calculate the total lesioned voxels (cc) for all participants’ scans.
Change in lesion volume was calculated by subtracting volume at initial scan from lesion volume at the follow-up scan with positive numbers indicating greater expansion. Change in lesion volume was used as the primary variable of interest in the analyses described below.
2.4. Evaluating longitudinal lesion expansion
A total of 104 participants (33 female; k = 301 observations) who had at least two scanning sessions (M = 2.89; SD = 0.93; range = 2–6 timepoints) were included in an analysis to evaluate longitudinal lesion expansion. A linear mixed effects model (LMEM) was conducted, with lesion volumes at each timepoint as the dependent variable, and days between scans as the independent variable. The random effect of participant ID and days since initial scan were specified. A significant effect of the independent variable was considered if p-value < 0.05.
2.5. Lesion expansion as a predictor of longitudinal behavior
To investigate the impact of lesion expansion on behavior, a general linear model (GLM) was created for a subset of participants who had longitudinal behavioral and imaging data available (N = 54). Participants were included in the present analysis if they were assessed using a standardized aphasia severity battery, the Western Aphasia Battery-Revised (WAB-R) (Kertesz, 2007), twice at least 3-months between assessments (M = 38.5; SD = 27 months) and received a high-resolution MRI scan within 10-days of the WAB-R assessment. Participants were included in this analysis if they tested as having aphasia at baseline WAB-R assessment (WAB AQ ≤ 93.8).
Independent variables included in the GLM were based on prior studies (Johnson et al., 2019, Plowman et al., 2012) which found significant relationships between the factors and the dependent variable: change in WAB AQ between assessments. The independent variables included: initial severity (NIH Stroke Scale; NIHSS), age at stroke, and days between assessments. A measure of lesion expansion (lesion volume at follow-up–lesion volume at initial scan) was also included as an independent variable. Relationships between independent variables and WAB AQ change were considered significant if p-values < 0.05.
3. Results
3.1. Evaluating longitudinal lesion changes
The mean time post stroke onset at the time of initial scan was 3.4 years (range = 6 mos–17.3 yrs), which is well within the range of what is considered the chronic stage of stroke recovery. Table 1 presents demographic information for participants included in the investigation of longitudinal expansion.
Table 1.
Descriptive statistics for cohort with at least 2 structural images used to evaluate longitudinal lesion expansion. Both Lesion Size Change and Days Between Scans were calculated using data from the final scan (Follow-up Lesion Size) and the initial scan (Initial Lesion Size) for each participant. Lesion sizes are measured in cubic centimeters (cc); Education measured in years (yrs).
|
Summary Statistics: Longitudinal Expansion (N = 104) | |||||
|---|---|---|---|---|---|
| Variable | N | Mean | Std. Dev. | Min | Max |
| Sex | 104 | ||||
| Female | 33 | 32 % | |||
| Male | 71 | 68 % | |||
| Education (yrs) | 104 | 15.11 | 2.32 | 10 | 20 |
| Initial Days Post-Stroke | 104 | 1231.24 | 1358.66 | 185 | 6329 |
| Initial Lesion Size (cc) | 104 | 95.9 | 69.68 | 1.3 | 355.39 |
| Follow-up Lesion Size (cc) | 104 | 110.27 | 73.4 | 1.11 | 353.3 |
| Lesion Size Change (cc) | 104 | 14.37 | 19.15 | −10.22 | 88.63 |
| Days Between Scans | 104 | 952.36 | 1067.52 | 20 | 4950 |
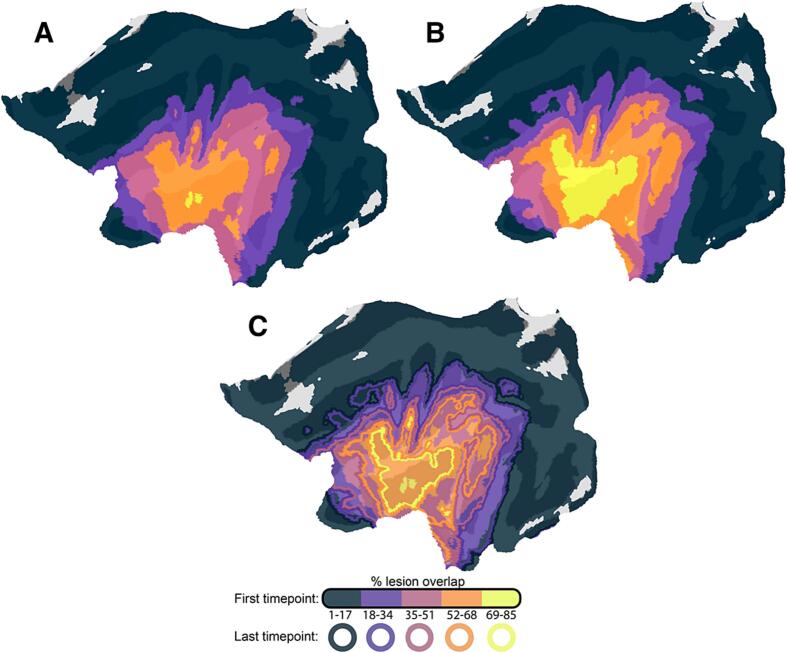
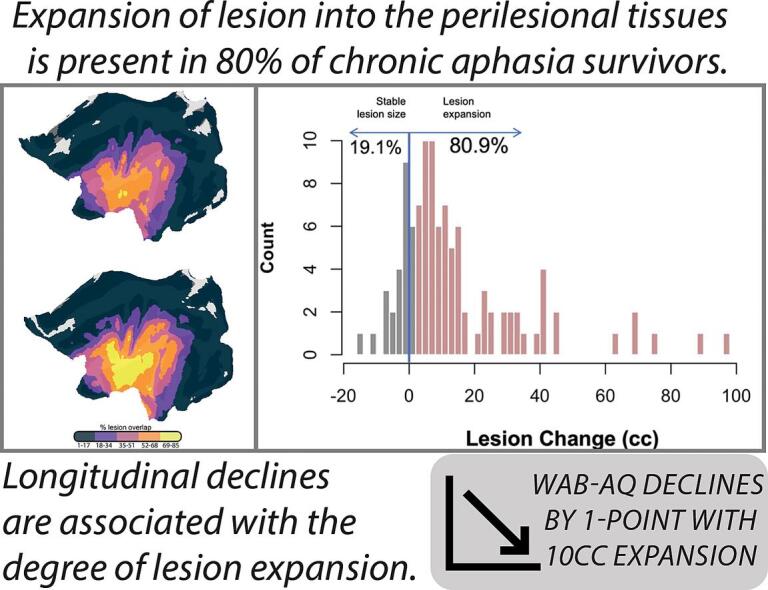
To better visualize GLE, Fig. 1 demonstrates lesion boundaries at timepoint 1, 2, and the group-level expansion of lesion between the two timepoints. In this figure, the percentage of the group that presents with a lesion at each location of the brain is independently shown for scans collected at the first (Fig. 1A) and last sessions (Fig. 1B). Both lesion overlap maps were segmented into five distinct bins, each representing a specific percentage range of overlap. When comparing identical bins between the two time points, a noticeable increase in the extent of lesion overlap was evident for the second time point. Across the group, the insula was the region most often damaged due to the stroke lesion and the most pronounced expansion of lesion overlap centered around this area.
Fig. 1.
(A) Flat map depicting group-level lesion overlap at timepoint 1; (B) Flat map depicting group-level lesion overlap at timepoint 2; and (C) Flat map depicting change in group-level lesion overlap. Solid-filled regions represent the percentage of participants (N = 104) with spatially overlapping lesions at the first time point (initial scan). Outlines indicate the percentage of participants with spatially overlapping lesions at the last (follow-up) scan. At the initial scan (A), participants showed maximal overlap in a small core of the insular cortex (two small yellow clusters). At their last follow up scan (B), the same percentage of participants demonstrated overlap across a much larger swathe of perisylvian cortex (yellow outline in figure C). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In a LMEM with the dependent variable of lesion size (cc) and independent variable of days between scan there was a significant effect of days between scan (p < 0.00001; Table 2). Random effects of participant ID and days since initial scan were used and assumptions for LMEM were inspected and met. The results from this model suggest that, for every day after the initial scanning session, the lesion increases by 0.02 cc (7.3 cc per year).
Table 2.
Results from the linear mixed effects model (LMEM) predicting lesion expansion across days since initial scan in 104 individuals with a total of 301 observations.
| LMEM: Lesion Expansion ∼ Days Since Initial Scan | |||||
|---|---|---|---|---|---|
| Fixed Effects: | |||||
| Predictors | Estimates | Std. Error | DF | t-value | p-value |
| (Intercept) | 96.77 | 6.85 | 196 | 14.14 | <0.001 |
| Days Since Initial Scan | 0.015 | 0.001 | 196 | 9.89 | <0.001 |
| Random Effects: | |||||
| Std Dev | Corr | ||||
| (Intercept) | 69.61 | (Intr) | |||
| Days Since Initial Scan | 0.009 | 0.27 | |||
| Residual | 6.95 | ||||
| Observations | 301 | ||||
| Marginal R (Holland et al., 2017) / Conditional R2 | 0.034 / 0.991 | ||||
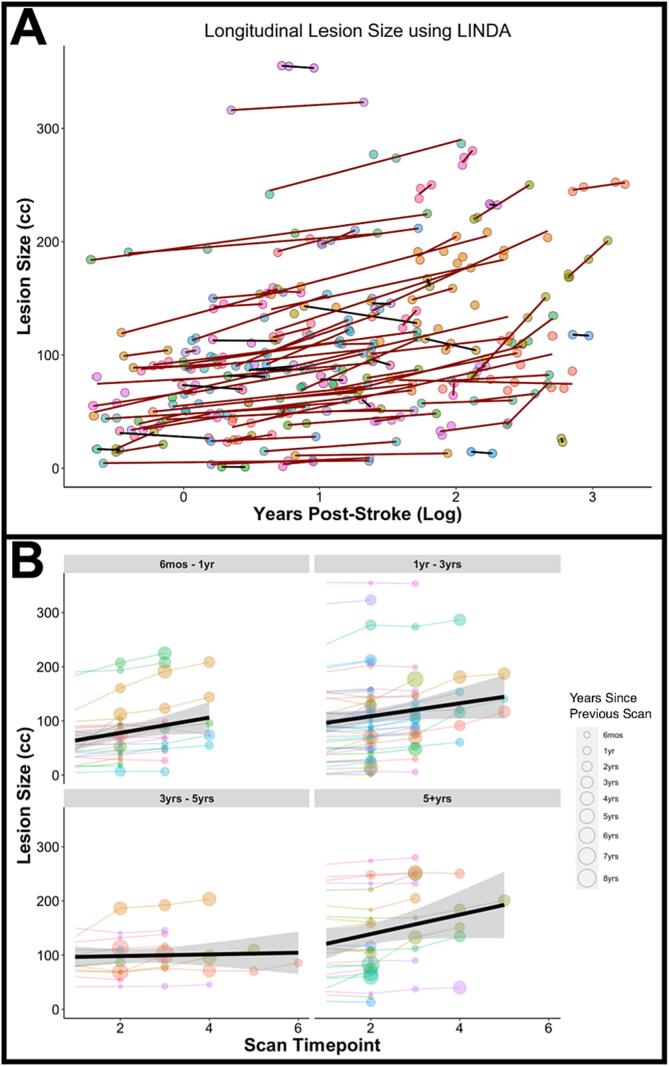
Fig. 2 presents the lesion volumes at each scanning timepoint available for each participant (indicated by circle color) in the chronic stage of stroke recovery. The slope of each participant’s lesion size trajectory is shown to detail participants with expanding lesions (red line) or participants with relatively stable lesion size (black line). For ease of visualization and interpretation of Fig. 2A, years post-stroke at time of scan (x-axis) was log transformed. Overall, the average change in lesion size was 14.4 cc (SD = 19.2 cc; range = -10.2 cc–88.6 cc), showing that the majority of participants’ chronic lesions (85/104, 81 %) expanded over time. To demonstrate that this effect is present even years beyond stroke occurrence, Fig. 2B presents the lesion volumes at each scanning timepoint with participants binned by the time post-stroke at the initial scanning timepoint.
Fig. 2.
(A) Change in lesion volume over time for 104 participants with chronic LH stroke and aphasia. Red lines indicate lesions that have increased in size between initial scan and final follow-up scan (positive slope); black lines indicate lesions that have remained stable over time (negative slope); (B) Change in lesion volume for participants grouped by the time post-stroke at initial scan. Size of the participant node indicates time between scanning sessions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
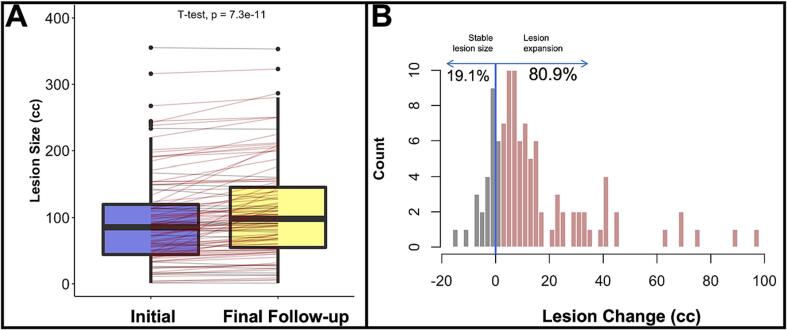
Finally, Fig. 3 provides a detailed observation of initial lesion size compared to the lesion size at the individual’s final scan (Fig. 3A). Though not accounting for time between scans, we observed 80% of our sample demonstrated an increase in lesion size between initial and final follow-up scans (Fig. 3B).
Fig. 3.
(A) Boxplot depicting lesion size at initial and final follow-up scan; (B) Histogram demonstrating proportion of expanders and non-expanders in the sample (N = 104).
3.2. Effect of longitudinal lesion changes on behavior
Table 3 presents demographic information for participants included in the analysis investigating how change in lesion volume may explain longitudinal WAB AQ change.
Table 3.
Descriptive statistics for subsample with both baseline and follow-up WAB AQ assessments as well as both baseline and follow up MRI examinations WAB = Western Aphasia Battery, AQ = Aphasia Quotient, NIHSS = National Institutes of Health Stroke Scale, Lesion sizes are measured in cubic centimeters (cc).
|
Summary Statistics: Longitudinal Behavior and Lesion Expansion Cohort (N = 54) | |||||
|---|---|---|---|---|---|
| Variable | N | Mean | Std. Dev. | Min | Max |
| Sex | 54 | ||||
| Female | 14 | 26 % | |||
| Male | 40 | 74 % | |||
| Education (yrs) | 54 | 15.31 | 2.38 | 10 | 20 |
| Initial Days Post-Stroke | 54 | 1228.35 | 1168.77 | 185 | 6127 |
| Initial Lesion Size (cc) | 54 | 105.17 | 58.75 | 4.44 | 277.03 |
| Age at Stroke | 54 | 54.44 | 10.84 | 31 | 75 |
| Initial NIHSS | 54 | 5.59 | 3.42 | 1 | 14 |
| Lesion Size Change (cc) | 54 | 21.55 | 24.83 | −10.22 | 118.58 |
| Days Between Scans | 54 | 1131.28 | 870.51 | 88 | 4539 |
| WAB AQ Change | 54 | 2.07 | 7.58 | –22.7 | 19.5 |
A GLM was conducted to investigate the effect of change in lesion volume on long-term behavioral performance in 54 participants with aphasia (mean baseline WAB AQ = 55.8; SD = 21.7; range = 17.8–91.8). In this model, WAB AQ was included as the dependent variable, and change in lesion size, initial stroke severity, age at stroke, and days between WAB assessments were included as independent variables. Assumptions for the GLM were visually inspected and were met.
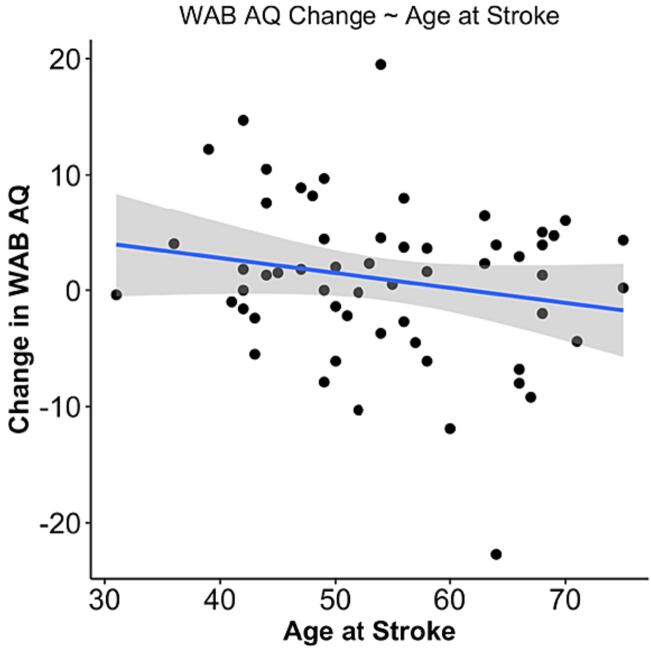
We observed a main effect of age at stroke on change in WAB AQ (p = 0.031), as well as a main effect of change in lesion volume on change in WAB AQ (p = 0.025). Results from the model demonstrate that every 10 cc of lesion expansion was associated with 0.9 points decrease on the WAB AQ, and for every 10-year increase at age of stroke, WAB AQ decreased by 1.9 points (Table 4). Fig. 4, Fig. 5 present the main effects of lesion size change and age at stroke on WAB AQ, respectively. Of note, we removed the individual outlier observed in both linear models. Importantly, removing this individual did not change the significance of the main effect between lesion size change and age at stroke in predicting WAB AQ change (p = 0.035; p = 0.033, respectively). Change in WAB AQ performance between assessments was not associated with time between assessments or initial stroke severity.
Table 4.
Results from GLM predicting change in WAB AQ performance. Bolded p-values indicate significant (p < 0.05) predictors.
|
Predicting Change in WAB AQ | |||
|---|---|---|---|
| Predictors | Estimates | CI | P |
| (Intercept) | 13.102 | 2.179 – 24.026 | 0.020 |
| Lesion Size Change | −0.088 | −0.165 – −0.012 | 0.025 |
| Initial NIHSS | 0.323 | −0.193 – 0.839 | 0.215 |
| Age at Stroke | −0.188 | −0.358 – −0.018 | 0.031 |
| Days between WABs | −0.002 | −0.004 – 0.001 | 0.147 |
| Observations | 54 | ||
| R2 / R2 adjusted | 0.238 / 0.175 | ||
Fig. 4.
Scatterplot demonstrating the significant main effect of change in lesion volume (cc) as a predictor of change in WAB AQ.
Fig. 5.
Scatterplot demonstrating the significant main effect of age at stroke as a predictor of change in WAB AQ.
4. Discussion
Previous research indicates that cortical damage associated with acute stroke, as measured by lesion size, continues to evolve with time and that this increase is due to both accelerated global atrophy and, more targeted decreases in cortical integrity at perilesional sites (Seghier et al., 2014, Naeser et al., 1998). However, the relationship between GLE and behavioral sequalae of stroke remains wholly unexplored. The current study addressed this gap by examining the relationship between GLE and language impairment in a large cohort of individuals with LH stroke and aphasia.
Consistent with a study by Seghier and colleagues (2014) that examined GLE, we report that lesion expansion was present in the chronic timeframe in the majority of participants, and we extend these results, for the first time, to individuals with chronic LH stroke and aphasia. Direct comparison of lesion size at two time points (mean separation between time points = 31 months) confirmed that lesion volumes were significantly larger at follow-up testing. One important contribution of the present study is the investigation of long-term GLE in individuals years after their stroke event (minimum 6 months post-stroke at initial scan). Seghier and colleagues’ paper included participants within the late subacute stages (≥3 months post-stroke), when stroke-related edema could still be present. Therefore, the present study not only validates the findings that lesion expansion is present in individuals beyond the acute stage but also provides evidence that individuals years post-stroke demonstrate GLE as well.
Finally, the impact of GLE on behavioral change was investigated in a subset of participants who had both MRI scans and WAB assessments available at both initial and follow-up assessments. A general linear model, with change in WAB AQ as the dependent variable, revealed that both change in lesion volume and age at stroke were significantly associated with change in WAB AQ (p = 0.025; p = 0.031, respectively). This is the first study, to our knowledge, to demonstrate a relationship between GLE and behavioral change in a sample of individuals with chronic stroke aphasia.
4.1. Pathophysiology of stroke lesions
While the pathophysiology of and neuronal recovery patterns associated with the acute stage of stroke have been well-defined (Kuriakose and Xiao, 2020, Alia et al., 2017, Seitz and Donnan, 2015), explanations of analogous changes in the chronic stage of recovery remain largely unexplored. One potential explanation for perilesional degradation observed in the current study is that GLE reflects persistent local Wallerian degeneration (WD) of short-range intracortical connections driven by lack of input from areas immediately surrounding the stroke lesion. Though typically discussed in terms of its impact on the acute to sub-acute recovery stages, there is evidence to suggest WD continues in some instances through the chronic stage of stroke recovery. For example, Werring and colleagues confirmed that degeneration can be observed in stroke patients 2–6 months post infarct, although it was noted that extent of WD was variable across individuals (Werring et al., 2000). Interestingly, there is evidence that other types of MRI scans, most notably diffusion tensor imaging (DTI), which measures white matter tract integrity, can be used as an alternative measure of changes due to WD, a fact which opens the interesting possibility of examining DTI in future chronic stroke studies (Pierpaoli et al., 2001).
An important consideration in the current study is the fact that our patients were specifically chosen for study inclusion based on the presence of aphasia. Aphasia typically results from strokes that damage brain sites in the putative left hemisphere language system. Damage to these areas is most often the result of ischemia or hemorrhage of the middle cerebral artery (MCA), which is situated between two watershed areas, areas that exist at the border between two major cerebral arteries. One hypothesis for observed lesion expansion is that watershed areas are more susceptible to the neural degeneration observed in GLE (Naeser et al., 1998). This may be due to the proximity between two adjacent arteries, causing watershed zones to be particularly susceptible to alterations of hemodynamic conditions (van der Zwan et al., 1992), which can result in hypoperfusion, eventually resulting in neural degeneration (Broughton et al., 2009). In animal models, chronic hypoperfusion has been shown to contribute to neuronal death, which can lead to expanding lesions in these areas post-stroke. Kudo and colleagues demonstrated that gerbil brains post-stroke had considerable hypoperfusion in watershed zones surrounding a stroke lesion, and all lesions expanded after 12 weeks, particularly in the middle frontal gyrus, which is adjacent to the inferior frontal gyrus that, when damaged, is associated with speech production deficits (Kudo et al., 1993). This reduced hypoperfusion can appear intact but can create what is described as a “functional lesion,” where neurons are viable but are not functioning properly (Thompson et al., 2017, Love et al., 2002, Richardson et al., 2011). Indeed, evidence from Thompson and colleagues (2017) demonstrates perilesional hypoperfusion in chronic stroke lesions (0–6mm surrounding the lesion), which is associated with behavioral scores in persons with aphasia (Thompson et al., 2017). Future studies might examine GLE and its relationship with behavioral improvement in alternative stroke populations in which watershed areas are not so directly implicated.
4.2. Gradual lesion expansion predicts change in behavioral performance
Language performance in the chronic stage of aphasia is dynamic with nearly 30% of individuals demonstrating significant gradual decline in linguistic performance, even after receiving intensive speech-language therapy. Measures of neural health have recently garnered attention in the stroke-aphasia literature due to their association with chronic behavioral declines in spared contralateral regions (Johnson et al., 2022, Basilakos et al., 2019, Wilmskoetter et al., 2019). Specifically, the presence and extent of markers of small vessel disease (i.e., white matter hyperintensities, lacunes, enlarged peri-vascular spaces) in the contralateral hemisphere have been proposed as a measure of overall brain health, which is significantly related to longitudinal performance. Evaluating the relative health of the ipsilateral hemisphere, however, poses a challenge due to the hyperintense tissues surrounding the lesion proper, and the often minimal, spared tissue that is able to be evaluated if a lesion is particularly large.
Our understanding of the relationship between brain health and behavioral outcomes in the ipsilesional hemisphere is less clearly developed. For example, one study found that fMRI changes in perilesional areas was not associated with behavioral change (de Haan et al., 2013). Another found that cerebral blood flow in perilesional areas was similarly unrelated to improvement (Walenski et al., 2022). In contrast, early evidence from our group (Fridriksson et al., 2012) and others have demonstrated the importance of perilesional integrity to predict overall severity and aphasia rehabilitation (Walenski et al., 2022). If we assume that perilesional tissue degraded most in individuals with greater GLE, then the current study can be interpreted as providing further support for the idea that health of perilesional tissue is critical to long-term recovery. Such an interpretation has clear implications for therapeutic interventions designed to prevent/slow brain atrophy in stroke survivors, highlighting the need to develop and test interventions directed at perilesional tissues.
5. Limitations and future directions
Though the present study presents evidence that perilesional necrosis is associated with long-term behavioral change in chronic stroke aphasia, there remains to be determined the cause of the underlying mechanism that triggers lesion expansion. One possibility could be due to genetic or health factors that could result in necrosis of the tissue(s) surrounding the lesion. Future studies should examine the relationship between comorbidities (including small vessel disease, diabetes, and activity level) and changes in aphasia severity as these associated health factors have been shown to influence overall aphasia severity and recovery. Unfortunately, these data were not available for all participants with longitudinal imaging and behavioral assessments in the current.
We were limited in the longitudinal behavioral data available for the cohort of participants with longitudinal imaging. The present results provide evidence that GLE is related to changes in performance on a language assessment, however, it is unclear if other behavioral tasks (i.e., measures of cognition) also demonstrate a similar association. Prior studies suggest observed GLE could be an early marker of vascular dementia (Seghier et al., 2014), therefore, future studies should investigate the impact of GLE on more global measures of cognition.
Although not affecting the interpretation of the present findings, future investigations would benefit from examining the impact of acute treatment (i.e., via pharmacological intervention and/or tissue plasminogen activator) and lesion expansion as a factor of overall response to treatment in the chronic stage. Given the nature of recruiting participants in the chronic stage of recovery and utilizing retrospectively collected data, we were unable to investigate these factors, but future longitudinal studies could shed light on a potential mechanism that predicts GLE in chronic recovery.
6. Conclusions
Conventional wisdom regarding chronic stroke lesions suggests that lesion borders do not change past the acute and subacute stages of injury. The results from the present study provide compelling evidence for GLE well into the chronic stages of left hemisphere MCA stroke-induced aphasia. Moreover, we show, for the first time, GLE is relevant to disease prognosis and potentially treatment response. GLE provides another mechanistic explanation for declines observed in chronic stroke recovery and these findings may have important implications for researchers and clinicians interested in maximizing performance and recovery by minimizing/slowing the evolution of post-insult damage to the brain.
CRediT authorship contribution statement
Lisa Johnson: . Roger Newman-Norlund: Writing – review & editing, Software, Data curation, Conceptualization. Alex Teghipco: Writing – review & editing, Writing – original draft, Visualization, Software, Investigation, Data curation, Conceptualization. Chris Rorden: Writing – original draft, Software, Data curation. Leonardo Bonilha: Writing – original draft, Validation. Julius Fridriksson: Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is partially supported by the Office for the Study of Aging Research Fellowship, awarded to Johnson, at the University of South Carolina. This work was also supported by the National Institute on Deafness and Other Communication Disorders (Fridriksson: R03 DC005915, R01 DC008355, R01 DC009571, R03 DC010262, R01 DC011739, R21 DC014170, P50 DC014664).
Data availability
This data is already available in ARCQuery (https://github.com/rordenlab/AphasiaResearchCohortQuery).
References
- Aftonomos L.B., Appelbaum J.S., Steele R.D. Improving outcomes for persons with aphasia in advanced community-based treatment programs. Stroke. 1999;30(7):1370–1379. doi: 10.1161/01.STR.30.7.1370. [DOI] [PubMed] [Google Scholar]
- Alia C., Spalletti C., Lai S., et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: Novel approaches in neurorehabilitation. Front. Cell. Neurosci. 2017;11(March):1–22. doi: 10.3389/fncel.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos A., Stark B.C., Johnson L., et al. Leukoaraiosis is associated with a decline in language abilities in chronic aphasia. Neurorehabil. Neural Repair. 2019;33(9) doi: 10.1177/1545968319862561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C., Grewe T., Flöel A., et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet. 2017;389(10078):1528–1538. doi: 10.1016/S0140-6736(17)30067-3. [DOI] [PubMed] [Google Scholar]
- Broughton B.R.S., Reutens D.C., Sobey C.G. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- de Haan B., Rorden C., Karnath H.O. Abnormal perilesional BOLD signal is not correlated with stroke patients’ behavior. Front. Hum. Neurosci. 2013;(OCT) doi: 10.3389/fnhum.2013.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez B., Cardebat D., Demonet J.F., et al. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35(9):2171–2176. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- Forkel S.J., Thiebaut de Schotten M., Dell’Acqua F., et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain. 2014;137(7):2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- Fridriksson J., Bonilha L., Baker J.M., Moser D., Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cereb. Cortex. 2010;20(5) doi: 10.1093/cercor/bhp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., Richardson J.D., Fillmore P., Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage. 2012;60(2):854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G., Spatt J. Influence of size and site of cerebral lesions on spontaneous recovery of aphasia and on success of language therapy. Brain Lang. 1994;47(4):684–698. doi: 10.1006/brln.1994.1063. [DOI] [PubMed] [Google Scholar]
- Harnish S.M., Rodriguez A.D., Blackett D.S., et al. Aerobic exercise as an adjuvant to aphasia therapy: theory, preliminary findings, and future directions. Clin. Ther. 2018;40(1):35–48.e6. doi: 10.1016/j.clinthera.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A., Fromm D., Forbes M., MacWhinney B. Long-term recovery in stroke accompanied by aphasia: a reconsideration. Aphasiology. 2017;31(2):152–165. doi: 10.1080/02687038.2016.1184221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T.M.H., Seghier M.L., Leff A.P., Price C.J. Predicting outcome and recovery after stroke with lesions extracted from MRI images. Neuroimage Clin. 2013;2(1):424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T.M.H., Leff A.P., Prejawa S., et al. Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain. 2017;140:1718–1728. doi: 10.1093/brain/awx086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Basilakos A., Yourganov G., et al. Progression of aphasia severity in the chronic stages of stroke. Am. J. Speech Lang. Pathol. 2019;28(2) doi: 10.1044/2018_AJSLP-18-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L., Nemati S., Bonilha L., et al. Predictors beyond the lesion: Health and demographic factors associated with aphasia severity. Cortex. 2022;154:375–389. doi: 10.1016/j.cortex.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Grune & Stratton; 2007. Western Aphasia Battery-R. [Google Scholar]
- Kristinsson S., Basilakos A., Elm J., et al. Individualized response to semantic versus phonological aphasia therapies in stroke. Brain Commun. 2021;3(3) doi: 10.1093/braincomms/fcab174. fcab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T., Takeda M., Tanimukai S., Nishimura T. Neuropathologic changes in the gerbil brain after chronic hypoperfusion. Stroke. 1993;24(2):259–264. doi: 10.1161/01.str.24.2.259. discussion 265. [DOI] [PubMed] [Google Scholar]
- Kümmerer D., Hartwigsen G., Kellmeyer P., et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136(2):619–629. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose D., Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int. J. Mol. Sci. 2020;21(20) doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Ko M.H., Park S.H., Kim G.W. Prediction of aphasia severity in patients with stroke using diffusion tensor imaging. Brain Sci. 2021;11(304) doi: 10.3390/brainsci11030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love T., Swinney D., Wong E., Buxton R. Perfusion imaging and stroke: A more sensitive measure of the brain bases of cognitive deficits. Aphasiology. 2002;16(9):873–883. doi: 10.1080/02687030244000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marebwa B.K., Fridriksson J., Yourganov G., Feenaughty L., Rorden C., Bonilha L. Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Sci. Rep. 2017;7(1):8188. doi: 10.1038/s41598-017-07607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser M.A., Palumbo C.L., Prete M.N., et al. Visible changes in lesion borders on CT scan after five years poststroke, and long-term recovery in aphasia. Brain Lang. 1998;62(1):1–28. doi: 10.1006/brln.1997.1866. [DOI] [PubMed] [Google Scholar]
- Nudo R.J. Recovery after damage to motor cortical areas. Curr. Opin. Neurobiol. 1999;9(6):740–747. doi: 10.1016/s0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C., Barnett A., Pajevic S., et al. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13(6):1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Plowman E., Hentz B., Ellis C. Post-stroke aphasia prognosis: A review of patient-related and stroke-related factors. J. Eval. Clin. Pract. 2012;18(3) doi: 10.1111/j.1365-2753.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- Pustina D., Coslett H.B., Turkeltaub P.E., Tustison N., Schwartz M.F., Avants B. Automated segmentation of chronic stroke lesions using LINDA: Lesion identification with neighborhood data analysis. Hum. Brain Mapp. 2016;37(4):1405–1421. doi: 10.1002/hbm.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J.D., Baker J.M., Morgan P.S., Rorden C., Bonilha L., Fridriksson J. Cerebral perfusion in chronic stroke: Implications for lesion-symptom mapping and functional MRI. Behav. Neurol. 2011;24:117–122. doi: 10.3233/BEN-2011-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey R.R. A meta-analysis of clinical outcomes in the treatment of aphasia. J. Speech Lang. Hear. Res. 1998;41(1):172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- Rosso C., Vargas P., Valabregue R., et al. Aphasia severity in chronic stroke patients: a combined disconnection in the dorsal and ventral language pathways. Neurorehabil. Neural Repair. 2015;29(3):287–295. doi: 10.1177/1545968314543926. [DOI] [PubMed] [Google Scholar]
- Seghier M.L., Ramsden S., Lim L., Leff A.P., Price C.J. Gradual lesion expansion and brain shrinkage years after stroke. Stroke. 2014;45(3):877–879. doi: 10.1161/strokeaha.113.003587. [DOI] [PubMed] [Google Scholar]
- Seitz R.J., Donnan G.A. Recovery potential after acute stroke. Front. Neurol. 2015;6:238. doi: 10.3389/fneur.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer R.P., Kent T.A., Hulsebosch C.E. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26(11):2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Thompson C.K., Walenski M., Chen Y., et al. Intrahemispheric perfusion in chronic stroke-induced aphasia. Neural Plast. 2017;2017 doi: 10.1155/2017/2361691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye M., Mirman D. Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. Neuroimage Clin. 2018;20:1129–1138. doi: 10.1016/j.nicl.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwan A., Hillen B., Tulleken C.A., Dujovny M., Dragovic L. Variability of the territories of the major cerebral arteries. J. Neurosurg. 1992;77(6):927–940. doi: 10.3171/jns.1992.77.6.0927. [DOI] [PubMed] [Google Scholar]
- Walenski M., Chen Y., Litcofsky K.A., et al. Perilesional perfusion in chronic stroke-induced aphasia and its response to behavioral treatment interventions. Neurobiol Lang (camb). 2022;3(2):345–363. doi: 10.1162/nol_a_00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol., Neurosurgery Psychiatry. 2000;69(2):269 LP - 272. 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed]
- Wierenga C.E., Maher L.M., Moore A.B., et al. Neural substrates of syntactic mapping treatment: an fMRI study of two cases. J. Int. Neuropsychol. Soc. 2006;12(1):132–146. doi: 10.1017/S135561770606019X. [DOI] [PubMed] [Google Scholar]
- Wilmskoetter J., Marebwa B., Basilakos A., et al. Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain. 2019;142(10) doi: 10.1093/brain/awz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This data is already available in ARCQuery (https://github.com/rordenlab/AphasiaResearchCohortQuery).