Abstract
Approximately one-half of Escherichia coli isolates from patients with cystitis or pyelonephritis produce the pore-forming cytotoxin hemolysin, a molecule with the capacity to lyse erythrocytes and a range of nucleated cell types. A second toxin, cytotoxic necrotizing factor 1 (CNF1), is found in approximately 70% of hemolytic, but rarely in nonhemolytic, isolates. To evaluate the potential interplay of these two toxins, we used epidemiological and molecular biologic techniques to compare the cytotoxicity of hemolytic, CNF1+, and CNF1− cystitis strains toward human T24 bladder epithelial cells in vitro. A total of 29 isolates from two collections of cystitis-associated E. coli were evaluated by using methylene blue staining of bladder monolayers at 1-h intervals after inoculation with each strain. Most (20 of 29) isolates damaged or destroyed the T24 monolayer (less than 50% remaining) within 4 h after inoculation. As a group, CNF1+ isolates from one collection (11 strains) were less cytotoxic at 4 h than the CNF1− strains in that collection (P = 0.009), but this pattern was not observed among isolates from the second collection (18 strains). To directly evaluate the role of CNF1 in cytotoxicity of hemolytic E. coli without the variables present in multiple clinical isolates, we constructed mutants defective in production of CNF1. Compared to the CNF1+ parental isolates, no change in cytotoxicity was detected in these cnf1 mutants. Our results indicate that CNF1 does not have a detectable effect on the ability of hemolytic E. coli to damage human bladder cell monolayers in vitro.
The urinary tract is defended against bacterial infection in part by the flow of urine and the antibacterial effects of the bladder mucosa (12, 29). These mechanisms are quite effective in removing colonic organisms that enter via an ascending route from the periurethral area through the urethra into the bladder lumen. Experimentally, the majority of Escherichia coli bacteria introduced into the bladder of human volunteers disappear within 72 h (12). Nonetheless, urinary tract infections (UTI) constitute one of the most common bacterial infections in the United States, resulting in 6 million to 7 million physician office visits per year (36). The majority of these manifest as dysuria, frequency, and urgency and are considered to be cystitis or bladder infections. The most common culprit, uropathogenic E. coli, is found in 80% of cases (35). However, most studies have focused on E. coli strains isolated from patients with fever and flank pain, i.e., pyelonephritis, considered to be kidney infection, and less is known about the pathogenesis of cystitis.
Certain factors are more often found in E. coli that cause UTI than in isolates from the feces of control patients and may contribute to the virulence of the UTI strains. These include adhesive fimbriae, the iron-scavenging siderophore aerobactin, certain capsular polysaccharides, serum resistance, and two toxins, hemolysin and cytotoxic necrotizing factor 1 (CNF1) (reviewed in references 17, 27, and 39). The first of these toxins, hemolysin, is a pore-forming cytotoxin (reviewed in references 2 and 9) with the capacity to lyse erythrocytes and a range of nucleated cell types including granulocytes (10), fibroblasts (11), and human kidney epithelial cells (28, 38). The second toxin, CNF1, is associated with isolates from extraintestinal infections (primarily from UTI) (1, 5, 8, 25) and has marked effects on eukaryotic cell function, causing alterations in the cell cytoskeleton and morphology (7, 14, 21) and triggering internalization of latex beads and noninvasive bacteria (20). CNF1 is a lethal toxin when administered intravenously to mice (16) or sheep (13) and is dermonecrotic in rabbit skin (14).
Several studies have noted a close association between these two toxins. CNF1 is found in approximately 70% of hemolytic strains but rarely in nonhemolytic isolates (1, 3–5, 7, 8, 25). Evidence suggests these toxins are genetically linked; both cnf1 and hly have been identified on a chromosomal gene block in seven E. coli isolates (18), and one uropathogenic strain (J96) has been shown to carry both cnf1 and hly on chromosomal pathogenicity island II (6).
The epidemiology, genetic linkage to other virulence factors, and in vitro and in vivo effects of CNF1 suggest that it is a potentially important virulence factor, but the interplay of CNF1 and its frequent associate hemolysin has not been investigated. If CNF1 is a virulence factor, it may directly or indirectly facilitate the effects of hemolysin. Alternatively, the association of CNF1 and hemolysin may result solely from their common carriage on a block of virulence genes. In this study we examined the hypothesis that production of CNF1 influences cytotoxicity of hemolytic E. coli isolated from cystitis cases toward bladder epithelial cells. Using both epidemiological and molecular biologic techniques, we compared 29 CNF1+ and CNF1− clinical isolates and isogenic CNF1+ and CNF1− derivatives of two strains. Our results indicate that CNF1 does not affect the cytotoxicity of hemolytic isolates toward human bladder cells in vitro.
MATERIALS AND METHODS
Cell lines and bacterial strains.
The T24 (HTB-4) human bladder transitional-cell carcinoma cell line (American Type Culture Collection, Rockville, Md.) was cultured at 37°C and 5% CO2 in McCoy’s 5A medium with glutamine containing 10% fetal bovine serum and antibiotic-antimycotic solution (final concentrations, 100 μg of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B per ml; Gibco-BRL, Gaithersburg, Md.).
E. coli isolates from patients with first-time cystitis were obtained from collections at the University of Washington (UW) and University of Michigan (UM). Twenty-nine of these isolates were evaluated in this study (see Results). Each strain was stored at −70°C in Luria broth (LB; 10 g of tryptone, 5 g of yeast extract, and 0.5 g of NaCl per liter) supplemented with 20% glycerol. Relevant characteristics of each strain were determined in the original studies and are indicated in Table 1. Stapleton et al. (37) evaluated isolates with probes for P-related fimbriae (papEFG), hemolysin (hlyA), aerobactin, and the diffuse adhesin family (daaC) and for phenotypic expression of hemolysin and of P-related adhesins by agglutination of α-d-Gal-(1,4)-β-d-Gal-O-(CH2)8-COOCH3-coated latex beads and mannose-resistant hemagglutination of human type O and sheep erythrocytes. Foxman et al. (22) probed isolates for sequences homologous to loci for 10 factors: aerobactin (aer), type II capsule (kpsMT), CNF1 (cnf1), hemolysin (hly), OmpT (ompT), and P-related (prf), S (sfa), and type 1 (fim) fimbriae. For this study, the cnf1 status of each isolate was confirmed by DNA dot blotting and bioassay for multinucleation of HeLa cells in vitro. Hemolytic phenotype was screened on LB plates containing 5% washed sheep erythrocytes with 20 mM CaCl2; hemolytic zones varied in size among isolates.
TABLE 1.
Cytotoxicity of E. coli cystitis isolates
| Strain | Relevant characteristicsa | % of T24 monolayer remainingb |
|---|---|---|
| UW isolates (37) | ||
| CNF1+ | ||
| F3 | hly+ papEFG+ (MRHA/H/S Gal-Gal−) daaC aer | 90 ± 8 |
| F11 | hly+ papEFG+ (MRHA/H/S Gal-Gal−) daaC aer | 37 ± 28 |
| F12 | hly+ papEFG+ (MRHA/H/S Gal-Gal−) daaC aer | 93 ± 9 |
| F24 | hly+ papEFG+ (MRHA/H/S Gal-Gal−) daaC aer | 92 ± 11 |
| F38 | hly+ papEFG+ (MRHA/H/S Gal-Gal−) daaC aer | 120 ± 11 |
| F63 | hly+ papEFG+ (MRHA/H/S Gal-Gal−) daaC aer | 94 ± 1 |
| CNF1− | ||
| F14 | hly+ papEFG+ (MRHA/H/S Gal-Gal+) daaC aer+ | 23 ± 15 |
| F27 | hly+ papEFG+ (MRHA/H Gal-Gal+) daaC aer+ | 8 ± 1 |
| F36 | hly+ papEFG (MRHA− Gal-Gal−) daaC aer+ | 40 ± 24 |
| F39 | hly+ papEFG+ (MRHA/H/S Gal-Gal+) daaC+ aer+ | 33 ± 5 |
| F40 | hly+ papEFG+ (MRHA/H/S Gal-Gal+) daaC aer+ | 22 ± 9 |
| UM isolates (22) | ||
| CNF1+ | ||
| BF284 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 4 ± <1 |
| BF250 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 4 ± <1 |
| BF270 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 22 ± 15 |
| BF248 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 12 ± 2 |
| BF235 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 21 ± 4 |
| BF233 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 18 ± 11 |
| BF292 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 2 ± 1 |
| BF280 | hly+ prf+ sfa+ drb aer kpsMT+ ompT+ | 95 ± 4 |
| BF1048 | hly+ prf+ sfa drb aer kpsMT+ ompT+ | 64 ± 20 |
| CNF1− | ||
| BF229 | hly+ prf sfa drb aer+ kpsMT+ ompT+ | 20 ± 8 |
| BF1028 | hly+ prf+ sfa+ drb aer+ kpsMT ompT+ | 6 ± <1 |
| BF1008 | hly+ prf+ sfa drb aer+ kpsMT+ ompT+ | 25 ± 16 |
| BF1061 | hly+ prf+ sfa+ drb aer+ kpsMT+ ompT+ | 4 ± 1 |
| BF1002 | hly+ prf+ sfa drb aer+ kpsMT+ ompT | 17 ± 2 |
| BF1060 | hly+ prf+ sfa drb+ aer+ kpsMT+ ompT+ | 127 ± 7 |
| BF1021 | hly+ prf+ sfa+ drb aer+ kpsMT+ ompT | 102 ± 8 |
| BF1003 | hly+ prf+ sfa drb aer+ kpsMT+ ompT+ | 3 ± 1 |
| BF1070 | hly+ prf+ sfa drb aer+ kpsMT+ ompT+ | 17 ± 14 |
Phenotypes and genotypes (see Materials and Methods) were determined in the original studies (22, 37). The cnf1 status of UW isolates was determined and that of UM isolates was confirmed by dot blotting for this study. MRHA, mannose-resistant hemagglutination; H, human type O erythrocytes; S, sheep erythrocytes; Gal-Gal, α-d-Gal-(1,4)-β-d-Gal-O-(CH2)8-COOCH3.
Bladder cell monolayers were assessed by methylene blue staining 4 h after inoculation. Values are means ± standard errors of eight replicate wells from two to three trials, expressed as percentage of staining of the uninoculated T24 monolayer.
Cytotoxicity assays.
The effect of growth of each bacterial isolate on T24 cell monolayers was determined by quantitating cell mass of the surviving monolayer colorimetrically after staining with the basic dye methylene blue (31, 40). Assays were initiated with cultures of each bacterial isolate that were inoculated from frozen stocks and grown overnight at 37°C in LB broth under static conditions. Strains F3.297 and F11.297 were cultured in the presence of 15 μg of tetracycline per ml. T24 cells were seeded at 104 cells/well in 96-well microtiter plates and incubated for 48 h to confluence. At time zero, a standardized inoculum (A600 = 0.13) of each strain was diluted 1:20 into fresh McCoy’s medium containing 10 mM HEPES and added to the monolayers (eight wells/strain). Plates were incubated at 37°C and 5% CO2, and at 1-h intervals the monolayers were washed twice with 200 μl of Dulbecco’s phosphate-buffered saline (PBS) and fixed in 10% formalin in PBS. Fixed monolayers were washed twice with 200 μl of borate buffer (10 mM, pH 8.4) and stained for 10 min with methylene blue (1% in 10 mM borate buffer). Excess stain was removed by five washes with borate buffer, and plates were dried overnight at room temperature. Bound methylene blue was extracted with 200 μl of 0.1 M HCl and quantitated at an optical density of 620 nm in a microtiter plate reader (Titertek Multiscan MCC; Flow Laboratories, Irvine, United Kingdom). Growth of each strain was monitored spectrophotometrically by inoculating parallel wells containing McCoy’s medium with the same stock culture.
Statistical comparisons of the cytotoxicity of CNF1+ and CNF1− isolates as groups were made by using the nonparametric Mann-Whitney test. Three isolates from the collections supplied were not included in the study since a limited number of cultures could be tested in a single trial. Those isolates were F15 (CNF1−), which left 4% of the T24 monolayer at 4 h in one trial conducted, and strains BF264 (CNF1+) and BF283 (CNF1+), which were not tested in methylene blue assays but had low hemolytic titers.
Detection of cnf1 DNA sequences.
Plasmid pISS392 is comprised of the vector pGEM3 (Promega, Madison, Wis.) and a 3.5-kb AccI-StuI fragment with the intact cnf1 gene from strain E-B35 (19). The presence of cnf1 sequences in each isolate was confirmed by dot blotting using as the probe an internal 0.9-kb HindIII fragment of the cnf1 gene derived from pISS392 (G fragment [19]). Briefly, overnight cultures of each strain in 96-well microtiter dishes were lysed with an equal volume of lysis solution (0.6 M NaCl, 0.2 N NaOH, 0.08% sodium dodecyl sulfate [SDS]), an aliquot was transferred to Qiabrane Nylon Plus membranes (Qiagen, Chatsworth, Calif.), and the membranes were dried and neutralized (10 min; 0.5 M Tris [pH 7.5]–1.5 M NaCl). Gel-purified cnf1 probe was labeled and detected by using an Amersham (Arlington Heights, Ill.) ECL nucleic acid labeling and detection kit as instructed by the manufacturer. Hybridization was overnight at 42°C in ECL Gold hybridization buffer. Posthybridization treatment consisted of three washes with 5× SSC (SSC is 0.15 M NaCl plus 0.015 M sodium citrate [pH 7.2]), three washes with 6 M urea–0.4% SDS–0.5× SSC at 42°C, and two washes at 22°C with 2× SSC.
Bioassay for CNF1 activity.
CNF1 activity was determined by bioassay for formation of enlarged multinucleate HeLa cells, an observation originally described by Caprioli et al. (7). Isolates F3 and F3.297 were cultured overnight in Trypticase soy broth, washed two times with PBS, and lysed in a French pressure cell (American Instrument Co., Silver Spring, Md.) twice at 18,000 psi. Cell debris was removed by centrifugation for 10 min at 10,000 rpm, and extracts were filtered through a 0.2-μm-pore-size polyethersulfone filter (Nalge, Rochester, N.Y.). Protein concentration was measured by the bicinchoninic acid method using a Sigma protein assay kit (Sigma, St. Louis, Mo.). Blinded twofold dilutions of each extract in PBS were added to 96-well microtiter plates containing T24 bladder or HeLa epithelial cells cultured in McCoy’s 5A or Eagle minimal essential medium, respectively. Plates were incubated for 72 h (37°C, 5% CO2) washed three times with 200 μl of PBS, fixed with 95% ethanol, and stained for 1 h with Giemsa to visualize nuclei. The fraction of cells that were multinucleate was determined with an ocular grid. CNF1 activity in F11, F11.297, and all strains in the study was assayed essentially as described above except that cultures were grown in LB (overnight or 24 h) and lysed with four freeze-thaw cycles shifting from a −70°C freezer or dry ice-ethanol bath to 37°C (32), followed by sonication (2 to 12 min). All isolates positive with a cnf1 DNA probe were phenotypically CNF1+ in the bioassay.
Construction of CNF1− mutants.
Plasmid pSE297 was provided by S. Elliott and contains a 1.4-kb internal BglII-PstI fragment of the cnf1 gene cloned in pJPS5608, a tetracycline-resistant version of the RK6 replicon-based suicide plasmid pJPS5603 (33). pSE297 was introduced into strains F3 and F11 by electroporation and selection for the tetracycline resistance marker carried on the vector. Six F3 and F11 transformants were screened for CNF1 activity by bioassay (data not shown), and disruption of the cnf1 locus was confirmed in one transformant from each background (designated F3.297 and F11.297) by amplifying the junctions between the cnf1 locus and the inserted plasmid sequence. Primers W1 (5′-TCCATGCTTCTTCCTCAGTAG; nucleotides [nt] 2655 to 2675 [cnf1 numbering from reference 19]) and W2 (5′-TTGGTATCAAATTTCCCTTCAC; nt 900 to 922) anneal within cnf1 but outside the internal BglII-PstI cnf1 fragment contained in pSE297. A second set of primers, W4 (5′-TTAGGCACCCCAGGCTTTACAC) and W91 (5′-CCAGGGTTTTCCCAGTCACGAC), anneal within vector sequences of pSE297, 64 and 123 nt, respectively, outside of the BamHI and PstI sites of the pUC19 polylinker flanking the cloned fragment. PCRs used PCR Supermix (Gibco BRL, Gaithersburg, Md.) according the following protocol: denaturation for 5 min at 95°C; 30 cycles of 1.5 min at 52°C, 3 min at 72°C, and 1 min at 95°C; and a final cycle of 1.5 min at 52°C and 5 min at 72°C. Primers were at a final concentration of 200 nM. Samples were analyzed on 1% agarose gels run in Tris-borate buffer.
RESULTS
Effect of hemolytic E. coli on bladder cells.
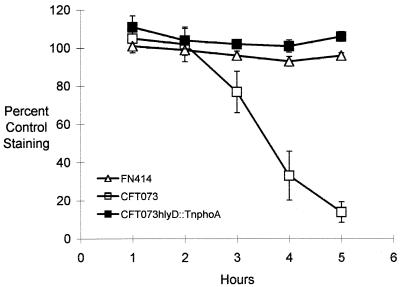
We used an in vitro assay of cell mass to determine the cytotoxicity of hemolytic E. coli toward bladder cell monolayers. In this protocol, T24 monolayers were inoculated with various E. coli strains and the fraction of the bladder monolayer remaining at 1-h intervals after inoculation was quantitated by staining with the basic dye methylene blue. Three strains from previous studies (28, 38) were used to evaluate the assay: CFT073, a hemolytic pyelonephritic isolate; CFT073hlyD::TnphoA, a hemolysin-deficient derivative of CFT073; and FN414, a nonhemolytic fecal isolate from a normal individual (24). When incubated with hemolytic CFT073, T24 cell monolayers were rapidly destroyed and methylene blue staining decreased precipitously at 3 to 4 h postinoculation (Fig. 1). However, no killing was observed with strain CFT073hlyD::TnphoA, in which hemolytic activity has been lost, or with the nonhemolytic fecal isolate FN414, suggesting that hemolysin was associated with these cytotoxic effects. Neither CFT073 nor FN414 carries cnf1 sequences, based on dot blot analysis (data not shown). Inoculation of parallel sets of wells containing McCoy’s medium showed that growth rates of the three strains were similar (data not shown).
FIG. 1.
Effects of hemolytic E. coli on survival of T24 bladder cell monolayers. T24 bladder cell monolayers were inoculated with E. coli CFT073, CFT073hlyD::TnphoA (a hemolysin-deficient derivative), or FN414 (a nonhemolytic fecal isolate). The fraction of the T24 cell monolayer remaining was determined at 1-h intervals by staining with methylene blue. Data for each strain are the means ± standard errors from a minimum of three experiments.
Effects of CNF1+ and CNF1− cystitis isolates on bladder cells.
Hemolysin-positive E. coli isolates from patients with first-time cystitis were obtained from collections at two geographic sites (29 strains) and compared in methylene blue cytotoxicity assays. Approximately one-half (i.e., 15) of the strains were CNF1+, based on DNA probe analysis and bioassay for multinucleation of HeLa cells in vitro (see Materials and Methods). As with isolate CFT073, damage to T24 monolayers after incubation with the majority of the hemolytic cystitis isolates was rapid (Table 1). However, the cytotoxicity varied among strains, and some isolates had only minimally damaged the monolayer by the 4-h point.
Among the UW isolates, five of five CNF1− strains damaged the bladder monolayer within 4 h (less than 50% of the monolayer remaining), whereas only one of six CNF1+ strains in this same collection was as cytotoxic (P = 0.009, Mann-Whitney test). However, in a trial where the assay was prolonged, three of the five less cytotoxic CNF1+ strains destroyed the monolayer by the 5-h point (data not shown); thus, the difference in those isolates constituted a lag of approximately 1 h in cytotoxic effects. No difference in cytotoxicity between CNF1+ and CNF1− isolates was observed among the 18 UM strains; in each of the CNF1+ and CNF1− groups from that collection, seven of the nine isolates destroyed the monolayer at 4 h (P = 0.78). Nor was a difference in cytotoxicity observed when CNF1+ and CNF1− strains from both collections combined were compared (P = 0.43). However, the CNF1+ isolates from the UW collection were also less cytotoxic as a group than the CNF1+ isolates from the UM collection (P = 0.012).
Effects of isogenic CNF1+ and CNF1− isolates on bladder cells.
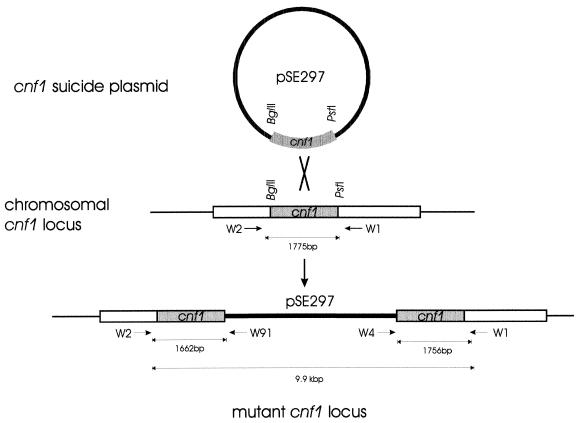
Since confounding differences occur among clinical isolates, we directly evaluated the influence of CNF1 on cytotoxicity by constructing isogenic strains differing in ability to produce CNF1. Two isolates (F3 and F11) were chosen for further study because they had different levels of cytotoxicity and were also virulent in a CBA mouse model of UTI (26). The chromosomal cnf1 gene in F3 and F11 was inactivated by integration of a plasmid containing an internal fragment of the cnf1 gene (pSE297). The resulting mutants, F3.297 and F11.297, contain two truncated versions of the cnf1 gene separated by the plasmid sequences (Fig. 2). Disruption of the cnf1 locus was confirmed by amplifying the junctions between the cnf1 locus and the inserted plasmid sequence (see Materials and Methods). Amplification of cnf1 sequences with primers W1 and W2 produced a 1.7-kb fragment (predicted 1,775 bp) from parental F3 or F11 chromosomal DNA but did not produce a product from F3.297 or F11.297 template (data not shown). The PCR product using these primers and template from F3.297 or F11.297 would contain the integrated vector and have a predicted size of 9.9 kb. However, amplification of F3.297 or F11.297 template with combinations of primers W91 and W4, which anneal within the plasmid vector, and primers W1 and W2, which anneal within cnf1, produced fragments of approximately 1.7 kb (predicted, 1,662 bp) and 1.8 kb (predicted, 1,756 bp), corresponding to the regions of cnf1 flanking the integrated vector (data not shown). These products were not amplified from template of the parental isolate F3 or F11.
FIG. 2.
Construction of cnf1 mutants. Plasmid pSE297 containing a 1.4-kb internal fragment of cnf1 on a suicide vector was introduced into E. coli F3 and F11 by electroporation and selection for the tetracycline resistance of the vector. The resulting, mutated cnf1 locus contains two truncated cnf1 genes flanking the integrated vector. Integration of pSE297 sequences at the chromosomal cnf1 locus was confirmed by amplifying fragments from the junctions between the integrated vector and cnf1 sequences (see Materials and Methods).
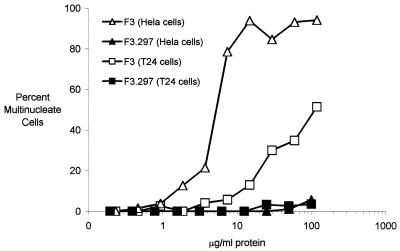
To determine whether CNF1 activity was absent from mutated strains, extracts from each strain were evaluated for the ability to induce multinucleation of HeLa and T24 bladder cells in vitro. Cell lysates or sonicates were prepared from overnight cultures of strains F3, F3.297, F11, and F11.297 and added to HeLa or T24 bladder cells in 96-well microtiter plates. After 72 h, F3 lysates containing 3.7 to 7.5 μg of protein/ml (F11 sonicates, 3 to 6 μg/ml [data not shown]) induced formation of 50% multinucleate cells in HeLa cells, whereas extracts from mutants F3.297 or F11.297 (data not shown) did not contain detectable CNF1 activity at protein concentrations of 100 μg/ml (Fig. 3). Results obtained with T24 bladder cells were similar, but the fraction of multinucleate cells was lower with this cell line (Fig. 3).
FIG. 3.
CNF1 activities in extracts from E. coli F3 and F3.297 (cnf1::pSE297). Twofold serial dilutions of cell extracts (Materials and Methods) from isolates F3 or F3.297 (cnf1::pSE297) were added to HeLa or T24 cells in 96-well microtiter plates. Plates were incubated for 72 h, fixed, and stained with Giemsa stain. Data are fraction of cells which were multinucleate, determined by using an ocular grid, and are from a representative experiment.
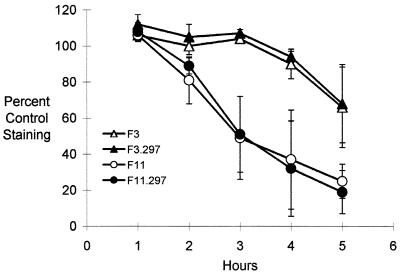
Comparing the CNF1-defective mutants and their parental counterparts, we found no effect of CNF1 status on cytotoxicity toward T24 cells. Neither F3.297 nor F11.297 differed from the parental CNF1+ isolates in ability to damage T24 bladder cell monolayers, based on methylene blue staining (Fig. 4). The growth rate of F3.297 and F11.297 was equivalent to that of the parent in Trypticase soy broth and in wells containing McCoy’s medium inoculated in parallel with cytotoxicity assays (data not shown).
FIG. 4.
Effects of isogenic CNF1+ and CNF1−, hemolytic E. coli on survival of T24 bladder cell monolayers. T24 bladder cell monolayers were inoculated with E. coli F3, F3.297 (cnf1::pSE297), F11, or F11.297 (cnf1::pSE297). The fraction of the T24 monolayer remaining was determined at 1-h intervals by staining with the dye methylene blue. Data for each strain are means ± standard errors from two or three experiments.
DISCUSSION
Approximately one-half of E. coli isolated from patients with cystitis (40%) or pyelonephritis (49%) produce the pore-forming cytotoxin hemolysin (27). Of hemolytic isolates from extraintestinal infections (primarily UTI strains), a large fraction (65 to 83%) encode the toxin CNF1, but CNF1 is rarely found in nonhemolytic isolates (1, 5, 8, 25). The tight genetic linkage of CNF1 to hemolysin (6, 18) and the rarity of nonhemolytic CNF1-producing isolates suggested that CNF1 and hemolysin may have an interactive role in pathogenesis of UTI.
If CNF1 and hemolysin acted in concert to damage bladder epithelial cells, we reasoned this might be reflected in vitro by altered cytolysis of monolayers inoculated with hemolytic, CNF1-producing strains of E. coli. CNF1 causes alteration of cytoskeletal organization in animal cells and can trigger the entry of latex beads or noninvasive bacteria in vitro. Cytoskeletal alterations can begin rapidly and at low concentrations of CNF1; purified CNF1 increased formation of actin stress fibers and membrane ruffles in HEp-2 cells in 2 h at 10−9 M (20). Hemolysin is a pore-forming cytolytic toxin (2, 9) that rapidly kills a spectrum of cell types (10, 11, 23), including renal tubular (28, 38) and T24 bladder epithelial (this study) cells. We envisioned that CNF1 might influence the action of hemolysin possibly by triggering the internalization or association of hemolytic E. coli with bladder cells, thereby increasing the effectiveness of hemolysin. It has been noted previously that digalactose-binding pili increase the in vitro lytic activity of hemolytic strains toward erythrocytes, suggesting that binding to target cells increases the effectiveness of toxin delivery (30).
One approach to the question was to evaluate the cytotoxicity of a range of hemolysin-producing cystitis isolates categorized by their CNF1 status. Interestingly, we found that CNF1+ isolates from one site (UW) exhibited a lag in destruction of T24 monolayers compared to CNF1− isolates from that collection. In contrast, among CNF1+ and CNF1− strains from the second collection (UM), no difference was observed. This discrepancy could be from sampling or could be actual differences in the populations of E. coli strains causing cystitis in the Seattle, Wash., and Ann Arbor, Mich., areas, e.g., different clones or pathogenicity islands.
Thus, to simplify the question of whether hemolysin and CNF1 interact, we used a genetic approach, constructing isogenic mutants defective in production of CNF1. Two UW CNF1+ isolates, F3 and F11, were selected for mutagenesis, the former with relatively low and the latter with relatively high cytotoxicity. Comparison of the CNF1+ and CNF1− versions of these isolates indicated that CNF1 status does not alter cytotoxicity in our in vitro assay.
These observations indicate that CNF1 does not greatly alter the capacity of hemolytic cystitis isolates to kill T24 bladder cells. However, these data reflect the end result of a potentially complex process involving expression of hemolysin and CNF1 and their effects on a specific cell type under in vitro conditions. The potential for subtler interactions in the expression and activity of these proteins and in cellular responses to them under different conditions or in a different cell type remains. For instance, the rapidity with which hemolytic isolates kill T24 bladder cells in vitro may obscure the impact of CNF1, but these findings may not reflect in vivo processes. Additionally, CNF1 may act in the pathogenesis of cystitis in some other way. De Rycke et al. (15) observed cytopathic effects on HeLa cells, progressing to lethality 5 days after a brief initial exposure to CNF1+ bacteria (hemolysin was inhibited by seroneutralization). Hence, while hemolysin may be the dominant determinant of bladder cell fate in the short term, there remains the possibility that in vivo CNF1 influences surviving cells (i.e., those adjacent or marginally affected by hemolysin) or acts in conjunction with other factors such as cytolethal distending toxin (34).
ACKNOWLEDGMENTS
This work was supported by Public Health Service research grant PO1 DK49720-01 from the National Institutes of Health.
We thank S. Elliott and V. Falbo for providing pSE297 and pISS392, respectively, R. Hebel for advice on statistical analysis, and H. L. T. Mobley for providing laboratory support for initiation of this study.
REFERENCES
- 1.Alonso P, Blanco J, Blanco M, Gonzalez E A. Frequent production of toxins by Escherichia coli strains from urinary tract infections: relation with hemagglutination. FEMS Microbiol Lett. 1987;48:391–396. [Google Scholar]
- 2.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Weller U, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 3.Bisicchia R C, Ciammarughi R, Caprioli A, Falbo V, Ruggeri F. Toxin production and haemagglutination in strains of Escherichia coli from diarrhoea in Brescia, Italy. J Hyg. 1985;95:353–361. doi: 10.1017/s002217240006277x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco J, Alonso M P, Gonzalez E A, Blanco M, Garabal J I. Virulence factors of bacteraemic Escherichia coli with particular reference to production of cytotoxic necrotizing factor (CNF) by P-fimbriate strains. J Med Microbiol. 1990;31:175–183. doi: 10.1099/00222615-31-3-175. [DOI] [PubMed] [Google Scholar]
- 5.Blanco J, Blanco M, Alonso M P, Blanco J E, Gonzalez E A, Garabal J I. Characteristics of haemolytic Escherichia coli with particular reference to production of cytotoxic necrotizing factor type 1 (CNF1) Res Microbiol. 1992;143:869–878. doi: 10.1016/0923-2508(92)90074-x. [DOI] [PubMed] [Google Scholar]
- 6.Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and α-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol Lett. 1995;126:189–196. doi: 10.1111/j.1574-6968.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli A, Falbo V, Roda L, Ruggeri F, Zona C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect Immun. 1983;39:1300–1306. doi: 10.1128/iai.39.3.1300-1306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprioli A, Falbo V, Ruggeri F, Baldassarri L, Bisicchia R C, Ippolito G, Romoli E, Donelli G. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J Clin Microbiol. 1987;25:146–149. doi: 10.1128/jcm.25.1.146-149.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalieri S J, Bohach G A, Snyder I S. Escherichia coli α-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984;48:326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalieri S J, Snyder I S. Effect of Escherichia coli α-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982;36:455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalieri S J, Snyder I S. Cytotoxic activity of partially purified Escherichia coli α-haemolysin. J Med Microbiol. 1982;15:11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- 12.Cox C, Hinman F., Jr Experiments with induced bacteriuria, vesical emptying and bacterial growth on the mechanism of bladder defense to infection. J Urol. 1961;86:739–748. doi: 10.1016/S0022-5347(17)65257-1. [DOI] [PubMed] [Google Scholar]
- 13.De Rycke J. Toxic effects for lambs of cytotoxic necrotizing factor from Escherichia coli. Res Vet Sci. 1990;49:349–354. [PubMed] [Google Scholar]
- 14.De Rycke J, Gonzalez E A, Blanco J, Oswald E, Blanco M, Boivin R. Evidence for two types of cytotoxic necrotizing factor in human and animal clinical isolates of Escherichia coli. J Clin Microbiol. 1990;28:694–699. doi: 10.1128/jcm.28.4.694-699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rycke J, Mazars P, Nougayrede J P, Tasca C, Boury M, Herault F, Valette A, Oswald E. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect Immun. 1996;64:1694–1705. doi: 10.1128/iai.64.5.1694-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Rycke J, Phan-Thanh L, Bernard S. Immunochemical identification and biological characterization of cytotoxic necrotizing factor from Escherichia coli. J Clin Microbiol. 1989;27:983–988. doi: 10.1128/jcm.27.5.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H, Warren J, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 135–174. [Google Scholar]
- 18.Falbo V, Famiglietti M, Caprioli A. Gene block encoding production of cytotoxic necrotizing factor 1 and hemolysin in Escherichia coli isolates from extraintestinal infections. Infect Immun. 1992;60:2182–2187. doi: 10.1128/iai.60.6.2182-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanie L, Oswald E, Boquet P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 21.Fiorentini C, Arancia G, Caprioli A, Falbo V, Ruggeri F, Donelli G. Cytoskeletal changes induced in Hep-2 cells by the cytotoxic necrotizing factor of Escherichia coli. Toxicon. 1988;26:1047–1056. doi: 10.1016/0041-0101(88)90203-6. [DOI] [PubMed] [Google Scholar]
- 22.Foxman B, Zhang L, Palin K, Tallman P, Marrs C F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J Infect Dis. 1995;171:1514–1521. doi: 10.1093/infdis/171.6.1514. [DOI] [PubMed] [Google Scholar]
- 23.Gadeberg O V, Orskov I, Rhodes J M. Cytotoxic effect of an α-hemolytic Escherichia coli strain on human blood monocytes and granulocytes in vitro. Infect Immun. 1983;41:358–364. doi: 10.1128/iai.41.1.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hostacka A. Production of enterotoxin, verotoxin, hemolysin, and cytotoxic necrotizing factor by Escherichia coli of intestinal and extraintestinal origin. Folia Microbiol. 1994;39:79–82. doi: 10.1007/BF02814536. [DOI] [PubMed] [Google Scholar]
- 26.Johnson D E, Lockatell C V, Russell R G, Hebel J R, Island M D, Stapleton A E, Stamm W E, Warren J W. Comparison of Escherichia coli strains recovered from human cystitis or pyelonephritis infections in transurethrally challenged mice. Infect Immun. 1998;66:3059–3065. doi: 10.1128/iai.66.7.3059-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobley H L T, Green D, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norden C W, Green G M, Kass E H. Antibacterial mechanisms of the urinary bladder. J Clin Invest. 1968;47:2689–2700. doi: 10.1172/JCI105952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Hanley P, Lalonde G, Ji G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficiency of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun. 1991;59:1153–1161. doi: 10.1128/iai.59.3.1153-1161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver M, Harrison N, Bishop J, Cole P, Laurent G. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci. 1989;92:513–518. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- 32.Oswald E, De Rycke J, Lintermans P, Van Muylem K, Mainil J, Daube G, Pohl P. Virulence factors associated with cytotoxic necrotizing factor type two in bovine diarrheic and septicemic strains of Escherichia coli. J Clin Microbiol. 1991;29:2522–2527. doi: 10.1128/jcm.29.11.2522-2527.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penfold R J, Pemberton J M. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene. 1992;118:145–146. doi: 10.1016/0378-1119(92)90263-o. [DOI] [PubMed] [Google Scholar]
- 34.Peres S Y, Marches O, Daigle F, Nougayrede J P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 35.Stamm W E, Hooton T M. Management of urinary tract infections in adults. N Engl J Med. 1993;329:1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 36.Stamm W E, Hooton T M, Johnson J R, Johnson C, Stapleton A E, Roberts P L, Moseley S, Fihn S D. Urinary tract infections: from pathogenesis to treatment. J Infect Dis. 1997;159:400–406. doi: 10.1093/infdis/159.3.400. [DOI] [PubMed] [Google Scholar]
- 37.Stapleton A, Moseley S, Stamm W. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163:773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- 38.Trifillis A L, Donnenberg M S, Cui X, Russell R G, Utsalo S J, Mobley H L, Warren J W. Binding to and killing of human renal epithelial cells by hemolytic P-fimbriated E. coli. Kidney Int. 1994;46:1083–1091. doi: 10.1038/ki.1994.370. [DOI] [PubMed] [Google Scholar]
- 39.Warren J W. Host-parasite interactions and host defense mechanisms. In: Schrier R W, Gottschalk C W, editors. Diseases of the kidney. Boston, Mass: Little Brown and Company; 1997. pp. 873–893. [Google Scholar]
- 40.Wilson A P. Cytotoxicity and cell viability assays. In: Frishney R I, editor. Animal cell culture, a practical approach. Oxford, England: IRL Press; 1996. pp. 263–303. [Google Scholar]