Abstract
Background:
Like other chronic, inflammatory skin disorders, hidradenitis suppurativa (HS) is increasingly recognized to be associated with various medical disorders.
Objective:
Using the Rochester Epidemiology Project (REP), we sought to conduct the first American population-based study examining the association between HS and various comorbid conditions.
Methods:
From the REP database we identified patients diagnosed with HS from 2003 through 2018 who were residents of Olmsted County, Minnesota, USA, along with age- and sex-matched controls. The frequency of a wide variety of comorbid conditions was compared between the groups.
Results:
A total of 1,160 patients with HS were identified during the study period. Compared with age- and sex-matched controls, patients with HS had a significantly higher frequency of several medical conditions, including depression, anxiety, hyperlipidemia, acne conglobata, dissecting cellulitis, pilonidal cysts, polycystic ovary syndrome, diabetes, chronic kidney disease, psoriasis, atopic dermatitis, obesity, and disordered substance use, among others.
Limitations:
Our study was limited by its retrospective design.
Conclusions:
Providers caring for patients with HS should consider these results, along with those of similar studies, and obtain a thorough history, comprehensive physical examination, and, potentially, laboratory testing and referral to other specialists.
Keywords: acne inversa, autoinflammatory disorders, comorbid conditions, follicular occlusion, hidradenitis suppurativa, screening
Introduction
Hidradenitis suppurativa (HS) is an inflammatory disorder of the hair follicle unit, characterized by deep tender nodules, abscesses, and sinus tracts.1 It can cause severe pain, malodor, drainage, and limitation of physical activities, resulting in impaired quality of life,2 increased health care costs, and substantial morbidity.3–5 Similar to atopic dermatitis and psoriasis, HS has been recently associated with various other medical conditions, including obesity, inflammatory bowel disease (IBD), and psychiatric disorders.6–9 Understanding these associations is essential to determine proper screening, laboratory testing, and/or referrals to other disciplines for patients with HS. The purpose of the current study was to use population-based data from a well-studied US county to better characterize the association between HS and other medical conditions.
Methods
The study was approved by the Mayo Clinic Institutional Review Board. STROBE guidelines were followed for the study. The Rochester Epidemiology Project (REP) resource makes available all medical records of patients from Olmsted County, Minnesota, USA, beginning in the 1960s.10–12 It is a frequently used resource because of the centralized location of individual medical records from patients in the county.13,14 It is particularly useful owing to the isolation of the population, utilization by most health care providers in the county, and all data points being entered directly by these providers.15 Olmsted County is in southeastern Minnesota and has a population of 158,293, of which 83.6% identify as White according to 2019 data.16
We searched the REP for the medical records of patients who had received an International Classification of Diseases, Ninth and Tenth Revisions, diagnostic code for HS from January 1, 2003, to December 31, 2018, and were residents of Olmsted County. For each HS case identified, 1 age- and sex-matched control without HS was identified from the REP database. The matched controls were chosen randomly from among the pool of residents with a visit date close to the time of the patient’s HS diagnosis (i.e., the index date for the case-control group). Given that most of the data were collected electronically, we initially sought to estimate the false-positive and false-negative rates of International Classification of Diseases ascertainment for HS. Of the identified patients, 10% of the cases and non-HS controls (n=115 of each group) were randomly selected and reviewed by the primary author (O.S.) for documentation supporting a diagnosis of HS, as defined by the modified Dessau criteria.17 This review confirmed the diagnosis of HS in our cohort of cases and the absence of HS in the control group.
For the HS patients and controls, comorbid diagnoses at the index date and at the last documented complete medical visit were recorded. Comorbid conditions that were abstracted are highlighted in Table 1 and Figure 1.
Table 1.
Demographics and Comorbid Conditions
| Valuea |
||||
|---|---|---|---|---|
| Variable | Cases (n=1,160) | Controls (n=1,160) | Odds ratio (95% CI) | P valueb |
|
| ||||
| Age, y | 35.4 (13.8) | 35.5 (13.7) | 1.00 (1.00–1.01) | .82 |
| Female | 847 (73.0) | 847 (73.0) | 1.00 (0.83–1.20) | >.99 |
| White | 895 (77.9) (n=1,149) | 1,082 (94.3) (n=1,147) | 4.72 (3.55–6.29) | <.001 |
| Acne vulgaris or conglobata | 88 (7.6) | 39 (3.4) | 0.42 (0.29–0.62) | <.001 |
| Alzheimer disease | 3 (0.3) | 4 (0.3) | 1.33 (0.30–5.96) | >.99 |
| Anxiety | 140 (12.1) | 95 (8.2) | 0.65 (0.49–0.85) | .002 |
| Atopic dermatitis | 18 (1.6) | 7 (0.6) | 0.39 (0.16–0.93) | .04 |
| Depression | 274 (23.6) | 177 (15.3) | 0.58 (0.47–0.72) | <.001 |
| Diabetes | 144 (12.4) | 66 (5.7) | 0.43 (0.31–0.58) | <.001 |
| Dissecting cellulitis | 71 (6.1) | 9 (0.8) | 0.12 (0.06–0.24) | <.001 |
| Down syndrome | 6 (0.5) | 2 (0.2) | 0.33 (0.07–1.65) | .29 |
| Herpes zoster | 9 (0.8) | 7 (0.6) | 0.78 (0.29–2.09) | .80 |
| Hyperlipidemia | 163 (14.1) | 106 (9.1) | 0.62 (0.48–0.80) | <.001 |
| Hypertension | 171 (14.7) | 102 (8.8) | 0.56 (0.43–0.72) | <.001 |
| IBD | 13 (1.1) | 5 (0.4) | 0.38 (0.14–1.08) | .10 |
| Lymphomas | 3 (0.3) | 7 (0.6) | 2.34 (0.60–9.07) | .34 |
| Major adverse cardiac event | 9 (0.8) | 1 (0.1) | 0.11 (0.01–0.87) | .02 |
| Metabolic syndrome | 4 (0.3) | 2 (0.2) | 0.50 (0.09–2.73) | .69 |
| NASH | 18 (1.6) | 9 (0.8) | 0.50 (0.22–1.11) | .12 |
| Obesity | 222 (19.1) | 72 (6.2) | 0.28 (0.21–0.37) | <.001 |
| Obstructive sleep apnea | 12 (1.0) | 7 (0.6) | 0.58 (0.23–1.48) | .36 |
| PCOS | 20 (1.7) | 6 (0.5) | 0.30 (0.12–0.74) | .009 |
| Periodontitis | 45 (3.9) | 41 (3.5) | 0.91 (0.59–1.40) | .74 |
| Pilonidal cyst/sinus | 9 (0.8) | 0 (0) | 19.15 (1.11–330) | .004 |
| Psoriasis | 24 (2.1) | 9 (0.8) | 0.37 (0.17–0.80) | .01 |
| Kidney disease | 52 (4.5) | 26 (2.2) | 0.49 (0.30–0.79) | .004 |
| Sexual dysfunction | 8 (0.7) | 4 (0.3) | 0.50 (0.15–1.66) | .39 |
| Sleep disturbance | 113 (9.7) | 53 (4.6) | 0.44 (0.32–0.62) | <.001 |
| Spondyloarthropathy | 5 (0.4) | 4 (0.3) | 0.80 (0.21–2.99) | >.99 |
| Substance use disorder | 137 (11.8) | 56 (4.8) | 0.38 (0.28–0.52) | <.001 |
| Suicidality | 21 (1.8) | 10 (0.9) | 0.47 (0.22–1.01) | .07 |
| Thyroid disease | 78 (6.7) | 54 (4.7) | 0.68 (0.47–0.97) | .04 |
| Tobacco smoking | 207 (17.8) | 78 (6.7) | 0.33 (0.25–0.44) | <.001 |
Abbreviations: IBD, inflammatory bowel disease; NASH, nonalcoholic steatohepatitis; PCOS, polycystic ovary syndrome.
Values are mean ( sd) or No. (%).
P values by Fisher exact test unless otherwise noted.
Figure 1.
Comorbid conditions significantly associated with hidradenitis suppurativa
Statistical Analysis
The frequency of comorbid conditions was compared between the HS group and the control group. Continuous variables were compared using T- test. Categorical variables were reported as number of patients (percentage) and compared with the Fisher exact test. Odds ratios (95% CIs) for each parameter were included from logistic regression models. P<.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Inc) was used for analysis.
Results
Patient Characteristics
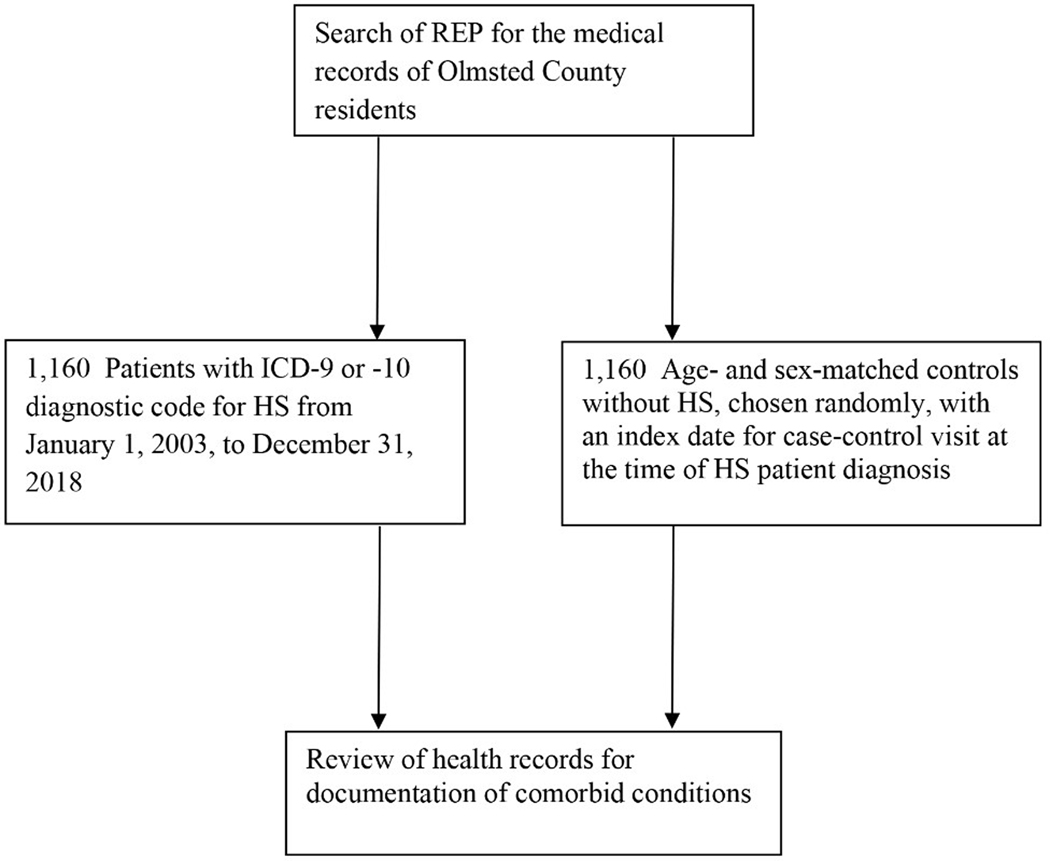
Our search of the REP identified 1,160 patients with a diagnosis of HS during the study period (Figure 2). Mean (sd) age at diagnosis was 35 (14 ) years, 73.0% were female (n=847), and 77.9% were White (895/1,149) (Table 1). The 1,160 HS patients were compared with 1,160 age- and sex-matched controls. The percentage of patients who were White was lower in the HS group than the control group (77.9% vs 94.3%; P<.001) (Table 1).
Figure 2.
STROBE Flow Chart. Selection of study cases of hidradenitis suppurativa (HS) and matched control patients. ICD indicates International Classification of Diseases; REP, Rochester Epidemiology Project.
Comorbid Conditions
Cutaneous
Compared with the control group, the HS cohort was significantly more likely to have dermatologic comorbid conditions. Acne vulgaris or conglobata (7.6% vs 3.4%; P<.001), atopic dermatitis (1.6% vs 0.6%; P=.04), dissecting cellulitis (6.1% vs 0.8%; P<.001), pilonidal cyst/sinus (0.8% vs 0%; P=.004), and psoriasis (2.1% vs 0.8%; P=.01) were all significantly more common in patients with HS.
Endocrinologic and Metabolic
Diabetes (12.4% vs 5.7%; P<.001), hyperlipidemia (14.1% vs 9.1%; P<.001), hypertension (14.7% vs 8.8%; P<.001), obesity (19.1% vs 6.2%; P<.001), PCOS (1.7% vs 0.5%; P=.009), and thyroid disease (6.7% vs 4.7%; P=.04) occurred more frequently in the HS group than in the control group.
Psychiatric and Behavioral
Anxiety (12.1% vs 8.2%; P=.002), depression (23.6% vs 15.3%; P<.001), sleep disturbances (9.7% vs 4.6%; P<.001), substance use disorder (11.8% vs 4.8%; P<.001), and tobacco smoking (17.8% vs 6.7%; P<.001) were also significantly more common in HS patients. However, suicidality (1.8% vs 0.9%; P=.07) was not significantly different between groups.
Inflammatory and Other
Diagnosis of IBD (1.1% vs 0.4%; P=.10) or spondyloarthropathy (0.4% vs 0.3%; P>.99) was not significantly higher in HS patients. Both kidney disease (4.5% vs 2.2%; P=.004) and major adverse cardiac events (0.8% vs 0.1%; P=.02) were significantly more likely in HS patients.
Comorbid Conditions by Sex
Comparison of comorbid conditions by sex among patients with HS showed several differences. Female patients were significantly younger than male patients (mean age, 34 vs 39 years; P<.001) (Table 2). Diagnosis of depression (25.9% vs 17.6%; P=.003), thyroid disease (7.7% vs 4.2%; P=.03), and psoriasis (2.6% vs 0.6%; P=.04) were more likely in female HS patients than male HS patients. Conversely, hyperlipidemia (11.2% vs 21.7%; P<.001), nonalcoholic steatohepatitis (1.1% vs 2.9%; P=.03), hypertension (13.0% vs 19.5%; P=.007), and obstructive sleep apnea (0.2% vs 3.2%; P<.001) were less likely in female HS patients than male HS patients. In male patients with HS, tobacco smoking (22.4% vs 16.2%; P=.02) and substance use disorders (15.7% vs 10.4%; P=.02) occurred more frequently than in female HS patients (Table 2).
Table 2.
Comparison of Female and Male Patients With Hidradenitis Suppurativa
| Valuea |
|||
|---|---|---|---|
| Variable | Female (n=847) | Male (n=313) | P valueb |
|
| |||
| Age, y | 34 (13) | 39 (15) | <.001 |
| White | 665 (79.1) (n=841) | 230 (74.7) (n=308) | .13 |
| Acne vulgaris or conglobata | 71 (8.4) | 17 (5.4) | .10 |
| Alzheimer disease | 2 (0.2) | 1 (0.3) | >.99 |
| Anxiety | 110 (13.0) | 30 (9.6) | .13 |
| Atopic dermatitis | 11 (1.3) | 7 (2.2) | .29 |
| Depression | 219 (25.9) | 55 (17.6) | .003 |
| Diabetes | 97 (11.5) | 47 (15.0) | .11 |
| Dissecting cellulitis | 52 (6.1) | 19 (6.1) | >.99 |
| Down syndrome | 5 (0.6) | 1 (0.3) | >.99 |
| Herpes zoster | 5 (0.6) | 4 (1.3) | .26 |
| Hyperlipidemia | 95 (11.2) | 68 (21.7) | <.001 |
| Hypertension | 110 (13.0) | 61 (19.5) | .007 |
| IBD | 7 (0.8) | 6 (1.9) | .13 |
| Lymphomas | 1 (0.1) | 2 (0.6) | .18 |
| Major adverse cardiac event | 6 (0.7) | 3 (1.0) | .71 |
| Metabolic syndrome | 3 (0.4) | 1 (0.3) | >.99 |
| NASH | 9 (1.1) | 9 (2.9) | .03 |
| Obesity | 174 (20.5) | 48 (15.3) | .053 |
| Obstructive sleep apnea | 2 (0.2) | 10 (3.2) | <.001 |
| PCOS | 20 (2.4) | N/A | N/A |
| Periodontitis | 30 (3.5) | 15 (4.8) | .39 |
| Pilonidal cyst/sinus | 5 (0.6) | 4 (1.3) | .26 |
| Psoriasis | 22 (2.6) | 2 (0.6) | .04 |
| Kidney disease | 34 (4.0) | 18 (5.8) | .20 |
| Erectile dysfunction | N/A | 8 (2.6) | N/A |
| Sleep disturbance | 79 (9.3) | 34 (10.9) | .44 |
| Spondyloarthropathy | 3 (0.4) | 2 (0.6) | .62 |
| Substance use disorder | 88 (10.4) | 49 (15.7) | .02 |
| Suicidality | 12 (1.4) | 9 (2.9) | .13 |
| Thyroid disease | 65 (7.7) | 13 (4.2) | .03 |
| Tobacco smoking | 137 (16.2) | 70 (22.4) | .02 |
Abbreviations: IBD, inflammatory bowel disease; N/A, not applicable; NASH, nonalcoholic steatohepatitis; PCOS, polycystic ovary syndrome.
Values are mean (sd) or No. (%).
P values by Fisher exact for categorical variables, T-Test for continuous variables.
Discussion
HS is an inflammatory condition that imposes a large burden on both patients and health care systems, being associated with several cutaneous and noncutaneous comorbid conditions. Identification of these comorbid conditions is important to optimize disease management and to improve patient outcomes. To date, no US population–based studies have examined comorbid conditions in patients with HS. With use of the REP, our population-based study characterized HS-associated comorbid conditions during a 15-year period.
HS is more common in females, with the highest prevalence in the third decade.18,19 Some studies, particularly from outside the US and Europe, have found that men are more likely to have HS,20,21 and males may also have increased disease severity.18,22 Our results were similar to those of published North American and European studies showing an increased prevalence of HS in females compared with males. Additionally, females in our study were more likely to have comorbid conditions than were their HS-free counterparts (Supplemental Table). However, in comparing females with HS with their male counterparts in our study, males with HS were more likely to smoke and have substance use issues in addition to having hyperlipidemia, nonalcoholic steatohepatitis, hypertension, and obstructive sleep apnea. In contrast, female HS patients were more likely to have depression, thyroid disease, and psoriasis. Although the association of HS with these varying comorbid conditions has been described in the literature, sex differences in association of these comorbid conditions have not been addressed in the literature.23
The association between HS and metabolic and endocrine conditions has been highlighted over the years.8,18,24–27 A 2019 systematic review and meta-analysis of 5 studies showed that HS patients have an increased risk of metabolic syndrome.24 Other studies have also found an association between metabolic syndrome and HS.26,27 Our study showed no correlation between metabolic syndrome and HS; however, the individual components of metabolic syndrome (ie, diabetes, obesity, hyperlipidemia, and hypertension) were all significantly more common in HS patients. This difference in findings may be related to the younger age group represented in our cohort (median age, 34 years) because metabolic syndrome is well recognized to be most prevalent in patients aged 60 through 69 years.28 In addition, the cross-sectional nature of our study precludes us from evaluating whether some patients would eventually meet diagnostic criteria for metabolic syndrome. Multiple studies have shown diabetes to be independently associated with HS,8,25–27 whereas 1 study did not.29 Our results also corroborated these findings. This may be explained by the inflammatory pathogeneses seen in both diseases. These results suggest that glucose screening could be part of the initial workup for patients with HS.
Obesity is a known risk factor associated with HS.8,27 We showed a strong association between HS and obesity. This link may be due to proinflammatory cytokine release by adipose tissue.30,31 Recent studies have addressed the potential role of surgical intervention in the management of HS.32–35 In a study of 383 patients, 49% had resolution of disease, 20% reported a decrease in symptoms, 20% reported stability, and 11% reported worsening after bariatric surgery.32 Physicians should counsel HS patients about weight loss, for decreasing cardiovascular risk factors and also possibly improving HS symptoms.
A systematic review and meta-analysis that included 9 studies showed HS to be significantly associated with hypertriglyceridemia, decreased high-density lipoprotein level, and metabolic syndrome.36 We also found that HS patients are more likely to have hyperlipidemia. Hypertension has been associated with HS in various studies,36 which we also observed. This could be explained, at least partially, by increased obesity and tobacco use in HS patients.
An association with PCOS37,38 has been described in female HS patients8,39 but not in all studies.40 We also observed this association in our female patients. The link between HS and PCOS may result from increased androgens and similar demographics between these conditions; both disorders also share comorbid conditions such as obesity, diabetes, and metabolic syndrome. Clinicians should have a low threshold for screening female HS patients with clinical signs of androgen excess for PCOS.
Thyroid dysfunction is associated with HS in some studies, but others have not established an association.8,41–43 Our results corroborate the association, which may result from the immunologic etiology of both disorders, as well as the role of the thyroid gland in metabolism. Our data did not separate thyroid disorders into hyperthyroidism or hypothyroidism, but hypothyroidism most likely represents most cases.
Various cutaneous comorbid conditions have been associated with HS. Classically, HS has been described as part of a follicular occlusion tetrad, which includes acne conglobata, pilonidal cysts, and dissecting cellulitis. In fact, in a recent multicenter study, 23.6% of patients with HS had concomitant pilonidal cysts.44 In a Korean population-based nationwide study, Lee et al21 showed significant associations between HS and acne conglobata, pilonidal cysts, and psoriasis. Our study had similar findings and also demonstrated a significant association with atopic dermatitis. Sherman et al45 described a 2-way association between HS and atopic dermatitis in a recent retrospective cohort study of 6,779 HS patients.
Psychiatric comorbid conditions, namely depression and anxiety, have also been associated with HS. A large meta-analysis found a strong association between HS and both anxiety and depression.46 Our study adds further evidence to this association. Interestingly, Wright et al47 reported that children, adolescents, and adults with HS are at higher risk for depression compared with controls. Thus, the emotional and psychological burden of HS may lead, or at least contribute, to development of anxiety and depression. A large systematic review and meta-analysis showed a significant association not only between HS and depression and anxiety, but also between HS and bipolar disorder, schizophrenia, substance-related disorders, alcohol use disorder, and suicide.48 Another recent systematic review also demonstrated a significant association between HS and depression, anxiety, and suicidality.49 Our study did not find an association with suicidality, but absolute numbers were low, which precludes drawing any firm conclusions.
Tobacco use is one of the most commonly associated behaviors linked to HS. In a large retrospective cohort analysis of nearly 4 million tobacco smokers, Garg et al50 demonstrated that the incidence of HS is twice as high in smokers than nonsmokers. A systematic review and meta-analysis of 25 studies showed that HS patients were 4 times more likely to smoke tobacco.51 We also demonstrated an association between HS and tobacco use; currently, it is unknown whether tobacco use is a trigger for HS or simply an associated disorder. We also found HS to be associated with substance use. In a cross-sectional population-based study of HS patients, Garg et al52 showed that HS patients were twice as likely to have a substance use disorder. There is a growing body of literature for this association that is important to recognize because HS causes a high pain burden, which could cause patients to turn to substance use for analgesia.53,54
We did not find an association between HS and IBD, although our overall numbers were small. In a meta-analysis of 8 studies, Chen and Chi55 found an association of HS and IBD; another meta-analysis of 6 studies found similar results.56 This association may also hold true in pediatric HS patients.57 A plausible reason for this includes the increased potential for misdiagnosis of HS as cutaneous Crohn disease in patients with Crohn disease. In addition, the cross-sectional nature of our study can lead to an underestimation of the association of HS and IBD because some patients with HS have undiagnosed Crohn disease. Similar to the case with IBD, we also found no association between HS and spondyloarthropathy. Lee et al21 showed a significant association in their large, nationwide, population-based study from Korea. Richette et al58 also demonstrated this association in their multicenter prospective study.
We found significant associations between HS and major adverse cardiac events and also kidney disease. Another population-based cohort study demonstrated a similar association between HS and major adverse cardiac events,59 and Bailey et al60 confirmed the association in a recent systematic review and meta-analysis of 8 studies. This may be the result of chronic systemic inflammation, and it may be prudent to discuss this risk with HS patients.60 There is scant literature on the association between HS and kidney disease, but Miller et al61 found higher estimated glomerular filtration rate values in HS patients than in controls.
Our study has several limitations. In the REP database, diseases are entered only after they are recognized and recorded by the physician. Thus, if a disorder was not brought to medical attention or was insufficiently documented, it would not be identified in the database. This could be particularly true for patients with mild HS or mild versions of the comorbid conditions we studied. Furthermore, HS is a clinical diagnosis, and other diseases may present similarly. In addition, the racial makeup of Olmsted County (ie, 83.6% White and 6.9% African American in 2019) is not representative of the United States overall, which is particularly important when considering that HS may have a racial predilection.16 Although our study demonstrated an increased and varied burden of comorbid conditions associated with HS, we were unable to stratify by severity and could not make conclusions regarding the association between HS severity and comorbid conditions.
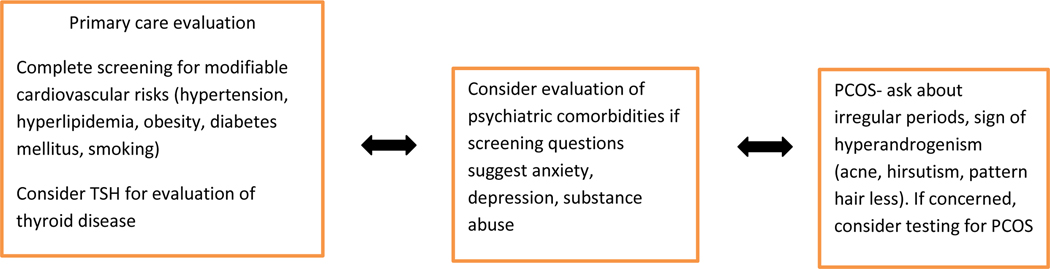
Our results are similar to those of prior studies demonstrating a significant burden of comorbid conditions in HS patients. These varied associations highlight the need for a thorough review of systems, comprehensive physical examination, and possible laboratory monitoring and specialty referrals when caring for HS patients. A discussion with the patient’s primary care physician regarding these comorbid conditions (Figure 3) may be prudent and could translate into early management and prevention of complications.
Figure 3.
Flow Chart. Consultations and investigations to consider in a patient with hidradenitis suppurativa (HS)
Supplementary Material
Funding:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Disclosure: None
References
- 1.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J. Am. Acad. Dermatol 2009; 60: 539–61; quiz 62–3. [DOI] [PubMed] [Google Scholar]
- 2.Alavi A, Anooshirvani N, Kim WB et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am. J. Clin. Dermatol 2015; 16: 61–5. [DOI] [PubMed] [Google Scholar]
- 3.Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol 2020; 82: 1045–58. [DOI] [PubMed] [Google Scholar]
- 4.Kirby JS, Miller JJ, Adams DR et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol 2014; 150: 937–44. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Strunk A, Garg A. Comparative Overall Comorbidity Burden Among Patients With Hidradenitis Suppurativa. JAMA Dermatol 2019; 155: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohorst JJ, Kimball AB, Davis MD. Systemic associations of hidradenitis suppurativa. J. Am. Acad. Dermatol 2015; 73: S27–35. [DOI] [PubMed] [Google Scholar]
- 7.Kimball AB, Sundaram M, Gauthier G et al. The Comorbidity Burden of Hidradenitis Suppurativa in the United States: A Claims Data Analysis. Dermatol Ther (Heidelb) 2018; 8: 557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlyankevich J, Chen AJ, Kim GE et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J. Am. Acad. Dermatol 2014; 71: 1144–50. [DOI] [PubMed] [Google Scholar]
- 9.Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin. Cutan. Med. Surg 2017; 36: 55–7. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin. Proc 1996; 71: 266–74. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin. Proc 2012; 87: 1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int. J. Epidemiol 2012; 41: 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Leibson CL et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin. Proc 2012; 87: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP et al. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. Am. J. Epidemiol 2011; 173: 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahi V, Alikhan A, Vazquez BG et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology 2014; 229: 154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Census Bureau. Quick facts: Olmsted County, Minnesota. In, Vol. 2021. 2019. [Google Scholar]
- 17.Zouboulis CC, Del Marmol V, Mrowietz U et al. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015; 231: 184–90. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez BG, Alikhan A, Weaver AL et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J. Invest. Dermatol 2013; 133: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J. Am. Acad. Dermatol 1996; 35: 191–4. [DOI] [PubMed] [Google Scholar]
- 20.Mebazaa A, Ben Hadid R, Cheikh Rouhou R et al. Hidradenitis suppurativa: a disease with male predominance in Tunisia. Acta Dermatovenerol Alp Pannonica Adriat 2009; 18: 165–72. [PubMed] [Google Scholar]
- 21.Lee JH, Kwon HS, Jung HM et al. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population-based study. J. Eur. Acad. Dermatol. Venereol 2018; 32: 1784–90. [DOI] [PubMed] [Google Scholar]
- 22.Canoui-Poitrine F, Revuz JE, Wolkenstein P et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J. Am. Acad. Dermatol 2009; 61: 51–7. [DOI] [PubMed] [Google Scholar]
- 23.Cartron A, Driscoll MS. Comorbidities of hidradenitis suppurativa: A review of the literature. Int J Womens Dermatol 2019; 5: 330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Zuniga MJM, Garcia-Perdomo HA, Ortega-Loayza AG. Association Between Hidradenitis Suppurativa and Metabolic Syndrome: A Systematic Review and Meta-analysis. Actas Dermosifiliogr (Engl Ed) 2019; 110: 279–88. [DOI] [PubMed] [Google Scholar]
- 25.Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and diabetes mellitus: updated systematic review and adjusted meta-analysis. Clin. Exp. Dermatol 2019; 44: e126–e32. [DOI] [PubMed] [Google Scholar]
- 26.Shalom G, Freud T, Harman-Boehm I et al. Hidradenitis suppurativa and metabolic syndrome: a comparative cross-sectional study of 3207 patients. Br. J. Dermatol 2015; 173: 464–70. [DOI] [PubMed] [Google Scholar]
- 27.Miller IM, Ellervik C, Vinding GR et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol 2014; 150: 1273–80. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356–9. [DOI] [PubMed] [Google Scholar]
- 29.Hughes R, Knudsen E, Kirthi S et al. Framingham risk assessment in hidradenitis suppurativa. Br. J. Dermatol 2017; 176: 1404–6. [DOI] [PubMed] [Google Scholar]
- 30.Lim ZV, Oon HH. Management of Hidradenitis Suppurativa in Patients with Metabolic Comorbidities. Ann Dermatol 2016; 28: 147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabat R, Chanwangpong A, Schneider-Burrus S et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS One 2012; 7: e31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kromann CB, Ibler KS, Kristiansen VB et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm. Venereol 2014; 94: 553–7. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin. Exp. Dermatol 2014; 39: 315–7; quiz 7–8. [DOI] [PubMed] [Google Scholar]
- 34.Golbari NM, Lee Porter M, Kimball AB. Response to: Remission of hidradenitis suppurativa after bariatric surgery. JAAD Case Rep 2018; 4: 278–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallagher C, Kirthi S, Burke T et al. Remission of hidradenitis suppurativa after bariatric surgery. JAAD Case Rep 2017; 3: 436–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzellos T, Zouboulis CC, Gulliver W et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br. J. Dermatol 2015; 173: 1142–55. [DOI] [PubMed] [Google Scholar]
- 37.Diamanti-Kandarakis E, Kouli CR, Bergiele AT et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J. Clin. Endocrinol. Metab 1999; 84: 4006–11. [DOI] [PubMed] [Google Scholar]
- 38.March WA, Moore VM, Willson KJ et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod 2010; 25: 544–51. [DOI] [PubMed] [Google Scholar]
- 39.Garg A, Neuren E, Strunk A. Hidradenitis Suppurativa Is Associated with Polycystic Ovary Syndrome: A Population-Based Analysis in the United States. J. Invest. Dermatol 2018; 138: 1288–92. [DOI] [PubMed] [Google Scholar]
- 40.Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J. Cutan. Med. Surg 2007; 11: 125–31. [DOI] [PubMed] [Google Scholar]
- 41.Miller IM, Vinding G, Sorensen HA et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin. Exp. Dermatol 2018; 43: 899–905. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Lopez MA, Blanco R, Mata C et al. Coexistence of Hidradenitis Suppurativa with Autoimmune Thyroiditis: Report of Three Cases. Dermatology 2016; 232: 162–4. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Lopez MA, Hernandez JL, Vilanova I et al. Thyroid autoimmunity in patients with hidradenitis suppurativa: a case-control study. Clin. Exp. Dermatol 2017; 42: 642–4. [DOI] [PubMed] [Google Scholar]
- 44.Ozkur E, Karadag AS, Ustuner P et al. Clinical and demographic features of hidradenitis suppurativa: a multicentre study of 1221 patients with an analysis of risk factors associated with disease severity. Clin. Exp. Dermatol 2021; 46: 532–40. [DOI] [PubMed] [Google Scholar]
- 45.Sherman S, Kridin K, Bitan DT et al. Hidradenitis suppurativa and atopic dermatitis: A 2-way association. J. Am. Acad. Dermatol 2020. [DOI] [PubMed] [Google Scholar]
- 46.Jalenques I, Ciortianu L, Pereira B et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: Systematic review and meta-analysis. J. Am. Acad. Dermatol 2020; 83: 542–53. [DOI] [PubMed] [Google Scholar]
- 47.Wright S, Strunk A, Garg A. New-onset depression among children, adolescents, and adults with hidradenitis suppurativa. J. Am. Acad. Dermatol 2020; 83: 1360–6. [DOI] [PubMed] [Google Scholar]
- 48.Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med 2020; 8: 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel KR, Lee HH, Rastogi S et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol 2020; 83: 737–44. [DOI] [PubMed] [Google Scholar]
- 50.Garg A, Papagermanos V, Midura M et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br. J. Dermatol 2018; 178: 709–14. [DOI] [PubMed] [Google Scholar]
- 51.Acharya P, Mathur M. Hidradenitis suppurativa and smoking: A systematic review and meta-analysis. J. Am. Acad. Dermatol 2020; 82: 1006–11. [DOI] [PubMed] [Google Scholar]
- 52.Garg A, Papagermanos V, Midura M et al. Opioid, alcohol, and cannabis misuse among patients with hidradenitis suppurativa: A population-based analysis in the United States. J. Am. Acad. Dermatol 2018; 79: 495–500 e1. [DOI] [PubMed] [Google Scholar]
- 53.Aldana PC, Driscoll MS. Is substance use disorder more prevalent in patients with hidradenitis suppurativa? Int J Womens Dermatol 2019; 5: 335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lesort C, Villani AP, Giai J et al. High prevalence of cannabis use among patients with hidradenitis suppurativa: results from the VERADDICT survey. Br. J. Dermatol 2019; 181: 839–41. [DOI] [PubMed] [Google Scholar]
- 55.Chen WT, Chi CC. Association of Hidradenitis Suppurativa With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol 2019; 155: 1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phan K, Tatian A, Woods J et al. Prevalence of inflammatory bowel disease (IBD) in hidradenitis suppurativa (HS): systematic review and adjusted meta-analysis. Int. J. Dermatol 2020; 59: 221–8. [DOI] [PubMed] [Google Scholar]
- 57.Lloyd-McLennan AM, Ali S, Kittler NW. Prevalence of inflammatory bowel disease among pediatric patients with hidradenitis suppurativa and the potential role of screening with fecal calprotectin. Pediatr. Dermatol 2021; 38: 98–102. [DOI] [PubMed] [Google Scholar]
- 58.Richette P, Molto A, Viguier M et al. Hidradenitis suppurativa associated with spondyloarthritis -- results from a multicenter national prospective study. J. Rheumatol 2014; 41: 490–4. [DOI] [PubMed] [Google Scholar]
- 59.Egeberg A, Gislason GH, Hansen PR. Risk of Major Adverse Cardiovascular Events and All-Cause Mortality in Patients With Hidradenitis Suppurativa. JAMA Dermatol 2016; 152: 429–34. [DOI] [PubMed] [Google Scholar]
- 60.Bailey AMJ, Oi-Yee Li H, Tan MG et al. Hidradenitis suppurativa and major adverse cardiac events: A systematic review and meta-analysis. J. Am. Acad. Dermatol 2021; 84: 844–8. [DOI] [PubMed] [Google Scholar]
- 61.Miller IM, Carlson N, Mogensen UB et al. A Population- and Hospital-based Cross-sectional Study of Renal Function in Hidradenitis Suppurativa. Acta Derm. Venereol 2016; 96: 68–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.