Abstract
Background:
Rumination syndrome is a functional gastroduodenal disorder characterised by effortless regurgitation of recently ingested food. Emerging evidence reports duodenal eosinophilic inflammation in a subset, suggesting a shared pathophysiology with functional dyspepsia (FD). We assessed the clinical features of rumination syndrome and functional dyspepsia in a community-based study.
Methods
A survey assessing gastrointestinal symptoms, diet and psychological symptoms was mailed to 9,835 residents of Olmsted County, MN, USA in 2017–2018 and diagnostic codes were obtained from linked clinical records. The two disorders were assessed as mutually exclusive in ‘pure’ forms with a separate overlap group, all compared to a control group not meeting criteria for either. Prevalence of associations, and univariate and independent associations with predictors were assessed by logistic regression.
Results:
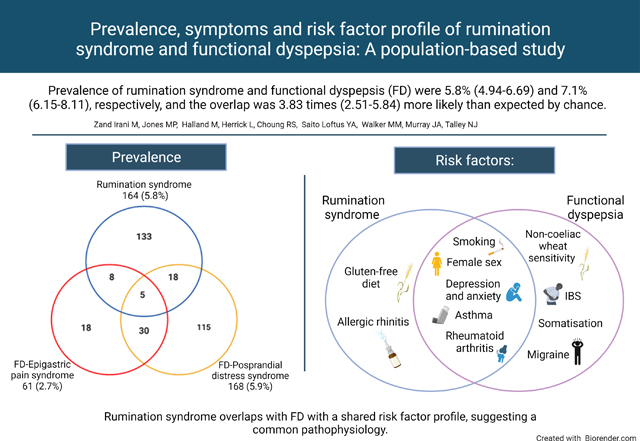
Prevalence of rumination syndrome and FD were 5.8% (4.94–6.69) and 7.1% (6.15–8.11), respectively, and the overlap was 3.83 times (2.51–5.84) more likely than expected by chance. Independent predictors for rumination were (OR, 95%CI) female gender (1.79, 1.21–2.63), smoking (1.89, 1.28–2.78), gluten free diet (1.58, 1.14–2.19), allergic rhinitis (1.45,1.01–2.08) and depression (1.10, 1.05–1.16). FD was independently associated with female gender, depression, non-coeliac wheat sensitivity, migraine, irritable bowel syndrome and somatic symptoms. A similar reported efficacy (≥54%) of low-fat or dairy free diets was found with both disorders (p=0.53 and p=1.00, respectively). The strongest independent associations with overlapping FD and rumination syndrome were history of rheumatoid arthritis (3.93 1.28–12.06) and asthma 3.02 (1.44–6.34).
Conclusion:
Rumination syndrome overlaps with FD with a shared risk factor profile, suggesting a common pathophysiology.
Keywords: Rumination syndrome, Functional dyspepsia
Graphical Abstract
“Left: Prevalence of rumination syndrome and its overlap with the two subtypes of functional dyspepsia (FD). Right: Independent risk factors of rumination syndrome and functional dyspepsia from a multivariate logistic regression model. “
Introduction
Rumination syndrome is a functional gastroduodenal disorder characterised by the involuntary regurgitation of recently ingested food, which is subsequently spat out or re-swallowed (1). The aetiology of the disorder is unknown, but there is a significant burden associated with symptoms and lack of effective treatment, exacerbated by the lack of recognition in clinical practice (2).
The global prevalence of rumination syndrome has recently been reported to be 2.8%, based on volunteer sampling (3). However, previous studies using random population samples have reported a wide prevalence from 0.8% up to 10.9% (4–6), and as yet there are no population-based studies reporting the association with other diseases.
Rumination syndrome is relatively unique among functional gastrointestinal disorders (FGIDs) in that objective manometric criteria exist, although symptoms are an excellent indicator of the disorder (7). On high-resolution manometry, a characteristic spike in intragastric pressure (termed the R-wave) is followed by the sudden elevation of the lower oesophageal junction into the thoracic cavity creating a false hiatal hernia that precedes the retrograde passage of food (2, 8–10). Some individuals with rumination syndrome are able to voluntarily replicate the manometric motor sequence (11), while most others report involuntary occurrence (12, 13). Previous studies have linked rumination syndrome to eating disorders with repetitive vomiting or purging behaviour (12, 13), and to children with developmental delay, suggesting a possible acquired or congenital insult to the gastro-oesophageal junction in these subgroups (14–16). However, rumination syndrome is now recognised to usually occur in the absence of those risk factors (2, 17, 18), although it is still classified as a feeding disorder by the diagnostic and statistical manual of mental disorders (DSM-5) after excluding an eating disorder (19). Two recent studies retrospectively analysed records of post-prandial high-resolution manometry in 94 and 542 individuals with gastro-oesophageal reflux disease (GORD), respectively, and observed the presence of manometric features of rumination syndrome in 20% of individuals with symptoms refractory to acid suppression, and 10% of those with reflux hypersensitivity (defined as normal 24-hour acid exposure time but high correlation of symptoms to reflux events (20)) (21, 22).
Emerging evidence suggests the pathophysiology of functional dyspepsia (FD) and rumination syndrome may overlap (23, 24). FD is a common functional gastrointestinal disorder (FGID) with a population prevalence of 6.9%−17.6% (25), and comprises upper gastrointestinal symptoms in the absence of visible mucosal ulcerations or obstructive lesions on upper endoscopy (26). Both rumination syndrome and FD are characterised by bothersome postprandial symptoms (1, 27, 28), with overlapping gastric viscero-sensory and motor abnormalities (29–35), leading to a lower threshold of visceral symptom generation upon distension compared to controls (30, 31). In rumination syndrome, this bothersome perception is accompanied by a motor sequence that results a reduction of lower oesophageal sphincter tone (30), which may serve to vent the stomach and provide symptom relief, and hence rumination syndrome has been hypothesised to comprise a behavioural response (either conscious or unconscious) to aversive digestive stimuli (36).
The duodenum is fundamentally involved in sensing and controlling the movement of chyme into the upper small intestine and synchronising gastric contractions, and hence duodenal mucosal pathology can alter gastric motility (29, 37–42). Recent studies examining duodenal biopsies in rumination syndrome, previously uncharacterised in this context, reported significantly higher eosinophil count compared to controls (23, 24). Eosinophilic and mast cell micro-inflammation is a recognised feature of FD (43), and is associated with structural and functional alterations in duodenal nerves (32, 44); when induced in animal models, eosinophilic inflammation leads to the development of gastric dysmotility (45).
There are two distinct but overlapping subtypes of FD (1); epigastric pain syndrome (EPS), characterised by upper abdominal pain, and postprandial distress syndrome (PDS), characterised by stasis and early satiety. An inconsistent risk factor profile for FD is reported in population studies (25) with regard to gender prevalence (46–50), and the prevalence of smoking and excessive alcohol intake (46–48, 50, 51), irritable bowel syndrome (IBS) (46, 47, 52) and mood disorders (50, 53).
In this study we aimed to assess the prevalence of rumination syndrome and FD, and overlap, in a US population. We also aimed to characterise the risk factors, symptom profile and dietary habits in rumination syndrome and FD.
Methods
Subjects and survey methods:
A survey with validated questionnaires was mailed to 9,835 residents of Olmsted County, MN, from November 2017 through August 2018 in phased, batched mailings of 2,000 to 4,000 in each mailing. The sample included adult respondents from a prior GI survey (n=3,831) and approximately 6,000 randomly selected new randomly selected Olmsted County adult residents. This yielded 2901 usable surveys with a response rate of 29.5%. The respondent age group in the prior GI survey was older than the current age distribution of Olmsted county, so the additional randomly selected individuals came from the Rochester Epidemiological Project (REP) database and were chosen to match the age distribution of the Olmsted County at the time of the initial surveys.
The REP, which is the medical record linkage system for health care providers to residents of Olmsted County, Minnesota, provides essentially a complete enumeration of the population from which random samples can be drawn (54, 55). Due to being geographically defined, it is not subject to referral biases present in other registries at tertiary care hospitals and is not limited to people aged 65 years and older as some governmental databases (54). The information stored in clinical records is prospectively collected and not subject to potential recall biases (55).
A modified Dillman method (56) was used with an initial letter and survey followed by a reminder postcard approximately two weeks later. For those who did not respond, a second letter and survey were mailed approximately 4 weeks after the initial letter. For those whose survey was returned without a forwarding address within the county or for those for whom the survey was forward but who lived outside the county, randomly selected replacements were selected for the first two mailings. Due to the number of addresses that were unavailable, a decision was made to replace those returned from the last two mailings. Mailings were sent to 10,143 people however, there were a number of inaccurate addresses resulting in an actual mailing of 9,868 residents with mailing addresses in Olmsted County.
Questionnaires and linked medical records
Talley Bowel Disease Questionnaire:
A validated and reliable instrument used to identify GI symptoms and those with FGIDs; the original Talley Bowel Disease Questionnaire (BDQ) was designed in 1988 (57) as a self-report instrument to measure symptoms experienced over the prior year and to collect past medical history data. Extensive reliability and validity testing has been conducted with a median kappa chance corrected measure of agreement for symptom items of 0.8 (range 0.5–1.0)(58). The questionnaire has been modified several times to meet the needs of new studies. For example, the gastroesophageal reflux questionnaire was developed to expand on the GI symptoms measurement (59). The BDQ has been updated to reflect the current Rome IV criteria (1, 60, 61). An abbreviated version with 22 gastrointestinal questions was used for this study, as well as the Somatic Symptom Checklist (SSC) which measures the frequency and burden of non-gastrointestinal symptoms on a five-point Likert scale each, allowing the overall mean SSC score to be computed for each subject (62), as well as the prevalence of those with frequent (daily or several times a week) and/or severely bothersome symptoms (4 or 5 on Likert scale).
Hospital Anxiety and Depression Scale (HADS):
A validated 14-item scale used to measure mood disorders in non-psychiatric outpatients and applied in patients with irritable bowel syndrome (IBS) and FD (63–66).
Food Elimination questions
Food elimination questions came from the public domain questions from the National Health and Nutritional Examination Survey (67).
Linked medical records:
Data on 17 a priori selected medical and psychiatric comorbidities as well as demographic and exposure risk factors were extracted from the REP medical record linkage system (54, 55).
Case and control definition:
Cases:
FD was defined according to the ROME IV criteria (1) by the presence of either PDS or EPS (or both). PDS was defined by the occurrence of (i) feeling uncomfortably full after a regular sized meal, interfering with usual activities, (ii) inability to finish a regular sized meal. EPS defined as epigastric abdominal pain occurring at least 2–3 days a month. rumination syndrome was defined as per the ROME IV criteria (1) as the presence of effortless regurgitation of recently ingested food (excluding vomiting) occurring for at least 1 day a week and not commonly preceded by retching.
Controls:
Survey respondents who do not meet the criteria for either FD or rumination syndrome were considered as controls.
To allow the creation of a consistent control group for comparisons, and to avoid overestimation of an overlap of risk factors and disease associations, rumination syndrome was treated as mutually exclusive from each of FD, PDS and EPS for the purpose of comparisons. Individuals with overlapping diagnoses of both rumination syndrome-FD, unless specified otherwise, were analysed separately.
Statistical analysis
Prevalence and univariate associations of the potential predictors of rumination syndrome, PDS and EPS were evaluated via unconditional logistic regression models and the results reported as odds ratios (OR) with 95% confidence interval (95% CI). Statistically independent predictors were identified via a backward elimination algorithm based on unconditional logistic regression, which included all risk factors. Unpaired t-test for was used to compare the dietary characteristics between FD and rumination syndrome.
Ethical Considerations
The proposal was approved by the Mayo Clinic and Olmsted Medical Centre Institutional Review Board.
Results
Overall, 3,001 usable surveys were obtained. There were slightly more female respondents (n=1,732, 59.7%) than male (1,169, 40.3%). The majority of the respondents were in the age group of 50–59 (1,269, 41.7%) with an age range of 25 years to over 90 years of age. 2573 subjects did not meet the criteria for rumination syndrome or FD and were considered as controls.
The prevalence of rumination syndrome, FD and FD subtypes:
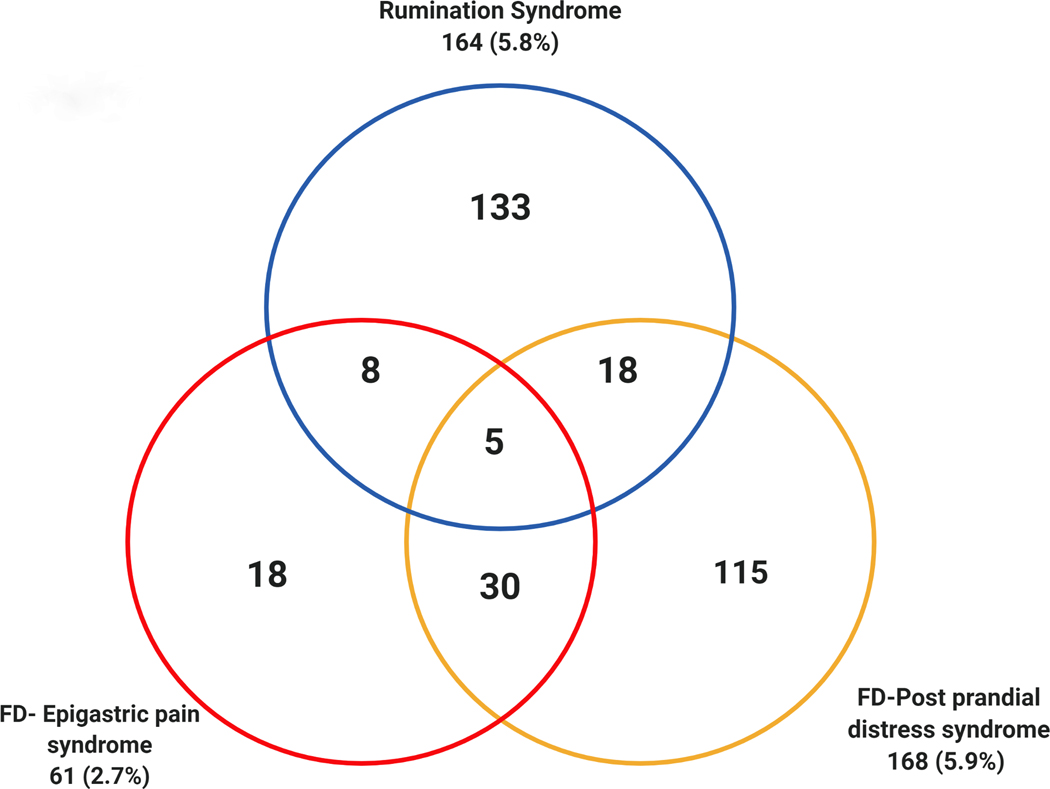
164 subjects met the criteria for rumination syndrome (prevalence 5.8% (95%CI 4.9–6.7)). 194 subjects met the criteria for FD (prevalence: 7.1% (95%CI 6.2 −8.1)). 31 subjects had both FD and rumination syndrome, equating to 16.0% (95%CI 11.1–21.9) of the total number of FD subjects. The odds of rumination syndrome was 3.83 times higher (95%CI 2.51–5.84) in those with FD compared to controls.
The prevalence of EPS and PDS were 2.2% (95% CI 1.7–2.8%) and 5.9% (95%CI 5.1–6.9%), respectively, and 35 individuals had both EPS and PDS (18.0% of FD) (Figure 1).
Figure 1.
The prevalence and overlap of rumination syndrome with functional dyspepsia (FD) and its subtypes
Risk factors for rumination syndrome, FD subtypes and rumination syndrome-FD overlap
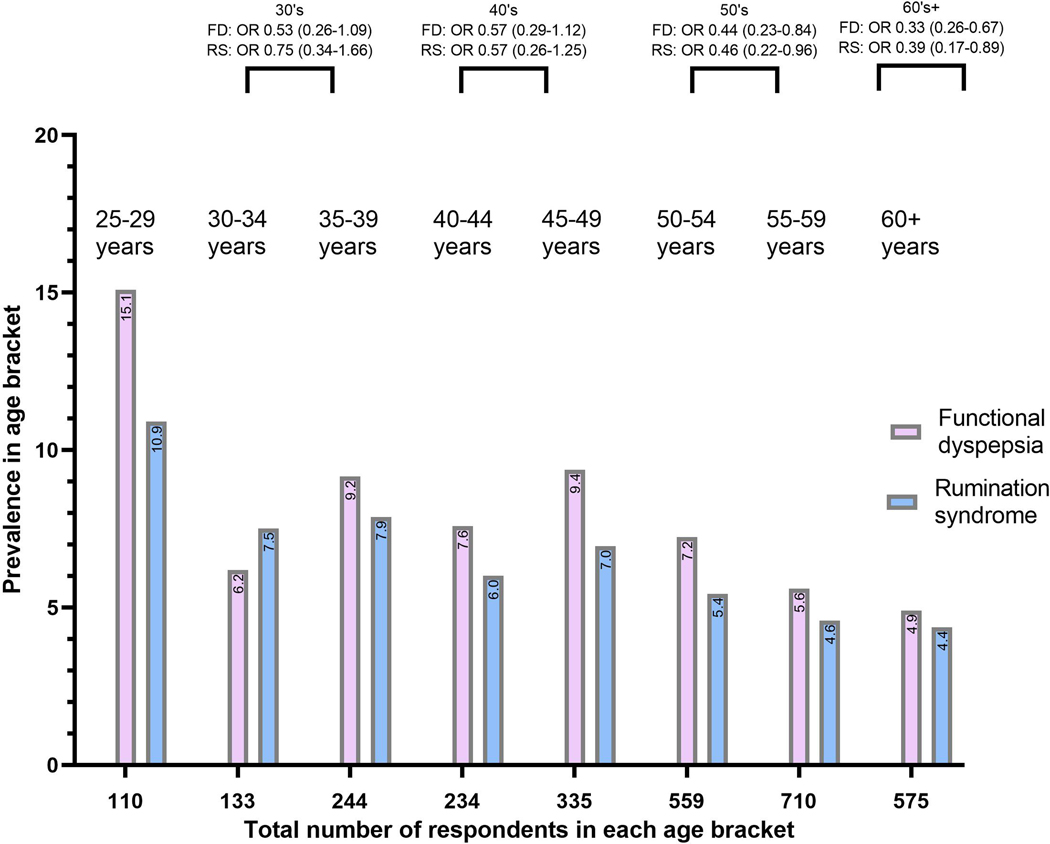
The prevalence of the assessed risk factors in rumination syndrome, FD and those meeting criteria for both, as well as the results of the unadjusted and age and gender adjusted regression are summarised in Tables 1–2. Independent risk factors are presented in Table 3. Both FD and rumination syndrome were more prevalent in the younger age groups (Figure 2), but the age and gender adjustment did not impact the level of statistical significance for most risk factors (Tables 1–2).
Table 1.
The prevalence of risk factors of functional dyspepsia, rumination syndrome and the overlap group of both, and the results of unadjusted, and age and gender adjusted associations in comparison to the control group.
| Risk factor | Group | |||
|---|---|---|---|---|
| Control (n=2573) | Functional dyspepsia (n=163) | Overlap (n=31) | Rumination syndrome (n=133) | |
| Demographics, diet, and exposures | ||||
| Female gender | 1483 (57.6%) |
A130 (79.8%) B2.90 (1.96– 4.27) <0.005 |
A26 (83.9%) B3.8 (1.46–10.0) 0.006 |
A92 (69.2%) B1.65 (1.142.41) 0.009 |
| Cigarette smoking | 548 (21.3%) |
A45 (27.6%) B1.41 (0.99–2.01) 0.06 C1.56 (1.08–2.24) |
A11 (35.5%) B2.03 (0.97–4.26) 0.061 C2.3 (1.09–4.89) |
A43 (32.3%) B1.76 (1.21–2.57) 0.03 C1.87 (1.27–2.72) |
| Harmful drinking | 160 (6.2%) |
A18 (11.0%) B1.87 (1.12–3.13) 0.017 C2.12 (1.25–3.59) |
A2 (6.5%) B1.04 (1.25–4.39) 0.96 C1.19 (1.01–1.14) |
A10 (7.5%) B1.23 (0.63–2.38) 0.55 C1.26 (0.64–2.46) |
| Illicit drug use | 34 (1.3%) |
A6 (3.7%), B2.85 (1.18–6.89) 0.020 C2.83 (1.15–6.96) |
A2 (6.5%), B5.15(1.18–22.43) 0.029 C5.15 (1.13–23.55) |
A3 (2.3%) B1.72 (0.52–5.68) 0.89 C1.57 (0.46–5.14) |
| Gluten free diet (current or previous) | 154 (6.0%) |
A23 (14.1%) B1.81 (1.37–2.38) <0.005 C1.63 (1.23–2.17) |
A3 (9.7%) B1.43 (0.71–2.89) 0.32 C1.25 (0.61–2.55) |
A15 (11.3%) B1.61 (1.17–2.23) 0.004 C1.48 (1.07–2.06) |
| BMI | ||||
| Mean, (SD) | 28.80 (6.15) | 29.59 (8.49), 0.12 | 30.59 (8.15) | 29.92 (6.38), 0.042 |
| <18.5 | 20 (0.8%) |
A2 (1.2%) B1.02 (1.00–1.04) 0.12 C1.02 (1.00–1.04) |
A1(3.2%) B4.25 (0.55–32.74) 0.16 C 2.70 (0.32–22.90) |
A1 (0.8%) B1.03 (1.00–1.05) 0.042 C1.03 (1.00–1.05) |
| 18.5–24.9 | 695 (27.0%) |
A52 (31.9%) B1.23 (0.90–1.78) 0.18 C1.06 (0.75–1.51) |
A8(25.8%) B0.97 (0.65–1.45) 0.88 C0.89 (0.59–1.35) |
A30 (22.6%) B0.79 (0.52–1.19) 0.26 C0.68 (0.45–1.04) |
| 24.9–29.9 | 912 (35.5%) |
A39 (23.9%) B0.59 (0.40–0.85) 0.004 C0.69 (0.48–1.01) |
A9 (29.0%) B0.75 (0.34–1.62) 0.46 C0.94 (0.43–2.01) |
A40 (30.1%) B0.80 (0.55–1.17) 0.25 C0.89 (0.61–1.31) |
| ≥30 | 926 (36.0%) |
A69 (42.3%) B1.31 (0.95–1.80) 0.10 C1.33 (0.95–1.84) |
A 13(41.9%) B1.28 (0.63–2.63) 0.49 C1.22 (0.56–2.54) |
A61 (45.9%) B1.51 (1.06–2.14) 0.022 C1.55 (1.09–2.21) |
| Any allergy to food or medications | 1222 (53.4%) |
A93 (62.8%), B1.48 (1.05–2.08) 0.026 C1.31 (0.93–1.86) |
A24 (77.2%), B3.00 (1.29–6.98) 0.011 C2.67 (1.14–6.26) |
A73 (61.3%) B1.39 (0.95–2.02) 0.09 C1.33 (0.91–1.94) |
| Medical comorbidities | ||||
| Asthma | 420 (16.3%) |
A39 (23.9%) B1.61 (1.11–2.34) 0.013 C1.38 (0.94–2.02) |
A 13(41.9%) B 3.70 (1.80–7.60) <0.005 C3.09(1.49–6.43) |
A28 (21.1%) B1.37 (0.90–2.10) 0.16 C1.23 (0.80–1.91) |
| Atopic dermatitis | 97 (3.8%) |
A7 (4.3%) B1.14 (0.52–2.51) 0.74 C0.92 (0.41–2.04) |
A1(3.2%) B0.85 (0.52–2.51) 0.87 C0.63 (0.08–4.76) |
A8 (6.0%) B 1.63 (0.78–3.43) 0.20 C1.46 (0.69–3.09) |
| Allergic rhinitis | 845 (32.9%) |
A60 (36.8%) B1.19 (0.86–1.65) 0.30 C1.12 (0.80–1.56) |
A13(41.9%) B1.47 (0.72–3.03) 0.29 C1.39 (0.67–2.86) |
A58 (43.6%) B1.58 (1.10–2.24) 0.011 C1.54 (1.08–2.19) |
| Migraine | 513 (20.0%) |
A71 (43.6%) B3.10 (2.24–4.28) <0.005 C2.46 (1.75–3.44) |
A12 (38.7%) B2.53 (1.22–5.25) 0.012 C1.88 (0.89–4.00) |
A43 (32.3%) B1.92 (1.32–2.79) <0.005 C1.71 (1.16–2.53) |
| Rheumatoid Arthritis | 77 (3.0%) |
A4 (2.5%) B0.81 (0.29–2.25) 0.69 C0.85 (0.30–2.36) |
A4 (12.9%) B4.80 (1.64–14.05) 0.004 C4.77 (1.59–14.31) |
A6 (4.5%) B1.53 (0.65–3.58) 0.98 C1.65(.070–3.88) |
| Diabetes Mellitus | 426 (16.6%) |
A25 (15.3%) B0.91 (0.59–1.41) 0.68 C1.15 (0.73–1.81) |
A10 (32.3%) B2.40 (1.12–5.12) 0.024 C3.19 (1.44–7.06) |
A16 (12.0%) B0.69 (0.40–1.17) 0.17 C0.79 (0.46–1.37) |
| Non-coeliac wheat sensitivity | 32 (1.3%) |
A14 (8.6%) B7.42 (3.89–14.20) <0.005 C5.96 (3.07–11.58) |
A2 (6.5%) B5.45 (1.25–23.80) 0.024 C4.26 (0.95–19.18) |
A2 (1.5%) B1.21 (0.29–5.09) 0.80 C1.01 (0.24–4.29) |
| Coeliac disease | 30 (1.2%) |
A5 (3.1%) B2.26 (1.02–6.96) 0.046 C2.27 (0.85–6.06) |
A 1 (3.2%) B2.81 (0.37–21.25) 0.32 C2.10 (0.27–16.58) |
A2 (1.5%) B1.29 (0.31–5.38) 0.35 C1.08 (0.25–4.65) |
| IBS (clinical diagnosis in medical record) | 219 (8.5%) |
A40 (24.5%) B3.49 (2.38–5.12) <0.005 C3.14 (2.13–4.64) |
A 6 (19.4%) B2.58 (1.05–6.35) 0.04 C2.37 (0.95–5.23) |
A15 (11.3%) B1.37 (0.78–2.38) 0.27 C1.30 (0.74–2.27) |
| Ulcerative colitis | 41 (1.6%) |
A3 (1.8%) B1.16 (0.35–3.78) 0.24 C1.36 (0.41–4.50) |
0 (0.0%) |
A2 (1.50%) B0.94 (0.23–3.94) 0.94 C1.03 (0.25–4.35) |
n (prevalence as a percentage)
Unadjusted odds ratio (95% confidence interval) p-value of having disease in comparison to the control group
Adjusted odds ratio (95% confidence interval) p-value of having disease in comparison to the control group
Abbreviations: BMI: Body Mass Index. IBS: Irritable Bowel Syndrome. FD: Functional Dyspepsia. SD: Standard deviation. HADS: Hospital Anxiety and Depression Scale. SSC: Somatic Symptom Checklist
Table 2.
The prevalence of psychiatric risk factors in functional dyspepsia and rumination syndrome, and the results of unadjusted, and age and gender adjusted associations.
| Risk factor | Group | |||
|---|---|---|---|---|
| Control (n=2573) | Functional dyspepsia (n=163) | Overlap (n=31) | Rumination syndrome (n=133) | |
| Panic disorder | 100 (3.9%) |
A10 (6.1%) B1.62 (0.83–3.16) 0.16 C1.24 (0.63–2.46) |
A 4 (12.0%) B3.66 (1.26–10.66) 0.17 C2.61 (0.86–7.93) |
A11 (8.2%) B 2.23 (1.16–4.26) 0.015 C1.86 (0.96–3.62) |
| Major depression | 672 (26.1%) |
A86 (52.8%) B3.15 (2.29–4.35) <0.005 C2.59 (1.87–3.60) |
A15 (48.40%) B2.65 (1.30–5.39) 0.007 C2.04 (0.98–4.22) |
A44 (33.1%) B1.40 (0.96–2.03) 0.078 C1.20 (0.82–1.76) |
| HADS depression, mean (SD) | 9.8 (3.5) | A12.0 (4.2) <0.005 | A12.0 (4.1) <0.005 | A10.8 (3.5) <0.005 |
| Generalised anxiety disorder | 310 (12.06%) |
A39 (23.9%) B2.29 (1.57–3.35) <0.005 C1.85 (1.25–2.74) |
A 11(35.5%) B4.01 (1.90–8.45) <0.005 C3.30 (1.51–7.20) |
A28 (21.1%) B1.94 (1.26–3.00) 0.003 C1.63 (1.04–2.55) |
| HADS anxiety, mean (SD) | 11.5 (3.4) | A14.2 (4.3) <0.005 | A14.9 (5.5) <0.005 | A12.7 (4.3) <0.005 |
| SSC, xmean (SD) | 5.5 (5.0) | A10.5 (6.8) <0.005 | A11.0 (6.12) <0.005 | A7.7 (5.5) <0.005 |
n (prevalence as a percentage)
Unadjusted odds ratio (95% confidence interval) p-value of having disease in comparison to the control group
Adjusted odds ratio (95% confidence interval) p-value of having disease in comparison to the control group
Abbreviations: HADS: Hospital Anxiety and Depression Scale, SD: Standard deviation, SSC: Somatic Symptom Checklist.
Table 3.
The association of functional dyspepsia, rumination syndrome and the overlap group of both with the examined risk factors in a multivariate regression model.
| Risk factor | Group | ||
|---|---|---|---|
| Functional dyspepsia | Overlap | Rumination syndrome | |
| Demographic and exposure risk factors | |||
| Female gender | 2.04 (1.33–3.10) | 3.22 (1.19–8.47) | 1.79 (1.21–2.63) |
| Smoking | - | - | 1.89 (1.28–2.78) |
| Diet risk factors | |||
| Gluten free diet (current or previous) | - | - | 1.58 (1.14–2.19) |
| Non-coeliac wheat sensitivity | 4.35 (2.15–9.80) | - | |
| Medical co-morbidities | |||
| Asthma | - | 3.02 (1.44–6.34) | - |
| Allergic rhinitis | - | 1.45 (1.01–2.08) | |
| Migraine | 1.53 (1.06–2.21) | - | |
| IBS (diagnostic code) | 1.85 (1.21–2.82) | - | |
| Rheumatoid Arthritis | - | 3.93 (1.28–12.06) | - |
| Diabetes Mellitus | 2.64 (1.20–5.81) | ||
| Psychiatric comorbidities | |||
| Major depression (diagnostic code) | 1.47 (1.02–2.11) | - | - |
| HADS- Depression | 1.10 (1.05–1.16) | - | 1.10 (1.05–1.16) |
| HADS- Anxiety | - | 1.22 (1.12–1.32) | - |
| SSC | 1.07 (1.04–1.10) | - | - |
Abbreviations: IBS: irritable bowel syndrome. HADS: Hospital Anxiety and Depression Scale. SSC: Somatic Symptom Checklist.
Figure 2.
The prevalence of rumination syndrome (rumination syndrome) and functional dyspepsia (FD), the odds ratio compared to the age bracket 25–29, and the corresponding total number of survey respondents in each age brackets.
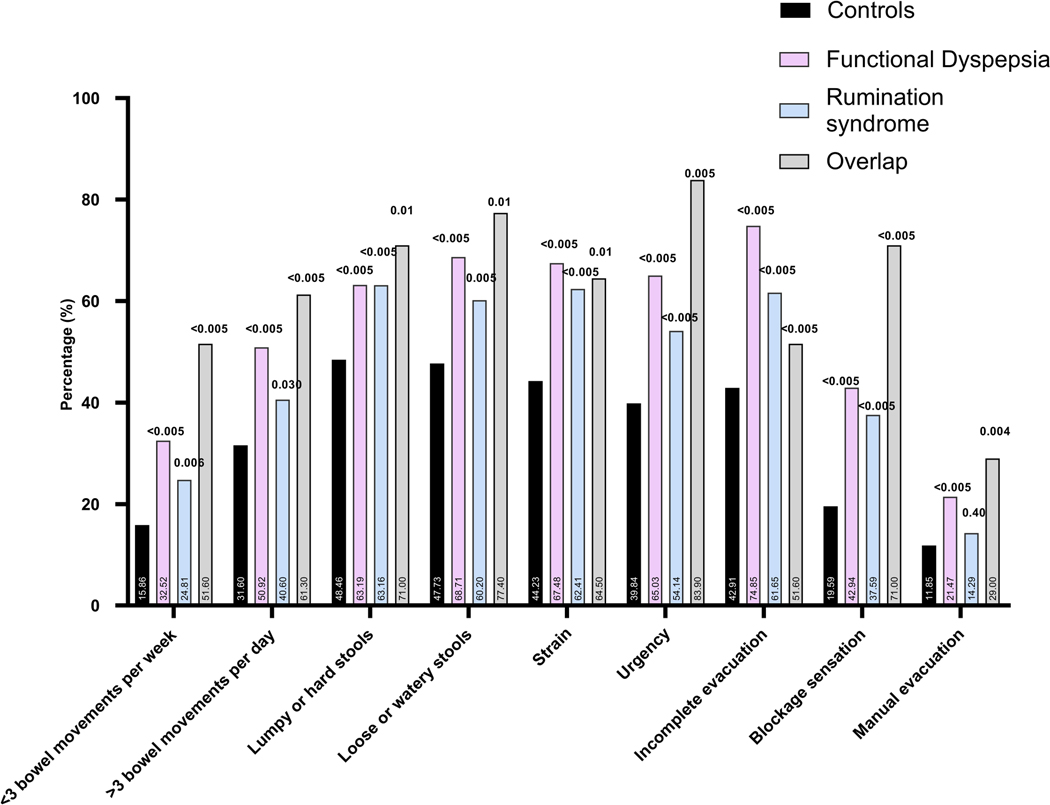
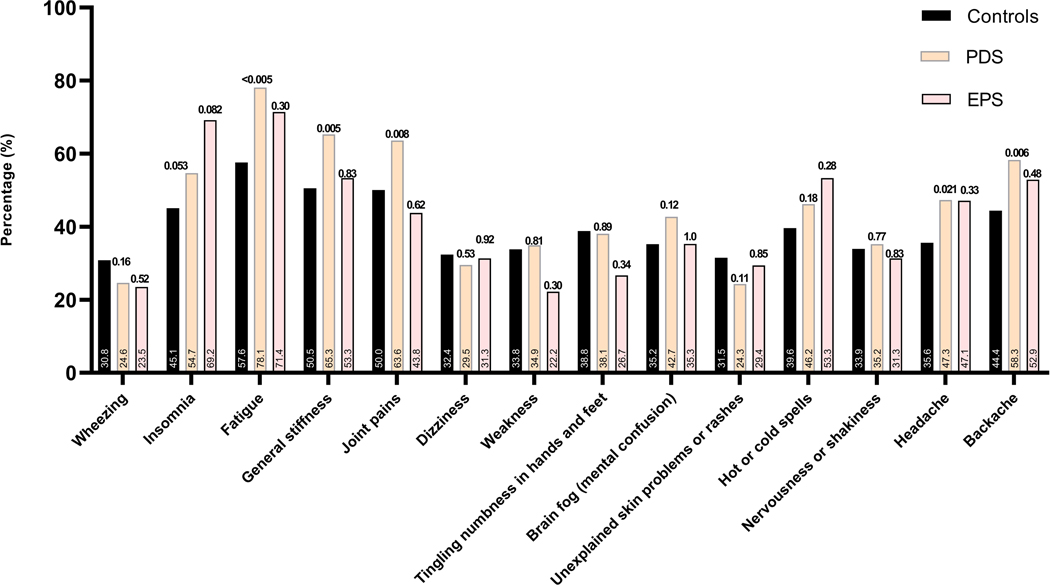
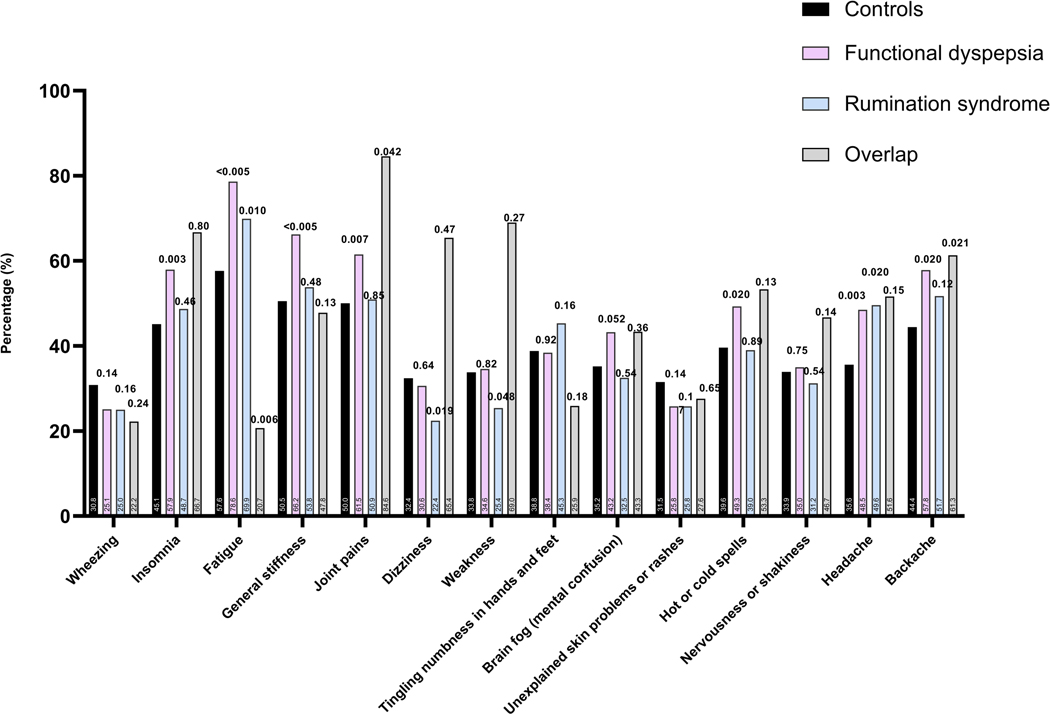
The prevalence of different bowel symptoms is outlined in Figure 3. At least three frequent (occurring 25% of the time or more) gastrointestinal symptoms were present in the majority of individuals with rumination syndrome and FD (105 of 133 (78.9%) and 145 of 163 (89.0%), respectively) compared to 1156 of 2706 (42.7%) of controls. A high prevalence of non-gastrointestinal somatic symptoms (assessed collectively as mean SSC score) was found in the entire sample of survey respondents, being most prevalent in FD (Figure 4). For most of the intestinal and extra-intestinal symptoms, the prevalence was highest in the rumination syndrome-FD overlap group although this failed to reach statistical significance for the majority of extra-intestinal symptoms in this group (Figure 3 and 4).
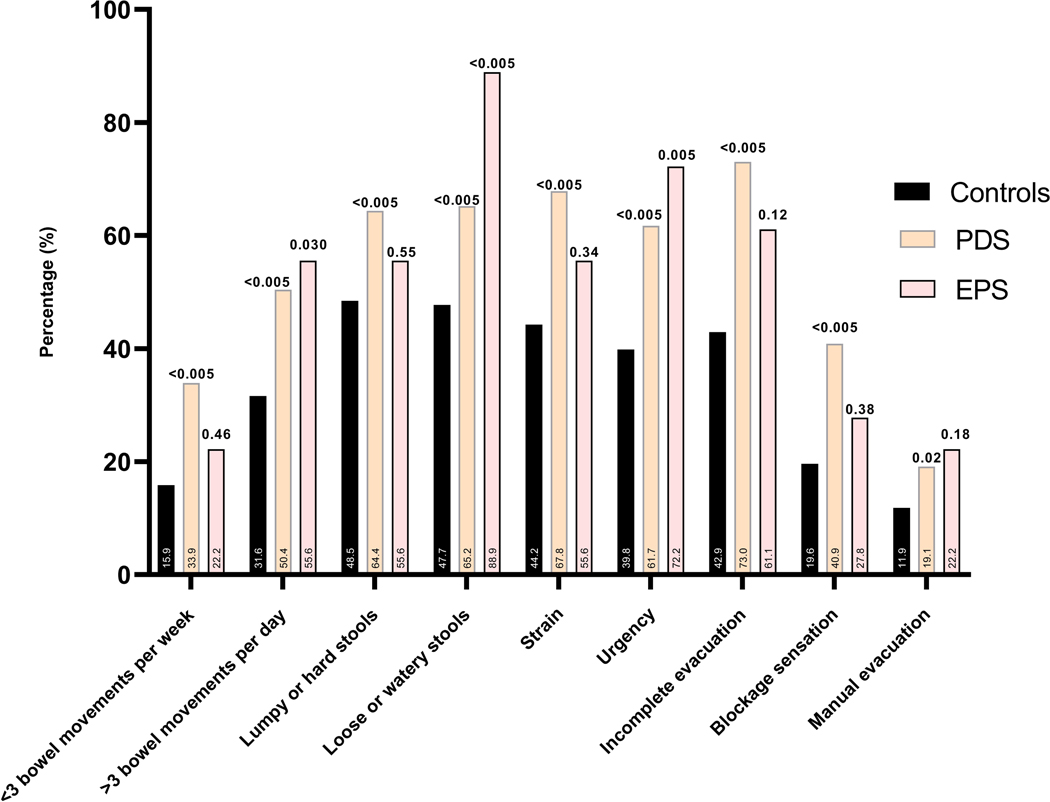
Figure 3.
(A) The prevalence of different bothersome (occurring 25% of the time or more) intestinal symptoms in individuals with rumination syndrome, functional dyspepsia and those with overlapping diagnosis in comparison to controls, and comparing the FD subtypes to controls (B).
Note: p-value over bar: compared to controls. PDS: Postprandial distress syndrome, EPS: Epigastric pain syndrome.
Figure 4.
(A) The prevalence of those with frequent (occurring daily or several times a week) and/or severely bothersome (4 or 5 on Likert scale) non-gastro-intestinal symptoms in individuals with rumination syndrome and functional dyspepsia and those with overlapping diagnosis in comparison to controls, and comparing the FD subtypes to controls (B).
Note: p-value over bar: compared to controls. PDS: Postprandial distress syndrome, EPS: Epigastric pain syndrome.
Onset and characteristics of rumination symptoms
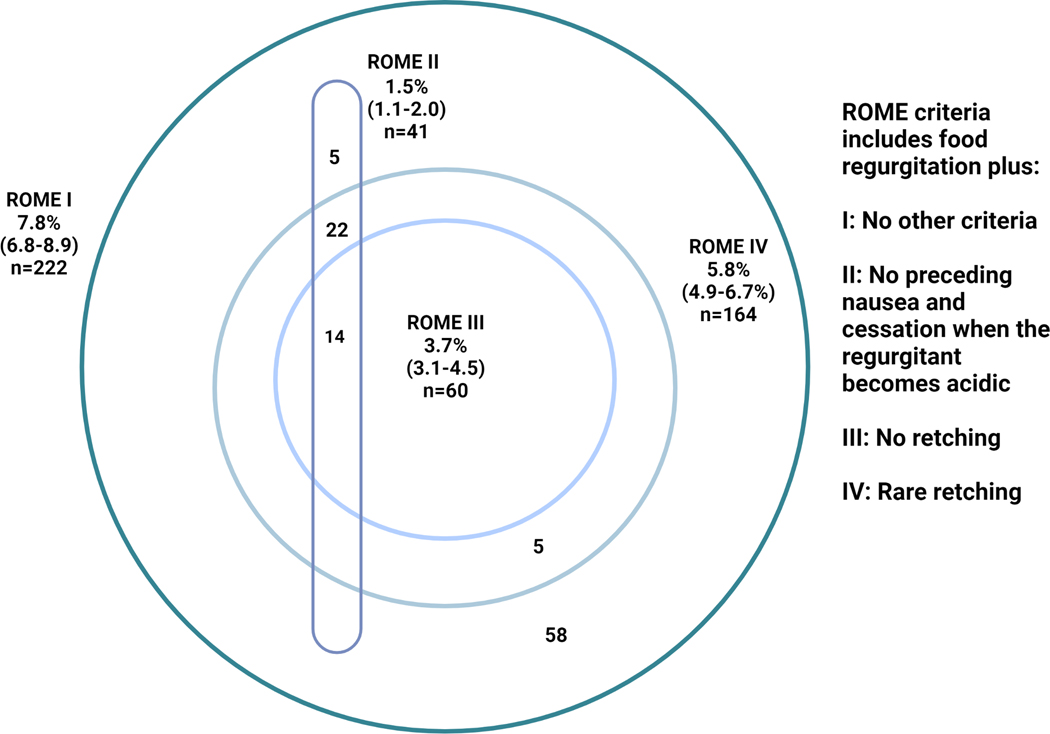
The prevalence of rumination syndrome was widely different when applying each of the ROME I-IV definitions (1, 4, 68) (Figure 5). Considering the 164 individuals with rumination syndrome (not excluding the overlap with FD), the majority (n=148, 90.2%) reported no or occasional (less than once a month) nausea preceding food regurgitation. 41 individuals (29.3%) reported a non-acidic and non-sour tasting food regurgitation. 9 (5.5%) reported a bout of gastroenteritis or having used antibiotics in the 3 months preceding the symptom onset. 13 (7.9%) reported inadvertent weight loss of less than 5kg, but none reported more significant weight loss.
Figure 5.
A comparative prevalence of rumination syndrome using different versions of ROME criteria in our sample.
Notes: (i) The frequency of rumination is defined as 2–3/month in ROME III and 1/week in ROME IV. (ii) The exact wording of food regurgitation is slightly different between different versions, and ROME IV uniquely includes re-swallowing without necessarily rechewing or spitting out (1, 4, 68). (iii): In our study, we have asked if the regurgitant is sour, in ROME II the question is “does it stop when it becomes sour”.
Dietary profile
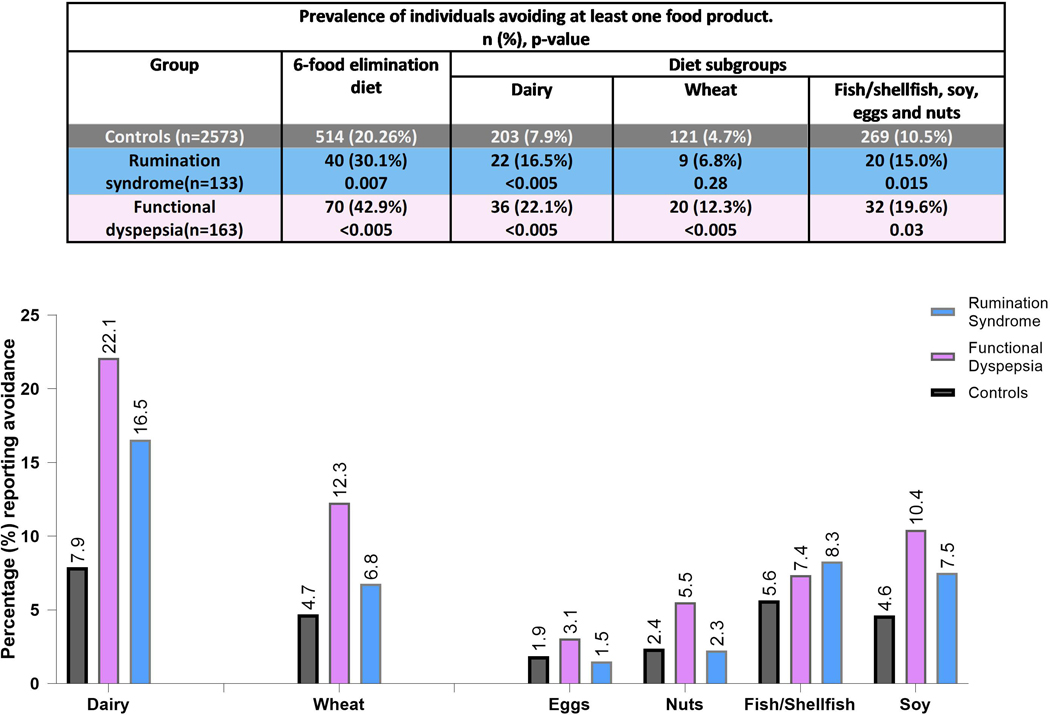
A similar proportion of FD and rumination syndrome (15 (11.2%) and 23 (14.1%), respectively) reported being currently or previously on a gluten free diet (p=0.44). Overall, 36.8% of rumination syndrome (n=49) and 46.0% of FD (n=75) had tried at least one of the three dietary modifications assessed (Table 3), a comparably high proportion of FD and rumination syndrome reported efficacy and adherence of a low-fat diet and dairy free diet. Very few had tried a low FODMAP diet. Having at least one form of dietary avoidance of the components of the 6-food elimination diet (fish, eggs, nuts, soy, wheat and milk) (69) was significantly more common in both FD and rumination syndrome compared to controls but not different between the two disorder groups (p=0.18). The prevalence of dietary avoidance in the two disorders compared to controls remained significantly higher even after excluding wheat and dairy from the components of the 6-food elimination (Figure 6).
Figure 6.
Self-reported dietary avoidance data in rumination syndrome, functional dyspepsia compared to controls, among the components of the 6-food elimination diet. (p-value: compared to controls)
Regarding gluten free diet, the reason of use and duration of use was asked but the numbers were few to characterise. Subjects were asked if they had received a diagnosis of non-coeliac sensitivity by a doctor. In FD, 14.1% (n=23) had tried gluten free diet, and 8.6% (n=14) were told they have non-coeliac wheat sensitivity. In rumination syndrome, a comparable proportion had tried a gluten free diet (11.3%, n=15) but the prevalence of non-coeliac wheat sensitivity was lower (1.5%, n=2) (table 1).
Discussion
The prevalence of rumination syndrome in this population sample was 6% and the disorder was three-fold more likely to overlap with FD, which itself had a prevalence of 7%. As the overlap was more than expected by chance, this suggests there may be a common underlying pathophysiology. There were overlapping but distinct risk factor profiles for FD and rumination syndrome, with atopic, psychiatric, and dietary risk factor associations. The overlap group with both rumination syndrome and FD shared risk factor profile of both disorders and was independently predicted by rheumatoid arthritis (OR 3.93, 95%CI 1.28–12.06) and asthma (OR 3.02, 95% 1.44–6.34). Subjects with either condition reported a similar efficacy and adherence to lactose free and low-fat diets, and a similar rate of avoidance of the components of the 6-food diets was found.
The prevalence of rumination syndrome in our study and other questionnaire-based studies (3–6), contrasts the much lower number of cases reported in studies relying on diagnostic codes in referral centres (36, 70, 71). This may be the result of lack of disclosure by patients including due to embarrassment and previous unhelpful encounters (12, 13, 72). A population-based study reported that more than 80% of individuals with rumination syndrome had not seen a doctor in the preceding year (6). Misdiagnosis of rumination syndrome as GORD or vomiting is not uncommon (73, 74), and participants in case-series commonly report never having been asked about rumination symptoms before (12, 13, 71).
The association between rumination syndrome, mood disorders and psychosomatic symptoms confirms earlier findings (75, 76). Prospective cohort studies concluded that psychological morbidity and disorders of the gut-brain axis each pose a risk factor for the future development of the other (77, 78). A small but significant subset of rumination syndrome in our study reported a history of gastroenteritis or antibiotic use (5.5% and 7.9%, respectively), an association similar to FD where the risk can be up to nine times higher following bacterial gastroenteritis (79). A recent large registry based study reported antibiotic use prior to the development of functional gastrointestinal symptoms in about a quarter of the cases, suggesting one causal direction (80). Preceding psychological trauma in 14% of rumination syndrome individuals has been reported in a retrospective study suggesting a bidirectional causation relationship akin to FD (76).
We observed the frequency of almost all of the assessed gastrointestinal symptoms was higher in both FD and rumination syndrome compared to controls, and was particularly high in those with FD-rumination syndrome overlap. These results were not unexpected and confirms the common overlap of FGIDs (81). A previous association between rumination syndrome and anorectal symptoms has been reported (76) as with our study. More than two thirds of rumination syndrome cases reported acidic food regurgitation; this may present as a symptom of rumination syndrome or overlap with GORD (36).
The historical data linking rumination syndrome to features of pleasant tasting regurgitant, rechewing and ruminating on demand is representative of only a subset of individuals, even when considering the psychiatric literature (82). Rumination can be misdiagnosed as refractory GORD (21, 22), and commonly overlaps with other forms of regurgitation (such as belch-regurgitation and belch-rumination (21, 36, 83)). The distinction between rumination syndrome, GORD and other forms of regurgitation has been attempted by various versions of the ROME criteria; ROME I had no additional criteria in addition to food regurgitation (84), while the absence of preceding nausea and the absence of acidic regurgitation (i.e. cessation of regurgitation when it becomes acidic) were uniquely exclusion criteria in ROME II (85). Hence not unexpectedly, and similar to our findings, Thompson et al (4), in a survey of 1149 individuals, found a 5-fold lower prevalence of rumination syndrome when ROME II was applied compared to ROME I (0.8% and 4.2%, respectively). ROME III and IV include the absence of retching as a criterion instead of exclusion based on nausea (4, 68), and 90% of individuals in our study defined by ROME IV as rumination syndrome did not report nausea, suggesting that this is not a discriminatory feature. Applying ROME II for rumination syndrome to our sample yielded the lowest prevalence of 1.5% (1.1–2.0), suggesting the disorder is not rare even when occurring in isolation to features of GORD.
We searched for an association of FD and rumination syndrome with disorders of immune activation including atopic conditions, as an association of atopy with FD was reported in a large UK general practitioner study (86, 87) and FD is also linked to duodenal eosinophilia (43, 88). Emerging evidence from animal models supports the onset of eosinophilic intestinal inflammation in predisposed individuals after skin or respiratory allergen exposure, referred to as an “atopic march” (89). In our sample, FD was univariately associated with asthma (OR 1.61, 95%CI 1.11–2.34) and rumination syndrome was independently associated with allergic rhinitis (OR 1.45 95%CI 1.01–2.08). The overlap of FD-rumination syndrome was more strongly associated with asthma (multivariate OR 3.02 95%CI 1.44–6.34). The stronger association of asthma with the overlap group, where more frequent gastrointestinal symptoms were found, was also observed in the UK GP study assessing different FGIDs (87).
Individuals with rumination syndrome and FD were significantly more likely to have tried a gluten free diet than controls, although the prevalence of reporting having received a diagnosis of non-coeliac gluten sensitivity by a health professional was only increased in FD. This suggests that non-coeliac wheat sensitivity, which is commonly associated with FD (90), may be under-recognised in rumination syndrome. Interestingly, both rumination syndrome and FD reported a similar efficacy of lactose free and low-fat diets (84.2–90.0%, p=0.53 and 54.32–54.4% p=1.0, respectively). In rumination syndrome, dietary studies are largely limited to reducing meal size or consistency (15). Dietary data in FD is largely derived from observational studies, with suggested benefits of lactose free, gluten free, low fat and low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diets (91, 92). Plausible mechanisms of benefit of restrictive diets include altering luminal osmotic contents (93, 94), but emerging evidence suggests a possible link to mucosal inflammation through microbiome mediated mast cell activation or directly through atypical food sensitivities (95–98).
Both rumination syndrome and FD were more likely to avoid one or more components of the 6-food elimination diets (fish, eggs, nuts, soy, wheat and milk) than controls, even when this was assessed independent of wheat and dairy (Figure 6) (69). This diet has been studied in eosinophilic oesophagitis (EoE), a non-IgE T helper-2 mediated inflammatory condition (99) that overlaps with eosinophilic duodenitis in up to half of the cases when extra-oesophageal symptoms are present (100).
There were insufficient number of individuals with EPS to make inferences regarding whether the risk factor profile is different to PDS. Stool urgency and loose stools were more prevalent in EPS, and anorectal symptoms were no different from controls, while PDS had a significantly higher prevalence of all gastrointestinal symptoms compared to controls.
A shared pathogenesis of FD and rumination syndrome indicated treatment options may overlap. Both disorders respond to psychological interventions (101, 102). Rumination syndrome responds to the increase lower oesophageal tone with diaphragmatic breathing or Gamma-aminobutyric acid receptor agonist (GABA) stimulation using baclofen in randomised controlled trials (103–105). More treatment options are available in FD including proton pump inhibitors (106), which have recently been shown to suppress duodenal mucosal micro-inflammation in a prospective study (107), prokinetics (108) neuromodulation (109) and altering dietary and probiotic therapies (91, 110).
The strengths of the current study include the investigation of a random community sample of non-healthcare seeking individuals with minimal selection bias, and the availability of accurate clinical linkage information. Limitations include the number of individuals with FD-rumination syndrome overlap was relatively small, and there was insufficient number of individuals with EPS to allow a comparison of the risk factor profile in relation to PDS. The prevalence of rumination syndrome in this sample may not apply to other populations with a different (non-white) ethnic composition, and the survey was restricted to adults noting that rumination syndrome was reported in up to 5% of 2163 school children in one survey (111). The questionnaire did not assess ROME IV criteria for IBS, but IBS as a diagnostic code was obtained linked medical records. We applied a question on the sourness of the regurgitant to examine a potential overlap with GORD, but other symptom data were not available. Endoscopic and manometric data of rumination syndrome subjects would have been of value, but symptoms are established to be an excellent indicator of rumination syndrome (7).
In conclusion, the overlapping prevalence and the shared symptom and risk profile of FD and rumination syndrome supports the presence of a common underlying pathophysiology. Based on the risk factors identified and the finding of intestinal inflammation in FD and rumination syndrome (23, 24, 43, 88), we speculate these conditions may represent in many cases atopic gut disorders. Our findings of similar dietary responses in rumination syndrome and FD suggests that diet may contribute to the pathogenesis and could have a therapeutic role in both disorders, and randomised trials are indicated.
Table 4.
The number of individuals with functional dyspepsia (FD) and rumination syndrome (rumination syndrome) with dietary restriction trials and the perceived efficacy and adherence.
| Lactose/Dairy free diet | Low fat diet | Low Fodmap diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FD | rumination syndrome | p-value | FD | rumination syndrome | p-value | FD | rumination syndrome | p-value | |
| Tried the diet n (% relative to the disease group) | 30 (18.4%) | 19 (14.3%) | 0.35 | 48 (29.4%) | 35 (26.3%) | 0.57 | 9 (5.5%) | 2 (1.5%) | N/A |
| Reported efficacy n (%) | 27 (90.0%) | 16 (84.2%) | 0.53 | 26 (54.2%) | 19 (54.3%) | 1.00 | 7 (77.8%) | 2 (100.0%) | N/A |
| Remain on this diet n (%) | 21 (70.0%) | 11 (57.9%) | 0.45 | 15 (31.2%) | 17 (48.6%) | 0.096 | 5 (55.6%) | 1 (50.0%) | N/A |
FD: Functional dyspepsia, rumination syndrome: Rumination syndrome. N/A: Not applicable due to small sample size.
% of those that have tried it. p-value: Compares rumination syndrome to FD.
Acknowledgments:
We acknowledge Professor Simon Keely for his support of Mudar Zand Irani as a PhD supervisor.
This study acknowledges support from the National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Digestive Health and an NHMRC investigator grant to Dr Talley.
Disclosures:
Dr Nicholas J. Talley reports personal fees from Allakos, from Aviro Health, from Antara Life Sciences, from Arlyx from Bayer, from Danone, from Planet Innovation, from Takeda, from Viscera Labs, from twoXAR, from Viscera Labs, from Dr Falk Pharma, from Censa, from Cadila Pharmaceuticals, from Progenity Inc, from Sanofi-aventis, from Glutagen, from ARENA Pharmaceuticals, from IsoThrive, from BluMaiden, from RosePharma, from Intrinsic Medicine, from OzSage, non-financial support from HVN National Science Challenge NZ, outside the submitted work; In addition, NJT has a patent Biomarkers of IBS licensed (#12735358.9 −1405/2710383 and (#12735358.9 −1405/2710384), a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, a patent Nestec European Patent licensed, and a patent Singapore Provisional Patent NTU Ref: TD/129/17 “Microbiota Modulation Of BDNF Tissue Repair Pathway” issued and copyright Nepean Dyspepsia Index (NDI) 1998 and Editorial: Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, Med (Journal of Cell Press). NJT participates Committees: Australian Medical Council (AMC) Council Member (2016–2019), MBS Review Taskforce (2016–2020), NHMRC Principal Committee, Research Committee (2016–2021), Asia Pacific Association of Medical Journal Editors (APAME) (current), GESA Board Member (2017–2019). NJT Misc: Avant Foundation (judging of research grants) (2019). NJT community and patient advocacy groups: Advisory Board, IFFGD (International Foundation for Functional GI Disorders). NJT acknowledges funding from the National Health and Medical Research Council (NHMRC) for the Centre for Research Excellence in Digestive Health. NJT holds an NHMRC Investigator grant.
Dr. Joseph Murray has received study grants from Nexpep/ImmusanT, National Institutes of Health, Immunogenics, Johnson & Johnson, Kanyos/Anakion, Takeda Pharmaceutical, Allakos, Oberkotter, and Cour; consultancy fees from Bionix, UKKO, Dren Bio, Dr. Schar USA, Chugai Pharma; holds patents licensed to Evelo Biosciences; and receives royalties from Torax Medical.
MZI, MMW, MPJ, LH, MH, RSC and YASL: no disclosures.
Common abbreviations:
- FD
Functional dyspepsia
- PDS
Postprandial distress syndrome
- EPS
Epigastric pain syndrome
- GORD
Gastro-oesophageal reflux disease
- FGID
Functional gastrointestinal disorders
References
- 1.Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–92. [DOI] [PubMed] [Google Scholar]
- 2.Absah I, Rishi A, Talley NJ, Katzka D, Halland M. Rumination syndrome: pathophysiology, diagnosis, and treatment. Neurogastroenterol Motil. 2017;29(4). [DOI] [PubMed] [Google Scholar]
- 3.Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology. 2021;160(1):99–114. e3. [DOI] [PubMed] [Google Scholar]
- 4.Thompson W, Irvine E, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Digestive diseases and sciences. 2002;47(1):225–35. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. US householder survey of functional gastrointestinal disorders. Digestive diseases and sciences. 1993;38(9):1569–80. [DOI] [PubMed] [Google Scholar]
- 6.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. Am J Gastroenterol. 2002;97(9):2290–9. [DOI] [PubMed] [Google Scholar]
- 7.Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. American Journal of Gastroenterology. 2014;109(1):52–9. [DOI] [PubMed] [Google Scholar]
- 8.Barba E, Burri E, Accarino A, Malagelada C, Rodriguez-Urrutia A, Soldevilla A, et al. Biofeedback-guided control of abdominothoracic muscular activity reduces regurgitation episodes in patients with rumination. Clin Gastroenterol Hepatol. 2015;13(1):100–6.e1. [DOI] [PubMed] [Google Scholar]
- 9.Amarnath RP, Abell TL, Malagelada JR. The rumination syndrome in adults. A characteristic manometric pattern. Ann Intern Med. 1986;105(4):513–8. [DOI] [PubMed] [Google Scholar]
- 10.Gourcerol G, Dechelotte P, Ducrotte P, Leroi AM. Rumination syndrome: when the lower oesophageal sphincter rises. Digestive and Liver Disease. 2011;43(7):571–4. [DOI] [PubMed] [Google Scholar]
- 11.Smout AM, Breumelhof R. Voluntary induction of transient lower esophageal sphincter relaxations in an adult patient with the rumination syndrome. The American journal of gastroenterology. 1990;85(12):1621–5. [PubMed] [Google Scholar]
- 12.Fairburn CG, Cooper PJ. Rumination in bulimia nervosa. British medical journal (Clinical research ed). 1984;288(6420):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckern M, Stevens W, Mitchell J. The relationship between rumination and eating disorders. International Journal of Eating Disorders. 1999;26(4):414–9. [DOI] [PubMed] [Google Scholar]
- 14.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- 15.Lang R, Mulloy A, Giesbers S, Pfeiffer B, Delaune E, Didden R, et al. Behavioral interventions for rumination and operant vomiting in individuals with intellectual disabilities: A systematic review. Research in developmental disabilities. 2011;32(6):2193–205. [DOI] [PubMed] [Google Scholar]
- 16.Fairburn CG, Cooper PJ. The clinical features of bulimia nervosa. The British Journal of Psychiatry. 1984;144(3):238–46. [DOI] [PubMed] [Google Scholar]
- 17.Levine D, Wingate D, Pfeffer J, Butcher P. Habitual rumination: a benign disorder. Br Med J (Clin Res Ed). 1983;287(6387):255–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S, Hyman PE, Cocjin J, Di Lorenzo C. Rumination syndrome in adolescents. The Journal of pediatrics. 2000;136(4):528–31. [DOI] [PubMed] [Google Scholar]
- 19.Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21. [Google Scholar]
- 20.Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional Esophageal Disorders. Gastroenterology. 2016. [DOI] [PubMed] [Google Scholar]
- 21.Sawada A, Guzman M, Nikaki K, Sonmez S, Yazaki E, Aziz Q, et al. Identification of different phenotypes of esophageal reflux hypersensitivity and implications for treatment. Clinical Gastroenterology and Hepatology. 2021;19(4):690–8. e2. [DOI] [PubMed] [Google Scholar]
- 22.Yadlapati R, Tye M, Roman S, Kahrilas PJ, Ritter K, Pandolfino JE. Postprandial high-resolution impedance manometry identifies mechanisms of nonresponse to proton pump inhibitors. Clinical Gastroenterology and Hepatology. 2018;16(2):211–8. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friesen HJ, Rosen J, Low Kapalu C, Singh M, Spaeth T, Cocjin JT, et al. Mucosal eosinophils, mast cells, and intraepithelial lymphocytes in youth with rumination syndrome. Neurogastroenterology & Motility. 2021:e14155. [DOI] [PubMed] [Google Scholar]
- 24.Halland M, Talley NJ, Jones M, Murray JA, Cameron R, Walker MM. Duodenal pathology in patients with rumination syndrome: duodenal eosinophilia and increased intraepithelial lymphocytes. Digestive diseases and sciences. 2019;64(3):832–7. [DOI] [PubMed] [Google Scholar]
- 25.Barberio B, Mahadeva S, Black CJ, Savarino EV, Ford AC. Systematic review with meta-analysis: global prevalence of uninvestigated dyspepsia according to the Rome criteria. Alimentary pharmacology & therapeutics. 2020;52(5):762–73. [DOI] [PubMed] [Google Scholar]
- 26.Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Official journal of the American College of Gastroenterology| ACG. 2017;112(7):988–1013. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien MD, Bruce BK, Camilleri M. The rumination syndrome: clinical features rather than manometric diagnosis. Gastroenterology. 1995;108(4):1024–9. [DOI] [PubMed] [Google Scholar]
- 28.Soykan I, Chen J, Kendall BJ, McCallum RW. The rumination syndrome: clinical and manometric profile, therapy, and long-term outcome. Dig Dis Sci. 1997;42(9):1866–72. [DOI] [PubMed] [Google Scholar]
- 29.Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–15. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thumshirn M, Camilleri M, Hanson RB, Williams DE, Schei AJ, Kammer PP. Gastric mechanosensory and lower esophageal sphincter function in rumination syndrome. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1998;275(2):G314–G21. [DOI] [PubMed] [Google Scholar]
- 31.Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121(3):526–35. [DOI] [PubMed] [Google Scholar]
- 32.Cirillo C, Bessissow T, Desmet A-S, Vanheel H, Tack J, Berghe PV. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. American Journal of Gastroenterology. 2015;110(8):1205–15. [DOI] [PubMed] [Google Scholar]
- 33.Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127(6):1685–94. [DOI] [PubMed] [Google Scholar]
- 34.Ardila-Hani A, Arabyan M, Waxman A, Ih G, Berel D, Pimentel M, et al. Severity of dyspeptic symptoms correlates with delayed and early variables of gastric emptying. Digestive diseases and sciences. 2013;58(2):478–87. [DOI] [PubMed] [Google Scholar]
- 35.Maselli DB, Park S-Y, Camilleri M. Gastric Motor Functions in Patients With Mood Disorders and Functional Gastroduodenal Symptoms. Psychosomatic Medicine. 2021;83(2):171–6. [DOI] [PubMed] [Google Scholar]
- 36.Tucker E, Knowles K, Wright J, Fox M. Rumination variations: aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Alimentary pharmacology & therapeutics. 2013;37(2):263–74. [DOI] [PubMed] [Google Scholar]
- 37.Azpiroz F, Malagelada J. Pressure activity patterns in the canine proximal stomach: response to distension. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1984;247(3):G265–G72. [DOI] [PubMed] [Google Scholar]
- 38.Shafik A, El Sibai O, Shafik AA. Study of the duodenal contractile activity during antral contractions. World Journal of Gastroenterology: WJG. 2007;13(18):2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heddle R, Collins P, Dent J, Horowitz M, Read N, Chatterton B, et al. Motor mechanisms associated with slowing of the gastric emptying of a solid meal by an intraduodenal lipid infusion. Journal of gastroenterology and hepatology. 1989;4(5):437–47. [DOI] [PubMed] [Google Scholar]
- 40.Cooke AR. Localization of receptors inhibiting gastric emptying in the gut. Gastroenterology. 1977;72(5):875–80. [Google Scholar]
- 41.Barker G, Cochrane GM, Corbett G, Hunt J, Roberts SK. Actions of glucose and potassium chloride on osmoreceptors slowing gastric emptying. The Journal of physiology. 1974;237(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunt J, Smith J, Jiang C. Effect of meal volume and energy density on the gastric emptying of carbohydrates. Gastroenterology. 1985;89(6):1326–30. [DOI] [PubMed] [Google Scholar]
- 43.Brown G, Duncanson K, Eslick GD, Jones MP, Walker MM, Keely S, et al. Fr280Gastroduodenal eosinophilia and mast cells in functional gastrointestinal diseases (functional dyspepsia and irritable bowel syndrome): A Meta-Analysis. Gastroenterology. 2021;160(6):S-282. [Google Scholar]
- 44.Giancola F, Volta U, Repossi R, Latorre R, Beeckmans D, Carbone F, et al. Mast cell-nerve interactions correlate with bloating and abdominal pain severity in patients with non-celiac gluten / wheat sensitivity. Neurogastroenterology & Motility. 2020;32(6):e13814. [DOI] [PubMed] [Google Scholar]
- 45.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nature immunology. 2001;2(4):353–60. [DOI] [PubMed] [Google Scholar]
- 46.Locke III GR, Schleck CD, Zinsmeister AR, Talley NJ. Do distinct dyspepsia subgroups exist in the community? A population-based study. American Journal of Gastroenterology. 2007;102(9):1983–9. [DOI] [PubMed] [Google Scholar]
- 47.Zagari RM, Law GR, Fuccio L, Cennamo V, Gilthorpe MS, Forman D, et al. Epidemiology of functional dyspepsia and subgroups in the Italian general population: an endoscopic study. Gastroenterology. 2010;138(4):1302–11. [DOI] [PubMed] [Google Scholar]
- 48.Aro P, Talley NJ, Ronkainen J, Storskrubb T, Vieth M, Johansson SE, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137(1):94–100. [DOI] [PubMed] [Google Scholar]
- 49.Perveen I, Rahman MM, Saha M, Rahman MM, Hasan MQ. Prevalence of irritable bowel syndrome and functional dyspepsia, overlapping symptoms, and associated factors in a general population of Bangladesh. Indian Journal of Gastroenterology. 2014;33(3):265–73. [DOI] [PubMed] [Google Scholar]
- 50.Piessevaux H, De Winter B, Louis E, Muls V, De Looze D, Pelckmans P, et al. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterology & Motility. 2009;21(4):378–88. [DOI] [PubMed] [Google Scholar]
- 51.Min B-H, Huh KC, Jung H-K, Yoon YH, Choi KD, Song KH, et al. Prevalence of uninvestigated dyspepsia and gastroesophageal reflux disease in Korea: a population-based study using the Rome III criteria. Digestive diseases and sciences. 2014;59(11):2721–9. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzaki J, Suzuki H, Asakura K, Fukushima Y, Inadomi J, Takebayashi T, et al. Classification of functional dyspepsia based on concomitant bowel symptoms. Neurogastroenterology & Motility. 2012;24(4):325–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mak A, Wu J, Chan Y, Chan F, Sung J, Lee S. Dyspepsia is strongly associated with major depression and generalised anxiety disorder-a community study. Alimentary pharmacology & therapeutics. 2012;36(8):800–10. [DOI] [PubMed] [Google Scholar]
- 54.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dillman DA. Mail and telephone surveys: The total design method: Wiley; New York; 1978. [Google Scholar]
- 57.Talley NJ, Phillips SF, Melton J 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. [DOI] [PubMed] [Google Scholar]
- 58.Talley N, Phillips S, Wiltgen C, Zinsmeister A, Melton LJ. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. [DOI] [PubMed] [Google Scholar]
- 59.Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Do distinct dyspepsia subgroups exist in the community? A population-based study. American Journal of Gastroenterology. 2007;102(9):1983–9. [DOI] [PubMed] [Google Scholar]
- 60.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology. 2016;150(6):1262–79.e2. [DOI] [PubMed] [Google Scholar]
- 61.Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel Disorders. Gastroenterology. 2016;150(6):1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 62.Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7:247–57. [DOI] [PubMed] [Google Scholar]
- 63.Crawford JR, Henry JD, Crombie C, Taylor EP. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40:429–34. [DOI] [PubMed] [Google Scholar]
- 64.Boyce P, Gilchrist J, Talley NJ, Rose D. Cognitive-behaviour therapy as a treatment for irritable bowel syndrome: a pilot study. Aust N Z J Psychiatry. 2000;34:300–9. [DOI] [PubMed] [Google Scholar]
- 65.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 66.Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.(CDC) CfDC. National Health and Nutritional Examination Survey 2016. [Google Scholar]
- 68.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada J-R, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–79. [DOI] [PubMed] [Google Scholar]
- 69.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Official journal of the American College of Gastroenterology| ACG. 2013;108(5):679–92. [DOI] [PubMed] [Google Scholar]
- 70.Chial HJ, Camilleri M, Williams DE, Litzinger K, Perrault J. Rumination syndrome in children and adolescents: diagnosis, treatment, and prognosis. Pediatrics. 2003;111(1):158–62. [DOI] [PubMed] [Google Scholar]
- 71.O’Brien MD, Bruce BK, Camilleri M. The rumination syndrome: clinical features rather than manometric diagnosis. Gastroenterology. 1995;108(4):1024–9. [DOI] [PubMed] [Google Scholar]
- 72.Parry-Jones B. Merycism or rumination disorder. The British Journal of Psychiatry. 1994;165(3):303–14. [DOI] [PubMed] [Google Scholar]
- 73.Malik R, Srivastava A, Yachha SK, Poddar U. Chronic vomiting in children: A prospective study reveals rumination syndrome is an important etiology that is underdiagnosed and untreated. Indian Journal of Gastroenterology. 2020;39:196–203. [DOI] [PubMed] [Google Scholar]
- 74.Nakagawa K, Sawada A, Hoshikawa Y, Nikaki K, Sonmez S, Woodland P, et al. Persistent Postprandial Regurgitation vs Rumination in Patients With Refractory Gastroesophageal Reflux Disease Symptoms: Identification of a Distinct Rumination Pattern Using Ambulatory Impedance-pH Monitoring. Am J Gastroenterol. 2019;114(8):1248–55. [DOI] [PubMed] [Google Scholar]
- 75.Almansa C, Rey E, Sanchez RG, Sanchez AA, Diaz-Rubio M. Prevalence of functional gastrointestinal disorders in patients with fibromyalgia and the role of psychologic distress. Clin Gastroenterol Hepatol. 2009;7(4):438–45. [DOI] [PubMed] [Google Scholar]
- 76.Vijayvargiya P, Iturrino J, Camilleri M, Shin A, Vazquez-Roque M, Katzka DA, et al. Novel Association of Rectal Evacuation Disorder and Rumination Syndrome: Diagnosis, Co-morbidities and Treatment. United European Gastroenterol J. 2014;2(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aro P, Talley NJ, Johansson S-E, Agréus L, Ronkainen J. Anxiety is linked to new-onset dyspepsia in the Swedish population: a 10-year follow-up study. Gastroenterology. 2015;148(5):928–37. [DOI] [PubMed] [Google Scholar]
- 78.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley N. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61(9):1284–90. [DOI] [PubMed] [Google Scholar]
- 79.Mearin F, Pérez-Oliveras M, Perelló A, Vinyet J, Ibañez A, Coderch J, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129(1):98–104. [DOI] [PubMed] [Google Scholar]
- 80.Jones MP, Walker MM, Holtmann GJ, Koloski NA, Shah A, Talley NJ. Fr071 ANTIBIOTICS FREQUENTLY PRECEDE FIRST DIAGNOSIS OF FUNCTIONAL GASTROINTESTINAL DISORDERS. Gastroenterology. 2021;160(6):S-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones MP, Walker MM, Holtmann GJ, Koloski NA, Shah A, Talley NJ. Fr074 OVERLAP BETWEEN HEARTBURN, FUNCTIONAL DYSPEPSIA AND IRRITABLE BOWEL SYNDROME, OCCURS MORE THAN CHANCE AND IMPACTS ON PSYCHOLOGICAL WELLBEING. Gastroenterology. 2021;160(6):S-208. [Google Scholar]
- 82.Birmingham C, Firoz T. Rumination in eating disorders: literature review. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity. 2006;11(3):e85–e9. [DOI] [PubMed] [Google Scholar]
- 83.DeLay K, Pandolfino JE, Roman S, Gyawali CP, Savarino E, Tye M, et al. Diagnostic yield and reliability of post-prandial high-resolution manometry and impedance-ph for detecting rumination and supragastric belching in PPI non-responders. Neurogastroenterology & Motility. 2021:e14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson W. Functional bowel disorders and functional abdominal pain. Gastroenterol Int. 1992;5:75–91. [Google Scholar]
- 85.Thompson W. Fuctional bowel disorders and functional abdominal pain. Gut. 1999;45:1143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ford AC, Talley NJ, Walker MM, Jones MP. Increased prevalence of autoimmune diseases in functional gastrointestinal disorders: case–control study of 23 471 primary care patients. Alimentary pharmacology & therapeutics. 2014;40(7):827–34. [DOI] [PubMed] [Google Scholar]
- 87.Jones MP, Walker MM, Ford AC, Talley NJ. The overlap of atopy and functional gastrointestinal disorders among 23 471 patients in primary care. Alimentary pharmacology & therapeutics. 2014;40(4):382–91. [DOI] [PubMed] [Google Scholar]
- 88.Talley NJ, Walker MM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clinical Gastroenterology and Hepatology. 2007;5(10):1175–83. [DOI] [PubMed] [Google Scholar]
- 89.Olbrich CL, Bivas-Benita M, Xenakis JJ, Maldonado S, Cornwell E, Fink J, et al. Remote allergen exposure elicits eosinophil infiltration into allergen nonexposed mucosal organs and primes for allergic inflammation. Mucosal immunology. 2020;13(5):777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Potter MD, Jones MP, Walker MM, Koloski NA, Keely S, Holtmann G, et al. Incidence and prevalence of self-reported non-coeliac wheat sensitivity and gluten avoidance in Australia. Medical Journal of Australia. 2020;212(3):126–31. [DOI] [PubMed] [Google Scholar]
- 91.Duncanson KR, Talley NJ, Walker MM, Burrows TL. Food and functional dyspepsia: a systematic review. Journal of human nutrition and dietetics. 2018;31(3):390–407. [DOI] [PubMed] [Google Scholar]
- 92.Staudacher HM, Nevin AN, Duff C, Kendall BJ, Holtmann GJ. Epigastric symptom response to low FODMAP dietary advice compared with standard dietetic advice in individuals with functional dyspepsia. Neurogastroenterology & Motility. 2021:e14148. [DOI] [PubMed] [Google Scholar]
- 93.Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. American Journal of Gastroenterology. 2008;103(10):2613–23. [DOI] [PubMed] [Google Scholar]
- 94.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. Journal of gastroenterology and hepatology. 2010;25(8):1366–73. [DOI] [PubMed] [Google Scholar]
- 95.Singh P, Grabauskas G, Zhou S-Y, Zhang Y, Owyang C. 377 MAST-CELL ACTIVATION LEADING TO COLONIC BARRIER DYSFUNCTION FOLLOWING HIGH-FODMAP DIET IS MEDIATED VIA LIPOPOLYSACCHARIDE. Gastroenterology. 2021;160(6):S-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pryor J, Burns GL, Duncanson K, Horvat JC, Walker MM, Talley NJ, et al. Functional Dyspepsia and Food: Immune Overlap with Food Sensitivity Disorders. Current gastroenterology reports. 2020;22(10):1–10. [DOI] [PubMed] [Google Scholar]
- 97.Duncanson K, Burns G, Pryor J, Keely S, Talley NJ. Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia. Nutrients. 2021;13(4):1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fritscher-Ravens A, Pflaum T, Mösinger M, Ruchay Z, Röcken C, Milla PJ, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology. 2019;157(1):109–18. e5. [DOI] [PubMed] [Google Scholar]
- 99.Simon D, Cianferoni A, Spergel J, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71(5):611–20. [DOI] [PubMed] [Google Scholar]
- 100.Peterson KA, Genta RM, Rasmussen HS, Youngblood B, Kamboj AP. Fr198 GASTRODUODENAL EOSINOPHILIA IS UNDER-APPRECIATED IN EOSINOPHILIC ESOPHAGITIS (EOE) PATIENTS WITH FUNCTIONAL BOWEL SYMPTOMS: A REAL LIFE EXPERIENCE. Gastroenterology. 2021;160(6):S-262. [Google Scholar]
- 101.Rodrigues DM, Motomura DI, Tripp DA, Beyak MJ. Are psychological interventions effective in treating functional dyspepsia: a systematic review and meta-analysis. Journal of Gastroenterology and Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 102.Murray H, Juarascio A, Call C, Hunt R, Keshishian A, Thomas J, editors. Feasibility, acceptability, and preliminary efficacy of cognitive-behavioral therapy for rumination disorder (CBT-RD). International Conference on Eating Disorders, Chicago, IL; 2019. [Google Scholar]
- 103.Barba E, Accarino A, Soldevilla A, Malagelada J-R, Azpiroz F. Randomized, placebo-controlled trial of biofeedback for the treatment of rumination. Official journal of the American College of Gastroenterology| ACG. 2016;111(7):1007–13. [DOI] [PubMed] [Google Scholar]
- 104.Pauwels A, Broers C, Van Houtte B, Rommel N, Vanuytsel T, Tack J. A randomized double-blind, placebo-controlled, cross-over study using baclofen in the treatment of rumination syndrome. Official journal of the American College of Gastroenterology| ACG. 2018;113(1):97–104. [DOI] [PubMed] [Google Scholar]
- 105.Blondeau K, Boecxstaens V, Rommel N, Farré R, Depeyper S, Holvoet L, et al. Baclofen improves symptoms and reduces postprandial flow events in patients with rumination and supragastric belching. Clinical Gastroenterology and Hepatology. 2012;10(4):379–84. [DOI] [PubMed] [Google Scholar]
- 106.Pinto-Sanchez MI, Yuan Y, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database of Systematic Reviews. 2017(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wauters L, Ceulemans M, Frings D, Lambaerts M, Accarie A, Toth J, et al. Proton pump inhibitors reduce duodenal eosinophilia, mast cells, and permeability in patients with functional dyspepsia. Gastroenterology. 2021;160(5):1521–31. e9. [DOI] [PubMed] [Google Scholar]
- 108.Ford AC, Moayyedi P, Black CJ, Yuan Y, Veettil SK, Mahadeva S, et al. Systematic review and network meta-analysis: efficacy of drugs for functional dyspepsia. Alimentary pharmacology & therapeutics. 2021;53(1):8–21. [DOI] [PubMed] [Google Scholar]
- 109.Zhou W, Li X, Huang Y, Xu X, Liu Y, Wang J, et al. Comparative efficacy and acceptability of psychotropic drugs for functional dyspepsia in adults: A systematic review and network meta-analysis. Medicine. 2021;100(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J, Wu HM, Wang X, Xie J, Li X, Ma J, et al. Efficacy of prebiotics and probiotics for functional dyspepsia: a systematic review and meta-analysis. Medicine. 2020;99(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rajindrajith S, Devanarayana NM, Perera BJC. Rumination syndrome in children and adolescents: a school survey assessing prevalence and symptomatology. BMC gastroenterology. 2012;12(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]