Abstract
Chimeric antigen receptor (CAR)-T cell therapy is an innovative approach to immune cell therapy that works by modifying the T cells of a patient to express the CAR protein on their surface, and thus induce their recognition and destruction of cancer cells. CAR-T cell therapy has shown some success in treating hematological tumors, but it still faces a number of challenges in the treatment of solid tumors, such as antigen selection, tolerability and safety. In response to these issues, studies continue to improve the design of CAR-T cells in pursuit of improved therapeutic efficacy and safety. In the future, CAR-T cell therapy is expected to become an important cancer treatment, and may provide new ideas and strategies for individualized immunotherapy. The present review provides a comprehensive overview of the principles, clinical applications, therapeutic efficacy and challenges of CAR-T cell therapy.
Keywords: chimeric antigen receptor T cell therapy, immunotherapy, malignant tumors, adverse effects
1. Introduction
Cancer is a major challenge to human health worldwide, and, while making some progress, traditional cancer treatments, such as chemotherapy, radiotherapy and surgery, often have a series of limitations and side effects (1). However, in recent years, chimeric antigen receptor (CAR)-T cell therapy, which is also known as the ‘living drug’, has emerged (2).
CAR-T cell therapy has garnered interest in the field of cancer treatment as a personalized cancer immunotherapy strategy (2,3). It works by altering the immune system of a patient, allowing it to recognize, attack and remove cancer cells (4). Among the immune system, CAR-T cells are a special subpopulation of T cells that are genetically engineered to express specific antigen receptors, and to effectively recognize and destroy cancer cells (5). However, this therapy also faces multifaceted challenges, such as antigen selection, treatment tolerance and safety (6,7). Tumor cells lacking specific antigens or displaying heterogeneity in antigen expression can impair the antigen selectivity of CAR-T cells (8). Moreover, tumor cells can develop resistance by downregulating antigen expression and enhancing the activity of immune inhibitory factors in response to CAR-T cell-induced cytotoxicity (9). Additionally, cytokine release syndrome (CRS) induced by CAR-T cell therapy, which manifests as fever and difficulty breathing, low blood pressure, nausea and vomiting, poses a notable safety challenge. Currently, progress has been made in addressing the aforementioned issues by examining multiple antigen targets, improving the design of CAR-T cells, adjusting drug dosages and enhancing the activity of CAR-T cells. However, these measures have not completely eliminated the challenges (9). Further research and efforts are required to solve these problems, and to improve the efficacy and safety of CAR-T cell therapy (10). It is hypothesized that with the continuous progression of science and technology, CAR-T cell therapy will serve an important role in the future and bring a revolutionary change in individualized cancer treatment.
Currently, the majority of review articles primarily focus on the side effects of CAR-T cell therapy, targeted therapies for solid tumors, current limitations and novel structural designs of CAR-T, providing a detailed and in-depth analysis and commentary on these aspects. However, there is a lack of comprehensive description of CAR-T cell therapy as a whole (4,11–13). Therefore, by summarizing the recent literature on CAR-T cell therapy, the present review provides a more comprehensive overview of the latest research status of CAR-T cell therapy in terms of the basic structure of CAR-T cells, the tumor-killing mechanism, clinical treatment steps, an overview of the current stage of clinical use and overview of marketed drugs, with an aim to assist researchers in quickly and comprehensively understanding the latest advancements in this field.
2. CAR-T cell structure
CAR-T cells are genetically modified T cells that express the CAR protein on their surface (14). The CAR protein is composed of an external recognition region and an internal signaling region (6). The external recognition region usually consists of a single-chain antibody (scFv) or antigen-binding domain that recognizes and binds to specific antigens on the surface of the target cancer cells (15). This recognition region can be genetically engineered to ensure that it binds the target antigen efficiently (16). The internal signaling region typically includes the signaling molecules and signaling modules required to activate T cells (6). When the CAR binds to the target antigen, the internal signaling region initiates signaling that prompts antigen-specific activation and proliferation of the CAR-T cell (17).
One of the specific components of CAR-T cells, the antigen recognition domain (18), which is also known as the external recognition region, is usually composed of a scFv that recognizes and binds to the target antigen (19). The scFv consists of an antigen-binding portion and structural domains connected to CD3ζ or other signaling domains (such as CD28, 4-1BB, CD19 and OX40 domains) (20). Single-chain antibodies are made up of variable regions of heavy and light chains joined together with high specificity and affinity (21). The scFv introduces the antigen to T cells by binding to it, thereby activating the antitumor effect of T cells (22). Selecting the appropriate scFv can ensure the highly specific recognition and killing ability of CAR-T cells for specific antigens (22). The transduction domain of CAR-T cells, which is also known as the internal signaling region or activation domain (23), is located inside the CAR-T cell, and helps transmit external antigen recognition signals to the inside of the cell to activate the T cell and trigger an immune response (23). The most commonly used transduction domain is the CD3ζ domain, which is involved in the signaling pathway for T-cell activation (24). The co-stimulatory domains are used to enhance the activation effect and proliferation of the CAR-T cells (25). Common co-stimulatory factors include CD28, 4-1BB (CD137) and OX40, which provide additional signaling to increase CAR-T-cell survival, function and antitumor response (26,27). In addition, to introduce the gene for CAR into T cells, it can either be introduced via a viral vector, such as a retrovirus or lentivirus, or a non-viral method, such as transfection (28).
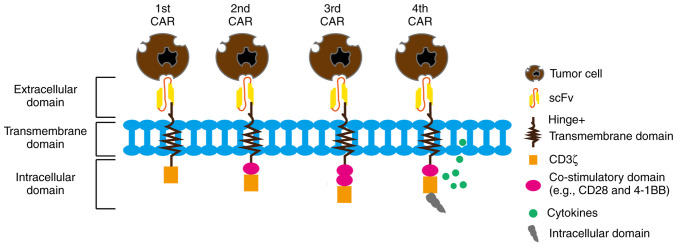
First-generation CARs typically contain an antigen recognition domain and a CD3ζ transduction domain (9). This simple structure provides only preliminary antigen recognition and T-cell activation signals (29), but has a limited effect and poor therapeutic efficacy for antigens with low-level expression and heterogeneous expression (30). To enhance the activation and persistence of CAR-T cells (31), and to improve the therapeutic efficacy, studies introduced second/third generation CARs (31,32). The second-generation CARs have the addition of one or more co-stimulatory factor domains, such as CD28 or 4-1BB, to the first generation in order to enhance T-cell activation and to improve cell proliferation and survival (31). The third-generation CARs have the addition of further co-stimulatory factor domains to the second generation (32). The fourth-generation CARs exhibit an improved CAR structure via the introduction of one or more stimulatory secretion cassettes or polyclonal antibody secretion systems (33). These additional secretion elements can secrete specific cytokines, such as IL-12 and IL-18, upon the binding of CAR-T cells to antigens, further enhancing T-cell activation and promoting the immune response and antitumor effects (34,35). To increase the initial activation state of CAR-T cells, preactivation domains, such as CD28 or CD137 preactivation domains, have been introduced in a number of CAR designs (36–38). These domains enhance the activation of CAR-T cells to a more favorable state prior to antigen binding (39). To avoid cross-reactivity with similar antigenic structures present in normal tissues, studies have begun designing CAR-T cells with narrower antigen recognition capabilities (39,40). Restricted antigen recognition domains are achieved by selecting specific fragile tumor-specific antigens or tumor-specific neoantigens to improve therapeutic efficacy and reduce adverse effects (40). These improved CAR designs aim to increase CAR-T-cell persistence, enhance cell-killing capacity and antitumor response, avoid unwanted toxicity, and improve selective and specific recognition (41). Furthermore, the improved designs can enhance cell proliferation and survival signaling, attenuate activation-induced inhibitory signaling and promote memory T-cell formation (Fig. 1) (42).
Figure 1.
CAR structures include an extracellular antigen binding domain, a hinge region, a transmembrane domain and one or more intracellular signaling domains. The first-generation CAR consists of a CD3ζ signaling domain. Based on the understanding of the importance of co-stimulatory domains for durable therapy, the second-generation CAR was developed with an additional co-stimulatory domain linked to the CD3ζ intracellular signaling domain. The third-generation CAR includes two co-stimulatory domains linked to the CD3ζ signaling domain. The fourth-generation CAR introduces an additional intracellular domain that co-expresses certain small molecules (such as IL-12, IL-18 and programmed cell death protein 1), which can trigger cytokine-induced signaling or block signaling pathways that affect CAR-T cell function, aiming to improve therapeutic effects. CAR, chimeric antigen receptor; scFv, single-chain antibody.
3. Targeted killing mechanism of CAR-T cells
The antigen used for CAR-T cells is a primary design consideration (43). Typically, CAR-T cell therapies target tumor-specific antigens or tumor-associated antigens that are upregulated on the surface of cancer cells or only on tumor cells (44). The CAR-T cell recognizes and binds to the target antigen, which is usually a specific protein or glycoprotein upregulated on the surface of cancer cells (2), through a single-chain variant antibody (scFv) on the CAR protein. The scFv is able to bind tightly to the target antigen, enabling specific recognition by the CAR-T cell (45). Once CAR-T cells recognize the target antigen, signaling domains within the CAR, such as CD3ζ, will be activated, triggering an intracellular signaling cascade (46). This process is similar to the activation of a normal T-cell receptor upon binding to an antigen (47). The activated CAR-T cells then target tumor cells expressing the target antigen for killing through a variety of mechanisms, including: i) Direct cytotoxin release where activated CAR-T cells release cytotoxins, such as perforin and targeting enzymes, which directly lead to tumor cell lysis and apoptosis (18); ii) cytokine release where activated CAR-T cells secrete cytokines, such as interferon γ and tumor necrosis factor α, to further stimulate immune cell activation and inflammatory response (48,49); and iii) immune cell alliance where activated CAR-T cells can activate and recruit other immune cells, such as natural killer (NK) cells and macrophages, to form an immune cell alliance to jointly attack tumor cells (5,50).
4. CAR-T cell therapy treatment process
First, doctors screen patients to determine if the patients are eligible to receive CAR-T cell therapy (51). This typically includes evaluating the disease type, stage of disease, physical health and immune system status of the patient (52). The peripheral blood of the patient is collected, and the T cells are isolated using centrifugation and immunomagnetic bead assay. In the laboratory, the T cells are genetically modified to introduce the CAR gene, which enables the T cells to recognize and attack specific tumor cells. The modified T cells are expanded and cultured in vitro to increase their number, which allows a sufficient number of CAR-T cells to be obtained for use in therapy (53). Before a patient receives CAR-T cell therapy, they may need to undergo treatment preparation, such as lymphodepletion and bridging therapy. Lymphodepletion is the conditioning treatment required for CAR-T cell therapy and the goal of this therapy is to reduce competing cell populations (including normal T cells, natural killer cells and macrophages) in the patient and increase the survival and therapeutic efficacy of the CAR-T cells (54). The number of immune cells in the body is reduced using chemotherapy drugs or radiation therapy. However, this approach has certain side effects, including temporarily weakening the immune response of the patient and increasing the risk of infection. Therefore, it is necessary to administer antibiotics to prevent bacterial infections and to monitor and treat any potential signs of infection in the patient (53). Bridging therapy often refers to the use of other treatments such as chemotherapy, targeted therapy or radiation therapy before CAR-T cell therapy, in order to control tumor progression or provide temporary therapeutic effects. The purpose of bridge therapy is to buy time for patients while waiting for the preparation and production of CAR-T cell therapy (54). Once the CAR-T cells are expanded to a sufficient number and the patient is prepared for treatment, the doctor injects the CAR-T cells into the patient through an intravenous infusion (55). Patients are closely observed and monitored after receiving CAR-T cell therapy; this includes monitoring for adverse reactions, evaluation of tumor response and monitoring CAR-T cell activity and survival in the body (55).
During the CAR-T therapy process, there are also a number of challenges in collecting T cells from the patient, and the key to manufacturing CAR-T cells lies in appropriate T cell collection and engineering. However, there may be manufacturing failures due to poor sample quality, low cell quantity or inadequate cell yield, and for certain patients with cancer, the suppression of their own immune system can lead to a reduction in the quantity or to an impairment of the function of the T cells in the patient (56). During the process of cell collection, external contamination from microorganisms, bacteria, viruses and other contaminants may occur. These contaminants can have a negative impact on the survival and function of CAR-T cells, leading to a decrease in their quality (54). Additionally, physical and chemical damage may be inflicted on the cells during separation, culture or transportation. Such damage can result in a decline in cell function or even cell death, ultimately affecting the therapeutic efficacy of the CAR-T cells (57–59). This can lead to delays in starting treatment and increase the difficulty and uncertainty of therapy. Manufacturing CAR-T cells requires a certain amount of time to expand the cells and test their quality. Typically, patients need to undergo other forms of treatment during the waiting period, which can increase burdens, require further medical resources and incur additional costs (60).
5. Clinical utilization of CAR-T cell therapy
CAR-T cell therapy has achieved notable application results in hematological tumors (61). For example, CAR-T cells designed against the CD19 antigen have achieved therapeutic effects by targeting and killing CD19+ leukemia cells (62). In addition, CAR-T cell therapy has shown notable efficacy in the treatment of relapsed/refractory B-cell non-Hodgkin's lymphoma (B-NHL) (63), B-cell acute lymphoblastic leukemia (B-ALL) and chronic lymphocytic leukemia, with ~40–60% of patients with B-NHL obtaining durable remission and survival after receiving CAR-T cell therapy, and ~80–90% of patients with B-ALL obtaining durable remission and survival or complete remission, after receiving CAR-T cell therapy (55,64). The application of CAR-T cell therapy in the treatment of solid tumors, as opposed to hematological tumors, continues to be investigated, and despite a number of challenges and limitations, positive advances have been made (65). CAR-T cells have been designed and applied to target antigens on the cell surface of neuroblastoma cells, such as GD2 (66). Preliminary clinical trial data have shown that CAR-T cells demonstrate some therapeutic efficacy in patients with high-risk and refractory neuroblastoma (6). CAR-T cells for prostate-specific memory acid phosphatase have also demonstrated some anti-prostate cancer efficacy, and clinical trials of CAR-T cell therapy for soft-tissue sarcoma have suggested some potential (67,68). CAR-T cell therapies in solid tumors face a number of challenges, such as antigenic diversity, immune escape due to the tumor microenvironment and achieving sufficient proliferation and infiltration (32,65,69). These factors limit the application of CAR-T cell therapy in the treatment of solid tumors (65).
6. Side effects and clinical challenges of CAR-T cell therapy
Although CAR-T cell therapy has shown notable efficacy in the treatment of hematological and solid tumors, it has also brought about a number of treatment-associated side effects and safety issues, and still faces a number of challenges in clinical application that may limit its widespread use (65).
CRS is one of the most common side effects associated with CAR-T cell therapy with an incidence of 20–50% (70). When CAR-T cells kill tumor cells, they release large amounts of cytokines, leading to the overactivation of the immune system and systemic inflammatory response (71,72). Mild CRS may manifest as symptoms such as fever, chills and headache, while severe CRS may lead to life-threatening conditions, such as hypotension, respiratory distress and organ insufficiency (73). In addition, CAR-T cells exhibit persistent cell proliferation, which can lead to organ function impairment, and CRS may also cause anemia, thrombocytopenia and leukopenia, which can induce spontaneous bleeding and increase the risk of infection (74). The release of cytokines can lead to a reduction in the number of lymphocytes and other immune cells in the immune system (53). This lymphodepletion results in an overall decrease in immune cells, including T cells, B cells and NK cells, leading to cytopenia (75). Additionally, the depletion of precursor cells in the hematopoietic system can reduce the production of mature red blood cells, white blood cells and platelets (75). Headache, coma and neurological dysfunction are also possible side effects (76). Furthermore, CAR-T cells may have on-target off-tumor effects when CAR-T cells attack non-tumor cells expressing the target antigen, but cells that do not express this antigen are spared (71). Non-specific effects of CAR-T cell therapy are likely due to inflammatory responses that can be activated by CAR-T cell therapy (26).
The efficacy of CAR-T cell therapy is limited by the selection and heterogeneity of target tumor antigens (77). Antigen expression varies between tumor types and patients, and a number of tumors may even lack specific antigens (78). In addition, intratumor heterogeneity can make it more difficult for CAR-T cells to recognize and attack antigens (79). Although CAR-T cell therapy has resulted in long-term remission and survival in a number of patients, not all patients will consistently benefit from this type of therapy (80). Patients may experience relapse or drug resistance, resulting in a less durable efficacy after treatment (81). Solid tumors typically have a complex tumor microenvironment, including the production of immunosuppressive factors (such as cytokines) and the interaction of tumor cells with other cells (such as macrophages, regulatory T cells and myeloid suppressor cells), so that the tumors may suppress the activity of CAR-T cells 32). This makes the infiltration and killing ability of CAR-T cells in solid tumors limited, with a higher chance of immune escape (42).
CAR-T cell therapy faces challenges in dealing with tonic signaling, antigen loss and low antigen density (82). Tonic signaling refers to the state where CAR-T cells remain active and release cytokines even in the absence of normal stimulation, potentially leading to cytotoxicity and unnecessary inflammation (83). Antigen loss occurs when tumor cells lose the antigens originally targeted by CAR-T cells, rendering the CAR-T cells unable to effectively kill these tumor cells, resulting in treatment failure or relapse (84). Low antigen density is also a notable challenge, as it means that there are fewer antigens on the surface of target tumor cells. This low antigen density may prevent CAR-T cells from accurately recognizing and attacking the target cells, thereby reducing treatment efficacy (85). It has been revealed that by improving the design of CAR-T cells and introducing switchable activation technology, they can remain silent or regulate their activity when lacking stimulation (22). To address the issues of antigen loss and low antigen density, multi-antigen-targeting CAR-T cells are being designed to simultaneously attack multiple antigens, reducing the impact of losing a single antigen (82). Additionally, co-stimulatory molecules are utilized to enhance CAR-T cell recognition and cytotoxic activity against tumor cells (84). The phenotype and functional characteristics of CAR-T cells is also affected by the selection of the co-stimulatory domain (26). Through pre-clinical investigations, the incorporation of a BBζ co-stimulatory domain has been shown to preserve a greater frequency of central memory CAR-T cells when compared with CD28ζ-containing CAR-T cells, which were enriched for effector memory phenotype cells (25,86). However, this mainly applies to the second and third generation CAR-T structure design. In comparison with terminally differentiated effector cells, central memory CAR-T cells that have been enriched with the BBζ co-stimulatory structural domain are less differentiated and can give rise to daughter cells that perform cytotoxic functions, whilst continuing to replenish the population of memory cells for a longer response duration, thus eliciting improved tumor control (86). Activation of the BB pathway has been shown to promote T cell proliferation through the regulation of cyclin-dependent kinases and to sustain the survival of activated T cells. In trials involving patients with B cell lymphoma subsets and chronic lymphocytic leukemia, the median progression free survival of patients treated with CD28ζ CAR-T cell products has in a number of cases spanned from 8–36 months (84). Furthermore, there is strong evidence in support of both CD28ζ and BBζ CAR-T cells, with subsets of treated patients remaining in ongoing remission 7–10 years post-treatment (25,87). Analogous to investigations into BBζ CAR-T cells, the addition of TNF-R superfamily molecules, such as CD27 and OX40, in CAR design has led to improved antigen-dependent memory formation and enhanced T cell survival (88).
7. CAR-T cell therapy representative drugs
Tisagenlecleucel (89) (Novartis International AG) and axicabtagene ciloleucel (89) (Kite Pharma; Gilead Sciences, Inc.) have been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency. They are used for the treatment of relapsed/refractory B-NHL in adults (90). In addition, Tecartus™ (91) is a CAR-T cell therapy drug, developed by Gilead Sciences, Inc., for the treatment of relapsed/refractory B-ALL in adults (Table I). Bristol-Myers Squibb Company developed Breyanzi (lisocabtagene maraleucel) for the treatment of adult relapsed/refractory large B-cell lymphoma (91). There are also a number of CAR-T drugs in clinical trials including bb2121, which is a CAR-T cell therapy that targets the B-cell maturation antigen (BCMA) that has been used for the treatment of multiple myeloma (92,93). Furthermore, CD22-CAR CAR-T cell therapies, in which CD22 is the target antigen are used for the treatment of B-ALL, HER2 CAR-T cell therapy for metastatic colorectal cancer and IL13Rα2/EGFRvIII CAR-T cell therapy for glioblastoma are all in clinical trials (94–97).
Table I.
Selected CAR-T drugs targeting CD19.
| Drug name | Company | Indications | Objective response rate, % | Time to market |
|---|---|---|---|---|
| Axicabtagene ciloleucel | Kite Pharma; Gilead Sciences, Inc. | DLBCL | 72 | January 2017 |
| FL | 91 | March 2021 | ||
| Tisagenlecleucel | Novartis International AG | ALL | 81 | August 2017 |
| DLBCL | 52 | May 2018 | ||
| Tecartus | Gilead Sciences, Inc. | MCL | 87 | July 2020 |
| Breyanzi | Bristol-Myers Squibb Company | DLBCL | 73 | February 2021 |
DLCBL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; ALL, anaplastic large cell lymphoma.
Anti-BCMA CAR-T cell therapy is a targeted treatment approach for multiple myeloma, a cancer of the plasma cells in bone marrow (98). BCMA is an antigen expressed on the surface of multiple myeloma cells and is considered a critical therapeutic target for the disease (99). The therapy uses genetic engineering techniques to modify the T cells of a patient to express CARs that recognize and attack the BCMA (100). Currently, two anti-BCMA CAR-T cell therapies (ide-cel and cilta-cel) have been approved by the FDA for clinical treatment and have shown significant efficacy in treating multiple myeloma (100).
The dosage and fractionation of CAR-T drugs are crucial factors that can influence key aspects such as drug efficacy and safety (98). The dosage of CAT-T drugs is generally personalized based on factors such as the weight and physical condition of the patient (98). Studies have revealed that anti-CD19 CAR-T cells achieved optimal clinical efficacy at a dose of 50–100 million cells/kg body weight, while anti-BCMA CAR-T cells demonstrated optimal efficacy at a dose >100 million cells/kg body weight, within a certain dose range (100,101). Increasing the dose may lead to an increase in objective response rates (ORRs) until a threshold is reached (98). However, when the ORR begins to stabilize, further dose escalation is unlikely to improve the ORR, but it may increase the incidence and/or severity of adverse events associated with the mechanism (98). Excessive dosage can potentially induce intense immune reactions and severe side effects, while inadequate dosage may result in poor therapeutic outcomes (99). However, in a logistic regression analysis concerning B-ALL, a higher CAR T-cell dose was associated with a higher probability of response, there was no increase in CRS incidence or severity across dose ranges and patients achieved comparable early response rates independently of dose, but, increasing the dose of CAR-T cells may lead to an increased risk of CRS or neurotoxicity, which is a common concern (100). Further research is warranted to elucidate the association between threshold dosing and post-CAR outcomes (100). Therefore, a comprehensive evaluation and personalized dosage adjustments are necessary to achieve optimal treatment effects. Additionally, dose fractionation is an important strategy for CAT-T drugs (100). Research has indicated that treatment efficacy is not adversely affected by dose fractionation. It has been suggested that, instead of a single dose infusion, dose fractions of CAR-T cells administered over 2–3 days may decrease the incidence and/or severity of CAR-T cell toxicity including CRS and neurotoxicity, especially in patients with a high tumor burden and for patients that require CAR-T cell therapy in higher doses for efficacy (101). The effects generated by a slow and continuous administration of drug often exhibit longer-lasting and more stable outcomes compared with a single high dose (101). This approach involves dividing the drug into several equal parts and administering them gradually over different time periods with the aim to enhance treatment efficacy and reduce side effects (100). Dose stratification of CAR T-cell treatment based on specific product characteristics or disease burden, the use of phase I trial designs that incorporate efficacy or pharmacokinetic data, and the development of CAR T-cells with decreased potential for toxicity, could all aid clinicians and researchers to optimize CAR T-cell dosing, expand the therapeutic window and improve the availability of this emerging cancer immunotherapy (99). These strategies could also minimize drug toxicity and resistance as well as reduce the frequency of adverse reactions during treatment.
8. Future development of CAR-T cell therapy
At present, CAR-T cell therapy is mainly applied in the treatment of certain hematological tumors. However, the future development direction aims to expand the range of applications, improve the therapeutic effects, reduce the serious side effects and lower the cost of treatment (69). Studies are currently working on further improvement measures to advance the effectiveness and safety of CAR-T cell therapy (77,102). Potential measures include: i) Introducing an adjustable switching system in order to start or stop the activity of CAR-T cells in a timely manner to mitigate the occurrence of adverse reactions (103); ii) exploring the use of multiple CAR structures to recognize multiple antigens (104) or the use of bispecific CARs to recognize two antigens at the same time to overcome immune escape (105); iii) and utilizing gene editing technology to precisely genetically modify CAR-T cells in order to enhance their cellular activation, viability and antitumor effects to mitigate antitumor immune escape mechanisms (106). Switching to CAR-NK cells, in addition to T cells, may also be beneficial, as there is a class of NK cells that also has antitumor effects (107). NK cells are one of the most important cells in the immune system; CAR-NK cells, also known as enhanced NK cells, can activate NK cells by breaking through the limitations of killer immunoglobulin-like receptors, which are a class of receptors expressed on the surface of NK cells that bind to HLA-C-like molecules, thereby inhibiting NK cell activity, in order to enhance the specific killing effect of NK cells on tumor cells (108). Several factors in the tumor microenvironment, such as immunosuppressive cells, cytokines and infiltrating cells, may affect the function and effectiveness of CAR-T cells (109). Therefore, studies are aiming to enhance the survival and antitumor effects of CAR-T cells in the tumor microenvironment using specific molecular targeting strategies, such as receptors or antibodies on the surface of CAR-T cells (9,102). These further improvements and strategies are all aimed at further enhancing the efficacy, durability and safety of CAR-T cell therapy. With in-depth research on CAR-T cells and continuous technological innovations, it is expected that CAR-T cell therapy will serve a greater role in the field of cancer treatment in the future.
9. Summary and outlook
CAR-T cell therapy is a revolutionary immunotherapy that has achieved notable success in treating a number of B cell-associated malignancies. By targeting specific antigens on the surface of tumors, CAR-T cells are able to identify and destroy malignant cells, providing a new treatment option for those patients for whom conventional therapies have failed. However, CAR-T cell therapy still faces a number of challenges and limitations. Serious adverse reactions, such as CRS and neurotoxicity, may occur during treatment. By contrast, immune escape mechanisms and suppression of the tumor microenvironment may limit the effectiveness and durability of CAR-T cells. Therefore, further improving the safety, specificity and durability of CAR-T cell therapies is one of the current research priorities. In the future, further development and application of CAR-T cell therapy is expected. Firstly, the design and construction of CAR-T cells will be continuously optimized, such as the introduction of adjustable switch systems, bispecific CARs and genetically modified CAR-T cells. Secondly, CAR-T cell therapy may be expanded to a wider range of diseases, such as other types of cancer, autoimmune diseases and infectious diseases. With the continuous research on CAR-T cell therapy and technological advancement, the authors of the present review are confident that CAR-T cell therapy will continue to make progress in the future and serve an even greater role in the field of cancer treatment and immune disease therapy.
Acknowledgements
Not applicable.
Funding Statement
The present review was funded by The Public Welfare Research Project (Qianjiang, China; grant no. 2023GYX002).
Availability of data and materials
Not applicable.
Authors' contributions
DS, XS, XY, SL and XW designed the theme of the review; DS, XS, SL, XW, XY and MW retrieved the relevant literature; and DS, XS, XY and MW wrote and reviewed the article. Data authentication is not applicable. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019. doi: 10.1016/j.ctrv.2020.102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu W, Zhou Q, Masubuchi T, Shi X, Li H, Xu X, Huang M, Meng L, He X, Zhu H, et al. Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy. Cell. 2020;182:855–871. e23. doi: 10.1016/j.cell.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, Haris M, Wilson NE, Liu F, Gabunia K, et al. Single-Cell analyses identify brain mural cells expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell. 2020;183:126–142. e17. doi: 10.1016/j.cell.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterner RC, Sterner RM. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: From CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41:119. doi: 10.1186/s13046-022-02327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad A. CAR-T Cell Therapy. Int J Mol Sci. 2020;21:4303. doi: 10.3390/ijms21124303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94((S1)):S3–S9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 8.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 9.Abreu TR, Fonseca NA, Gonçalves N, Moreira JN. Current challenges and emerging opportunities of CAR-T cell therapies. J Control Release. 2020;319:246–261. doi: 10.1016/j.jconrel.2019.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Labanieh L, Majzner RG, Klysz D, Sotillo E, Fisher CJ, Vilches-Moure JG, Pacheco KZB, Malipatlolla M, Xu P, Hui JH, et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell. 2022;185:1745–1763. e22. doi: 10.1016/j.cell.2022.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, Abou-El-Enein M. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20:49–62. doi: 10.1038/s41571-022-00704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chohan KL, Siegler EL, Kenderian SS. CAR-T Cell Therapy: The efficacy and toxicity balance. Curr Hematol Malig Rep. 2023;18:9–18. doi: 10.1007/s11899-023-00687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YJ, Abila B, Mostafa Kamel Y. CAR-T: What Is Next? Cancers (Basel) 2023;15:663. doi: 10.3390/cancers15030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, Dreger P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 15.Bao C, Gao Q, Li LL, Han L, Zhang B, Ding Y, Song Z, Zhang R, Zhang J, Wu XH. The application of nanobody in CAR-T therapy. Biomolecules. 2021;11:238. doi: 10.3390/biom11020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, Myklebust JH, Kadapakkam M, Weber EW, Tousley AM, et al. Tuning the antigen density requirement for CAR T-cell Activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 18.Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S. Killing mechanisms of chimeric antigen receptor (CAR) T Cells. Int J Mol Sci. 2019;20:1283. doi: 10.3390/ijms20061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Donk NWCJ, Usmani SZ, Yong K. CAR T-cell therapy for multiple myeloma: State of the art and prospects. Lancet Haematol. 2021;8:e446–e461. doi: 10.1016/S2352-3026(21)00057-0. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava S, Riddell SR. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan Y, Chen R, Huang Y, Meng X, Chen J, Liao C, Tang Y, Zhou C, Gao X, Sun J. Tuning the ignition of CAR: optimizing the affinity of scFv to improve CAR-T therapy. Cell Mol Life Sci. 2021;79:14. doi: 10.1007/s00018-021-04089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, Good Z, Belk JA, Daniel B, Klysz D, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372:eaba1786. doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tousley AM, Rotiroti MC, Labanieh L, Rysavy LW, Kim WJ, Lareau C, Sotillo E, Weber EW, Rietberg SP, Dalton GN, et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature. 2023;615:507–516. doi: 10.1038/s41586-023-05778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honikel MM, Olejniczak SH. Co-Stimulatory receptor signaling in CAR-T Cells. Biomolecules. 2022;12:1303. doi: 10.3390/biom12091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, Gao L, Wen Q, Zhong JF, Zhang C, Zhang X. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13:86. doi: 10.1186/s13045-020-00910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh N, Frey NV, Engels B, Barrett DM, Shestova O, Ravikumar P, Cummins KD, Lee YG, Pajarillo R, Chun I, et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat Med. 2021;27:842–850. doi: 10.1038/s41591-021-01326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smole A, Benton A, Poussin MA, Eiva MA, Mezzanotte C, Camisa B, Greco B, Sharma P, Minutolo NG, Gray F, et al. Expression of inducible factors reprograms CAR-T cells for enhanced function and safety. Cancer Cell. 2022;40:1470–1487. e77. doi: 10.1016/j.ccell.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drougkas K, Karampinos K, Karavolias I, Koumprentziotis IA, Ploumaki I, Triantafyllou E, Trontzas I, Kotteas E. Comprehensive clinical evaluation of CAR-T cell immunotherapy for solid tumors: A path moving forward or a dead end? J Cancer Res Clin Oncol. 2023;149:2709–2734. doi: 10.1007/s00432-022-04547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: A paradigm shift? Blood. 2022;139:2737–2746. doi: 10.1182/blood.2022015789. [DOI] [PubMed] [Google Scholar]
- 31.Roselli E, Boucher JC, Li G, Kotani H, Spitler K, Reid K, Cervantes EV, Bulliard Y, Tu N, Lee SB, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J Immunother Cancer. 2021;9:e003354. doi: 10.1136/jitc-2021-SITC2021.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez M, Moon EK. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barros LRC, Couto SCF, da Silva Santurio D, Paixão EA, Cardoso F, da Silva VJ, Klinger P, Ribeiro PDAC, Rós FA, Oliveira TGM, et al. Systematic review of available CAR-T Cell Trials around the World. Cancers (Basel) 2022;14:2667. doi: 10.3390/cancers14112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agliardi G, Liuzzi AR, Hotblack A, De Feo D, Núñez N, Stowe CL, Friebel E, Nannini F, Rindlisbacher L, Roberts TA, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12:444. doi: 10.1038/s41467-020-20599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glienke W, Dragon AC, Zimmermann K, Martyniszyn-Eiben A, Mertens M, Abken H, Rossig C, Altvater B, Aleksandrova K, Arseniev L, et al. GMP-Compliant Manufacturing of TRUCKs: CAR T Cells targeting GD(2) and Releasing Inducible IL-18. Front Immunol. 2022;13:839783. doi: 10.3389/fimmu.2022.839783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etxeberria I, Glez-Vaz J, Teijeira Á, Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020;4((Suppl 3)):e000733. doi: 10.1136/esmoopen-2020-000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilikueta A, Etxeberria I, Bolaños E, Lang V, Rodriguez M, Aznar MA, Jure-Kunkel M, Melero I. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur J Immunol. 2016;46:513–522. doi: 10.1002/eji.201445388. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, An L, Huang R, Xiong J, Yang H, Wang X, Zhang X. Strategies to enhance CAR-T persistence. Biomark Res. 2022;10:86. doi: 10.1186/s40364-022-00434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Q, Zhang X, Tu L, Cao J, Hinrichs CS, Su X. Size-dependent activation of CAR-T cells. Sci Immunol. 2022;7:eabl3995. doi: 10.1126/sciimmunol.abl3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng N, Fang J, Xue G, Wang Z, Li X, Zhou M, Jin G, Rahman MM, McFadden G, Lu Y. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell. 2022;40:973–985. e7. doi: 10.1016/j.ccell.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, Porter DL, Maloney DG, Grupp SA, Mackall CL, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: Clinical perspective and significance. J Immunother Cancer. 2018;6:137. doi: 10.1186/s40425-018-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, Bailey SR, Boroughs AC, Frigault MJ, Leick MB, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 44.Marei HE, Althani A, Afifi N, Hasan A, Caceci T, Pozzoli G, Cenciarelli C. Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme. Cancer Med. 2021;10:5019–5030. doi: 10.1002/cam4.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu H, Fang X, Tuhin IJ, Tan J, Ye J, Jia Y, Xu N, Kang L, Li M, Lou X, et al. CAR T cells equipped with a fully human scFv targeting Trop2 can be used to treat pancreatic cancer. J Cancer Res Clin Oncol. 2022;148:2261–2274. doi: 10.1007/s00432-022-04017-x. [DOI] [PubMed] [Google Scholar]
- 46.Feng Q, Sun B, Xue T, Li R, Lin C, Gao Y, Sun L, Zhuo Y, Wang D. Advances in CAR T-cell therapy in bile duct, pancreatic, and gastric cancers. Front Immunol. 2022;13:1025608. doi: 10.3389/fimmu.2022.1082984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Entezam M, Sanaei MJ, Mirzaei Y, Mer AH, Abdollahpour-Alitappeh M, Azadegan-Dehkordi F, Bagheri N. Current progress and challenges of immunotherapy in gastric cancer: A focus on CAR-T cells therapeutic approach. Life Sci. 2023;318:121459. doi: 10.1016/j.lfs.2023.121459. [DOI] [PubMed] [Google Scholar]
- 48.Davenport AJ, Jenkins MR, Cross RS, Yong CS, Prince HM, Ritchie DS, Trapani JA, Kershaw MH, Darcy PK, Neeson PJ. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol Res. 2015;3:483–494. doi: 10.1158/2326-6066.CIR-15-0048. [DOI] [PubMed] [Google Scholar]
- 49.Chen C, Gu YM, Zhang F, Zhang ZC, Zhang YT, He YD, Wang L, Zhou N, Tang FT, Liu HJ, Li YM. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-Met CAR-T in gastric cancer. Oncoimmunology. 2021;10:1901434. doi: 10.1080/2162402X.2021.1901434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K, Nelson A, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasqui DM, Latorraca CDOC, Pacheco RL, Riera R. CAR-T cell therapy for patients with hematological malignancies. A systematic review. Eur J Haematol. 2022;109:601–618. doi: 10.1111/ejh.13851. [DOI] [PubMed] [Google Scholar]
- 52.Fabrizio VA, Boelens JJ, Mauguen A, Baggott C, Prabhu S, Egeler E, Mavroukakis S, Pacenta H, Phillips CL, Rossoff J, et al. Optimal fludarabine lymphodepletion is associated with improved outcomes after CAR T-cell therapy. Blood Adv. 2022;6:1961–1968. doi: 10.1182/bloodadvances.2021006418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bupha-Intr O, Haeusler G, Chee L, Thursky K, Slavin M, The B. CAR-T cell therapy and infection: a review. Expert Rev Anti Infect Ther. 2021;19:749–758. doi: 10.1080/14787210.2021.1855143. [DOI] [PubMed] [Google Scholar]
- 54.Roddie C, Neill L, Osborne W, Iyengar S, Tholouli E, Irvine D, Chaganti S, Besley C, Bloor A, Jones C, et al. Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. 2023;7:2872–2883. doi: 10.1182/bloodadvances.2022009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangal JL, Handlos JL, Esrafili A, Inamdar S, Mcmillian S, Wankhede M, Gottardi R, Acharya AP. Engineering metabolism of chimeric antigen receptor (CAR) cells for developing efficient immunotherapies. Cancers (Basel) 2021;13:1123. doi: 10.3390/cancers13051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19:775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martino M, Alati C, Canale FA, Musuraca G, Martinelli G, Cerchione C. A review of clinical outcomes of CAR T-Cell Therapies for B-Acute lymphoblastic leukemia. Int J Mol Sci. 2021;22:2150. doi: 10.3390/ijms22042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geldres C, Savoldo B, Dotti G. Chimeric antigen receptor-redirected T cells return to the bench. Semin Immunol. 2016;28:3–9. doi: 10.1016/j.smim.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Zhang H, Lan H, Wu J, Xiao Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. 2023;14:1101495. doi: 10.3389/fimmu.2023.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haslauer T, Greil R, Zaborsky N, Geisberger R. CAR T-Cell therapy in hematological malignancies. Int J Mol Sci. 2021;22:8996. doi: 10.3390/ijms22168996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, Xiao L, Han Y, Lu L. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2021;18:1896–1903. doi: 10.1038/s41423-020-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for B-cell lymphoma. Curr Probl Cancer. 2022;46:100826. doi: 10.1016/j.currproblcancer.2021.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shalabi H, Qin H, Su A, Yates B, Wolters PL, Steinberg SM, Ligon JA, Silbert S, DéDé K, Benzaoui M, et al. CD19/22 CAR T cells in children and young adults with B-ALL: Phase 1 results and development of a novel bicistronic CAR. Blood. 2022;140:451–463. doi: 10.1182/blood.2022015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, Ye Z, Qian Q. Current Progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15:2548–2560. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, Richards RM, Jiang L, Barsan V, Mancusi R, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zarrabi KK, Narayan V, Mille PJ, Zibelman MR, Miron B, Bashir B, Kelly WK. Bispecific PSMA antibodies and CAR-T in metastatic castration-resistant prostate cancer. Ther Adv Urol. 2023;15:17562872231182219. doi: 10.1177/17562872231182219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, Hwang WT, Lal P, Carpenter EL, Maude SL, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: A phase 1 trial. Nat Med. 2022;28:724–734. doi: 10.1038/s41591-022-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller IC, Zamat A, Sun LK, Phuengkham H, Harris AM, Gamboa L, Yang J, Murad JP, Priceman SJ, Kwong GA. Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control. Nat Biomed Eng. 2021;5:1348–1359. doi: 10.1038/s41551-021-00781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu Y, Li J, Ni F, Yang Z, Gui X, Bao Z, Zhao H, Wei G, Wang Y, Zhang M, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun. 2022;13:5313. doi: 10.1038/s41467-022-32960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, Li Y. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40:367. doi: 10.1186/s13046-021-02148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, Xu J, Zhuang Y, Zhang W, Weng XQ, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA. 2019;116:9543–9551. doi: 10.1073/pnas.1819745116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin D, Guo T, Kou H, Liu L, Tang L, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14:161. doi: 10.1186/s13045-021-01170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR T-cell therapy. Blood. 2023;141:2460–2469. doi: 10.1182/blood.2022017415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corti C, Venetis K, Sajjadi E, Zattoni L, Curigliano G, Fusco N. CAR-T cell therapy for triple-negative breast cancer and other solid tumors: preclinical and clinical progress. Expert Opin Investig Drugs. 2022;31:593–605. doi: 10.1080/13543784.2022.2054326. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, Zhang L, Wei G, Tian Y, Zhao K, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609:369–374. doi: 10.1038/s41586-022-05140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mensali N, Köksal H, Joaquina S, Wernhoff P, Casey NP, Romecin P, Panisello C, Rodriguez R, Vimeux L, Juzeniene A, et al. ALPL-1 is a target for chimeric antigen receptor therapy in osteosarcoma. Nat Commun. 2023;14:3375. doi: 10.1038/s41467-023-39097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: What we know so far. Nat Rev Clin Oncol. 2023;20:359–371. doi: 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jayaraman J, Mellody MP, Hou AJ, Desai RP, Fung AW, Pham AHT, Chen YY, Zhao W. CAR-T design: Elements and their synergistic function. EBioMedicine. 2020;58:102931. doi: 10.1016/j.ebiom.2020.102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Qiu S, Li W, Wang K, Zhang Y, Yang H, Liu B, Li G, Li L, Chen M, et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res. 2023;33:341–354. doi: 10.1038/s41422-023-00789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, Eyquem J, Zhao Z, Whitlock BM, Miele MM, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heitzeneder S, Bosse KR, Zhu Z, Zhelev D, Majzner RG, Radosevich MT, Dhingra S, Sotillo E, Buongervino S, Pascual-Pasto G, et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40:53–69.e9. doi: 10.1016/j.ccell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, Mehta B, Zhang H, Dakhova O, Carrum G, et al. In Vivo Fate and Activity of Second-versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin's Lymphomas. Mol Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 89.Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, Beauvais D, Roulin L, Gros FX, Rubio MT, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–2154. doi: 10.1038/s41591-022-01969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han D, Xu Z, Zhuang Y, Ye Z, Qian Q. Current Progress in CAR-T cell therapy for hematological malignancies. J Cancer. 2021;12:326–334. doi: 10.7150/jca.48976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason J, Kipps TJ, Gillenwater HH, Gong L, Yang L, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139:1794–1806. doi: 10.1182/blood.2021011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng D, Sun J. Overview of anti-BCMA CAR-T immunotherapy for multiple myeloma and relapsed/refractory multiple myeloma. Scand J Immunol. 2020;92:e12910. doi: 10.1111/sji.12910. [DOI] [PubMed] [Google Scholar]
- 93.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curran E, O'Brien M. Role of blinatumomab, inotuzumab, and CAR T-cells: Which to choose and how to sequence for patients with relapsed disease. Semin Hematol. 2020;57:157–163. doi: 10.1053/j.seminhematol.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu J, Meng Q, Sun H, Zhang X, Yun J, Li B, Wu S, Li X, Yang H, Zhu H, et al. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021;12:1109. doi: 10.1038/s41419-021-04100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maggs L, Cattaneo G, Dal AE, Moghaddam AS, Ferrone S. CAR T Cell-Based immunotherapy for the treatment of glioblastoma. Front Neurosci. 2021;15:662064. doi: 10.3389/fnins.2021.662064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rotte A, Frigault MJ, Ansari A, Gliner B, Heery C, Shah B. Dose-response correlation for CAR-T cells: A systematic review of clinical studies. J Immunother Cancer. 2022;10:e005678. doi: 10.1136/jitc-2022-005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dasyam N, George P, Weinkove R. Chimeric antigen receptor T-cell therapies: Optimising the dose. Br J Clin Pharmacol. 2020;86:1678–1689. doi: 10.1111/bcp.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stefanski HE, Eaton A, Baggott C, Rossoff J, Verneris MR, Prabhu S, Pacenta HL, Phillips CL, Talano JA, Moskop A, et al. Higher doses of tisagenlecleucel are associated with improved outcomes: A report from the pediatric real-world CAR consortium. Blood Adv. 2023;7:541–548. doi: 10.1182/bloodadvances.2022007246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frigault M, Rotte A, Ansari A, Gliner B, Heery C, Shah B. Dose fractionation of CAR-T cells. A systematic review of clinical outcomes. J Exp Clin Cancer Res. 2023;42:11. doi: 10.1186/s13046-022-02540-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn BC. CAR T-Cell-Based gene therapy for cancers: New perspectives, challenges, and clinical developments. Front Immunol. 2022;13:925985. doi: 10.3389/fimmu.2022.925985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wagner DL, Koehl U, Chmielewski M, Scheid C, Stripecke R. Review: Sustainable Clinical Development of CAR-T Cells-switching from viral transduction towards CRISPR-Cas Gene Editing. Front Immunol. 2022;13:865424. doi: 10.3389/fimmu.2022.865424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gagelmann N, Riecken K, Wolschke C, Berger C, Ayuk FA, Fehse B, Kröger N. Development of CAR-T cell therapies for multiple myeloma. Leukemia. 2020;34:2317–2332. doi: 10.1038/s41375-020-0930-x. [DOI] [PubMed] [Google Scholar]
- 105.Zeng W, Zhang Q, Zhu Y, Ou R, Peng L, Wang B, Shen H, Liu Z, Lu L, Zhang P, Liu S. Engineering Novel CD19/CD22 Dual-Target CAR-T cells for improved anti-tumor activity. Cancer Invest. 2022;40:282–292. doi: 10.1080/07357907.2021.2005798. [DOI] [PubMed] [Google Scholar]
- 106.Ghaffari S, Khalili N, Rezaei N. CRISPR/Cas9 revitalizes adoptive T-cell therapy for cancer immunotherapy. J Exp Clin Cancer Res. 2021;40:269. doi: 10.1186/s13046-021-02076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong M, Chen YY. Killer fatigue: Transition to NK-cell-like phenotype is a signature of CAR-T cell exhaustion. Cell. 2021;184:6017–6019. doi: 10.1016/j.cell.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 108.Good CR, Aznar MA, Kuramitsu S, Samareh P, Agarwal S, Donahue G, Ishiyama K, Wellhausen N, Rennels AK, Ma Y, et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell. 2021;184:6081–6100. e26. doi: 10.1016/j.cell.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allen GM, Frankel NW, Reddy NR, Bhargava HK, Yoshida MA, Stark SR, Purl M, Lee J, Yee JL, Yu W, et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science. 2022;378:eaba1624. doi: 10.1126/science.aba1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.