Abstract
Although nasal vaccination has emerged as an interesting alternative to systemic or oral vaccination, knowledge is scarce about the immune responses after such immunization in humans. In the present study, we have compared the kinetics and organ distribution of the antibody responses after nasal and oral vaccination. We immunized female volunteers nasally or orally with cholera toxin B subunit (CTB) and determined the specific antibody levels in serum and nasal and vaginal secretions, as well as the number of circulating antibody-secreting cells, before immunization and 1, 2, 3, 6, and 26 weeks thereafter. Nasal vaccination induced 9-fold CTB-specific immunoglobulin A (IgA) and 56-fold specific IgG antibody increases in nasal secretions, whereas no significant IgA increase was seen after oral vaccination. Both oral and nasal vaccination resulted in 5- to 6-fold CTB-specific IgA and 20- to 30-fold specific IgG increases in vaginal secretions. Strong serum responses to CTB were also induced by both routes of vaccination. A notable difference between nasal and oral vaccination was that the nasal route elicited a specific antibody response with a later onset but of much longer duration than did the oral route. We conclude from this study that the nasal route is superior to the oral route for administering at least nonliving vaccines against infections in the upper respiratory tract, whereas either oral or nasal vaccination might be used for eliciting antibody responses in the female genital tract.
Many pathogens cause disease by first colonizing or penetrating through the mucosa of the gastrointestinal, respiratory, or genital tract. Local antibodies at these mucosal surfaces play a central role in the primary defense against these pathogens by preventing the binding of the microbes and their produced toxins to the epithelium (8, 37, 38). Mucosal vaccination is often a prerequisite for induction of an efficient local immune response, since systemic vaccination does not usually increase the nasal or intestinal antibody levels, unless the individual has received initial priming by a mucosal route (28, 35). The vaccine antigens stimulate lymphocytes in the mucosa-associated lymphoid tissues in the different organs, and the activated cells are transported via the peripheral blood to effector sites in the mucosae. The distribution of the antibody classes varies on different mucosal surfaces; for example, immunoglobulin A (IgA) is the major isotype in the gut, whereas IgA and IgG are found in comparable amounts in nasal and vaginal secretions (4, 7, 31).
For reasons of simplicity, the two most attractive routes of mucosal vaccination in humans would be the oral and nasal routes. Several oral vaccines have recently been developed, and a few have been licensed for human use, one example being an oral cholera vaccine containing cholera toxin B subunit (CTB) together with a whole-cell vaccine component (15). CTB is a well-characterized nontoxic yet potent mucosal immunogen, partly because of its high-affinity binding to the receptor GM1 ganglioside (14). In order to induce a strong local immune response after vaccination, a whole-cell preparation or a protein with mucosa-binding properties is required (2). Consistent with this concept, several studies in animals have shown that CTB used as a carrier for various protein or carbohydrate antigens can markedly enhance mucosal immunogenicity for nonbinding antigens (3, 16). The conclusions drawn from using CTB as an immunogen would probably also hold true for conjugate vaccines with CTB as a carrier (32).
Earlier experiments in animal models showed that lymphocytes activated by oral vaccination are transported not only to the intestinal tract but also to other tissues, such as the mammary and salivary glands, the respiratory tract, and the genital mucosa (26). More recent investigations with immunized mice or humans indicate that the antibody response is stronger on the mucosa on which the vaccine was administered than at more distal sites (17, 31). Thus, while the oral route remains the preferred way of administering vaccines to protect against infections in the gastrointestinal tract, it may not be ideal for vaccination against respiratory or urogenital tract infections. Recent studies have shown that vaginal vaccination may be better than oral vaccination for inducing specific antibodies in vaginal and cervical secretions (12, 20, 36), and nasal immunization in animals has been superior to the oral route for stimulating local antibody production in the airway mucosa (3, 17). Notably, nasal immunization has also induced substantial antibody responses in the vagina in both animals and humans, which has made it an attractive route for future vaccination against sexually transmitted diseases (4, 12, 17, 20, 32).
Although no vaccines are currently given by the nasal route, a few experimental studies with nasal vaccines in humans have recently been performed (1, 4, 27). Basic information on the characteristics of the immune response after such vaccination is still lacking, however, partly because it has been supposed that nasal immune responses share many important features with intestinal responses. In our initial study of nasal vaccination of humans with CTB, the increases in specific nasal antibodies were unexpectedly low (4). Since this could conceivably be due to kinetics of the antibody response which are different from that after oral vaccination, in the present study we compared the kinetics and magnitudes of the antibody responses in serum and in secretions from the respiratory and the genital tracts after nasal or oral vaccination, with CTB as the model immunogen.
MATERIALS AND METHODS
Vaccines.
The vaccine used for oral administration was the licensed oral cholera vaccine (Dukoral) produced by SBL Vaccine (Stockholm, Sweden). This vaccine consists of 1.0 mg of CTB and 1011 heat- and formalin-killed vibrios administered in 150 ml of a sodium bicarbonate solution. The vaccine for nasal use consisted of CTB, also supplied by SBL Vaccine, which was diluted in phosphate-buffered saline (PBS) to a concentration of 0.625 mg/ml. The CTB in both vaccines was produced and purified from a recombinant strain of Vibrio cholerae lacking the cholera toxin A subunit gene but harboring a CTB-overexpressing multicopy plasmid (33). The endotoxin content was 10 ng/mg of CTB protein.
Subjects and vaccination.
Twenty-seven Swedish female volunteers aged 19 to 36 years gave informed consent to participate in the study, which was approved by the Swedish Medical Products Agency and the local Human Research Ethical Committee of the Medical Faculty, Göteborg University, Göteborg, Sweden. Exclusion criteria for participation included previous vaccination against cholera or enterotoxigenic Escherichia coli, travel in the last 3 years to a country where cholera or enterotoxigenic E. coli is prevalent, ongoing allergic reactions or a history of severe atopic disease, or signs of infectious respiratory disease at the time of the first vaccination.
One group of volunteers (n = 10) received a single 250-μg dose of the nasal CTB vaccine, and a second group of volunteers (n = 8) were given two 250-μg doses of the nasal vaccine with a 2-week interval. The nasal vaccine was administered as a 100-μl spray given twice in both nostrils, i.e., a total volume of 400 μl, using an atomizer (Apoteksbolaget AB, Stockholm, Sweden). A third group of volunteers (n = 9) received two doses of the licensed oral cholera vaccine, with a 2-week interval.
Follow-up for adverse reactions.
Surveillance for possible side effects was performed during five consecutive days after each vaccination. A questionnaire inquiring about the occurrence of symptoms that might be related to vaccination (itching, sneezing, nasal congestion, increased nasal secretions, nasal bleeding, systemic reactions, or any other symptoms) was completed each day by each of the volunteers.
Sampling of serum and secretions.
Blood and nasal and vaginal secretion samples were taken immediately before the first vaccination and 1, 2, 3, and 6 weeks after the second vaccination. Samples were also taken 6 months after vaccination from six individuals in the group vaccinated orally and from eight individuals from each of the groups vaccinated nasally. No vaginal secretion samples were taken at times of ongoing menstruation. The nasal secretions were sampled by a validated tampon method (5). A dry double cotton gauze tampon (1.5 by 20 cm) (Scholl, Solna, Sweden) was inserted into one of the nostrils and left in place for approximately 2 h. After removal, the tampon was placed in 1.0 ml of PBS and stored at −70°C. The tampons were thawed, and the fluid was squeezed out by centrifugation (3,500 × g) in a pierced Eppendorf tube placed on top of another tube, and after additional centrifugation at 9,500 × g, the samples were immediately analyzed. The vaginal secretions were sampled with a Polywick tampon, 10 by 30 mm, obtained from Polyfiltronics Group Inc. (Rockland, Mass.), which was inserted deep into the vagina by the female volunteer herself. After 2 h it was taken out by the volunteer with the aid of a thread attached to the Polywick tampon, placed in 1 ml of PBS, and stored at −70°C. The Polywick tampons were thawed and centrifuged for 10 min at 3,500 × g in pierced Eppendorf tubes as described above, and the samples were treated with bromelain from Sigma Chemical Company (St. Louis, Mo.) to solubilize the mucus (36). Twenty-five micrograms of bromelain per ml of sample was added, and the samples were incubated for 60 min at 37°C, followed by centrifugation for 10 min at 9,500 × g.
Determination of total Ig and specific antibodies.
The total IgA and IgG antibody contents in secretions were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (4). Briefly, the plates were coated with goat anti-human IgA, α chain specific, from Jackson ImmunoResearch Laboratories (West Grove, Pa.) and goat anti-human IgG F(ab)2 (Jackson). Thereafter, the samples and standards (polyclonal human plasma IgA and IgG; Calbiochem Corp., La Jolla, Calif.) were added in duplicate and serially diluted. Bound total IgA and total IgG antibodies were determined by using horseradish peroxidase (HRP)-conjugated goat anti-human serum IgA, α chain specific (Jackson), and HRP-conjugated goat anti-human IgG, Fcγ specific (Jackson), followed by the enzyme chromogen substrate. The endpoint titers were determined as the reciprocal dilutions giving an absorbance at 450 nm of 0.4 above background. The concentrations of total IgA or IgG antibody in the secretion samples were then calculated by using the standards.
CTB-specific antibodies were measured by a modified GM1-ELISA (34) as previously described (4). Briefly, plates were coated first with GM1 ganglioside (Sigma) and then with CTB. The samples and a positive serum reference (a serum pool from Swedish volunteers vaccinated orally with the whole-cell–CTB cholera vaccine) were added in duplicate and serially diluted. Bound CTB-specific IgA and IgG antibodies were determined with HRP-conjugated rabbit anti-human serum IgA, α chain specific (Jackson), and HRP-conjugated goat anti-human IgG, Fcγ specific (Jackson), and developed as described above.
The ELISA was repeated if the endpoint titers determined in duplicate for the reference on the plate varied more than twofold. Samples with titers below the detection limit were assigned a titer of half the lowest dilution. The specific antibody content in secretions was expressed as arbitrary units per milliliter. The CTB-specific IgA and IgG antibody contents were divided by the total IgA and IgG concentrations (micrograms per milliliter) in the nasal and vaginal secretion samples to adjust for variations in the Ig content in secretion sample eluates collected from different volunteers or on different days. The fold increases were calculated by dividing the adjusted postvaccination value by the adjusted prevaccination value from each individual.
Determination of ASC numbers.
Mononuclear cells (MNC) in peripheral blood were analyzed for numbers of total and specific IgA and IgG antibody-secreting cells (ASCs) by the enzyme-linked immunospot assay (11) essentially as previously described (18). Briefly, nitrocellulose-bottomed wells were coated with GM1 ganglioside, with affinity-purified goat anti-human IgG F(ab)2 (Jackson) for total IgA and IgG determination, and with bovine serum albumin for control purposes. After the GM1-coated wells were blocked and CTB (SBL Vaccine) was added, the MNCs isolated by gradient centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) were incubated in the wells for 3 to 4 h in numbers per well ranging from 5 × 104 to 1 × 106. The spots were visualized by incubating them with HRP-conjugated goat anti-human IgA and IgG from Southern Biotechnology Associates (Birmingham, Ala.) and the enzyme chromogen substrate. The ASCs were enumerated in quadruplicate wells, and the results were transformed into numbers of spot-forming cells per 106 MNCs.
Statistical methods.
Before calculations, all specific antibody titers in nasal or vaginal secretions were adjusted for variations in total Ig content and all values were log10 transformed. Analysis of the significance of the differences between the prevaccination titers and the maximal postvaccination titers was performed with a paired Student’s t test, using the Bonferroni method for correction for multiple tests (three tests per group). Analysis of variance was used for analysis of the significance of differences in kinetics of serum and nasal secretion titers, and post hoc comparisons of the individual groups were performed with Scheffé’s test. The software Statistica 4.0 for Windows (Softstat, Tulsa, Okla.) was used for these calculations. Differences were considered statistically significant at P values of <0.05. For analysis of the significance of differences in kinetics of the titers in the vaginal samples, a different method had to be used, since there were missing observations caused by menstruation. A new statistical method was developed that used the model of compound symmetry to obtain the variance-covariance estimates more efficiently (13). The results were expressed as global 95% confidence intervals of the differences in log titers.
RESULTS
Safety.
Since we had not previously given the 250-μg dose of CTB nasally to humans, we carefully recorded all symptoms of possible adverse reactions. In the group receiving one nasal dose, three of eight subjects experienced occasional sneezing and slightly increased nasal secretion occurring a few hours after vaccination and disappearing after at most 24 h whereas five individuals had no adverse reactions at all. The two additional subjects in this group contracted upper respiratory infections during the follow-up period, which precluded the possibility of evaluating adverse reactions in these individuals. In the group vaccinated twice nasally, four of seven volunteers experienced symptoms similar to those described above and three volunteers had no adverse reactions. The additional subject in this group could not be evaluated because of respiratory infection during the follow-up period. The individuals vaccinated orally with the licensed cholera vaccine, containing 1,000 μg of CTB, served as controls with respect to adverse reactions in the nose. In this group, three of nine volunteers reported occasional sneezing and slightly increased nasal secretions during the follow-up period. Thus, there was no difference in the frequency and degree of adverse nasal reactions between the group vaccinated orally and the group vaccinated once nasally. In the group of volunteers that received two nasal vaccinations, a small increase in the frequency of mild local side effects in the nose was recorded. No systemic or more serious local adverse reactions were reported in any of the groups.
Antibody responses in serum and nasal and vaginal secretions.
Serum samples were taken from all individuals before vaccination and 1, 2, 3, and 6 weeks after vaccination and from most subjects after 6 months, and the specific IgA and IgG antibody titers to CTB were analyzed by ELISA. The individual values of the IgA and IgG titers in serum and nasal and vaginal secretion samples before vaccination and at peak level after vaccination are shown in Fig. 1, and the fold increases are shown in Table 1. The specific antibody titers in the nasal and vaginal secretions were adjusted to the total amounts of IgA and IgG to correct for variations in the levels of total IgA and IgG. Importantly, the specific titer increases in the secretions were not due to variations in the total Ig levels, since they were also seen in the unadjusted titers (data not shown). We did not adjust the individual serum titers for total antibody content because of the stability of the total antibody levels in serum.
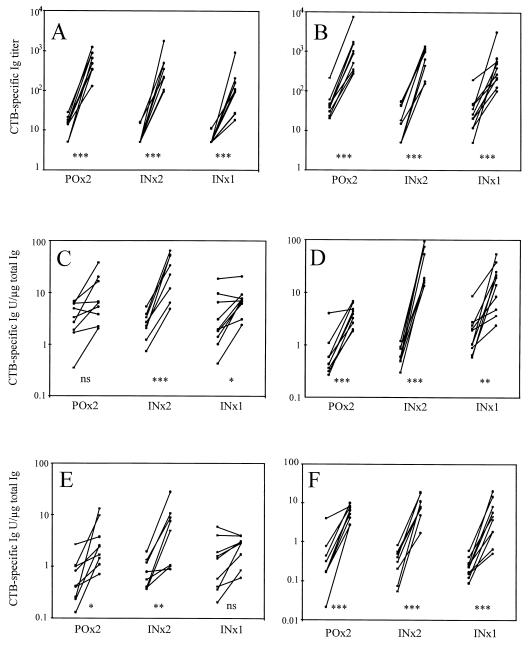
FIG. 1.
CTB-specific increases in serum IgA (A) and IgG (B), nasal secretion IgA (C) and IgG (D), and vaginal secretion IgA (E) and IgG (F) after two oral (POx2), two nasal (INx2), or a single nasal (INx1) vaccination. The individual titers, or titers adjusted to the total Ig content, from the time points before and at peak level after vaccination are shown. The statistical significance of the increases is shown as follows: ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05; and ns, P > 0.05.
TABLE 1.
Maximal fold increases of antibody titers against CTB in sera and nasal and vaginal secretions from different vaccination groups
| Vaccination | Fold increase in:
|
|||||
|---|---|---|---|---|---|---|
| Seruma
|
Nasal secretionsb
|
Vaginal secretionsb
|
||||
| IgA | IgG | IgA | IgG | IgA | IgG | |
| Oral × 2 | 37 | 19 | 2.5 | 6.2 | 4.9 | 20 |
| Nasal × 2 | 46 | 38 | 9.3 | 56 | 5.7 | 30 |
| Nasal × 1 | 18 | 14 | 2.6 | 8.1 | 2.0 | 16 |
Geometric means.
Geometric means of fold increases calculated from the titers adjusted for total Ig content in the sample.
Although very strong IgA and IgG anti-CTB responses were induced in sera in all vaccination groups, two nasal vaccinations resulted in the greatest fold increases, i.e., about 40-fold for both IgA and IgG (Fig. 1A and B and Table 1). To elicit specific IgA and IgG responses in nasal secretions, administration of two nasal doses of CTB was by far the most efficient vaccination method, resulting in substantial antibody increases in all vaccinees (Fig. 1C and D and Table 1). In contrast, oral vaccination or a single nasal vaccination induced an increase in specific IgA in only about half of the vaccinees and the mean fold increases of IgA or IgG were considerably lower than after two intranasal doses. The mean increases of specific IgA and IgG in nasal secretions were 4 and 10 times higher, respectively, after nasal vaccination than after oral vaccination, although the mean fold increases of serum IgA and IgG were similar after both types of vaccination (Table 1). The IgA and IgG anti-CTB responses in vaginal secretions were similar in frequency and magnitude after vaccination with either two nasal or two oral doses (Fig. 1E and F and Table 1). A single nasal dose was not sufficient to evoke an anti-CTB IgA response in the genital tract. It is noteworthy that the IgG response dominated over the IgA response both in nasal and vaginal secretions.
ASCs.
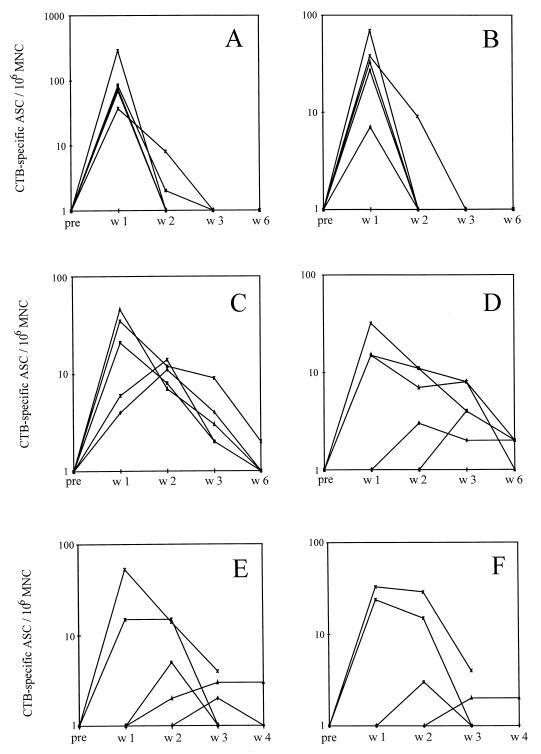
From each of the three vaccination groups, five volunteers were randomly selected for enzyme-linked immunospot assay analysis of the number of specific ASCs in their blood. The blood samples were taken from each individual before vaccination and 1, 2, 3, 4, and 6 weeks and 6 months after vaccination. The numbers of CTB-specific IgA and IgG ASCs are shown in Fig. 2. The peak response in the group vaccinated twice orally was 1 week after vaccination in all subjects, and in the majority of the individuals the ASCs had disappeared by 2 weeks after vaccination (Fig. 2A and B). The kinetics of the response was different in the volunteers vaccinated twice nasally with CTB. Although three of the volunteers had their peak responses of CTB-specific IgA and IgG 1 week after vaccination, the circulating ASCs could be detected for at least 3 weeks in all subjects (Fig. 1C and D). In the group that was given a single nasal dose, only two of five volunteers responded with levels of circulating CTB-specific ASCs comparable to those of the volunteers in the group vaccinated twice nasally (Fig. 1E and F). However, these individuals had the same pattern of prolonged responses as the individuals in the group vaccinated twice nasally. After 6 months no circulating CTB-specific ASCs could be detected in any of the volunteers (data not shown).
FIG. 2.
CTB-specific ASCs per 106 MNCs before (pre) and 1 (w1), 2 (w2), 3 (w3), and 4 (w4) or 6 (w6) weeks after two oral vaccinations, IgA (A) and IgG (B); two nasal vaccinations, IgA (C) and IgG (D); and a single nasal vaccination, IgA (E) and IgG (F).
Kinetics of antibody titers in serum and nasal and vaginal secretions.
The kinetics of the antibody responses in serum and nasal and vaginal secretions were analyzed by determining the CTB-specific IgA and IgG titers before vaccination and 1, 2, 3, 6, and 26 weeks after vaccination. The fold increases of the serum titers from prevaccination to postvaccination at the different time points are shown in Table 2. The IgA and IgG titers of the volunteers vaccinated twice orally increased significantly by 1 week after the second vaccination (P < 0.001 in both cases), and thereafter the titers gradually decreased (Table 2). The IgA and IgG titers of the volunteers vaccinated twice nasally also increased significantly by 1 week after the second vaccination (P < 0.001), but they continued to increase during the following weeks and remained high for at least 6 months (Table 2). The IgA and IgG titers in the samples from the volunteers vaccinated with a single nasal dose started to augment later than the other groups, but the increases were significant 2 weeks after the vaccination both for IgA and IgG (P < 0.001 and P = 0.01, respectively) (Table 2). As for the group vaccinated twice nasally, the IgA and IgG levels remained elevated 6 months after vaccination. The different kinetics of the antibody responses in serum in the oral and the nasal groups, with a later onset but longer duration of the responses after nasal vaccination, reflect the kinetics of the circulating ASCs in the different groups (Fig. 2).
TABLE 2.
Kinetics of fold increases in antibody titers against CTB in sera from different vaccination groups
| Antibody | Vaccination | Fold increasea
|
||||
|---|---|---|---|---|---|---|
| Wk 1 | Wk 2 | Wk 3 | Wk 6 | 6 Mo | ||
| IgA | Oral × 2 | 34 | 28 | 16 | 6.6 | 4.5 |
| Nasal × 2 | 15 | 28 | 30 | 23 | 16 | |
| Nasal × 1 | 2.1 | 8.8 | 11 | 12 | 7.6 | |
| IgG | Oral × 2 | 19 | 14 | 13 | 9.0 | 5.9 |
| Nasal × 2 | 11 | 21 | 25 | 30 | 30 | |
| Nasal × 1 | 1.7 | 6.0 | 8.6 | 10 | 14 | |
Geometric means.
The fold increases of the titers in nasal secretions from prevaccination to postvaccination at the different time points are shown in Table 3. Two nasal vaccinations resulted in high specific IgA titers in nasal secretions, peaking as late as 6 weeks after the second vaccination (P > 0.001) (Table 3). However, the nasal IgA response in the groups vaccinated twice orally or once nasally was poor at all time points, the means of the fold increases for these two groups being less than 2 during the study period. The IgG titer rise after two nasal vaccinations was significant at 2 weeks after the second vaccination (P = 0.001) but did not peak until 6 weeks after the second vaccination, and after 6 months the titer levels were still maintained (Table 3). Although oral vaccination did not induce an increase in specific nasal IgA titers, the IgG titers did increase significantly. The maximal rise was attained by 2 weeks (P = 0.003) after vaccination and had started to decline when examined 6 weeks after the last vaccination. From 3 weeks after vaccination onwards the IgG increases after oral vaccination were considerably smaller than those of the group vaccinated twice nasally (Table 3). The kinetics after a single nasal vaccination was similar to that after two nasal vaccinations, but the magnitudes of the responses were lower.
TABLE 3.
Kinetics of fold increases in antibody titers against CTB in nasal secretions from different vaccination groups
| Antibody | Vaccination | Fold increasea
|
||||
|---|---|---|---|---|---|---|
| Wk 1 | Wk 2 | Wk 3 | Wk 6 | 6 Mo | ||
| IgA | Oral × 2 | 1.5 | 1.9 | 1.7 | 1.1 | 0.6 |
| Nasal × 2 | 2.4 | 1.8 | 3.9 | 9.3 | 3.9 | |
| Nasal × 1 | 1.4 | 1.7 | 1.6 | 1.7 | 1.1 | |
| IgG | Oral × 2 | 4.1 | 5.4 | 4.8 | 3.3 | 2.3 |
| Nasal × 2 | 3.7 | 7.0 | 14 | 35 | 34 | |
| Nasal × 1 | 0.9 | 2.0 | 2.0 | 2.8 | 6.2 | |
Geometric means of fold increases calculated from the titers adjusted for total Ig content in the sample.
The fold increases of the titers in vaginal secretions from prevaccination to postvaccination at the different time points are shown in Table 4. At variance with the kinetics in nasal secretions and in serum, the maximal increases of specific IgA titers in vaginal secretions in the group given two nasal doses appeared by 1 week after vaccination (Table 4). The kinetics of the specific IgG titers in the nasally and orally vaccinated groups differed significantly. Thus, the titers in the group vaccinated twice nasally were maximal at 6 weeks after vaccination, whereas the titers in the group vaccinated orally had a peak response at 2 and 3 weeks after vaccination (Table 4). At 6 months the fold increases in the groups vaccinated twice orally or nasally were more similar, since the levels in the group vaccinated nasally had decreased. The rise in vaginal secretion IgG in the group vaccinated once nasally was significantly slower than those of the other two groups, but the levels were maintained at 6 months.
TABLE 4.
Kinetics of fold increases in antibody titers against CTB in vaginal secretions from different vaccination groups
| Antibody | Vaccination | Fold increasea
|
||||
|---|---|---|---|---|---|---|
| Wk 1 | Wk 2 | Wk 3 | Wk 6 | 6 Mo | ||
| IgA | Oral × 2 | 2.9 | 4.8 | 2.5 | 1.4 | 0.6 |
| Nasal × 2 | 4.5 | 2.5 | 3.1 | 1.9 | 0.6 | |
| Nasal × 1 | 1.1 | 0.9 | 1.5 | 1.5 | 0.6 | |
| IgG | Oral × 2 | 9.0 | 15 | 13 | 5.4 | 5.7 |
| Nasal × 2 | 6.9 | 12 | 13 | 26 | 7.8 | |
| Nasal × 1 | 0.7 | 1.5 | 8.4 | 12 | 13 | |
Geometric means of fold increases calculated from the titers adjusted for total Ig content in the sample.
DISCUSSION
In recent years nasal vaccination has evolved as an interesting new route of vaccine administration, and we and others have shown its efficiency in inducing immune responses in the respiratory and genital tracts in animal models (3, 17, 20, 32). Although a few clinical studies with nasal vaccination have been performed, information is still lacking on fundamental aspects of the induction of an immune response in the upper respiratory tract of humans (1, 4, 27). In this study, we aimed at characterizing the antibody response in humans after nasal vaccination and comparing it with that after vaccination by the oral route, which is the only mucosal vaccination route that is in general use today. We show that both the circulating specific ASCs and the specific antibody responses in serum and secretions appear later and persist for a longer time after nasal vaccination than after oral vaccination. Moreover, in contrast to oral vaccination, nasal vaccination induced specific IgA antibodies in the nasal secretions from all vaccinees. On the other hand, and in contrast to recent findings in animals (20), oral vaccination was comparable to nasal vaccination in inducing specific IgA and IgG in vaginal secretions.
Oral vaccination with either the nonliving whole-cell cholera vaccine used in this study or the live Salmonella typhi Ty 21a vaccine induces specific circulating ASCs, with the peak response occurring approximately 1 week after vaccination and then rapidly declining (21, 25). This pattern, which was confirmed in our study, contrasts with the later but much longer response after nasal vaccination with CTB. This pronounced difference in the kinetics of ASCs between oral and nasal vaccination was also indicated after vaccination of humans with live attenuated influenza virus (27). Another difference between oral and nasal vaccination, shown by our study, was in the durations of the antibody responses in serum and secretions. Studies of the CTB-specific antibody responses after oral cholera vaccination have shown that the specific IgA contents in both serum and fecal extracts decreased by 70 to 90% after 6 months compared with those 9 days after vaccination (19, 22). In the present study, nasal vaccination resulted in persistent specific serum and nasal IgA and IgG for at least 6 months after vaccination whereas the serum titers after oral vaccination began to wane early. One possible explanation for the more persistent immune response after nasal vaccination than after oral vaccination is that the antibody response in the intestine is, and needs to be, more tightly regulated than that in the upper respiratory tract, in order to minimize the risk of reacting against the heavy load of food antigens and commensal bacteria. Another possibility could be that the antigen is trapped more efficiently and is presented for a longer time in the upper respiratory tract than in the intestinal tract. Finally, the reason could be that we used a lower vaccine dose nasally than orally, but this is less likely, since we also found indications of the different kinetics after higher doses of CTB in a previous study (4).
It is generally inferred from the concept of the common mucosal immune system that oral vaccination results in migration of antibody-producing cells not only back to the intestine but also to the respiratory tract, genital tract, and mammary glands (26, 27). Our results here, however, show that on a group basis oral vaccination with CTB did not induce a significant specific IgA response in the nose. This discrepancy between our findings and those of previous studies might be due to the fact that in the earlier experiments live virus was used as the immunogen whereas we vaccinated with a nonreplicating antigen (27, 29). However, we too found a small increase in specific IgG in the nose after oral vaccination, part of which might originate from serum. In contrast, we believe that the major part of the nasal IgG found after nasal vaccination is locally produced, since such vaccination induced fold increases in nasal IgG that were 10 times higher than those after oral vaccination, even though the serum antibody levels were comparable for the two immunization routes. The arguments for a compartmentalization between the mucosal antibody responses in the upper respiratory tract and those in the gut are also reinforced by the fact that nasal vaccination did not induce CTB-specific IgA responses in fecal extracts (unpublished observations), whereas most subjects responded with specific IgA in fecal extracts after oral vaccination with CTB (19).
In a previous clinical study of nasal vaccination with CTB we determined the presence of secretory component (SC) by ELISA in both nasal and vaginal secretion samples to confirm that the IgA was secretory (4). In that study we detected SC in virtually all postvaccination samples. However, since this ELISA for quantifying secretory IgA was not good enough to be useful in a vaccine study, we did not analyze the presence of SC in this study. To obtain direct evidence for local production of specific IgA and IgG, we adjusted the mean specific serum antibody titers to the normal levels of total IgA and IgG in serum (3.5 and 13 mg/ml, respectively) and expressed the CTB-specific titers as units per microgram of total Ig. The mean adjusted serum IgA and IgG values at peak levels were 1.4 and 0.66, respectively, after oral vaccination; 0.86 and 0.45, respectively, after two nasal vaccinations; and 0.3 and 0.28, respectively, after one nasal vaccination. If these values are compared with the postvaccination levels in nasal and vaginal secretions, it is evident that, except for the vaginal IgA titers after oral vaccination, the mean levels in the secretions are substantially higher than those in serum (Fig. 1). These comparisons further strengthen our view that IgA and IgG in nasal and vaginal secretions are locally produced. The exceptionally high fold increases of specific IgG in nasal secretions suggests that a mechanism also exists for active transport of IgG onto the mucosal surface.
In recent years many groups have compared the relative efficiencies of different routes of vaccination in inducing antibodies in the genital tract in animals. The majority of these studies have shown that vaginal or nasal immunization is the most efficient route for inducing specific antibodies and antibody-producing cells in the genital tract, although oral immunization with live organisms has also proven effective (10, 12, 20, 32). However, there are major differences between rodents and humans in the organization of the genital immune system. For example, in humans, the cervix is the predominant immune effector organ, whereas in rodents the uterus is known to harbor the majority of the genital antibody-producing cells (24, 30). In addition, at variance with human serum IgA, the major part of the rodent serum IgA is dimeric and may be transported onto the genital mucosa via the SCs (30). In the development of vaccines against genital tract infections it is therefore necessary to explore how to stimulate an optimal immune response in the human female genital tract.
In a previous study we showed that nasal vaccination elicits an antibody response in vaginal secretions in human volunteers (4). Two studies that have recently compared oral and vaginal vaccination in humans concluded that the vaginal route is superior to the oral route in evoking a genital antibody response (23, 36). The present study is the first to compare the nasal and oral routes with respect to genital antibody responses in humans. We show that both the oral and the nasal routes of vaccination induce specific antibodies in vaginal secretions, which does not preclude the possibility that vaginal vaccination may be more efficient in inducing such a response in humans. Although specific IgA and IgG antibodies to CTB in vaginal secretions could be derived both from immigrated cells and from transudated serum antibodies (6), there are several reasons for believing that the IgA and IgG might originate from cells in the genital tissue. Firstly, there are many ASCs in the normal human cervix producing both IgA and IgG (9). Secondly, in our study the specific IgA and IgG levels relative to total Ig are substantially lower in serum than those in vaginal secretions (except for IgA after oral vaccination). Thirdly, the specific IgG levels decrease in vaginal secretions from 6 weeks to 6 months after vaccination, while the IgG levels in serum did not change during this time.
It was apparent that a single nasal vaccination was unsatisfactory for obtaining a good local immune response in the nasal secretions. The analysis of the circulating ASCs, which is often used to reflect the local antibody response after mucosal vaccination, confirmed that the induction of local immunity was less efficient than after two nasal vaccinations. These results are in accordance with the responses after one oral vaccination with CTB (18).
The findings from the present study suggest that the optimal time point for sampling nasal secretions after nasal vaccination is considerably later than expected from studies of the kinetics of the intestinal mucosal response after oral vaccination (18, 19). Thus, in future studies of nasal vaccination it will be important to take this new information about the slower kinetics into consideration. In addition, it is evident that the nasal route is superior to the oral route in evoking an antibody response against nonreplicating antigens in the upper respiratory tract. It still remains to be determined whether nasal vaccination also elicits specific antibodies in the lower respiratory tract. Moreover, based on the results of this and previous studies (23, 36), it is important to directly compare nasal and vaginal vaccination in humans with reference to specific immune responses in the genital tract.
ACKNOWLEDGMENTS
This work was supported by Maxim Pharmaceuticals, the Swedish Medical Research Council (grant 16x-3383), Åke Wibergs stiftelse, Stiftelsen Lars Hiertas minne, Göteborg Medical Society, the Commission of European Communities for Biomedical and Health Research (Biomed), and SIDA-SAREC, Sweden (Special Program for AIDS and Related Diseases).
We gratefully acknowledge all volunteers who participated in the study, SBL Vaccine for providing us with the CTB vaccine preparation, and Astra Draco for their gift of the nasal spray devices. We also thank Maja Berg for excellent technical assistance and Sture Holm, Department of Mathematical Statistics, Göteborg University, for statistical advice, including development of a new method for multiple comparisons when there are missing values.
Anna Rudin and Eva-Liz Johansson contributed equally to the study.
REFERENCES
- 1.Aggerbeck H, Gizurarson S, Wantzin J, Heron I. Intranasal booster vaccination against diphtheria and tetanus in man. Vaccine. 1997;15:307–316. doi: 10.1016/s0264-410x(96)00175-2. [DOI] [PubMed] [Google Scholar]
- 2.Aizpurua H J, Russell-Jones G J. Oral vaccination. Identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988;167:440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist C, Lagergård T, Lindblad M, Holmgren J. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–2025. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergquist C, Johansson E-L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist, C., E.-L. Johansson, and A. Rudin. New methods for sampling nasal and vaginal secretions in humans. Submitted for publication.
- 6.Bouvet J P, Bélec L, Pirès R, Pillot J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect Immun. 1994;62:3957–3961. doi: 10.1128/iai.62.9.3957-3961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandtzaeg P. Cells producing immunoglobulins and other immune factors in human nasal mucosa. Protides Biol Fluids Proc Colloq. 1985;32:363–366. [Google Scholar]
- 8.Cotter T W, Meng Q, Shen Z-L, Zhang Y-X, Su H, Caldwell H. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley-Nowick P A, Bell M, Edwards R P, McCallister D, Gore H, Kanbour-Shakir A, Mestecky J, Partridge E E. Normal uterine cervix: characterization of isolated lymphocyte phenotypes and immunoglobulin secretion. Am J Reprod Immunol. 1995;34:241–247. doi: 10.1111/j.1600-0897.1995.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 10.Cui Z-D, Tristam T D, LaScolea L J, Kwiatkowski T, Jr, Kopti S, Ogra P. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect Immun. 1991;59:1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czerkinsky C, Nilsson L-Å, Nygren H, Ouchterlony Ö, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 12.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm, S. Estimation and multiple comparisons when there are missing values with an application to immunology. Biometrical J., in press.
- 14.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, Svennerholm A-M. Bacterial enteric infections and vaccine development. Gastroenterol Clin N Am. 1992;21:283–302. [PubMed] [Google Scholar]
- 16.Holmgren, J., C. Czerkinsky, N. Lycke, and A.-M. Svennerholm. 1994. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen carrier and adjuvant. Am. J. Trop. Med. Hyg. 50(Suppl.):42–54. [PubMed]
- 17.Hopkins S, Kraehenbuhl J-P, Schödel F, Potts A, Peterson D, De Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jertborn M, Svennerholm A-M, Holmgren J. Immunological memory after immunization with oral cholera B subunit-whole-cell vaccine in Swedish volunteers. Vaccine. 1994;12:1078–1081. doi: 10.1016/0264-410x(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 19.Jertborn M, Svennerholm A-M, Holmgren J. Intestinal and systemic immune responses in humans after oral immunizations with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine. 1996;14:1459–1465. doi: 10.1016/s0264-410x(96)00071-0. [DOI] [PubMed] [Google Scholar]
- 20.Johansson E-L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantele A. Antibody-secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine. 1990;8:321–326. doi: 10.1016/0264-410x(90)90088-4. [DOI] [PubMed] [Google Scholar]
- 22.Kilhamn J, Jertborn M, Svennerholm A-M. The kinetics of local and systemic immune responses to an oral cholera vaccine given alone or together with acetylcysteine. Clin Diagn Lab Immunol. 1998;5:247–250. doi: 10.1128/cdli.5.2.247-250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutteh W H, Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Lewis D J M, Novotny P, Dougan G, Griffin G E. The early cellular and humoral immune response to primary and booster oral immunisation with cholera toxin B subunit. Eur J Immunol. 1991;21:2087–2094. doi: 10.1002/eji.1830210917. [DOI] [PubMed] [Google Scholar]
- 26.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 27.Moldoveanu Z, Clements M L, Prince S J, Murphy B R, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 28.Ogra P L, Karzon D T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969;102:1423–1430. [PubMed] [Google Scholar]
- 29.Ogra P L, Karzon D T, Righthand F, MacGillivray M. Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N Engl J Med. 1968;279:893–900. doi: 10.1056/NEJM196810242791701. [DOI] [PubMed] [Google Scholar]
- 30.Parr M B, Parr E L. Mucosal immunity in the female and male reproductive tracts. In: Ogra P L, Lamm M-E, McGhee J R, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 677–689. [Google Scholar]
- 31.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in the human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholera as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svennerholm A-M, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 35.Svennerholm A-M, Jertborn M, Gothefors L, Karim A M M M, Sack D A, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit whole cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 36.Wassén L, Schön K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44:408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- 38.Winner L S, III, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J-P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholera infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]