Abstract
Human granulocytic ehrlichiosis (HGE) is an emerging tick-borne infection with a specific tropism for granulocytes. We previously isolated and cultivated the HGE agent in the promyelocytic leukemia cell line HL-60 and have also demonstrated the susceptibility of both granulocytic and monocytic human marrow progenitors. Circulating monocytes have not been observed to be infected, suggesting that cell susceptibility may be differentiation specific. To evaluate this hypothesis, HL-60 cells were differentiated towards granulocytes (with dimethyl sulfoxide or all-trans retinoic acid) or toward monocytes-macrophages (with 12-O-tetradecanoylphorbol-13-acetate [TPA], gamma interferon, or 1,25-dihydroxyvitamin D3) and then challenged with HGE. HGE binding, internalization, and proliferation were compared in differentiated and untreated control HL-60 cells by immunofluorescence, electron microscopy, and Giemsa staining. Granulocytic differentiation resulted in a doubling of HGE binding and enhanced infection consistent with the agent’s clinical tropism for neutrophils. Granulocytic cells were unable to kill internalized ehrlichiae even after activation induced by N-formyl-Met-Leu-Phe alone or together with tumor necrosis factor alpha. In contrast, monocyte-macrophage differentiation with TPA resulted in complete resistance to infection through at least two distinct mechanisms: (i) reduction in binding and uptake and (ii) killing of any internalized organisms. Diminished binding in TPA-treated cells correlated with their reduced expression of sialyl Lewis x (CD15s), a putative cellular receptor component for HGE. The degree of monocytic differentiation and activation induced (i.e., TPA > gamma interferon > vitamin D3) correlated with resistance to HGE. Thus, HL-60 cells exhibit a striking differentiation-specific susceptibility to HGE. Differentiation-induced changes in bacterial adhesion and killing capacity underlie the tropism of HGE for granulocytic HL-60 cells and, conversely, the resistance of activated macrophages to infection.
The ehrlichiae are obligate intracellular tick-borne pathogens with specific cellular tropisms. Two distinct ehrlichial species have recently emerged as important human pathogens: Ehrlichia chaffeensis, which infects monocytes (17), and the agent of human granulocytic ehrlichiosis (HGE), which is unique for its preferential growth within granulocytes (2, 7). HGE causes an acute febrile illness associated with cytopenias and the presence of bacterial inclusions (morulae) within peripheral blood neutrophils. Infection can be complicated by renal, pulmonary, and neurologic manifestations (1).
We previously isolated and cultivated the etiologic agent of HGE by using the human promyelocytic leukemia cell line HL-60 (9). The short life span of peripheral blood granulocytes and the susceptibility of HL-60 cells to infection also led us to investigate whether human bone marrow progenitors might be targets of HGE infection. Indeed, we found that both granulocytic and monocytic marrow progenitors were susceptible to infection, suggesting the presence of a common receptor and/or pathway of entry in the two cell types (14). However, peripheral blood monocytes have not been observed to be infected in vivo, raising the possibility that the susceptibility of monocytic cells to HGE is determined by their state of differentiation or activation.
Like marrow progenitors, HL-60 cells have the potential to differentiate along both granulocytic and monocytic pathways. HL-60 cells incubated in the presence of dimethyl sulfoxide (DMSO) undergo progressive and terminal differentiation toward mature granulocytes (5, 8, 20). Monocytic differentiation of HL-60 cells can be induced by using a variety of agents (3, 15, 18, 21). For example, the protein kinase C activator 12-O-tetradecanoylphorbol-13-acetate (TPA) is a potent inducer of terminal macrophage differentiation (3, 21). Therefore, we performed detailed studies of the effects of HL-60 cell differentiation upon cellular susceptibility to infection with the agent of HGE.
MATERIALS AND METHODS
Cultivation of the HGE agent.
We used two Midwestern isolates (HGE 2 and 6) made by our laboratory from the blood of patients with acute ehrlichiosis, confirmed to be HGE by genospecies-specific PCR, 16S rDNA sequencing, and serologic testing against Ehrlichia equi and HGE antigens. The HGE isolates were propagated continuously in the HL-60 cell line (CL240; American Type Culture Collection) in RPMI 1640 medium (Celox Laboratories, Hopkins, Minn.) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) and 2 mM l-glutamine as previously described (9). Infection was monitored by examining cytocentrifuged preparations for the presence of intracellular bacteria by both immunofluorescence (see below) and Giemsa staining.
Differentiation of HL-60 cells.
To induce granulocytic differentiation, uninfected HL-60 cells (5 × 105 cells/ml) were incubated for 6 days with 1.25% DMSO (Sigma, St. Louis, Mo.) or 1 μM all-trans retinoic acid (RA; Sigma). RA was freshly made from a 1 mM stock solution prepared in 95% (vol/vol) ethanol and stored at −80°C (final ethanol concentration, 0.01%). Ethanol (0.01%) alone had no effect on growth or differentiation of HL-60 cells.
TPA (Sigma) was prepared as a 1.6 × 10−4 M stock in DMSO and stored at −80°C for ≤6 months. To induce monocytic differentiation, HL-60 cells were incubated in 25-cm2 tissue culture flasks with TPA (1.6 × 10−7 to 1.6 × 10−10 M) freshly prepared from the stock solution in growth medium (final DMSO concentration, 0.01%). Cells (5 × 105/ml) were incubated with TPA for periods ranging from 1 h to 6 days. The resulting adherent cells were harvested for study by vigorous pipetting and/or gentle scraping.
Recombinant gamma interferon (rIFN-γ; (105-U/ml stock solution; Genentech, South San Francisco, Calif.) was prepared in Dulbecco’s phosphate-buffered saline (PBS) (without Ca or Mg) and stored at 4°C. HL-60 cells (3 × 105/ml) were exposed to concentrations ranging from 200 to 2,000 U/ml in growth medium in 24-well tissue culture plates for 4 to 6 days. 1,25-Dihydroxyvitamin D3 (VD3; Calbiochem, La Jolla, Calif.) was prepared as a 10−4 M stock solution by dissolving in 95% ethanol and stored at −20°C. HL-60 cells (3 × 105/ml) were exposed to VD3 for 6 days at concentrations ranging from 1 μM to 1 nM. All manipulations involving VD3 were carried out under subdued light.
Myeloperoxidase and nonspecific esterase staining were performed as markers of granulocytic and monocytic differentiation, respectively, as described previously (12, 16). Nitroblue tetrazolium (NBT) reduction was assayed by incubating 2 × 106 cells in 1 ml of medium with an equal volume of 0.2% NBT (Sigma) dissolved in PBS (pH 7.5) in the presence or absence of 200 ng of freshly diluted TPA for 20 min at 37°C (8). The percentage of cells containing intracellular reduced blue-black formazan deposits was determined by microscopy following Giemsa staining.
Infection of HL-60 cells.
Prior to experiments, cells were washed free from any inducing agent with fresh RMPI 1640 or PBS. Both early-passage (<7) and laboratory-adapted (passage >40) HGE isolates were used, and each experiment was repeated at least three times to confirm results. Cell-free bacterial suspensions were prepared from cultures containing 106 HL-60 cells/ml (>99% infected), passaged through a 27-gauge needle three times to aid in lysis, and then centrifuged at 125 × g for 10 min to pellet cell debris. The resulting supernatant was centrifuged at 1,236 × g for 15 min at 4°C to produce a bacterial pellet that was used to inoculate differentiated and untreated HL-60 cells (approximate inoculum, 10 organisms/cell).
Assays of binding, CD15s expression, and infection.
Binding assays were performed both in the presence and in the absence of serum (heat-inactivated fetal bovine serum) with similar results. Cells and bacteria were coincubated for 15 min on ice to allow binding to take place. Cells were then vigorously washed with PBS at 4°C (to remove unbound bacteria) and brought to a final concentration of 3 × 105 cells/ml in growth medium. Samples (100 μl) were immediately removed, and cytospin slides were prepared and fixed in a 1:1 solution of methanol and acetone. For the detection of HGE antigens, serum from a patient who had recovered from culture-proven HGE (HGE immunofluorescence assay titer, 1:5,120) was diluted 1:500 in Tris-buffered saline with 3% bovine serum albumin and detected by secondary labeling with rhodamine-conjugated anti-human immunoglobulin G (Organon Teknika, West Chester, Pa.). Slides were examined for epifluorescence by an observer blinded as to treatment group. The number of rhodamine-fluorescing bacteria adhering to cells was counted for >200 cells to derive mean binding for control and differentiated cells. In other experiments, double labeling of HGE antigens and CD15s was performed as described above with the addition of the anti-CD15s monoclonal antibody CSLEX-1 (Becton Dickinson, San Jose, Calif.) followed by secondary labeling with fluorescein-conjugated anti-mouse immunoglobulin M (Organon Teknika). The degree of cell surface CD15s expression was graded as follows: 4+, brilliant; 3+, bright; 2+, moderate; 1+, faint; and 0, none. To study the course of infection, cells remaining after binding assays were transferred to a 24-well tissue culture plate and incubated at 37°C and 5% CO2. Samples were taken 2, 4, 6, 24, and 48 h after HGE inoculation.
Electron microscopy.
DMSO-treated, TPA-treated, and uninduced HL-60 cells (106 cells/ml) were inoculated with cell-free bacterial preparations (see above), washed in RPMI 1640 to remove unbound bacteria, and then transferred to 25-cm2 tissue culture flasks for incubation. Samples (1 ml) were centrifuged at 200 × g to pellet cells, medium was removed, and cell pellets were overlaid with a primary fixative comprised of 0.1 M sodium phosphate buffer with 2.5% glutaraldehyde, 4% paraformaldehyde, and 0.1 M sucrose (pH 7.2). Following shipment to the Rocky Mountain Laboratory, cells were processed as previously described (19). Briefly, samples were postfixed with reduced osmium (0.5 to 1%), subjected to a mordanting process using 0.1% tannic acid, and then stained en bloc with 1% aqueous uranyl acetate (pH 3.9) overnight. Samples were next infiltrated with resin, sectioned, and further stained with 1% uranyl acetate and lead citrate or alternately with 1% KMnO4.
Stimulation of DMSO-treated cells.
The following stock solutions were prepared: tumor necrosis factor alpha (TNF-α; Gibco BRL, Grand Island, N.Y.), 105 U/ml in PBS; granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, Calif.), 1 μg/ml in PBS with 0.03% bovine serum albumin; and N-formyl-Met-Leu-Phe (FMLP; Sigma), 0.02 M in DMSO. DMSO-treated HL-60 cells (3.5 × 105 cells/ml) were stimulated with either G-CSF (50 ng/ml), FMLP (10−7 M), TNF-α (100 U/ml), or a combination of FMLP and TNF (6, 13) either immediately after HGE challenge or 48 h later (once infection was well established) and compared with unstimulated controls. The number of cells containing intracellular inclusions was determined 48 to 72 h after stimulation.
Statistics.
Data were entered into a Microsoft Excel database, and groups were compared by using the two-tailed Student t test with α = 0.05.
RESULTS
Granulocytic differentiation enhances susceptibility to infection with the HGE agent.
After 6 days of exposure to 1.25% DMSO, 40 to 50% of HL-60 cells demonstrated granulocytic morphology and were myeloperoxidase positive. Greater than 90% of DMSO-treated cells reduced NBT (compared with <5% of untreated controls), indicating that functional differentiation had also taken place. In repeated experiments, DMSO-treated cells were ≥2 times more susceptible to HGE infection than untreated controls (compare Fig. 1E and D). The mean proportions of cells infected at 48 h were 70% DMSO-treated cells and 29% untreated HL-60 controls (P < 0.001) (Table 1). Infection also progressed more rapidly in DMSO-differentiated cells; DMSO-treated cells reached terminal stages of infection 3 to 4 days after inoculation, compared with 7 days for controls. Treatment with RA (1 μM), which also induced granulocytic differentiation of HL-60 cells, similarly resulted in enhanced susceptibility to HGE infection, although its effect was less pronounced (Table 1). DMSO treatment was therefore used to induce granulocytic differentiation in subsequent studies.
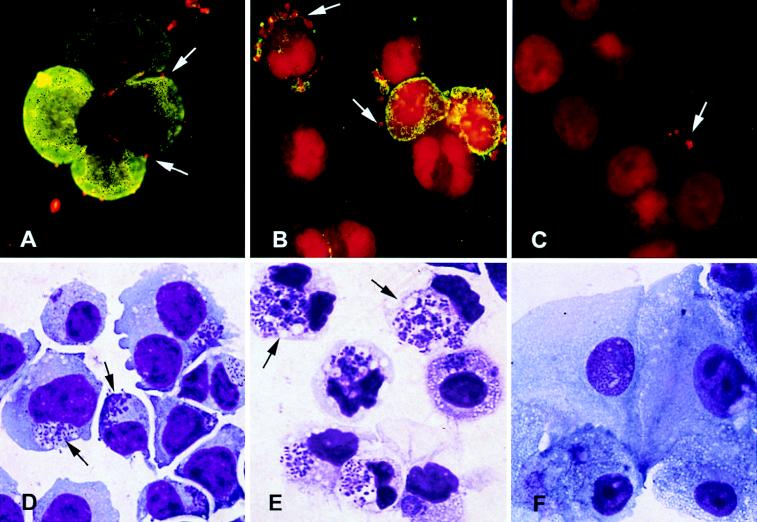
FIG. 1.
Photomicrographs of untreated and differentiated HL-60 cells incubated with the agent of HGE. (A to C) Immunofluorescence with rhodamine labeling of HGE organisms and fluorescein labeling of CD15s 15 min after HGE inoculation (magnification, ×3,300). (D to F) Giemsa stain 48 h after inoculation with HGE (magnification, ×3,300). (A) HGE binding to uninduced HL-60 cells which express high levels of CD15s; (B) increased binding of HGE to granulocytic DMSO-treated cells, particularly to those cells which most express CD15s; (C) reduced bacterial binding to TPA-treated monocytic cells which are devoid of CD15s expression; (D) infection of control uninduced HL-60 cells; (E) increased susceptibility to infection of DMSO-treated cells, as evidenced by larger and more abundant morulae; (F) absence of bacterial replication in TPA-treated cells.
TABLE 1.
Binding and infection of untreated and differentiated HL-60 cells following HGE inoculation
| Inducing agent | Bacteria bound/cella | Cells infected (%)b
|
|
|---|---|---|---|
| 48 h | 7 days | ||
| None | 4.2 ± 1.4 | 29 | >99 |
| Granulocytic inducers | |||
| DMSO | 8.6 ± 3.2c | 70d | >99 |
| RA | NDe | 38 | >99 |
| Monocytic inducers | |||
| TPA | 2.0 ± 0.8d | 0d | 0d |
| γ-IFN | 3.0 ± 2.9 | 10d | 1d |
| VD3 | 3.4 ± 2.6 | 21f | >99 |
Mean ± standard deviation (four samples per group).
Mean percentage of cells infected (four samples per group).
P = 0.07 compared with untreated controls, Student’s t test.
P < 0.05 compared with untreated controls, Student’s t test.
ND, not done.
P = not significant compared with other monocytic inducers and control uninduced cells.
Stimulation of DMSO-treated HL-60 cells with G-CSF, TNF-α, FMLP, or the combination of TNF-α and then FMLP immediately after HGE inoculation did not prevent the development of infection. Furthermore, 48 h after infection was established, such stimulation did not induce killing of the HGE. Despite their inability to kill HGE, infected DMSO-treated cells continued to reduce NBT normally.
Monocytic differentiation results in resistance to HGE infection.
Greater than 95% of cells treated with TPA became clumped, adherent, and strongly nonspecific esterase positive within 24 h. Forty percent of TPA-treated cells reduced NBT. In contrast to the enhancement of infection observed following granulocytic differentiation with DMSO or RA, as little as a 1-h exposure to TPA (>1 nM) rendered HL-60 cells resistant to HGE infection. When examined 3 days following inoculation, none of the TPA-treated HL-60 cells contained bacterial colonies (Fig. 1F), compared with 29% of untreated controls. Seven days following inoculation, TPA-treated cells remained completely uninfected whereas >99% of untreated HL-60 cells were infected. Occasionally, some TPA-treated cells (i.e., 1 to 5%) contained solitary intracellular bacilli 24 to 48 h after inoculation but never developed classic membrane-enveloped ehrlichial colonies (morulae), suggesting that bacterial replication did not take place. Treatment of HL-60 cells with rIFN-γ in concentrations of ≥1,000 U/ml also resulted in differentiation along the monocytic pathway. However, rIFN-γ-treated cells, unlike those treated with TPA, continued to proliferate and generally remained nonadherent. In addition, only 60% demonstrated monocytic morphology, indicating that many cells still retained undifferentiated features. rIFN-γ-treated cells demonstrated intermediate susceptibility to HGE: up to 23% of cells supported ehrlichial growth initially (48 h) but the infection diminished over time, with fewer than 10% of cells observed to be infected at 7 days postinoculation. Interestingly, although treatment with VD3 in concentrations as high as 1 μM resulted in some features of monocytic differentiation such as adherence (20 to 40%) and NBT reduction (65%), VD3-treated cells remained susceptible to HGE (Table 1).
Susceptibility to infection in differentiated cells correlates with bacterial adhesion and invasion.
To explore potential mechanisms for the differentiation-specific susceptibility to HGE, bacterial adherence to differentiated and untreated control HL-60 cells was assayed (four independent experiments). DMSO-treated granulocytic cells bound a mean of 8.6 ± 3.2 bacteria/cell, compared with 4.2 ± 1.4 bacteria/cell bound by untreated controls (P = 0.07). Approximately 20% of DMSO-treated cells were “hyperbinders,” binding >20 bacteria/cell, while such cells were rarely noted in untreated controls (Fig. 1A and B). In contrast, TPA-treated monocytic HL-60 cells exhibited a twofold reduction in binding compared with controls (mean of 2.0 ± 0.8 bacteria/cell; P = 0.04). Many TPA-treated cells exhibited no bacterial binding (Fig. 1C). HGE adhesion to rIFN-γ- and VD3-treated cells did not differ significantly from that observed in control HL-60 cells (means of 3.0 ± 2.9 and 3.4 ± 2.6 bacteria/cell, respectively).
Electron microscopy revealed that DMSO-treated cells rapidly ingested ehrlichiae; many cells contained bacterial inclusions as early as 20 min postinoculation (Fig. 2B). In contrast, untreated HL-60 cells rarely exhibited internalized organisms until 4 to 6 h following inoculation (Fig. 2A). By 24 h, both DMSO-treated and untreated HL-60 cells demonstrated typical bacterial replication within endosomes. Again, DMSO-treated cells became more heavily infected than controls (not shown).
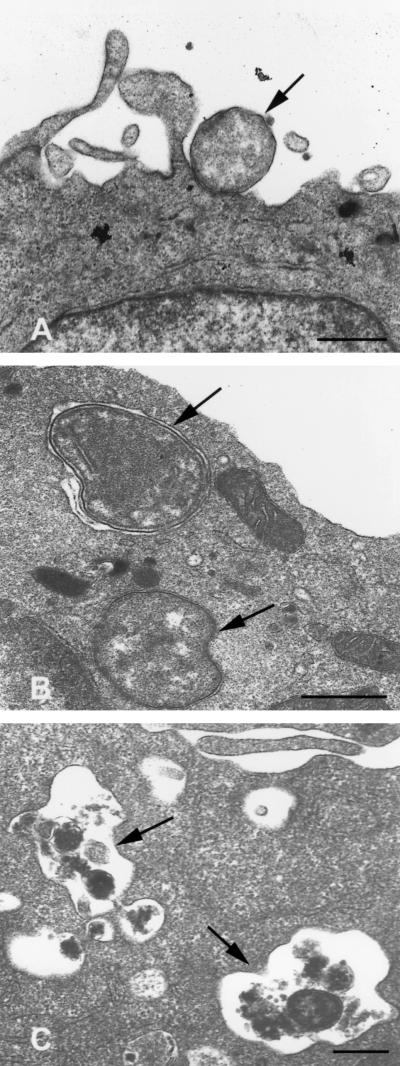
FIG. 2.
Transmission electron micrographs of untreated and differentiated HL-60 cells incubated with the agent of HGE. These micrographs were derived from the same experiment and were chosen to illustrate the altered interactions between HL-60 cells and the HGE agent resulting from differentiation. (A) Untreated HL-60 cells exhibit binding, but no internalization, of ehrlichiae 4 h after inoculation. (B) DMSO-treated granulocytic cells more rapidly internalize ehrlichiae, shown 20 min postinoculation. (C) TPA-treated monocytoid cells destroy internalized ehrlichiae, shown 2 h following inoculation. Scale bars = 0.5 μm.
Bacterial binding to differentiated cells correlates with their surface CD15s expression.
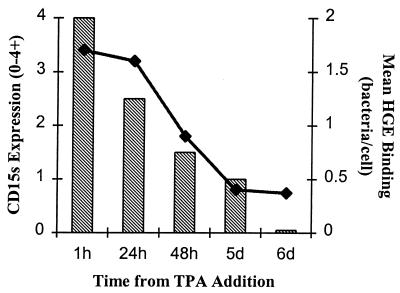
Recently we demonstrated that the leukocyte cell surface carbohydrate CD15s (sialyl Lewis x) plays an important role in HGE binding to and infection of susceptible cells (10). To evaluate whether differentiation-specific changes in bacterial binding correlated with cellular CD15s expression, we treated HL-60 cells with DMSO or TPA for 6 days and compared CD15s expression and bacterial adherence with results for uninduced controls. DMSO-treated cells expressed CD15s at levels similar to those for untreated HL-60 cells but with a greater degree of heterogeneity. Cells with the highest amount of expression clearly bound the most bacteria (Fig. 1B). In contrast, the low-binding TPA-treated cells were devoid of CD15s expression (Fig. 1C). In other experiments, we exposed HL-60 cells to TPA for various periods of time from 1 h through 6 days and evaluated CD15s expression and bacterial binding. Both CD15s expression and bacterial adhesion diminished gradually over time (Fig. 3). Both rIFN-γ-treated and VD3-treated cells continued to express CD15s and bound HGE normally (see above).
FIG. 3.
CD15s expression and binding of the agent of HGE relative to duration of TPA exposure in HL-60 cells. CD15s expression is graded as follows: 4+, brilliant; 3+, bright; 2+ moderate; 1+, faint; and 0, none. The data represent mean numbers of bacteria adherent per cell as determined by counting >200 cells.
Monocytic differentiation results in killing of the HGE agent.
Although diminished in number, those bacteria which bound to TPA-treated cells were rapidly internalized (<2 h), as observed both by indirect immunofluorescence and transmission electron microscopy. However, unlike susceptible cells, any organisms which entered TPA-treated cells were noted to undergo degradation within 2 to 4 h (Fig. 2C). No microscopic evidence of infection remained by 24 h. Furthermore, the addition of TPA to undifferentiated HL-60 cells which already contained well-established infection (i.e., 60% of cells exhibiting morulae) led to the prompt inhibition of further bacterial replication and resulted in the eradication of existing infection within 72 h. As a control to exclude direct toxicity of TPA for the HGE agent, bacteria were incubated with 10−7 M TPA for 30 min. These bacteria remained viable and fully capable of establishing infection in susceptible cells.
DISCUSSION
We have demonstrated differentiation-specific susceptibility of HL-60 cells to infection with the agent of HGE—remarkable in that it parallels closely the clinical tropism of this unique organism. Differentiation of HL-60 cells toward granulocytes enhances susceptibility to infection, consistent with the observed tropism of the HGE agent for neutrophils. Granulocytic HL-60 cells exhibit increased binding and uptake of the organism, which they are then unable to kill. Enhanced adhesion and invasion alone may account for increased susceptibility of these cells to infection. It is also possible that additional factors such as alterations in the intracellular environment or available nutrients render granulocytic cells more supportive of ehrlichial growth.
Similar to mature granulocytes, DMSO-treated HL-60 cells have been shown to become chemotactic, undergo degranulation, produce oxidative products in response to stimulation, ingest latex beads, and kill Staphylococcus aureus (5, 8, 20) and therefore should be well armed to kill the HGE agent. The DMSO-treated cells used in these experiments were indeed able to reduce NBT (both when infected and when uninfected). However, even when stimulated with G-CSF or TNF and/or FMLP, DMSO-treated cells were unable to kill the organism, suggesting that the HGE agent inhibits, or is resistant to, the cell’s microbicidal armamentarium. Neutrophils from sheep experimentally infected with Ehrlichia phagocytophila (genetically closely related, if not identical, to HGE) have been reported to have diminished phagocytosis and killing of S. aureus, suggesting that ehrlichial infection may globally impair neutrophil function (25).
Treatment of HL-60 cells with RA also enhanced HGE infection, confirming that increased susceptibility occurred independently of the agent used to induce granulocytic differentiation. Recently, it was reported that diagnostic culture of granulocytic ehrlichiae from both equine and human sources could be enhanced by the addition of RA to HL-60 cells in a culture system that used PCR as a means of detection (11).
Monocyte-macrophage differentiation, most strikingly that induced by TPA, resulted in restriction of infection through at least two mechanisms: (i) diminished bacterial binding and entry and (ii) growth inhibition and subsequent killing of internalized organisms. Recently we have demonstrated that CD15s, a carbohydrate expressed on the surface of leukocytes that normally functions as a ligand for endothelial selectins, also is critical for HGE binding and subsequent infection of leukocytes (10). Bacterial adhesion in TPA-treated cells correlated with their degree of cell surface CD15s expression. Thus, the observed twofold reduction in HGE binding to TPA-differentiated cells may well be related to the modification or down regulation of CD15s. Diminished binding alone, however, was not the sole mechanism of TPA-induced resistance to HGE infection. First, as little as 1 h of exposure to TPA, which did not immediately alter either cell surface CD15s expression or HGE binding, rendered cells resistant to infection. Furthermore, even after HGE infection of uninduced HL-60 cells was well established, the addition of TPA resulted in inhibition of further bacterial replication and the eradication of existing infection. Similarly, rIFN-γ treatment did not reduce binding of the HGE agent or significantly alter CD15s expression, but it did substantially inhibit subsequent infection. Taken together, these findings suggest that in monocytic-differentiated cells, regulation of HGE binding and uptake may be dissociated from the cell’s bacteriostatic and bactericidal properties. For the complete prevention of HGE infection, diminished binding and uptake and the ability to kill HGE all appear to be required.
Both granulocytic and monocytic differentiated HL-60 cells possess potent antimicrobial defenses similar to those of peripheral blood granulocytes and monocytes. Both express Fc receptors and are capable of phagocytosis, degranulation, and oxidative killing (3, 5, 8, 15, 20, 21). What accounts for the ability of monocytic, but not granulocytic, cells to kill the HGE agent therefore remains enigmatic. TPA-induced phosphorylation of surface receptors, such as transferrin or other important signalling proteins, could have profound effects on the growth of an intracellular organism such as HGE (23, 24).
In contrast to the inhibition of HGE growth by both TPA and rIFN-γ, VD3 in concentrations as high as 10−6 M did not result in resistance to infection. Treatment of HL-60 cells with VD3 induces many features of monocytic differentiation including phagocytosis, α-naphthyl acetate esterase activity, and the release of superoxide anions (15, 18). However VD3-treated cells continue to express granulocyte-specific markers such as CD44, FMC10, and FMC12, demonstrate less adherence, and continue to proliferate (4, 18). Thus, VD3 treatment results in a less differentiated phenotype than TPA treatment—a feature that has been attributed to the induction of distinct protein kinase C signal pathways (22).
The greater degree of terminal macrophage differentiation achieved with TPA was accompanied by the highest degree of resistance to HGE infection. Similarly, marrow-derived monocytic cells support the growth of the HGE agent (14) but peripheral blood monocytes have not been observed to be infected in vivo. Our observations suggest that through differentiation and/or activation, permissive monocytes can acquire the ability to kill the organism. Activated monocytes are therefore likely to be important effectors in the host response to HGE.
The observed differentiation-specific susceptibility of HL-60 cells to infection by the HGE agent is remarkable in that it closely parallels events in natural infection. In addition, differentiation of HL-60 cells provides a model for further studies to define critical cellular and molecular determinants underlying the remarkably evolved and restricted tropism of this organism. A greater understanding of how, on the one hand, HGE is able to survive within the hostile environment of the granulocyte while, on the other hand, it can be killed by differentiated monocytic cells may help shed new light both on phagocyte function and on the mechanisms that intracellular pathogens adopt to evade host defenses.
ACKNOWLEDGMENTS
This work was supported by grants 1R01AI40952-01 to J.L.G. and A107421 to M.B.K. from NIH-NIAID.
We are grateful to Curt Nelson, Janet Larson, and Jeffrey Miller for helpful input.
REFERENCES
- 1.Bakken J S, Kreuth J, Wilson-Nordskog C, Tilden R L, Asanovitch K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 2.Bakken J S, Dumler J S, Chen S-M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper Midwest United States. JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 3.Ball E D, Guyre P E, Shen L, Glynn J M, Maliszewski C R, Baker P E, Fanger M W. Gamma interferon induced monocytoid differentiation in the HL-60 cell line. J Clin Invest. 1984;73:1072–1077. doi: 10.1172/JCI111292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brackman D, Lund-Johansen F, Aarskog D. Expression of leukocyte differentiation antigens during differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3: comparison with the maturation of normal monocytic and granulocytic bone marrow cells. J Leukocyte Biol. 1995;58:547–555. doi: 10.1002/jlb.58.5.547. [DOI] [PubMed] [Google Scholar]
- 5.Brietman T E, Selonick S E, Collins S J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning D D, Pan Z K, Prossnitz E R, Ye R D. Cell type- and developmental stage-specific activation of NF-kB by fmet-leu-phe in myeloid cells. J Biol Chem. 1997;272:7995–8001. doi: 10.1074/jbc.272.12.7995. [DOI] [PubMed] [Google Scholar]
- 7.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;23:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins S J, Ruscetti F N, Gallagher R E, Gallo R C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation with dimethylsulfoxide. J Exp Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman J L, Nelson C N, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 10.Goodman, J. L., C. N. Nelson, M. B. Klein, S. F. Hayes, and B. W. Weston. Submitted for publication.
- 11.Heimer R, Van Andel A, Wormser G P, Wilson M L. Propagation of granulocytic Ehrlichia spp. from human and equine sources from HL-60 cells induced to differentiate into functional granulocytes. J Clin Microbiol. 1997;35:923–927. doi: 10.1128/jcm.35.4.923-927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplow L S. Simplified myeloperoxidase staining using benzidine dihydrochloride. Blood. 1965;26:21–29. [PubMed] [Google Scholar]
- 13.Khwaja A, Carver J E, Linch D C. Interactions of granulocyte-macrophage colony-stimulating factor (CSF), granulocyte CSF, and tumor necrosis factor alpha in priming of the neutrophil respiratory burst. Blood. 1992;79:745–753. [PubMed] [Google Scholar]
- 14.Klein M B, Miller J S, Nelson C N, Goodman J L. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J Infect Dis. 1997;176:1405–1409. doi: 10.1086/517332. [DOI] [PubMed] [Google Scholar]
- 15.Kreutz M, Adreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990;76:2457–2461. [PubMed] [Google Scholar]
- 16.Li C, Lam K, Yi L T. Esterase in human leukocytes. J Histochem Cytochem. 1973;21:1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Maeda K, Markowitz N, Hawely R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rikettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy D M, San Miguel J F, Freake H C, Green P M, Zola H, Catovsky D, Goldman J M. 1,25-Dihydroxyvitamin D3 inhibits proliferation of human promyelocytic leukemia (HL-60) cells and induces monocyte-macrophage differentiation in HL-60 cells and normal bone marrow cells. Leuk Res. 1983;7:51–55. doi: 10.1016/0145-2126(83)90057-7. [DOI] [PubMed] [Google Scholar]
- 19.Munderloh U G, Madigan J E, Dumler J S, Goodman J L, Hayes S F, Barlough J E, Nelson C N, Kurtti T J. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J Clin Microbiol. 1996;34:664–760. doi: 10.1128/jcm.34.3.664-670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newberger P E, Chaovaniec M E, Greenberger J S, Cohen H J. Functional changes in the human leukemic cell line (HL-60) J Cell Biol. 1979;82:315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovera G, Santoli D, Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with phorbol diester. Proc Natl Acad Sci USA. 1979;76:2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and by 1,25-dihydroxyvitamin D3. J Leukocyte Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- 23.Testa U, Titeux M, Louache F, Thomopoulos P, Rochant H. Effect of phorbol esters on iron uptake in human hematopoietic cell lines. Cancer Res. 1984;44:4981–4986. [PubMed] [Google Scholar]
- 24.Vandenbark G R, Niedel J E. Phorbol diesters and cellular differentiation. J Natl Cancer Inst. 1984;73:1013–1018. [PubMed] [Google Scholar]
- 25.Woldehiwet Z. The effects of tick-borne fever on some functions of polymorphonuclear cells of sheep. J Comp Pathol. 1987;97:481–485. doi: 10.1016/0021-9975(87)90026-0. [DOI] [PubMed] [Google Scholar]