Abstract
Background
Artificial tendons may be an effective alternative to autologous and allogenic tendon grafts for repairing critically sized tendon defects. The goal of this study was to quantify the in vivo hindlimb biomechanics (ground contact pressure and sagittal-plane motion) during hopping gait of rabbits having a critically sized tendon defect of the tibialis cranialis and either with or without repair using an artificial tendon.
Methods
In five rabbits, the tibialis cranialis tendon of the left hindlimb was surgically replaced with a polyester, silicone-coated artificial tendon (PET-SI); five operated control rabbits underwent complete surgical excision of the biological tibialis cranialis tendon in the left hindlimb with no replacement (TE).
Results
At 8 weeks post-surgery, peak vertical ground contact force in the left hindlimb was statistically significantly less compared to baseline for the TE group (p = 0.0215). Statistical parametric mapping (SPM) analysis showed that, compared to baseline, the knee was significantly more extended during stance at 2 weeks post-surgery and during the swing phase of stride at 2 and 8 weeks post-surgery for the TE group (p < 0.05). Also, the ankle was significantly more plantarflexed during swing at 2 and 8 weeks postoperative for the TE group (p < 0.05). In contrast, there were no significant differences in the SPM analysis among timepoints in the PET-SI group for the knee or ankle.
Conclusions
Our findings suggest that the artificial tibialis cranialis tendon effectively replaced the biomechanical function of the native tendon. Future studies should investigate (1) effects of artificial tendons on other (e.g., neuromuscular) tissues and systems and (2) biomechanical outcomes when there is a delay between tendon injury and artificial tendon implantation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-04581-7.
Keywords: Gait, Kinematics, Kinetics, Animal model, Biomaterials
Background
Injuries to tendons and ligaments account for at least 4% of all musculoskeletal trauma cases [1]. Critically sized tendon defects (i.e., those too large to heal spontaneously), or gaps, are especially debilitating because tendons perform essential biomechanical functions during movement, including storing elastic energy and transmitting forces between muscles and bones. Such defects may form at the time of severe trauma [2, 3] or with chronic tendon ruptures for which the ruptured ends cannot be approximated due to muscle retraction [4]. Autologous tendon grafts represent the current gold-standard and are the most common clinical treatment [5], although allogenic grafts are also used [6]. Clinical use of autologous grafts is limited by donor site morbidity [7], while allografts present biosafety concerns and depend on a sufficient supply of donor tendons. Functional outcomes with tendon grafts are modest, with less than half of patients achieving “excellent” function based on standardized clinical assessments [5, 8, 9].
Artificial tendons that permanently replace part or all of a biological tendon may be an effective alternative to tendon grafts for critically sized tendon defects. Many types of artificial tendons of varying designs and materials have been tested [10–13]. One recent polyester, silicone-coated (PET-SI) artificial tendon was tested in rabbits [14] and goats [15–17] for up to 180 days; the artificial tendon integrated closely with the muscle fibers with no apparent scarring; the muscle-artificial tendon interface was stronger than the muscle itself. Similar artificial tendons have been used in humans [18, 19]. However, despite the critical biomechanical role of tendons during movement, the effect of the artificial tendons on movement biomechanics has not been rigorously quantified.
We recently reported the hindlimb biomechanics of rabbits with surgical replacement of either the tibialis cranialis or Achilles biological tendon with a PET-SI artificial tendon [20]. For both groups, ankle kinematics and vertical ground contact forces during the stance phase of hopping gait recovered from 2–6 weeks postoperative toward those measured pre-surgery. Three key limitations of this previous study, which motivated the study presented herein, were small treatment groups (n = 2), measurements limited to hindlimb biomechanics during the stance phase of hopping gait, and failure of the Achilles artificial tendon at the point of attachment of the artificial tendon to the bone anchor prior to the study endpoint.
The present study was performed to focus on the effect of critically sized defects of the tibialis cranialis tendon on functional mechanics of the hind limb. Our objective was to quantify unilateral hindlimb biomechanics during the entire hopping gait cycle of rabbits having loss of the tibialis cranialis tendon as compared with rabbits having the defect repaired with an artificial tibialis cranialis tendon. Since the tibialis cranialis is an ankle dorsiflexor muscle, our a priori hypotheses were that compared to rabbits with tibialis cranialis tendon excision, rabbits with the artificial tendon would have greater (1) maximum ankle dorsiflexion angle and (2) ankle range of motion during the swing phase of gait.
Methods
Artificial Tendon
The artificial tendon, adapted from a previously reported design [14–17], consisted of two custom double-armed strands of size 0 braided polyester suture (RK Manufacturing, Danbury, CT, USA) (Fig. 1). The strands were folded in half and braided for the desired length of the artificial tendon; tendons of varying lengths were fabricated in 2 to 4 mm increments to accommodate variation in lengths of the biological tendons they replaced. The folded distal end formed a loop to facilitate attachment to bone with a suture anchor; the proximal end had swaged needles on each strand for suturing the tendon to muscle. The braid was coated with biocompatible silicone (LSR BIO M340, Elkem, Oslo, Norway) to discourage tissue adhesion.
Fig. 1.
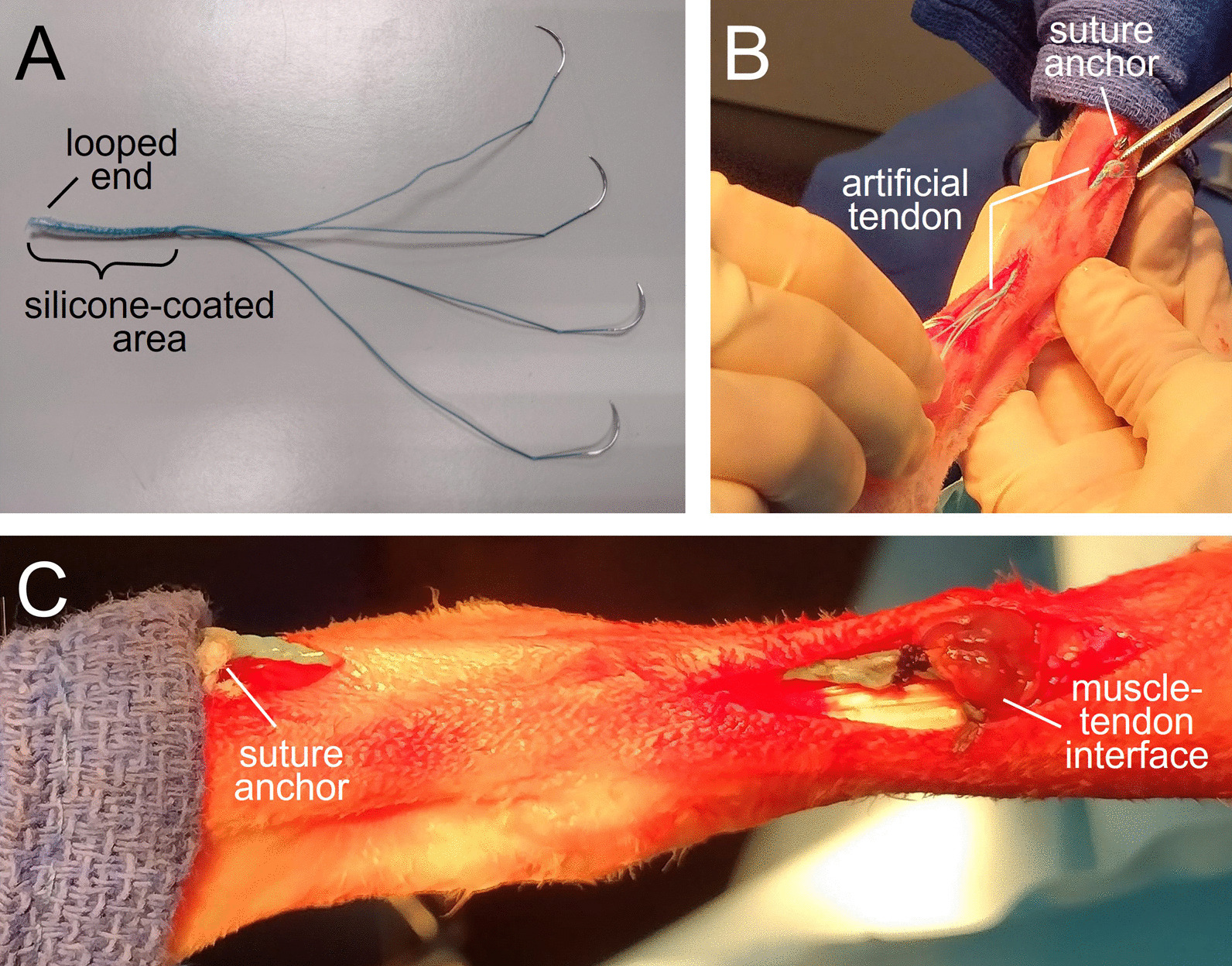
Polyester, silicone-coated (PET-SI) artificial tendon. A Artificial tendon prior to implantation. The looped end was tied to a suture anchor, while the needle ends were sewn to the distal end of the tibialis cranialis muscle. B Intraoperative placement of the artificial tendon using two separate skin incisions. C Completed implantation of artificial tendon prior to closure of incisions
Tendon replacement model
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Tennessee-Knoxville (protocol #2726). All procedures were performed at the University of Tennessee-Knoxville. The study included ten healthy female New Zealand White rabbits (Robinson Services Inc, USA), ranging from 17 to19 weeks old weighing an average of 3.64 ± 0.31 kg (Table 1). Rabbits were housed individually, acclimatized for a minimum of 2 weeks prior to surgery, fed ad libitum (standard laboratory diet, Timothy hay, daily greens), and given daily positive human interaction and enrichment. In addition, rabbits received playpen time twice weekly for at least 10 min prior to surgery and starting 2 weeks post-surgery.
Table 1.
Rabbit demographics and biological and artificial tendon lengths for the tendon excision only and tendon excision with replacement groups
| Age (wks) | Weight at surgery (kg) | Weight at euthanasia (kg) | Biological tendon length (mm) | Artificial tendon length (mm) | Percent biological tendon (%) | |
|---|---|---|---|---|---|---|
| Tendon excision only group | ||||||
| TE1 | 17.4 | 3.08 | 3.9 | 62 | NA | NA |
| TE2 | 17.4 | 3.62 | 4.63 | 60 | NA | NA |
| TE3 | 17.4 | 3.74 | 4.74 | 58 | NA | NA |
| TE4 | 19.4 | 4.02 | 4.87 | 43 | NA | NA |
| TE5 | 19.4 | 3.85 | 4.61 | 51 | NA | NA |
| Mean (std) | 18.2 (1.1) | 3.66 (0.36) | 4.55 (0.38) | 54.8 (7.8) | ||
| Tendon excision and replacement group | ||||||
| PET-SI1 | 19.1 | 4 | 4.65 | 50 | 44 | 88.0 |
| PET-SI2 | 17.4 | 3.69 | 4.36 | 50 | 45 | 90.0 |
| PET-SI3 | 17.4 | 3.21 | 4.26 | 50 | 46 | 92.0 |
| PET-SI4 | 19.4 | 3.75 | 4.56 | 55 | 51 | 92.7 |
| PET-SI5 | 19.4 | 3.42 | 4.47 | 53 | 45 | 84.9 |
| Mean (std) | 18.6 (1.0) | 3.6 (0.31) | 4.5 (0.16) | 51.6 (2.3) | 46.2 (2.8) | 89.5 (3.2) |
The rabbits were randomly assigned to either the excision only group (TE, n = 5) or the group with replacement of the tendon with a polyester, silicone-coated artificial tendon (PET-SI, n = 5). The randomization sequence was generated using a custom script in MATLAB (MathWorks, Inc., Natick, MA, USA). To reduce the number of animals, no control group was used; values of variables measured pre-surgery were considered reference or control values. An experimental unit was defined as one rabbit. Potential confounders (e.g., time of surgery, housing location) were not controlled.
The results reported herein are part of a larger overall study that also included measurement of muscle properties (results forthcoming). Thus, the number of animals per group, n = 5 (Fig. 5), was computed a priori based on a power analysis that considered muscle property values reported in the literature. Specifically, following surgical tenotomy, rabbit soleus muscle mass decreased by 25% compared to intact muscle at four weeks post-tenotomy [21]. We conducted a power analysis (G*Power 3.1, Heinrich-Heine-Universität Düsseldorf, DE) to compute the sample size required to detect recovery of 20% over time in the PET-SI group. For a between-timepoint comparison using a two-tailed Student’s t-test, a per-group sample size of n = 4 is needed to detect an effect size of 3.46 (i.e. 20% recovery) with power and significance . We increased the per-group sample size to n = 5 to conservatively account for potentially higher within-group variation of muscle mass and other outcome measures.
Fig. 5.
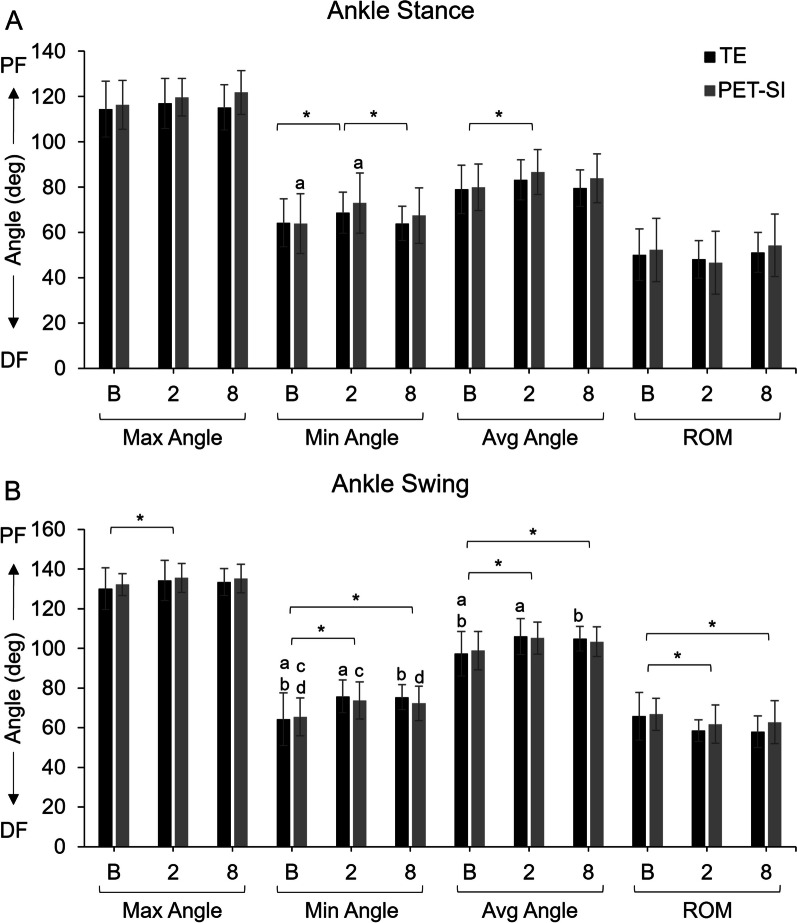
Mean maximum, minimum, and average ankle joint angles and ankle range of motion (ROM) and standard deviation for the tendon excision (TE) only group and tendon excision and replacement (PET-SI) group at baseline (B, pre-surgery), 2 weeks post-surgery, and 8 weeks post-surgery during A stance phase of gait, and B swing phase of gait. PF – Plantarflexion; DF – Dorsiflexion. A larger angle indicates greater plantarflexion/less dorsiflexion. * indicates significant (p < 0.05) differences between timepoints across groups. a, b indicate significant (p < 0.05) differences between timepoints within a group. Table 3 gives specific p-values for each comparison
Rabbits were given hydromorphone (0.1 mg/kg IM) as a preoperative analgesic, sedated with midazolam (1 mg/kg IM), and induced into general anesthesia with isoflurane via face mask. Rabbits were intubated and positioned in right lateral dorsal oblique recumbency. General anesthesia was maintained with isoflurane gas vaporized in 100% oxygen. A loading dose of lidocaine (2 mg/kg IV) was given, followed by a lidocaine CRI (50 mcg/kg/min IV) with isotonic fluids at a rate of 30 ml/h IV throughout the procedure. The left hind limb was clipped, suspended, and aseptically prepared for surgery. A second dose of hydromorphone (0.05 mg/kg IM) was given just prior to the start of surgery.
In the TE group, 1 cm and 2 cm incisions were made over the point of insertion and musculotendinous junction, respectively, of the tibialis cranialis muscle. The tendon was excised from the enthesis to the musculotendinous junction.
In the PET-SI group, a 2 cm incision was made over the point of insertion of the tibialis cranialis muscle. The tendon was released at its insertion. A guide hole was pre-drilled with a 1.5 mm drill bit in the proximal metatarsus at the insertion point. A 2 mm × 6 mm bone suture anchor (Jorgenson Laboratories, Loveland, CO, USA) was screwed into the hole until finger tight. A 3 cm incision was made over the musculotendinous junction of the tibialis cranialis muscle. From among the available artificial tendon lengths, we selected a length that was approximately 85—95% of the length of each rabbit’s biological tendon (Table 1) as measured when the foot was in full plantarflexion; the shorter length was selected to offset the length added by the bone suture anchor. The artificial tendon was passed underneath the skin from the proximal incision to the distal incision. The distal loop of the artificial tendon was sutured to the anchor using #2 Fiberwire suture with two passes through the anchor and loop. The needle ends of the artificial tendon were sutured to the distal end of the tibialis cranialis muscle using a single suture loop pattern for each strand. Adjacent suture strands were tied together using six throws, and excess suture was cut and removed. The tibialis cranialis tendon was sharply excised at the musculotendinous junction.
In both groups, the incisions were closed with a double-layer closure. The subcutaneous layer was closed with a simple continuous pattern with 3–0 PDS (synthetic absorbable monofilament). The skin was closed with an intradermal pattern using 3–0 PDS. An incisional block with 0.15 ml of lidocaine (2 mg/ml SC) was performed. The rabbits received meloxicam (1 mg/kg SC) and enrofloxacin (5 mg/kg SC diluted in 6 ml of sterile saline) immediately post-surgery. As part of a standard protocol for management of postoperative pain and inflammation, all rabbits had laser therapy (MultiRadiance ACTIVet Pro, Solon, OH, USA; 1000 Hz for 1 min, 50 Hz for 1 min, and 1000–3000 Hz for 1 min) performed, once, immediately post-surgery. Left lateral and craniocaudal radiographic views were acquired immediately post-operatively and then every 2 weeks post-surgery.
The operated limb was bandaged for three days post-surgery, and silver sulfadiazine topical cream was applied to the incisions. Each rabbit received hydromorphone (1 mg/kg IM; q 6 h for 3 days), enrofloxacin (5 mg/kg PO; q 12 h for 7 days), and meloxicam (1 mg/kg PO; q 24 h for 7 days). Lactated ringer’s solution (150 ml SC) was administered twice daily starting the day after surgery and continuing for 5 doses. Rabbits were weighed at least twice per week for two weeks post-surgery, then at least every other week for the remainder of the study. Our Institutional Animal Care and Use Committee protocol established a priori that a rabbit would be removed from the study by humane euthanasia if (1) its body weight decreased by at least 20% from the pre-surgery and showed no signs of improvement with intervention; (2) there was dehiscence, tissue breakdown, or infection that could not be treated or repaired; or (3) other signs of distress were present that could not be managed.
Prior to surgery and after recovery from surgery, biomechanics and imaging data were collected every other week until the end of the study. During off weeks, in a single session, the rabbits hopped along the walkway six times in each direction (see below). At 8 weeks post-surgery, the rabbits were humanely euthanized by intravenous overdose of pentobarbital (390 mg/ml, minimum 1 ml/10 lbs).
Biomechanics testing
Prior to surgery, rabbits were trained to hop along a 2.6 m-long walkway with an active high-resolution pressure sensing area of 1.3 m (2-Tile High-Resolution Strideway System, Tekscan, Norwood, MA, USA). Reflective 7.5 mm flat circular markers were placed on the lateral aspect of the left limb at the hip (greater trochanter), knee, ankle (lateral malleolus), and 5th metatarsophalangeal (MTP) joint (Fig. 2). Marker trajectories were recorded with three high-speed cameras (Prime 13, OptiTrack, NaturalPoint, Inc, Corvallis, OR, USA) that were placed equidistant from each other and parallel to one side of the walkway in order to capture sagittal plane motion. Pressure and video data were recorded synchronously at 240 Hz. The researchers were not blinded to rabbit group assignment during the biomechanics test sessions.
Fig. 2.
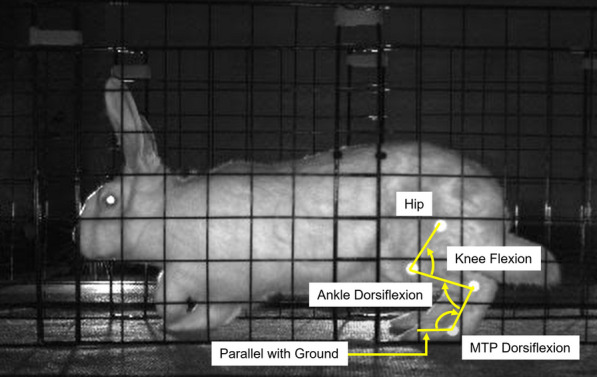
Image of a subject hopping in the walkway. Bright dots are the 7.5 mm flat circular markers located at the hip, knee, ankle, and 5th metatarsophalangeal (MTP) joints. Arrows indicate direction for flexion of a given joint
Pressure data were processed using pressure analysis software (Strideway 7.80, Tekscan, Norwood, MA, USA) and custom written MATLAB scripts (MATLAB 2022a, MathWorks, Natick, MA, USA). Videos were exported from the motion capture software (Motive:Tracker 1.9, OptiTrack, NaturalPoint, Inc. Corvallis, OR, USA) and marker position data was initially obtained using DeepLabCut [22]. Custom written MATLAB scripts were used to manually verify and adjust the marker positions, calculate sagittal plane joint angles for the knee, ankle, and MTP joints, and combine the joint angle data from the 3 cameras into one time-series curve for the entire length of the walkway. MTP angle during stance was calculated using the ankle marker and a ground plane defined as a horizontal line on the video frames (Fig. 2). The researchers who processed the data were not involved in the surgery or data collection and were blinded to rabbit group assignment.
Data analysis
Statistical analysis software (SAS 9.4, SAS Institute, Cary, NC, USA) was used to perform a two-factor (group, timepoint, and group*timepoint) analysis of variance (ANOVA) with repeated measures (rabbit). A p-value < 0.05 was used to determine any significant differences. The factor “group” included levels “TE” and “PET-SI”, and the factor “timepoint” included levels “baseline” (pre-surgery), “2 weeks post-surgery”, and 8 weeks post-surgery”. Normality of data was assessed using a Shapiro–Wilk test, and non-normal data were corrected using rank data transformation if necessary. Gait velocity was included as a covariate in the model. Least squared means with a Tukey–Kramer adjustment was used for post-hoc pairwise comparisons, ten in total:
- Weeks Post-Surgery
-
oBaseline vs. 2 weeks post-surgery
-
oBaseline vs. 8 weeks post-surgery
-
o2 weeks post-surgery vs. 8 weeks post-surgery
-
o
- Group x Weeks Post-Surgery
-
oTE baseline vs PET-SI baseline
-
oTE baseline vs. TE 2 weeks post-surgery
-
oTE baseline vs. TE 8 weeks post-surgery
-
oTE 2 weeks post-surgery vs. TE 8 weeks post-surgery
-
oPET-SI baseline vs. PET-SI 2 weeks post-surgery
-
oPET-SI baseline vs. PET-SI 8 weeks post-surgery
-
oPET-SI 2 weeks post-surgery vs. PET-SI 8 weeks post-surgery
-
o
Three trials for each timepoint for each rabbit were selected to include in the analysis (3 trials × 3 timepoints × 10 rabbits = 90 trials). In order to choose the three trials, each video initially was assessed qualitatively; a video was excluded if the rabbit was hopping abnormally (i.e., play hopping or flicking of feet while hopping) at any point during the trial. For the remaining videos, the gait velocity was calculated for each rabbit and video and averaged across timepoints. At each timepoint, the three trials that were closest to the average gait velocity for the rabbit were selected for further analysis. Comparisons were made for the operated limb only. The independent variables for pressure mat data were peak vertical force, vertical impulse, vertical impulse distribution, and average ground contact area. The independent variables for kinematics data were stance percent of stride, range of motion, and maximum, minimum, and average joint angle for the knee and ankle during the stance and swing phase of gait and for the MTP during stance phase of gait. Stance and swing phases of gait were determined using the pressure mat data. Peak vertical force and vertical impulse were normalized by body weight.
Statistical parametric mapping (SPM) [23, 24] open-source MATLAB software [25] was used to compare the kinematic curves for each group, joint, and gait phase. A two-factor (group, timepoint, and group*timepoint) ANOVA with repeated measures (rabbit) was performed first. Then, post-hoc tests were performed using a SPM two-tailed paired t-test to compare joint angles between timepoints within each group. A Bonferroni correction was used to account for multiple comparisons.
Results
No rabbit was excluded or removed prematurely from the study; all rabbits in each group (n = 5 per group) were included in the data analysis. There were no significant differences in age or weight at time of surgery, weight at euthanasia, or length of the biological tendon between the two groups (Table 1). The length of the artificial tendons ranged from 84.9 to 92.7% (mean 89.5 ± 3.2%) of the length of the biological tendon (Table 1), which was within the targeted range.
Biomechanics: pressure data
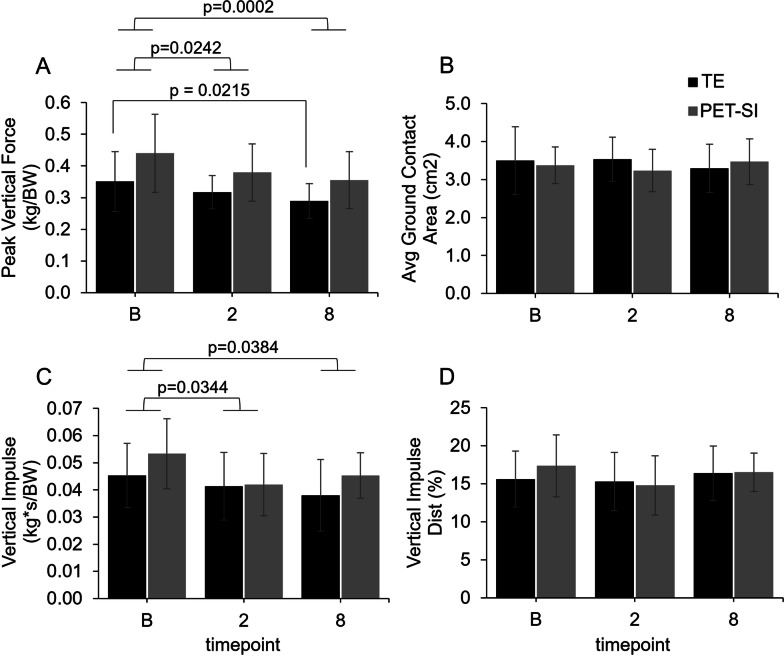
Timepoint was significant for peak vertical force (p = 0.0002) and vertical impulse (p = 0.0180). More specifically, across groups, both peak vertical force and vertical impulse were significantly less at 2 and 8 weeks post-surgery compared to baseline (Fig. 3A and C). In addition, peak vertical force was significantly less at 8 weeks post-surgery compared to baseline for the TE group (p = 0.0215), but not the PET-SI group (p = 0.0621). Faster gait velocity was significantly associated with greater peak vertical force (p = 0.0028) and vertical impulse (p = 0.0191).
Fig. 3.
Mean values and standard deviation at baseline (pre-surgery), and 2 weeks, and 8 weeks post-surgery for the operated limb for the tendon excision only (TE) and tendon excision and replacement (PET-SI) groups for A Peak vertical force normalized by body weight (kg/BW); B Average ground contact area (cm2); C Impulse normalized by body weight (kg*s/BW); D Vertical impulse distribution (%)
Biomechanics: kinematics
Of the main factors, treatment group was not significant, but timepoint had a significant effect on many of the kinematics variables for both groups (Figs. 3, 4, 5, Table 2). The p-values for main effects, their interaction, and the by-week comparisons are in Table 2 and 3. Across groups, there was an increase in ankle plantarflexion and knee extension and a decrease in ankle dorsiflexion and knee flexion at 2 and 8 weeks post-surgery compared to baseline, especially during swing phase. Across groups, the overall range of motion during swing phase was significantly less for the knee at 2 weeks post-surgery and the ankle at 2 and 8 weeks post-surgery compared to baseline. There was significant recovery of range of motion in the knee and average angle of the MTP by 8 weeks post-surgery. There was no difference in stance percent of stride across groups or timepoints (Table 4). However, gait velocity was significantly correlated with the stance percent of stride (p < 0.0001; Table 2).
Fig. 4.
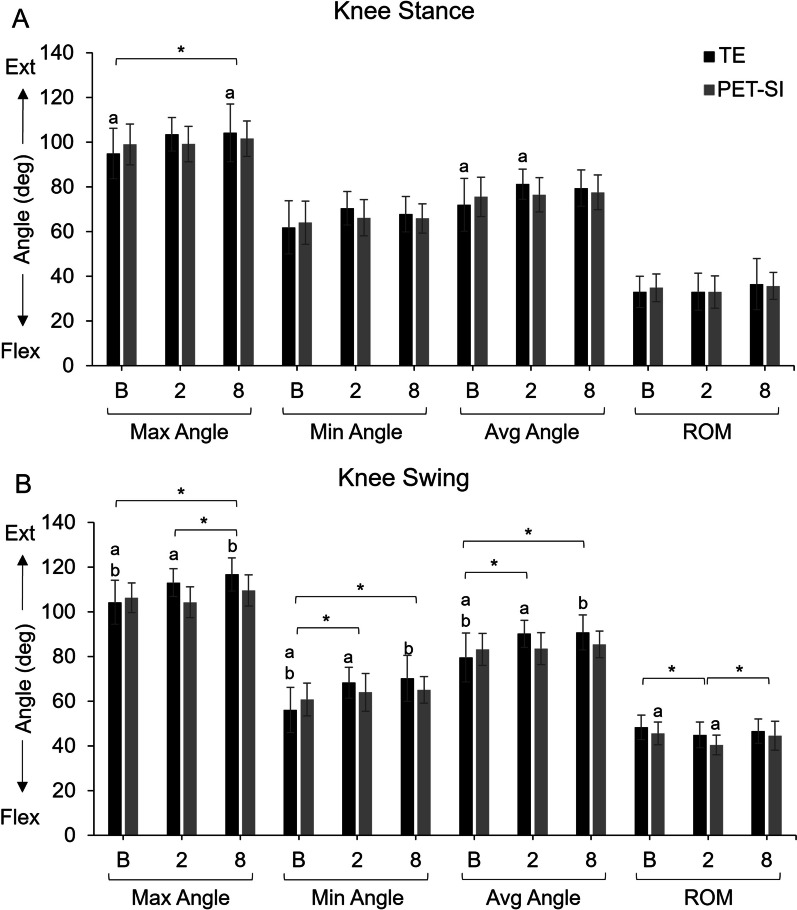
Maximum, minimum, and average knee joint angles and knee range of motion (ROM) for the tendon excision (TE) only group and tendon excision and replacement (PET-SI) group at baseline (B, pre-surgery), 2 weeks post-surgery, and 8 weeks post-surgery during A stance phase of gait, and B) swing phase of gait. Error bars represent one standard deviation. Ext – Extension; Flex – Flexion. A larger angle indicates greater extension/less flexion. * indicates significant (p < 0.05) differences between timepoints across groups. a, b indicate significant (p < 0.05) differences between timepoints within a group. Table 3 gives specific p-values for each comparison
Table 2.
Statistical results (p-values) from the 2-factor ANOVA with repeated measures for each kinematic variable for knee, ankle, and MTP joints during stance and swing phase of gait
| Variable | Gait phase | Normality | Group | Weeks post | GxW | Gait velocity |
|---|---|---|---|---|---|---|
| Knee | ||||||
| Max angle | Stance | NS | NS | 0.027 | NS | NS |
| Swing | NS | NS | < 0.0001 | 0.0008 | NS | |
| Min angle | Stance | Transformed | NS | 0.0932 | NS | NS |
| Swing | NS | NS | < 0.0001 | 0.0108 | NS | |
| Avg angle | Stance | Transformed | NS | 0.0809 | 0.0497 | NS |
| Swing | Transformed | NS | 0.0004 | 0.0066 | NS | |
| ROM | Stance | NS | NS | NS | NS | NS |
| Swing | NS | NS | 0.0017 | NS | NS | |
| Ankle | ||||||
| Max angle | Stance | Transformed | NS | NS | NS | 0.0575 |
| Swing | Transformed | NS | 0.0145 | NS | NS | |
| Min angle | Stance | NS | NS | 0.0012 | NS | NS |
| Swing | Transformed | NS | < 0.0001 | NS | NS | |
| Avg angle | Stance | Transformed | NS | 0.0097 | NS | NS |
| Swing | Transformed | NS | < 0.0001 | NS | NS | |
| ROM | Stance | NS | NS | 0.0825 | NS | NS |
| Swing | NS | NS | 0.0055 | NS | NS | |
| MTP | ||||||
| Max Angle | Stance | NS | NS | 0.0005 | NS | NS |
| Swing | Transformed | NS | < 0.0001 | NS | NS | |
| Min Angle | Stance | NS | NS | NS | NS | NS |
| Swing | NS | NS | 0.0588 | NS | NS | |
| Avg Angle | Stance | NS | NS | 0.0398 | NS | NS |
| Swing | NS | NS | < 0.0001 | NS | NS | |
| ROM | Stance | NS | NS | 0.08 | NS | NS |
| Swing | Transformed | NS | NS | NS | NS |
The normality column indicates whether the data needed to be transformed or not. Group was TE or PET-SI. Weeks post was baseline, 2 or 8-weeks post-surgery. GxW is the group by weeks post-surgery interaction term. Gait velocity was included as a covariate in the model. ROM – range of motion; NS – not significant. p < 0.05 considered significant. p < 0.1 also included.
Table 3.
Statistical results (p-values) for least-square means with Tukey–Kramer multiple-comparison adjustment for each kinematic variable for the knee, ankle, and MTP joints during stance and swing phase of gait for each timepoint
| Knee | Ankle | MTP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Variable | Gait Phase | B vs 2 | B vs 8 | 2 vs 8 | B vs 2 | B vs 8 | 2 vs 8 | B vs 2 | B vs 8 | 2 vs 8 |
| Combined (averaged over TE and PET-SI) | Max angle | Stance | NS | 0.0262 | NS | NS | NS | NS | 0.0006 | NS | 0.0078 |
| Swing | 0.0971 | < 0.0001 | 0.0098 | 0.0111 | NS | NS | < 0.0001 | NS | 0.0001 | ||
| Min Angle | Stance | 0.0754 | NS | NS | 0.0013 | NS | 0.0167 | NS | NS | NS | |
| Swing | 0.0002 | < 0.0001 | NS | < 0.0001 | < 0.0001 | NS | 0.0759 | NS | NS | ||
| Avg Angle | Stance | 0.0979 | NS | NS | 0.0085 | NS | 0.0871 | NS | NS | 0.034 | |
| Swing | 0.0053 | 0.0005 | NS | < 0.0001 | 0.0007 | NS | < 0.0001 | NS | 0.0003 | ||
| ROM | Stance | NS | NS | NS | NS | NS | 0.0795 | 0.0736 | NS | NS | |
| Swing | 0.0014 | NS | 0.0367 | 0.0117 | 0.0161 | NS | NS | NS | NS | ||
| TE | Max angle | Stance | 0.0787 | 0.0494 | NS | NS | NS | NS | NS | NS | NS |
| Swing | 0.0012 | < 0.0001 | NS | NS | NS | NS | 0.0101 | NS | NS | ||
| Min angle | Stance | 0.0527 | NS | NS | NS | NS | NS | NS | NS | NS | |
| Swing | < 0.0001 | < 0.0001 | NS | 0.0025 | 0.0038 | NS | NS | NS | NS | ||
| Avg angle | Stance | 0.0196 | NS | NS | NS | NS | NS | NS | NS | NS | |
| Swing | 0.0004 | 0.0002 | NS | 0.0018 | 0.0216 | NS | 0.0149 | NS | NS | ||
| ROM | Stance | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Swing | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| PET-SI | Max angle | Stance | NS | NS | NS | NS | NS | NS | 0.0317 | NS | NS |
| Swing | NS | NS | NS | NS | NS | NS | 0.0021 | NS | 0.0059 | ||
| Min angle | Stance | NS | NS | NS | 0.0145 | NS | NS | NS | NS | NS | |
| Swing | NS | NS | NS | 0.0051 | 0.046 | NS | NS | NS | NS | ||
| Avg angle | Stance | NS | NS | NS | 0.07 | NS | NS | NS | NS | NS | |
| Swing | NS | NS | NS | 0.0531 | NS | NS | 0.0222 | NS | 0.0141 | ||
| ROM | Stance | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Swing | 0.0251 | NS | NS | NS | NS | NS | NS | NS | NS |
Group was combined (averaged over TE and PET-SI), TE or PET-SI. Timepoints were baseline (B), 2-, or 8-weeks post-surgery. ROM – range of motion; NS – not significant. p < 0.05 considered significant. p < 0.1 also included.
Table 4.
The percentage of the gait cycle that was the stance phase for both groups over time
| Weeks post-surgery/group | Stance percent stride (SD) | ||
|---|---|---|---|
| Baseline | 2 | 8 | |
| TE | 48.1 (6.0) | 47.6 (4.7) | 48.2 (4.9) |
| PET-SI | 48.6 (3.5) | 45.9 (6.9) | 49.7 (5.0) |
There were no significant differences between groups or over time
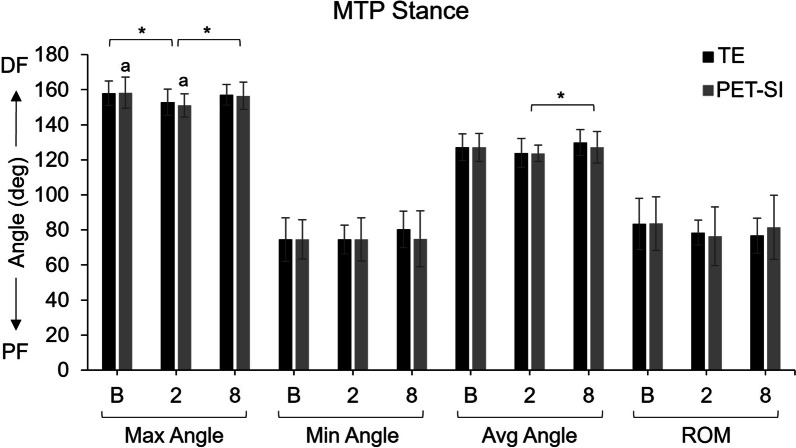
For the group-by-timepoint interactions, during stance (Figs. 3a, 4a, and 5a, Table 3), the TE group's knee extension significantly increased from baseline to 8 weeks post-surgery, and the knee was significantly more extended overall at 2 weeks post-surgery compared to baseline. In contrast, the PET-SI group had no significant differences among timepoints for the knee during stance. However, the PET-SI group had significantly less ankle and MTP dorsiflexion at 2 weeks post-surgery compared to baseline.
There were more between-factor differences in kinematics variables during the swing phase of gait compared to the stance phase (Figs. 3b, 4b, 5b, Table 3). At 2 and 8 weeks post-surgery compared to baseline, the TE group maintained the knee and ankle in a significantly more extended/plantarflexed position overall, with significantly greater knee extension, less knee flexion, and less ankle dorsiflexion. For the PET-SI group, the knee range of motion was significantly less at 2 weeks post-surgery compared to baseline, but partly recovered by 8 weeks post-surgery. Similar to the TE group, there was a significant decrease in maximal ankle dorsiflexion at 2 and 8 weeks post-surgery compared to baseline. However, the overall posture (average angle) of the ankle was not significantly affected.
Statistical parametric mapping analysis
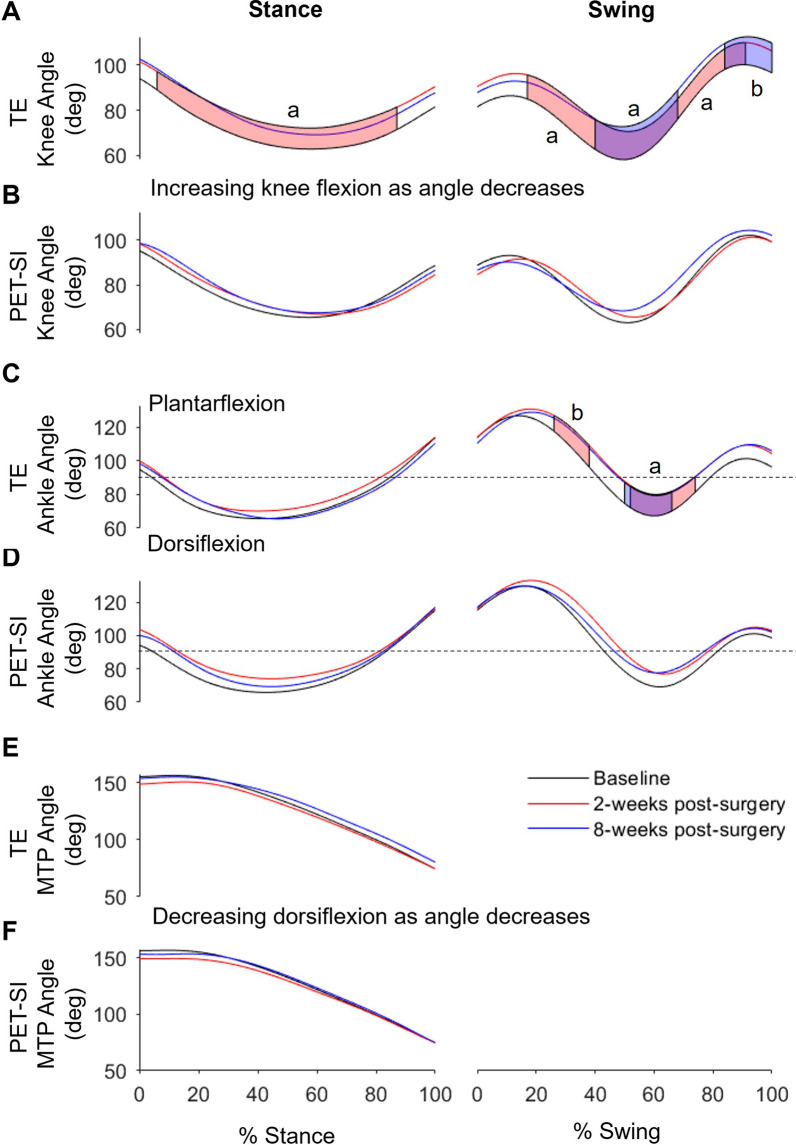
There was a significant difference in joint angle among the three timepoints between ~ 44–58% of the swing phase for the knee and ~ 38–80% of the swing phase for the ankle. Post-hoc paired t-tests showed that knee flexion was significantly less during stance at 2 weeks post-surgery and during swing at 2 and 8 weeks post-surgery for the TE group (Fig. 6, 7a). Ankle dorsiflexion was also significantly less during swing at 2 and 8 weeks post-surgery for the TE group (Fig. 7c). There were no significant differences among timepoints in the PET-SI group for the knee or ankle.
Fig. 6.
Maximum, minimum, and average MTP joint angles and MTP range of motion (ROM) for the tendon excision (TE) only group and tendon excision and replacement (PET-SI) group at baseline (B, pre-surgery), 2 weeks post-surgery, and 8 weeks post-surgery during stance phase of gait. Error bars represent one standard deviation. PF – Plantarflexion; DF – Dorsiflexion. A larger angle indicates greater dorsiflexion/less plantarflexion. * indicates significant (p < 0.05) differences between timepoints across groups. a indicates significant (p < 0.05) differences between timepoints within a group. Table 3 gives specific p-values for each comparison
Fig. 7.
Mean joint angles for TE and PET-SI groups over the gait cycle for the A, B knee, C, D ankle, and E, F MTP. The horizontal dashed line in C and D indicates 90° where the ankle is at neutral dorsi/plantar-flexion. Red shaded areas indicate significant differences between baseline and 2 weeks post-surgery. Blue shaded areas indicate significant differences between baseline and 8 weeks post-surgery. Purple shaded areas are the overlap of red and blue shaded areas. a: p = 0.001; b: p = 0.002
Discussion
The results supported our hypotheses that compared to the TE group, the PET-SI group would have greater peak ankle dorsiflexion angle and ankle range of motion during the swing phase of gait. Additionally, compared to the TE group, the hindlimb kinematics of the PET-SI group were more similar to baseline kinematics measured pre-surgery. Our results were consistent with those of our previous preliminary study [20] and suggest that the artificial tendons effectively performed the biomechanical function of the native tendons they replaced.
Non-intuitively, peak vertical force at 8 weeks post-surgery was significantly less than at pre-surgery for the TE group and nearly so for the PET-SI group. This was unexpected since the tibialis cranialis muscle, based on its moment arm, does not generate torque that is expected to contribute to vertical ground contact force. A possible explanation for the difference is that the rabbits could have shifted biomechanical loads from the operated limb to the sound limb and spend less time on the operated limb, potentially due to discomfort or perceived functional impairment of the operated limb; further analysis of bilateral pressure data is needed to confirm this. In addition, a longer study duration would help determine if peak vertical force normalizes with additional recovery time.
An important anatomical feature that allows biological tendons to slide relative to surrounding tissues is the tendon sheath. In the PET-SI group, it appeared that a sheath formed around the artificial tendon; immediately post-mortem, we observed the artificial tendon sliding relative to surrounding tissues during passive ankle motion (Additional file 1: video). Thus, the silicone coating performed the intended function of preventing tissues from adhering to the surface of the artificial tendon. These findings are qualitative, visual inspections of the tissues; the extent to which the structure and function of the tissues surrounding the artificial tendons mimic the native epitenon or tendon sheath surrounding the biological tendons is unknown. Future studies will evaluate histology samples to assess the cellular structure of the tissues ensheathing the artificial tendons. Functionally, it would also be useful to quantify the effective mechanical friction and damping coefficients at the tendon-sheath interface.
Though we observed statistically significant differences between the PET-SI and TE groups, the TE group still appeared to have substantial ankle dorsiflexion function. Notably, the TE group had an average maximum dorsiflexion angle during swing phase of gait of 75.4°, which was only 3.1° less than that of the PET-SI group. One possible explanation is that, in the TE group, other muscles crossing the ankle, such as extensor digitorum longus and the peroneus muscle group (longus, brevis, tertius) [26], may have compensated substantially for the lost contribution of the tibialis cranialis muscle to ankle dorsiflexion torque and movement. The extensor digitorum longus muscle also crosses the knee and ankle [27] and, thus, may generate an ankle dorsiflexion torque passively as the knee is flexed. Finally, we suspect that the ankle joint is at least partly dorsiflexed passively during both stance and swing phases of hopping gait: during stance as the torso and proximal hindlimb move cranially and near the end of the cranial swing of the hindlimb due to the inertia of the foot.
There are several possible reasons for the modest biomechanical declines from pre- to post-surgery of rabbits with the PET-SI artificial tendons. For one, the PET-SI artificial tendon did not interface with the muscle as seamlessly as a biological tendon does. Specifically, biological tendons integrate with muscles over a large portion of their length via aponeuroses, which facilitates force transmission between muscle and tendon [28]; conversely, a relatively small number (four) of suture strands of the artificial tendon were tied to the distal end of the muscle. Second, the mechanical properties (e.g., stiffness) may be different between biological and artificial tendons; such differences have not been quantified but will be investigated in a future study. Third, though surgical interventions may have caused pain and discomfort in both groups, these may have been greater in the PET-SI group due to the implantation of the artificial tendons. Fourth, the biologic tendon passes under the extensor retinaculum, whereas the artificial tendon, due to its size, cannot be placed under the retinaculum. This results in a change in the moment arm and torque direction between the biologic and artificial tendons. Finally, both groups were bandaged for 3 days post-surgery, which partly immobilized the ankle joint; bandaging may have had modest adverse effects (e.g., disuse muscle atrophy) leading to impaired biomechanical function [29].
Previous animal studies of PET-SI artificial tendons did not quantify movement biomechanics, noting only qualitatively that animals resumed “normal gait by 3 [weeks post-surgery]” [16]. Our results contradict previous observations, as rabbits in the PET-SI group had significantly different kinetics and kinematics at 2 and 8 weeks post-surgery compared to baseline. Additionally, comparison of our results to other tendon repair methods (e.g. tendon grafting) is challenged by the fact that few previous animal studies of tendon repair consider movement biomechanics [30, 31]. This is surprising and unfortunate given the important role of tendons in movement production. In a canine model, autografts were used to reconstruct the failed repair of the 2nd and 5th flexor digitorum profundus tendons; the authors generally found no difference in ground contact kinetics between normal and repaired paws [32]. Another study quantified foot strike patterns and ankle kinematics in a rat model of tenocyte/hyaluronic acid therapy for Achilles tendon injury [33]. Some studies report effects of repairs on range of motion [34, 35]. To facilitate clinical translation, future studies should compare biomechanics among PET-SI artificial tendons and current treatments, such as tendon grafts, for criticially sized tendon defects.
An important consideration for future research is the effect of the PET-SI artificial tendon on the interfacing muscle tissue. Previous histology showed that polyester microfibers integrated amicably with muscle fibers, with no evidence of tissue damage or scar tissue formation [15–17]. However, the extent of tissue examined and the types of histology stains used were limited. Additionally, the artificial tendon may adversely affect other aspects of muscle physiology, such as blood flow [36] or tissue loads [37], potentially leading to tissue necrosis.
There were several limitations of our study. First, the number of samples per group was relatively small, which may have underpowered our statistical comparisons for some variables; however, the group size was similar to those of the previous in vivo studies of the PET-SI tendons [14–17]. Second, the study duration was relatively short but about the same as a previous in vivo study of a polyester artificial tendon [17] and greater than a study of muscle changes following tenectomy [21]. To support clinical translation, future studies should investigate biomechanical function with long-term use of PET-SI artificial tendons. Third, the study did not include a control group of healthy, non-operated rabbits to account for potential changes in biomechanics as the rabbits aged or gained more experience with our experiment protocol from pre- to post-surgery. However, since the rabbits had already reached skeletal maturity by the start of the study [38], and since the study duration was relatively short, we did not expect biomechanics to change significantly during the study due to aging. Therefore, we considered that the benefit of a control group was outweighed by the ethical cost of using additional animals. Fourth, we surgically replaced a healthy biological tendon with an artificial tendon in a single surgery; future studies should model the common clinical scenario that a tendon defect is present for some period prior to surgical replacement with an artificial tendon.
Conclusions
In conclusion, our in vivo study provided the most substantial quantitative evidence to date of a positive treatment effect of PET-SI artificial tendons on biomechanical motor function. Therefore, PET-SI artificial tendons potentially could be an effective alternative treatment option for critically sized tendon defects and other severe tendon pathologies.
Supplementary Information
Additional file 1. A video of the rabbit hindlimb (skin removed) showing the tibialis cranialis artificial tendon (green) sliding relative to surrounding tissues during passive ankle motion. The video was recorded immediately post-mortem with no tissue fixation.
Acknowledgements
Thank you to Dr. Bryce Burton, Dr. Kelsey Finnie, and Chris Carter for veterinary care and support, and to the Animal Housing Facility staff for animal care assistance. Thank you to Kellie McGhee, Ivy Milligan, and Raina Desai for their help with data collection and analysis.
Abbreviations
- ANOVA
Analysis of variance
- BW
Body weight
- CRI
Constant-rate infusion
- DF
Dorsiflexion
- IM
Intramuscular
- IV
Intravenous
- MTP
Metatarsophalangeal
- NS
Not Significant
- PET-SI
Polyester, silicone-coated artificial tendon
- PF
Plantarflexion
- PDS
Polydioxanone
- PO
Per OS (by mouth)
- ROM
Range of motion
- SC
Subcutaneous
- SPM
Statistical parametric mapping
- TE
Tendon excision
Authors contributions
KLE, DEA, and DLC contributed to the study design. KLE managed all aspects of the study. KLE, KMB, CB, CBG, and DEA performed the animal surgeries. CH, KS, DM, and OF assisted with surgery support tasks, post-surgery animal care, data collection, and data processing. KLE and XS performed data and statistical analysis. KLE and DLC wrote the draft of the manuscript. All authors read and approved the final manuscript.
Funding
Research was funded by the National Institutes of Health Award #R61AR078096. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animal study was approved by the University of Tennessee-Knoxville Institutional Animal Care and Use Committee (Protocol #2726–0220).
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338–1344. doi: 10.1016/j.injury.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Jepegnanam TS, Nithyananth M, Boopalan PR, Cherian VM, Titus VT. Reconstruction of open contaminated achilles tendon injuries with soft tissue loss. J Trauma. 2009;66(3):774–779. doi: 10.1097/TA.0b013e31817c96c7. [DOI] [PubMed] [Google Scholar]
- 3.Iorio ML, Han KD, Evans KK, Attinger CE. Combined Achilles tendon and soft tissue defects: functional outcomes of free tissue transfers and tendon vascularization. Ann Plast Surg. 2015;74(1):121–125. doi: 10.1097/SAP.0b013e31828bb353. [DOI] [PubMed] [Google Scholar]
- 4.Schachter AK, White BJ, Namkoong S, Sherman O. Revision reconstruction of a pectoralis major tendon rupture using hamstring autograft: a case report. Am J Sports Med. 2006;34(2):295–298. doi: 10.1177/0363546505278697. [DOI] [PubMed] [Google Scholar]
- 5.Sarzaeem MM, Lemraski MM, Safdari F. Chronic Achilles tendon rupture reconstruction using a free semitendinosus tendon graft transfer. Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1386–1391. doi: 10.1007/s00167-011-1703-x. [DOI] [PubMed] [Google Scholar]
- 6.Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354–1361. doi: 10.2106/00004623-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971–980. doi: 10.1053/jars.2001.28979. [DOI] [PubMed] [Google Scholar]
- 8.LaSalle WB, Strickland JW. An evaluation of the two-stage flexor tendon reconstruction technique. J Hand Surg Am. 1983;8(3):263–267. doi: 10.1016/S0363-5023(83)80155-5. [DOI] [PubMed] [Google Scholar]
- 9.Frakking TG, Depuydt KP, Kon M, Werker PM. Retrospective outcome analysis of staged flexor tendon reconstruction. J Hand Surg Br. 2000;25(2):168–174. doi: 10.1054/jhsb.1999.0338. [DOI] [PubMed] [Google Scholar]
- 10.Murray GA, Semple JC. A review of work on artificial tendons. J Biomed Eng. 1979;1(3):177–184. doi: 10.1016/0141-5425(79)90040-2. [DOI] [PubMed] [Google Scholar]
- 11.Rawlins R. The role of carbon fibre as a flexor tendon substitute. Hand. 1983;15(2):145–148. doi: 10.1016/S0072-968X(83)80004-7. [DOI] [PubMed] [Google Scholar]
- 12.Howard CB, McKibbin B, Ralis ZA. The use of Dexon as a replacement for the calcaneal tendon in sheep. J Bone Joint Surg Br. 1985;67(2):313–316. doi: 10.1302/0301-620X.67B2.3980547. [DOI] [PubMed] [Google Scholar]
- 13.Mendes DG, Iusim M, Angel D, Rotem A, Mordehovich D, Roffman M, Lieberson S, Boss J. Ligament and tendon substitution with composite carbon fiber strands. J Biomed Mater Res. 1986;20(6):699–708. doi: 10.1002/jbm.820200604. [DOI] [PubMed] [Google Scholar]
- 14.Melvin DB, Klosterman B, Gramza BR, Byrne MT, Weisbrode SL, Litsky AS, Clarson SJ. A durable load bearing muscle to prosthetic coupling. ASAIO J. 2003;49(3):314–319. doi: 10.1097/01.MAT.0000065369.46216.9C. [DOI] [PubMed] [Google Scholar]
- 15.Melvin AJ, Litsky AS, Mayerson JL, Stringer K, Juncosa-Melvin N. Extended healing validation of an artificial tendon to connect the quadriceps muscle to the Tibia: 180-day study. J Orthop Res. 2012;30(7):1112–1117. doi: 10.1002/jor.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melvin A, Litsky A, Mayerson J, Stringer K, Melvin D, Juncosa-Melvin N. An artificial tendon to connect the quadriceps muscle to the tibia. J Orthop Res. 2011;29(11):1775–1782. doi: 10.1002/jor.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melvin A, Litsky A, Mayerson J, Witte D, Melvin D, Juncosa-Melvin N. An artificial tendon with durable muscle interface. J Orthop Res. 2010;28(2):218–224. doi: 10.1002/jor.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdullah S. Usage of synthetic tendons in tendon reconstruction. BMC Proc. 2015;9(3):A68. doi: 10.1186/1753-6561-9-S3-A68. [DOI] [Google Scholar]
- 19.Talia AJ, Tran P. Bilateral patellar tendon reconstruction using LARS ligaments: case report and review of the literature. BMC Musculoskelet Disord. 2016;17(1):302. doi: 10.1186/s12891-016-1161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall PT, Stubbs C, Pedersen AP, Billings C, Stephenson SM, Greenacre CB, Anderson DE, Crouch DL. Effect of polyester-based artificial tendons on movement biomechanics: a preliminary in vivo study. J Biomech. 2023;151:111520. doi: 10.1016/j.jbiomech.2023.111520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry JA, Cotter MA, Cameron NE, Pattullo MC. The effect of immobilization on the recovery of rabbit soleus muscle from tenotomy: modulation by chronic electrical stimulation. Exp Physiol. 1994;79(4):515–525. doi: 10.1113/expphysiol.1994.sp003784. [DOI] [PubMed] [Google Scholar]
- 22.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 2018;21(9):1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- 23.Pataky TC. One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Engin. 2012;15(3):295–301. doi: 10.1080/10255842.2010.527837. [DOI] [PubMed] [Google Scholar]
- 24.Pataky TC. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J Biomech. 2010;43(10):1976–1982. doi: 10.1016/j.jbiomech.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Pataky TC. 2022; https://spm1d.org. Accessed February 3, 2023.
- 26.Lieber RL, Blevins FT. Skeletal muscle architecture of the rabbit hindlimb: functional implications of muscle design. J Morphol. 1989;199(1):93–101. doi: 10.1002/jmor.1051990108. [DOI] [PubMed] [Google Scholar]
- 27.Grover DM, Chen AA, Hazelwood SJ. Biomechanics of the rabbit knee and ankle: muscle, ligament, and joint contact force predictions. J Biomech. 2007;40(12):2816–2821. doi: 10.1016/j.jbiomech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Bojsen-Moller J, Magnusson SP. Mechanical properties, physiological behavior, and function of aponeurosis and tendon. J Appl Physiol. 2019;126(6):1800–1807. doi: 10.1152/japplphysiol.00671.2018. [DOI] [PubMed] [Google Scholar]
- 29.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol. 2013;45(10):2200–2208. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res. 2014;3(6):193–202. doi: 10.1302/2046-3758.36.2000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottagisio M, Lovati AB. A review on animal models and treatments for the reconstruction of Achilles and flexor tendons. J Mater Sci - Mater Med. 2017;28(3):45. doi: 10.1007/s10856-017-5858-y. [DOI] [PubMed] [Google Scholar]
- 32.Cheng W, Cornwall R, Crouch DL, Li Z, Saul KR. Contributions of muscle imbalance and impaired growth to postural and osseous shoulder deformity following brachial plexus birth palsy: a computational simulation analysis. J Hand Surg Am. 2015;40(6):1170–1176. doi: 10.1016/j.jhsa.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Liang J-I, Lin P-C, Chen M-Y, Hsieh T-H, Chen J-JJ, Yeh M-L. The effect of tenocyte/hyaluronic acid therapy on the early recovery of healing Achilles tendon in rats. Journal of Materials Science: Materials in Medicine. 2014;25(1):217–227. [DOI] [PubMed]
- 34.He M, Gan AW, Lim AY, Goh JC, Hui JH, Chong AK. Bone marrow derived mesenchymal stem cell augmentation of rabbit flexor tendon healing. Hand Surg. 2015;20(3):421–429. doi: 10.1142/S0218810415500343. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Hou Z, Liu P, Lee Y, Ding Z, Zheng X. An experimental study comparing active mobilization to passive flexion-active extension–active flexion after flexor tendon repair in zone 2. J Hand Surg. 2013;38(4):672–676. doi: 10.1016/j.jhsa.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Sagi HC, Papp S, DiPasquale T. The effect of suture pattern and tension on cutaneous blood flow as assessed by laser doppler flowmetry in a pig model. J Orthopaed Trauma. 2008;22(3). [DOI] [PubMed]
- 37.Gawlitta D, Li W, Oomens CWJ, Baaijens FPT, Bader DL, Bouten CVC. The relative contributions of compression and hypoxia to development of muscle tissue damage: an in vitro study. Ann Biomed Eng. 2007;35(2):273–284. doi: 10.1007/s10439-006-9222-5. [DOI] [PubMed] [Google Scholar]
- 38.Masoud I, Shapiro F, Kent R, Moses A. A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J Orthop Res. 1986;4(2):221–231. doi: 10.1002/jor.1100040211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. A video of the rabbit hindlimb (skin removed) showing the tibialis cranialis artificial tendon (green) sliding relative to surrounding tissues during passive ankle motion. The video was recorded immediately post-mortem with no tissue fixation.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.