Abstract
Humans rely heavily on facial expressions for social communication to convey their thoughts and emotions and to understand them in others. One prominent but controversial view is that humans learn to recognize the significance of facial expressions by mimicking the expressions of others. This view predicts that an inability to make facial expressions (e.g., facial paralysis) would result in reduced perceptual sensitivity to others’ facial expressions. To test this hypothesis, we developed a diverse battery of sensitive emotion recognition tasks to characterize expression perception in individuals with Moebius Syndrome (MBS), a congenital neurological disorder that causes facial palsy. Using computer-based detection tasks we systematically assessed expression perception thresholds for static and dynamic face and body expressions. We found that while MBS individuals were able to perform challenging perceptual control tasks and body expression tasks, they were less efficient at extracting emotion from facial expressions, compared to matched controls. Exploratory analyses of fMRI data from a small group of MBS participants suggested potentially reduced engagement of the amygdala in MBS participants during expression processing relative to matched controls. Collectively, these results suggest a role for facial mimicry and consequent facial feedback and motor experience in the perception of others’ facial expressions.
Keywords: facial expressions, facial experience, facial feedback, facial mimicry, emotion perception
1. INTRODUCTION
Long before children are able to communicate verbally, their facial expressions convey their thoughts and emotions. Babies learn to portray their needs via the language of facial expressions from the first appearance of the social smile at about two months (Soderling, 1959). In the same way that babies learn the names of people and objects around them, they also learn the motor sequences for smiling and making other facial expressions during development (Tautermannova, 1973; Wolff, 1963). Facial mimicry, facial feedback, and motor experience, are considered to be three critical components of how humans learn to make facial expressions, feel the associated emotions, and perceive the same in others. For example, some evidence suggests that humans learn to express emotions via facial mimicry (Dimberg & Thunberg, 1998) which can be thought of as the human version of monkey see, monkey do (Gallese & Sinigaglia, 2011; Keysers & Gazzola, 2009; Rizzolatti et al., 2001; Ross & Atkinson, 2020). Thus, as an infant sees its parent smile, the visual cues of the observed motion are converted into motor actions of the baby’s own facial muscles (Isomura & Nakano, 2016; Soussignan et al., 2018) (but see Davis et al. (Davis et al., 2021)). Further, according to the facial feedback hypothesis, this motor action in turn provides proprioceptive feedback to the brain, that helps encode the sensation of the motor action (Oberman et al., 2007; Soderkvist et al., 2018). Eventually, babies learn the emotional tags of these motor actions as ‘happiness, ‘sadness’, ‘anger’, etc. and associate them with the corresponding mental and physiological states. In addition to facial mimicry and facial feedback, some evidence suggests that motor experience also influences perception, such that expertise in performing a particular action, improves one’s perception of the same action in others. For example, professional actors have been shown to be better at explicit recognition of facial expressions (Conson et al., 2013), while dancers who have expertise portraying emotion with their bodies, tend to be better at perceiving portrayed body expressions (Christensen et al., 2016; Orlandi et al., 2017). Consequently, facial movement could be a contributing factor in efficient facial motion perception and its absence could lead to impairment in the perception of facial expressions.
In this study, we investigated the role that facial movement, and thus facial mimicry, facial feedback, and motor experience have on facial expression perception. We achieved this with a comprehensive battery of emotion detection tasks in a group of individuals with Moebius Syndrome (MBS). MBS is a rare congenital disorder characterized by limited lateral gaze and non-progressive facial paralysis due to underdevelopment or absence of the VIth (abducens) and VIIth (facial) cranial nerves or nuclei. As a result, individuals with MBS are unable to smile, frown, grimace, or make other facial expressions from birth. Thus, this condition provides an ideal model to investigate how the inability to express emotions with one’s face, affects the ability to process and recognize emotions expressed by others.
The few prior studies of individuals with MBS have produced conflicting results, with some studies concluding that the inability to make facial expressions does not impact expression perception (Calder et al., 2000; Rives Bogart & Matsumoto, 2010; Vannuscorps et al., 2020), while others have reported deficits in emotional expression recognition in individuals with MBS (Bate et al., 2013; De Stefani, Ardizzi, et al., 2019; De Stefani, Nicolini, et al., 2019; Giannini et al., 1984). However, these prior studies had critical limitations. First, some studies reporting a deficit (Calder et al., 2000; Rives Bogart & Matsumoto, 2010) did not include appropriate control tasks to account for differences in vision and general perceptual difficulties in MBS due to limited abduction of the eyes (i.e., inability to move eyes laterally because of the VIth nerve palsy). Second, some of the previous studies finding no impairment used only full-blown facial expressions in the emotion recognition tasks (Rives Bogart & Matsumoto, 2010), which limit their sensitivity. Since individuals with MBS do not routinely report major difficulties with social communication, we expect that any impairment in emotion recognition would be subtle and require sensitive measurement methods. Third, none of the previous studies has examined the neural correlates of facial expression processing in individuals with MBS and how they differ from healthy controls.
To address these issues, our study had three goals. First, we sought to systematically characterize facial expression processing in MBS in the context of carefully designed perceptual control tasks. We hypothesized that if MBS participants could perform difficult non-emotional perceptual tasks similar to controls, but not emotional perceptual tasks, then this would provide strong evidence that facial expression processing is affected in MBS. Second, we used a battery of sensitive emotion detection tasks to measure individual psychometric functions and detection thresholds. We did this by having participants detect emotion in images and videos containing varying levels of facial and body expressions and determining their perceptual sensitivity to emotional expressions by computing their thresholds for 50% detection accuracy. We hypothesized that while MBS participants may show no difference in recognition accuracy for full-blown facial expressions, we would see subtle differences in emotion detection thresholds in MBS compared to controls for facial expressions (but not body expressions), since the inability to make facial expressions would likely affect their sensitivity to low levels of emotional information in a face. Third, we used functional magnetic resonance imaging (fMRI) to explore whether individuals with MBS engage the same underlying neural circuitry for facial expression processing, and to the same extent, as healthy controls. To do this, we used task-based fMRI in a small group of participants to explore the neural circuits involved in facial expression processing in MBS, and how they differ from brain networks normally engaged in the task.
2. RESULTS
To systematically characterize expression processing in MBS, we enrolled individuals with MBS who had no documented intellectual or social disabilities and a group of age- and gender-matched control individuals (see Methods and Supplemental Table A for details). Each participant completed all or a subset a comprehensive series of computer-based facial and body emotion detection and recognition tasks (see Methods and Supplemental Table B for details). A small subset of participants also completed exploratory task-based fMRI scans.
2.1. Impaired detection of emotion from static facial expressions
First, we characterized emotion detection thresholds for happy and fearful faces in a static facial expression task by presenting morphs that contained different levels of the two facial expressions (Figures 1A and 1B). Briefly, participants were shown happy and fearful morphs in separate runs and asked to indicate if they thought the face was happy or neutral, or fearful or neutral, respectively, during emotion detection task runs, and to indicate if the mouth was open or closed during feature detection control task runs. The latter served as a critical perceptual control task to measure participants’ ability to process individual facial features. Morph levels yielding 50% detection accuracy were estimated as a percentage of expression information (0% morph level representing neutral expression and 100% morph level representing full-blown happy or fearful expression) by building, for each task, the psychometric curves for each participant and each emotion.
Figure 1. Static Facial Expression Task.
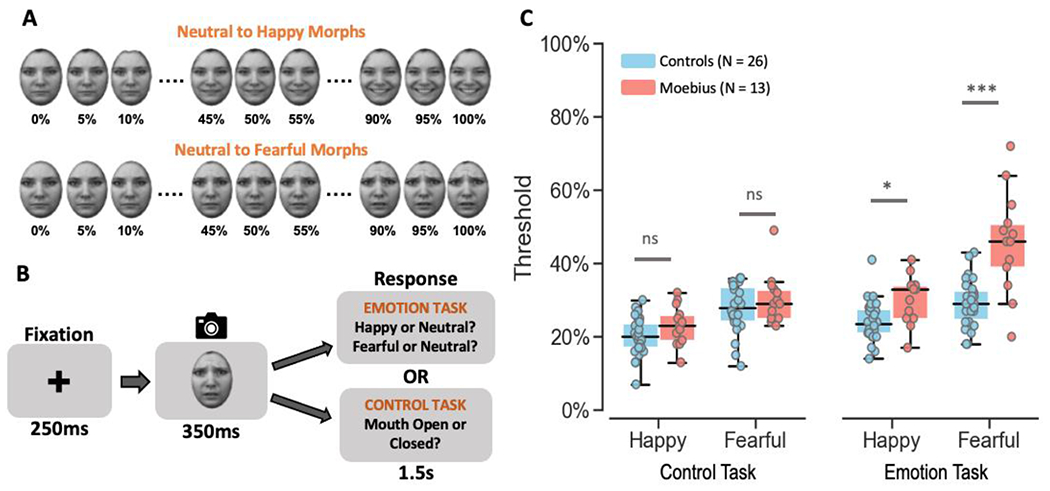
A. Illustration of how stimuli were created by morphing a neutral face to its corresponding happy or fearful face in increments of 5% yielding 21 images for each emotion from 100% neutral to 100% happy or fearful. B. Depiction of the trial structure – each trial began with a 250ms fixation cross, followed by the happy or fearful morph image (in separate runs) for 350ms, and a 1.5s response window during which participants were to indicate if they thought the face was happy or neutral (or fearful or neutral in separate runs) during emotion task runs, or whether the mouth was open or closed during control task runs. C. Box and whisker plots showing the thresholds for each task for control participants in blue and MBS individuals in red. MBS individuals had similar thresholds as control participants on the control task but higher thresholds for the emotion task (*p < 0.01; *** p < 10−6; ns: no significant difference). KDEF images used in Figure 1 panels A and B are reproduced from KDEF stimulus database - Lundqvist et al., 1998 (https://www.kdef.se/home/aboutKDEF.html), with permission from Karolinska Institutet, Psychology section, Copyright year: 1998, Copyright holder: Karolinska Institutet, Psychology section.
Overall, MBS individuals exhibited higher emotion detection thresholds than control participants, while feature detection thresholds did not differ between the two groups (Figure 1C). A mixed-effects beta regression analysis of threshold values showed the expected main effects of Task (Z = 6.80, p < 1.02 x 10−11) and Expression (Z = 8.70, p < 2.0 x 10−16), and more importantly a main effect of Group (Z = 3.92, p < 8.72 x 10−5), a two-way Task x Group (Z = 3.54, p < 0.0004) interaction, and a three-way Task x Expression x Group interaction (Z = 2.23, p < 0.025). Post-hoc Bonferroni-corrected between-group comparisons showed that for both happy and fearful face morphs, emotion detection thresholds for the emotion task were significantly higher for MBS individuals compared to controls (happy: t124 = 3.07, p < 0.003 with 50% thresholds for MBS: 30.3 ± 6.7% SD and Controls: 24.2 ± 5.6% SD; fearful: t124 = 7.65, p < 2.02 x 10−14 with 50% thresholds for MBS: 45.7 ± 13.9% SD and Controls: 29.2 ± 6.5% SD). By contrast, feature detection (open or closed mouth) thresholds for the control task were similar between the two groups for both happy (t124 = 1.64, p > 0.10; 50% thresholds for MBS: 23.2 ± 5.6% SD and Controls: 20.1 ± 5.2% SD) and fearful expressions (t124 = 1.39, p > 0.17; 50% thresholds for MBS: 30.3 ± 6.7% SD and Controls: 27.8 ± 6.4% SD).
These results indicate that individuals with MBS can reliably detect changes in facial features from static facial expressions (such as open or closed mouth) but are unable to efficiently process these changes to extract emotional information from them.
Having demonstrated a significant impairment in detecting and recognizing emotion from static facial expressions, we next asked whether these deficits were limited to emotion detection tasks, or whether the impairment transcends into other types of more complex tasks such as expression matching. In addition, since the static face experiment employed simple feature detection as a control task, in the next experiment we used the more complex visual process of facial identity matching as a control task. This served to identify any generalized high-level processing deficits in MBS individuals relative to controls.
2.2. Selective impairment in higher order face processing: facial identity vs. facial expression
In this task, participants were shown three faces in a triangular formation (Figure 2A and 2B) and asked to indicate which of the two lower faces (or neither) matched the top face based on the facial identity of the actor (during identity task runs) or based on facial expression of the actor (during expression task runs). Overall, MBS individuals performed similar to controls on the identity matching but worse on expression matching (Figure 2C). A Task x Group mixed-effects beta regression analysis of accuracy rates (percent correct) showed a main effect of Task (Z = 4.26, p < 2.05 x 10−5), no main effect of Group (Z = 1.02, p > 0.31), and a significant Task x Group interaction (Z = 2.14, p < 0.03). Post-hoc between group comparisons for each of the tasks showed that MBS individuals (mean ± SD: 60.2 ± 17.3%) performed similar to controls (mean ± SD: 60.5 ± 18.6%) on the identity task (t24.3 = 0.10, p > 0.93), but were much worse on the expression task (MBS: 67.5 ± 8.3% SD; controls: 79.1 ± 8.1% SD; t24.3 = 2.998, p < 0.006). Additionally, to rule out any difference in response confidence between the two groups, we examined the proportion of match trials where participants chose “no match” and found no significant differences between the two groups for either the identity task (MBS: 10.9 ± 3.6% SE; controls: 10.5 ± 2.0% SE; t22.2 = 0.44, p > 0.97) or expression (MBS: 15.9 ± 4.5% SE; controls: 15.2 ± 2.3% SE; t22.2 = 0.74, p > 0.88). Overall, these data indicate that individuals with MBS have difficulty processing emotional cues while other higher order face processing functions such as identity processing are not impacted by the facial paralysis (participants performed well above chance level of 33%).
Figure 2. Identity and Expression Matching Task.
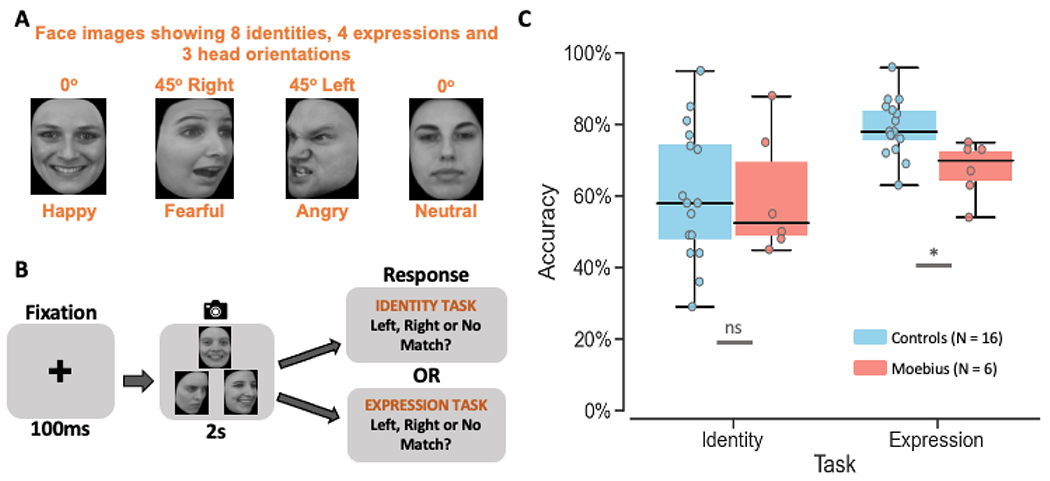
A. Images of 8 different facial identities and 4 different expressions (happy, fearful, angry, and neutral) from 3 different viewpoints (left, front, and right) were used as stimuli. B. Depiction of the trial structure – each trial began with a 100ms fixation cross, followed by the presentation of three faces in a triangle format for 2s, during which participants were to indicate which of the two bottom faces (or neither) matched the top face either on identity or expression (in separate runs). C. Box and whisker plots showing performance accuracy for identity and expression matching for control participants in blue and MBS individuals in red. MBS individuals had similar performance as control participants on the identity matching task but worse performance on the expression matching task (*p < 0.01; ns: no significant difference; chance performance on this task = 33%). KDEF images used in Figure 2 panels A and B are reproduced from KDEF stimulus database - Lundqvist et al., 1998 (https://www.kdef.se/home/aboutKDEF.html), with permission from Karolinska Institutet, Psychology section, Copyright year: 1998, Copyright holder: Karolinska Institutet, Psychology section.
Taken together, results from the two static face tasks above (emotion detection and expression matching) provide strong evidence for a selective impairment in MBS individuals for facial expression processing, while other aspects of face processing such as feature detection and identity processing remain intact.
Although static facial expressions are predominantly used in the literature to study emotion perception and most previous studies in MBS have used static faces, in real life social interactions, we invariably encounter facial expressions in their dynamic form. Accordingly, we next sought to examine whether individuals with MBS who appear to have difficulty extracting emotion information from static faces, can accurately detect emotion from dynamic faces. We hypothesized that due to facial paralysis MBS individuals would show impairment in processing facial motion and expressions from dynamic faces.
2.3. Impaired perception of facial motion and facial emotion
Participants were shown video clips of happy, fearful, and angry faces (Figure 3A, 3B) of different durations in a randomized order, and asked to indicate if they thought the video clip depicted a happy, fearful, angry, or neutral expression (during the emotion task runs), or if they thought the mouth in the video moved or not (during the motion control task runs). The latter served as a perceptual control to establish whether individuals with MBS have an intact ability to perceive facial motion. We computed the psychometric function for facial motion detection and facial emotion categorization (for happy, fearful and angry expressions) for the perceptual control and emotion tasks, respectively, and then estimated the 50% threshold for detecting motion and categorizing each emotion in the face.
Figure 3. Dynamic Facial Expression Task.
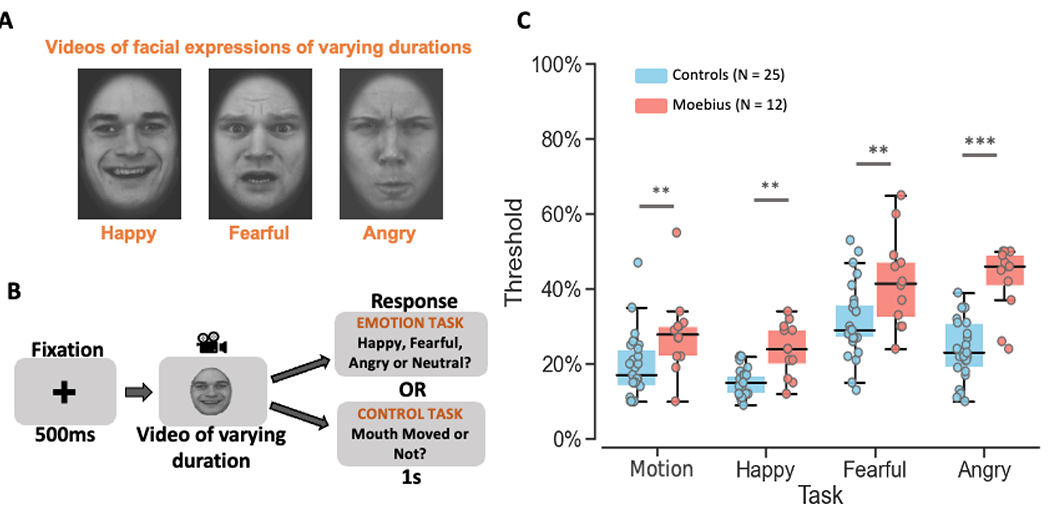
A. Videos of 8 different durations ranging from 200ms to 1.6s depicting dynamic facial expressions of happiness, fear, and anger were used as stimuli. B. Depiction of the trial structure – each trial began with a 500ms fixation cross, followed by a happy, fearful, or angry video of varying duration (200ms, 400ms, 600ms, 800ms, 1s, 1.2s, 1.4s, or 1.6s), and a 1s response window during which participants were to indicate if they thought the video depicted a happy, fearful, angry, or neutral expression during the emotion task runs, or whether the mouth moved or not during the facial motion control task runs. C. Box and whisker plots showing the thresholds for each task for control participants in blue and MBS individuals in red. MBS individuals had higher thresholds than controls participants for both facial motion detection and emotion categorization tasks (**p < 0.001; *** p < 10−6). KDEF images used in Figure 3 panels A and B are reproduced from KDEF stimulus database - Lundqvist et al., 1998 (https://www.kdef.se/home/aboutKDEF.html), with permission from Karolinska Institutet, Psychology section, Copyright year: 1998, Copyright holder: Karolinska Institutet, Psychology section.
Overall, MBS individuals showed significantly higher thresholds than controls for both the emotion categorization and perceptual control tasks, indicating that facial paralysis dampens the ability to extract both motion and emotion information from a face (Figure 3C). A Task (Control, Happy, Fearful, Angry) x Group (Controls, MBS) mixed-effects beta regression analysis of facial motion and emotion thresholds showed not only an expected main effect of Task (p < 2 x 10−16), but also a strong main effect of Group (p = 6.17 x 10−8), and no significant Task x Group interaction (p = 0.069), such that MBS individuals were uniformly worse than controls on all tasks including facial motion and facial expressions detection.
Based on our a priori hypothesis, planned between-group pairwise tests revealed significantly higher thresholds for MBS individuals compared to controls for the control task and all three expression tasks, i.e., control (t118 = 3.63, p < 0.0004; 50% thresholds for MBS: 28.0 ± 10.8% SD, Controls: 19.3 ± 8.7% SD), happy (t118 = 3.77, p < 0.0003; 50% thresholds for MBS: 24.0 ± 6.9% SD vs. Controls: 15.3 ± 3.9% SD), fearful (t118= 3.94, p < 0.0001; MBS: 42.0 ± 12.3% SD vs. Controls: 31.3 ± 10.0% SD), and angry expressions (t118= 7.11, p < 9.60 x 10−11; MBS: 42.8 ± 9.1% SD vs. Controls: 24.0 ± 8.0% SD), with the latter yielding the largest difference in thresholds between the two groups. Thus, not only did MBS individuals have difficulty extracting emotion information from a moving face compared to controls, but they were also impaired at detecting facial motion from the dynamic face videos. These results suggest that facial paralysis in MBS impairs perception of facial motion more generally and impacts perception of facial expressions. Next, to confirm that this deficit in detecting motion and recognizing emotion was limited to facial expressions, we examined the ability of MBS individuals to extract motion and emotion information from body expressions. We predicted that MBS individuals would perform similarly to controls on body motion and body expression tasks.
2.4. Intact perception of body motion and body emotion
In this task, participants were shown video clips of happy, fearful, and angry bodies of different durations (Figures 4A and 4B) in a randomized order and asked to indicate if they thought the actor’s body in the video clip depicted a happy, fearful, angry or neutral expression (during the emotion categorization task runs), or if they thought the actor’s arms in the video moved or not (during the motion detection task runs). The latter served as a perceptual control to establish whether individuals with MBS have an intact ability to perceive body motion. We computed the psychometric function for body motion detection and body emotion categorization (for happy, fearful and angry expressions) for the perceptual control and emotion tasks, respectively, and then estimated the 50% threshold for detecting motion and categorizing each emotion in the body.
Figure 4. Dynamic Body Expression Task.
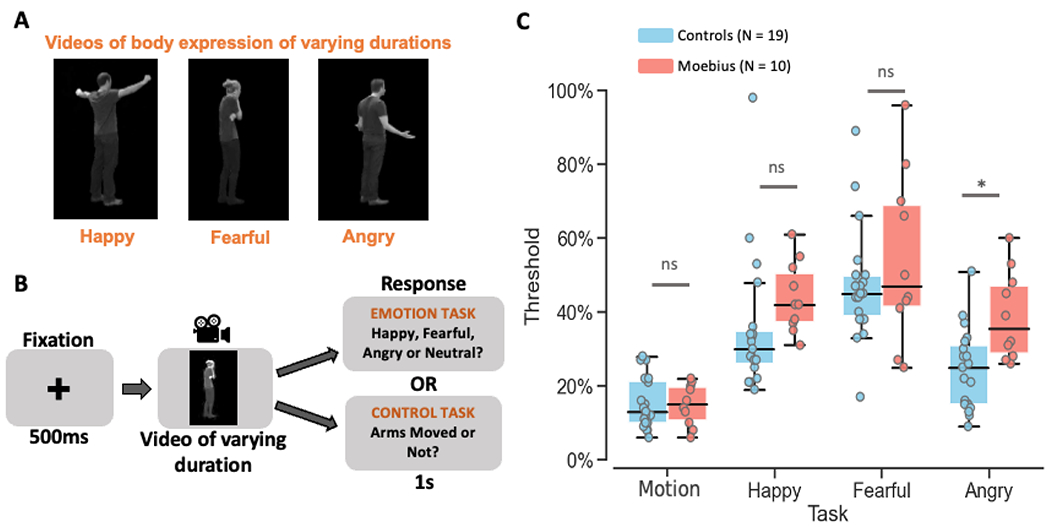
A. Videos of 12 different durations ranging from 200ms to 2.4s depicting dynamic body expressions of happiness, fear, and anger were used as stimuli. B. Depiction of the trial structure – each trial began with a 500ms fixation cross, followed by a happy, fearful, or angry video of varying duration (200ms, 400ms, 600ms, 800ms, 1s, 1.2s, 1.4s, 1.6s, 1.8s, 2.0s, 2.2s, or 2.4s), and a 1s response window during which participants were to indicate if they thought the video depicted a happy, fearful, angry, or neutral expression during the emotion task runs, or whether the arms moved or not during the body motion control task runs. C. Graph showing the emotion thresholds for each task for control participants in blue and MBS individuals in red. MBS individuals had similar thresholds as control participants for body motion detection (control task) and for two of the three body emotion detection tasks (*p < 0.01; ns: no significant difference). Figure 4 panels A and B use reproduced images from the Action Database – Keefe et al., 2014, with permission from the authors (copyright year: 2014, copyright holder: Keefe et al.).
Overall, MBS individuals showed thresholds similar to controls for both the control and emotion tasks (Figure 4C) indicating that facial paralysis does not affect body motion perception or the ability to extract emotion information from body expressions. A Task (Control, Happy, Fearful, Angry) x Group (Controls, MBS) mixed-effects beta regression analysis of body motion and emotion thresholds showed only a main effect of Task (p < 2.0 x 10−16), and critically no main effect of Group (p = 0.09), or Task x Group interaction (p = 0.27).
Based on our a priori hypothesis, planned between-group pairwise tests revealed slightly higher thresholds for MBS individuals than controls only for the angry expression task (t92.5 = 2.87, p = 0.005; 50% thresholds for MBS: 38.9 ± 12.1% SD, Controls: 24.5 ± 11.1% SD) but not for the motion control task (t92.5 = 0.11, p = 0.91; 50% thresholds for MBS: 14.8 ± 5.8% SD, Controls: 15.3 ± 7.0% SD) or the happy (t92.5 = 1.16, p = 0.25; 50% thresholds for MBS: 43.8 ± 9.6% SD, Controls: 35.6 ± 18.5% SD) and fearful (t92.5 = 1.58, p = 0.12; 50% thresholds for MBS: 54.3 ± 23.2% SD, Controls: 47.4 ± 15.7% SD) body expression task. Thus, although MBS individuals showed similar thresholds as controls for three of the four task conditions (i.e., control, happy and fearful conditions) there appears to be a trend for MBS thresholds to be somewhat higher than controls (especially for the angry condition). This suggests that there might be some subtle differences between the two groups for this task, which could be further investigated in the future. Overall, in contrast to the consistently higher thresholds seen for MBS individuals relative to controls in the facial expression task, our results suggest that MBS individuals do not have a clear and general difficulty extracting motion or emotion information from body expressions.
2.5. Behavioral Summary
Overall, the results from our comprehensive battery of behavioral tasks combined across all 4 static and dynamic facial and body expression tasks (i.e., higher thresholds for facial emotion detection and recognition and reduced performance on facial expression matching), together suggest that facial paralysis in MBS significantly affects perception of facial motion and facial expression but has limited impact on body motion and body expression perception (but the latter may warrant further study). Thus, the inability to portray emotion on their own face appears to dampen the ability of MBS individuals to extract emotional information only from facial expressions.
But what brain changes, if any, subserve this dampening in facial expression processing in MBS? To answer this question, we conducted exploratory fMRI scans to examine the potential neural correlates of this behavioral impairment in a small subset of MBS individuals and age- and gender-matched controls, focusing on face-selective regions of the brain(Kanwisher et al., 1997; Pitcher et al., 2017; Zhang et al., 2020) such as fusiform face area (FFA), posterior superior temporal sulcus (pSTS) and the amygdala (AMG). These regions were chosen because they are considered core parts of the face-processing network and are thought to be involved in processing invariant and changeable aspects of a face such as facial identity and facial expressions (Bruce & Young, 1986; Haxby et al., 2000).
2.6. Potentially reduced amygdala engagement during facial expression processing
Using a functional face localizer, we first identified face-selective regions of interest (ROIs) in the right FFA and right pSTS that responded significantly more to static and dynamic faces than objects (see Methods for details) for each participant. In addition, using a standard neuroanatomical atlas (TT_N27 in AFNI), we defined an anatomical ROI in the right AMG for each participant. Within these three ROIs, we then compared the average fMRI activity during identity vs. expression tasks for controls and individuals with MBS.
Overall, the right FFA and right pSTS showed similar fMRI activity profiles for the two tasks between controls and MBS, while activity profiles in the amygdala appeared different between the two groups. A Task x Group linear-mixed effects analysis of right FFA data showed no significant main effect of Task (p > 0.77) or Group (p > 0.97), or Task x Group interaction (p > 0.22). fMRI activity in right pSTS expectedly showed a significant main effect of Task (p < 0.01), but no main effect of Group (p > 0.97) or Task x Group interaction (p > 0.89).
Due to the small sample size for the MBS group, we also compared the fMRI activity within each group between the two tasks. These paired t-tests revealed no difference in activity between Identity and Expression conditions in right FFA for both controls (p > 0.23) and MBS (p > 0.23). By contrast, in right pSTS, controls showed higher fMRI activity during expression than identity task runs, while in the MBS group this comparison did not reach statistical significance (Controls: t14 = 2.46, p < 0.01; MBS: t5 = 1.87, p < 0.06; see Figure 5A and 5B). In contrast to right FFA and right pSTS, a different pattern was observed in right AMG. While no significant main effect of Task (p > 0.78), main effect of Group (p > 0.49) or Task x Group interaction (p < 0.07) was found in right AMG, the two groups showed seemingly opposite fMRI activity profiles. Although these effects were not statistically significant, in contrast to control participants who on the average showed numerically higher activity during expression than identity task runs (10 out of 15 controls showed this pattern; paired sampled t-test: t14 = 1.41, p < 0.09), individuals with MBS predominantly showed the opposite pattern, i.e., numerically lower activity during expression task runs than during identity task runs (4 out of 6 MBS individuals showed this opposite pattern; paired sample t-test: t5 = −1.81, p < 0.07; see Figure 5C). Due to the low number of MBS participants, we also ran non-parametric Wilcoxon signed rank tests for the right AMG and found similar results (controls: Z = 1.48, p < 0.07; MBS: Z= −1.57, p < 0.06; see also Supplemental Material F for additional Bayesian prevalence analysis (Ince et al., 2021) of these data). Thus, taken together, although the fMRI results are statistically weak and should be interpreted with caution due to low statistical power, they show qualitatively that while MBS individuals show relatively normal activation patterns in face-selective regions such as FFA and pSTS, the amygdala which is known to be a core region for processing emotion in general and facial expressions in particular, may exhibit a different pattern of activation compared to controls.
Figure 5. Neural correlates of expression processing in a small group of MBS individuals relative to controls.
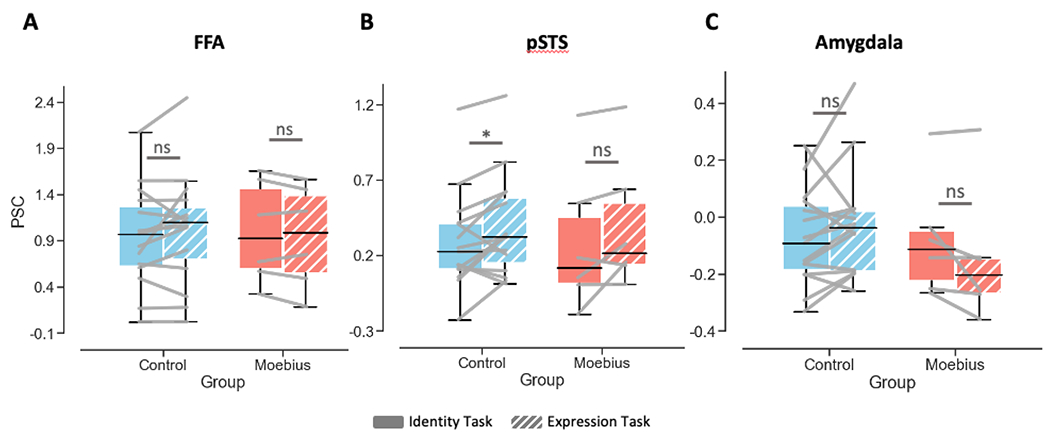
A. fMRI activity plots showing no difference in BOLD percent signal change in right FFA during identity (solid) vs. expression (patterned) matching for Controls (N = 15) in blue and MBS individuals (N = 6) in red. B. fMRI activity plots showing significantly higher BOLD percent signal change in right pSTS for expression compared to identity matching for Controls (*p < 0.01) but not MBS (p = 0.06). C. BOLD percent signal change in right amygdala was not significantly different during expression matching relative to identity matching for Controls or MBS, although numerically the two groups showed opposite patterns (Controls: expression > identity while MBS: expression < identity).
3. DISCUSSION
Using a battery of behavioral tasks, we found that individuals with Moebius Syndrome, who from birth are unable to move their facial muscles to express emotions, were impaired at detecting emotion in others’ facial expressions. This impairment in facial emotion detection was accompanied by an impairment in facial motion perception. Importantly, while the facial paralysis affected the sensitivity of MBS individuals to facial motion and facial expressions, it did not impact their ability to perceive body motion or extract emotional information from body expressions. Thus, the inability to move a particular body part (in this case one’s face) appears to impact the perception of motion and emotional information in the same body part of others (here facial expressions vs. body expressions), resulting in a selective dampening of facial expression perception. Additionally, our MBS participants although impaired at facial expression processing, could accurately perform other challenging tasks such as feature detection and identity matching from static faces and body motion detection and expression recognition from dynamic bodies. These behavioral findings were supported by preliminary fMRI data in a small subgroup of MBS individuals that suggested reduced engagement of the amygdala during an expression processing task. Taken together, these convergent findings suggest that the inability to make facial expressions from birth, results in a slight dampening of perception of others’ facial expressions and may be associated with changes in the level of engagement of the neural circuitry underlying emotion processing.
Our results of dampened facial expression perception align well with those of others who have previously reported generalized face processing difficulties in Moebius Syndrome (Bate et al., 2013) as well as those reporting reduced autonomic modulation during emotion processing (De Stefani, Ardizzi, et al., 2019; De Stefani, Nicolini, et al., 2019; Nicolini et al., 2019). Our results are, however, at odds with the conclusion of some studies that have reported no impact of facial paralysis on emotion perception in Moebius Syndrome (Calder et al., 2000; Rives Bogart & Matsumoto, 2010; Vannuscorps et al., 2020). However, the use of full-blown facial expression stimuli, lack of appropriate control tasks and small sample size in these previous studies may account, in part, for their negative results. Vannuscorps et al., (Vannuscorps et al., 2020) did limit the amount of emotion information in one of their facial expression tasks (40% of full-blown facial expressions), but our data suggest that this may not have been low enough to pick up the subtle emotion deficit in MBS. In our study, most MBS individuals were, on average, able to perform the task at 40% morph level (even though on the average their thresholds were higher than controls). Thus, the task used by Vannuscorps et al. (Vannuscorps et al., 2020) likely was not sensitive enough to pick up subtle deficits in emotion processing. Individuals with MBS do not typically report significant difficulties in social interactions (Rives Bogart & Matsumoto, 2010), and thus it is not surprising that their facial expression perception deficits are subtle and that they are able to efficiently recognize full blown emotional expressions. Affected individuals have likely developed compensatory strategies during development to not only convey their own emotions, but also to understand another person’s emotional state by using other social cues such as voice tonality (Bogart et al., 2014). Although we did not collect physiological measures of emotion perception in the current study, future studies using autonomic skin conductance (as a proxy for emotional reactivity), eye tracking and electromyographic data, will help to further explicate the link between emotion perception and emotional experience. Future work could also focus on the generalizability of the dampening that we observed in the visual domain in our current study by examining the effect of facial paralysis on emotion perception in other modalities, such as perception of emotional vocalizations.
In addition to the use of challenging perceptual control tasks, a major advantage of our study is the use of dynamic body expressions as a control for the dynamic face task. The lack of impairment on the body motion and emotion tasks suggests a selective impairment in MBS for facial expressions. While the direct comparison of thresholds between the two dynamic tasks using a combined 3-way Modality (Dynamic faces, Dynamic bodies) x Task (Control, Happy, Fearful, Angry task) x Group (Controls, Moebius) mixed-effects beta regression analysis did not yield a significant three-way Modality x Task x Group interaction (likely due to low statistical power), the presence of a strong Group effect in the dynamic face task coupled with its absence in the dynamic bodies task, supports the idea of a selective impairment in facial motion and emotion processing but not body motion and emotion processing in MBS individuals.
Further, the difference in behavioral results between the static and dynamic face control tasks, i.e., MBS individuals performed similarly to controls on the feature detection task but not on the facial motion control task, suggests that MBS individuals are unimpaired at processing static facial features, but impaired at processing dynamic motion cues from the face. A potential criticism of our study could be that the control tasks may have been too easy, resulting in similar performance for MBS and Control groups due to a floor effect. For example, the absence of a difference in body movement thresholds between controls and MBS individuals could be related to the low task difficulty, while in fact MBS individuals might have trouble with this task if it were harder. This is unlikely, however, since the results from the identity and expression tasks show that even for a reasonably difficult control task (identity matching), MBS participants performed similarly to controls. This indicates that MBS individuals are able to reliably detect body motion, but not facial motion. Another potential criticism of our study could be that in addition to less motor experience, MBS individuals may also have less visual experience with expressions in general (the people around them may not express emotion on their faces as much as they would when interacting with typically developing children), resulting in higher expression thresholds. Future studies could examine the relative contribution of visual and motor experience, by quantifying the level of visual experience with facial expressions that MBS individuals encounter in their social interactions during development. Another interesting aspect for future research could be the impact of other types of facial paralysis on facial expression perception. For example, one might predict minimal or no impact of facial paralysis on facial expression perception in individuals with congenital hemifacial microsomia (who are unable to move only one side of their face; (Cousley & Calvert, 1997)). In addition, future studies could characterize facial expression perception before and after temporary facial paralysis such as that seen in Bell’s palsy (Ahmed, 2005). These studies would help identify the extent and duration of paralysis that is needed to significantly impact facial expression perception.
With regard to the choice of task type for our questions of interest, we used a yes-no detection task for static faces, and a 4-way emotion categorization task for dynamic faces and bodies; tasks that are susceptible to user bias. Typically, a two-interval forced choice (2-IFC) task is often recommended for such psychophysical experiments due to its limited susceptibility to response bias (Delicato, 2020; Marneweck et al., 2014) (but also see Yeshurun et al. (Yeshurun et al., 2008) for why 2-IFC procedures may not be completely bias-free). However, we chose our tasks in this way for their simplicity and short administration times, and so that we could limit task demand and cognitive load confounds. We also confirmed that the bias inherent in the task was not significantly different between the two groups by performing signal detection theory analysis on the catch trials (neutral - 0% morphs and emotional faces −100% morphs) in the static face task (see Supplemental Material C). Importantly, we also found that the sensitivity (d’) to 100% emotional faces did not vary between the two groups, which further supports the notion that the expression recognition deficits in MBS are subtle, and that individuals do not experience difficulty in detecting emotion from full-blown static facial expressions as has been used in several prior studies. While we were able to show that response bias could not explain the differences between the two groups for the static face task, a similar analysis for the dynamic face and body tasks was not possible due to the absence of neutral videos in these tasks. Another potential limitation of the static-face task was the estimation of thresholds based on psychometric fitting of staircase data which results in sub-optimal sampling of data along the psychometric range. This issue was alleviated by the use of the method of constant stimuli in the dynamic face and body tasks. Further, to rule out the possibility that systematic differences between Controls and MBS in the quality of psychometric fitting might affect the results, we examined the average sum of squares error (SSE) of the fit for the two groups for each condition in each of the three thresholding tasks (total of 12 conditions across the three tasks - static face task, dynamic face task and dynamic bodies task). We found no significant differences in the SSE between the two groups for 11 out of the 12 task conditions (all ps > 0.1; only the static face fearful emotion detection task condition showed slightly higher SSEs for MBS than Controls, p = 0.008). Thus, the quality of psychometric fitting could not explain the group effects seen in our study.
While the results from the planned between-group comparisons for the dynamic face and body tasks suggest a selective deficit in MBS for facial expression recognition, it is important to use caution in interpreting these results in the absence of a significant Group x Task interaction (likely due to the small sample size). Future work in a larger cohort of MBS participants could help explicate the subtle differences between the two groups for these tasks. Another potential limitation of our study was the choice of control task for the dynamic facial expression task. One might expect that MBS individuals would have difficulty detecting movement in the face, and thus an additional perceptual task that relied on facial features instead of facial motion might have been good to include. For example, in a future study participants could be asked to indicate whether they saw teeth being exposed during the video – this would provide an additional valuable measurement of whether MBS individuals are able to perceive featural changes in dynamic stimuli.
Although our study was focused on detection thresholds and performance accuracy, it is possible that response speed may be impacted in patient populations compared to controls. To examine whether this might be a factor to consider in our data, we compared the average reaction time (RT) between the two groups for each of the four tasks and found significant differences in RT only for the dynamic face control and emotion tasks, and the dynamic body emotion task, such that MBS individuals had longer RTs than controls (see Supplemental Table E). Thus, not only did MBS individuals perform worse than controls on the dynamic face tasks, but they were also slower to make their responses on this task, providing further evidence that the extraction of facial motion and facial expression information from dynamic movies is impacted in MBS individuals. Similar results were seen for the body expression task; critically we did not see a difference in RT between the two groups for the body motion control task, where thresholds for MBS individuals were similar to controls.
While we have linked facial expression perception deficits in our MBS cohort to their inability to move their face, we acknowledge that there might be several other factors not explicitly considered here that might also impact perceptual abilities in MBS. For example, many individuals with MBS encounter physical, cognitive, and social challenges. While we did not see evidence for any substantial issues related to these domains in our MBS participants (see Supplemental Table A for detailed breakdown of neuropsychological measures), our cohort might be biased as we only tested individuals who were able to travel and able to follow our task instructions. Regardless, these developmental, social, and quality of life issues are important factors to consider in the broader interpretation of the results reported here and should be studied further.
Overall, our results also align well with the general notion of experience influencing perception as has been demonstrated by studies in professional actors (Conson et al., 2013) and dancers (Christensen et al., 2016; Orlandi et al., 2017) showing enhanced ability to recognize facial and body expressions, respectively. Results from these studies on how experience enhances perception therefore predict that the lack of facial experience in MBS would adversely impact the ability to recognize others’ facial expressions. In fact, these results provide direct support for Darwin’s intuitive observation (Darwin, 1872) that suppressing one’s expressions of an emotion also suppresses the underlying emotion (facial feedback). Darwin also held that people tend to expressively imitate others (facial mimicry), and the current study in individuals with congenital facial palsy, who have never been able to imitate or express their emotions, finds what Darwin had predicted – a dampened sensitivity to facial emotional information in such individuals. Our results are in line with a recent study describing the role of motor control in visual body perception that reported impaired performance on a body judgement task in a group of congenital one-handers (Maimon-Mor et al., 2020). In fact, the crucial role of motor experience in emotion processing has been clinically leveraged in the treatment of depression by using muscle inactivation to manipulate emotional proprioception (Finzi & Rosenthal, 2016).
In contrast to previous studies of expression processing in Moebius Syndrome, we were also able to obtain some exploratory neuroimaging data to examine the neural correlates underlying the impact of facial paralysis on facial expression perception. Although the fMRI data are limited in terms of sample size (only 6 MBS participant completed the identity and expression tasks in the scanner), they suggest that a more robust investigation of the amygdala and related emotion processing circuitry in MBS is warranted in the future. Additionally, the trend for the expression vs. identity effect in pSTS to be slightly weaker in MBS than controls, should also be further studied as it may be linked to the potentially diminished involvement of the amygdala in MBS and reflect their behavioral deficit. Since it appears that the degree of engagement of these regions (especially the amygdala) may be impacted in MBS relative to controls, future studies could examine the use of visual training to improve the engagement of these networks during facial expression processing.
In summary, our behavioral and neuroimaging findings together provide support for a critical role of facial movement (and relatedly facial mimicry, motor experience, and facial feedback) in facial motion perception such that its disruption can lead to impairment in the perception of facial expressions. These results predict the effectiveness of visual training paradigms to enhance facial expression perception and improve social interactions in situations involving absent motoric experiences, such as the congenital facial palsy seen in Moebius Syndrome.
4. MATERIALS AND METHODS
4.1. Participants
Twenty-three individuals with Moebius Syndrome (17-64 years) and 51 normal volunteers (20-63 years) (or a legal guardian where applicable) provided written informed to participate in the study. MBS participants were enrolled under a protocol approved by the Institutional Review Board of the National Human Genome Research Institute at the National Institutes of Health (NCT02055248). MBS individuals (see Supplemental Material A for clinical information) were recruited to the study at the NIH Clinical Center (N=14) or at the 2018 MBS Foundation Conference in St. Petersburg, FL (N=10). Some of the MBS participants (N=16) completed survey questionnaires about their medical history and facial disability, some (N=10) completed additional neuropsychological testing, and some (N=14) completed a rehabilitation evaluation. Healthy volunteers for the study were recruited under an NIH IRB approved protocol of the National Institute of Mental Health (NCT00001360). No part of the study procedures or analyses was pre-registered prior to the research being conducted. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Study sample size was not predetermined as recruitment of MBS participants was difficult due to the rarity of the disease, and thus we enrolled as many participants as possible. Data were excluded based on predetermined criteria for each task separately as listed below. MBS and control participants completed a series of computer tasks involving static and dynamic facial and body expressions (see Supplemental Material B). Due to time and availability constraints, not all participants completed all tasks (3 MBS participants completed all 4 behavioral tasks; 1 participant completed 3 tasks; 9 participants completed 2 tasks; while 11 MBS participants completed only one behavioral task). In general, two age- and gender-matched controls were recruited for each MBS participant (5 Control participants completed all 4 behavioral tasks; 3 participants completed 3 tasks; 19 participants completed 2 tasks; while 20 Control participants completed only one behavioral task). Experimental conditions were kept similar between the two testing venues (NIH Clinical Center and MBS Foundation Conference). Eight MBS individuals (all 8 completed one or more behavioral tasks) and 16 control participants (12 completed one or more behavioral tasks) also completed functional magnetic resonance imaging (fMRI) scans at the NIH Clinical Center.
4.2. Static facial expression task
Fourteen MBS participants (mean age 35.2 ± 17.7 SD, 6 males) and 26 age- and gender-matched controls (mean age 32.1 ± 13.3 SD, 10 males) completed 2 runs of an emotion detection task and 2 runs of a feature detection task (that served as a perceptual control task) shown in Figure 1. Using FantaMorph software (Abrosoft) we first created a series of morphs with varying levels of happiness and fear using a neutral face and the corresponding happy or fearful face, respectively. Neutral and full-blown facial expression images for 14 male and 13 female actors were taken from the Karolinska Directed Emotional Faces set (KDEF (Lundqvist D, 1998)). For each actor, 19 intervening morphs of increasing expressional content in 5% increments were created by morphing the neutral image to the corresponding happy and fearful images. Starting at the 50% morph, participants were shown images of increasing or decreasing emotion depending on their performance in a 3-down/1-up adaptive staircase fashion. Each run contained a total of 169 trials consisting of 130 morph face trials (dependent on participant’s performance), 26 neutral face catch trials, and 13 full expression catch trials. Each trial began with a fixation cross for 250ms followed by the morphed face. The face appeared at the center of the screen for 350ms and subtended 4 and 6 degrees of visual angle in the horizontal and vertical dimensions, respectively. The face was followed by a fixation cross for 1.5s during which participants were asked to press the “yes” button if they thought the image showed a happy or fearful expression (in separate runs) or press the “no” button if they thought the image was neutral (see Figure 1A and 1B for stimuli and task details). In separate controls runs, using a similar procedure, participants were asked to press the “yes” button if they thought the image showed an open mouth (lips apart) or press the “no” button if they thought the image showed a closed mouth (lips touching). Responses were recorded using a response box (Current Designs, Inc.) and participants were asked to respond as quickly and as accurately as possible. Detection accuracies for each morph level for fearful and happy faces were used to generate each participant’s psychometric curves. A standard logistic function was used to fit each participant’s psychometric curve for each emotion using the fminsearch function available in the MATLAB Optimization Toolbox (MathWorks, Inc.). The α parameter of this fit (which denotes the point of subjective equality for a yes-no detection task (Klein, 2001; Wichmann & Hill, 2001)) was then used to identify the morph level threshold at which each participant achieved 50% detection accuracy. One MBS participant’s data were excluded from further analysis because the fitting procedure did not converge to yield appropriate parameter estimates (due to high sum of squares error of the fit). Morph level thresholds for the remaining participants were then entered into a mixed-effects beta regression model (using the R package mgcv) with Task and Expression as within-subjects factors and Group as a between-subjects factor (see results in Figure 1C and Supplemental Material D). Additionally, where appropriate, post-hoc Bonferroni-corrected pairwise comparisons were conducted to compare average thresholds between Controls and MBS groups for each emotion.
4.3. Facial identity and expression matching task
Six MBS participants (mean age 46.2 ± 18.1 SD, 3 males) and 16 age- and gender-matched controls (mean age 38.1 ± 14.3 SD, 8 males) completed 2 runs each of a facial identity matching task and a facial expression matching task shown in Figure 2. Stimuli for this task were prepared by selecting images of 8 actors depicting happy, fearful, angry, and neutral facial expressions from 3 viewpoints (front view and 45° to left and right) from the KDEF (Lundqvist D, 1998) database. On each trial, participants were shown 3 faces in a triangular formation for 2s (preceded and followed by a 100ms fixation cross; see Figure 2A and 2B) and were asked to indicate which of the two lower faces (or neither) matched the top face based on the facial identity of the actor (during identity task runs) or based on facial expression of the actor (during expression task runs). The top face was one of the 8 actors depicting one of the 4 expressions (from one of the 3 viewpoints), and one of the bottom faces matched the top face on identity (same actor, different expression) and the other matched the top on expression (different actor, same expression). On some trials neither of the bottom faces matched the top on identity or expression, and these served as catch trials to prevent participants from performing this task without explicitly detecting identity or expression. Each face in the triangular display subtended 2 (horizontal) and 3 (vertical) degrees of visual angle at 3-degree eccentricity about the central fixation cross. Each run consisted of 8 blocks of 12 trials, with each trial lasting 2.2s. Group differences in performance (accuracy rates) on each task were assessed using a mixed-effects beta regression analysis with Task (2 levels: identity and expression) as a within-subjects factor and Group as a between-subjects factor, as well as post-hoc Bonferroni-corrected pairwise comparisons (see results in Figure 2C and Supplemental Material D).
4.4. Dynamic facial expression task
Thirteen MBS participants (mean age 33.5 ± 16.5 SD, 6 males) and 25 age- and gender-matched controls (mean age 31.9 ± 11.8 SD, 11 males) completed 3 runs of a facial emotion task and 1 run of a facial motion control task shown in Figure 3 (one MBS participant completed only 2 runs of the emotion task due to time constraints). Stimuli for this task were created by stringing together the 21 morph images for each expression (created for the static facial expression task described above) into a slow-motion movie depicting the actor making a happy, fearful, or angry facial expression. The movie was then clipped to create 8 different lengths of the expression, such that each clip showed either 200ms, 400ms, 600ms, 800ms, 1s, 1.2s, 1.4s and 1.6s from the start of the movie. Shorter clips contained less emotion information than longer clips since the expression on the face in the video had not yet evolved into the full-blown expression. In this way, we limited the amount of emotion information in the dynamic facial expression movies. Using a method of constant stimuli, videos of varying lengths of the three different expressions were presented to participants in a randomly inter-mixed order within each run. Each trial began with a 500ms fixation cross, followed by the video clip presented at the center of the screen subtending 4 (horizontal) and 6 (vertical) degrees of visual angle. The video was in turn followed by a response window lasting 1s, during which participants were asked to indicate their response (see Figure 3 A and 3B for stimuli and task details). Each run contained 6 trials of each video length of each emotion, totaling 144 trials per run. During emotion categorization task runs, participants were asked to press one of four buttons to indicate whether the video clip depicted a happy, fearful, angry, or neutral expression. During facial motion detection task runs participants were asked to press one of two buttons to indicate whether the actor’s mouth moved during the video clip. Responses were recorded using a four-button response box (Current Designs Inc.) and participants were asked to respond as quickly and as accurately as possible. Data were analyzed similar to the static facial expression task such that detection and categorization accuracies for each video duration for each emotion were first used to generate each participant’s psychometric curves. The α parameter of a standard logistic function fit to the psychometric curves was then used to identify the video duration threshold at which each participant achieved 50% accuracy for each emotion. One MBS participant’s data were excluded from further analysis because the fitting procedure did not converge to yield appropriate parameter estimates. Duration thresholds for the facial motion detection and facial emotion categorization tasks were entered into a mixed-effects beta regression analysis with Task (4 levels: control, happy, fearful and angry task) as a within-subjects factor and Group (2 levels: Controls, MBS) as a between-subjects factor. Additionally, where appropriate, Bonferroni-corrected post-hoc t-tests were conducted to compare average thresholds between Controls and MBS groups for each task condition (see results in Figure 3C and Supplemental Material D).
4.5. Dynamic body expression task
Eleven MBS participants (mean age 37.7 ± 16.0 SD, 4 males) and 20 age- and gender-matched controls (mean age 35.1 ± 12.8 SD, 6 males) completed 3 runs of a body emotion task and 1 run of a body motion control task shown in Figure 4 (one MBS participant completed only 2 runs of the emotion task due to time constraints). Stimuli for this task were chosen from the Action Database of whole-body action videos (Keefe et al., 2014) of 10 actors making happy, fearful, and angry body movements (with their backs towards the camera to obscure the face). Each video was then clipped to create 12 different lengths of the body expression, such that each clip showed either 200ms, 400ms, 600ms, 800ms, 1s, 1.2s, 1.4s, 1.6s. 1.8s, 2s, 2.2s or 2.4s from the start of the video. Shorter clips contained less emotion information than longer clips since the movement of the body in the video had not yet evolved into a full-blown expression. In this way we limited the amount of emotion information in the dynamic body expression videos. Using a method of constant stimuli, videos of varying lengths of the three different expressions were presented to participants in a randomly inter-mixed order within each run. Each trial began with a 500ms fixation cross, followed by the video clip presented at the center of the screen subtending 6 (horizontal) and 4 (vertical) degrees of visual angle. The video was in turn followed by a response window lasting 1s, during which participants were asked to indicate their response (see Figure 4A and 4B for stimuli and task details). Participants completed 10 trials for each video length for each emotion, over 3 runs of the emotion task, with each run containing 120 trials. During emotion categorization task runs, participants were asked to press one of four buttons to indicate whether the video clip depicted a happy, fearful, angry, or neutral expression. During body motion detection task runs participants were asked to press one of two buttons to indicate whether the actor’s arms moved during the video clip. Responses were recorded using a four-button response box (Current Designs Inc.) and participants were asked to respond as quickly and as accurately as possible. Data were analyzed similar to the dynamic facial expression task such that categorization accuracies for each video duration for each emotion were first used to generate each participant’s psychometric curves. The α parameter of a standard logistic function fit to the psychometric curves was then used to identify the video duration threshold at which each participant achieved 50% accuracy for each emotion. One MBS and one Control participant’s data were excluded from further analysis because the fitting procedure did not converge to yield appropriate parameter estimates. Duration thresholds for the body motion detection and body emotion categorization tasks were entered into a mixed-effects beta regression analysis with Task (4 levels: control, happy, fearful and angry task) as a within-subjects factor and Group as a between-subjects factor. Additionally, where appropriate, Bonferroni-corrected post-hoc t-tests were conducted to compare average thresholds between Controls and MBS groups for each task condition (see results in Figure 4C and Supplemental Material D).
4.6. Functional MRI data acquisition
Eight individuals with MBS (mean age 37.1 ± 22.2 SD, 4 males) and 16 age- and gender-matched controls (mean age 32.9 ± 14.1 SD, 8 males) were scanned using a GE-MR750 3 Tesla MRI scanner. While in the MRI scanner, participants completed two blocked-design functional face localizer task runs, and 2 runs each of the identity and expression matching task (one MBS individual did not complete this task due to time constraints). fMRI scans were performed using a BOLD-contrast sensitive multi-echo echo-planar (EPI) sequence with three echo times (TEs: 12.5ms, 27.6ms and 42.7ms). Scanning parameters used were typical of whole brain fMRI studies (Array Spatial Sensitivity Encoding Technique [ASSET] acceleration factor = 2; TR = 2.2s; 33 interleaved AC-PC aligned 3.5mm thick slices with 3.2 x 3.2mm in-plane resolution). One participant (Patient 1) was scanned using a single echo EPI sequence (TE: 27.6ms) due to unavailability of the multi-echo sequence at the time, but all other parameters remained the same. A high-resolution T1 structural MP-RAGE scan (172 sagittal slices with 1mm x 1mm x 1mm voxel resolution, TE = 3.47ms, TR = 2.53ms, TI = 900ms, flip angle = 7°) was also collected for each participant.
During each Face localizer run, participants were shown in a random order, 2 blocks each of videos of moving faces, moving objects, static faces, static objects, or scrambled images. Each block within the run lasted 26.4s and contained 12 stimuli presented for 2s each with 200ms fixation between stimuli. Between blocks of stimuli participants were asked to fixate during baseline blocks lasting 13.2s each, resulting in each run lasting about 7min. The stimuli appeared at the center of the screen subtending 6 and 8 degrees of visual angle in the horizontal and vertical dimensions, respectively. Participants were instructed to perform a simple one-back matching task during the stimulus blocks and indicate with a button press whether the current image was the same as the previous image, or not. Participants also completed two each of the identity and expression matching tasks (presented in an alternating fashion) similar to the task outside the scanner (see Figure 2A and 2B). Each run consisted of 8 blocks of 12 trials each that lasted 28.8s (2.2s each for each trial within a block), followed by 13.2s of intervening baseline fixation, resulting in each run lasting about 6min 48s. Prior to the experiment participants practiced the tasks outside the scanner and were instructed to respond as quickly and accurately as possible while fixating at the central fixation cross.
4.7. Functional MRI data analysis
Each participant ’s fMRI data were preprocessed and analyzed using multiple programs in AFNI (Analysis of Functional Neuroimages (Cox, 1996; Cox & Hyde, 1997)). Preprocessing of fMRI data was performed using methods similar to those previously reported using AFNI’s afni_proc.py program (Taylor PA, 2018). In short, fMRI data were first despiked, the first four volumes of each run were discarded, and the remaining volumes were distortion corrected, slice-timing corrected, and registered to each other. Structural and functional data were first aligned and then spatially normalized to a Talairach-space aligned version of the ICBM_152_2009c atlas template (VS Fonov, 2009) using a non-linear warping procedure. For the task runs, data for the three echoes were optimally combined using standard methods (Kundu et al., 2012), then smoothed using a 4-mm FWHM smoothing kernel. The resulting time series were then normalized by the mean signal intensity of each voxel to reflect percent signal change, which served as the input for subsequent regression analysis using a General Linear Model (GLM).
4.7.1. Amygdala and face-selective ROI definition:
Data from both localizer runs were concatenated into one GLM analysis which modeled five conditions of interest (Dynamic faces, Dynamic objects, Static faces, Static objects, and Scrambled images) using standard hemodynamic response functions of 26.4s duration, 3 baseline parameters (second-order polynomial) per run, and six motion regressors of no-interest per run. The localizer GLM results were used to functionally define face-selective regions of interest (ROIs) in right fusiform face area (rFFA) and right posterior superior temporal sulcus (rpSTS) for each participant, by first thresholding the statistical maps of fMRI activity of faces relative to objects (t-value of contrast between dynamic and static faces vs. dynamic and static objects) using an individual voxel threshold of p < 0.001 and a family wise error cluster-corrected α of 0.05 (resulting in cluster size thresholds of 11 to 20 voxels per participant). Then, to locate right FFA and pSTS, activation clusters in the fusiform gyrus and posterior portions of the superior temporal sulcus, respectively, were identified, and the location of their peak voxels noted. For right amygdala, a standard Talairach atlas (Talairach & Tournoux, 1988) (TT_N27) was used to anatomically define a ROI in right amygdala (AMG).
4.7.2. Identity vs. expression task fMRI analysis:
Data from the 4 identity and expression matching runs were concatenated into one GLM analysis which modeled 2 conditions of interest (Identity and Expression) using standard hemodynamic response functions of 28.8s duration, 3 baseline parameters (second-order polynomial) per run, and six motion regressors of no-interest per run. Data for one control participant and one MBS individual were excluded from further analysis because of significant loss of degrees of freedom due to movement-and noise-related censoring of timepoints.
Using the anatomically defined amygdala ROI and a 4mm radius sphere drawn around the peak activated voxel in the right FFA and right pSTS face-selective ROIs, we extracted out the mean fMRI percent signal change (beta coefficients from GLM analysis) within these regions, during the Identity and Expression task conditions, separately. We performed a linear mixed-effects Task x Group analysis and paired t-tests within each group of participants to determine whether these regions were engaged to the same degree during identity and expression processing.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the NIH Division of Intramural Research under NHGRI protocol 14-HG-0055 (ZIA# HG200389; NCT02055248) and NIMH protocol 93-M-0170 (ZIA# MH002918; NCT00001360). The Moebius Syndrome Research Consortium (members detailed in Supplemental Material G) received research funding from the National Institutes of Health (Grant Number: U01HD079068). We thank Dr. Kathleen Rives Bogart for granting us use of the Moebius Syndrome survey, as well as for thought provoking discussions. Many thanks to Flavia Facio, Carol Van Ryzin, and Jose Salas for their help in recruiting and scheduling participants, and to the Moebius Syndrome Foundation for their assistance and support. We are grateful to all the Moebius Syndrome individuals and their families for their generous participation. Thanks to Shivani Goyal and Hannah Wild for their help with survey data compilation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
DATA AND CODE AVAILABILITY
Source data for figures and analyses are available on Open Science Framework at: https://osf.io/x437p/. Analysis code (Japee, 2022) is available on GitHub at: https://github.com/sjapee/MBS-Study-2021.
Stimuli used in this study cannot be shared due to legal copyright restrictions that prevent public archiving. However, they may be obtained directly from the references cited in the text for each task.
BIBLIOGRAPHY:
- Ahmed A. (2005). When is facial paralysis Bell palsy? Current diagnosis and treatment. Cleve Clin J Med, 72(5), 398–401, 405. 10.3949/ccjm.72.5.398 [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, & Taylor GJ (1994). The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res, 38(1), 23–32. 10.1016/0022-3999(94)90005-1 [DOI] [PubMed] [Google Scholar]
- Bate S, Cook SJ, Mole J, & Cole J (2013). First report of generalized face processing difficulties in mobius sequence. PLoS One, 8(4), e62656. 10.1371/journal.pone.0062656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra R, Preece D, Campitelli G, & Scott-Pillow G (2019). The Assessment of Emotional Reactivity Across Negative and Positive Emotions: Development and Validation of the Perth Emotional Reactivity Scale (PERS). Assessment, 26(5), 867–879. 10.n77/1073191117694455 [DOI] [PubMed] [Google Scholar]
- Bogart K, Tickle-Degnen L, & Ambady N (2014). Communicating without the Face: Holistic Perception of Emotions of People with Facial Paralysis. Basic Appl Soc Psych, 36(4), 309–320. 10.1080/01973533.2014.917973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart KR, & Matsumoto D (2010). Living with Moebius syndrome: adjustment, social competence, and satisfaction with life. Cleft Palate Craniofac J, 47(2), 134–142. 10.1597/08-257_1 [DOI] [PubMed] [Google Scholar]
- Brasseur S, Gregoire J, Bourdu R, & Mikolajczak M (2013). The Profile of Emotional Competence (PEC): development and validation of a self-reported measure that fits dimensions of emotional competence theory. PLoS One, 8(5), e62635. 10.1371/journal.pone.0062635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce V, & Young A (1986). Understanding face recognition. Br J Psychol, 77 (Pt 3), 305–327. 10.1111/j.2044-8295.1986.tb02199.x [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Cole J, Campbell R, & Young AW (2000). Facial expression recognition by people with mobius syndrome. Cogn Neuropsychol, 17(1), 73–87. 10.1080/026432900380490 [DOI] [PubMed] [Google Scholar]
- Christensen JF, Gomila A, Gaigg SB, Sivarajah N, & Calvo-Merino B (2016). Dance expertise modulates behavioral and psychophysiological responses to affective body movement. J Exp Psychol Hum Percept Perform, 42(8), 1139–1147. 10.1037/xhp0000176 [DOI] [PubMed] [Google Scholar]
- Conson M, Ponari M, Monteforte E, Ricciato G, Sara M, Grossi D, & Trojano L (2013). Explicit recognition of emotional facial expressions is shaped by expertise: evidence from professional actors. Front Psychol, 4, 382. 10.3389/fpsyg.2013.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousley RR, & Calvert ML (1997). Current concepts in the understanding and management of hemifacial microsomia. Br J Plast Surg, 50(7), 536–551. 10.1016/s0007-1226(97)91303-5 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, & Hyde JS (1997). Software tools for analysis and visualization of fMRI data. NMR Biomed, 10(4-5), 171–178. [DOI] [PubMed] [Google Scholar]
- Darwin C. (1872). The expression of the emotions in man and animals. J. Murray. [Google Scholar]
- Davis J, Redshaw J, Suddendorf T, Nielsen M, Kennedy-Costantini S, Oostenbroek J, & Slaughter V (2021). Does Neonatal Imitation Exist? Insights From a Meta-Analysis of 336 Effect Sizes. Perspect Psychol Sci, 16(6), 1373–1397. 10.1177/1745691620959834 [DOI] [PubMed] [Google Scholar]
- De Stefani E, Ardizzi M, Nicolini Y, Belluardo M, Barbot A, Bertolini C, Garofalo G, Bianchi B, Coude G, Murray L, & Ferrari PF (2019). Children with facial paralysis due to Moebius syndrome exhibit reduced autonomic modulation during emotion processing. J Neurodev Disord, 11(1), 12. 10.1186/s11689-019-9272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani E, Nicolini Y, Belluardo M, & Ferrari PF (2019). Congenital facial palsy and emotion processing: The case of Moebius syndrome. Genes Brain Behav, 18(1), e12548. 10.1111/gbb.12548 [DOI] [PubMed] [Google Scholar]
- Delicato LS (2020). A robust method for measuring an individual’s sensitivity to facial expressions. Atten Percept Psychophys, 82(6), 2924–2936. 10.3758/s13414-020-02043-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U, & Thunberg M (1998). Rapid facial reactions to emotional facial expressions. Scand J Psychol, 39(1), 39–45. 10.1m/1467-9450.00054 [DOI] [PubMed] [Google Scholar]
- Finzi E, & Rosenthal NE (2016). Emotional proprioception: Treatment of depression with afferent facial feedback. J Psychiatr Res, 80, 93–96. 10.1016/jjpsychires.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Gallese V, & Sinigaglia C (2011). What is so special about embodied simulation? Trends Cogn Sci, 15(11), 512–519. 10.1016/j.tics.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Tamulonis D, Giannini MC, Loiselle RH, & Spirtos G (1984). Defective response to social cues in Mobius’ syndrome. J Nerv Ment Dis, 172(3), 174–175. 10.1097/00005053-198403000-00008 [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2000). The distributed human neural system for face perception. Trends Cogn Sci, 4(6), 223–233. 10.1016/s1364-6613(00)01482-0 [DOI] [PubMed] [Google Scholar]
- Ince RA, Paton AT, Kay JW, & Schyns PG (2021). Bayesian inference of population prevalence. Elife, 10. 10.7554/eLife.62461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura T, & Nakano T (2016). Automatic facial mimicry in response to dynamic emotional stimuli in five-month-old infants. Proc Biol Sci, 283(1844). 10.1098/rspb.2016.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S. (2022). MBS study code github.com/sjapee/MBS-Study-2021
- Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, & McKenna MJ (2001). Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope, 111(3), 387–398. 10.1097/00005537-200103000-00005 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci, 17(11), 4302–4311. https://www.ncbi.nlm.nih.gov/pubmed/9151747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe BD, Villing M, Racey C, Strong SL, Wincenciak J, & Barraclough NE (2014). A database of whole-body action videos for the study of action, emotion, and untrustworthiness. Behav Res Methods, 46(4), 1042–1051. 10.3758/s13428-013-0439-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, & Gazzola V (2009). Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol, 19(6), 666–671. 10.1016/j.conb.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Klein SA (2001). Measuring, estimating, and understanding the psychometric function: a commentary. Percept Psychophys, 63(8), 1421–1455. 10.3758/bf03194552 [DOI] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh WM, & Bandettini PA (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage, 60(3), 1759–1770. 10.1016/j.neuroimage.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D FA, Öhman A (1998). The Karolinska directed emotional faces (KDEF). [Google Scholar]
- Maimon-Mor RO, Schone HR, Moran R, Brugger P, & Makin TR (2020). Motor control drives visual bodily judgements. Cognition, 196, 104120. 10.1016/j.cognition.2019.104120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneweck M, Palermo R, & Hammond G (2014). Discrimination and recognition of facial expressions of emotion and their links with voluntary control of facial musculature in Parkinson’s disease. Neuropsychology, 28(6), 917–928. 10.1037/neu0000106 [DOI] [PubMed] [Google Scholar]
- Nicolini Y, Manini B, De Stefani E, Coude G, Cardone D, Barbot A, Bertolini C, Zannoni C, Belluardo M, Zangrandi A, Bianchi B, Merla A, & Ferrari PF (2019). Autonomic Responses to Emotional Stimuli in Children Affected by Facial Palsy: The Case of Moebius Syndrome. Neural Plast, 2019, 7253768. 10.1155/2019/7253768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Winkielman P, & Ramachandran VS (2007). Face to face: blocking facial mimicry can selectively impair recognition of emotional expressions. Soc Neurosci, 2(3-4), 167–178. 10.1080/17470910701391943 [DOI] [PubMed] [Google Scholar]
- Orlandi A, Zani A, & Proverbio AM (2017). Dance expertise modulates visual sensitivity to complex biological movements. Neuropsychologia, 104, 168–181. 10.1016/j.neuropsychologia.2017.08.019 [DOI] [PubMed] [Google Scholar]
- Pitcher D, Japee S, Rauth L, & Ungerleider LG (2017). The Superior Temporal Sulcus Is Causally Connected to the Amygdala: A Combined TBS-fMRI Study. J Neurosci, 37(5), 1156–1161. 10.1523/JNEUROSCI.0114-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives Bogart K, & Matsumoto D (2010). Facial mimicry is not necessary to recognize emotion: Facial expression recognition by people with Moebius syndrome. Soc Neurosci, 5(2), 241–251. 10.1080/17470910903395692 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, & Gallese V (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci, 2(9), 661–670. 10.1038/35090060 [DOI] [PubMed] [Google Scholar]
- Ross P, & Atkinson AP (2020). Expanding Simulation Models of Emotional Understanding: The Case for Different Modalities, Body-State Simulation Prominence, and Developmental Trajectories. Front Psychol, 11, 309. 10.3389/fpsyg.2020.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderkvist S, Ohlen K, & Dimberg U (2018). How the Experience of Emotion is Modulated by Facial Feedback. J Nonverbal Behav, 42(1), 129–151. 10.1007/s10919-017-0264-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling B. (1959). The first smile; a developmental study. Acta Paediatr Suppl, 48(Supp 117), 78–82. https://www.ncbi.nlm.nih.gov/pubmed/13660814 [PubMed] [Google Scholar]
- Soussignan R, Dollion N, Schaal B, Durand K, Reissland N, & Baudouin JY (2018). Mimicking emotions: how 3-12-month-old infants use the facial expressions and eyes of a model. Cogn Emot, 32(4), 827–842. 10.1080/02699931.2017.1359015 [DOI] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imaging. Georg Thieme. Publisher description http://www.loc.gov/catdir/enhancements/fy1006/2010286519-d.html Contributor biographical information http://www.loc.gov/catdir/enhancements/fy1009/2010286519-b.html [Google Scholar]
- Tautermannova M. (1973). Smiling in infants. Child Dev, 44(3), 701–704. https://www.ncbi.nlm.nih.gov/pubmed/4730544 [PubMed] [Google Scholar]
- Taylor PA CG, Glen DR, Rajendra JK, Reynolds RC, Cox RW. (2018). FMRI processing with AFNI: Some comments and corrections on “Exploring the Impact of Analysis Software on Task fMRI Results”. [Google Scholar]
- Vannuscorps G, Andres M, & Caramazza A (2020). Efficient recognition of facial expressions does not require motor simulation. Elife, 9. 10.7554/eLife.54687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSwearingen JM, & Brach JS (1996). The Facial Disability Index: reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys Ther, 76(12), 1288–1298; discussion 1298-1300. 10.1093/ptj/76.12.1288 [DOI] [PubMed] [Google Scholar]
- VS Fonov AE, McKinstry RC, Almli CR, Collins DL. (2009). Unbiased nonlinear average age-appropriate brain templates from birth to adulthood [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol, 54(6), 1063–1070. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wichmann FA, & Hill NJ (2001). The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys, 63(8), 1293–1313. 10.3758/bf03194544 [DOI] [PubMed] [Google Scholar]
- Wolff P. (1963). Observations on the early development of smiling. In Determinants of infant behavior. [Google Scholar]
- Yeshurun Y, Carrasco M, & Maloney LT. (2008). Bias and sensitivity in two-interval forced choice procedures: Tests of the difference model. Vision Res, 48(17), 1837–1851. 10.1016/j.visres.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Japee S, Stacy A, Flessert M, & Ungerleider LG (2020). Anterior superior temporal sulcus is specialized for non-rigid facial motion in both monkeys and humans. Neuroimage, 218, 116878. 10.1016/j.neuroimage.2020.116878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for figures and analyses are available on Open Science Framework at: https://osf.io/x437p/. Analysis code (Japee, 2022) is available on GitHub at: https://github.com/sjapee/MBS-Study-2021.
Stimuli used in this study cannot be shared due to legal copyright restrictions that prevent public archiving. However, they may be obtained directly from the references cited in the text for each task.


