Abstract
Metabolic dysfunction-associated fatty liver disease or metabolic dysfunction-associated steatotic liver disease (MAFLD/MASLD), is a common chronic liver condition affecting a substantial global population. Beyond its primary impact on liver function, MAFLD/MASLD is associated with a myriad of extrahepatic manifestations, including cognitive impairment. The scope of cognitive impairment within the realm of MAFLD/MASLD is a matter of escalating concern. Positioned as an intermediate stage between the normal aging process and the onset of dementia, cognitive impairment manifests as a substantial challenge associated with this liver condition. Insights from studies underscore the presence of compromised executive function and a global decline in cognitive capabilities among individuals identified as being at risk of progressing to liver fibrosis. Importantly, this cognitive impairment transcends mere association with metabolic factors, delving deep into the intricate pathophysiology characterizing MAFLD/MASLD. The multifaceted nature of cognitive impairment in the context of MAFLD/MASLD is underlined by a spectrum of factors, prominently featuring insulin resistance, lipotoxicity, and systemic inflammation as pivotal contributors. These factors interplay within the intricate landscape of MAFLD/MASLD, fostering a nuanced understanding of the links between hepatic health and cognitive function. By synthesizing the available evidence, exploring potential mechanisms, and assessing clinical implications, the overarching aim of this review is to contribute to a more complete understanding of the impact of MAFLD/MASLD on cognitive function.
Keywords: Abdominal Obesity Metabolic Syndrome, Cognitive Dysfunction, Insulin Resistance, Metabolic Syndrome, Non-Alcoholic Fatty Liver Disease, Oxidative Stress
Background
Metabolic dysfunction-associated fatty liver disease, or metabolic dysfunction-associated steatotic liver disease (MAFLD/MASLD), is the most common cause of chronic liver disease, affecting a large part of the global population [1]. The global prevalence of MAFLD/MASLD is estimated at 25–30% globally, likely influenced by the escalating epidemics of type 2 diabetes mellitus (T2DM) and obesity [2,3].
Beyond its main involvement in liver function, MAFLD/MASLD is closely related to a spectrum of extrahepatic manifestations that affect several organs and systems, underscoring the complexity of MAFLD/MASLD [4]. Individuals with MAFLD/MASLD often develop a constellation of metabolic abnormalities, such as insulin resistance (IR), dyslipidemia, and obesity, which form a complex interplay that contributes to disease progression [5,6]. In addition, MAFLD/MASLD has been associated with an increased risk of cardiovascular disease, T2DM, and chronic kidney disease [7,8]. In this regard, poor cognitive performance might be an extrahepatic manifestation in MAFLD/MASLD individuals.
Mild cognitive impairment is an intermediate stage between typical aging and dementia. This transitional phase can evolve into dementia, frequently manifesting as Alzheimer disease [9]. Observational studies have revealed that individuals at high risk of liver fibrosis have impaired executive function, abstract reasoning, and global cognitive function (Table 1) [10–16]. The underlying mechanisms of this impairment have not been fully elucidated, but may be related to the pathophysiology of MAFLD/MASLD, led by insulin resistance (IR), lipotoxicity, progressive lipid deposition, lipid peroxidation, and systemic inflammation; all of which have been associated with intestinal dysbiosis, leaky gut, and progressive liver fibrosis [17]. Through a comprehensive review of published research, this review aims to provide a detailed understanding of the intricate relationship between MAFLD/MASLD and cognitive impairment. By synthesizing the available evidence, exploring possible mechanisms, and assessing clinical implications, the aim is to contribute to a more complete understanding of the impact of MAFLD/MASLD on cognitive function.
Table 1.
Insights from studies MAFLD/MASLD and cognitive impairment.
| Author | Study design | Group | Purpose | Measuring instrument for cognitive impairment | Results |
|---|---|---|---|---|---|
| Elliott et al (2013) [12] | Cohort | 224 NAFLD 100 controls |
To assess functional physical capacity and cognitive abilities | Psychological Health Assessment Questionnaire (PHAQ) y Cognitive Failures Questionnaire (CFQ) | NAFLD patients:
|
| Seo et al (2016) [13] | Cross sectional | 4472 participants from 20 to 59 years old | To evaluate liver enzyme activity and cognitive assessment | SRRT SDLT SDST |
NAFLD patients:
|
| Tuttolomondo et al (2018) [14] | Case-control | 80 NAFLD 83 without liver or cardiovascular disease |
To assess cognitive performance in patients with NAFLD | MMSE (74 with MAFL y 65 controls) | NAFLD patients:
|
| Weinstein et al (2019) [10] | Cross sectional | 1287 participants 378 (29%) with NAFLD |
To assess the relationship of NAFLD and its severity, using the NAFLD Fibrosis Score (NFS) with cognitive performance. | Trail – making test Abstract reasoning tests Hooper’s Visual Organization Test (visual perception) |
NAFLD and cognitive performance were not associated; however patients with poorer cognitive performance on tests were associated with higher risk of advanced fibrosis in patients with NAFLD (β=-0.11±0.05; P=0.028) |
| Moretti et al (2019) [15] | Cross sectional | 671 NAFLD 687 controls |
To assess the relationship between NAFLD and performance with frontal, executive functions, behavior changes (mood, apathy, and anxiety) | FAB Test (Frontal Assessment Battery) Becks test HAM-A test AES-C |
NAFLD patients:
|
| Celikbilek et al (2018) [16] | Cross sectional | 70 NAFLD and 73 controls matched by age and sex from 18 to 70 years | To assess cognitive functions | Turkish version of the MoCa test | NAFLD patients:
|
AES-C – Apathy Evaluation Scale – Clinician Version; CFQ: Cognitive Failures Questionnaire; FAB Test – Frontal Assessment Battery Test; HAM-A Test – Hamilton Anxiety Rating Scale; MAFLD – metabolic dysfunction-associated fatty liver disease; MMSE – mini-mental state examination; MoCA – Montreal Cognitive Assessment; NAFLD – non-alcoholic fatty liver disease, NFS – NAFLD fibrosis score; PHAQ – Psychological Health Assessment Questionnaire; SDLT – Stroop Color and Word Test – Delayed Recall; SDST – Stroop Color and Word Test – Sustained Attention; SRRT – Symbol Digit Modalities Test.
Epidemiology
Observational studies have provided insights into the relationship between MAFLD/MASLD and cognitive impairment [10,11,13,18,19]. Currently there are no reliable data on the prevalence and incidence of cognitive impairment in individuals with MAFLD/MASLD due to the relatively recent recognition of this condition and the continuing evolution of diagnostic criteria. However, emerging research suggests a potential association between metabolic dysfunction and cognitive impairment in individuals with MAFLD. Seo et al found that people with MAFLD/MASLD had inferior learning, recall, and concentration functions in comparison to those without the liver condition. Importantly, these cognitive deficits were observed independently of the metabolic factors typically associated with MAFLD/MASLD [13]. While Seo et al highlighted the independent cognitive effects of MAFLD/MASLD, later investigations brought attention to the cumulative impact of underlying metabolic factors associated with MAFLD/MASLD [10,11,20]. Weinstein et al observed that individuals with MAFLD/MASLD and T2DM had impaired visuospatial function, while individuals with MAFLD/MASLD without T2DM had no impaired performance on any of the cognitive tests [20]. Additionally, the association with cognitive impairment was found to be more pronounced in individuals at higher risk of liver fibrosis, introducing the concept that the severity of liver involvement could amplify cognitive consequences [10,11]. Cognitive impairment typically manifests at the age of 60 years or older and the risk increases with age [21]. Recent findings from a systematic review have added a temporal dimension to this association, revealing that cognitive impairment in individuals with MAFLD/MASLD tends to exacerbate with age. In particular, cognitive impairment is more pronounced in those aged 37–61 years, suggesting that this disease can contribute to the early onset of cognitive impairment [17].
Risk Factors: The Interplay of MetS, MAFLD/MASLD, and Cognitive Function
The association between MAFLD/MASLD and cognitive impairment is influenced by various risk factors, predominately those associated with metabolic syndrome (MetS) [22]. MetS is a complex and increasingly prevalent disease that encompasses a set of interconnected metabolic risk factors such as obesity, hypertension, dyslipidemia, and insulin resistance (IR) [23].
Insulin Resistance
IR poses a substantial risk as it not only affects glucose metabolism but also exerts influences on cognitive processes [24]. Although insulin can pass the blood–brain barrier (BBB), insulin receptors are not uniformly distributed across the brain; instead, they are concentrated in specific regions such as the olfactory bulb, hypothalamus, hippocampus, cerebral cortex, and cerebellum [25]. The presence of hyperglycemia associated with IR triggers detrimental effects on brain function, involving the accumulation of advanced glycation end products, oxidative stress, and glucose neurotoxicity [26]. Furthermore, the disruption of insulin signaling in the brain can lead to cognitive impairment by downregulating BBB insulin receptors and reducing the transport of insulin into the brain [27].
Hypertension
Cognitive impairment is commonly associated with hypertension [28]. Recognized as a well-established risk factor for cerebrovascular disease, recent findings emphasize the significant impact of hypertension on the progression of cognitive impairment, vascular dementia, and Alzheimer disease [29]. The adverse effects of hypertension extend to the structural and functional aspects of the cerebral microcirculation, leading to microvascular rarefaction, cerebromicrovascular endothelial dysfunction, and neurovascular uncoupling. These changes collectively compromise the integrity of the cerebral blood supply [30].
Obesity
Obesity is a well-established risk factor for cognitive impairment [31]. Adipose tissue-derived inflammatory signals and adipokines can impact the brain, leading to neuroinflammation and cognitive dysfunction [32–34]. Moreover, obesity is often accompanied by systemic inflammation, which is recognized as a key player in the development of cognitive disorders [35,36].
Systemic Atherosclerosis
MAFLD/MASLD is marked by systemic atherosclerosis, manifesting as a procoagulation state accompanied by inflammation, endothelial dysfunction, reactive oxidative stress, and heightened platelet activity. These factors collectively contribute to the development of atherosclerotic lesions and microvascular disease [37–39]. Additionally, MAFLD/MASLD is associated with asymptomatic brain lesions and changes in cerebral perfusion, which contribute to the risk of vascular dementia [37].
Mechanisms Underlying Cognitive Impairment in MAFLD/MASLD
MAFLD/MASLD has been proposed as a “multiple-hit pathogenesis” due to its various causative factors, such as genetic and epigenetic factors, gut microbiota, metabolic pathways, nutritional factors, and IR [40–42]. Nevertheless, the pathological mechanisms by which MAFLD/MASLD affects other systems, especially the central nervous system (CNS), have not yet been fully elucidated [43]. The key components contributing to cognitive dysfunction in MAFLD/MASLD include IR, systemic inflammation, lipotoxicity, vascular dysfunction, and dysbiosis [37,38]. Recent studies have helped broaden the perspective to fully understand the relationship between MAFLD/MASLD and cognitive dysfunction [19,44].
Vascular Alterations and Inflammation
Individuals with MAFLD/MASLD typically exhibit chronic low-grade inflammation, which extends systemically [45]. The association between inflammatory status and cognitive decline was shown in a cross-sectional study including 50–64-year-old patients with MAFLD/MASLD who had high indicators of inflammation: white blood cell count (WBC) and high-sensitivity C-reactive protein (hsCRP) [46]. This association may be attributed to the accumulation of intrahepatic fat, causing damaged hepatocytes to release abundant proinflammatory cytokines, leading to recruitment of macrophages to the liver [47]. The ongoing proinflammatory cycle of macrophage recruitment in the liver is maintained in a positive feedback loop by the secretion of cytokines and chemokines into the systemic circulation, contributing to the development of persistent low-grade systemic inflammation [48]. Under normal conditions, the CNS is functionally segregated from the systemic circulation by the BBB [49]. Nevertheless, cytokines and other proinflammatory factors can penetrate the brain through active transportation or by directly entering circumventricular regions, unique communication points between the bloodstream, brain tissue, and cerebrospinal fluid (CSF), characterized by absence of the BBB [50]. Once damage-associated molecular patterns (DAMPs) and proinflammatory cytokines reach the CNS, they activate microglia, increasing BBB permeability and thus promoting release of proinflammatory cytokines and initiating a complex immune response in the CNS. This process leads to migration of immune cells and inflammatory factors, resulting in a neurotoxic effect [51]. Together, these factors promote chronic neuroinflammation and formation of beta-amyloid plaques, which trigger cognitive deterioration due to neuronal death and injury [52].
Insulin Resistance
Another important metabolic indicator that has been associated with cognitive impairment in patients with MAFLD/MASLD is IR, as demonstrated in an observational study that evaluated the determinants of memory function in obese middle-aged patients with T2DM or newly diagnosed prediabetes with MAFLD/MASLD [44]. The findings indicated a direct correlation between memory performance and the extent of IR. This can be attributed to reduced expression of insulin receptors in the brain, leading to a subsequent decline in neuronal plasticity, neuroprotection, neuronal growth, and energy metabolism –functions typically supported by insulin under normal physiological conditions [53]. Additionally, lower memory function was linked to increased ectopic liver fat, which is itself associated with exposure to a high-fat diet (HFD), thereby negatively impacting memory function dependent on the hippocampus [44].
To comprehend the metabolic processes, it is essential to position the liver as the central organ responsible for metabolizing sugars such as fructose and glucose [54]. Additionally, once hepatic glycogen stores are saturated, the metabolic pathways of fructose lead to de novo synthesis of triglycerides [5]. Consequently, the impact of high-fructose diets (HFr) on hepatic metabolism mirrors that of a high-fat diet (HFD), amplifying the progression of MAFLD/MASLD. This escalation is attributed to disruptions such as oxidative stress, IR, lipid peroxidation, mitochondrial dysfunction, proinflammatory cytokines, and adipokines [55,56]. Furthermore, hippocampal-dependent and -independent memory disorders have been described in fructose-fed rodents, which is caused by hippocampal neuronal expression of GLUT4 and 5, which are specific transporters for fructose [57] by increasing the intake of fructose by neurons, neutralization mechanisms and redox balance decay, producing an overall increase in free radicals, advanced glycation products, glycoxidation, and oxidative stress. These biochemical effects are related to mitochondrial dysfunction, lipid peroxidation, posttranslational alterations of proteins, reduction of acetylcholinesterase, brain dysfunction, and reduced plasticity [58].
Lipotoxicity
The concept of lipotoxicity emerges as a crucial factor in understanding the pathophysiological mechanisms associated with metabolic diseases such as MAFLD/MASLD [59]. Lipotoxicity, defined as adverse effects resulting from accumulation of lipids in non-adipose tissues, particularly manifests in the liver, leading to 2 distinct phenotypes: MAFLD/MASLD and metabolic dysfunction-associated steatohepatitis (MASH) [60]. This process involves the release of free fatty acids (FFAs) from insulin-resistant adipocytes, triggering inflammatory pathways, cellular dysfunction, and lipoapoptosis. The liver, unable to eliminate excess FFAs, undergoes hepatocyte dysfunction and injury, contributing to the progression of lipotoxic conditions [61]. Lipotoxicity extends its impact beyond the liver, affecting various organs due to factors like HFr and HFD, leading to obesity and IR [62]. In the context of HFr and HFD, lipid accumulation increases intrahepatic triglycerides, activating enzymes like 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1), and promoting inflammation. Furthermore, SFAs like palmitic acid from HFD induce chronic low-grade inflammation, leading to cognitive decline and neurodegenerative diseases [63]. Furthermore, the dysregulation caused by lipotoxicity in the brain involves orexin, a neuropeptide crucial for cognitive functions, executive function, and learning. Loss of orexin signaling, particularly type A, is associated with memory impairment, obesity, learning deficits, and neuroinflammation. Targeting the orexin system may offer a potential avenue for mitigating diet-induced cognitive decline and addressing neurodegenerative diseases characterized by orexin loss [64].
Dysbiosis
Another pathophysiological aspect identified in MAFLD/MASLD-related cognitive impairment is alteration of the intestinal microbiota (dysbiosis). The intricate balance of the microbiota is influenced not only by dietary choices but also by various lifestyle factors, including exercise and sleep [65]. In fact, physical activity is crucial to maintain gut microbiota equilibrium, nourishing its diversity, which increases hippocampal volume [66]. Dysbiosis is associated with increased intestinal permeability to pathogen-associated molecular patterns (PAMPS), such as lipopolysaccharides (LPS), and other bacterial products [43]. The heightened influx of PAMPs through the portal vein initiates activation of TLR4 receptors located in Kupffer liver cells (KC) and hepatic stellate cells (HSC). This activation sets off the generation of proinflammatory cytokines implicated in the development of metabolic-dysfunction-associated steatohepatitis (MASH) [67]. The impact of dysbiosis extends beyond the liver, as TLR expression and activation are also observed in neurons, which experience the systemic inflammatory conditions associated with MAFLD/MASLD [68]. Additionally, low levels of short-chain fatty acids (SCFAs), along with hyperammonemia and microbial taxa alterations, lead to gut neuroinflammation and dysbiosis, impacting brain metabolism through the microbiota-gut–brain axis [69].
The Brain–Gut–Liver Axis
The gut–brain axis is a bidirectional communication system that involves information exchange between the CNS and the gastrointestinal tract. This intricate network incorporates neural, endocrine, and immune systems (Figure 1) [70]. Communication between the gut microbiome and the CNS is facilitated by microbiome-derived intermediates, which include compounds such as short-chain fatty acids, secondary bile acids, tryptophan metabolites, glutamate, γ-aminobutyric acid (GABA), dopamine, norepinephrine, serotonin, and histamine [71]. These bioactive substances act as messengers, playing a crucial role in modulating various physiological and neurological processes [72]. Conversely, communication from the brain to the gastrointestinal tract is facilitated by the vagal nerve, the autonomic nervous system, and the hypothalamic–pituitary–adrenal (HPA) axis [73].
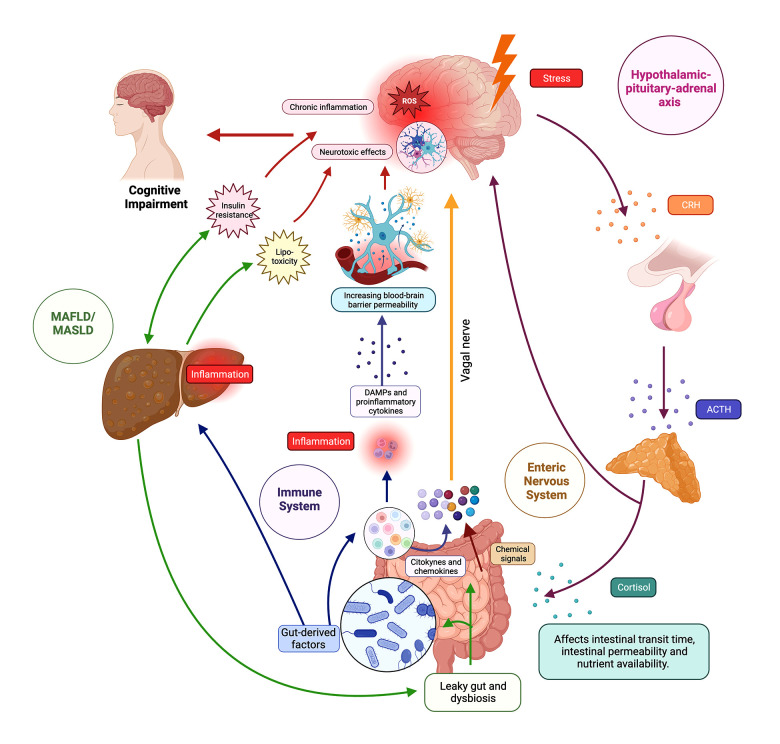
Figure 1. The brain–gut–liver axis.
The figure illustrates the intricate network of the brain–gut–liver axis, showing bidirectional communication pathways between the central nervous system (CNS), gastrointestinal tract, and liver. The axis involves neurological, endocrine, and immune components, influencing physiological and cognitive processes. In the neurological pathways, the enteric nervous system (ENS) senses the intestinal microenvironment, transmitting signals via vagal sensory nerves to the afferent neurons that express receptors for gut peptides (eg, CCK, ghrelin, leptin), influencing reactions and gut microbiota modulates gut peptide levels. In endocrine pathways, the hypothalamic–pituitary–adrenal (HPA) axis responds to stress, releasing corticotropin-releasing hormone (CRH) and stimulating cortisol production; cortisol impacts physiological processes, gut microbiota, and binds to glucocorticoid receptors (GR) in the brain. In immune pathways, gut-associated lymphoid tissue (GALT) communicates with the CNS through signaling molecules, influencing neuroimmune responses. Microbiome-derived products modulate immune cells and inflammation locally and systemically. Regarding the liver and cognitive impairment, the liver is connected to the gut via the portal vein and receives signals affecting liver health and the gut–brain axis. Disruptions in the axis, as seen in metabolic-associated fatty liver disease (MAFLD) and metabolic-associated steatosis of liver disease (MASLD), lead to “leaky gut”, which contributes to systemic inflammation, impacting cognitive function through interconnected pathways such as insulin resistance (IR) and lipotoxicity. ACTH – adrenocorticotropic hormone; CRH – corticotropin-releasing hormone, DAMPS – damage-associated molecular patterns; MAFLD – metabolic-associated fatty liver disease; MASLD – metabolic-associated steatotic liver disease. Created using Biorender.
Neurological Pathways
Physiologically, the enteric nervous system (ENS), through vagal sensory nerves and sympathetic neurons, directly or indirectly senses the intestinal microenvironment and consequently convert chemical signals from the environment into nerve impulses, which spread to the entire intestine and the CNS through the vagal nerve [74,75]. Vagal afferent neurons play a crucial role in this regulatory process by expressing receptors for several intestinal peptides, such as cholecystokinin (CCK), ghrelin, leptin, peptide tyrosine tyrosine (PYY), glucagon-like peptide-1 (GLP-1), and 5-hydroxytryptamine (5-HT), among others. These peptides are secreted by enteroendocrine cells (EEC) of the gastrointestinal tract [76]. Once specific gut peptides are detected by vagal afferent neurons, the relevant gut information is transmitted to the CNS, triggering various reactions [11]. The gut microbiota also plays a key role in modulating the levels of these gut peptides, such as CCK, ghrelin, leptin, PYY, GLP-1 and 5-HT., and this microbial influence extends to the vagal afferent pathway [77].
Endocrine Pathways
The HPA-axis is a crucial component of the endocrine pathways within the brain–gut–liver axis, playing a central role in the body’s response to stress [78]. When the brain detects stress, the hypothalamus releases corticotropin-releasing hormone (CRH), which signals the pituitary gland to release adrenocorticotropic hormone (ACTH) [79]. ACTH, in turn, stimulates the adrenal glands to produce cortisol and other stress-related hormones. These hormones not only influence various physiological processes throughout the body, but also affect the gut [80].
Cortisol receptors are expressed on various cells in the intestine, which directly influences their function [81]. Additionally, cortisol can influence the gut microbiota by modifying factors such as intestinal transit time, intestinal permeability, and nutrient availability. These alterations subsequently contribute to changes in the composition and diversity of the gut microbiome [82,83]. In the brain, cortisol exerts its influence by binding to glucocorticoid receptors (GR) located in the hippocampus, amygdala, and prefrontal cortex [84]. The proposed mechanism within the gut–brain axis suggests that gut microbes trigger stress circuits in the CNS via the vagus nerve and sensory neurons of the ENS [85,86].
Immune Pathways
Immune pathways are another integral dimension of the complex interconnections within the gut–brain axis. The gut, the main interface with the external environment, is endowed with a robust immune system, including an intricate network of immune cells, mucosal surfaces, and immunomodulatory molecules [87]. Communication between the intestinal immune system and the CNS involves both direct and indirect mechanisms [88]. Gut-associated lymphoid tissue (GALT) communicates with the CNS through release of signaling molecules, such as cytokines and chemokines, which influence neuroimmune responses [89–91]. Additionally, microbiome-derived products, such as short-chain fatty acids and various metabolites, can influence immune cell function and modulate inflammation both locally in the gut and systemically [92–94].
Liver and Cognitive Impairment
In the overall framework of the brain–gut–liver axis, the liver emerges as a central player in the orchestration of physiological responses, especially regarding MAFLD/MASLD and its effects on cognitive function. The liver, intimately connected to the gut via the portal vein, receives signals from gut-derived microbial molecules and products, creating a direct link between liver health and the gut–brain axis [95]. Disruptions of this axis, as occurs in MAFLD/MASLD, can increase intestinal permeability and allow translocation of harmful substances into the bloodstream. This phenomenon, known as „leaky gut”, can contribute to systemic inflammation and affect cognitive processes through the previously discussed pathways [96,97].
Neurological Manifestations
Multiple neurological manifestations of MAFLD/MASLD have been described, including decreased brain volume, subclinical or clinical cerebrovascular disease, and cognitive impairment [20,46,98]. These findings underscore the existence of brain signatures, which are distinct patterns or indicators within the brain that reflect the impact of MAFLD/MASLD on neurological health.
Brain Volume
The Framingham Study measured brain volume by brain MRI in patients with MAFLD/MASLD and found that this disease is associated with lower total brain volume, corresponding to brain aging in the overall sample at 7.3 years, in patients younger than 60 years, independent of visceral adipose tissue and cardiovascular risk factors [98].
Vascular Disease
Furthermore, a brain magnetic resonance spectroscopy study employing perfusion techniques was conducted to identify subclinical vascular damage in individuals with non-alcoholic fatty liver disease (NAFLD) who did not exhibit overt cardiovascular risk factors such as hypertension, diabetes, hypercholesterolemia, and obesity. The findings indicated that NAFLD patients have diminished cerebral perfusion (CBFr) in the semioval center and posterior cingulate cortex, but when the NAFLD cohort was categorized into subgroups based on factors like NAS score, presence/absence of NASH/fibrosis, and degree of steatosis, no statistically significant differences in CBFr values were observed [99]. Additionally, patients with stroke displayed a heightened prevalence of MAFLD/MASLD, characterized by greater severity according to the National Institutes of Health Stroke Scale, and poorer outcomes in terms of functionality at discharge as per the modified Ranking scale [100]. Exploring the relationship between MASLD and stroke with respect to its location, it was noted that patients with brainstem damage exhibited worsening of the initial NIHSS, along with a higher incidence of stroke progression and severity [101].
White Matter Lesions
Examining white matter lesions in MAFLD/MASLD patients, it was found that while the prevalence of these lesions was comparable between patients with and without MAFLD/MASLD, it was higher in those with MASH compared to non-MASLD individuals. Moreover, a direct correlation emerged between the number of white matter lesions and the degree of liver fibrosis in MASLD patients. However, multivariate analysis suggested that the presence of white matter lesions was closely linked to metabolic diseases in general [46].
Challenges and Treatment
As we have discussed throughout the review, currently there is not a single phenotype of cognitive impairment in individuals with MAFLD/MASLD [10,13,20]. The existing psychometric and neuropsychological test batteries, while valuable, may fall short in capturing the extensive spectrum of cognitive dysfunction implied by the complex interplay of metabolic and liver factors [51]. The multifactorial nature of cognitive impairment in the context of MAFLD/MASLD underscores the need for specialized assessment tools that can provide a more nuanced understanding of the diverse cognitive challenges faced by affected individuals.
Furthermore, the cognitive and functional challenges observed carry significant consequences for both healthcare systems and society. The increased demand for medical services, diagnostic procedures, and therapeutic interventions places a substantial burden on healthcare infrastructure. Additionally, the societal implications are profound, encompassing aspects such as reduced quality of life, diminished work productivity, and an increased need for caregiving suport [102].
Potential Treatments
Various treatments for MAFLD/MASLD have been explored, and some may influence cognitive function. Glucagon-like peptide (GLP-1) exhibits neuroprotective and anti-inflammatory properties, potentially reducing neuroinflammation in neurodegenerative diseases [103–105]. Insulin and liraglutide can improve brain damage through the Wnt/β-catenin signaling pathway, offering therapeutic potential for T2DM patients with cognitive impairment in [106]. A clinical trial with liraglutide in AD patients increased glucose transport in the blood–brain barrier, increasing the cerebral glucose metabolic rate [107]. Long-term liraglutide treatment in diabetic mice protected against impaired motor function and dopaminergic neuron loss [108]. Neuroprotective effects of liraglutide on diabetes-induced cognitive impairments were associated with enhanced hippocampal synapses and reduced neuronal apoptosis [109]. Repurposing FDA-approved GLP-1R agonists for neuroinflammation and neurodegeneration treatment requires human clinical trials to assess safety, tolerability, and efficacy [110].
In rats, FGF21 and vildagliptin attenuated IR, brain mitochondrial dysfunction, apoptosis, and cognitive impairment [111]. A study in HFD-fed rats with vildagliptin and dapagliflozin demonstrated improved peripheral insulin sensitivity, reduced weight gain, and prevention of cognitive impairment [112]. Empagliflozin protected cognitive functions in obese mice [12]. PPARγ agonists exhibited protective activity against oxidative damage, mitochondrial dysfunction, and apoptosis in various animal models [113].
Microinjection of synthetic CUR improved spatial memory in rats with multiple sclerosis [114]. Alpha linoleic acid (ALA) showed promise in inhibiting neuroinflammation, apoptotic cell loss, amyloidogenesis, and memory dysfunction [115]. Omega-3 supplementation in controlled clinical trials demonstrated positive outcomes in cognitive function [116]. A study with high-dose omega-3 and omega-6 fatty acid supplementation, combined with antioxidant vitamins, showed favorable improvements in cognitive function, functional capacity, fatigue, physical health, and daily sleepiness in elderly individuals with mild cognitive impairment [117].
Future Directions
The intricate interconnection between MAFLD/MASLD and cognitive impairment underscores the complexity of their impact on both metabolic and neurological well-being. The convergence of insulin resistance with NAFLD appears to exert systemic effects, possibly mediated through chronic inflammation, oxidative stress, and adipokine imbalance. This systemic perturbation may extend its influence to the brain, where the intricate crosstalk between metabolic health and cognitive function becomes apparent (Figure 2). Ongoing exploration of the complicated mechanisms linking MAFLD/MASLD with cognitive impairment is imperative. Anticipated advancements in diagnostic tools, treatment strategies, and preventive measures are poised to improve clinical outcomes and enrich the lives of individuals navigating these health challenges. Beyond mere acknowledgment of their coexistence, comprehending the relationship between MAFLD/MASLD and cognitive impairment necessitates a transformative approach to integrated care. This holistic perspective addresses the nuanced interplay between metabolic and cognitive health, holding significant potential to enhance patient outcomes, improve quality of life, and pave the way for future breakthroughs in managing these intertwined conditions.
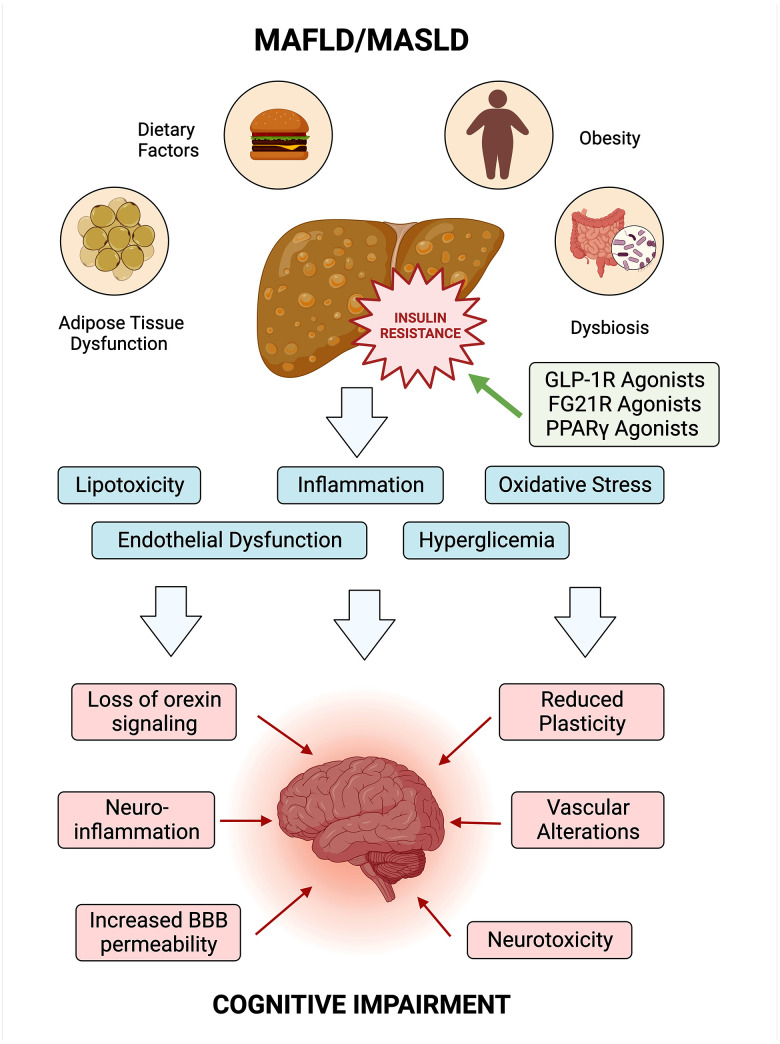
Figure 2. Exploring the link between MAFLD/MASLD, insulin resistance, and cognitive impairment.
Insulin resistance serves as a central node linking MAFLD/MASLD to systemic effects, influencing both metabolic and cognitive health. The figure shows potential pathways and shared risk factors. Understanding these intricate relationships is vital for advancing holistic approaches to health and potential interventions. BBB – blood–brain barrier. Created using Biorender.
Conclusions
In conclusion, the intricate relationship between MAFLD/MASLD and cognitive impairment involves a multifaceted interplay of metabolic and neurological factors. The prevalence of MAFLD/MASLD globally, driven by epidemics of T2DM and obesity, highlights the urgent need for a comprehensive understanding of its systemic implications. Beyond its primary impact on liver function, MAFLD/MASLD is associated with a spectrum of extrahepatic manifestations, including cognitive impairment.
Acknowledgments
The scholarships granted by Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) made it possible for David Medina-Julio and Mariana M Ramírez-Mejía to undertake postgraduate studies, enriching their academic and professional development.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of figures’ authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Chan KE, Koh TJL, Tang ASP, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: A meta-analysis and systematic review of 10739607 Individuals. J Clin Endocrinol Metab. 2022;107(9):2691–700. doi: 10.1210/clinem/dgac321. [DOI] [PubMed] [Google Scholar]
- 3.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The dtate of the disease. Gastroenterology. 2020;158(7):1851–64. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Pipitone RM, Ciccioli C, Infantino G, et al. MAFLD: A multisystem disease. Ther Adv Endocrinol Metab. 2023;14:20420188221145549. doi: 10.1177/20420188221145549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal SC, Eslam M, Mendez-Sanchez N. Detangling the interrelations between MAFLD, insulin resistance, and key hormones. Hormones (Athens) 2022;21(4):573–89. doi: 10.1007/s42000-022-00391-w. [DOI] [PubMed] [Google Scholar]
- 6.Kaya E, Yilmaz Y. Metabolic-associated fatty liver disease (MAFLD) : A multi-systemic disease beyond the liver. J Clin Transl Hepatol. 2022;10(2):329–38. doi: 10.14218/JCTH.2021.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun DQ, Jin Y, Wang TY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi: 10.1016/j.metabol.2020.154433. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y, Chen H, Liu Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: A 4.6-year cohort study in China. J Clin Endocrinol Metab. 2022;107(1):88–97. doi: 10.1210/clinem/dgab641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: A review article. Am J Alzheimers Dis Other Demen. 2018;33(8):500–7. doi: 10.1177/1533317518791401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein G, Davis-Plourde K, Himali JJ, et al. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int. 2019;39(9):1713–21. doi: 10.1111/liv.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Q, He R, Jiang H, et al. Association between metabolic dysfunction-associated fatty liver disease and cognitive impairment. J Clin Transl Hepatol. 2022;10(6):1034–41. doi: 10.14218/JCTH.2021.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott C, Frith J, Day CP, et al. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Dig Dis Sci. 2013;58(8):2383–91. doi: 10.1007/s10620-013-2657-2. [DOI] [PubMed] [Google Scholar]
- 13.Seo SW, Gottesman RF, Clark JM, et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology. 2016;86(12):1136–42. doi: 10.1212/WNL.0000000000002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuttolomondo A, Petta S, Casuccio A, et al. Reactive hyperemia index (RHI) and cognitive performance indexes are associated with histologic markers of liver disease in subjects with non-alcoholic fatty liver disease (NAFLD) : A case control study. Cardiovasc Diabetol. 2018;17(1):28. doi: 10.1186/s12933-018-0670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretti R, Caruso P, Gazzin S. Non-alcoholic fatty liver disease and neurological defects. Ann Hepatol. 2019;18(4):563–70. doi: 10.1016/j.aohep.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Celikbilek A, Celikbilek M, Bozkurt G. Cognitive assessment of patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2018;30(8):944–50. doi: 10.1097/MEG.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 17.George ES, Sood S, Daly RM, Tan SY. Is there an association between non-alcoholic fatty liver disease and cognitive function? A systematic review. BMC Geriatr. 2022;22(1):47. doi: 10.1186/s12877-021-02721-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cushman M, Callas PW, Alexander KS, et al. Nonalcoholic fatty liver disease and cognitive impairment: A prospective cohort study. PLoS One. 2023;18(4):e0282633. doi: 10.1371/journal.pone.0282633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S, Kim E, Cho H, et al. Associations between non-alcoholic fatty liver disease and cognitive impairment and the effect modification of inflammation. Sci Rep. 2022;12(1):12614. doi: 10.1038/s41598-022-16788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein AA, de Avila L, Paik J, et al. Cognitive performance in individuals with non-alcoholic fatty liver disease and/or type 2 diabetes mellitus. Psychosomatics. 2018;59(6):567–74. doi: 10.1016/j.psym.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn) 2016;22(2 Dementia):404–18. doi: 10.1212/CON.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira JR, Elkind MS, Moon YP, et al. The metabolic syndrome and cognitive performance: The Northern Manhattan Study. Neuroepidemiology. 2011;37(3–4):153–59. doi: 10.1159/000332208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Abbasi F, Robakis TK, Myoraku A, et al. Insulin resistance and accelerated cognitive aging. Psychoneuroendocrinology. 2023;147:105944. doi: 10.1016/j.psyneuen.2022.105944. [DOI] [PubMed] [Google Scholar]
- 25.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci Biobehav Rev. 2000;24(8):855–72. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 26.Hamed SA. Brain injury with diabetes mellitus: Evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol. 2017;10(4):409–28. doi: 10.1080/17512433.2017.1293521. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Tang TY, Lu CQ, Ju S. Insulin resistance and cognitive impairment: Evidence from neuroimaging. J Magn Reson Imaging. 2022;56(6):1621–49. doi: 10.1002/jmri.28358. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124(7):1025–44. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J, He Z, Wu L, et al. Prevalence of mild cognitive impairment in patients with hypertension: A systematic review and meta-analysis. Hypertens Res. 2021;44(10):1251–60. doi: 10.1038/s41440-021-00704-3. [DOI] [PubMed] [Google Scholar]
- 30.Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17(10):639–54. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc. 2017;76(4):443–54. doi: 10.1017/S0029665117002014. [DOI] [PubMed] [Google Scholar]
- 32.Miller AA, Spencer SJ. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Flores-Cordero JA, Pérez-Pérez A, Jiménez-Cortegana C, et al. Obesity as a risk factor for dementia and Alzheimer’s disease: The role of leptin. Int J Mol Sci. 2022;23(9):5202. doi: 10.3390/ijms23095202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leigh SJ, Morris MJ. Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165767. doi: 10.1016/j.bbadis.2020.165767. [DOI] [PubMed] [Google Scholar]
- 35.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease – the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89(3–4):144–49. doi: 10.1016/j.brainresbull.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Yu J, Shi YC, et al. The role of inflammation and endoplasmic reticulum stress in obesity-related cognitive impairment. Life Sci. 2019;233:116707. doi: 10.1016/j.lfs.2019.116707. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi R, Fargion S, Fracanzani AL. Brain involvement in non-alcoholic fatty liver disease (NAFLD): A systematic review. Dig Liver Dis. 2019;51(9):1214–22. doi: 10.1016/j.dld.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Robea MA, Balmus IM, Girleanu I, et al. Coagulation dysfunctions in non-alcoholic fatty liver disease-oxidative stress and inflammation relevance. Medicina (Kaunas) 2023;59(9):1614. doi: 10.3390/medicina59091614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valencia-Rodríguez A, Vera-Barajas A, Barranco-Fragoso B, et al. New insights into the association between non-alcoholic fatty liver disease and atherosclerosis. Ann Transl Med. 2019;7(Suppl 8):S300. doi: 10.21037/atm.2019.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–48. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Eslam M, George J. Genetic contributions to NAFLD: Leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol. 2020;17(1):40–52. doi: 10.1038/s41575-019-0212-0. [DOI] [PubMed] [Google Scholar]
- 42.Eslam M, Fan JG, Mendez-Sanchez N. Non-alcoholic fatty liver disease in non-obese individuals: The impact of metabolic health. Lancet Gastroenterol Hepatol. 2020;5(8):713–15. doi: 10.1016/S2468-1253(20)30090-X. [DOI] [PubMed] [Google Scholar]
- 43.Cheon SY, Song J. Novel insights into non-alcoholic fatty liver disease and dementia: Insulin resistance, hyperammonemia, gut dysbiosis, vascular impairment, and inflammation. Cell Biosci. 2022;12(1):99. doi: 10.1186/s13578-022-00836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vadini F, Simeone PG, Desideri G, et al. Insulin resistance and NAFLD may influence memory performance in obese patients with prediabetes or newly-diagnosed type 2 diabetes. Nutr Metab Cardiovasc Dis. 2021;31(9):2685–92. doi: 10.1016/j.numecd.2021.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Fricker ZP, Pedley A, Massaro JM, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the framingham heart study. Clin Gastroenterol Hepatol. 2019;17(6):1157–64e4. doi: 10.1016/j.cgh.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petta S, Tuttolomondo A, Gagliardo C, et al. The presence of white matter lesions is associated with the fibrosis severity of nonalcoholic fatty liver disease. Medicine (Baltimore) 2016;95(16):e3446. doi: 10.1097/MD.0000000000003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gehrke N, Schattenberg JM. Metabolic inflammation – a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158(7):1929–47e6. doi: 10.1053/j.gastro.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Narayanan S, Surette FA, Hahn YS. The immune landscape in nonalcoholic steatohepatitis. Immune Netw. 2016;16(3):147–58. doi: 10.4110/in.2016.16.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daneman R, Prat A. The blond–brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Ran M, Li H, et al. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front Mol Neurosci. 2022;15:1013933. doi: 10.3389/fnmol.2022.1013933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kjærgaard K, Mikkelsen ACD, Wernberg CW, et al. Cognitive dysfunction in non-alcoholic fatty liver disease-current knowledge, mechanisms and perspectives. J Clin Med. 2021;10(4):673. doi: 10.3390/jcm10040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guzman-Martinez L, Maccioni RB, Andrade V, et al. Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol. 2019;10:1008. doi: 10.3389/fphar.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craft S, Watson GS. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–78. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 54.Pal SC, Méndez-Sánchez N. Insulin resistance and adipose tissue interactions as the cornerstone of metabolic (dysfunction)-associated fatty liver disease pathogenesis. World J Gastroenterol. 2023;29(25):3999–4008. doi: 10.3748/wjg.v29.i25.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–71. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sellmann C, Priebs J, Landmann M, et al. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem. 2015;26(11):1183–92. doi: 10.1016/j.jnutbio.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Yonamine CY, Michalani MLE, Moreira RJ, Machado UF. Glucose transport and utilization in the hippocampus: from neurophysiology to diabetes-related development of dementia. Int J Mol Sci. 2023;24(22):16480. doi: 10.3390/ijms242216480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera DS, Lindsay CB, Codocedo JF, et al. Long-term, fructose-induced metabolic syndrome-like condition is associated with higher metabolism, reduced synaptic plasticity and cognitive impairment in Octodon degus. Mol Neurobiol. 2018;55(12):9169–87. doi: 10.1007/s12035-018-0969-0. [DOI] [PubMed] [Google Scholar]
- 59.Engin AB. What is lipotoxicity? Adv Exp Med Biol. 2017;960:197–220. doi: 10.1007/978-3-319-48382-5_8. [DOI] [PubMed] [Google Scholar]
- 60.Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, et al. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int J Mol Sci. 2018;19(7):2034. doi: 10.3390/ijms19072034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Wasilewska N, Lebensztejn DM. Non-alcoholic fatty liver disease and lipotoxicity. Clin Exp Hepatol. 2021;7(1):1–6. doi: 10.5114/ceh.2021.104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasiljević A, Bursać B, Djordjevic A, et al. Hepatic inflammation induced by high-fructose diet is associated with altered 11βHSD1 expression in the liver of Wistar rats. Eur J Nutr. 2014;53(6):1393–402. doi: 10.1007/s00394-013-0641-4. [DOI] [PubMed] [Google Scholar]
- 64.Duffy CM, Hofmeister JJ, Nixon JP, Butterick TA. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol Learn Mem. 2019;157:41–47. doi: 10.1016/j.nlm.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ge X, Cheng L, Liu Y, et al. Regulation of the gut microbiota by diet and exercise: Improvements in cognition and emotion. Future Foods. 2023;8:100256. [Google Scholar]
- 66.Saiyasit N, Chunchai T, Prus D, et al. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition. 2020;69:110576. doi: 10.1016/j.nut.2019.110576. [DOI] [PubMed] [Google Scholar]
- 67.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–303. doi: 10.1007/s10620-016-4049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohammed SK, Magdy YM, El-Waseef DA, Nabih ES, et al. Modulation of hippocampal TLR4/BDNF signal pathway using probiotics is a step closer towards treating cognitive impairment in NASH model. Physiol Behav. 2020;214:112762. doi: 10.1016/j.physbeh.2019.112762. [DOI] [PubMed] [Google Scholar]
- 69.Jeong MY, Jang HM, Kim DH. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci Lett. 2019;698:51–57. doi: 10.1016/j.neulet.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Ding JH, Jin Z, Yang XX, et al. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. 2020;26(40):6141–62. doi: 10.3748/wjg.v26.i40.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osadchiy V, Martin CR, Mayer EA. The gut–brain axis and the microbiome: Mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17(2):322–32. doi: 10.1016/j.cgh.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayer EA, Nance K, Chen S. The gut–brain axis. Annu Rev Med. 2022;73:439–53. doi: 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- 73.Asadi A, Shadab Mehr N, Mohamadi MH, et al. Obesity and gut–micro biota–brain axis: A narrative review. J Clin Lab Anal. 2022;36(5):e24420. doi: 10.1002/jcla.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583(7816):441–46. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoo BB, Mazmanian SK. The enteric network: Interactions between the immune and nervous systems of the gut. Immunity. 2017;46(6):910–26. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S, Liu M, Cao S, et al. The mechanism of the gut–brain axis in regulating food intake. Nutrients. 2023;15(17):3728. doi: 10.3390/nu15173728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhry TS, Senapati SG, Gadam S, et al. The impact of microbiota on the gut–brain axis: Examining the complex interplay and implications. J Clin Med. 2023;12(16):5231. doi: 10.3390/jcm12165231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith SM, Vale WW. The role of the hypothalamic–pituitary–adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–21. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mázala-de-Oliveira T, Silva BT, Campello-Costa P, Carvalho VF. The role of the adrenal–gut–brain axis on comorbid depressive disorder development in diabetes. Biomolecules. 2023;13(10):1504. doi: 10.3390/biom13101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tena-Garitaonaindia M, Arredondo-Amador M, Mascaraque C, et al. Modulation of intestinal barrier function by glucocorticoids: Lessons from preclinical models. Pharmacol Res. 2022;177:106056. doi: 10.1016/j.phrs.2022.106056. [DOI] [PubMed] [Google Scholar]
- 82.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124–36. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma L, Yan Y, Webb RJ, et al. Psychological stress and gut microbiota composition: A systematic review of human studies. Neuropsychobiology. 2023;82(5):247–62. doi: 10.1159/000533131. [DOI] [PubMed] [Google Scholar]
- 84.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geng ZH, Zhu Y, Li QL, et al. Enteric nervous system: The bridge between the gut microbiota and neurological disorders. Front Aging Neurosci. 2022;14:810483. doi: 10.3389/fnagi.2022.810483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome–brain–gut axis communication. Adv Exp Med Biol. 2014;817:115–33. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 87.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gwak MG, Chang SY. Gut-brain connection: Microbiome, gut barrier, and environmental sensors. Immune Netw. 2021;21(3):e20. doi: 10.4110/in.2021.21.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3(4):331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 90.Cahenzli J, Balmer ML, McCoy KD. Microbial-immune cross-talk and regulation of the immune system. Immunology. 2013;138(1):12–22. doi: 10.1111/j.1365-2567.2012.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mou Y, Du Y, Zhou L, et al. Gut microbiota interact with the brain through systemic chronic inflammation: Implications on neuroinflammation, neurodegeneration, and aging. Front Immunol. 2022;13:796288. doi: 10.3389/fimmu.2022.796288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Zhu N, Su X, et al. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12(5):793. doi: 10.3390/cells12050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krishnan S, Ding Y, Saedi N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23(4):1099–111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li D, Lu Y, Yuan S, et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: An umbrella review and updated meta-analysis. Am J Clin Nutr. 2022;116(1):230–43. doi: 10.1093/ajcn/nqac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu CL, Schnabl B. The Gut–liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. 2023;21(11):719–33. doi: 10.1038/s41579-023-00904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan M, Man S, Sun B, et al. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):443. doi: 10.1038/s41392-023-01673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song Q, Zhang X. The role of gut–liver axis in gut microbiome dysbiosis associated nafld and NAFLD-HCC. Biomedicines. 2022;10(3):524. doi: 10.3390/biomedicines10030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weinstein G, Zelber-Sagi S, Preis SR, et al. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham Study. JAMA Neurol. 2018;75(1):97–104. doi: 10.1001/jamaneurol.2017.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Airaghi L, Rango M, Maira D, et al. Subclinical cerebrovascular disease in NAFLD without overt risk factors for atherosclerosis. Atherosclerosis. 2018;268:27–31. doi: 10.1016/j.atherosclerosis.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Abdeldyem SM, Goda T, Khodeir SA, et al. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J Clin Lipidol. 2017;11(4):915–19. doi: 10.1016/j.jacl.2017.04.115. [DOI] [PubMed] [Google Scholar]
- 101.Li H, Hu B, Wei L, et al. Non-alcoholic fatty liver disease is associated with stroke severity and progression of brainstem infarctions. Eur J Neurol. 2018;25(3):577–e34. doi: 10.1111/ene.13556. [DOI] [PubMed] [Google Scholar]
- 102.Balp MM, Krieger N, Przybysz R, et al. The burden of non-alcoholic steatohepatitis (NASH) among patients from Europe: A real-world patient-reported outcomes study. JHEP Rep. 2019;1(3):154–61. doi: 10.1016/j.jhepr.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui QN, Stein LM, Fortin SM, Hayes MR. The role of glia in the physiology and pharmacology of glucagon-like peptide-1: Implications for obesity, diabetes, neurodegeneration and glaucoma. Br J Pharmacol. 2022;179(4):715–26. doi: 10.1111/bph.15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li QX, Gao H, Guo YX, et al. GLP-1 and underlying beneficial actions in Alzheimer’s disease, hypertension, and NASH. Front Endocrinol (Lausanne) 2021;12:721198. doi: 10.3389/fendo.2021.721198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Athauda D, Foltynie T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson’s disease. Neuropharmacology. 2018;136(Pt B):260–70. doi: 10.1016/j.neuropharm.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 106.Zhao Y, Yu J, Ping F, et al. Insulin and liraglutide attenuate brain pathology in diabetic mice by enhancing the Wnt/β-catenin signaling pathway. Exp Ther Med. 2022;24(1):439. doi: 10.3892/etm.2022.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gejl M, Brock B, Egefjord L, Vang K, et al. Blood–brain glucose transfer in Alzheimer’s disease: Effect of GLP-1 analog treatment. Sci Rep. 2017;7(1):17490. doi: 10.1038/s41598-017-17718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma D, Liu X, Liu J, et al. Long-term liraglutide ameliorates nigrostriatal impairment via regulating AMPK/PGC-1a signaling in diabetic mice. Brain Res. 2019;1714:126–32. doi: 10.1016/j.brainres.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 109.Yan W, Pang M, Yu Y, et al. The neuroprotection of liraglutide on diabetic cognitive deficits is associated with improved hippocampal synapses and inhibited neuronal apoptosis. Life Sci. 2019;231:116566. doi: 10.1016/j.lfs.2019.116566. [DOI] [PubMed] [Google Scholar]
- 110.Kopp KO, Glotfelty EJ, Li Y, Greig NH. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186:106550. doi: 10.1016/j.phrs.2022.106550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomed Pharmacother. 2018;97:1663–72. doi: 10.1016/j.biopha.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 112.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol. 2017;333:43–50. doi: 10.1016/j.taap.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 113.Eslami H, Sharifi AM, Rahimi H, Rahati M. Protective effect of telmisartan against oxidative damage induced by high glucose in neuronal PC12 cell. Neurosci Lett. 2014;558:31–36. doi: 10.1016/j.neulet.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 114.Barzegarzadeh B, Hatami H, Dehghan G, et al. Conjugated linoleic acid-curcumin attenuates cognitive deficits and oxidative stress parameters in the ethidium bromide-induced model of demyelination. Neurotox Res. 2021;39(3):815–25. doi: 10.1007/s12640-020-00310-0. [DOI] [PubMed] [Google Scholar]
- 115.Ali W, Ikram M, Park HY, et al. Oral administration of alpha linoleic acid rescues Aβ-induced Glia-mediated neuroinflammation and cognitive dysfunction in C57BL/6N mice. Cells. 2020;9(3):667. doi: 10.3390/cells9030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martí Del Moral A, Fortique F. Omega-3 fatty acids and cognitive decline: A systematic review. Nutr Hosp. 2019;36(4):939–49. doi: 10.20960/nh.02496. [DOI] [PubMed] [Google Scholar]
- 117.Stavrinou PS, Andreou E, Aphamis G, et al. The effects of a 6-month high dose omega-3 and omega-6 polyunsaturated fatty acids and antioxidant vitamins supplementation on cognitive function and functional capacity in older adults with mild cognitive impairment. Nutrients. 2020;12(2):325. doi: 10.3390/nu12020325. [DOI] [PMC free article] [PubMed] [Google Scholar]