Abstract
Objective:
Cochlear implantation of prelingually deaf infants provides auditory input sufficient to develop spoken language; however, outcomes remain variable. Inability to participate in speech perception testing limits testing device efficacy in young listeners. In postlingually implanted adults (aCI), speech perception correlates with spectral resolution an ability that relies independently on frequency resolution (FR) and spectral modulation sensitivity (SMS). Correlation of spectral resolution to speech perception is unknown in prelingually implanted children (cCI). In this study, FR and SMS were measured using a spectral ripple discrimination (SRD) task and were correlated with vowel and consonant identification. It was hypothesized that prelingually deaf cCI would show immature SMS relative to postlingually deaf aCI and that FR would correlate with speech identification.
Study Design:
Cross-sectional study
Setting:
In-person, booth testing
Methods:
SRD was used to determine highest spectral ripple density perceived at various modulation depths. FR and SMS were derived from spectral modulation transfer functions. Vowel and consonant identification were measured; SRD performance and speech identification were analyzed for correlation.
Results:
Fifteen prelingually implanted cCI and thirteen postlingually implanted aCI were included. FR and SMS were similar between cCI and aCI. Better FR was associated with better speech identification for most measures.
Conclusion:
Prelingually implanted cCI demonstrated adult-like FR and SMS; additionally, FR correlated with speech identification. FR may be a measure of CI efficacy in young listeners.
Keywords: cochlear implant, pediatric otology, speech perception, spectral resolution
Introduction
Cochlear implantation (CI) plays a critical role in spoken language development in young listeners with severe to profound hearing loss, with the goal of providing auditory input sufficient to understand speech. Speech perception is an important metric of device efficacy, and implantation before age 2 has demonstrated improved vocabulary, language, and speech understanding outcomes 1–5. Several patient-specific and environmental factors have been identified as contributors to implant performance, including socioeconomic standing, access to aural habilitation, early identification of hearing loss, and timely implantation 4,6,7. Despite successes of early intervention, spoken language outcomes in pediatric CI listeners remain variable 3,8, in part due to challenges with testing device efficacy in children too young to participate in speech perception tasks 8–10. Unrecognized suboptimal device performance may delay implementation of patient-specific interventions – including implant re-programming – and can result in deficient language abilities and hinder development of social skills, highlighting the need for age-appropriate measures of CI efficacy 11–13.
An important predictor of speech perception with a CI is a listener’s spectral resolution – the ability to perceive differences in intensity across the frequency spectrum in a complex sound. Relative to normal-hearing (NH) listeners, reduced spectral resolution makes it more difficult for CI listeners to discern spectral patterns that distinguish vowels and consonant place-of-articulation, amongst other characteristics of speech 14–16. Spectral resolution is a non-linguistic ability that improves with age in NH listeners and relies independently on two factors: frequency resolution (FR) and spectral modulation sensitivity (SMS). FR reflects how precisely the auditory system encodes place-of-stimulation across the cochlea and matures in infancy. SMS refers to the ability to detect changes in sound intensity across a spectrum and develops gradually, reaching maturity around 9 – 12 years of age 17. FR is limited in CI listeners due to the reduced number of cochlear electrodes, incomplete neural survival, and coarse place-of-stimulation due to current spread 18–20; it is less clear how SMS is affected in CI listeners 20. Spectral resolution can be measured by performance on tasks of spectral ripple discrimination (SRD). Tasks use broadband noise stimuli with amplitude-modulated spectral envelopes and typically involve detection of modulation from un-rippled noise or discriminating ripple stimuli based on differences in the spectral envelope. In adults with CIs (aCI), performance on SRD correlates with vowel, consonant, and speech identification, highlighting the value of using the SRD task as a proxy measure for device efficacy. However, the relationship of SRD performance to speech understanding is not consistently observed in pediatric CI studies 18–25. This may be because standard tests of spectral resolution do not independently assess FR and SMS, and their different maturation trajectories may obscure interpretation of young listener performance. Isolation of FR and SMS could provide a more accurate understanding of the development of spectral resolution in children with CIs (cCI). Recognizing how FR and SMS contribute to speech perception in cCI is an important step toward earlier identification of poor performers, widening the window of opportunity for interventions such as device re-programming, modified auditory habilitation and individualized speech processing strategies 26,27.
In this study, FR and SMS were derived using a SRD task to better understand development of spectral resolution in cCI and to study the relationship between FR and speech identification. Although older cCI in this study could be tested using speech-based tasks, SRD was selected because it does not rely on spoken language proficiency and can therefore be employed when testing infants and younger children. In listeners able to partake in speech and language testing, use of vowel and consonant identification tasks is an important measure of spectral proficiency. Vowel discrimination relies on processing of spectral cues 28 and develops early in life 29, making it ideal for use in this study. The first aim of this study was to determine the effect of listener age on SRD thresholds, FR and SMS. It was hypothesized that cCI would perform worse on the SRD task than aCI due to immature SMS while FR would be adult-like. The second aim was to evaluate the relationship between FR and speech identification tasks; it was hypothesized that speech identification performance would correlate positively with FR.
Methods
Participants
This study was conducted at the Virginia Merrill Bloedel Hearing Research Center at the University of Washington. Procedures were approved by the Institutional Review Board of Seattle Children’s Hospital. Recruitment occurred through the Communication Studies Participant Pool and Seattle Children’s Hospital and University of Washington Cochlear Implant programs between 2019 – 2021 using a consecutive sampling scheme. Testing occurred over that time with an 8-month pause due to the pandemic. Patients with neurocognitive or developmental impairments were excluded. All cCI were implanted prior to age 2; aCI were post-lingually implanted and tested at least one year after device activation (Table 1). Participants reported consistent, daily use of their CI.
Table 1.
Participant demographics
| Listener | Sex | Age | Ear T ested | Implantation | Hearing Loss Type | Hearing Loss Etiology | 1st Ear Implanted | 1st Device | Age at 1st Activation (months) | 2nd Ear Implanted | 2nd Device | Age at 2nd Activation (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | F | 16 | Right | Bilateral | Congenital, bilateral | Prematurity | Right | AB | 11 | Lett | AB | 47 |
| C2 | F | 8 | Right | Bilaterali† | Congenital, bilateral | Unknown | Right | Cochlear | 9 | Left | Cochlear | 9 |
| C3 | M | 11 | Right | Bilateral | Congenital, bilateral | Unknown | Right | Cochlear | 12 | Left | Cochlear | 20 |
| C4 | F | 5 | Right | Bilateral | Congenital, bilateral | Unknown | Right | Cochlear | 14 | Left | Cochlear | 39 |
| C5 | M | 10 | Right | Bilateral | Congenital, bilateral | EVA | Right | Cochlear | 17 | Left | Cochlear | 38 |
| C6 | F | 11 | Right | Bilateral | Congenital, bilateral | Unknown | Right | Cochlear | 9 | Left | Cochlear | 14 |
| C7 | F | 13 | Right | Bilateral | Congenital, bilateral | Unknown | Right | Cochlear | 13 | Left | Cochlear | 41 |
| C8 | M | 9 | Right | Bilateral | Congenital, bilateral | Genetic, non-syndromic | Left | Cochlear | 9 | Right | Cochlear | 18 |
| C9 | M | 11 | Left | Bilateral | Congenital, bilateral | Unknown | Left | Cochlear | 24 | Right | Cochlear | 48 |
| C10 | F | 5 | Left | Unilateral | Congenital, bilateral | Unknown | Left | Cochlear | 19 | NA | NA | NA |
| C11 | M | 6 | Left | Bilateral | Congenital, bilateral | Unknown | Left | Cochlear | 12 | Right | Cochlear | 18 |
| C12 | M | 13 | Left | Bilateral | Sudden, bilateral | Ototoxicity | Right | Cochlear | 15 | Left | Cochlear | 38 |
| C13 | M | 8 | Left | Bilateral | Congenital, bilateral | Unknown | Left | Cochlear | 18 | Right | Cochlear | 31 |
| C14 | M | 6 | Right | Bilateral† | Congenital, bilateral | Genetic, non-syndromic | Right | Cochlear | 12 | Left | Cochlear | 12 |
| C15 | M | 8 | Right | Bilateral† | Congenital, bilateral | Genetic, non-syndromic | Right | AB | 16 | Left | AB | 16 |
| Listener | Sex | Age | Ear Tested | Implantation | Hearing Loss Type | Hearing Loss Etiology | 1st Ear Implanted | 1st Device | Age at 1st Activation (years) | 2nd Ear Implanted | 2nd Device | Age at 2nd Activation (years) |
|
| ||||||||||||
| A1 | M | 56 | Right | Bilateral | Congenital, bilateral | Rubella | Left | Med-El | 36 | Right | Med-El | 44 |
| A2 | M | 51 | Right | Unilateral | Progressive bilateral | Genetic, non-syndromic | Right | AB | 45 | NA | NA | NA |
| A3 | F | 73 | Right | Bilateral | Congenital, bilateral | Unknown | Left | Cochlear | 55 | Right | Cochlear | 61 |
| A4 | F | 57 | Right | Bilateral | Progressive, bilateral | Ototoxicity | Left | AB | 45 | Right | AB | 47 |
| A5 | F | 21 | Right | Unilateral | Congenital, bilateral | Genetic, non-syndromic | Right | Cochlear | 10 | NA | NA | NA |
| A6 | M | 64 | Right | Unilateral | Unknown | Ototoxicity | Right | Cochlear | 63 | NA | NA | NA |
| A7 | F | 76 | Left | Unilateral | Progressive, bilateral | Genetic, non-syndromic | Left | Cochlear | 70 | NA | NA | NA |
| A8 | F | 76 | Right | Bilateral | Progressive, bilateral | Genetic, non-syndromic | Right | Cochlear | 50 | Left | Cochlear | 63 |
| A9 | M | 82 | Left | Unilateral | Progressive, bilateral | Unknown | Left | Cochlear | 68 | NA | NA | NA |
| A10 | M | 73 | Right | Unilateral | Progressive, bilateral | Other | Right | Cochlear | 70 | NA | NA | NA |
| A11 | F | 73 | Right | Unilateral | Progressive, bilateral | Unknown | Right | AB | 70 | NA | NA | NA |
| A12 | F | 56 | Right | Unilateral | Progressive, unilateral | Genetic, Waardenburg | Right | Med-El | 54 | NA | NA | NA |
| A13 | M | 73 | Right | Unilateral | Progressive, bilateral | Genetic, non-syndromic | Right | AB | 71 | NA | NA | NA |
Note: AB = Advanced Bionics, EVA = Enlarged vestibular aqueduct
Implanted simultaneously
Stimuli
Dynamic SRD stimuli were generated using MATLAB 30,31 scripts based on the “spectral-temporally modulated ripple test” (SMRT), described by Aronoff and Landsberger 32 with several modifications. One-second-long stimuli were generated using a phase-randomized sinusoidal carrier (100/octave) with frequencies spaced within 100 – 6500 Hz 31. “Target” ripple densities ranged from 0.125 ripples-per-octave (RPO) to 19.027 RPO and the “no-target” reference stimulus was a highly ripple-dense 20 RPO. Stimuli were generated with four starting ripple phases (0, 90, 180, 270 degrees) at four modulation depths (3-dB, 7-dB, 10-dB and 15-dB).
Speech stimuli consisted of 10 vowels (/i/, /I/, /eI/, /ɛ/, /æ/, /ɑ/, /u/, /ʊ/, /o/, /ʌ/), presented in an /h/-vowel-/d/ context and 14 consonants (/b/, /d/, /f/, /k/, /n/, /p/, /s/, /t/, /v/, /z/, /ð/, /ʃ/, /dʒ/ /g/) presented in an /a/-consonant-/a/ context. Stimuli were presented using Advanced Bionics ListPlayer speech test presentation software (ListPlayer, Version 2.2.11.52, Advanced Bionics, Valencia, CA) using the Parallels® Desktop for Mac. Consonant speech stimuli were spoken by a digitized female voice; vowels were spoken by a digitized male voice from the Pacific Northwest, calibrated to 65–70 dB SPL. For testing speech identification in noise, the same speech stimuli were presented in Auditech 4-talker babble with a +10 dB signal-to-noise ratio.
Procedure
Listeners performed testing in a double-walled sound-treated booth. Stimuli were played from a Mac Mini with output to a Crown D-45 amplifier and an external D/A device (SIIF USB SoundWave 7.1) and were presented through a Bowers & Wilkins (B&W) speaker placed at 0° azimuth 1 meter away. Unilateral CI subjects were tested using an earplug/earmold in the contralateral ear. In bilaterally implanted listeners, the better hearing ear with preferred processor settings and clinical maps was selected; if ears were symmetrical, the right ear was used.
Dynamic SRD Task –
Listeners were tested on their ability to identify rippled target stimuli that varied in ripple density from a highly rippled, no-target referent (20 RPO). One target stimulus and two non-target stimuli were presented in randomized order in each trial using a 3-interval 3-alternative forced choice method. Stimuli were presented at 65 dB SPL in soundfield and listeners were asked to select the “different” sound using a computer interface via mouse click. No feedback was given during testing. Ripple density varied adaptively, in a 2-up 1-down fashion, beginning at 1 RPO in quarter-log2 steps until 10 reversals occurred. SRD threshold was calculated as average ripple density (in RPO) for the last 6 reversals; higher thresholds indicated better performance. cCI performed one test run yielding a threshold. In aCI, thresholds from two runs were averaged; if the difference was greater than one RPO step size, a third run was completed, and mean SRD threshold was used. Thresholds were obtained for each modulation depth in random order. Following completion of the SRD task, listeners were given a ten-minute break to reduce fatigue.
Speech Identification Task –
Prior to testing, participants were given a written list of speech stimuli; listeners demonstrated the ability to read aloud or – with young participants C4, C10, C11 – verbally repeat the speech stimuli. During testing, each speech token was presented 3 times in randomized order. Using a computer graphical interface, listeners identified the speech stimulus via mouse click (or for young listeners C4, C10, C11 by repeating the word for an observer to click). Participants could repeat speech tokens with no effect on score. No feedback was given during testing. Listeners who correctly identified >80% speech stimuli in quiet were presented the same task with stimuli in noise. All listeners completed two test runs for each speech task; if difference in percentage correct was >10%, a third test run was completed. Percent correct scores were converted to rationalized arcsine units (RAU) to normalize error variance and facilitate correlational analysis 33.
Spectral Modulation Transfer Functions
SRD thresholds (in RPO) were calculated for each listener at four modulation depths (in dB). Individual listener SRD thresholds were fit to a function of ripple depth using non-linear least-squares regression in Microsoft Excel (version 16.23) with the equation, f(x) = B × ln(x/A) where f(x) represents ripple density threshold at modulation depth x 34. The slope of this function, (B) represents FR, the x-intercept (A) defines SMS. Functions were rejected if there was poor fit to observed data (r2 < 0.5; no (B) or (A) obtained for listener) or if all mean SRD thresholds were at floor (no (A) was obtained for listener, (B) set to 0.1). The effect of age group on SRD threshold was analyzed using 2-way repeated-measures ANOVA (modulation depth as within-subjects variable). The effect of age group on FR, SMS, and RAU were analyzed using one-way ANOVAs. Correlations between FR and RAU were analyzed using one-tailed bivariate correlations. Significance level was p < 0.05.
Results
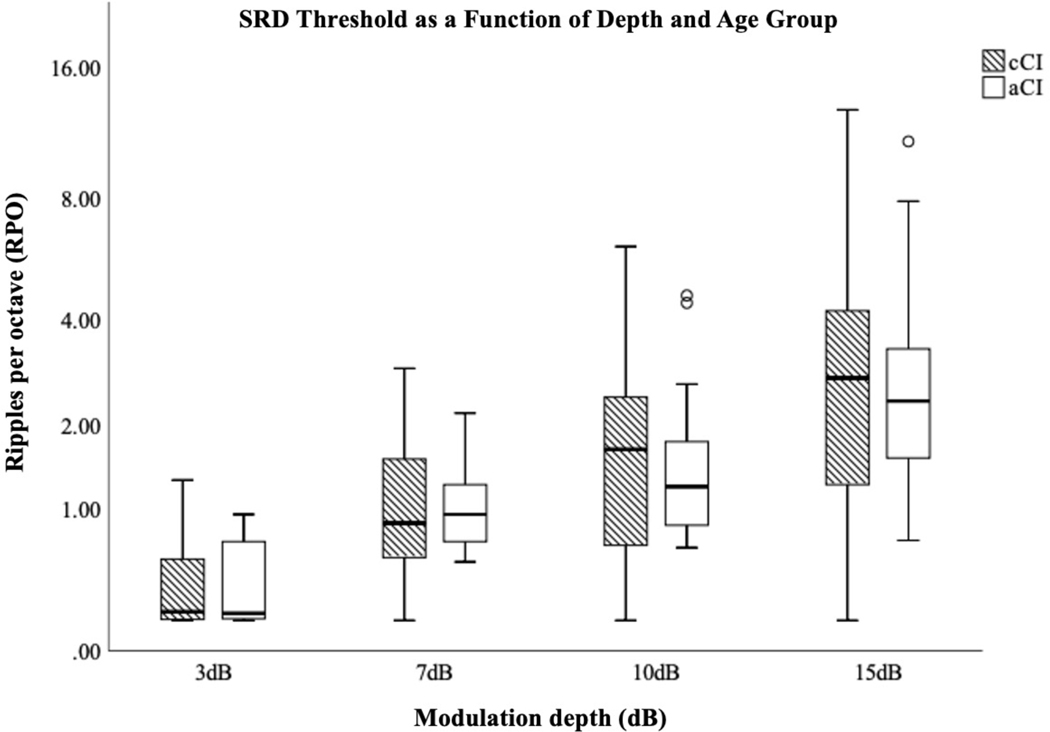
Fifteen cCI (5 – 16 years; mean 9.7, standard deviation [SD] 3.5) and 13 aCI (51 – 73 years; mean 59, SD 10) were included. Speech software failed for one cCI participant. All others completed speech identification tasks in quiet. Mean SRD thresholds for cCI at 3-, 7-, 10- and 15-dB were 0.4 (95% confidence interval [95%CI] 0.24 – 0.57), 1.1 (95%CI 0.68 – 1.46), 1.8 (95%CI 1.06 – 2.62) and 3.4 (95%CI 1.69 – 5.02) RPO, respectively. For aCI, mean SRD thresholds at 3-, 7-, 10- and 15-dB were 0.4 (95%CI 0.24 – 0.56), 1.1 (95%CI 0.79 – 1.37), 1.7 (95%CI 1.0 – 2.47) and 3.3 (95%CI 1.71 – 4.96) RPO, respectively. Thresholds increased significantly with modulation depth in both cCI and aCI groups (Figure 1). Results of 2-way ANOVA revealed a significant main effect of depth (greenhouse-geisser F(1.09,28.353) = 25.05; p < 0.0001; η2 = 0.0491). Neither effect of age nor the interaction reached significance.
Figure 1.
Box-whisker plot showing spectral ripple discrimination (SRD) threshold as a function of spectral modulation depth and age group. SRD threshold is in ripples per octave on a log base 2 scale. cCI = prelingually implanted school-aged children. aCI = postlingually implanted adults.
SMTF thresholds were derived directly from raw SRD thresholds for 23 participants. Performance at high ripple depths is thought to involve temporal processing. Therefore, for two participants with 15-dB thresholds more than two standard deviations above the mean, SMTF was derived from the 3-, 7- and 10-dB SRD thresholds only. For three other cCI, SMTF was flat due to floor performance across all modulation depths; no function was fit to these data and the (B) was set to 0.01 for use in correlational analyses. Mean SMTF coefficients are shown in Table 2. There was no significant correlation between SMTF coefficients (r = 0.207, 2-tail p = 0.333).
Table 2.
Mean Spectral Modulation Transfer Function Coefficients
| A | B | |
|---|---|---|
| cCI | 2.771 (0.53) | 1.565 (1.342) |
| aCI | 2.716 (0.435) | 1.424 (0.824) |
Note: Mean spectral modulation transfer coefficients: A = intercept, B = slope, SD in parentheses, cCI = prelingually implanted children, aCI = postlingually implanted adults
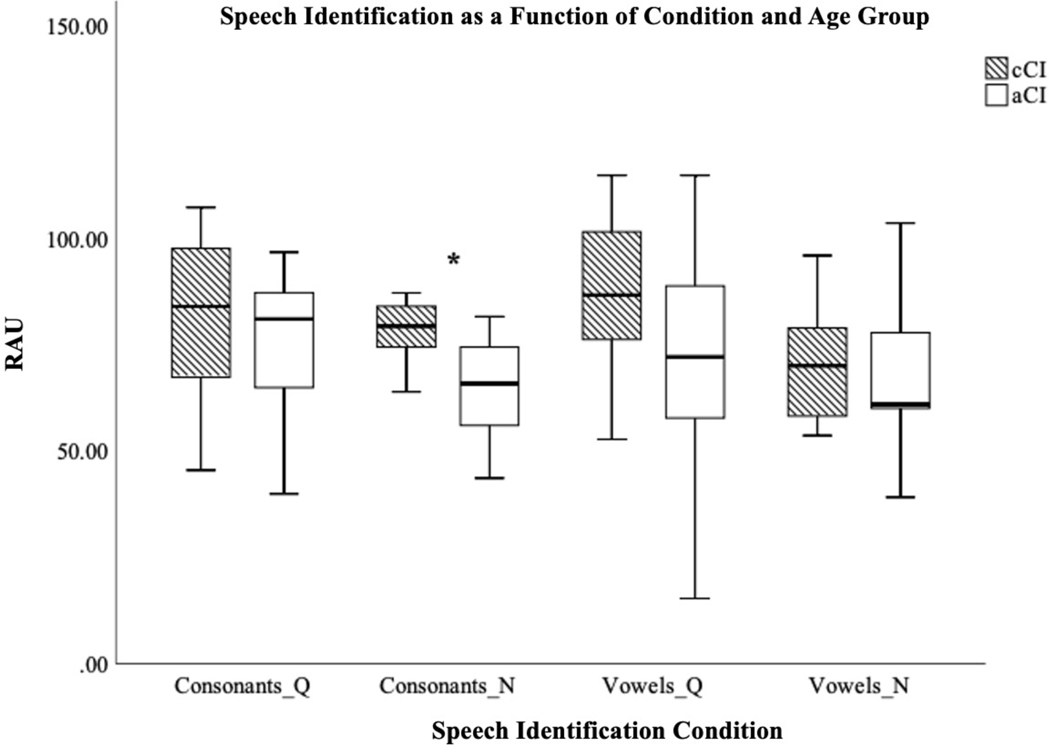
For the speech task, mean performance is shown for cCI and aCI in Table 3. In quiet, mean performance was slightly better in cCI than aCI for consonant and vowel recognition. Eight cCI (53%) and 7 aCI (58%) were tested in noise for consonants and 9 cCI (60%) and 5 aCI (42%) were tested on vowels in noise. Mean performance in noise tended to be better in cCI than aCI (Figure 2). One-way ANOVA revealed a significant effect of age group, favoring cCI, on identification of consonants in noise (F(1,14) = 6.167, p = 0.027). No significant effect was found for other conditions.
Table 3.
Mean Consonant and Vowel Identification Scores
| Consonant Identification | Vowel Identification | |||
|---|---|---|---|---|
| Quiet | Noise** | Quiet | Noise | |
| cCI | 81.2 (19.3) | 78.2 (7.6) | 86.4 (19.0) | 71.3 (14.2) |
| N = 15 | N = 8 | N = 15 | N = 9 | |
| aCI | 74.4 (18.3) | 64.5 (13.5) | 71.8 (25.7) | 68.2 (24.0) |
| N = 12 | N = 7 | N = 12 | N = 5 | |
Note: Mean RAU scores shown with SD in parentheses and sample size = N. cCI = prelingually implanted children, aCI = postlingually implanted adults.
statistically significant effect of age group.
Figure 2.
Box-whisker plot showing speech identification score in rationalized arcsine units (RAU) as a function of test condition and age group. Q = testing in quiet, N = testing in Auditech 4-talker babble with a +10 dB signal-to-noise ratio. cCI = prelingually implanted school-aged children. aCI = postlingually implanted adults.
Results of bivariate correlations between SMTF coefficients and speech scores are shown in Table 4. For all listeners, speech in quiet significantly improved with higher (B), suggesting that better FR is associated with better vowel and consonant recognition in quiet. The relationship between (B) and speech in noise was less clear: although vowel identification in noise improved with higher (B), this only approached significance and no trend was noted for consonants in noise. Interestingly, when only cCI data were considered, (B) was significantly correlated with all speech identification measures except consonants in noise. Taken together, these data suggest that for cCI, FR is a predictor of better vowel recognition in quiet and in noise, and better consonant recognition in quiet.
Table 4.
Bivariate correlations between spectral modulation transfer function slope B and speech identification scores (RAU)
| Consonants | Vowels | |||
|---|---|---|---|---|
| Quiet | Noise | Quiet | Noise | |
| r = 0.477** | 0.116 | 0.486** | 0.42 | |
| All Participants | p = 0.006 | 0.34 | 0.005 | 0.068 |
| N = 27 | 15 | 27 | 14 | |
| 0.464* | 0.221 | 0.560* | 0.676* | |
| cCI only | 0.041 | 0.299 | 0.015 | 0.023 |
| 15 | 8 | 15 | 9 | |
Note: Bivariate pearson correlations shown with one-tailed p values and sample sizes. cCI = prelingually implanted children.
and
indicate significant results at p <0.01 and 0.05, respectively.
Discussion
Spectral resolution was measured in aCI and cCI using a dynamic SRD task in this study. Results indicate that, by at least 5–12 years of age, spectral resolution in pre-lingually deaf cCI is mature with similar FR and SMS compared to post-lingually implanted adults. These results are consistent with earlier work showing mature static SRD performance in cCI with a similar age range 20. In NH listeners, SMS maturation occurs around 5–10 years old, reflecting concomitant development of the central auditory system and psychoacoustic abilities 14,16,17,23,35. The role of age on SRD in cCI is less clear, with several studies showing no difference in performance between age groups 15,24,25,36. When isolated, SMS correlated with chronological age of cCI in a study by Horn et al. (2017), despite no main effect of age on performance 20. The role of hearing (rather than chronologic) age has been similarly elusive in studies of cCI: Davidson et al. (2021) found that hearing age predicted dynamic SRD thresholds of cCI 36 whereas this relationship was not seen in a similar study by Landsberger et al. (2018) 15.
In this study, cCI performed significantly better than or comparably to aCI across all speech tasks. Due to heterogeneity of CI listeners in this study, firm conclusions about development of speech understanding in prelingually-implanted cCI cannot be drawn. Nonetheless, these results suggest that young implantees are able to benefit from spectrally impoverished auditory input provided by a CI at least as much as post-lingual implantees. Importantly, cCI in this study tended to show better speech identification than adults despite not having better spectral resolution than adults. This finding could suggest that cCI are better able than aCI to utilize non-spectral cues – namely, those not measured by a SRD task, such as temporal and intensity resolution. Further research is necessary to investigate this question which could have implications on optimization of audiological mapping, auditory habilitation and spoken-language instruction designed for prelingually-deaf CI listeners.
Research has shown that correlations between spectral resolution measures and speech perception may vary depending on the spectral task and speech stimuli employed 37. For example, a few studies found that in cCI, performance on SRD correlated with vowels and spondees-in-quiet, but not with monosyllabic words in quiet 20,23,25; however, these data are limited due to relatively small sample sizes. Some studies in aCI have suggested that spectral modulation perception at low ripple depths is particularly important for vowel and consonant identification 38,39. In contrast, a study in school-aged cCI and aCI showed that SRD at higher modulation depths were more correlated with spondee identification in noise 20. In this study, FR robustly correlated with three of four speech measures in cCI. The weaker, non-significant correlation with consonants in noise could reflect the fact that only a subset of consonants depend strongly on spectral place cues and that there is a strong reliance on formant frequencies for accurate vowel identification 28.
Despite the correlation between FR and speech perception in cCI implanted in infancy, significant work is needed to establish that FR measured in infancy is clinically useful. As a potential measure of device efficacy for infants, FR does not appear to rely on central auditory development as it matures in infancy in normal hearing children 14,17,40–42. Studies are currently underway to investigate how implanted infants’ FR, as well as temporal resolution, develops after CI activation and how this predicts later speech perception development. If these capacities prove to be predictive of clinical speech perception measures, measurement of device efficacy in infancy would hold potential to inform clinical management of these patients years before they are old enough to participate in speech perception testing. Earlier optimization of mapping, habilitation strategies, and language interventions years before development would potentially lead to improved long-term outcomes.
Ultimately, speech perception outcomes in cCI depend on a complex interplay of early-maturing sensory, and later-maturing non-sensory factors that remain poorly understood 34,38,39,43. This limits the utility of previous research employing spectral and/or temporal modulation perception tasks to study outcomes in young cCI. The advantage of the approach employed by this study is the ability to isolate the sensory capacity of interest. While the present study investigated the relationship between FR and speech identification, this could be applied to study other sensory capacities such as temporal and intensity resolution. Arguably, a battery of auditory capacities (rather than a singular focus) would be more illustrative and, perhaps, predictive of CI efficacy and long-term outcomes.
There are two main limitations of the present study. First, the small sample size requires repetition in order to strengthen confidence in the results. Second, comparison of pre-lingual to post-lingually deafened CI listeners is not ideal for studying development in prelingually-implanted CI users. Moving forward, a longitudinal study and/or a pre-lingually implanted aCI cohort would be ideal. Fortunately, with many of the first pre-lingually implanted infants in the U.S. having reached adulthood, such studies are becoming more feasible. Differences between the etiology, variability of hearing loss progression, and degree of sensorineural degradation likely confounded the basic age difference between the two groups – and could potentially even explain why cCI showed better speech identification in some measures than aCI.
Conclusion
Data from this study suggest that pre-lingually implanted school-aged children have adult-like spectral resolution – with similar FR, SMS, and equivocal or better speech perception when compared to post-lingually implanted adults. Early development of FR in infancy and its correlation to speech identification could be capitalized on to measure CI efficacy in young listeners. Studies to isolate FR in CI infants are currently underway, with the goal of serving as a proxy for speech perception before development of spoken language.
Funding:
NIH/NIDCD K23 DC013055, T32 DC000018; 2019 AAO-HNSF Resident Research Award
Footnotes
Conflicts of Interest: None
References
- 1.Tomblin JB, Barker BA, Spencer LJ, et al. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of speech, language, and hearing research : JSLHR. 2005;48(4):853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyu J, Kong Y, Xu TQ, et al. Long-term follow-up of auditory performance and speech perception and effects of age on cochlear implantation in children with pre-lingual deafness. Chin Med J (Engl). 2019;132(16):1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell R, Christianson E, Ramirez R, et al. Auditory comprehension outcomes in children who receive a cochlear implant before 12 months of age. The Laryngoscope (In Press). 2019. [DOI] [PubMed] [Google Scholar]
- 5.Dettman SJ, Dowell RC, Choo D, et al. Long-term Communication Outcomes for Children Receiving Cochlear Implants Younger Than 12 Months: A Multicenter Study. Otol Neurotol. 2016;37(2):e82–95. [DOI] [PubMed] [Google Scholar]
- 6.Colletti L, Mandalà M, Colletti V. Cochlear implants in children younger than 6 months. Otolaryngol Head Neck Surg. 2012;147(1):139–146. [DOI] [PubMed] [Google Scholar]
- 7.Kirkham E, Sacks C, Baroody F, et al. Health disparities in pediatric cochlear implantation: an audiologic perspective. Ear Hear. 2009;30(5):515–525. [DOI] [PubMed] [Google Scholar]
- 8.Geers AE, Strube MJ, Tobey EA, et al. Epilogue: factors contributing to long-term outcomes of cochlear implantation in early childhood. Ear Hear. 2011;32(1 Suppl):84S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear Hear. 2003;24(1 Suppl):24S–35S. [DOI] [PubMed] [Google Scholar]
- 10.Geers AE. Speech, language, and reading skills after early cochlear implantation. Arch Otolaryngol Head Neck Surg. 2004;130(5):634–638. [DOI] [PubMed] [Google Scholar]
- 11.McConkey Robbins A, Koch DB, Osberger MJ, et al. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg. 2004;30(5):570–574. [DOI] [PubMed] [Google Scholar]
- 12.Ganek H, McConkey Robbins A, Niparko JK. Language outcomes after cochlear implantation. Otolaryngol Clin North Am. 2012;45(1):173–185. [DOI] [PubMed] [Google Scholar]
- 13.Wake M, Hughes EK, Poulakis Z, et al. Outcomes of children with mild-profound congenital hearing loss at 7 to 8 years: a population study. Ear Hear. 2004;25(1):1–8. [DOI] [PubMed] [Google Scholar]
- 14.Peter V, Wong K, Narne VK, et al. Assessing spectral and temporal processing in children and adults using temporal modulation transfer function (TMTF), Iterated Ripple Noise (IRN) perception, and spectral ripple discrimination (SRD). J Am Acad Audiol. 2014;25(2):210–218. [DOI] [PubMed] [Google Scholar]
- 15.Landsberger DM, Padilla M, Martinez AS, et al. Spectral-Temporal Modulated Ripple Discrimination by Children With Cochlear Implants. Ear Hear. 2018;39(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayes H, Sheft S, Shafiro V. Discrimination of static and dynamic spectral patterns by children and young adults in relationship to speech perception in noise. Audiol Res. 2014;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby BJ, Browning JM, Brennan MA, et al. Spectro-temporal modulation detection in children. J Acoust Soc Am. 2015;138(5):EL465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118(2):1111–1121. [DOI] [PubMed] [Google Scholar]
- 19.Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007;8(3):384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn DL, Dudley DJ, Dedhia K, et al. Effects of age and hearing mechanism on spectral resolution in normal hearing and cochlear-implanted listeners. J Acoust Soc Am. 2017;141(1):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou N. Deactivating stimulation sites based on low-rate thresholds improves spectral ripple and speech reception thresholds in cochlear implant users. J Acoust Soc Am. 2017;141(3):EL243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawler M, Yu J, Aronoff JM. Comparison of the Spectral-Temporally Modulated Ripple Test With the Arizona Biomedical Institute Sentence Test in Cochlear Implant Users. Ear Hear. 2017;38(6):760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiNino M, Arenberg JG. Age-Related Performance on Vowel Identification and the Spectral-temporally Modulated Ripple Test in Children With Normal Hearing and With Cochlear Implants. Trends in Hearing. 2018;22:2331216518770959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeddi Z, Lotfi Y, Moossavi A, et al. Correlation between Auditory Spectral Resolution and Speech Perception in Children with Cochlear Implants. Iran J Med Sci. 2019;44(5):382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung KH, Won JH, Drennan WR, et al. Psychoacoustic Performance and Music and Speech Perception in Prelingually Deafened Children with Cochlear Implants. Audiology and Neurotology. 2012;17(3):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brochier T, McKay C, McDermott H. Encoding speech in cochlear implants using simultaneous amplitude and rate modulation. The Journal of the Acoustical Society of America. 2018;144:2042–2051. [DOI] [PubMed] [Google Scholar]
- 27.Nogueira W, Rode T, Büchner A. Spectral contrast enhancement improves speech intelligibility in noise for cochlear implants. The Journal of the Acoustical Society of America. 2016;139(2):728–739. [DOI] [PubMed] [Google Scholar]
- 28.DiNino M, Wright RA, Winn MB, et al. Vowel and consonant confusions from spectrally manipulated stimuli designed to simulate poor cochlear implant electrode-neuron interfaces. J Acoust Soc Am. 2016;140(6):4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson CE. Children’s phoneme identification in reverberation and noise. Journal of speech, language, and hearing research : JSLHR. 2000;43(1):144–157. [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resnick JM, Horn DL, Noble AR, et al. Spectral aliasing in an acoustic spectral ripple discrimination task. J Acoust Soc Am. 2020;147(2):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronoff JM, Landsberger DM. The development of a modified spectral ripple test. J Acoust Soc Am. 2013;134(2):EL217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28(3):455–462. [DOI] [PubMed] [Google Scholar]
- 34.Supin A, Popov VV, Milekhina ON, et al. Ripple depth and density resolution of rippled noise. J Acoust Soc Am. 1999;106(5):2800–2804. [DOI] [PubMed] [Google Scholar]
- 35.Moore JK, Linthicum FH, Jr. The human auditory system: a timeline of development. Int J Audiol. 2007;46(9):460–478. [DOI] [PubMed] [Google Scholar]
- 36.Davidson LS, Geers AE, Uchanski RM. Spectral Modulation Detection Performance and Speech Perception in Pediatric Cochlear Implant Recipients. Am J Audiol. 2021;30(4):1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gifford RH, Noble JH, Camarata SM, et al. The Relationship Between Spectral Modulation Detection and Speech Recognition: Adult Versus Pediatric Cochlear Implant Recipients. Trends Hear. 2018;22:2331216518771176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson ES, Oxenham AJ, Nelson PB, et al. Assessing the role of spectral and intensity cues in spectral ripple detection and discrimination in cochlear-implant users. J Acoust Soc Am. 2012;132(6):3925–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saoji AA, Litvak L, Spahr AJ, et al. Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners. J Acoust Soc Am. 2009;126(3):955–958. [DOI] [PubMed] [Google Scholar]
- 40.Wightman F, Allen P, Dolan T, et al. Temporal resolution in children. Child Dev. 1989;60(3):611–624. [PubMed] [Google Scholar]
- 41.Horn DL, Won JH, Rubinstein JT, et al. Spectral Ripple Discrimination in Normal-Hearing Infants. Ear Hear. 2017;38(2):212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble AR, Resnick J, Broncheau M, et al. Spectrotemporal Modulation Discrimination in Infants With Normal Hearing. Ear Hear. 2023;44(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isarangura S, Eddins AC, Ozmeral EJ, et al. The Effects of Duration and Level on Spectral Modulation Perception. Journal of speech, language, and hearing research : JSLHR. 2019;62(10):3876–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]