Abstract
Background:
Adverse Childhood Experience (ACE) has been shown to have detrimental impact on amygdala structure. Prior research found that adaptive psychological changes after Mindfulness-Based Interventions (MBI) were associated with amygdala volumetric changes. The present study aims to further investigate whether such effects also occur among ACE survivors and whether the effects are unique to MBI.
Methods:
A total of 64 young adult childhood adversity survivors were randomized to an eight-week MBI or Stress Management Education (SME) as an active control condition. Anatomical MRI and questionnaires on mindfulness, stress and psychological health were collected at baseline and post-intervention. Due to subject dropout, the final sample included 39 subjects (MBI:20, SME:19).
Results:
Both groups showed increased mindfulness levels, reduced stress, and improved psychological symptoms (depression, anxiety, and somatization), with no significant group by time interaction effect. There was no significant group difference on amygdala volumetric changes. Within the MBI group, childhood maltreatment severity was a significant mediator between changes of mindfulness levels and right amygdala volumetric changes. Across pooled sample of both groups, childhood maltreatment was a significant moderator for the effect of trait anxiety level changes on left amygdala volumetric changes.
Limitations:
Modest sample size, relatively low retention rates, suboptimal monitoring of home practice.
Conclusions:
MBI did not demonstrate overall better clinical effects than SME. Psychological-change-dependent amygdala volumetric change was not specific to MBI. Childhood maltreatment severity modulated the relationships between adaptive psychological changes and amygdala volumetric changes.
Keywords: Meditation, Stress, Depression, Anxiety, MRI, Childhood maltreatment
1. Introduction
Childhood adversity refers to adverse experiences during the first 18 years of life. The pervasive long-lasting impact of Adverse Childhood Experience (ACE) on various health conditions was investigated by a large-scale study in the 1990s later known as the “ACE study”, which established that more ACEs were associated with increased risks for alcoholism, drug abuse, depression, suicide attempts and other high risk health conditions (Felitti et al., 1998). ACE has further been shown to alter the trajectory of psychological and neural development, leading to recurrent and persistent psychopathology that are less likely to respond to pharmacological interventions or traditional cognitive behavioral therapy techniques (Nanni et al., 2012; Nemeroff et al., 2003).
Compelling preclinical and human research has demonstrated the detrimental impacts of childhood adversity on neural development (Teicher et al., 2003; Vyas et al., 2002), with abnormality in brain structure and function that are still present in adulthood (Teicher et al., 2016), especially with the amygdala. The amygdala is an important structure for processing fear-related information in mammals (LeDoux, 2007). The human amygdala undergoes rapid growth during the first year of life, with continued structural changes into early adulthood (Fareri and Tottenham, 2016). Severe stress in early life can delay, accelerate, or prolong typical growth patterns of the amygdala (Weems, 2017), leading to hypersensitivity or hypoactivity of the amygdala that underlies common psychological symptoms such as depression, anxiety, PTSD and dissociation (LeDoux, 2007). Preclinical research studies have demonstrated the direct associations among early life stress, amygdala structural abnormality and maladaptive anxious behavioral patterns that persists into adulthood (Dutcher et al., 2023; Mitra et al., 2005). A meta-analysis on human neuroimaging studies demonstrated reduction of bilateral amygdala volumes among adults with childhood maltreatment-related PTSD (Ahmed-Leitao et al., 2016). Smaller amygdala volumes were also observed in the overall PTSD population compared to trauma-exposed non-PTSD controls (Del Casale et al., 2022). Depressed patients with higher severity of childhood trauma were found to have smaller volumes of the right amygdala (Aghamohammadi-Sereshki et al., 2021). Another study suggested reduced amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma (Weissman et al., 2020).
Preclinical research has demonstrated that amygdala structures are malleable to environmental manipulation, e.g., pre-weaning sensory enrichment blunted the effects of maternal separation stress on amygdala neurons when tested later in adulthood (Hegde et al., 2020). Environmental enrichment in adulthood was also shown to be able to reverse basolateral amygdala hypertrophy induced by maternal separation (Koe et al., 2016). A meta-analysis on amygdala volumes among depressed patients (Hamilton et al., 2008) found that unmedicated depressed individuals had smaller amygdala volumes compared to controls, while medicated depressed patients had larger amygdala volumes compared to healthy controls, confirming the formulation that antidepressant medications increases levels of brain-derived neurotrophic factor which promotes neurogenesis and protects against glucocorticoid toxicity in the amygdala. Another study found increased left amygdala volume among PTSD patients after “Eye Movement Desensitization and Reprocessing (EMDR)” therapy (Laugharne et al., 2016). Increased left amygdala volumes were also observed among PTSD patients following light therapy treatments (Killgore et al., 2022). Such prior knowledge collectively demonstrates the malleability of amygdala structure with downstream effects on improving adaptive behaviors and clinical symptoms. However, since ACE survivors don’t typically have favorable outcome in response to mainstream pharmacological interventions or cognitive behavioral therapy (Nanni et al., 2012), we are in search of alternative behavioral interventions that can bring about clinical improvement as well as adaptive amygdala structural changes.
Mindfulness-Based Interventions (MBI) such as Mindfulness-Based Stress Reduction (MBSR) (Kabat-Zinn, 1982, 1990; Kabat-Zinn et al., 1992) and Mindfulness-Based Cognitive Therapy (Teasdale et al., 2000), have been shown to be effective for a wide range of clinical symptoms including depression (Hofmann and Gómez, 2017; Teasdale et al., 2000), anxiety (Evans et al., 2008; Hofmann et al., 2010) and PTSD (Boyd et al., 2018), all of which are common psychological symptoms among ACE survivors (Nelson et al., 2020). Considering the complexity of the clinical symptoms and the versatile effects of MBIs, mindfulness training can be an effective behavioral intervention for this population (Joss et al., 2019; Kuyken et al., 2015). MBI’s clinical benefits have been substantiated by neural and physiological changes (Afonso et al., 2020; Kwak et al., 2019; Lazar et al., 2005; Yang et al., 2019). Increasing amounts of research have demonstrated MBI-induced neural changes in various brain areas such as the hippocampus, amygdala, insula, superior and inferior frontal gyri, anterior and posterior cingulate cortex, fusiform gyrus, corpus callosum, somatosensory cortex, and cerebellum (Afonso et al., 2020). Based on the preclinical knowledge about the impact of early life stress on amygdala structure, this study is particularly interested in possible amygdala volumetric changes after MBI.
Three prior studies have reported MBI-related amygdala structural changes (Hölzel et al., 2010; Joss et al., 2021; Kral et al., 2022). The earliest study (Hölzel et al., 2010) evaluated amygdala gray matter density before and after the 8-week MBSR program among moderately stressed healthy adults. This study found no significant post-MBI gray matter changes at bilateral amygdala, however, there was a significant correlation between changes in perceived stress and gray matter density changes at the right amygdala, with more reduction in perceived stress associated with more decreases in right amygdaloid gray matter density (Hölzel et al., 2010). A recent study (Kral et al., 2022) found no significant group difference on amygdala gray matter volume between MBSR, active control and waitlist control, however, more home meditation practice was significantly correlated with more volumetric reduction of the right amygdala. Similar to the lack of group level significance in terms of post-MBI amygdala volumetric changes (Hölzel et al., 2010) or group differences in amygdala volumetric changes (Kral et al., 2022), our prior study also did not find significant post-MBI amygdala volumetric change nor a significant group difference comparing MBI to waitlist controls (Joss et al., 2021); however, we found that within the MBI group (1) more reduction of perceived stress was associated with more left amygdala volumetric increase; (2) attending more MBI sessions was associated with more right amygdala volumetric increase; and (3) higher ACE severity was associated with more bilateral amygdala volumetric increase. The lack of group level significance (Hölzel et al., 2010; Joss et al., 2021; Kral et al., 2022) could be reflective of the cross-individual variability in amygdala volumetric changes, which was better captured in correlative analyses. However, it’s worth noting that the two previous studies found that higher amount of MBI-induced stress reduction (Hölzel et al., 2010) or more MBI practice (Kral et al., 2022) were associated with reduction of right amygdala volumes, whereas in our previous study these adaptive behavioral features were associated with increase of right amygdala volumes (Joss et al., 2021). Such discrepancy can be attributed to multiple factors: (1) the detrimental impact of ACE on amygdala development (Aghamohammadi-Sereshki et al., 2021; Ahmed-Leitao et al., 2016) causing the ACE survivors to have post-MBI amygdala morphological changes that are related to ACE-related amygdala abnormality (Joss et al., 2021); (2) the different subject ages in the previous studies (~35(Hölzel et al., 2010), ~45(Kral et al., 2022) vs. ~25(Joss et al., 2021)) probe into the different developmental stages of the amygdala across the human lifespan. A large (N = 406) human neuroimaging study (Narvacan et al., 2017) has demonstrated the human amygdala volume reaches the peak around age 22.6 and starts to decline after age 40. Therefore, the MBI-related amygdala volumetric changes shall be considered within the larger context of lifetime human amygdala development. While there is no simple conclusion as to which direction of amygdala volumetric changes is desirable, changes consistent with the natural trend of amygdala development are likely adaptive, especially when they are associated with positive behavioral changes.
To further investigate this phenomenon, we conducted the present study to answer two primary questions: (1) can the psychological-change-dependent amygdala volumetric changes be replicated in another independent sample of ACE survivors? (2) is this neural effect unique to MBI? Therefore, unlike the waitlist control used in our previous study (Joss et al., 2020a,b, 2021), the present study used an active control condition called Stress Management Education (SME) which was used in several prior studies (Hoge et al., 2013; Holzel et al., 2013; Sevinc et al., 2019) as a control condition for MBSR. Based on our recent research (Joss et al., 2019, 2020a,b, 2021), our primary hypotheses included: (1) the previous findings on amygdala volumetric changes associated with stress reduction, MBI dosage and ACE severity (Joss et al., 2021) will be replicated. (2) these associations can only be observed in the MBI group, but not in the control group. Due to lack of significance of group level statistics in prior studies (Hölzel et al., 2010; Joss et al., 2021; Kral et al., 2022), we did not expect significant group differences in amygdala volumetric changes, but rather focused on studying the associations between psychological changes and amygdala volumetric changes.
In addition to amygdala volumetric changes, we also investigated changes in psychological symptoms to understand the behavioral context of neural changes. Common psychological symptoms among ACE survivors include depression, anxiety (Nelson et al., 2020), along with elevated levels of perceived stress (Joss et al., 2019).Therefore we focused on measurements of these symptoms with the Perceived Stress Scale (PSS) (Cole, 1999), Beck Depression Inventory (BDI) (Beck et al., 1988) and Spielberger State-Trait Anxiety Inventory-Trait subscale (STAI-t) (Barnes et al., 2002; Spielberger et al., 1971) respectively. Our previous study (Joss et al., 2019) revealed significant post-MBI score reduction of PSS, STAI, but not BDI. To account for the possible limitation of a particular instrument for measuring psychological symptoms, in this study, we also administered the Kellner’s Symptom Questionnaire (KSQ) (Kellner, 1987), which includes assessment for depression, anxiety, anger-hostility, and somatic symptoms with four respective subscales. KSQ is known for its sensitivity for detecting psychological abnormality and was used in previous ACE studies to evaluate psychological symptoms among young adult ACE survivors (Teicher et al., 2006). Finally, we measured changes in mindfulness levels with the Mindful Attention Awareness Scale (MAAS) (Brown and Ryan, 2003), which is widely used in MBI research (Osman et al., 2016). Based on prior knowledge on the clinical effects of MBI, we hypothesized that MBI, compared to the active control condition, would lead to improvement in symptoms of perceived stress, depression and anxiety, as well as trait mindfulness levels.
2. Methods
2.1. Subject enrollment
This study was approved by the Institutional Review Board (IRB) of Mass General Brigham, which serves the Massachusetts General Hospital, McLean Hospital and several other hospitals. ACE was assessed with the Adverse Childhood Experience questionnaire (Felitti et al., 1998) and the Maltreatment and Abuse Chronology of Exposure (MACE) Scale (Teicher and Parigger, 2015). Clinical profiles were determined through structured clinical interview based on DSM-IV-TR. Subjects were screened with the following inclusion criteria: (1) Current age between 21 and 35 years old. (2) Right-handed. (3) Having ACE as determined by any of the following: (a) MACE score >=3, or (b) ACE score >=1; or (c) as determined through structured clinical interview using the Traumatic Antecedents Interview (TAI). Such multimodal approach was to overcome the limitation associated with a particular instrument. (4) Meeting MRI eligibility criteria. Exclusion criteria include pregnancy, recent suicidality, psychosis, neurological disorders, use of psychotropic medications and supplements, routine use of illicit drugs during the past month, ongoing psychotherapy, prior participation of systematic meditation or mindfulness programs within the past 2 years or routine meditation practice during the past 6 months.
A total of sixty-four subjects were enrolled into the study, with 35 randomly allocated to MBI while the other 29 allocated to SME at a ratio of 1.2:1 stratified with gender. Twenty-one subjects in the MBI group and 19 subjects in the SME control group completed the study. One subject in the MBI group was excluded from data analyses due to incidental neurological findings from the MRI.
2.2. Research procedures
2.2.1. Overall procedure
Eligible subjects were instructed to fill out online questionnaires and complete an MRI visit within a month before the intervention starts. At the MRI visit, all subjects underwent a urine drug test and female subjects also completed a urine pregnancy test. The MRI procedures included a 6-minute anatomical scan and a 7-minute resting state fMRI scan. A computer task on episodic memory was administered outside of the MRI, which will be reported in a future manuscript. After the MRI visit, subjects were randomized to attend either MBI or SME. Both intervention programs lasted 8 continuous weeks, which consisted of eight two-hour long in-person weekly meetings plus one 6-hour “all day retreat” session. Within a month after the 8-week intervention programs were finished, subjects were administered the same research procedures.
Within a month before and after the beginning and the end of the 8-week intervention programs, subjects were instructed to fill out a battery of online questionnaires through a secure online platform REDCap (Harris et al., 2009). After the end of the intervention programs, follow-up online questionnaires were administered at 6-, 12- and 18-month post-intervention, which will be reported in a future manuscript.
2.2.2. MRI parameters
MRIs were acquired on a Siemens 3T Prisma system at McLean Imaging Center of McLean hospital. A 64-channel head coil was used to acquire all MRI images. High resolution anatomical image was acquired using a T1-weighted multi-echo MPRAGE (MEMPRAGE) sequence (van der Kouwe et al., 2008), which acquires 4 separate structural scans with different TE values ranging from 1.69 to 7.27 ms, but in the same time as a conventional scan, and then the 4 separate images were averaged to increase the signal to noise ratio. Voxel size is 1.0 × 1.0 × 1.0 mm, Field of View (FOV) read is 256 mm, base resolution is 256, and there are 176 slices per slab. Phase encoding direction is A>>P. TR=2530 ms, TI=1100 ms, TE-1 = 1.69 ms, TE-2 = 3.55 ms, TE-3 = 5.41, TE4=7.27 ms. Flip angle =7.0°.
2.3. Interventions
2.3.1. Mindfulness based intervention (MBI)
The MBI program was modeled after the MBSR program (Kabat--Zinn, 1982, 1990; Kabat-Zinn et al., 1992), which covered a wide range of topics such as mindfulness and awareness, perception and perspectives, being present, responding vs. reacting to stress, stress coping strategies, dealing with difficult emotions, handling difficult communications, and using mindfulness in everyday life. Based on prior research on specific difficulties faced by trauma survivors (Vallejo and Amaro, 2009), we modified the MBSR program into a trauma-sensitive MBI with emphasis on empowering the participants through using suggestive instead of directive language, incorporating more mindful movements, and providing options and choices whenever possible (Joss et al., 2019). Guided meditation audios of various incremental lengths were provided for home practice throughout the program, and subjects were instructed to practice as much as they could, which is a major difference with the 45 min per day home practice requirement of the original MBSR. This MBI also included the “3-minute breathing space” from MBCT (Teasdale et al., 2000) which was shown to be particularly beneficial for trauma survivors (King et al., 2013). Various forms of mindful movements were taught during MBI, including gentle mindful movements and mindful walking, as well as mindful yoga with the emphasis on cultivating body awareness during the movements rather than the physical exercise aspects. Instructions for chair yoga were provided for those having difficulties with floor yoga. In-between the weekly sessions, subjects were encouraged to practice at home as much as possible the skills learned from weekly group sessions, including body scan meditation, breathing meditation, open awareness meditation, loving kindness meditation, mindful eating, mindful yoga, mindful walking, mindful movements, etc.
2.3.2. Stress management education (SME)
The SME program was adapted from our previous studies that used this program as the active control condition for MBSR (Hoge et al., 2013; Holzel et al., 2013; Sevinc et al., 2019), it was designed to closely match with the psycho-education components of the MBSR program except for the mindfulness element. Both MBI and SME groups were taught by licensed clinical social workers with extensive experience in providing mental health services, with the MBI instructor specialized in mindfulness meditation whereas the SME instructor specialized in cognitive behavioral therapy. The SME program includes psychoeducation about stress and stress physiology, the health benefits of physical exercise, sleep hygiene, time management, nutrition and healthy diet, as well as resilience and altruism. Subjects were instructed to practice the learned skills throughout the week and were encouraged to engage in physical exercise in the form that they were naturally familiar with, e.g., walking, running, biking, exercising in the gym etc.
2.4. Data analysis
2.4.1. Statistical analysis of questionnaire scores
We used Linear Mixed Effects Models (LME) (Lindstrom and Bates, 1988) to evaluate the treatment effects on questionnaire scores. LME analyses were conducted with the “nlme” and “MuMIn” packages in statistical software R. The scores of each questionnaire were used as the dependent variable for each model, with “group” (i.e., MBI or SME) and “time point” (i.e., before or after the intervention) as independent variables, and group by time interaction was the effect of interest, with age, sex, ethnicity, and time-interval between the two measurements as covariates; separate variance for each group and time point was used, and “REML” method was chosen to maximize the restricted log-likelihood. Pearson correlation analyses were conducted among score change (Δ) of each questionnaire (ΔMAAS, ΔPSS, ΔSTAI-t, ΔBDI, ΔKSQ-Anxiety, ΔKSQ-Depression, ΔKSQ-Somatization, ΔKSQ-Hostility) in order to reveal the associations among psychological changes, especially to understand which symptoms changes were associated with changes in mindfulness levels (ΔMAAS).
2.4.2. Analyses of amygdala volumes
MRI data was analyzed with the longitudinal module of the FreeSurfer 6.0 software, which performs automated segmentation and extract measures of gray matter volume for subcortical regions (Iglesias et al., 2015) (Saygin et al., 2017). As recommended by FreeSurfer developers (Reuter and Fischl, 2011), Symmetrized Percent Change (SPC) of bilateral amygdala volumes for each subject was calculated, representing the rate of change with respect to the average of both pre- and post-intervention volumes, which is a more robust measure than regular percent change calculation that only considers the amount of change with respect to the baseline volumes.
The left and right amygdala SPC values of each group were tested against 0 in a one-sample t-test to determine whether there was significant volumetric change in each group. Then an independent two samples t-test was conducted to determine whether there was significant group difference in the amounts of amygdala volumetric changes.
To further investigate the relationship between clinical changes and amygdala changes, Pearson correlation analyses was conducted between bilateral amygdala SPC and score change (Δ) of each questionnaire among the whole sample consisted of both groups.
To particularly investigate the possible influence of childhood maltreatment on amygdala structural changes in response to MBI, scores of each childhood maltreatment measurement were entered into Pearson correlation analysis with bilateral amygdala SPC values within the MBI group. Independent two-sample t-test was also conducted to compare amygdala SPC values between subjects with ACE score ≥ 3 versus those with ACE score < 3.
2.4.3. Mediation and moderation analyses
To obtain additional mechanistic understanding, post hoc mediation and moderation analyses were conducted to further test hypothesized relationships among variables of interest. Mediation analysis was conducted using the Causal Mediation Analysis package in R (Tingley et al., 2014), with the right amygdala SPC as the dependent variable, ΔMAAS as independent variable, with the mediator being MACE overall severity score, in order to test whether MACE overall severity score was a significant mediator for the relationship between ΔMAAS and right amygdala SPC.
Moderation analysis was conducted using the lme function in R (Yu and Li, 2022), by testing whether the interaction of ΔSTAI-t and MACE overall severity score was a significant predictor for left amygdala SPC, in order to test whether MACE overall severity score moderated the relationship between ΔSTAI-t and left amygdala SPC.
2.4.4. Additional statistical considerations
For each set of analysis, correction for multiple comparison was conducted with the “p.adjust” function in R using the Benjamini & Hochberg method (Benjamini and Hochberg, 1995). The -bh parameter was used to correct for False Discovery Rate (FDR). Corrected p-values are reported as p’.
Effect size were calculated with Cohen’s d for all t-tests and Pearson’s r for all correlation analyses (Cohen, 1992). Based on Cohen’s guideline, the thresholds for small, medium and large effect sizes are 0.2, 0.5, and 0.8 respectively for d and 0.1, 0.3 and 0.5 respectively for r.
Post hoc power analyses were conducted with the software G*Power 3.1.9.7 for all the findings that were still significant (p’< 0.05) after FDR correction.
3. Results
3.1. Demographics and intervention compliance
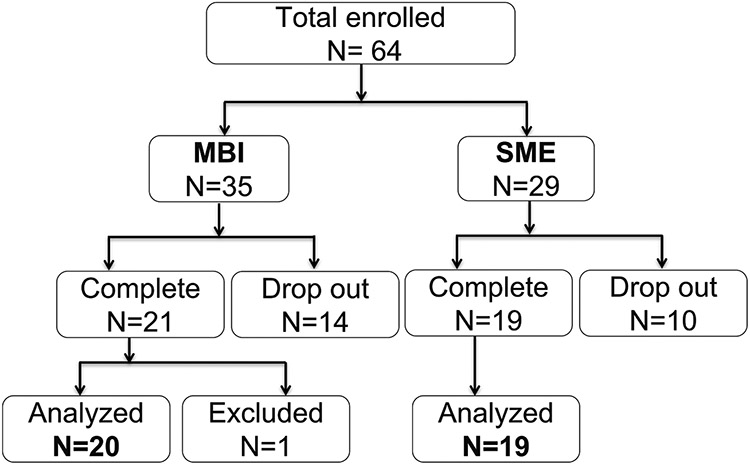
A total 64 subjects were enrolled and randomized to MBI (N = 35) and SME (n = 29) at 1.2:1 ratio, among which 24 subjects (14 in MBI and 10 in SME group) dropped out and did not complete post-intervention assessments. The overall subject retention rate is 62.5 %, with 60 % in the MBI group and 65.52 % in the SME group, with no significant group difference (X2 = 0.04, p = 0.85, Fig. 1). Furthermore, one subject in the MBI group was excluded from further analyses due to incidental findings of neurological abnormality in MRI. Therefore, a total of 20 subjects in MBI and 19 subjects in SME group were included in the data analyses. The two groups did not differ significantly on age, sex, race, childhood adversity, lifetime DSM diagnoses or hours of intervention session attendance (Table 1).
Fig. 1.
Flow chart of subject enrollment and retention. There was no significant group difference with subject dropout rates. One subject was excluded from analysis due to incidental neurological findings.
Table 1.
Demographic and clinical information.
| MBI | SME | Group Difference | |
|---|---|---|---|
| Sample Size (N) | 21 | 19 | |
| Sex: Female(F); Male(M) | F: 17; M: 4 | F: 14; M: 5 | Fisher’s exact test: p = 0.71 |
| Average Age (in years) (SE, range) | 26.71 (0.88, 22–33) | 25.58 (0.74, 22–34) | t = 0.97, p = 0.34 |
| Race (Frequencies) | |||
| White | 8 | 10 | Fisher’s exact |
| Black/African American | 3 | 0 | test: p = 0.21 |
| Asian | 8 | 8 | |
| Hispanic | 0 | 2 | |
| Unknown | 0 | 1 | |
| Childhood Maltreatment Assessments (Mean (SE)) | One-Way ANOVA |
||
| ACE Scores | 2.14 (0.48) | 2.42 (0.46) | F = 0.17, p = 0.68 |
| MACE-number of types of maltreatment | 2.00 (0.58) | 2.95 (0.66) | F = 1.17, p = 0.29 |
| MACE-overall severity scores | 21.10 (3.69) | 27.16 (4.06) | F = 1.22, p = 0.28 |
| MACE-sexual abuse | 0.52 (0.31) | 1.21 (0.52) | F = 1.29, p = 0.27 |
| MACE-parental verbal abuse | 3.67 (0.83) | 4.74 (0.84) | F = 0.82, p = 0.37 |
| MACE-non-verbal emotional abuse | 3.10 (0.54) | 3.21 (0.53) | F = 0.023, p = 0.88 |
| MACE-parental physical maltreatment | 3.19 (0.56) | 4.05 (0.72) | F = 0.90, p = 0.35 |
| MACE-witnessing violence between parents | 1.14 (0.45) | 2.21 (0.73) | F = 1.55, p = 0.22 |
| MACE-witnessing siblings abused by parents | 1.00 (0.32) | 1.11 (0.34) | F = 0.051, p = 0.82 |
| MACE-peer verbal abuse | 4.10 (0.75) | 4.11 (0.67) | F = 0.000099, p = 0.99 |
| MACE-peer physical abuse | 1.24 (0.38) | 1.37 (0.51) | F = 0.042, p = 0.84 |
| MACE-emotional neglect | 2.48 (0.66) | 3.26 (0.78) | F = 0.59, p = 0.45 |
| MACE-physical neglect | 0.67 (0.25) | 1.89 (0.56) | F = 3.98, p = 0.057 |
| DSM-IV-TR lifetime diagnosis (Frequencies) | Fisher’s exact test |
||
| Depressive Disorders | 10 | 13 | p = 0.21 |
| Anxiety Disorders | 15 | 15 | p = 0.72 |
| Total hours of intervention class attendance (mean (SE)) | 16.64 (1.01) | 14.43 (0.84) | t = 1.68, p = 0.10 |
| Total minutes of reported physical exercise (SE, range) | 191.45 (47.39) | 558.89 (126.73) | T = −2.77, p < 0.01 |
| Total minutes of non-exercise home practice | 742.70 (154.69) | 1071.58 (144.11) | T = −1.55, p = 0.13 |
3.2. Self-report questionnaires
LME analyses showed that there was no significant group by time intervention effect with any of the self-report questionnaires (p’ > 0.6). All questionnaires, except for STAI, showed significant main effect of time (p’ < 0.001) but no significant main effect of group. STAI had no significant main effect of either time or group. Correlation analyses demonstrated that increased mindfulness levels were associated with reduced depression and hostility (p < 0.05, medium to large effect sizes, Table 2), but not perceived stress. Symptom reduction of perceived stress, depression, somatization and hostility are closely correlated with each other (p < 0.05, medium to large effect sizes). STAI-t score change was not significantly correlated with other symptom changes. The range of post hoc power was 63.86–100 %, with a median of 92.09 %.
Table 2.
Correlation matrix among symptom questionnaire score changes and amygdala SPC among the whole sample. ($$: adjusted p’ < 0.01; $: adjusted p’ < 0.05, ^: adjusted p’ < 0.1. Raw p-value indicators: **: p < 0.01, *: p ≤ 0.05, #: p < 0.1. N = 39; N = 38 for right amygdala SPC due to outlier exclusion).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. ΔMAAS | 1.00 | ||||||||
| 2. ΔPSS | −0.21 | 1.00 | |||||||
| 3. ΔSTAI-t | −0.03 | 0.04 | 1.00 | ||||||
| 4. ΔBDI | $−0.47** | $0.40* | 0.03 | 1.00 | |||||
| 5. Δ KSQ-Anxiety | −0.30# | $0.44** | −0.01 | $$0.72** | 1.00 | ||||
| 6. Δ KSQ-Depression | $$−0.54** | $0.44** | 0.01 | $$0.76** | $$0.78** | 1.00 | |||
| 7. Δ KSQ-Somatization | −0.14 | 0.32* | 0.05 | $$0.57** | $$0.69** | $$0.48** | 1.00 | ||
| 8. KSQ-Hostility | ^−0.33* | $$0.48** | −0.18 | $$0.49** | $$0.59** | $$0.70** | ^0.35* | 1.00 | |
| 9. Left amygdala SPC | 0.31* | 0.02 | $−0.44** | −0.16 | 0.023 | 0.002 | −0.11 | 0.02 | 1.00 |
| 10. Right amygdala SPC | ^0.36* | 0.29# | 0.037 | 0.021 | −0.010 | −0.19 | 0.28# | −0.10 | −0.0077 |
3.3. Amygdala volumetric changes and contributing factors
Analyses of bilateral amygdala SPC values revealed no significant post-intervention change within either group, nor was there a significant group difference. The right amygdala SPC value of one MBI subject turns out to be a significant outlier (4.9 SD below the whole sample mean), thus was excluded from further analysis. Removing this outlier did not change the non-significance of group statistics. After excluding the outlier, both left and right amygdala SPC values were normally distributed.
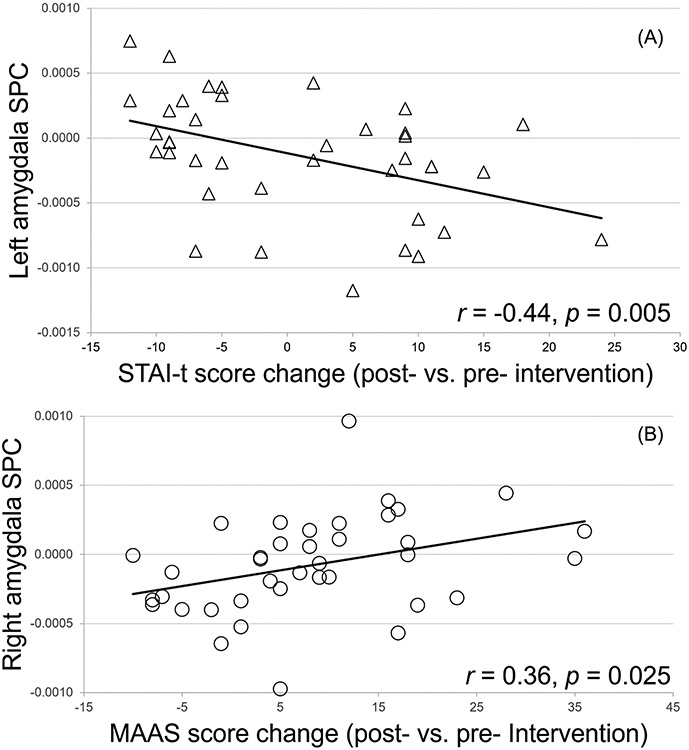
Correlation analyses between bilateral amygdala SPC and symptom changes (Table 2) found that left amygdala SPC was also significantly (FDR corrected) correlated with ΔSTAI-t (r = −0.44, p = 0.005, p’ = 0.015, Fig. 2.A). Changes of mindfulness levels (ΔMAAS) were associated with changes of amygdala volumes with r = 0.31, p = 0.05 and r = 0.36, p = 0.025 for left and right amygdala SPC respectively, although after FDR correction, only the right amygdala SPC was marginally significant (p’ = 0.066, Fig. 2.B). There was a marginally significant correlation (r = 0.29, p = 0.078, Table 2) between ΔPSS and right amygdala SPC, which did not survive FDR correction.
Fig. 2.
Correlations between left amygdala SPC and STAI-t score changes (A) and between right amygdala SPC and MAAS score changes (B) across the whole sample. Raw p values are displayed in the figure, with FDR corrected p’ = 0.015 and 0.066 for A and B respectively.
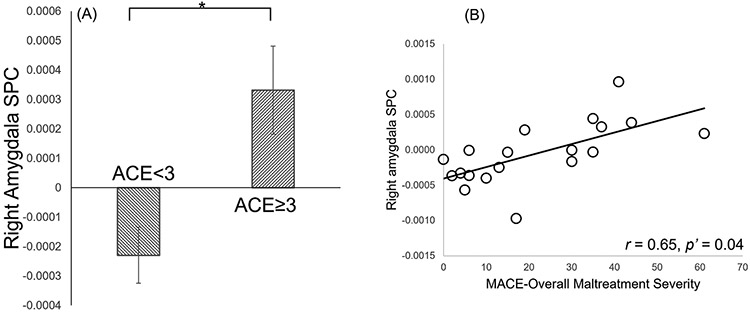
Within the MBI group, subjects with ACE ≥ 3 (N = 6) had significantly higher right amygdala SPC than those with ACE<3 (N = 13) (t = 3.25, p < 0.01, p’ = 0.03, Cohen’s d = 1.60, Fig. 3.A). Right amygdala SPC values were significantly correlated with MACE overall severity scores (r = 0.65, p < 0.01, p’ = 0.04, Fig. 3.B), number of types of maltreatment experienced (r = 0.61, p < 0.01, p’ = 0.04), as well as the severity of parental verbal abuse (r = 0.62, p < 0.01, p’ = 0.04), and marginally significantly correlated with peer verbal abuse (r = 0.54, p < 0.05, p’ = 0.07), peer physical abuse (r = 0.54, p < 0.05, p’ = 0.06), and sexual abuse (r = 0.55, p < 0.05, p’ = 0.07). The left amygdala SPC was not significantly correlated with any childhood maltreatment measures (p’ > 0.7). Bilateral amygdala SPCs were not significantly correlated with the amount of MBI session attendance or the amount of home practice (p > 0.31). The significant correlations had post hoc power ranging from 88.51 % to 93.95 %.
Fig. 3.
Associations between childhood maltreatment severity and post-MBI right amygdala volumetric changes within the MBI group. (A) Significant group difference between subjects with ACE scores above (N = 6) or below 3 (N = 13) (*: p’ = 0.03). (B) Correlation between MACE overall severity score and right amygdala SPC (p’ = 0.04, N = 19).
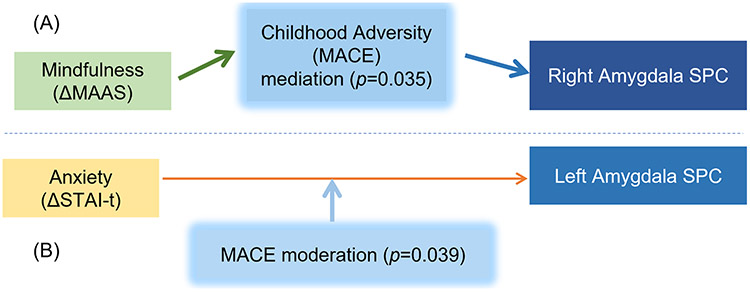
To obtain further mechanistic understanding, post hoc mediation and moderation analyses were conducted to test hypothesized mechanisms. Based on the above finding of the correlations between MACE overall severity score and right amygdala SPC within the MBI group (Fig. 3.B), a mediation analysis was conducted to test whether MACE severity was a significant mediator between ΔMAAS (as an indicator of therapeutic effect of the MBI) and right amygdala SPC, which found a significant mediation effect (p = 0.035, Fig. 4.A) within the MBI group. Based on our finding of a significant correlation between ΔSTAI-t and left amygdala SPC within the pooled sample (Fig. 2.A), we also tested whether MACE overall severity score might be a moderator for the effect of ΔSTAI-t on left amygdala SPC and found a significant moderation effect (p = 0.039, Fig. 4.B) in the pooled sample of all subjects from both groups. Because only one hypothesis was tested for each analysis, no FDR correction was needed (Chen et al., 2017).
Fig. 4.
Childhood adversity severity modulated the relationship between clinical improvements and amygdala volumetric changes. (A) Childhood adversity severity, as measured by MACE overall severity score, was a significant (p = 0.035) mediator between changes in mindfulness levels (ΔMAAS) and right amygdala SPC, within the MBI group. (B) MACE overall severity score was a significant (p = 0.039) moderator for the effect of trait anxiety levels (ΔSTAI-t) on left amygdala SPC, within pooled sample of both groups.
4. Discussion
Findings from this study replicated our prior finding that childhood maltreatment severity affects post-MBI right amygdala volumetric changes (Joss et al., 2021). Among the three factors (amount of stress reduction, intervention dosage and ACE severity) that had significant association with post-MBI amygdala volumetric changes in our prior study (Joss et al., 2021), only childhood maltreatment severity was replicated to have a significant correlation with post-MBI amygdala volumetric changes. Childhood maltreatment severity was also further elucidated to be a significant mediator between mindfulness level elevation and right amygdala volumetric changes. Several secondary findings include (1) clinical improvements were not unique to MBI, both groups had overall reduced psychological symptoms and improved mindfulness levels; (2) in the pooled sample of both groups, childhood maltreatment severity is a significant moderator for the effect of ΔSTAI-t on left amygdala SPC.
4.1. Amygdala volumetric changes
Human neuroimaging studies have demonstrated complex impact of ACE on amygdala structural development, with some neuroimaging studies reported ACE associated with larger amygdala volumes (Howell et al., 2014; Lupien et al., 2011; Mehta et al., 2009; Pechtel et al., 2014; Tottenham and Sheridan, 2010) while others reported smaller volumes (Aghamohammadi-Sereshki et al., 2021; Ahmed-Leitao et al., 2016; Driessen et al., 2000; Schmahl et al., 2003; Veer et al., 2015). For examples, adolescents (age 10–19) with ACE history, such as orphanage experience (Mehta et al., 2009; Tottenham and Sheridan, 2010), parental physical abuse (Howell et al., 2014), or maternal depression (Lupien et al., 2011), were observed with larger amygdala volumes compared to age-matched controls. In contrast, there are also several MRI studies reporting ACE survivors having smaller amygdala volumes compared to age matched controls, particularly among those with psychopathology such as major depressive disorder (Aghamohammadi--Sereshki et al., 2021), PTSD (Ahmed-Leitao et al., 2016; Veer et al., 2015) or borderline personality disorder (Driessen et al., 2000; Schmahl et al., 2003). In summary, the literature has no clear conclusion on whether ACE is associated with smaller or larger amygdala volumes. The present study did not observe significant group-level post-intervention amygdala volumetrics, which is likely due to the complexity of ACE impact on baseline amygdala volumes, e.g., if a subject had “smaller than normal” baseline amygdala volume, then increased volume would be an adaptive change, and vice versa. Since the pool of subjects are consisted of individuals with opposite directions of changes with amygdala volumes (see scatter plots in Fig. 2), no significant post-intervention changes were observed at the group level, which is similar to the two prior MBI studies that also reported no significant post-MBI amygdala volumetric change at the group level (Hölzel et al., 2010; Kral et al., 2022) despite their findings of significant correlations between amygdala volumetric changes and adaptive psychological changes. Therefore, we further conducted in-depth investigation on the brain-behavior association of amygdala volumetric changes as discussed below.
To reiterate, prior MBI studies found no significant post-intervention amygdala volumetric change (Hölzel et al., 2010; Joss et al., 2021; Kral et al., 2022) as well as no significant group difference on amygdala volumetric change comparing MBI to waitlist (Joss et al., 2021) or active control (Kral et al., 2022). Similar patterns are also found in the present study with no significant post-intervention amygdala volumetric change or group differences. Nevertheless, the three prior studies all reported that the individual variability in amygdala volumetric changes were significantly correlated with behavioral characteristics such as the amount of stress reduction (Hölzel et al., 2010; Joss et al., 2021) or the amount of mindfulness meditation home practice (Joss et al., 2021; Kral et al., 2022). Similarly, the present study found a significant correlation between left amygdala volumetric changes and changes in anxiety levels (ΔSTAI-t, Fig. 2A) and a marginally significant correlation between right amygdala volumetric changes and changes in mindfulness levels (ΔMAAS, Fig. 2.B). Such statistical phenomenon, in terms of the lack of significance with group comparisons accompanied by significant cross-individual brain-behavior associations, can be attributed to the large cross-individual variability with amygdala volumetric changes in either direction (Kral et al., 2022).
Our previous study found that adaptive behavioral changes, such as more stress reduction and higher MBI dosage, were associated with more increase of amygdala volumes (Joss et al., 2021). In light of those findings, the present study was conducted to address two primary questions: can the psychological-change-dependent amygdala volumetric changes be replicated in another independent sample of ACE survivors? (2) is this neural effect unique to MBI? The answers to these two questions are intertwined. Findings from the present study suggests the neural effects are not specific to MBI, but rather more dependent on psychological changes regardless how these psychological changes were achieved (e.g., stress reduction without practicing mindfulness meditation as in the SME group), because a significant correlation (FDR corrected p’ = 0.015) was found in the pooled sample of both groups between left amygdala volumetric changes and changes in anxiety levels, but this effect was not significant in the MBI group alone. There was also a marginally significant correlation in the pooled sample between right amygdala volumetric changes and changes in mindfulness levels, which was also not significant in the MBI group alone. The prior findings on the associations of amygdala volumetric changes with stress reduction or MBI dosage were not exactly replicated in this new sample. There was only a marginally significant correlation (r = 0.29, p = 0.078, Table 2) between PSS score change and right amygdala SPC which did not survive FDR correction. Replicability is a common issue with psychology research (Youyou et al., 2023). Nevertheless, the present study still found psychological-change-dependent amygdala volumetric changes, suggesting such associations are worth further investigation. The present study also elucidated that such behavior-brain associations are not unique to MBI. Prior findings (Hölzel et al., 2010; Joss et al., 2021) indicate that MBI affects amygdala volumetric changes through stress reduction, but findings from the present study does not necessarily support this possible mechanism, but rather points to the possibility that elevations of mindfulness levels (regardless whether it’s achieved through MBI) could affect amygdala volumetric changes directly.
While findings from the present study are consistent with our prior findings (Joss et al., 2021) that adaptive behavioral changes were associated with a trend of amygdala volumetric increase, the direction of these associations are in the opposite direction of the other two prior MBI studies that found the amount of stress reduction (Hölzel et al., 2010) or home meditation practice (Kral et al., 2022) were associated with a trend for right amygdala volume reduction. Multiple factors shall be taken into consideration when interpreting the differences in these findings: (1) It has not been established in the literature with regard to which direction of amygdala volumetric changes is desirable. Although a previous MBI study found the amount of stress reduction was associated with decreased post-MBI amygdala volume (Hölzel et al., 2010), it was contradictory with a prior cross-sectional study (Gianaros et al., 2008) that showed smaller amygdala volume predicted greater stress-related amygdala activation, thus implying reduced amygdala volume was an undesirable direction of change; the authors attributed the contradiction to limitations with MRI resolution and data analyses methodology (Hölzel et al., 2010). Nevertheless, a later study also found decreased amygdala volume being associated with the amount of meditation practice (Kral et al., 2022), which appears consistent with the prior finding (Hölzel et al., 2010) albeit not an exact replication. But beyond MBI, other types of therapy have found adaptive behavioral changes being associated with increased amygdala volumes (Killgore et al., 2022; Laugharne et al., 2016). In conclusion, the literature has not provided a consistent indication on whether amygdala volumetric increase or decrease being a desirable direction of change. The only consistency is the “brain-behavior association” aspect, i.e., when amygdala volumetric changes were associated with an adaptive psychological changes, it was interpreted as an adaptive neural change, regardless whether it was volumetric increase or decrease. (2) ACE has complex impact on amygdala development and may continue to influence amygdala volumetric changes during adulthood. While several studies revealed increased amygdala volumes among ACE-impacted adolescents or young adults (Teicher et al., 2016), several studies also revealed decreased amygdala volumes compared to age matched healthy controls among ACE survivors with psychopathology such as borderline personality disorder (Driessen et al., 2000; Schmahl et al., 2003), PTSD (Ahmed-Leitao et al., 2016) and dissociative identity disorder (Vermetten et al., 2006). Therefore, the trend for amygdala volumetric increase associated with symptom improvement (e.g., reduced trait anxiety, Fig. 2.A) could be an indicator of adaptive neural changes while recovering from psychopathology. (3) Different dosage of behavioral intervention could lead to different neural changes. To make the MBI program more trauma-sensitive, the home practice requirement in our modified MBI program was very flexible compared to the traditional 45 min per day home practice requirement of MBSR. As a result, the average amount of home practice in our two studies were ~12 h, which is much less compared to the 20 h (Hölzel et al., 2010) or 32 h (Kral et al., 2022) in the other studies. Dosage difference of skill building have explained different patterns of neural changes in other domains. For example, a neuroimaging study investigated motor cortex gray matter volume change after 6-weeks of juggling practice, and found that within the group that was prescribed the low intensity practice regimen, better jugging performance was associated with more gray matter reduction, whereas within the group with high intensity practice regimen, better performance was associated with more gray matter increase (Sampaio-Baptista et al., 2014). It is possible that there are different cellular level neuronal changes with different amount of skill learning or practice, leading to opposite directions of behavioral-brain association in neuroimaging findings. (4) In-vivo animal studies have observed that learning of motor skills was associated with both formation of new dendritic spines and elimination of older dendritic spines, with both processes associated with behavioral performance (Yang et al., 2009). Therefore, while the exact mechanism is still unclear, it is possible that either amygdala volumetric increase or decrease can be adaptive neural changes, especially when it’s associated with adaptive behavioral changes such as reduced anxiety or increased mindfulness levels.
A major finding from the present study is the positive association between severity of childhood maltreatment and right amygdala SPC within the MBI group, suggesting that subjects with more severe childhood maltreatment had more increase of right amygdala volumes. This finding replicates our prior finding of a positive correlation between ACE severity and right amygdala volumetric changes, in that subjects with more severe ACE demonstrated a trend for more volumetric increase of the right amygdala (Joss et al., 2021). The mediation analysis in the present study further elucidated that within the MBI group, childhood maltreatment severity was a significant mediator between changes of mindfulness levels and right amygdala volumetric changes. This finding points to the possibility that MBI may have unique effects for childhood maltreatment survivors; e.g., a previous clinical trial (N = 274) found that MBCT was only more effective than treatment as usual or the psychoeducation active control condition in preventing relapse of recurrent depression for patients with childhood trauma (Williams et al., 2014). While the exact mechanism of MBI’s unique benefits for childhood maltreatment survivors is still unknown, our preliminary findings suggest amygdala volumetric changes can be a potential neural mechanism for future investigations.
The different psychological associations of bilateral amygdala volumetric changes are also very interesting. A few prior ACE studies also found that the volumes of the right amygdala, compared to the left amygdala, had stronger associations with the severity of childhood maltreatment (Buss et al., 2012; Mehta et al., 2009; Pechtel et al., 2014; Tottenham and Sheridan, 2010). Two previous MBI studies also reported the adaptive behavioral changes being associated with right amygdala volumetric changes, but not the left amygdala (Hölzel et al., 2010; Kral et al., 2022). Research on human amygdala development suggests the right amygdala has a longer course of growth than the left amygdala (Uematsu et al., 2012), it is possible that the right amygdala may be particularly malleable to both environmental impact and behavioral changes. This study also found that post-MBI right amygdala volumetric changes were correlated with scores of several types of abuse, but not the neglect aspect of ACE. This finding is consistent with the well-known role of the right amygdala on threat detection (Dannlowski et al., 2012), especially with detecting angry faces (Morris et al., 1998), to which ACE survivors have been reported to exhibit greater right amygdala reactivity (da Silva Ferreira et al., 2014; McCrory et al., 2013). The exact mechanism of why and how childhood abuse affects post-MBI volumetric changes requires further investigation. The theoretical background is that the right amygdala is involved in rapid and automatic detection of possible danger (Hardee et al., 2008), while a major focus of MBI is practicing wisely “responding” to stressors instead of resorting to automatic stress reactivity (Joss et al., 2021). It’s possible that such meditation practice eventually leads to right amygdala volumetric changes. The observed mediating effect from childhood maltreatment severity likely operates either through ACE’s impact on pre-intervention baseline amygdala volumes or through impacting amygdala activity patterns as a result of the intervention. In addition, childhood maltreatment severity also had significant moderation effect on the relationship between changes of anxiety symptoms and left amygdala volumetric change in the pooled sample of both groups, indicating that ACE’s impact on left amygdala volumetric change was not specific to MBI. In our previous study (Joss et al., 2021), left amygdala volumetric changes were associated with the amount of stress reduction while right amygdala volumetric changes were associated with MBI dosage. Although the present study did not exactly replicate these findings, the general pattern of left amygdala volumetric changes being associated with anxiety reduction whereas right amygdala volumetric changes being associated with mindfulness level elevation are fairly similar to the our prior findings on the different behavioral associations of bilateral amygdala volumetric changes (Joss et al., 2021).
In conclusion, the present study confirmed the existence of psychological-change-dependent amygdala volumetric changes, although such brain-behavior association was not unique to MBI. Changes in trait anxiety levels were negatively correlated with changes in left amygdala volumes across the pooled sample of both groups, and childhood maltreatment severity was a significant moderator for this association. Within the MBI group, severity of childhood maltreatment was positively correlated with right amygdala volume change, and the association between changes of mindfulness levels and right amygdala volumes were mediated by childhood maltreatment severity. These findings inform future research on psychological-change-dependent amygdala volumetric changes, as well as further investigation on potential mechanisms of MBI’s benefits for childhood maltreatment survivors.
4.2. Both groups showed increased mindfulness levels and symptom reductions
ACE is an important variable in mental health research due to its impact on psychological and neural development (Teicher et al., 2016). Multiple researchers have suggested consideration of ACE survivors as a clinically and neurobiologically distinct ecophenotypic variant (Jamshidi et al., 2022; Teicher and Samson, 2013), because ACE survivors have distinct response patterns to pharmacological and behavioral interventions (Nanni et al., 2012; Nemeroff et al., 2003). In the mindfulness meditation research literature over the past two decades, the ACE or childhood trauma variable has not received sufficient attention. Most prior studies on the clinical effects of MBI focused on specific diagnostic categories rather than the developmental causes, with occasional reports of ACE as a confounding factor (Cash et al., 2015; Kuyken et al., 2015; Ma and Teasdale, 2004; Teasdale et al., 2000; Williams et al., 2014). In particular, one large clinical trial (N = 274) demonstrated that the MBCT was only more effective than control interventions among childhood trauma survivors for preventing relapse of recurrent depression (Williams et al., 2014), highlighting the importance of recognizing the contribution of the ACE factor in treatment response of MBIs. Although the primary objective of the present study was to investigate the neural effects of MBI, our findings still provided useful information on investigating MBI’s clinical effects for addressing various comorbid symptoms among ACE survivors.
The present study found that both MBI and SME group had significant reduction of various psychological symptoms. While it replicates our previous finding of MBI-induced clinical benefits among ACE survivors compared to waitlist controls (Joss et al., 2019), it did not support the hypothesis that these clinical benefits were unique to MBI, but rather demonstrates that MBI was not more effective than a generic group intervention for stress management. This could be attributed to the samples having relatively low ACE severity (with average ACE score of 1.7 in the previous study (Joss et al., 2019) and 2.3 in the present study) and moderate symptomology. For example, in the current study, the baseline PSS score is 19.56 which is within the moderate bracket (Cole, 1999); the baseline BDI score is 14.82 which is in the mild bracket (Beck et al., 1988), the baseline STAI-t score is 45.87 which is barely above the clinical cut-off score of 44 (Ercan et al., 2015). The previous clinical trial on recurrent depression also found subjects with below median severity of childhood trauma didn’t have better clinical effects from MBI compared to treatment as usual or the psychoeducation active control condition (Williams et al., 2014). Furthermore, previous studies that used the SME program as an active control for MBI also showed comparable effectiveness in reducing stress and anxiety (Hoge et al., 2013; Holzel et al., 2013). In the present study, the SME group also self-reported significantly higher amounts of physical exercise during the course of the 8-week intervention. Exercise has been shown to be particularly effective for reducing psychological symptoms (Chekroud et al., 2018) and for promoting neural plasticity (Fernandes et al., 2017), therefore the clinical effects of the SME intervention in the current study could also be attributed to the physical exercise component.
The fact that the SME group also had increased mindfulness levels is intriguing, but it is consistent findings from a prior study that reported similar post-intervention MAAS scores between MBSR and SME (Sevinc et al., 2019). A recent study also found increased mindfulness levels after “Health Enhancement Program (HEP)” that was used as a control condition for MBSR (Kral et al., 2022) and there was no significant group difference between MBSR and HEP on increased mindfulness levels (Kral et al., 2022). The SME program focuses on psychoeducation topics such as healthy diet, sleep hygiene, time management, and positive perspectives, which could lead to elevated trait mindfulness due to the following reasons: (1) comprehensive psychoeducation on various self-care topics could improve self-awareness throughout daily lives, which could lead to increased self-report mindfulness levels on MAAS items such as “I snack without being aware that I’m eating”. Heightened awareness for self-care also increases opportunities for “mindful moments” throughout the daily life, even if the experience was not explicitly labeled as “mindfulness” in the control group, the accumulation of these self-care-oriented self-awareness can lead to increased trait mindfulness levels. (2) it is possible that stress reduction achieved through non-meditation approaches can naturally lead to increased trait mindfulness levels. Just like there are natural individual variability on levels of trait mindfulness without any formal meditation training, certain experiences such as exposure to natural scenery, has been shown to increase mindfulness levels without explicitly learning or practicing mindfulness meditation (Olszewska-Guizzo et al., 2022). The elevated mindfulness levels in the SME group indicate the possibility of improving mindfulness levels through stress reduction without formal meditation practices.
Another minor nuances with the clinical finding from the present study is the lack of significant reduction with the STAI-t scores in either group, which is contradictory with the significant score reductions on the KSQ-anxiety subscale. This could be related to the differences in the instruments themselves in terms of how anxiety is measured. The KSQ has been shown to be highly sensitive for detecting symptomatology differences in cross-sectional and longitudinal clinical studies (Benasi et al., 2020) while STAI-t is widely used in psychology literature. Such difference in score change between the two instruments should be noted in future psychometric studies to better understand how the two instruments measure anxiety differently. Despite lack of score changes at the group level, the individual variability in STAI-t score change was significantly correlated with the individual variability in left amygdala volume change, suggesting that STAI-t could be sensitive for capturing the individual variability of neural-based anxiety level changes in a longitudinal trial.
4.3. Limitations and future directions
There are several major limitations with the present study that shall be addressed in future research: (1) The sample size in this study is comparable to many prior MBI neuroscience studies, e.g., N = 26 of the previous report on MBI-induced amygdala volumetric changes (Hölzel et al., 2010), N = 14 of an fMRI study on the effect of compassion training on neural responses to evoked pain (Berry et al., 2020), N = 27 of an MRI study on the neural effects of MBI for Parkinson’s disease (Pickut et al., 2013) as well as N = 34 of our previous study on amygdala volumetric changes after MBI among ACE survivors (Joss et al., 2021). Nevertheless, a larger sample may be able to yield more robust findings. (2) This study included subjects with a wide range of ACE severity and types, as well as various current psychological symptoms. Such wide range enabled the investigation of ACE severity on post-MBI amygdala volumetric changes, which led to the finding of significant group difference on post-MBI right amygdala volumetric changes among those with ACE scores above vs. below 3 (Fig. 3). Alternatively, purposeful selection of a relatively homogenous patient population may be more suitable for illuminating the effects of MBI for healing specific neural impacts of specific childhood adversity experiences with specific current psychopathology (e.g., complex PTSD patients with childhood sexual abuse history). (3) Although the two groups did not have significant difference in subject retention rate, it is still worth noting that the overall subject retention rate is relatively low (62.5 %) compared to prior MBI studies with retention rate ranging from 72 % to 100 % (Joss and Teicher, 2021). A lot of factors could affect the retention rates of a longitudinal trial, such as recruitment strategies, the amount of staff support, and a myriad of logistical challenges. Most cited reasons for dropouts are transportation difficulties and scheduling conflicts, while some subjects did not provide dropout reasons. Throughout the course of the study, a psychiatrist was on-call to respond to any psychiatric emergencies, but the psychiatrist did not receive any emergency calls. Our previous study with a higher retention rate of 76 % (Joss et al., 2019) had one or two research staff members attend each intervention session with the subjects. In comparison, the present study was understaffed and was not able to provide such support. It is possible that group interventions for the ACE population could benefit from additional support to improve subject retention. (4) Unlike previous reports (Joss et al., 2021; Kral et al., 2022), the present study did not find significant correlation between the amount of home practice and amygdala volumetric changes. This could be due to the suboptimal research methodology for monitoring home practice. In the current study, this information was gathered through a weekly daily-practice report paper form, which is susceptible to inaccuracy. Emerging research techniques such as Ecological Momentary Assessments (EMA), which sends short prompts to subjects’ mobile devices several times a day for subjects to report activity and subjective experience, could significantly improve the frequency and accuracy of home practice reporting, as well as improving compliance with home practice requirements. Findings from the present study also raised interesting questions such as whether mindfulness level increase can directly induce neural changes, and whether stress reduction through non-MBI approaches can also lead to elevation of trait mindfulness, which shall be further investigated in future MBI research.
Acknowledgments
We thank Lauri Klein and Colette Coleman for teaching the intervention classes. We thank all research subjects for participating in this study.
Funding
This study was funded by the NIH (Grant No: 5K01AT009085). The funding source played no role in the study design, data collection, data analysis or manuscript writing.
Footnotes
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. DJ and SWL are practitioners of mindfulness meditation.
CRediT authorship contribution statement
Diane Joss: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Junjie Lu: Data curation. Martin H Teicher: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Sara W. Lazar: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
References
- Afonso RF, Kraft I, Aratanha MA, Kozasa EH, 2020. Neural correlates of meditation: a review of structural and functional MRI studies. Front. Biosci. Scholar 12, 92–115. [DOI] [PubMed] [Google Scholar]
- Aghamohammadi-Sereshki A, Coupland NJ, Silverstone PH, Huang Y, Hegadoren KM, Carter R, Seres P, Maiykhin NV, 2021. Effects of childhood adversity on the volumes of the amygdala subnuclei and hippocampal subfields in individuals with major depressive disorder. J. Psychiatry Neurosci 46, E186–E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed-Leitao F, Spies G, van den Heuvel L, Seedat S, 2016. Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: a systematic review. Psychiatry Res. Neuroimaging 256, 33–43. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Harp D, Jung WS, 2002. Reliability generalization of scores on the Spielberger state-trait anxiety inventory. Educ. Psychol. Meas 62, 603–618. [Google Scholar]
- Beck AT, Steer RA, Carbin MG, 1988. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev 8, 77–100. [Google Scholar]
- Benasi G, Fava GA, Rafanelli C, 2020. Kellner’s symptom questionnaire, a highly sensitive patient-reported outcome measure: systematic review of clinimetric properties. Psychother. Psychosom 89, 74–89. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol 57, 289–300. [Google Scholar]
- Berry MP, Lutz J, Schuman-Olivier Z, Germer C, Pollak S, Edwards RR, Gardiner P, Desbordes G, Napadow V, 2020. Brief self-compassion training alters neural responses to evoked pain for chronic low back pain: a pilot study. Pain Med. 21, 2172–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JE, Lanius RA, McKinnon MC, 2018. Mindfulness-based treatments for posttraumatic stress disorder: a review of the treatment literature and neurobiological evidence. J. Psychiatry Neurosci 43, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, 2003. The benefits of being present: mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol 84, 822–848. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA, 2012. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. P. Natl. Acad. Sci. U. S. A 109, E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash E, Salmon P, Weissbecker I, Rebholz WN, Bayley-Veloso R, Zimmaro LA, Floyd A, Dedert E, Sephton SE, 2015. Mindfulness meditation alleviates fibromyalgia symptoms in women: results of a randomized clinical trial. Ann. Behav. Med 49, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, Chekroud AM, 2018. Association between physical exercise and mental health in 1· 2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry 5, 739–746. [DOI] [PubMed] [Google Scholar]
- Chen SY, Feng Z, Yi X, 2017. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis 9, 1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., 1992. A power primer. Psychol. Bull 112, 1155–1159. [DOI] [PubMed] [Google Scholar]
- Cole SR, 1999. Assessment of differential item functioning in the perceived stress scale-10. J. Epidemiol. Community Health 53, 319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira GC, Crippa JAS, de Lima Osório F, 2014. Facial emotion processing and recognition among maltreated children: a systematic literature review. Front. Psychol 5,1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, 2012. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry 71, 286–293. [DOI] [PubMed] [Google Scholar]
- Del Casale A, Ferracuti S, Barbetti AS, Bargagna P, Zega P, Iannuccelli A, Caggese F, Zoppi T, De Luca GP, Parmigiani G, 2022. Grey matter volume reductions of the left hippocampus and amygdala in PTSD: a coordinate-based meta-analysis of magnetic resonance imaging studies. Neuropsychobiology 81, 257–264. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D, 2000. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch. Gen. Psychiatry 57, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Dutcher EG, Lopez-Cruz L, Pama EAC, Lynall ME, Bevers ICR, Jones JA, Khan S, Sawiak SJ, Milton AL, Clatworthy MR, Robbins TW, Bullmore ET, Dalley JW, 2023. Early-life stress biases responding to negative feedback and increases amygdala volume and vulnerability to later-life stress. Transl. Psychiatry 13, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan I, Hafizoglu S, Ozkaya G, Kirli S, Yalcintas E, Akaya C, 2015. Examining cut-off values for the state-trait anxiety inventory. Rev. Argent. Clin. Psicol 24, 143–148. [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D, 2008. Mindfulness-based cognitive therapy for generalized anxiety disorder. J. Anxiety Disord 22, 716–721. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Tottenham N, 2016. Effects of early life stress on amygdala and striatal development. Dev. Cogn. Neurosci 19, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am. J. Prev. Med 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Arida RM, Gomez-Pinilla F, 2017. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev 80, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR, 2008. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci 28, 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH, 2008. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry 13, 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Thompson JC, Puce A, 2008. The left amygdala knows fear: laterality in the amygdala response to fearful eyes. Soc. Cogn. Affect Neurosci 3, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde A, Suresh S, Mitra R, 2020. Early-life short-term environmental enrichment counteracts the effects of stress on anxiety-like behavior, brain-derived neurotrophic factor and nuclear translocation of glucocorticoid receptors in the basolateral amygdala. Sci. Rep 10, 14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Gómez AF, 2017. Mindfulness-based interventions for anxiety and depression. Psychiatr. Clin. North Am 40, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D, 2010. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol 78, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, Worthington JJ, Pollack MH, Simon NM, 2013. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry 74, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW, 2010. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect Neurosci 5, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Hoge EA, Greve DN, Gard T, Creswell JD, Brown KW, Barrett LF, Schwartz C, Vaitl D, Lazar SW, 2013. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D, Styner MA, Sanchez MM, 2014. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev. Psychobiol 56, 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, 2015. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi J, Schofield PR, Gatt JM, Fullerton JM, 2022. Phenotypic and genetic analysis of a wellbeing factor score in the UK Biobank and the impact of childhood maltreatment and psychiatric illness. Transl. Psychiatry 12, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Khan A, Lazar SW, Teicher MH, 2019. Effects of a mindfulness-based intervention on self-compassion and psychological health among young adults with a history of childhood maltreatment. Front. Psychol 10, 2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Khan A, Lazar SW, Teicher MH, 2021. A pilot study on amygdala volumetric changes among young adults with childhood maltreatment histories after a mindfulness intervention. Behav. Brain Res 399, 113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Lazar SW, Teicher MH, 2020a. Effects of a mindfulness based behavioral intervention for young adults with childhood maltreatment history on hippocampal morphometry: a pilot MRI study with voxel-based morphometry. Psychiatry Res. Neuroimaging 301, 111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Lazar SW, Teicher MH, 2020b. Nonattachment predicts empathy, rejection sensitivity, and symptom reduction after a mindfulness-based intervention among young adults with a history of childhood maltreatment. Mindfulness 11, 975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Teicher MH, 2021. Clinical effects of mindfulness-based interventions for adults with a history of childhood maltreatment: a scoping review. Curr. Treat. Options Psychiatry 8, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J., 1982. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 4, 33–47. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J., 1990. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. Delta. [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, et al. , 1992. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am. J. Psychiatry 149, 936–943. [DOI] [PubMed] [Google Scholar]
- Kellner R., 1987. A symptom questionnaire. J. Clin. Psychiatry 48, 268–274. [PubMed] [Google Scholar]
- Killgore WD, Vanuk JR, Dailey NS, 2022. Treatment with morning blue light increases left amygdala volume and sleep duration among individuals with posttraumatic stress disorder. Front. Behav. Neurosci 16, 910239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Erickson TM, Giardino ND, Favorite T, Rauch SA, Robinson E, Kulkarni M, Liberzon I, 2013. A pilot study of group mindfulness-based cognitive therapy (MBCT) for combat veterans with posttraumatic stress disorder (PTSD). Depress. Anxiety 30, 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe AS, Ashokan A, Mitra R, 2016. Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl. Psychiatry 6 e729–e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral TR, Davis K, Korponay C, Hirshberg MJ, Hoel R, Tello LY, Goldman RI, Rosenkranz MA, Lutz A, Davidson RJ, 2022. Absence of structural brain changes from mindfulness-based stress reduction: two combined randomized controlled trials. Sci. Adv 8, eabk3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S, 2015. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet 62222–62224. S0140–6736. [DOI] [PubMed] [Google Scholar]
- Kwak S, Lee TY, Jung WH, Hur JW, Bae D, Hwang WJ, Cho KIK, Lim KO, Kim SY, Park HY, 2019. The immediate and sustained positive effects of meditation on resilience are mediated by changes in the resting brain. Front. Hum. Neurosci 13, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugharne J, Kullack C, Lee CW, McGuire T, Brockman S, Drummond PD, Starkstein S, 2016. Amygdala volumetric change following psychotherapy for posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci 28, 312–318. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B, 2005. Meditation experience is associated with increased cortical thickness. Neuroreport 16, 1893–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J., 2007. The amygdala. Curr. Biol 17, R868–R874. [DOI] [PubMed] [Google Scholar]
- Lindstrom MJ, Bates DM, 1988. Newton—Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J. Am. Stat. Assoc 83, 1014–1022. [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Séguin JR, 2011. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl. Acad. Sci. U. S. A 108, 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD, 2004. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J. Consult. Clin. Psychol 72, 31–40. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, Samuel S, Viding E, 2013. Amygdala activation in maltreated children during preattentive emotional processing. Br. J. Psychiatry 202, 269–276. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ, 2009. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J. Child Psychol. Psychiatry 50, 943–951. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S, 2005. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A 102, 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ, 1998. Conscious and unconscious emotional learning in the human amygdala. Nature 393, 467–470. [DOI] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A, 2012. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169, 141–151. [DOI] [PubMed] [Google Scholar]
- Narvacan K, Treit S, Camicioli R, Martin W, Beaulieu C, 2017. Evolution of deep gray matter volume across the human lifespan. Hum. Brain Mapp 38, 3771–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Scott RD, Bhutta ZA, Harris NB, Danese A, Samara M, 2020. Adversity in childhood is linked to mental and physical health throughout life. BMJ 371, m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr, Weiss PM, Dunner DL, 2003. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc. Natl. Acad. Sci. U. S. A 100, 14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska-Guizzo A, Sia A, Fogel A, Ho R, 2022. Features of urban green spaces associated with positive emotions, mindfulness and relaxation. Sci. Rep 12, 20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Lamis DA, Bagge CL, Freedenthal S, Barnes SM, 2016. The mindful attention awareness scale: further examination of dimensionality, reliability, and concurrent validity estimates. J. Pers. Assess 98, 189–199. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH, 2014. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 97, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickut BA, Van Hecke W, Kerckhofs E, Mariën P, Vanneste S, Cras P, Parizel PM, 2013. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: a randomized controlled longitudinal trial. Clin. Neurol. Neurosurg 115, 2419–2425. [DOI] [PubMed] [Google Scholar]
- Reuter M, Fischl B, 2011. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 57, 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Scholz J, Jenkinson M, Thomas AG, Filippini N, Smit G, Douaud G, Johansen-Berg H, 2014. Gray matter volume is associated with rate of subsequent skill learning after a long term training intervention. Neuroimage 96, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJ, Boyd E, Reuter M, Stevens A, Van Leemput K, McKee A, Frosch MP, 2017. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage 155, 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner JD, 2003. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. Neuroimaging 122, 193–198. [DOI] [PubMed] [Google Scholar]
- Sevinc G, Hölzel BK, Greenberg J, Gard T, Brunsch V, Hashmi JA, Vangel M, Orr SP, Milad MR, Lazar SW, 2019. Strengthened hippocampal circuits underlie enhanced retrieval of extinguished fear memories following mindfulness training. Biol. Psychiatry 86, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gonzalez-Reigosa F, Martinez-Urrutia A, Natalicio LF, Natalicio DS, 1971. The state-trait anxiety inventory. Rev. Interam. Psicol. Interam. J. Psychol 5, 3–4. [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA, 2000. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J. Consult. Clin. Psychol 68, 615–623. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM, 2003. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev 27, 33–44. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Parigger A, 2015. The ‘Maltreatment and Abuse Chronology of Exposure’(MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One 10, e0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2013. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 170, 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17, 652–666. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE, 2006. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am. J. Psychiatry 163, 993–1000. [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K, 2014. Mediation: R package for causal mediation analysis. J. Stat. Softw 59, 1–38.26917999 [Google Scholar]
- Tottenham N, Sheridan MA, 2010. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H, 2012. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One 7, e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo Z, Amaro H, 2009. Adaptation of mindfulness-based stress reduction program for addiction relapse prevention. Humanist. Psychol 37, 192–206. [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B, 2008. Brain morphometry with multiecho MPRAGE. Neuroimage 40, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Oei NY, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SA, 2015. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res. Neuroimaging 233, 436–442. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD, 2006. Hippocampal and amygdalar volumes in dissociative identity disorder. Am. J. Psychiatry 163, 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BS, Chattarji S, 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci 22, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, 2017. Severe stress and the development of the amygdala in youth: a theory and its statistical implications. Dev. Rev 46, 44–53. [Google Scholar]
- Weissman DG, Lambert HK, Rodman AM, Peverill M, Sheridan MA, McLaughlin KA, 2020. Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depress. Anxiety 37, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, Hackmann A, Krusche A, Muse K, Von Rohr IR, Shah D, Crane RS, Eames C, Jones M, Radford S, Silverton S, Sun Y, Weatherley-Jones E, Whitaker CJ, Russell D, Russell IT, 2014. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J. Consult. Clin. Psychol 82, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Barrós-Loscertales A, Li M, Pinazo D, Borchardt V, Ávila C, Walter M, 2019. Alterations in brain structure and amplitude of low-frequency after 8 weeks of mindfulness meditation training in meditation-naïve subjects. Sci. Rep 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB, 2009. Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youyou W, Yang Y, Uzzi B, 2023. A discipline-wide investigation of the replicability of psychology papers over the past two decades. Proc. Natl. Acad. Sci. U. S. A 120, e2208863120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Li B, 2022. Statistical Methods for mediation, Confounding and Moderation Analysis Using R and SAS. CRC Press. [Google Scholar]