Abstract
Introduction:
Point-of-care ultrasound is becoming increasingly popular, and we sought to examine its role in evaluating ocular and periocular structures and facial vasculature. With the large number of point-of-care ultrasound devices available, it is difficult to determine which devices may be best suited for ophthalmic and facial aesthetic applications. This study compares five popular handheld point-of-care ultrasound devices to help guide clinicians in choosing the device best suited for their needs.
Methods:
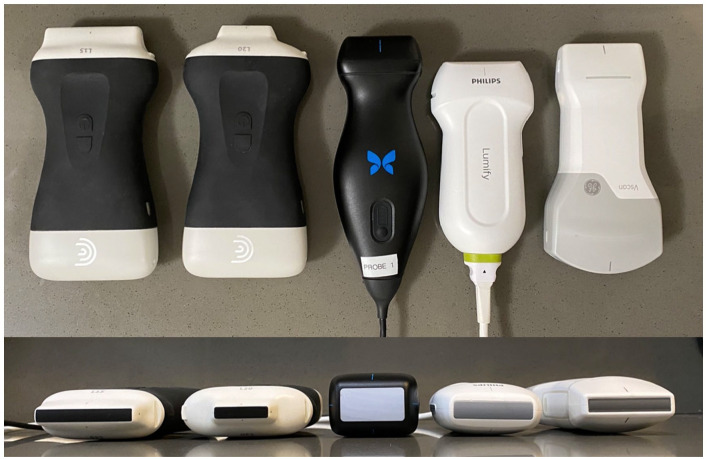
We compared five point-of-care ultrasound devices: Butterfly IQ+ (Butterfly, Burlington, MA), L15 (Clarius Mobile Health, Vancouver, British Columbia, Canada), L20 (Clarius Mobile Health, Vancouver, British Columbia, Canada), Lumify (Philips, Amsterdam, Netherlands) and Vscan Air (GE, Boston, MA). Three ophthalmologists obtained the following views on three volunteers: eight arteries, four ocular and periocular structures and areas of filler injections. The image quality of each view was graded on a four-point Likert-type scale. In addition, graders filled out a survey. The data were analysed using analysis of variance tests with the significance level set to p < 0.05.
Results:
In terms of overall image quality, the L20 received the highest mean rating, followed by the L15, Vscan Air, Butterfly IQ+ and the Lumify (p < 0.05). With further stratification for structure type, the L20 was ranked first for filler, artery and orbital imaging (p < 0.05).
Conclusions:
The L20 received the highest image quality rankings. While image quality is an important aspect of point-of-care ultrasound device selection, other factors such as cost, wireless capabilities, range of presets and battery life should also be considered.
Keywords: Handheld ultrasound, point-of-care ultrasound, ultrasound-guided filler injection, Butterfly IQ+, Clarius, Vscan Air, Lumify
Introduction
Point-of-care ultrasound (POCUS) devices have become increasingly popular in a wide range of healthcare specialties in recent years. Their portability, accessibility and lack of radiation have made them especially attractive among available imaging modalities.
In the field of ophthalmology, ultrasound is useful for a variety of applications, including A-scans, B-scans and ultrasound biomicroscopy (UBM) to evaluate for intraocular pathologies such as retinal detachments, tumours, vitreous inflammation, intraocular foreign bodies and extraocular pathologies such as giant cell arteritis. 1 POCUS has become a valuable tool in the emergency room in the evaluation of retinal or vitreous detachments given its diagnostic reliability. 2
Injectable fillers have become a mainstay procedure in facial aesthetics but can unfortunately result in infrequent but severe adverse effects. These adverse effects include ischaemia or vision loss, which can occur through vascular compression by extravascular filler or direct injection into vessels. 3 Visualisation of a patient’s vascular anatomy during filler injection has been shown to have utility in preventing adverse effects, and ultrasound-guided filler injections can help guide safe and appropriate filler placement.4 –7
While multiple studies have found reasonable agreement between POCUS devices and standard ultrasound machines in terms of clinical outcomes and image quality, none of these studies included ocular or aesthetic imaging.8 –10 Furthermore, there is limited research comparing the different POCUS devices to each other. The authors found only two studies doing so, with one study comparing three devices for gynaecological imaging while another compared four devices with three non-ophthalmological views: right upper quadrant focused assessment with sonography, transverse view of the neck with the internal jugular vein and carotid artery, and the parasternal long-axis view of the heart.11,12 We found only one abstract pertaining to the use of POCUS devices in ophthalmology that compared the Butterfly IQ+ to the Accutome B-Scan Pro and found no differences in overall image quality between the two. 13 There is unfortunately a lack of research on the application of these devices in ophthalmology, where visualising extremely small structures pose a significant challenge.
Our aim was to compare five commercial poses devices specifically regarding ophthalmological and facial aesthetic applications, fields in which there is utility in expanded usage of POCUS devices. We feel that this area of research is needed to help guide ophthalmologists and aesthetic clinicians towards POCUS devices that are best suited for their practices, whether that may be an aesthetics-focused clinic, emergency room or resource-limited area.
Materials and methods
The five commercially available ultrasound devices included in this study are the Butterfly IQ+ (Butterfly, Burlington, MA), L15 (Clarius Mobile Health, Vancouver, British Columbia, Canada), L20 (Clarius Mobile Health, Vancouver, British Columbia, Canada), Lumify (Philips, Amsterdam, Netherlands) and Vscan Air (GE, Boston, MA). These devices were selected based on their availability and frequency of mention in this literature.
All five devices were loaned from the manufacturers or other hospital departments at our request. No financial incentives were offered by the manufacturers. All devices were within their warranty periods; the Butterfly IQ+ was new, the L15 and L20 were less than a year old and the Vscan and Lumify were less than 2 years old. The Butterfly IQ+ was connected to an Apple iPad with a cable; the L15, L20 and Vscan Air were connected to an Apple iPad wirelessly; and the Lumify was connected to a Samsung Galaxy S6 with a cable.
All five devices were tested by three ophthalmologists on three volunteers. The volunteers were female and were, on average, 45 years old. For two of the volunteers, videos and images were taken in the areas of filler injections prior to, during and after the injections. All devices were set to the vascular preset with or without the colour-flow Doppler activated, with the exception of the L15 and L20, which were set to the ‘Aesthetic’ preset. In addition, 12 structures were evaluated on the right side of the face of one volunteer. Eight arteries were included: zygomaticotemporal artery, zygomaticofacial artery, supraorbital artery, supratrochlear artery, infraorbital artery, dorsal nasal artery, angular artery at the nasolabial fold and facial artery at the jaw. Four orbital and periorbital structures were also included: retina, meibomian glands, lacrimal gland, and orbicularis oculi muscle. Images and videos of the arteries of interest were obtained using the vascular preset with colour-flow Doppler activated. For the orbital and periorbital structures, the devices were set to their respective ophthalmic settings: ‘Ophthalmic’ for the Butterfly IQ+ and Vscan Air, ‘Ocular’ for the L15 and L20, and ‘Superficial’ for the Lumify. While obtaining images and videos, the three physicians graded the image quality of each view on a four-point Likert-type scale: excellent (4), good (3), poor (2) and not visible (1). Grades were kept confidential to minimise bias among the other graders.
In addition, the three graders filled out a survey immediately after trialling the devices. The survey was modelled after the one created by Le et al. 11 and graded each device in three categories: ease of use, image quality and overall satisfaction. Ease of use statements inquired about physical characteristics, image quality, manoeuvrability and overall satisfaction. Image quality statements inquired about detail resolution, contrast resolution, clutter and overall satisfaction. Graders rated their agreement with each statement using a five-point Likert-type scale (1 indicating ‘strongly disagree’ or ‘very dissatisfied’ and 5 indicating ‘strongly agree’ or ‘very satisfied’). In addition, qualitative feedback for each device was gathered.
The image quality score data were analysed using analysis of variance (ANOVA) tests with the significance level set to p < 0.05. Free responses were compiled and sorted. This study was approved by the University of Southern California Institutional Review Board. All patients provided written informed consent for the acquisition and publication of images.
Results
Table 1 describes image quality rankings and scores for each of the devices. In terms of overall image quality, the L20 received the highest mean rating, followed by the L15, Vscan Air, Butterfly IQ+ and the Lumify (p < 0.05). When further stratified by category of structures, the L20 was ranked first for filler, artery and orbital imaging (p < 0.05). While the L15 was ranked second for filler imaging, it was tied at last for arterial imaging.
Table 1.
Device rankings by image quality scores.
| Filler image quality ranking* | Mean score a (n = 7) | Artery image quality ranking* | Mean score a (n = 8) | Orbital image quality ranking* | Mean score a (n = 4) | Overall image quality ranking* | Mean score a (n = 19) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Clarius L20 | 3.79 | Clarius L20 | 2.79 | Clarius L20 | 2.83 | Clarius L20 | 3.15 |
| 2 | Clarius L15 | 3.32 | GE Vscan Air | 1.88 | GE Vscan Air | 2.50 | Clarius L15 | 2.24 |
| 3 | GE Vscan Air | 2.21 | Butterfly IQ+ | 1.79 | Philips Lumify (tie) | 2.08 | GE Vscan Air | 2.13 |
| 4 | Butterfly IQ+ | 2.05 | Philips Lumify (tie) | 1.46 | Clarius L15 (tie) | 2.08 | Butterfly IQ+ | 1.87 |
| 5 | Philips Lumify | 1.68 | Clarius L15 (tie) | 1.46 | Butterfly IQ+ | 1.75 | Philips Lumify | 1.67 |
Scores were ranked from 1 (not visible) to 4 (excellent).
p < 0.05 based on ANOVA test.
Table 2 compares key features of the devices. All are small, handheld devices that weigh less than 0.5 kg and can easily be transported. They are all compatible with a wide variety of iOS and Android devices. The Butterfly IQ+ and Lumify are wired devices while the Vscan Air, L15 and L20 are wireless. While the Vscan Air, L15 and L20 all have battery lives under an hour, the Butterfly IQ+ can scan for over 2 hours, and the Lumify can scan for the length of the battery life of the connected device. Finally, while the Vscan Air has two probes on either end and the Butterfly IQ+ has one probe that functions as a linear, curvilinear or phased probe depending on the setting, the L15, L20 and Lumify only have a linear probe, and to access other probe types, a separate device must be purchased.
Table 2.
Comparison of devices.
| GE Vscan Air | Clarius L20 | Clarius L15 | Butterfly IQ+ | Philips Lumify L12-4 | |
|---|---|---|---|---|---|
| Physical characteristics | |||||
| Probe dimensions (mm) | 131 × 64 × 40 | 147 × 76 × 32 | 147 × 76 × 32 | 163 × 56 × 35 | 114 × 45 |
| Weight (g) | 205 | 290 | 290 | 309 | 108 (without cable) |
| Number of probes | (2) Linear and curved | (1) Linear | (1) Linear | (1) Linear / curvilinear / phased | (1) Linear |
| Battery life (minutes) | 50 | 60 | 60 | 144 | Powered by device it is connected to |
| Device compatibility | iOS, Android | iOS, Android | iOS, Android | iOS, Android | iOS, Android (but certain features only available with Android devices) |
| Technology | |||||
| Transducer technology | Piezoelectric crystal | Piezoelectric crystal | Piezoelectric crystal | Silicon chip containing a 2D array of 9000 capacitive micromachined ultrasound transducers | Piezoelectric crystal |
| Ultrasound frequency (MHz) | Curved: 2–5 Linear: 3–12 |
8–20 | 5–15 | 1–10 | 4–12 |
| Min–Max depth (cm) | Curved: 2–8 Linear: 5–24 |
0.5–4 | 7 (max) | 1–30 | 1–12 |
| Presets | Curved: Abdominal, Cardiac, OB/GYN, Vascular, MSK, Lung Linear: Vascular, MSK, Nerves, Lung, Small Parts, Ophthalmic, Neo Head |
Aesthetics, Dermatology, Lung, MSK, Nerve/Pain, Ocular, Plastic Surgery, Small Organs, Vascular | Aesthetics, Arterial, Breast, Carotid, Dermatology, Diagnostic Breast, Elbow, Foot/Ankle, Hand/Wrist, Interventional Breast, Knee, Lung, MSK, Nerve/Pain, Ocular, Plantar, Plastic Surgery, Shoulder, Small Organs, Thyroid, Vascular, Venous | Abdomen, Abdomen Deep, Aorta and Gallbladder, Bladder, Cardiac, Cardiac Deep, Coherence Imaging, FAST, Lung, Lung Tissue, MSK-Soft Tissue, MSK, Nerve, OB 1/GYN, OB 2/3, Ophthalmic, Paediatric Abdomen, Paediatric Cardiac, Paediatric Lung, Small Organ, Vascular: Access, Vascular: Carotid, Vascular: Deep Vein, Vascular: Face | Lung, MSK, Soft Tissue, Superficial, Vascular |
| Pulsed-wave Doppler availability | No | Only withmembership | Only with membership | Only with membership | Yes |
| Purchase details | |||||
| Cost (US$) | 4765 (no membership offered) | 5395 + 595 annual membership fee or 6900 one-time purchase | 3395 + 595 annual membership fee or 4900 one-time purchase | 2399 + 420 annual membership fee | 6000 (no membership offered) |
| Subscription required? | No | No (but additional features included with membership) | No (but additional features included with membership) | Yes | No |
| Standard warranty period (years) | 3 | 3 | 3 | 1 | 5 |
OB: obstetrics; GYN: gynaecology; MSK: musculoskeletal; FAST: focused assessment with sonography for trauma.
It should be noted that all the devices had specific ophthalmic presets available other than the Lumify, which only offers a superficial or soft tissue setting. All devices had vascular presets available. The L20 and L15 have an additional Aesthetics preset available with the purchase of a membership, and the Butterfly IQ+ has a ‘Needle Viz’ tool that is optimised for needle visualisation. It was noted that the Lumify had a large amount of visual noise when colour-flow Doppler was activated.
Discussion
In ophthalmology, ultrasound devices are used for imaging of the anterior segment with UBM, A-scanning for intraocular lens calculations in cataract surgery and A- and B-scanning for ocular tumour evaluations. The eyelid and periorbital structures can also be evaluated. Emergency room physicians commonly use ultrasound to identify retinal detachments. With the rise in filler injections, ultrasound has also found a new utility in guiding injections to prevent ischemic complications.
Although the L20 received the highest ratings for orbital structure image quality (p < 0.05), we found that all five devices were able to sufficiently visualise the orbital structures, which included the retinal B-scan, orbicularis oculi muscle and lacrimal gland, with the exception of the much smaller meibomian glands, which range from 2.0 to 5.5 mm in length (Figure 1). 14 We were especially interested in the Butterfly IQ+, as it utilises a unique imaging technology that uses a single silicon chip containing 9000 capacitive micromachined ultrasound transducers instead of the traditional crystal-based transducer, which the other four devices use. This method decreases cost and allows its single probe to be used as a linear, curved and phased transducer. However, it received the second-lowest image quality rating in our study and the lowest rating in the study by Le et al. 11 Nevertheless, it may be a viable option for those who already have the device and are interested in using it for orbital imaging, as it was able to sufficiently visualise the orbicularis oculi, lacrimal gland and retina B-scan.
Figure 1.
B-scans taken from each device: (a) Butterfly IQ+ (Butterfly), (b) L15 (Clarius), (c) L20 (Clarius), (d) Lumify (Philips) and (e) Vscan Air (GE).
For those whose practices are more aesthetics-focused, the L20 may be the most suitable device. For visualisation of the facial arteries, the L20 received a mean score of 2.8 out of 4, while the other four devices all received scores under 2.0 (p < 0.05). It has the highest range of ultrasound frequencies of the devices tested, enabling higher resolution of superficial structures. In addition, an aesthetics package that is specifically designed to be used for facial injections is included for both the L15 and L20 with the purchase of a membership. As image quality seems to be the most important factor in evaluating ultrasound devices, the L20 may be superior in this regard. 11 Of note, all five devices were able to visualise larger arteries such as the angular and facial arteries (Figure 2). Smaller arteries, on the contrary, may depend more on patient facial anatomy and user skill. The L15, in particular, was not highly rated for visualisation of arterial or orbital structures, mainly because of its large probe, which was difficult to place onto the curvatures of the face (Figures 3 and 4).
Figure 2.
Supratrochlear artery as viewed by each device: (a) Butterfly IQ+ (Butterfly), (b) L15 (Clarius), (c) L20 (Clarius), (d) Lumify (Philips) and (e) Vscan Air (GE).
Figure 3.
Placement of each device on the eye for a B-scan: (a) Butterfly IQ+ (Butterfly), (b) L15 (Clarius), (c) L20 (Clarius), (d) Lumify (Philips) and (e) Vscan Air (GE).
Figure 4.
Each device is shown from a bird-eye and lateral view. From left to right: Butterfly IQ+ (Butterfly), L15 (Clarius), L20 (Clarius), Lumify (Philips) and Vscan Air (GE).
With the ever-increasing popularity of filler injections, having access to a reliable ultrasound device is a valuable tool, and the relative affordability of POCUS devices may allow more clinicians to purchase them. In addition to allowing the clinician to avoid injecting filler intravascularly, ultrasound can similarly aid clinicians in the case of adverse events caused by fillers. When adverse events such as vascular occlusion occur during filler injection, having access to an ultrasound device can reliably and clearly identify the vessel(s) involved is important in helping the clinician properly diagnose ischaemia and address the event.7,15
As for other characteristics, all are similarly sized, connect to both Android and iOS devices and can be used for a variety of presets, allowing various teams or departments to share devices, if needed. Battery life is much higher for the Butterfly IQ+ and Lumify than the other three devices, which can only scan for about 1 hour (Table 2). In addition, all of the devices heated up with prolonged use; the L15 and L20 software is programmed to automatically shut down to avoid overheating. This may warrant consideration for busy practices where multiple clinicians may be sharing devices or where there is not enough downtime to allow the devices to recharge and cool down between uses. Finally, while the wireless nature of the L15, L20 and Vscan Air make the device more compact and portable, it also caused noticeably longer pairing times with the connecting device, and the graders reported some delays with establishing connectivity.
Further barriers to use include cost and training. Retail prices of these devices range from US $2399 for the Butterfly IQ+ to US $6900 for the L20 (Table 2). Annual membership fees should also be accounted for: the Vscan Air and Lumify do not offer associated memberships, the Butterfly IQ+ requires a membership and the L20 and L15 offer memberships that grant additional features but do not require them. Warranty ranges from 1 year for the Butterfly IQ+ to 5 years for the Lumify, and repair services are offered by all manufacturers (Table 2). Quality assurance tests are not offered by any of the manufacturers other than the Butterfly IQ+, which can be performed anytime through their application. In addition, although these devices come with training courses and educational resources, further comprehensive ultrasound training may be helpful in accessing these devices’ full potentials. 16
For safety reasons, users should be mindful that the maximum levels for thermal index, mechanical index and spatial-peak temporal average determined by the US Food and Drug Administration are lower for ophthalmic scanning due to differences in the way the eye experiences ultrasound’s thermal effects. For reference, thermal index should be ⩽ 1, spatial-peak temporal average ⩽ 50 mW/cm2 and mechanical index ⩽ 0.23. 17 Although the L15, L20 and Vscan Air have these limits programmed into their respective ophthalmic presets, the Lumify and Butterfly IQ+ do not, and the user is responsible for ensuring that the limits are not exceeded. For the Lumify, which does not have an ophthalmic preset but only a ‘Superficial’ preset, its use in the ocular area is considered an off-label use. As a best practice for all these devices, clinicians should take care to limit exposure time and note these indices, which are shown onscreen for all devices. 18
Our study has several limitations. There were only three graders and three volunteers, which limited the data that could be statistically analysed, such as image resolution and contrast grades. In addition, it was impossible to blind graders to which device they were testing at any given time. None of the graders had previously used any of the devices tested or received extensive training prior to testing the devices, which may have resulted in different experiences than that of someone who is very familiar with them. However, it does ensure that none of the graders had pre-existing biases towards or against any particular devices and also helps us present how these devices are experienced by new users. Although performance should not vary significantly between units, of, we only tested one unit per model in this study. Finally, there is a lack of data from objective tests performed on test phantoms available from the manufacturers, which would have been interesting to compare.
In conclusion, it is important for ophthalmologists and aesthetic clinicians to continue to adopt the rapidly evolving technology in the field of ultrasound to enhance clinical practice. In our study, the Clarius L20 received the highest image quality rankings overall, as well as for filler, orbital and arterial imaging. While image quality is an important aspect of POCUS device selection, other factors such as cost, wireless capability, range of presets and battery life should also be considered to find the device best tailored to one’s needs. Future studies regarding ultrasound applications to the ophthalmology and aesthetic fields will continue to be necessary as new technologies emerge.
Acknowledgments
The authors acknowledge the unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, New York, NY.
Footnotes
Contributors: KEP and SZ-N conceived the study. KEP conducted a literature review, wrote the initial draft of the manuscript and conducted data analysis. KEP, AOP, PM, CT and SZ-N collected data. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: University of Southern California Institutional Review Board.
Informed consent: Written informed consent was obtained from the patient for publishing the case details and images.
Guarantor: Dr. Sandy Zhang-Nunes.
ORCID iD: Sandy Zhang-Nunes  https://orcid.org/0000-0001-5435-3424
https://orcid.org/0000-0001-5435-3424
References
- 1. Silverman R. Focused ultrasound in ophthalmology. Clin Ophthalmol 2016; 10: 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottlieb M, Holladay D, Peksa GD. Point-of-care ocular ultrasound for the diagnosis of retinal detachment: a systematic review and meta-analysis. Acad Emerg Med 2019; 26: 931–939. [DOI] [PubMed] [Google Scholar]
- 3. Cohen BE, Bashey S, Wysong A. The use of hyaluronidase in cosmetic dermatology: a review of the literature. J Clin Investig Dermatol 2015; 3: 7. [Google Scholar]
- 4. Rocha PS, Guerra TA, Teixeira DA. Description of a safe Doppler ultrasound-guided technique for hyaluronic acid filler in the face – a method to avoid adverse vascular events. J Cosmet Dermatol 2022; 21: 2783–2787. [DOI] [PubMed] [Google Scholar]
- 5. Huang YL, Chang SL, Cheng CY. Two-step, imaging-device–guided, precise filler-injection technique. J Am Acad Dermatol 2020; 83: e119–e120. [DOI] [PubMed] [Google Scholar]
- 6. Schelke LW, Cassuto D, Velthuis P, et al. Nomenclature proposal for the sonographic description and reporting of soft tissue fillers. J Cosmet Dermatol 2020; 19: 282–288. [DOI] [PubMed] [Google Scholar]
- 7. Mehta P, Kaplan JB, Zhang-Nunes S. Ischemic complications of dermal fillers. Plast Aesthetic Res 2022; 9: 57. [Google Scholar]
- 8. Jung EM, Dinkel J, Verloh N, et al. Wireless point-of-care ultrasound: first experiences with a new generation handheld device. Clin Hemorheol Microcirc 2021; 79: 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newhouse SM, Effing TW, Dougherty BD, et al. Is bigger really better? Comparison of ultraportable handheld ultrasound with standard point-of-care ultrasound for evaluating safe site identification and image quality prior to pleurocentesis. Respiration 2020; 99: 325–332. [DOI] [PubMed] [Google Scholar]
- 10. Rykkje A, Carlsen JF, Nielsen MB. Hand-held ultrasound devices compared with high-end ultrasound systems: a systematic review. Diagnostics 2019; 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le MPT, Voigt L, Nathanson R, et al. Comparison of four handheld point-of-care ultrasound devices by expert users. Ultrasound J 2022; 14: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toscano M, Szlachetka K, Whaley N, et al. Evaluating sensitivity and specificity of handheld point-of-care ultrasound testing for gynecologic pathology: a pilot study for use in low resource settings. BMC Med Imaging 2020; 20: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dzihic E, To JK, Horton D, et al. Clinical comparison of ophthalmic ultrasound with a portable multipurpose ultrasound. Invest Ophthal Vis Sci 2021; 62: 2307. [Google Scholar]
- 14. Greiner JV, Glonek T, Korb DR, et al. Volume of the human and rabbit meibomian gland system. In: Sullivan DA, Dartt DA, Meneray MA. (eds) Lacrimal gland, tear film, and dry eye syndromes 2. Vol. 438 (Advances in experimental medicine and biology). New York: Springer US, 1998, pp. 339–343. [DOI] [PubMed] [Google Scholar]
- 15. Schelke LW, Velthuis P, Kadouch J, et al. Early ultrasound for diagnosis and treatment of vascular adverse events with hyaluronic acid fillers. J Am Acad Dermatol 2023; 88: 79–85. [DOI] [PubMed] [Google Scholar]
- 16. Baribeau Y, Sharkey A, Chaudhary O, et al. Handheld point-of-care ultrasound probes: the new generation of POCUS. J Cardiothorac Vasc Anesth 2020; 34: 3139–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Food and Drug Administration. Marketing clearance of diagnostic ultrasound systems and transducers, 2019, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/marketing-clearance-diagnostic-ultrasound-systems-and-transducers
- 18. Harris GR. Safety considerations for diagnostic ultrasound in the eye: editorial. J Ultrasound Med 2019; 38: 1163–1165. [DOI] [PubMed] [Google Scholar]