Abstract
Background
Serum gamma-glutamyltransferase (GGT) activity has been proposed as a promising predictor of atherosclerosis-related complications and a prognostic marker for cardiovascular diseases. The objective of this study was to investigate the potential correlation between serum levels of GGT and early-onset coronary artery disease (EOCAD).
Methods
A retrospective, hospital-based case-control study was conducted, which included 860 patients with EOCAD and gender- and age-matched controls. Serum levels of GGT were measured using the reference measurement procedure on an automatic biochemistry analyser.
Results
The serum GGT levels of patients with EOCAD (34.90 ± 31.44 U/L) were significantly higher than those of the control group (21.57 ± 16.44 U/L, p < .001). Elevated serum levels of GGT were found to be an independent risk factor for EOCAD, with an odds ratio (OR) of 1.021 (95% confidence interval (CI): 1.014–1.029). Additionally, for every quartile increase in serum GGT levels, the risk of developing EOCAD increased by 1.6-fold. Moreover, serum GGT levels were significantly associated with disease severity, with lower GGT levels observed in patients without significant vascular disease (31.74 ± 24.06 U/L) compared to those with two-vessel disease (33.06 ± 25.00 U/L, p = .002) and three-vessel disease (37.75 ± 36.76 U/L, p = .001).
Conclusions
The results of this study suggest that elevated serum GGT levels are associated with the development of EOCAD, and GGT may be implicated in the pathogenesis of the disease. Further large-scale prospective studies are needed to explore the potential relationship between serum GGT levels and the dynamic development of EOCAD.
Keywords: Gamma-glutamyltransferase, cardiovascular disease, early-onset, coronary artery disease
Introduction
Gamma-glutamyltransferase (GGT) is located in the proximal renal tubule, liver, pancreas and intestine. Although present in the cytoplasm, the majority of the enzyme is located within the cell membrane, where it can transport amino acids and peptides as γ-glutamyl peptides across the cell membrane. GGT plays a critical role in maintaining appropriate intracellular concentrations of reduced glutathione (GSH) [1].
Serum GGT activity is primarily produced by the liver, where it predominantly localizes to the biliary pole of the hepatocyte. The joint analysis of GGT and alkaline phosphatase has become a common diagnostic tool for hepatobiliary-related diseases, particularly alcoholic liver disease. Nonetheless, the relationship between serum levels of GGT and other diseases have been widely investigated in recent decades [2–4].
A well-established correlation exists between serum levels of GGT and cardiovascular diseases (CVDs) risk factors, such as body mass index (BMI), hypertension, type 2 diabetes, glucose intolerance and blood lipids [5–7]. Furthermore, as a promoter of oxidative stress and lipid peroxidation, GGT may potentially contribute to the pathogenesis of atherosclerosis [8]. Intense GGT activity has been detected in the intimal layers of atherosclerotic plaques, and catalytically active GGT has also been found in microthrombi that adhere to the surface of the plaque [9]. GGT has been extensively studied in relation to coronary artery disease (CAD). Numerous population-based studies have established a positive correlation between serum GGT levels and CAD, highlighting it as a risk factor for CVD mortality [10,11]. Additionally, normal-range GGT levels have been strongly linked to all-cause mortality, especially in the elderly [12,13].
CAD traditionally affects the elderly, but modern lifestyles have increased its incidence among younger populations. Early-onset coronary artery disease (EOCAD) is more commonly associated with genetic factors and a higher prevalence of traditional cardiovascular risk factors [14,15]. Clinically, EOCAD is generally considered more severe than later-onset CAD and more likely to manifest as acute coronary syndromes. Patients diagnosed with EOCAD have a poorer long-term prognosis [16,17]. Thus, recognizing risk factors is crucial for early diagnosis and screening of EOCAD. Despite the potential significance of serum GGT levels in relation to EOCAD, particularly in the Chinese population, studies in this area remains limited. Therefore, the objective of our study was to explore the potential correlation between serum GGT levels and EOCAD.
Materials and methods
Participants
We conducted a retrospective case-control study and recruited all patients who had visited the cardiology clinic at the Affiliated Hospital of Qingdao University between August 2013 and September 2022. The study included 860 patients with CAD who met the following criteria: (i) patients aged younger than 50 years; (ii) diagnosed with CAD via coronary angiography (iii) clinical diagnosis of CAD was based on presenting angina symptoms, cardiac troponin level changes and/or electrocardiographic changes. Exclusion criteria included patients with liver or kidney diseases, tumours, and other severe illnesses that could potentially confound the study outcomes. Additionally, 860 hospital-based controls who were matched for age and gender, had no history of CVD, and whose absence of CVD signs or symptoms was confirmed using electrocardiography (ECG) were also included in the study. The study was conducted in compliance with the ethical standards outlined in the 1975 Declaration of Helsinki and received approval from the Research Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY-WZLL-27918). Because the study was retrospective in nature and the study subjects’ data were anonymized to prevent any potential harm or infringement of their rights, the ethical committee approved a waiver of informed consent.
Clinical parameters
Recorded baseline characteristics included gender, age, BMI, smoking and drinking history, as well as hypertension and diabetes status. Automated oscillometric device was used to measure diastolic and systolic blood pressure twice, 30 min apart, and the average of these readings was calculated. Hypertension was defined as blood pressure readings of 140/90 mmHg or higher, or by an individual’s use of antihypertensive medication. Diagnosis of diabetes mellitus (DM) was based on the criteria established by the American Diabetes Association, and it required venous samples and laboratory testing. The diagnostic criteria for DM in this study were a fasting plasma glucose level of 7.0 mmol/L or higher, a 2-h plasma glucose level of 11.1 mmol/L or higher during a 75-g oral glucose tolerance test, or a glycated haemoglobin (HbA1c) level of 6.5% or higher.
Coronary angiography
Two senior cardiologists independently assessed coronary angiograms while being blinded to the clinical data. A third cardiologist was engaged in resolving any disputes that arose between the two senior cardiologists. To minimize intra-observer variability, assessments were conducted as efficiently as possible.
The coronary angiogram evaluation comprised the left main coronary artery, right coronary artery, left anterior descending and left circumflex. Lesions with a luminal stenosis degree of 50% or greater were deemed significant. Patients were classified into subgroups of single-, double- or triple-vessel disease based on the number of significantly stenosed vessels. The Gensini score is a tool that provides useful information regarding the severity of vascular lesions, including both the degree and location of arterial stenosis. For each patient, the severity of coronary stenosis was evaluated from the coronary angiogram using a scoring method that considered both the degree of luminal narrowing and geographic location in the coronary artery tree [18]. Specifically, stenosis was quantitatively assessed by assigning scores of 1, 2, 4, 8, 16 or 32 for luminal narrowings of 0–25%, 25–50%, 50–75%, 75–90%, 90–99% or 99–100%, respectively. To account for the influence of the location of the lesion, each score was then multiplied by its corresponding geographical importance score ranging from 0.5 to 5.
Laboratory measurements
In this study, the fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum creatinine (SCr) and serum alanine aminotransferase (ALT) were quantified using an automatic biochemistry analyser (Hitachi HCP-7600, Chiyoda City, Japan). The serum GGT levels were measured using the reference measurement procedure from the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) [19]. l-γ-Glutamyl-3-carboxy-4-nitroanilide acted as the substrate, glycylglycine acted as the acceptor, and glycylglycine provided buffering. The temperature of the reaction was 37 °C, and the 5-amino-2-nitrobenzoate reaction product was measured at a wavelength of 410 nm.
Statistical analysis
Statistical analyses were conducted using SPSS version 16.0 (IBM Corp., Armonk, NY) and GraphPad Prism Software version 8 (GraphPad Software Inc., La Jolla, CA). We presented continuous variables as mean ± standard deviation (SD) and categorical variables as proportions. Normality of data was assessed using the Kolmogorov–Smirnov test. The Chi-square or Fisher’s exact tests were used to compare categorical variables, while independent sample t-tests were used to compare means between two independent groups. To compare means among three or more independent groups that were not related, one-way analysis of variance (ANOVA) was used. The least significant difference (LSD) method was used for post hoc testing with multiple comparisons. To compare non-normally distributed variables, the Kolmogorov–Smirnov Z test was used for two groups and the Kruskal–Wallis test was used for multiple groups. The strength of linear relationship between two variables was assessed using either Pearson’s correlation coefficient (r) or Spearman’s rank correlation coefficient (rs), based on the normality of the data. Logistic regression analysis was used to investigate the potential association between serum GGT levels and EOCAD, and the results were reported as odds ratios (ORs) with their corresponding 95% confidence intervals (CIs). The statistical significance threshold was set at .05.
Results
For the present study, we recruited 860 patients diagnosed with EOCAD. The mean age of the patients was 43.91 ± 4.64, and 88.60% of the participants were male. These 860 participants were matched with sex and age controls, resulting in a sample size of 1720 individuals. The EOCAD cohort exhibited significant increases in BMI, FBG, TG, TC, LDL-C, SCr and ALT activity/levels compared to the control group. Furthermore, EOCAD participants exhibited higher rates of BMI, hypertension, DM and smoking than the control group. Among the EOCAD patient group, 354 patients were diagnosed with myocardial infarction (MI). The EOCAD patients were categorized as follows: 247 individuals with disease in a single-vessel disease, 210 individuals with double-vessel disease, 187 individuals with triple-vessel disease, and 216 patients with no significant vascular disease. A summary of the clinical characteristics of all participants is provided in Table 1.
Table 1.
Demographic and clinical characteristics of EOCAD patients and controls.
| Variable | CAD (n = 860) | Control (n = 860) | p Value |
|---|---|---|---|
| Gender, male n (%)a | 762 (88.60) | 762 (88.60) | 1.000 |
| Age, yearsb | 43.91 ± 4.64 | 43.91 ± 4.64 | 1.000 |
| BMI (kg/m2)b | 27.54 ± 13.28 | 26.57 ± 19.11 | <.001 |
| Hypertension, n (%)a | 252 (29.30) | 189 (21.97) | .001 |
| Diabetes, n (%)a | 121 (14.07) | 45 (5.23) | <.001 |
| Smoking, n (%)a | 404 (46.98) | 269 (31.28) | <.001 |
| Drinking, n (%)a | 263 (30.58) | 231 (26.86) | .098 |
| FBG, mmol/Lb | 5.82 ± 2.14 | 5.27 ± 1.59 | <.001 |
| TG, mmol/Lb | 2.30 ± 2.76 | 1.43 ± 1.23 | <.001 |
| TC, mmol/Lb | 4.18 ± 1.66 | 4.18 ± 1.15 | .004 |
| HDL-C, mmol/Lb | 1.09 ± 0.28 | 1.37 ± 0.32 | <.001 |
| LDL-C, mmol/Lb | 2.68 ± 1.04 | 2.67 ± 0.72 | <.001 |
| SCr, μmol/Lb | 84.53 ± 25.01 | 81.19 ± 16.85 | .001 |
| ALT, U/Lb | 25.86 ± 10.42 | 20.92 ± 9.98 | <.001 |
| Lp(a), μmol/Lb | 246.37 ± 256.81 | 190.11 ± 213.79 | <.001 |
| Myocardial infarction, n (%) | 354 (41.16) | – | – |
| Severity of CAD | – | – | – |
| Single-vessel disease, n (%) | 247 (28.72) | – | – |
| Double-vessel disease, n (%) | 210 (24.42) | – | – |
| Triple-vessel disease, n (%) | 187 (21.74) | – | – |
| GGT, U/Lb | 34.90 ± 31.44 | 21.57 ± 16.44 | <.001 |
EOCAD: early-onset coronary artery disease; BMI: body mass index; FBG: fasting blood glucose; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SCr: serum creatinine; ALT: alanine aminotransferase; GGT: gamma-glutamyltransferase.
Categorical variables are expressed as percentages. p Values of the categorical variables were calculated by χ2 test.
Continuous variables are expressed as the mean ± SD. p Values of the continuous variables were calculated using independent sample t-test or Mann–Whitney’s U test.
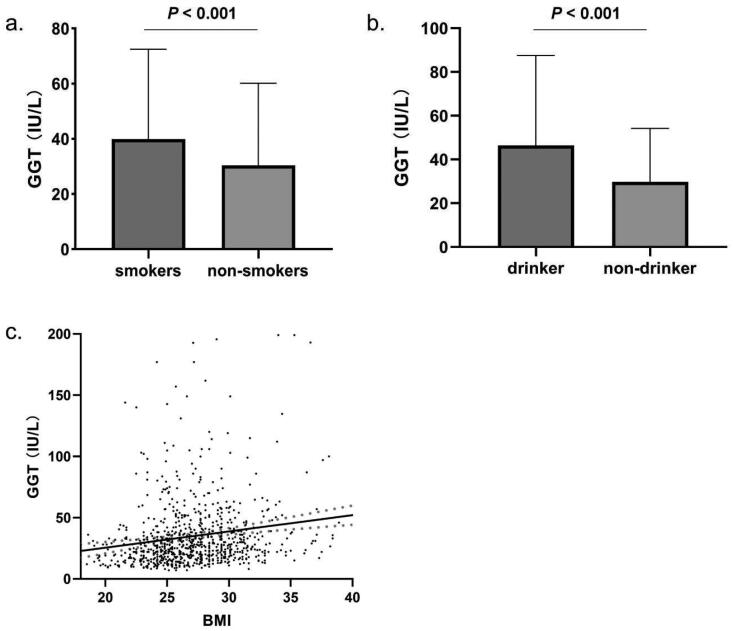
In patients with EOCAD, smoking status significantly increased serum GGT levels (39.91 ± 32.58 U/L) compared to non-smokers (30.45 ± 29.73 U/L, p < .001, Figure 1(a)). Alcohol consumption significantly increased serum GGT levels (p < .001, Figure 1(b)). The mean GGT levels in drinkers were 46.47 ± 41.11 U/L, while the non-drinkers’ group had the mean GGT levels of 29.80 ± 24.41 U/L. Furthermore, a significant positive correlation was observed between BMI and serum GGT levels (p < .001, Figure 1(c)). The serum levels of GGT were significantly correlated with the traditional risk factors for CAD. GGT levels were positively correlated with FBG (rs = 0.171), TG (rs = 0.417), TC (rs = 0.202) and LDL-C (rs = 0.170), while a negative correlation was found between serum levels of GGT and HDL-C (rs = −0.144), with all p values less than .001.
Figure 1.
Factors influencing serum GGT levels in EOCAD patients. (a) Serum GGT levels in patients with smoking status (39.91 ± 32.58 U/L, n = 404) were significantly higher than non-smokers (30.45 ± 29.73 U/L, n = 456, p < .001). (b) Compared with non-drinker EOCAD patients (29.80 ± 24.41 U/L, n = 263), the drinker had higher serum GGT levels (46.47 ± 41.11 U/L, n = 597, p < .001). (c) BMI was positively correlated with serum GGT level (rs = 0.220, n = 860, p < .001).
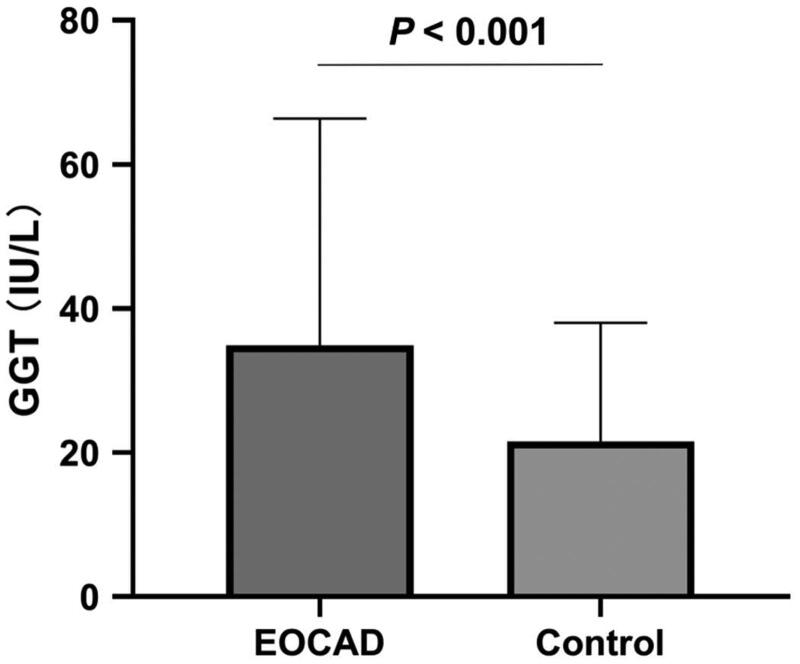
The serum levels of GGT were significantly higher in the EOCAD group (34.90 ± 31.44 U/L) than in the control group (21.57 ± 16.44 U/L) (p < .001, Figure 2). Our binary logistic regression analysis, which adjusted for confounding variables such as gender, age, drinking and smoking status, DM and hypertension status, BMI, TG, TC, LDL-C, HDL-C, ALT and SCr, identified elevated serum GGT levels as an independent risk factor for EOCAD (OR = 1.021, 95% CI: 1.014–1.029). Specifically, the risk of EOCAD increased by 1.021-fold for every one-unit rise in serum GGT levels. The OR adjustment models that included various conventional factors showed comparable results (Table 2).
Figure 2.
Comparison of serum GGT levels between EOCAD patients and controls. Serum GGT levels in EOCAD patients (34.90 ± 31.44 U/L) were significantly increased when compared with the non-EOCAD controls (21.57 ± 16.44 U/L, p < .001).
Table 2.
Associations between serum GGT levels and presence of EOCAD.
| Adjustment models | OR (95% CI) | p | |
|---|---|---|---|
| Model 1 | Crude, no adjustment | – | <.001 |
| Model 2 | Adjusting for age, gender, BMI, smoking, drinking, hypertension and diabetes statues | 1.033 (1.026–1.040) | <.001 |
| Model 3 | Adjusting for FBG, TG, TC, HDL-C, LDL-C, SCr and ALT | 1.020 (1.013–1.027) | <.001 |
| Model 4 | Adjusting for smoking, hypertension, diabetes statues, BMI, FBG, TG, TC, HDL-C, LDL-C, UA and ALT | 1.019 (1.012–1.026) | <.001 |
| Model 5 | Adjusting for cofactors | 1.021 (1.014–1.029) | <.001 |
EOCAD: early-onset coronary artery disease; BMI: body mass index; FBG: fasting blood glucose; LDL-C: low-density lipoprotein cholesterol; UA: uric acid; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; ALT: alanine aminotransferase; TC: total cholesterol; SCr: serum creatinine; GGT: gamma-glutamyltransferase.
The study population was divided into four distinct groups based on their baseline GGT levels, with each group representing one quartile of the distribution. The quartile boundaries were defined as follows: 1st quartile (<11 U/L), 2nd quartile (11–16 U/L), 3rd quartile (16–25 U/L) and 4th quartile (serum GGT levels >25 IU/L). With each increase in serum GGT level by one quartile, the risk of EOCAD increased by more than 1.60-fold (OR = 1.602, 95% CI: 1.406–1.825). The general clinical characteristics for each quartile of GGT at baseline are presented in Table 3.
Table 3.
General clinical characteristics according to quartiles of GGT at baseline.
| Q1 (<11 U/L) | Q2 (11–16 U/L) | Q3 (16–25 U/L) | Q4 (>25 U/L) | p | |
|---|---|---|---|---|---|
| Gender, male n (%)a | 25 (49.02) | 92 (75.41) | 209 (89.32) | 436 (96.25) | <.001 |
| Age, yearsb | 44.59 ± 5.19 | 44.32 ± 4.39 | 43.98 ± 4.78 | 43.69 ± 4.56 | .118 |
| BMI (kg/m2)b | 24.67 ± 2.63 | 26.06 ± 3.22 | 27.07 ± 3.54 | 27.68 ± 3.55 | <.001 |
| Hypertension, n (%)a | 11 (7.28) | 23 (18.85) | 49 (20.94) | 106 (23.40) | .711 |
| Diabetes, n (%)a | 4 (7.84) | 14 (11.48) | 29 (12.39) | 74 (16.34) | .189 |
| Smoking, n (%)a | 7 (13.73) | 41 (33.61) | 100 (42.74) | 256 (56.51) | <.001 |
| Drinking, n (%)a | 6 (11.76) | 25 (20.49) | 43 (17.70) | 189 (41.72) | <.001 |
| FBG, mmol/Lb | 5.52 ± 2.42 | 5.57 ± 1.94 | 5.63 ± 2.06 | 5.88 ± 1.90 | .001 |
| TG, mmol/Lb | 1.39 ± 1.35 | 1.43 ± 1.05 | 1.88 ± 1.99 | 2.86 ± 3.35 | <.001 |
| TC, mmol/Lb | 3.64 ± 1.81 | 3.75 ± 1.41 | 4.09 ± 1.74 | 4.40 ± 1.63 | <.001 |
| HDL-C, mmol/Lb | 1.24 ± 0.28 | 1.15 ± 0.28 | 1.09 ± 0.26 | 1.06 ± 0.27 | <.001 |
| LDL-C, mmol/Lb | 2.38 ± 1.17 | 2.36 ± 1.81 | 2.66 ± 1.11 | 2.82 ± 1.02 | <.001 |
| SCr, μmol/Lb | 78.00 ± 28.65 | 84.73 ± 21.86 | 87.16 ± 33.82 | 83.85 ± 19.44 | .174 |
| ALT, U/Lb | 15.47 ± 8.93 | 20.23 ± 9.64 | 24.28 ± 9.65 | 29.37 ± 6.50 | <.001 |
| Chronic CAD, n (%) | 5 (9.80) | 6 (4.92) | 19 (8.12) | 27 (5.96) | .462 |
| UA, n (%) | 27 (52.94) | 69 (56.56) | 120 (51.28) | 233 (51.43) | .774 |
| MI, n (%) | 19 (37.25) | 47 (38.52) | 95 (40.59) | 193 (42.60) | .778 |
| Single-diseased vessels, n (%) | 13 (25.49) | 42 (38.52) | 67 (28.63) | 125 (27.59) | .48 |
| Double-diseased vessels, n (%) | 7 (13.73) | 23 (18.85) | 60 (25.64) | 120 (26.79) | .092 |
| Triple-diseased vessels, n (%) | 8 (15.69) | 19 (15.57) | 47 (20.09) | 113 (24.94) | .073 |
| Gensini scores | 31.51 ± 34.67 | 39.25 ± 45.19 | 44.88 ± 45.51 | 43.14 ± 39.96 | .046 |
CAD: coronary artery disease; UA: unstable angina; MI: myocardial infarction; BMI: body mass index; FBG: fasting blood glucose; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SCr: serum creatinine; ALT: alanine aminotransferase.
Categorical variables are expressed as percentages. p Values of the categorical variables were calculated by χ2 test.
Continuous variables are expressed as the mean ± SD. p Values of the continuous variables were calculated using Kruskal–Wallis test.
The serum GGT levels in male patients (37.16 ± 32.59 U/L) were significantly higher than those in male control group without CAD (22.19 ± 17.11 U/L, p < .001). Following adjustments for all relevant factors, increased serum levels of GGT was an independent risk factor for EOCAD in males (OR = 1.021, 95% CI: 1.013–1.028). For each increase in serum GGT levels by one quartile, the risk of EOCAD increased by 1.65-fold (OR = 1.650, 95% CI: 1.432–1.901). Although serum GGT levels were lower in the control group (16.81 ± 8.32 U/L) compared to the EOCAD group (17.27 ± 8.17 U/L) in females, the difference was not statistically significant (p = .942).
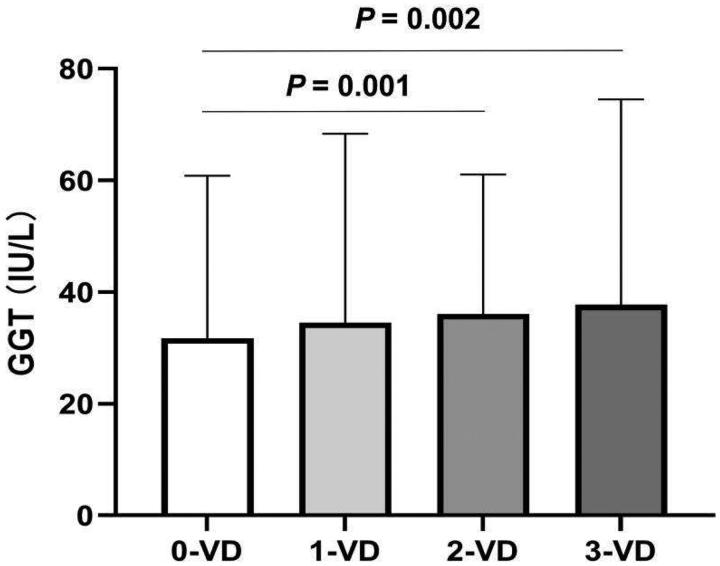
Although serum GGT levels exhibited a gradual increase in the chronic CAD, unstable angina and MI groups, the increase was not statistically significant (p = .246). The mean GGT levels for each group were 27.96 ± 15.64 U/L, 35.23 ± 34.61 U/L and 35.68 ± 28.94 U/L, respectively. The analysis demonstrated that patients with double-vessel (33.06 ± 25.00 U/L, p = .002) and triple-vessel disease (37.75 ± 36.76 U/L, p = .001) had significantly higher serum GGT levels than those without significant diseased vessels (31.74 ± 24.06 U/L, Figure 3). No significant difference in serum GGT levels was observed among the other groups. Serum GGT levels demonstrated a weak correlation with Gensini scores (rs = 0.66, p = .054). The correlation trend between serum GGT levels and the severity of EOCAD is almost entirely derived from males.
Figure 3.
Comparing serum GGT levels according to number of diseased coronary arteries in EOCAD patients. Levels of GGT in the group with 0-vessel disease (31.74 ± 24.06 U/L, n = 216) were significantly lower than those in groups with double-vessel disease (33.06 ± 25.00 U/L, n = 210) and triple-vessel disease (37.75 ± 36.76 U/L, n = 187).
Discussion
In this study, we conducted a comprehensive analysis of the relationship between serum levels of GGT and the risk of EOCAD. The results showed that: (1) the EOCAD group exhibited significantly higher serum levels of GGT compared to the control group, (2) elevated serum GGT levels were independently associated with an increased risk of EOCAD, (3) there was a weak but significant correlation between serum GGT levels and the disease’s severity and (4) serum GGT levels could be affected by several variables, including gender, smoking and drinking status, BMI, DM and hypertension status, as well as blood glucose and lipid levels.
GGT is an enzyme that is present in the kidneys, liver, pancreas, spleen and vascular endothelium of the body [20]. An elevation in its levels can be in response to various liver and biliary tract diseases, alcohol abuse and certain medications. As a result, GGT levels are often measured along with other liver enzymes such as ALT and AST for assessing liver function and detecting possible liver illness.
The correlation between liver dysfunction and dyslipidaemias is receiving increasing attention, reinforcing the emerging paradigm that regards the liver as a potentially novel factor in CVD or as an active contributor to its pathogenesis [21]. Several reports have provided substantial evidence for the heightened risk of CVD in individuals with liver disease [22,23]. Hepatic enzymes, among which GGT is included, function as reliable indicators of hepatic function. It is reasonable to hypothesize that young individuals with elevated GGT activity may exhibit an increased degree of liver dysfunction and CVD, partly attributable to the liver’s role in lipid metabolic processes, including cholesterol reverse transport. MicroRNAs in macrophages play a pivotal role in maintaining cholesterol homeostasis and are involved in the initial phases of atherosclerosis development. In this context, inhibiting miR-33a can lead to a reduction in plasma cholesterol levels and a decrease in plaque burden, whereas miR-33b can elevate plasma HDL cholesterol levels [24,25].
GSH is a crucial antioxidant that is involved in several important defence mechanisms, and GGT activity is essential for maintaining adequate intracellular levels of GSH. The intracellular levels of GSH are dependent on a balance between its synthesis and consumption, as it cannot pass through cell membranes easily. This balance necessitates a sufficient cysteine supply for synthesis [26]. In most cells, the required cysteine for GSH synthesis is obtained through membrane-bound GGT-catalysed cycloglutathione degradation. Stark et al. suggested that GGT-mediated GSH cleavage could lead to the production of reactive oxygen species (ROS) and stimulation of oxidative reactions [27]. Extensive evidence suggests that oxidative stress plays a critical role in the development and progression of endothelial dysfunction and CAD [28,29]. ROS are highly reactive molecules that can damage cellular components such as proteins, lipids and DNA. Elevated ROS levels can cause damage to the endothelium, resulting in decreased production of nitric oxide (NO), a molecule that regulates blood flow and pressure. NO maintains normal blood vessel function by promoting vasodilation and inhibiting platelet aggregation and adhesion to the endothelium [30]. Without adequate NO production, blood flow is impaired, and platelets can adhere to the endothelium, initiating the formation of atherosclerotic plaques. ROS can activate pro-inflammatory signalling pathways and promote the expression of adhesion molecules, leading to the recruitment of inflammatory cells to the site of endothelial injury [31], which exacerbates endothelial dysfunction and promotes the development of atherosclerotic plaques. Moreover, the generation of cysteinyl-glycine at the sites of GGT activity could lead to favourable conditions for promoting LDL oxidation [32]. Oxidized LDL is a critical factor in atherosclerotic plaque formation [33]. As atherosclerotic plaques continue to accumulate within the coronary arteries, they can narrow the lumen of the artery, reducing blood flow to the heart muscle. A plaque rupture can trigger the formation of a blood clot, leading to a complete blockage of the artery and potentially resulting in MI. Theories on the association between GGT levels and CVD have been the subject of research for nearly three decades. Numerous studies have shown a close relationship between serum GGT levels and various CVD, such as CAD, angina pectoris, MI, stroke and metabolic syndrome [34,35]. Several population-based studies and meta-analyses have revealed a positive and dose-dependent association between serum GGT levels within the reference range and an increased risk of mortality from CVD and all-cause mortality [10–13]. Patients with EOCAD are typically under 50 years of age and may have distinct etiological factors compared to those with late-onset CAD. Our earlier research revealed a significant correlation between the ALMS1 and MTHFR variants and the likelihood of EOCAD development [36,37]. The prevalence and severity of EOCAD were associated with serum levels of uric acid, asymmetric dimethylarginine, as well as some fatty acids and amino acids [38–41]. Furthermore, we evaluated the serum lipoprotein metabolism in individuals with EOCAD using differential proteomic analysis of apolipoproteins A-I, A-IV and C-I, which confirmed abnormal metabolism [42]. However, studies investigating the relationship between serum GGT levels and EOCAD are relatively limited. Sheikh et al. conducted a study of 367 patients and found that serum GGT levels were predictive of premature CAD [43]. In addition, Huang et al. demonstrated that serum levels of GGT were correlated with the risk of acute coronary syndrome among relatively young patients in a study of 216 patients [44].
We expanded the sample size in our study to 1720 participants, which included 860 patients diagnosed with EOCAD. Among all patients, 6.63% had chronic CAD, 52.21% had unstable angina and 41.16% had MI, which is consistent with the higher severity of EOCAD compared to late-onset CAD. The findings of our study revealed that the patient group had significantly elevated serum GGT levels in comparison to the control group. Furthermore, the GGT levels were found to be correlated with several traditional risk factors associated with CAD in the patient cohort. Smoking, alcohol consumption and high BMI have been identified as factors that may increase serum GGT levels. Additionally, patients with EOCAD and comorbid hypertension and/or DM exhibited higher serum GGT levels. We found a significant correlation between GGT and serum lipid levels. Specifically, serum LDL-C, TC and TG levels were significantly and positively correlated with GGT levels, while serum HDL-C levels showed a negative correlation. Our regression analysis revealed that increased serum GGT levels were an independent risk factor for EOCAD, with each unit increase in GGT levels associated with a 1.021-fold increase in the risk of EOCAD. Although patients with chronic CAD, unstable angina and MI tended to have higher serum GGT levels, this difference was not statistically significant. Moreover, there was only a weak relationship between Gensini scores of coronary artery stenosis and serum GGT levels. This may be related to the fact that this study primarily focuses on patients with EOCAD. However, notable differences in serum GGT levels were found between patients without significant coronary artery stenosis, those with double-vessel disease, and those with triple-vessel disease. Furthermore, other related studies also suggest that serum GGT levels are related to the severity of CAD [45,46].
Our study has some notable limitations. First, while serum levels of GGT have a significant relationship with traditional risk factors for EOCAD, we found that the correlation between serum levels of GGT and disease severity is weaker than anticipated. Due to the retrospective and case-control design of our study, we were unable to establish a causal relationship between serum levels of GGT and the EOCAD dynamic progression. This issue warrants further investigation through prospective research. Second, the cardio-protective effects of oestrogen and other factors lead to females being underrepresented among EOCAD patients. This demographic limitation constrains the examination of the connection between serum GGT levels and EOCAD in female patients. We encountered a similar issue in our study. Third, we acknowledge that coronary angiography is crucial for excluding CAD among control participants. However, younger individuals without symptoms of CVD rarely undergo coronary angiography. Therefore, we selected control participants based on symptoms and resting ECG alone. Nonetheless, this approach is not foolproof as ischemia in asymptomatic, young and active adults with a normal ECG is uncommon but not impossible.
Conclusions
In conclusion, our study demonstrates a significant elevation of serum levels of GGT in EOCAD patients, highlighting its association with multiple risk factors for the disease. Moreover, elevated serum GGT levels were found to be an independent risk factor for EOCAD. However, the weak relationship between serum GGT levels and the severity of EOCAD suggests a complex pathogenesis that requires further investigation. Further prospective studies are required to confirm whether elevated serum GGT levels precede the occurrence of EOCAD or whether there is a reciprocal relationship or interaction.
Funding Statement
The study was supported by grants from the National Natural Science Foundation of China (No. 81672073) and Shandong Provincial Natural Science Foundation, China (Nos. ZR2022MH200 and ZR2020MH034).
Author contributions
CX designed the study; JL, RHL, CZ and JJG acquired the data; CZ, TTZ and YW analysed and interpreted the data; CX wrote the manuscript; GWH, LML and CX reviewed and revised the manuscript; all authors approved the final manuscript.
Consent form
Informed consent form for publication was obtained from all authors.
Disclosure statement
The authors declare no competing interests.
Data availability statement
The datasets used and/or analysed during this current study are available from the corresponding author on reasonable request.
References
- 1.Couto N, Wood J, Barber J.. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:1–9. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Ha MH, Kim JH, et al. Gamma-glutamyltransferase and diabetes – a 4 year follow-up study. Diabetologia. 2003;46(3):359–364. doi: 10.1007/s00125-003-1036-5. [DOI] [PubMed] [Google Scholar]
- 3.Grimm C, Hofstetter G, Aust S, et al. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. Br J Cancer. 2013;109(3):610–614. doi: 10.1038/bjc.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunutsor SK, Laukkanen JA.. Gamma-glutamyltransferase and risk of chronic kidney disease: a prospective cohort study. Clin Chim Acta. 2017;473:39–44. doi: 10.1016/j.cca.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DA, Sattar N, Smith GD, et al. The associations of physical activity and adiposity with alanine aminotransferase and gamma-glutamyltransferase. Am J Epidemiol. 2005;161(11):1081–1088. doi: 10.1093/aje/kwi125. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Xin X, Luo XP.. The relationship between the ratio of gamma-glutamyltransferase to high-density lipoprotein cholesterol and the risk of diabetes mellitus using publicly available data: a secondary analysis based on a longitudinal study in Japan. Lipids Health Dis. 2023;22(1):7. doi: 10.1186/s12944-023-01772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet F, Gastaldelli A, Pihan-Le Bars F, et al. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. J Hypertens. 2017;35(3):493–500. doi: 10.1097/HJH.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 8.Corti A, Belcastro E, Dominici S, et al. The dark side of gamma-glutamyltransferase (GGT): pathogenic effects of an ‘antioxidant’ enzyme. Free Radic Biol Med. 2020;160:807–819. doi: 10.1016/j.freeradbiomed.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Franzini M, Corti A, Martinelli B, et al. Gamma-glutamyltransferase activity in human atherosclerotic plaques – biochemical similarities with the circulating enzyme. Atherosclerosis. 2009;202(1):119–127. doi: 10.1016/j.atherosclerosis.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Kengne AP, Czernichow S, Stamatakis E, et al. Gamma-glutamyltransferase and risk of cardiovascular disease mortality in people with and without diabetes: pooling of three British Health Surveys. J Hepatol. 2012;57(5):1083–1089. doi: 10.1016/j.jhep.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Breitling LP, Claessen H, Drath C, et al. Gamma-glutamyltransferase, general and cause-specific mortality in 19,000 construction workers followed over 20 years. J Hepatol. 2011;55(3):594–601. doi: 10.1016/j.jhep.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Koehler EM, Sanna D, Hansen BE, et al. Serum liver enzymes are associated with all-cause mortality in an elderly population. Liver Int. 2014;34(2):296–304. doi: 10.1111/liv.12311. [DOI] [PubMed] [Google Scholar]
- 13.Du G, Song Z, Zhang Q.. Gamma-glutamyltransferase is associated with cardiovascular and all-cause mortality: a meta-analysis of prospective cohort studies. Prev Med. 2013;57(1):31–37. doi: 10.1016/j.ypmed.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Hauser ER, Crossman DC, Granger CB, et al. A genomewide scan for early-onset coronary artery disease in 438 families: the GENECARD study. Am J Hum Genet. 2004;75(3):436–447. doi: 10.1086/423900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cipriani V, Mannucci PM, Ardissino D, et al. Familial aggregation of early-onset myocardial infarction. Eur J Intern Med. 2010;21(6):511–515. doi: 10.1016/j.ejim.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Cole JH, Miller JI 3rd, Sperling LS, et al. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol. 2003;41(4):521–528. doi: 10.1016/s0735-1097(02)02862-0. [DOI] [PubMed] [Google Scholar]
- 17.Schoenenberger AW, Radovanovic D, Stauffer JC, et al. Acute coronary syndromes in young patients: presentation, treatment and outcome. Int J Cardiol. 2011;148(3):300–304. doi: 10.1016/j.ijcard.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Rampidis GP, Benetos G, Benz DC, et al. A guide for Gensini score calculation. Atherosclerosis. 2019;287:181–183. doi: 10.1016/j.atherosclerosis.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 6. Reference procedure for the measurement of catalytic concentration of gamma-glutamyltransferase. Clin Chem Lab Med. 2002;40(7):734–738. doi: 10.1515/CCLM.2002.126. [DOI] [PubMed] [Google Scholar]
- 20.Perrin-Sarrado C, Pongas M, Dahboul F, et al. Reduced activity of the aortic gamma-glutamyltransferase does not decrease S-nitrosoglutathione induced vasorelaxation of rat aortic rings. Front Physiol. 2016;7:630. doi: 10.3389/fphys.2016.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Møller S, Bernardi M.. Interactions of the heart and the liver. Eur Heart J. 2013;34(36):2804–2811. doi: 10.1093/eurheartj/eht246. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Lee YH, Kim SU, et al. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19(10):2138–2147.e10. doi: 10.1016/j.cgh.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Duell PB, Welty FK, Miller M, et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(6):e168–e185. [DOI] [PubMed] [Google Scholar]
- 24.Kabłak-Ziembicka A, Badacz R, Przewłocki T.. Clinical application of serum microRNAs in atherosclerotic coronary artery disease. J Clin Med. 2022;11(22):6849. doi: 10.3390/jcm11226849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Márquez AB, van der Vorst E, Maas SL.. Key chemokine pathways in atherosclerosis and their therapeutic potential. J Clin Med. 2021;10(17):3825. doi: 10.3390/jcm10173825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oestreicher J, Morgan B.. Glutathione: subcellular distribution and membrane transport. Biochem Cell Biol. 2019;97(3):270–289. doi: 10.1139/bcb-2018-0189. [DOI] [PubMed] [Google Scholar]
- 27.Stark AA, Zeiger E, Pagano DA.. Glutathione metabolism by gamma-glutamyltranspeptidase leads to lipid peroxidation: characterization of the system and relevance to hepatocarcinogenesis. Carcinogenesis. 1993;14(2):183–189. doi: 10.1093/carcin/14.2.183. [DOI] [PubMed] [Google Scholar]
- 28.Incalza MA, D'Oria R, Natalicchio A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Daiber A, Chlopicki S.. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: evidence for redox-based therapies. Free Radic Biol Med. 2020;157:15–37. doi: 10.1016/j.freeradbiomed.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Gresele P, Momi S, Guglielmini G.. Nitric oxide-enhancing or -releasing agents as antithrombotic drugs. Biochem Pharmacol. 2019;166:300–312. doi: 10.1016/j.bcp.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Volpe C, Villar-Delfino PH, Dos Anjos P, et al. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paolicchi A, Minotti G, Tonarelli P, et al. Gamma-glutamyl transpeptidase-dependent iron reduction and LDL oxidation – a potential mechanism in atherosclerosis. J Investig Med. 1999;47(3):151–160. [PubMed] [Google Scholar]
- 33.Jiang H, Zhou Y, Nabavi SM, et al. Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front Cardiovasc Med. 2022;9:925923. doi: 10.3389/fcvm.2022.925923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung HS, Lee JS, Kim JA, et al. γ-glutamyltransferase variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based cohort study. J Clin Med. 2019;8(6):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasapoglu B, Turkay C, Bayram Y, et al. Role of GGT in diagnosis of metabolic syndrome: a clinic-based cross-sectional survey. Indian J Med Res. 2010;132:56–61. [PubMed] [Google Scholar]
- 36.Xuan C, Bai XY, Gao G, et al. Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677T and risk of myocardial infarction: a meta-analysis for 8,140 cases and 10,522 controls. Arch Med Res. 2011;42(8):677–685. doi: 10.1016/j.arcmed.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SY, Xuan C, Wang Y, et al. Association between ALMS 1 variants and early-onset coronary artery disease: a case-control study in Chinese population. Biosci Rep. 2020;40(9):BSR20193637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xuan C, Li H, Tian QW, et al. Quantitative assessment of serum amino acids and association with early-onset coronary artery disease. Clin Interv Aging. 2021;16:465–474. doi: 10.2147/CIA.S298743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xuan C, Liu ZF, Wang Q, et al. Increased serum concentrations of asymmetric dimethylarginine (ADMA) in patients with early-onset coronary artery disease. Clin Chim Acta. 2017;464:195–199. doi: 10.1016/j.cca.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Tian TT, Li H, Chen SJ, et al. Serum uric acid as an independent risk factor for the presence and severity of early-onset coronary artery disease: a case-control study. Dis Markers. 2018;2018:1236837. doi: 10.1155/2018/1236837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xuan C, Tian QW, Li H, et al. Serum fatty acids profile and association with early-onset coronary artery disease. Ther Adv Chronic Dis. 2021;12:20406223211033102. doi: 10.1177/20406223211033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xuan C, Li H, Li LL, et al. Screening and identification of pregnancy zone protein and leucine-rich alpha-2-glycoprotein as potential serum biomarkers for early-onset myocardial infarction using protein profile analysis. Proteomics Clin Appl. 2019;13(3):e1800079. [DOI] [PubMed] [Google Scholar]
- 43.Sheikh M, Tajdini M, Shafiee A, et al. Association of serum gamma-glutamyltransferase and premature coronary artery disease. Neth Heart J. 2017;25(7–8):439–445. doi: 10.1007/s12471-017-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Luo J, Liu X, et al. Gamma-Glutamyltransferase and risk of acute coronary syndrome in young Chinese patients: a case-control study. Dis Markers. 2018;2018:2429160. doi: 10.1155/2018/2429160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh KK, Kapoor A, Khanna R, et al. Serum gamma glutamyltransferase (GGT) in coronary artery disease: exploring the Asian Indian connection. Ann Card Anaesth. 2022;25(4):408–413. doi: 10.4103/aca.aca_62_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aksakal E, Tanboga IH, Kurt M, et al. The relation of serum gamma-glutamyl transferase levels with coronary lesion complexity and long-term outcome in patients with stable coronary artery disease. Atherosclerosis. 2012;221(2):596–601. doi: 10.1016/j.atherosclerosis.2012.01.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during this current study are available from the corresponding author on reasonable request.