Abstract
Aotus lemurinus monkeys were immunized with pools of either lipid-tailed peptides injected in PBS or peptides in Montanide ISA-51, all derived from four Plasmodium falciparum pre-erythrocytic antigens, namely, LSA1, LSA3, SALSA, and STARP. These formulations were well tolerated. Their immunogenicity was demonstrated by the induction of both B- and T-cell responses to most of the peptides studied (of the 12, 10 induced antibody production, 9 induced T-cell proliferative responses, and all 12 induced gamma interferon secretion). Immune responses proved to be long lasting, since some were still detectable 210 days after immunization. Of particular importance is the fact that B- and T-cell responses elicited in this way by synthetic peptides were specific for native parasite proteins on P. falciparum sporozoites and liver stage parasites.
The possibility of developing malaria vaccines based on pre-erythrocytic antigens was first considered following the observation that immunization with X-radiation-attenuated sporozoites could induce protective immunity (17, 30). However, more recent studies carried out in parallel under in vivo and in vitro conditions have shown in both humans and rodents that protection depends on the abilities of irradiated sporozoites to penetrate hepatocytes and, further, to transform into uninucleate liver trophozoites (14). The indication that persistent liver form parasites are required to induce protection (14, 38, 46) was confirmed recently (34, 44).
Based on this rationale, we have focused our recent work on the identification and characterization of liver stage antigens (24, 35). Four of them, namely, LSA1, LSA3, SALSA, and STARP, were recently characterized (4, 12a, 21, 22). The B- and T-cell antigenicity of several regions of these four molecules was established by epidemiological studies (3a, 4, 12a, 21, 22), and the corresponding synthetic peptides were produced to study their immunogenicity.
Taking into account, on the one hand, the known potential of Aotus lemurinus as a model for erythrocytic stages of Plasmodium falciparum malaria (8, 10) and, on the other hand, the susceptibility of monkeys in the family Cebidae to P. falciparum liver stage development (11, 12, 13a, 14, 16), the aim of the present study was to gather preliminary indications about their capacity to develop an immune response to these antigens compared to mice, chimpanzees, and humans before embarking on systematic studies involving larger numbers of monkeys.
Immunization.
Four A. lemurinus griseimembra monkeys (from northern Colombia) with karyotype II or III were enrolled in immunization experiments using 12 synthetic peptides derived from the above-described four pre-erythrocytic-stage antigens, together with one control. Each of the four animals was immunized with one of the four molecules by using a mixture of peptides as described in Table 1. Immunizations were performed subcutaneously three times at intervals of 20 days. The final volume per injection was 500 μl containing 200 μg of each peptide. Six of the peptides were lipid-tailed peptides coupled with a palmitic acid at the carboxyl-terminal end using a lysine residue as a linker, which, on the basis of previous good immunogenicity results (3, 36), were injected in saline only, i.e., without an adjuvant. The remaining six peptides (without a lipidic component) were emulsified in Montanide ISA-51. All were produced by the stepwise solid-phase tert-butoxycarbonyl technique (39) in a 430A automated peptide synthesizer (Applied Biosystems, Foster City, Calif.) and checked for homogeneity by analytical reverse-phase high-pressure liquid chromatography and for identity by amino acid analysis (3).
TABLE 1.
Immunization schemea
| Protein (Aotus monkey) | Peptide or compound | Sequence |
|---|---|---|
| LSA1 (M21) | LSA1-J/Lipo | ERRAKEKLQEQQSDLEQRKADTKK K(Pam)-NH2b |
| LSA1-NR | DTKKNLERKKEHGDILAEDLYGRLEIP | |
| LSA1-REP | LAKEKLQEQQSDLEQERLAKEKLQEQQSDLEQERLAKEKLQ | |
| LSA1-TER | NSRDSKEISIIEKTNRESITTNVEGRRDIHKGHKGHL | |
| LSA3 (M23) | LSA3-NRI | DELFNELLNSVDVNGEVKENILEESQ |
| LSA3-NRII/Lipo | LEESQVNDDIFNSLVKSVQQEQQHNV K(Pam)-NH2 | |
| LSA3-RE | VESVAPSVEESVAPSVEESVAENVEESV | |
| LSA3-CT1/Lipo | LLSNIEEPKENIIDNLLNNI K(Pam)-NH2 | |
| SALSA (M4) | SALSA-1/Lipo | SAEKKDEKEASEQGEESHKKENSQESA K(Pam)-NH2 |
| SALSA-2 | NGKDDVKEEKKTNEKKDDGKTDKVQEKVLEKSPK | |
| STARP (M48) | STARP-R/Lipo | STDNNNTKTISTDNNNTKTI K(Pam)-NH2 |
| STARP-M/Mixotope/Lipo | STDNNNTKTISTDNNNTNTI K(Pam)-NH2 | |
| L---T-NTIKA---S-IT-N | ||
| ----D--D-NL------D-T | ||
| -------K---------K-K | ||
| Adjuvant (V63) | Montanide ISA-51 |
Aotus monkeys were immunized with combinations of (i) lipopeptides (Lipo) injected in phosphate-buffered saline without an adjuvant at one location (pooled when there was more than one lipopeptide from each molecule) and (ii) pools of peptides emulsified with Montanide ISA-51 adjuvant (SEPPIC, Paris, France) injected on the same day at another site. STARP-M/Mixotope/Lipo consists of a mixed-epitope degenerated sequence as indicated above and described in reference 20.
Peptide linked to palmitic acid (Pam) via a lysine residue.
Antibody production in response to peptide immunization.
A high level of production of antibodies against 10 of the 12 peptides tested was observed. Sera collected from Aotus monkeys 15 and 210 days after the third immunization were tested in parallel by using standard enzyme-linked immunosorbent assay (ELISA) procedures described previously (6), except that rabbit anti-Cebidae monkey immunoglobulin G (IgG) (a gift of T. Fandeur, Institut Pasteur de Guyane, Cayenne, French Guiana), diluted 1/2,000, was used as the second antibody and revealed by peroxidase-conjugated anti-rabbit IgG (Biosys, Compiègne, France) at a dilution of 1/4,000.
As shown in Table 2, detectable antibodies against peptides LSA1-NR, LSA1-TER, and LSA1-REP were induced by the immunization scheme and, interestingly, found to increase thereafter, despite the fact that no further boosting had been performed. Only LSA1-J/Lipo did not induce antibodies; however, we observed in chimpanzees that the antibody response to this peptide was one which varied the most from one animal to another (3a). Responses to both SALSA peptides, which do not share cross-reactive epitopes (4), were elicited, and that against SALSA-2 was much stronger than that against SALSA-1. This is similar to what has been observed in immunized mice and chimpanzees, as well as in exposed African humans (4), confirming that SALSA-2 contains a potent B-cell epitope(s). Antibody responses to both STARP-R and STARP-M, two related peptides, were obtained; however, the response was strikingly stronger for STARP-M, which is a convergent combinatorial library of peptides, or mixotope, obtained in a single synthesis by introducing degenerated sequences into the 10-amino-acid repeat region of the protein (see Table 1). This result is in keeping with epidemiological observations (21) and with previous studies using viral models in which the mixotope strategy was found to enhance immunogenicity (18, 23). The antibody responses to STARP peptides are also of interest because in vitro studies have indicated that they can significantly reduce sporozoite invasion in human hepatocytes and therefore may play a role in protection (20). Antibodies to three of the four LSA3 peptides, LSA3-CT1, LSA3 NR-II, and LSA3-RE, were produced, although at low titers only. These were nevertheless specific, since preimmunization sera were negative even when tested at 1:20, as were postimmunization samples tested with the control antigens RESA (47) and MSP3 (40). Although titers were low, they persisted for a long time, i.e., up to 7 months, after immunization. This lower immunogenicity of LSA3 peptides in the single Aotus monkey studied contrasts with the high titers induced in mice of various breeds and in outbred chimpanzees (3, 3a). It is noteworthy that we obtained strong antibody responses not only to repetitive motifs, as is frequently the case (31), but also to nonrepetitive regions of the molecules (e.g., LSA1-NR, LSA1-TER, and SALSA-2).
TABLE 2.
Antibody responsesa
| Monkey | Antigen | ELISA titer on post- immunization day:
|
|
|---|---|---|---|
| 15 | 210 | ||
| M21 | LSA1-J | <100b | <100 |
| LSA1-NR | 2,700 | 8,100 | |
| LSA1-REP | 100 | 300 | |
| LSA1-TER | 2,700 | 8,100 | |
| M23 | LSA3-NRI | <100 | <100 |
| LSA3-NRII | 100 | 100 | |
| LSA3-RE | 100 | 100 | |
| LSA3-CT1 | 100 | 100 | |
| M4 | SALSA-1 | 100 | 100 |
| SALSA-2 | 2,700 | 8,100 | |
| M48 | STARP-R | 2,700 | 2,700 |
| STARP-M | 24,300 | 8,100 | |
Titers determined in samples taken 15 and 210 days postimmunization correspond to the dilution of the test sera whose optical density at 450 nm was above the mean of control Aotus sera plus 2 standard deviations. Controls included (i) sera from 10 Aotus monkeys with no history of exposure to malaria, as well as preimmunization sera from the immunized monkeys, and (ii) peptides RESA and MSP3. The mean optical densities of the control Aotus sera against each of the peptides, used to define the cutoff values, ranged between 0.02 and 0.18.
Negative at a dilution of 1:100.
Production of T-cell responses.
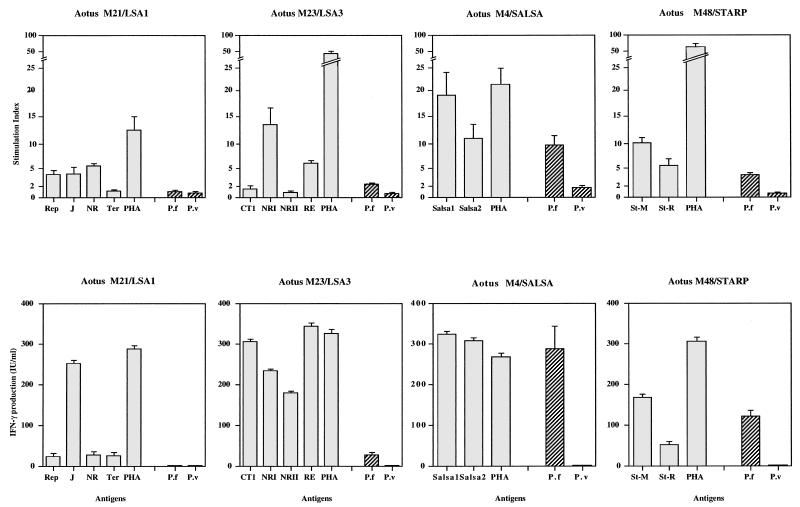
Remarkably, all of the 12 peptides derived from the four P. falciparum pre-erythrocytic molecules contained T-cell epitopes capable of inducing either proliferation, high gamma interferon, (IFN-γ) production, or, more frequently, both in Aotus lymphocytes (Fig. 1). In this case, production of IFN-γ has particular significance, since it is recognized as a major mechanism of defense against liver stage parasites (45). Aotus cells collected on day 0 (preimmunization) and 15 days after the third immunization were tested by lymphoproliferation with 10 μg of each peptide per ml as described elsewhere (26). IFN-γ concentrations in supernatants collected from triplicate wells on day 5 were assessed by a two-site capture ELISA (3, 4, 22) using a combination of anti-human IFN-γ monoclonal antibodies identified as able to react with Aotus IFN-γ. Each of the four LSA1 peptides contained T-cell determinants capable of stimulating IFN-γ production, and three of the four peptides induced a significant proliferative response. When tested 210 days postimmunization, a strong lymphoproliferation in response to two of the four peptides, LSA1-NR and LSA1-REP, was still detectable (data not shown). We found secretion of IFN-γ in response to each of the four LSA3 peptides and T-cell proliferation in response to two of the four peptides, LSA3-NRI and LSA3-RE. This is reminiscent of studies with rodents in which LSA3-CT1/Lipo was capable of inducing a T-cell response that could only be revealed in terms of IFN-γ production (41a). The presence of T-cell epitopes for Aotus lymphocytes within both SALSA-1 and SALSA-2 was shown by the induction of both proliferative and IFN-γ responses to these peptides. Cellular responses were also positive with the two STARP peptides. They were also long lasting, as they remained detectable 210 days postimmunization (data not shown). As was the case for antibody production, both lymphoproliferation and IFN-γ production were slightly higher with the multiepitope peptide STARP-M. The specificity of the above-described responses was ascertained by negative results recorded for two control monkeys tested in parallel, as well as with preimmunization lymphocyte samples from immunized Aotus monkeys.
FIG. 1.
T-cell proliferative responses and IFN-γ secretion. The stimulation index was calculated as the mean number of counts per minute of triplicate test cultures divided by the mean number of counts per minute of triplicate control cultures (nonstimulated cells) and was considered positive when >2. IFN-γ concentrations were calculated from a standard curve included in each plate and made by using culture medium containing known amounts of a standard IFN-γ (NIH-IFN-γ Gp23-901-530). Negative and positive controls (unstimulated cells and cells stimulated with phytohemagglutinin [PHA]) were included in each assay. Supernatants from human and Aotus phytohemagglutinin-stimulated blasts yielded similar values in the assay. Synthetic peptides and sonicated P. falciparum (P.f) sporozoite extracts (NF54 strain; a gift of W. Eling) were adjusted to a final protein concentration of 10 μg/ml. P. vivax (P.v) sporozoites (obtained from Anopheles albimanus mosquitoes fed on human blood samples) and noninfected A. albimanus mosquito salivary gland extracts were used as controls.
Relevance to native parasite proteins.
Synthetic peptides may not always properly mimic the conformation of epitopes within the whole parasite protein. This question is of particular importance, as it can affect firstly the protective properties of the immune responses and secondly the ability of the parasite to boost it upon challenge. It was thus addressed at both the B- and T-cell levels. Antibody titers were determined by testing twofold serial dilutions in a “wet” indirect fluorescent-antibody test (IFAT) as previously described for sporozoites (15) and using Carnoy-fixed P. falciparum liver schizont sections as previously described for liver stage parasites (16).
Antibodies induced in the four immunized monkeys recognized the corresponding P. falciparum native proteins expressed on the sporozoite surface and/or in liver stage schizonts (Table 3). In agreement with our ELISA results, anti-STARP antibodies were the most reactive.
TABLE 3.
IFAT determination of stage-specific antibody responsesa
| Monkey | Source of immunizing peptides | Sporozoites
|
Liver stage parasites
|
||
|---|---|---|---|---|---|
| Day 15 | Day 210 | Day 15 | Day 210 | ||
| M21 | LSA1 | <20b | <20 | 100 | 100 |
| M23 | LSA3 | 100 | 100 | 100 | 100 |
| M4 | SALSA | 100 | 100 | <20 | <20 |
| M48 | STARP | 3,200 | 3,200 | 200 | 100 |
Twofold serial dilutions in phosphate-buffered saline of serum from each monkey were tested in an IFAT, starting at 1:20. Each value is the reciprocal of the highest positive antibody dilution. Rabbit anti-Saimiri monkey IgG diluted 1/200 was employed, as in an ELISA, as the second antibody and detected with fluorescein-conjugated goat anti-rabbit IgG (heavy and light chains; Diagnostic Pasteur France) diluted at 1/100. Sera from 10 Aotus monkeys with no history of malaria were used as controls and found to be negative at a dilution of 1:20.
Negative at a dilution of 1:20.
Aotus sera did not react with P. vivax sporozoites (titers of <1/20; data not shown), which do not share any homologous protein with the four P. falciparum antigens studied. The serum of Aotus monkey M21, immunized with LSA1 peptides, reacted specifically with liver schizonts but, as expected, not with other parasite stages, thus confirming the strict liver stage expression of this molecule (22).
For technical reasons, T-cell responses can be assessed only with sporozoites (the output of in vitro and in vivo methods of liver stage P. falciparum production is too low). Nevertheless, it is interesting that an in vitro challenge with P. falciparum sporozoites, but not with P. vivax (used as a control), could induce specific lymphoproliferation and IFN-γ production in cells from each of the three animals immunized with the three molecules expressed at the sporozoite stage, but not with LSA1, which is expressed only at the liver stage (Fig. 1). In our previous studies with chimpanzees (3a), each animal was immunized with LSA3 together with each of the other three molecules, and this impeded the precise determination of which pre-erythrocytic molecule was responsible for the T-cell proliferation observed with sporozoite extracts. The present results obtained with animals immunized with single molecules indicate that, in addition to LSA3, both synthetic peptides STARP and SALSA can also induce T-cell responses specific to native T-cell epitopes on sporozoites. Thus, the immune responses induced by artificial immunization were stage specific and relevant to native proteins.
Although the number of monkeys used in this study was small, the immune responses observed confirm the high immunogenicity of our molecules and stress the interest of the lipopeptide strategy. In recent years, synthetic lipopeptide technology has received more consideration for vaccine delivery (5, 13, 36, 49). Although the design of the experiment does not permit immunogenicity comparisons between identical peptides in adjuvants versus lipopeptides, the present results obtained with Aotus monkeys support previous indications (3). We tested six lipopeptides in Aotus monkeys, and, remarkably, all of them were able to induce high T-cell and/or humoral responses without an adjuvant. This contrasts with another study in which human immunodeficiency virus-derived lipopeptides were injected into macaques and the animals responded to this formulation only when it was mixed with incomplete Freund’s adjuvant (5) and is in favor of the immunogenicity of the pre-erythrocytic molecules under study. We cannot exclude the possibility that the Montanide adjuvant injected with the peptides into the same animal, albeit at a different site, indirectly influenced the immunogenicity of the lipopeptides. However, it is noteworthy that the strongest immune responses in our experiments were generated in the Aotus monkey immunized with lipopeptides only, and the same has been previously observed in mice and chimpanzees (3, 3a).
Indeed, the results sound promising when compared to those of other trials performed with Saimiri and Aotus monkeys and antigens derived from Plasmodium organisms at various stages of the life cycle. These were done by using different antigen formulations and different adjuvants and therefore cannot be strictly compared. Nevertheless, it is striking that strong immune responses were obtained in the above studies only with powerful adjuvants, the majority of the studies relying on Freund’s adjuvant (7, 19, 25, 27, 32, 41–43). Despite this fact, only 10% of the animals developed strong immune responses, 44% developed moderate responses, 35% developed weak responses, and 9% did not respond at all (1, 7, 9, 19, 25, 27, 32, 33, 41–43). In contrast, the substantial and long-lasting responses obtained by using lipopeptides without any adjuvant or a mixture of peptides with an adjuvant which can be used in humans compare favorably with those in previous studies with the same animal species.
The strategy employed to identify both the four antigens and the specific peptides may have particularly favored the selection of more immunogenic molecules (35). The screening process of an initial set of 120 clones encoding P. falciparum pre-erythrocytic molecules included several steps which involved the selection of immunodominant B- and T-cell epitopes. Strong B-cell epitopes were identified by screening with human antibodies obtained from different sources: (i) a set of 15 African human sera from areas of endemicity, (ii) antibodies affinity purified on each recombinant protein and tested upon sporozoites and liver stage parasites, and (iii) sera from “postimmune” individuals who had left the area of endemicity several years before to determine if the immune response were long lasting. It is our belief that this strategy favored the selection of more conserved and immunodominant antigens containing not only B-cell epitopes but also strong T-helper epitopes associated with them. The T-cell epitopes were selected by taking advantage of the observation that they are frequently localized in unstable regions close to regularly organized structures susceptible to proteolysis and therefore susceptible to being processed and associated with major histocompatibility complex molecules (3). Since it has been shown that T-cell epitopes could overlap and segregate within a relatively small area of a given molecule (2, 28, 37, 48), the synthesis of medium-size peptides (20 to 41 amino acids) was chosen to increase the chance of getting several T-cell epitopes recognized by various class II antigens in one given peptide.
We have found that the immunization of Aotus monkeys with the 12 peptides described above corroborated data about their antigenicity obtained with individuals exposed to malaria: among the 12 peptides, 11 were found to define B-cell epitopes in human populations with high antibody production, and all 12 defined T-cell epitopes with high prevalence to 9 of the 12 peptides (4, 12a, 21, 22). Moreover, the value of each of the four molecules was supported by the identification of numerous cytotoxic T-lymphocyte epitopes (3, 3a, 4, 29). Data obtained with Aotus monkeys therefore confirm the previous indications about the good antigenicity of these four molecules in various species (3, 3a, 4, 6a, 12a, 21, 22) and show that these medium-size peptides can associate with major histocompatibility complex class II antigens of the Aotus species as they do with those of humans (22).
So far, Aotus monkeys have been used mainly for assessment of the immunogenicity and protective efficacy of several blood stage antigens (7, 9, 10, 27, 32, 42) but not much for pre-erythrocytic vaccine development; hence, the reproducibility of this model, in parasitological terms, has yet to be defined. Nevertheless, preliminary studies conducted with Aotus, Saimiri, and Cebus monkeys have indicated that, in contrast to blood stage parasites, liver schizogony can be readily obtained with P. falciparum strains without the need for previous adaptation to these monkeys (11, 12, 13a, 14, 16). This, together with the good antigenicity and immunogenicity of the pre-erythrocytic-stage molecules described in this paper and the relevance of responses to native proteins, suggests that Aotus monkeys have potential for preclinical steps in the development of pre-erythrocytic-stage malaria vaccines.
Acknowledgments
We gratefully acknowledge the pertinent advice of J.-L. Pérignon. We also thank B. E. Ferro and S. Hurtado for technical support and V. M. Salazar and L. A. Ruiz for handling the Aotus monkey colony. The NF54 P. falciparum sporozoites were kindly provided by W. Eling (Nijmegen, The Netherlands), and anti-human IFN-γ monoclonal antibodies were provided by M. A. Cousin (Roussel Uclaf, Paris, France).
This work was supported by Life Sciences and Technologies for Developing Countries (STD3-CT-920053-CEC) and the Fondo Colombiano de Investigaciones Cientificas y Proyectos Especiales “Francisco Jose de Caldas” (COLCIENCIAS).
REFERENCES
- 1.Barr P J, Green K M, Gibson H L, Bathurst I C, Quakyi I A, Kaslow D C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumhuter S, Wallace C J, Proudfoot A E, Bron C, Corradin G. Multiple T cell antigenic determinants identified within a limited region of the horse cytochrome c molecule. Eur J Immunol. 1987;17:651–656. doi: 10.1002/eji.1830170511. [DOI] [PubMed] [Google Scholar]
- 3.BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, Thomas A, Druilhe P. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol. 1997;27:1242–1253. doi: 10.1002/eji.1830270528. [DOI] [PubMed] [Google Scholar]
- 3a.BenMohamed, L. et al. Submitted for publication.
- 4.Bottius E, BenMohamed L, Brahimi K, Gras-Masse H, Lepers J-P, Raharimalala L, Aikawa A, Meis J F G M, Slierendregt B, Tartar A, Thomas A, Druilhe P. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J Immunol. 1996;156:2874–2884. [PubMed] [Google Scholar]
- 5.Bourgault I, Chirat A, Tartar A, Levy J P, Guille J G, Venet A. Simian immunodeficiency virus as a model for vaccination against HIV: induction in rhesus macaques of GAG or NEF specific cytotoxic T lymphocytes by lipopeptides. J Immunol. 1994;152:2530–2537. [PubMed] [Google Scholar]
- 6.Brahimi K, Pérignon J-L, Bossus M, Gras H, Tartar A, Druilhe P. Fast immunopurification of small amounts of specific antibodies on peptides bound to ELISA plates. J Immunol Methods. 1993;162:69–75. doi: 10.1016/0022-1759(93)90408-y. [DOI] [PubMed] [Google Scholar]
- 6a.Brasseur, P., et al. Submitted for publication.
- 7.Chang S P, Case S E, Gosnell W L, Hashimoto A, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee-Ng C T, Barr P J, Yokota B T, Hui G S N. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins W E. South American monkeys in the development and testing of malarial vaccines. Mem Inst Oswaldo Cruz Rio de J. 1992;87:401–406. doi: 10.1590/s0074-02761992000700068. [DOI] [PubMed] [Google Scholar]
- 9.Collins W E, Anders R F, Ruebuesh I, Kemp D J, Woodrow G C, Campbell G H, Brown G V, Irving O, Goss N, Filipski V K, Coppel R L, Broderson J R, Thomas H, Pye D, Skinner J C, Wilson C, Stanfill P S, Procell P M. Immunization of owl monkeys with the ring-infected erythrocyte surface antigen of Plasmodium falciparum. Am J Trop Med Hyg. 1991;44:34–41. doi: 10.4269/ajtmh.1991.44.34. [DOI] [PubMed] [Google Scholar]
- 10.Collins W E, Galland G G, Sullivan J S, Morris C L. Selection of Aotus monkey model for testing Plasmodium falciparum blood-stage vaccine. Am J Trop Med Hyg. 1994;51:224–232. doi: 10.4269/ajtmh.1994.51.224. [DOI] [PubMed] [Google Scholar]
- 11.Collins W E, Galland G G, Sullivan J S, Morris C L, Richardson B B, Roberts J M. The Santa Lucia strain of Plasmodium falciparum as a model for vaccine studies. I. Development in Aotus lemurinus griseimembra. Am J Trop Med Hyg. 1996;54:372–379. doi: 10.4269/ajtmh.1996.54.372. [DOI] [PubMed] [Google Scholar]
- 12.Collins W E, Warren M, Skinner J C, Chin W, Richardson B B. Studies on the Santa Lucia (El Salvador) strain of Plasmodium falciparum in Aotus trivirgatus monkeys. J Parasitol. 1977;63:52–56. [PubMed] [Google Scholar]
- 12a.Daubersies, P., et al. Unpublished data.
- 13.Deres K, Schild H, Wiesmuller K, Jung G, Rammensee H. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 13a.Druilhe, P. Unpublished data.
- 14.Druilhe P, Marchand C. From sporozoite to liver stages: the saga of the irradiated sporozoite vaccine. In: McAdam K, editor. New strategies in parasitology. Edinburgh, Scotland: Churchill Livingstone; 1989. pp. 39–48. [Google Scholar]
- 15.Druilhe P, Pradier O, Marc J P, Miltgen F, Mazier D, Parent D. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfections. Infect Immun. 1986;53:393–397. doi: 10.1128/iai.53.2.393-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druilhe P, Puebla R M, Miltgen F, Perrin L, Gentilini M. Species- and stage-specific antigens in exoerythrocytic stages of Plasmodium falciparum. Am J Trop Med Hyg. 1984;33:336–341. doi: 10.4269/ajtmh.1984.33.336. [DOI] [PubMed] [Google Scholar]
- 17.Edelman R, Hoffman S L, Davis J R, Beier M, Sztein M B, Losonsky G, Herrington D A, Eddy H A, Hollingdale M R, Gordon D M, Clyde D F. Long-term persistence of sterile immunity in a volunteer immunized with X-irradiated Plasmodium falciparum sporozoites. J Infect Dis. 1993;168:1066–1070. doi: 10.1093/infdis/168.4.1066. [DOI] [PubMed] [Google Scholar]
- 18.Estaquier J, Gras-Masse H, Boutillon C, Ameisen J C, Capron A, Tartar A, Auriault C. The mixotope: a combinatorial peptide library as a T cell and B cell immunogen. Eur J Immunol. 1994;24:2789–2795. doi: 10.1002/eji.1830241132. [DOI] [PubMed] [Google Scholar]
- 19.Etlinger H M, Caspers P, Matile H, Schoenfeld H J, Stueber D, Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991;59:3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidock D, Pasqueto V, Gras H, Badell E, Eling W, Ballou W R, Belghiti J, Tartar A, Druilhe P. Plasmodium falciparum sporozoite invasion is inhibited by naturally acquired or experimentally induced polyclonal antibodies to the STARP antigen. Eur J Immunol. 1997;27:2502–2513. doi: 10.1002/eji.1830271007. [DOI] [PubMed] [Google Scholar]
- 21.Fidock D A, Bottius E, Brahimi K, Moelans I I M, Aikawa M, Konings R N H, Certa U, Olafsson P, Kaidoh T, Asavanich A, Guerin-Marchand C, Druilhe P. Cloning and characterization of a novel Plasmodium falciparum sporozoite surface antigen, STARP. Mol Biochem Parasitol. 1994;64:219–232. doi: 10.1016/0166-6851(94)00012-3. [DOI] [PubMed] [Google Scholar]
- 22.Fidock D A, Gras-Masse H, Lepers J P, Brahimi K, BenMohamed L, Mellouk S, Guerin-Marchand C, Londono A, Raharimalala L, Meis J F G, Langsley G, Roussilhon C, Tartar A, Druilhe P. Plasmodium falciparum liver stage-specific antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]
- 23.Gras-Masse H, Ameisen J C, Rouaix B C, F, Bossus M, Deprez B, Neyrink J L, Capron A, Tartar A. Synthetic vaccines and HIV-1 hypervariability: a “mixotope” approach. Pept Res. 1992;5:211–216. [PubMed] [Google Scholar]
- 24.Guérin-Marchand C, Druilhe P, Galey B, Londono A, Patarapotikul J, Beaudoin R, Dubeaux C, Tartar A, Mercereau-Puijalon O, Langsley G. A liver stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987;329:164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- 25.Herrera M, Rosero F, Herrera S, Caspers P, Rotmann D, Sinigaglia F, Certa U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect Immun. 1992;60:154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera S, De Plata C, Gonzalez J M, Perlaza B L, Bettens F, Corradin G, Arevalo-Herrera M. Antigenicity and immunogenicity of multiple antigen peptides (MAP) containing P. vivax CS epitopes in Aotus monkeys. Parasite Immunol. 1997;19:161–170. doi: 10.1046/j.1365-3024.1997.d01-193.x. [DOI] [PubMed] [Google Scholar]
- 27.Herrera S, Herrera M A, Perlaza B L, Burki Y, Caspers P, Döbelli H, Rotmann D, Certa U. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc Natl Acad Sci USA. 1990;87:4017–4021. doi: 10.1073/pnas.87.10.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J A, Thorpe C J, Hayball J D, O’Hehir R E, Lamb J R. Overlapping T-cell epitopes in the group I allergen of Dermatophagoides species restricted by HLA-DP and HLA-DR class II molecules. J Allergy Clin Immunol. 1994;93:891–899. doi: 10.1016/0091-6749(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 29.Hill A V S, Elvin J, Willis A C, Aidoo M, Allsopp C E M, Gotch F M, Gao X M, Takiguchi M, Greenwood B M, Townsend A R M, McMichael A J, Whittle H C. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992;360:434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman S L, Franke E D, Hollingdale M R, Druilhe P. Attacking the infected hepatocyte. Washington, D.C: ASM Press; 1996. pp. 35–37. [Google Scholar]
- 31.Hollingdale M R, Nardin E H, Tharavanij S, Schwartz A L, Nussenzweig R S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells: an in vitro assay of protective antibodies. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 32.Inselburg J, Bathurst I C, Kansopon J, Barr P J, Rossan R. Protective immunity induced in Aotus monkeys by a recombinant SERA protein of Plasmodium falciparum: further studies using SERA 1 and MF75.2 adjuvant. Infect Immun. 1993;61:2048–2052. doi: 10.1128/iai.61.5.2048-2052.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp B, Hundt E, Enders B, Küpper H A. Protection of Aotus monkeys from malaria infection by immunization with recombinant hybrid proteins. Infect Immun. 1992;60:2397–2401. doi: 10.1128/iai.60.6.2397-2401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Londono J A, Sedegah M, Charoenvit Y, Baudoin R L, Druilhe P. Antigenic analysis of Plasmodium yoelii liver stages by fluorescence antibody assays. Trop Med Parasitol. 1991;42:381–385. [PubMed] [Google Scholar]
- 35.Marchand, C., and P. Druilhe. 1990. How to select Plasmodium falciparum pre-erythrocytic antigens in an expression library without defined probe. Bull. W. H. O. 68(Suppl.):158–164. [PMC free article] [PubMed]
- 36.Martinon F, Gras-Masse H, Boutillon C, Chirat F, Deprez B, Guillet J-G, Gomard E, Tartar A, Levy J-P. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J Immunol. 1992;149:3416–3422. [PubMed] [Google Scholar]
- 37.Meister G E, Roberts C G P, Berzofsky J A, De Groot A S. Two novel T cell epitope prediction algorithms based on MHC-binding motifs: comparison of predicted and published epitopes from Mycobacterium tuberculosis and HIV protein sequences. Vaccine. 1995;13:581–591. doi: 10.1016/0264-410x(94)00014-e. [DOI] [PubMed] [Google Scholar]
- 38.Mellouk S, Lunel F, Sedegah M, Beaudoin R L, Druilhe P. Protection against malaria induced by irradiated sporozoites. Lancet. 1990;335:721. doi: 10.1016/0140-6736(90)90832-p. [DOI] [PubMed] [Google Scholar]
- 39.Merrifield R B. Solid-phase peptide synthesis. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2152. [Google Scholar]
- 40.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira M-C, Tartar A, Druilhe P. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood. 1994;84:1594–1602. [PubMed] [Google Scholar]
- 41.Patarroyo M E, Romero P, Torres M L, Clavijo P, Martinez A, Rodriguez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 41a.Perlaza, B. L., et al. Unpublished data.
- 42.Rodriguez R, Moreno A, Guzman F, Calvo M, Patarroyo M E. Studies of owl monkeys leading to the development of a synthetic vaccine against the asexual blood stages of Plasmodium falciparum. Am J Trop Med Hyg. 1990;43:339–354. doi: 10.4269/ajtmh.1990.43.339. [DOI] [PubMed] [Google Scholar]
- 43.Ruebush T K I, Campbell G H, Moreno A, Patarroyo M E, Collins W E. Immunization of owl monkeys with a combination of Plasmodium falciparum asexual blood-stage synthetic peptide antigens. Am J Trop Med Hyg. 1990;43:355–366. doi: 10.4269/ajtmh.1990.43.355. [DOI] [PubMed] [Google Scholar]
- 44.Scheller L F, Azad A F. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc Natl Acad Sci USA. 1995;92:4066–4068. doi: 10.1073/pnas.92.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schofield L, Ferreira A, Altszuler R, Nussenzweig V, Nussenzweig R S. Interferon-γ inhibits intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987;139:2020–2025. [PubMed] [Google Scholar]
- 46.Suhrbier A, Winger L A, Castellano E, Sinden R. Survival and antigenic profile of irradiated malarial sporozoites in infected liver cells. Infect Immun. 1990;58:2834–2839. doi: 10.1128/iai.58.9.2834-2839.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troye-Blomberg M, Riley E M, Perlmann H, Andersson G, Larsson A, Snow R W, Allen S J, Houghten R A, Olerup O, Greenwood B M, Perlmann P. T and B cell responses of Plasmodium falciparum malaria-immune individuals to synthetic peptides corresponding to sequences in different regions of the P. falciparum antigen Pf155/RESA. J Immunol. 1989;143:3043–3048. [PubMed] [Google Scholar]
- 48.van Binnendijk R S, Versteeg-van Oosten J P M, Poelen M C M, Brugghe H F, Hoogerhout P, Osterhaus A D M E, Uytdehaag F G C M. Human HLA class I- and HLA class II-restricted cloned cytotoxic T lymphocytes identify a cluster of epitopes on the measles virus fusion protein. J Virol. 1993;67:2276–2284. doi: 10.1128/jvi.67.4.2276-2284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitiello A, Ishioka G, Grey H M, Rose R, Farnass P, LaFond R, Yuan L, Chisari F V, Furze J, Bartholomeuz R, Chestnut R W. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. Induction of a primary cytotoxic T lymphocyte response in humans. J Clin Invest. 1995;95:341–349. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]