Abstract
Introduction
Cisplatin-induced nephrotoxicity (CIN) can be prevented by fluid hydration, electrolyte supplementation, or forced diuresis; however, the best way to prevent CIN is still unknown. The aim of this study was to provide objective evidence on the optimal design of hydration schemes to prevent CIN based on an update of the literature.
Methods
A Pubmed and Embase search were conducted in December 2021 and repeated in April 2022 and March 2023. Two independent reviewers screened the articles. The included articles were categorized and reviewed per category.
Results
Twenty-seven articles met the inclusion criteria. The included studies varied widely. Four out of seven studies investigating diuretics found a protective effect of adding mannitol to the hydration scheme. All six studies investigating duration and amount of volume of hydration found that a short-hydration scheme resulted in less CIN than a longer hydration scheme. Seven out of nine articles evaluating the role of electrolytes found that magnesium supplementation reduced the risk of nephrotoxicity. Three studies investigated the safety of oral hydration and concluded that nephrotoxicity did not occur more frequently after oral hydration.
Conclusion
The hydration scheme of cisplatin should be short and consist of a relatively small amount of volume. The scheme should include mannitol and magnesium supplementation. Head-to-head studies are needed to investigate the safety of furosemide compared with mannitol and the dose of mannitol and magnesium.
Keywords: cisplatin, kidney/drug effects, fluid therapy, diuretics, electrolytes, magnesium
This study aimed to provide objective evidence on the optimal design of hydration schemes to prevent cisplatin-induced nephrotoxicity based on an update of the literature.
Implications for Practice.
This study showed that hydration schemes should be short with a volume of 2 to 4 L administered in 4 to 5 hours. This short-hydration scheme prevents cisplatin-induced nephrotoxicity (CIN). Hospitalization is no longer necessary for fluid hydration in cisplatin treatment resulting in shorter hospital stays. The addition of mannitol and magnesium to hydration schemes resulted in lower incidence of CIN. The possibility of oral hydration is promising but could not be used in daily practice yet due to the absence of large studies confirming the preventive effect of oral hydration.
Introduction
Cisplatin or cisdiamminedichloroplatinum (II) (CDPP) is a well-known chemotherapeutic drug used for the treatment of solid and hematologic malignancies including leukemia, lymphomas, head and neck, bladder, ovarian, and lung cancers.1-6 As a platinum-based compound, one of the main dose-limiting side effects of cisplatin is nephrotoxicity,7,8 with an incidence of 20%-30%. Nephrotoxicity might already occur after one single dose of cisplatin.9,10 Cisplatin-induced nephrotoxicity (CIN) manifests as both chronic kidney injury and acute kidney injury (AKI), loss of electrolytes, such as magnesium and potassium,11 or polyuria.12 In addition, cisplatin is associated with other important side effects, such as ototoxicity and neurotoxicity.13
Different mechanisms underlie CIN.9 First, cisplatin is uptaken into kidney cells. Different transporters are involved in the uptake of cisplatin in kidney cells, such as organic cation transporter 2 (OCT2), copper transporter 1 (CTR1), and volume-regulated anion channels (VRAC).13,14 Second, cisplatin increases the expression of these uptake transporters while decreasing the expression of efflux transporters, resulting in accumulation of cisplatin. Then, this high concentration of cisplatin in the kidneys results in a cascade reaction of reactive oxygen species, inflammation, oxidative stress, vascular injury, necrosis, and apoptosis.14,15 In addition, cisplatin causes renal vasoconstriction resulting in a lower renal blood flow causing even more renal damage.16 Various mechanisms are described for ototoxicity, which overlap the mechanisms of CIN and could lead to permanent loss of hearing.17
Several strategies have been studied to reduce CIN of which hyperhydration is one of the most well-known interventions. The protective mechanism of hyperhydration is unclear, but a hypothesis is that volume expansion results in an increase in renal cisplatin excretion.16 The administration of electrolytes like sodium chloride (NaCl) is suggested to also have a protective effect on CIN by preventing decreased osmolality and thereby preventing osmotic stress responses.18 In addition, forced diuresis by adding furosemide or mannitol in a hydration scheme might lead to less nephrotoxicity due to faster elimination of cisplatin. Hypomagnesemia is a common adverse effect of cisplatin treatment due to damage to renal tubular cells and damage to the Ca2+/Mg2+ sensing receptor. Magnesium supplementation is suggested to have a protective effect on CIN by influencing renal transporters such as OCT2 and multidrug and toxin extrusion protein 1 resulting in less accumulation of cisplatin in the kidneys.19 The combination of these measures are hereafter referred to as “hydration schemes.”
As exploratory part of the study from Niggebrugge et al, a questionnaire among Dutch hospitals was conducted to gain insight into the differences of measures applied to prevent CIN.20 This survey was conducted to select 2 hospitals that differ the most in terms of hydration duration but were similar in terms of other variables. This survey revealed a large variety in hydration schemes used among hospitals and even within hospitals. Hydration schemes within hospitals varied depending on the indication of cisplatin. All hospitals added magnesium to the hydration fluid, but there were substantial differences between hospitals in the duration and volume of pre- and posthydration. Prehydration was defined as hydration before the cisplatin administration, where posthydration was defined as hydration after the cisplatin hydration. For example, the total duration of treatment ranged from 6.7 hours to 40 hours, and the volume of total treatment ranged from 3.2 to 6.5 Liters. Half of the hospitals used NaCl 0.9% as hydration fluid, most other hospitals used glucose 2.5%/NaCl 0.45% as hydration fluid.20 The European Society of Clinical Pharmacy Special Interest Group on Cancer Care (ESCP) is not clear about the optimal hydration scheme.21 A hydration scheme should consist of hydration before or after cisplatin or a combination of both and different types of fluid could be used. Also, diuretics or electrolytes could be added to the hydration scheme. Since the publication of the ESCP recommendations in 2008, several reviews have been published focusing on measures to prevent CIN. These reviews investigated different aspects of hydration schemes, such as the duration of hydration, adding electrolytes such as magnesium to hydration schemes, antioxidant treatment, and the preventive role of mannitol. A summary of the previously published reviews is shown in Table 1.22-27
Table 1.
Summery of previously published reviews.
| Author and year of publication | Inclusion period | Aim of the review | Conclusions |
|---|---|---|---|
| Launay-Vacher et al (2008)21 | Up to 2007 | To evaluate the prevention of cisplatin-induced nephrotoxicity. | Use saline solution infusion for an urine flow of 3-4 L/24 hours for 2 to 3 days. Do not use diuretics. Monitor magnesium levels and give magnesium if necessary. |
| Crona et al (2017)22 | 1966 to October 2015 | To evaluate clinical studies that have examined hydration and supplementation strategies to prevent CIN. | Short-duration low-volume hydration with magnesium supplementation is the best practice to prevent CIN. Mannitol could be used in selected patients. |
| Casanova et al (2020)23 | Up to December 2017 | To evaluate the efficacy of compounds that could be used to protect against CIN. | 1 g of magnesium intravenous seems to be the best compound to prevent CIN. |
| Danwilai et al (2021)24 | Up to February 2018 | To determine the clinical effect of magnesium supplementation to prevent CIN. | Magnesium supplementation should be used to prevent CIN. |
| Kandhare et al (2019) 25 | Up to February 2017 | To evaluate the efficacy of antioxidants against CIN. | Treatment with antioxidants results in a reduced risk of CIN. |
| Hamroun et al (2019)26 | January 1, 1978 to June 1, 2018 | To evaluate methods that will reduce the occurrence of cisplatin-induced acute kidney injury (CIA) | Magnesium supplementation is a protective method to reduce CIA. |
| Li et al (2021)27 | Up to May 2021 | To evaluate efficacy and safety of application of mannitol in hydration schemes of cisplatin. | Mannitol is effective and safe in reducing CIN but could result in adverse effects. |
Even after publication of the abovementioned reviews and ESCP recommendations, the best way to prevent CIN is still not clear. The substantial differences in hydration schemes within the Netherlands and absence of clear guidelines prompted us to write this narrative review. The aim of our study is to provide an objective update of the literature on the optimal design of hydration schemes to prevent CIN based on an update of the literature.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.28 The study protocol was registered with PROSPERO International Prospective Register of systematic reviews, registration number CRD42022321500.
Search Strategy
A search strategy was built in collaboration with a librarian and reviewed by a second librarian. The search strategy is presented in Supplementary material S1. A Pubmed and Embase search were performed in December 2021 and repeated in April 2022 and March 2023. Articles were screened and selected for exclusion by keywords by an online web application for exploring and filtering searches for eligible studies (Rayyan and Endnote).29 The keywords are presented in Supplementary material S2. The actual exclusion was done manually by 2 independent reviewers (CS and ES) based on title and abstract. Afterward, titles, abstracts, and full texts were screened based on the inclusion and exclusion criteria by 2 independent reviewers (CS and ES). Disagreements were resolved through discussion. The reasons of exclusion were recorded.
Study Selection
Studies were included if published since January 1, 2018, which was the end of the inclusion period of most previous published reviews.21-27 The studies had to be written in English or Dutch. Animal studies were excluded, as well as case reports, case series with less than 10 patients, reviews, congress abstracts, abstract-only publications, comments or letters, and in vitro studies. Studies were not excluded based on the quality of the study.
Data Extraction
A single reviewer (CS) conducted the data extraction and a second reviewer (ES) checked the data extraction to reduce the risk of bias. Collected data included first author, year of publication, aim of the study, study design, number of patients, indication of cisplatin, in- and exclusion criteria, cisplatin dose, number of cisplatin cycles, time interval between cycles, characteristics of the hydration regimen (volume, time of administration, and type of fluid), addition of electrolytes, time points at which kidney function was measured, use of diuretics, use of other drugs, definition of nephrotoxicity, endpoints, results, limitations, and strengths of the study. The included articles were categorized and reviewed per category.
Results
Search Results
The search yielded 5396 articles in Pubmed and Embase. Removing results by computerized tools (Endnote and Rayyan) based on language, publication date, or duplicates before screening of title and abstract resulted in 908 eligible articles. Of these, 831 articles were marked for exclusion based on keywords and manually excluded based on title or abstract. Titles and abstracts of the remaining 77 articles were screened, leading to 32 articles being assessed for inclusion by means of in-depth reading. This assessment resulted in the exclusion of another five records: one exclusion was based on endpoints, three exclusions were based on publication type, and one exclusion was based on the number of patients. As a result, 27 articles were included in the review (Fig. 1). Subsequently, the included articles were grouped into the following categories: diuretics, hydration duration and volume, addition of electrolytes, route of administration, and others. One article has been assigned to multiple categories.30
Figure 1.
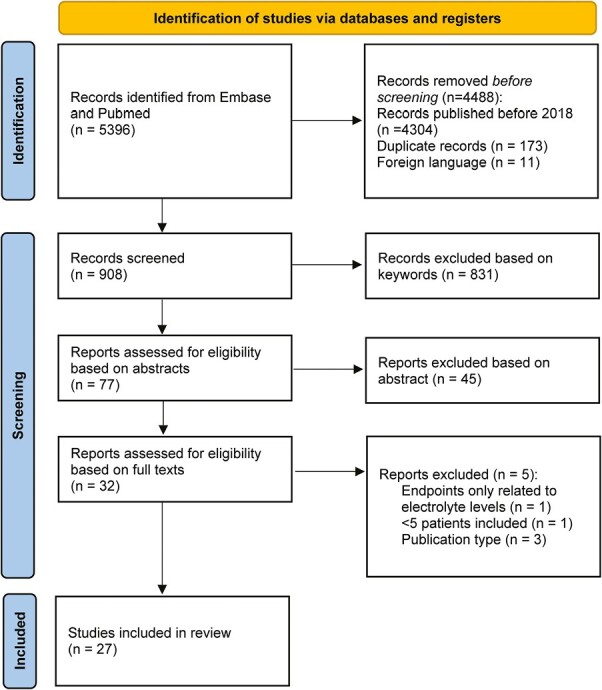
PRISMA flow chart.
Diuretics
Seven of the included articles investigated the protective effect of diuretics.31-37 All trials used mannitol as a diuretic, and one study compared the use of mannitol with furosemide.35 The studies varied widely regarding the mannitol dose, time interval between cycles, cisplatin dose, definition of nephrotoxicity and in the way nephrotoxicity was determined. Four studies found a protective effect of adding mannitol to the hydration scheme.31-33,37 The dose of mannitol in these studies ranged from 12.5 g to 100 g. Another study found a significant protective effect of mannitol 12 g in schemes with cisplatin doses of 50 mg/m2. In schemes with cisplatin doses higher than 50 mg/m2, all patients received mannitol, and only the dose of mannitol was investigated. There was no association found between the mannitol dosage and nephrotoxicity in schemes with cisplatin doses higher than 50 mg/m2,34 and in one study, no statistic difference was found.36 In contrast, more patients developed AKI in the mannitol group; however, this was not statistically significant, and more patients received a cisplatin dose of 100 mg/m2 in the mannitol group (84%) compared with the control group (45%).36 One study compared the renal protective effects of furosemide and mannitol and concluded that there were no statistical differences between the 2 diuretics.35
Duration and Hydration Volume
Six articles investigated the impact of the duration of hydration and the hydration volume.20,30,38-41 In all studies, cisplatin doses were ≥60 mg/m2, and one was a single arm study with a small study population.30 It should be noted that in one study, the dose and administration of diuretics were different for the intervention and control group.41 Five studies compared 2 groups: a short-hydration group and a conventional hydration group as a control group.20,38-41 In the short-hydration group, the duration of fluid administration was shorter and consisted of a smaller hydration volume, ranging from 1.9 to 4.3 L for the short-hydration group and 4.5 to 7.8 L for the conventional hydration group. In the studies describing both a short and a conventional hydration group, other variables with potential impact on the degree of nephrotoxicity, such as the administration of a diuretic or cisplatin dose, were comparable. Patients in the short-hydration groups mostly received hydration during 4 or 5 hours, except for one study, in which the short-hydration group received intermittent intravenous hydration on days 1, 2, and 3 (6 hours on day 1, 4 hours on days 2 and 3).39 Patients in the conventional hydration group received hydration during 24 hours or more (range 24 hours to 4 days). All studies found that a short-hydration scheme resulted in less CIN than a conventional hydration scheme based on creatinine evaluation or eGFR decline. The cutoff values of nephrotoxicity were different in the studies, see Table 2 for details.
Table 2.
Overview of included studies.
| Author and year of publication | Purpose of study and type of intervention to reduce CIN | Study type | Cisplatin dose + number and time interval cycles | Hydration regimen (volume, time of administration, and type of fluid) | Formula used for renal function estimation and definition of nephrotoxicity | Results |
|---|---|---|---|---|---|---|
| Andarsari et al (2021)33 | To investigate the renoprotective effect of mannitol 20% 500 mL in patients with head and neck cancer. Type of intervention: diuretics |
Observational single-arm cohort study (n = 52) | 35 mg/m2-100 mg/m2 (3-6 cycles) Time interval per cycle: not described. |
Mannitol (20% 500 mL) and NS after cisplatin. | Renal function estimation: CG equation. Definition of nephrotoxicity: not described |
No significant differences between the cisplatin-5 fluorouracil group and cisplatin-paclitaxel group. The average value of serum creatinine and BUN were within the normal limit. Mannitol can maintain the value of creatinine levels in patients within normal limit |
| Aoyama et al (2020)39 | To compare the efficacy of CH vs. SH scheme’s on the degree of kidney function Type of intervention: duration and hydration volume |
Retrospective cohort study (n = 26) | Majority of the patients 80 mg/m² Time interval per cycle: not described |
SH scheme: n = 7 Day 1: 2200 mL NS in 6 hours Day 2 + 3: 1050 mL NS in 4 hours KCl 20 mEq, MgSO4 20 mEq and 20 mg furosemide. CH scheme: n = 19 Day 1: 3200 mL NS in 24 hours Day 2 + 3: 1000 mL RS and 500 mL AC in 6 hours KCl 20 mEq and 20 mg furosemide |
Renal function estimation: Not calculated Definition of nephrotoxicity: Increase of serum creatinine >0.3 mg/dL or more than 50% of the basal value. |
More nephrotoxicity in the CH group(42.1% vs. 0%). Higher treatment completion rate for the SH regimen (100% vs. 73.6%) Less-dose reduction in the SH group (42.8% vs. 35.7%). |
| Ashrafi et al (2019)42 | To compare the influence of magnesium on nephrotoxicity Type of intervention: addition of electrolytes |
Retrospective multicenter cohort study (n = 46) | 50-100 mg/m2 every 3 weeks | Cisplatin in 500 mL NS in 2h. Group 1 n = 22: 1L NS with 10 mEq KCl 15% and 1g MgSO4 50% in 1 hour before and after cisplatin. Group 2 n = 24: 1L NS with 10 mEq KCl. Patients < 1.8 mg/dL serum Mg level received Mg. |
Renal function estimation: CG equation Definition of nephrotoxicity: increase of serum creatinine >0.5 mg/dl and of GFR reduction >50% |
Significant difference in serum creatinine. Risk of nephrotoxicity in group 1, 19% more than in group 2. No significant difference in serum magnesium levels. |
| Ashrafi et al (2020)43 | To evaluate the effect of vitamin E on nephrotoxicity due to cisplatin. Type of intervention: others |
Prospective randomized double-blind placebo controlled study (n = 51) | 50-75 mg/m2 every 3 weeks for 4 cycles | Treatment group n = 26: One tablet 400 IU vitamin E/day Control group n = 25: Placebo tablet once daily Cisplatin in 1L NS + 20 mEq KCl + 1g of MgSO4 in 3 hours + 500 mL NS in 2 hours. |
Renal function estimation: MDRD formula Definition of nephrotoxicity: not given. |
Serum creatinine was significant decreased in the vitamin E group compared with the control group in the first, second, fourth cycle and 1 month after chemotherapy. (P < .05) The GFR was not reduced in the vitamin E group. (P = .001) |
| Bégin et al (2021)31 | To compare nephrotoxicity between hydration with or without mannitol. Type of intervention: diuretics |
Retrospective single-center study (n = 1821) | <75 mg/m² and ≥75 mg/m², (1-6 cycles) Time interval per cycle: not described |
Cisplatin dose <75 mg/m²: 2L + 1L Cisplatin dose ≥75 mg/m²: 3L + 1L Mannitol group: n = 658 1L NS with mannitol + 1L dextrose 5% NaCl 0.45%. Mannitol dose: cisplatin <75 mg/m²: 12.5 g cisplatin ≥75mg/m²: 25 g D5%-0.45% NaCl group: n = 732 NS altering with D5%-0.45 NaCl NS group: n = 431 NS alone |
Renal function estimation: CG equation Definition of nephrotoxicity: AKI according to CTCAE version 4.03. |
Risk of all grade cisplatin associated AKI lower for the mannitol group (HR = 0.62; 95%CI, 0.42-0.89. The effect of mannitol on AKI is different for patients with different malignancies. No statistical difference in nephrotoxicity between hydration with or without mannitol in patients receiving cisplatin doses ≥75 mg/m2 for most malignancies |
| Chantharakhit et al (2022)41 | To evaluate the efficacy of short hydration for nephrotoxicity for short hydration in comparison with conventional hydration in intermediate to high-dose cisplatin-based chemotherapy regimen. Type of intervention: duration and hydration volume |
Prospective non-randomized controlled cohort study (n = 30) | Medium to high dose every 3 weeks | SH scheme: n = 14 Pre-hydration: 1L NS with KCl 10 mEq + 50% MgSO4 2 mL + 20% mannitol 200 mL Posthydration: 500 mL + 10 mEq KCl Total: 1.5-2 L hydration in 6 hours CH scheme: n = 16 Prehydration: 3L NS in 24 hours on day 0, 20% mannitol 65 mL on day 1. Posthydration: 1L D5NS + 20% mannitol 135 mL + 10% MgSO4 10 mL + KCL 20 mEq Total: 4-4.5L in 30 hours |
Renal function estimation: unknown Definition of nephrotoxicity: eGFR decline >20% from the baseline after cisplatin administration |
SH before intermediate to high dose cisplatin administration reduce the risk of acute kidney injury significantly (OR 0.06; 95%CI, 0.003, 0.990. P-value .049). The levels of eGFR were stable in the SH group and declined in the CH group with a significant difference (P-value = .001). |
| Dhillon et al (2019)34 | To investigate the association of the mannitol dose on the development of nephrotoxicity and to determine an optimal mannitol dosing schedule. Type of intervention: diuretics |
Retrospective case-control study (n = 1245) | ≥50 mg/m2, ≥1 cycle Time interval per cycle: not described |
For cisplatin doses of >50 mg/m2:12 g or 20 g mannitol postcisplatin or 20 g mannitol pre and postcisplatin. For cisplatin doses of 50 mg/m2: 12 g postcisplatin or mannitol is not given. The hydration fluid and addition of electrolytes is unknown. 237 patients were matched to 1008 controls. |
Renal function estimation: MDRD formula Definition of nephrotoxicity: AKI according to CTCAE for grades 1-3. Grades 4 or 5: NCI CTCAE |
237/1462 developed nephrotoxicity. Cases: 31% hypomagnesemia Control: 20% hypomagnesemia Overall, no association between mannitol dosage and nephrotoxicity. For cisplatin doses of 50 mg/m2: Less nephrotoxicity after 12 g mannitol in comparison with no mannitol administration. |
| El Hamamsy et al (2018)44 | To investigate the effect of acetazolamide compared with mannitol in reducing nephrotoxicity. Type of intervention: others |
Prospective controlled pilot study (n = 35) | Mean 75 mg/m² every 3 weeks for 3 cycles | ACTZ group n = 15: 250 mg acetazolamide 30 minutes before cisplatin and NS administration. Mannitol group n = 20: Mannitol 20% 100 mL 30 minutes before cisplatin and NS administration |
Renal function estimation: CG equation Definition of nephrotoxicity: RIFLE-criteria |
ACTZ group: increased creatinine clearance Mannitol group: creatinine clearance decreased and more dose reductions. A statistic difference in creatinine clearance for the first (P = .014), second (P = .001) cycle but not for the third cycle (P = .18). |
| Hägerström et al (2019)32 | To compare nephrotoxicity between hydration with or without mannitol in patients with head and neck cancer. Type of intervention: diuretics |
Single center retrospective cohort study (n = 78) | Weekly 40 mg/m2 for 5 till 6 weeks | Mannitol group n = 39: 2.5L NS + 500 mL of 15% mannitol infusion during chemotherapy Control group n = 39: 2.5L NS only |
Renal function estimation: Unknown Definition of nephrotoxicity: Unknown |
Mean value of 51Cr-EDTA after 3 cycles: 96.4 mL/minutes (mannitol group) vs. 92.3 mL/minute (control group). The largest decline of 51Cr-EDTA in the control group. A significant smaller decrease of 51Cr-EDTA in the mannitol group. Decline in eGFR was not significant. |
| Hase et al (2020)30 | To investigate the feasibility of a SH regimen with 20 mEq of Mg for patients with lung cancer. Type of intervention: duration and volume of hydration and addition of electrolytes |
Prospective single-arm single-center study (n = 40) | ≥60 mg/m², 2 or 4 cycles every 3-4 weeks | Mannitol 20% 300 mL + 20mEq MgSO4 before cisplatin infusion Cisplatin in 500 mL NS. Solita T1 2L (containing Na (90 mEq/L), Cl (70 mEq/L), L-lactate (20 mEq/L), and glucose (26 g/L)) Total duration: 5 hours Total volume: 2.3 L |
Renal function estimation: CG equation Definition of nephrotoxicity: CTCAE version 4.0 based on creatinine values. |
Patients with ≥ grade 2 Cr elevation: 97.5%. |
| Horinouchi et al (2018)45 | To investigate the effect of oral hydration with OS-1 on nephrotoxicity in patients with lung cancer. Type of intervention: method of administration |
Multi-center single-arm prospective study (n = 46) | ≥60 mg/m² every 3-4 weeks. (1-5 cycles) | 500 mL NS with 8 mEq MgSO4 and 10 mEq KCl + 200 mL 20% mannitol + cisplatin 250 mL NS in 1 hour + 500 mL oral OS-1 500 mL OS-1 consist of: Na 25 mEq, Cl 25 mEq, Ka 10 mEq, MgSO4 1 mEq, lactate 15.5 mEq, glucose 9 g |
Renal function estimation: CG equation Definition of nephrotoxicity: CTCAE V.4.0 |
Patients with ≥ grade 2 Cr elevation: 2.2% Patients with grade 1 Cr elevation: 54.3%-85% completed the planned cycles |
| Karvan et al (2022)46 | To investigate the effect of melatonin on the prevention of CIN. Type of intervention: other |
Randomized controlled clinical study (n = 66) | 50-150 mg/m2 for 1 cycle | Melatonin group: n = 36 Melatonin administration 24 hours before cisplatin continued for 5 days at a dose of 20 mg Control group: n = 30 Both: 1L NS with 10 mL KCl 15% w/v and MgSO4 50% w/v + 500 mL NS |
Renal function estimation: Unknown Definition of nephrotoxicity: AKIN criteria |
AKI stage 1: 3 (8.3%) in melatonin and 8 (26.7%) in control group. (P = .096) GFR after 24 hours: increased and then decreased significantly after 5 days |
| Kimura et al (2018)47 | To analyze the effect of Mg on nephrotoxicity in patients with head and neck cancer. Type of intervention: addition of electrolytes |
Retrospective cohort study (n = 121) | 80 mg/m² on day 6 every 3 weeks for 2 cycles | Both groups: 20 mg furosemide Mg group n = 56: 1L NS with 20 mEq MgSO4 3 hours + cisplatin + 2 L of Soldem 3A (Na 35 mEq/L, K 20 mEq/L, L-lactate 20 mEq/L, glucose 43.0 g/L) 3 days Crystalloid-only group n = 65: 1L ringer lactate + 2 L NS + cisplatin + 2 L of Soldem 3A (Na 35 mEq/L, K 20 mEq/L, L-lactate 20 mEq/L, glucose 43.0 g/L) 3 days |
Renal function estimation: CG equation. Definition of nephrotoxicity: 20% reduction in Ccr after 2 cycles. |
No significant difference in CCr after 1 cycle Lower incidence of nephrotoxicity in Mg group. Decrease CCr 4.9 mL/minute for Mg group and 15.0 mL/minute for conventional group. |
| Konishi et al (2018)48 | To investigate the effect of Mg in relation to PTH and PTH-rP levels. Type of intervention: addition of electrolytes |
Single center prospective observational study (n = 55) | 70 or 80 mg/m² every 3 weeks | 1L NS in 2-3 hours + 2 L electrolyte liquid (including sodium, potassium, and calcium), 60 g of D-mannitol and 20 mg of furosemide. Mg group n = 37: 8 mEq of MgSO4 on day 1 Control group n = 18: no MgSO4 |
Renal function estimation: Not calculated Definition of nephrotoxicity: a > 1.1-fold increase creatinine levels according to CTCAE. |
Mg group: significant lower creatinine levels. 8.1% grade 1 creatinine increases Control group: 22.2% grade 1 and 5.6%, grade 2 creatinine increases |
| Kubo et al (2019)49 | To investigate the effect of Mg premedication on nephrotoxicity in patients with esophageal cancer. Type of intervention: addition of electrolytes |
Single center retrospective cohort study (n = 105) | 70 mg/m² every 3 weeks (1-3 cycles) | Day 0: 2000 mL NS. Day 1: Cisplatin in 2 hours in combination with 5-FU, docetaxel, mannitol, and 2500 mL fluid. Day 2 till 5: 5-FU + 2 L fluid Mg group n = 65: MgSO4 in 100 mL NS 1 hour before cisplatin administration. Control group n = 40: no MgSO4 |
Renal function estimation: CG equation. Definition of nephrotoxicity: Increase in serum Cr level of grade 2 or higher, according to the CTCAE. |
Less change in serum creatinine for the Mg group (0.094) versus the control group (0.247). (P = .0116) Less grade 2 and higher nephrotoxicity in the Mg group |
| Makimoto et al (2021)35 | To compare the effect on nephrotoxicity of furosemide and mannitol in patients with advanced NSCLC. Type of intervention: diuretics |
Two-arm, prospective, randomized single-center phase II study (n = 44) | ≥75 mg/m², every 3-4 weeks (1-6 cycles) | 1.7 L fluid in 3h + 4 mEq MgSO4 before and after a cycle of cisplatin. FUR group n = 22: Bolus furosemide after the cisplatin administration. MAN group n = 22: Mannitol infusion over 30 minutes just before cisplatin administration |
Renal function estimation: Unknown. Definition of nephrotoxicity: grade 1 ≥ according to CTCAE version 4.0. |
Creatinine elevation: no significant difference FUR group: n = 2 MAN group: n = 4 Median worst creatinine score: FUR group: 0.79 mg/dL (0.52-1.07) MAN group 0.71 mg/dl (0.48-1.38) |
| Minzi et al (2020)50 | To investigate the effect of supplementation with K, Mg, and Ca in patients with solid tumors. Type of intervention: addition of electrolytes |
Non-randomized interventional study (n = 99) | Cisplatin dose ≥50 mg/week Number of doses: not described. |
Cisplatin in 1 L NS in 90 minutes. Intervention group n = 49 3 L NS with 1.5 g KCl, 1 g MgSO4 and 1 g C12H22CaO14 in 6 hours. Control group n = 50 3 L NS in 6 hours. |
Renal function estimation: Not calculated. Definition of nephrotoxicity: Serum creatinine elevation >1.5 times that at baseline (grade = 1) |
Day 7, 14, and 21 significant lower serum creatinine levels in the intervention group. The baseline and serum creatinine level at day 28 were comparable |
| Miyoshi et al (2022)51 | To investigate the difference of 20 mEq Mg with 8 mEq Mg in nephrotoxicity. Type of intervention: addition of electrolytes |
Multicentre, retrospective, observational study (n = 272) | Not described, patients were matched based on the dose | Hydration methods (>2.5 L): the conventional high-volume hydration with 20 mEq Mg or 8 mEq Mg. 20 mEq Mg: n = 136 8 mEq Mg: n = 136 |
Renal function estimation: CG equation. Definition of nephrotoxicity: increase in SCr of 1 grade according to CTCAE ver. 4.0 for AKI. |
≥Grade 1: 18.4% in the 8 mEq group and 20.6% in the 20 mEq group. Grade 2 elevations: 2.9% in the 8 mEq group and 3.7% in the 20 mEq group. No significant difference (P = .926) |
| Naiki et al (2020)38 | To investigate nephrotoxicity and electrolyte abnormalities in SH regimen. Type of intervention: duration and hydration volume |
Retrospective study (n = 101) | 70 mg/m² (2-12 cycles) Time interval per cycle: not described |
SH regimen n = 48: 1900 mL on day 1. Patients received KCl 20 mEq, 8 mEq MgSO4 and 200 mL mannitol. CH regimen n = 53: Day 1: 1.5-2 L in 4-6 hours followed by gemcitabine treatment. Day 2: 2 L followed by cisplatin treatment + 3 L. |
Renal function estimation: Not calculated. Definition of nephrotoxicity: not described. |
No difference in median total cycles Nephrotoxicity was unchanged in both groups The incidence of severe hyponatremia was higher in the CH group |
| Niggebrugge-Mentink et al (2020)20 | To compare the nephrotoxicity in a SH or LH scheme for patients with NSCLC. Type of intervention: duration and hydration volume |
Retrospective cohort study (n = 100) | Dose: unknown, 1 till 4 cycles. Time interval per cycle: not described |
SH scheme n = 50: 1 L NS in 2 hours + 1 L NS in 2 hours Cisplatin: in 1 L in 2 hours KCl 20 mmol and MgSO4 24.36 mmol. LH scheme n = 50: 1 L NS in 2 hours + 4L in 24 hours. Cisplatin: 0.5 L in 2 hours KCl 80 mmol and MgSO4 9.8 mmol. |
Renal function estimation: CKD-EPI equation Definition of nephrotoxicity: AKI grades 1 to 3 according to KDIGO guideline |
LH group: less cisplatin cycles, more discontinuation due to nephrotoxicity and more AKI grade 1 and 2 LH group: eGFR after 2 cycles: −9 (−22 to −2) and -13 (−22 to − 4) after 4 cycles SH group: eGFR after 2 cycles 1 (−6 to 5) and −6 (−16 to − 1) after 4 cycles |
| Puisset et al (2019)52 | To investigate nephrotoxicity after oral hydration post-cisplatin. Type of intervention: method of administration |
Single-center retrospective study (n = 517) | 75 mg/m2 (50-81 mg/m2) (1-3 cycles) Time interval per cycle: not described |
Before: 2 L NS with 2 g KCl and 2 g MgSO4 in 6 hours. After: IV/IV group n = 241: 1 L NS, 1 g KCl, and 1 g MgSO4 in 2 hours. IV/PO group n = 276 Drink as much water as possible, at least 1 L/day. |
Renal function estimation: MDRD formula Definition of nephrotoxicity: CTCAE version 4.0 and the RIFLE criteria |
More grade ≥ 1 nephrotoxicity in the IV/IV group (39.4%) vs. IV/PO group (25.7%, P = .001) Grade ≥ 2 nephrotoxicity in 3.4% in the IV/IV group and 1.8% in the IV/PO group (P = .27) No significant difference in switching to carboplatin |
| Rachman et al (2022)36 | To compare the risk of AKI in patients receiving high-dose cisplatin with and without addition of mannitol. Type of intervention: diuretics |
Ambispective study: combination of prospective and retrospective study (n = 110) | ≥75 m/m2 for one cycle (first, second, third, or fourth cycle) | Treatment group: n = 63 Addition of mannitol to 1-2 L 0.9% NS in 1-2 hours prior to cisplatin. Mannitol dose: 20 g in 0.9% 100 mL NS Control group: n = 47 1-2 L 0.9% NS in 1-2 hours prior to cisplatin Cisplatin: in 500 mL 0.9% NS in 2-3 hours |
Renal function estimation: unknown Definition of nephrotoxicity: grade 1-4 of AKI according to CTCAE v 4.0. Grade 1 AKI: 0.3 mg/dl or 1.5 times from baseline creatinine. |
More AKI was observed in the mannitol group but not statistically different. (22.6% vs. 10.4%, P = .076) The increase in serum creatinine was 29% in the mannitol vs 16.7% in the control group. |
| Sainamthip et al (2022)37 | To evaluate the benefit of mannitol combined with hydration to prevent cisplatin-induced nephrotoxicity. Type of intervention: diuretics |
Randomized, double-blind placebo controlled phase II trial (n = 48) | 40-100 mg/m2 for one cycle | 2 L NS in 25 hours prehydration followed by cisplatin administration. Posthydration: 1 L NS + 10 mL 1-% magnesium sulfate + 20 mEq KCl in 6h, followed by 100 mL 20% mannitol (20 g) or placebo in 30 m. Thereafter 2 L NS in 25 hours. Mannitol group (n = 23): 100 mL 20% mannitol (20 g) Control group (n = 25): Placebo |
Renal function estimation: GFR was calculated based on 24 hours urine. Definition of nephrotoxicity: AKIN criteria: serum creatinine level of ≥0.3 mg/dL or ≥50% urine output of <0.5 mL/kg/h> 6 hours. |
After 48 hours after receiving cisplatin: no statistically significant difference in AKI between the mannitol and control group. At 7 days: 4.5% of the mannitol group had a reduced eGFR compared with 36.4% of the control group which was significant different. (P = .021) A low eGFR was developed in 13.6% of the mannitol group vs. 48% in the control group (P = .012). |
| Saito et al (2022) 53 | To determine if ORS affects CIN and electrolyte imbalance in a real-world setting. Type of intervention: method of administration |
Retrospective control study (n = 200) | ≥75 mg/m2 mean treatment cycles: 3. Time interval per cycles: not described |
ORS group n = 133: 1 L ORS on days 1-3 Control group n = 67: 1 L beverage other than ORS on day 1-3 Prehydration: 500 mL 0.25% NS + 10 mEq KCl in 1 hours. Mannitol 20% + 8mEQ MgSO4. Cisplatin: 250 mL NS in 1 hour. Posthydration: 500 mL 0,25% NS + 10 mEq KCl in 1 hour |
Renal function estimation: Not calculated Definition of nephrotoxicity: grade ≥2 SCr elevation (1.5× baseline or 1.5× upper limit of normal) |
Incidence of CIN was 9.8% in the ORS group and 7.5% in the control group but not statistical significant (P = .31). The variation of SCr levels was similar between the groups. |
| Sugisaki et al (2022)54 | To evaluate the correlation between preloading with 20 mEq Mg and CIN in patients with esophageal cancer. Type of intervention: addition of electrolytes |
Retrospective single-center study (n = 160) | 70-80 mg/m2 per cycle ever 3-4 weeks for 1 till 2 cycles | The Mg group n = 84 1.5 L + 500 mL + 1L. MgSO4 20 mEq (2.46 g) and 20 mg of furosemide just before cisplatin. Control group n = 76 1.3 L + 0.3 L + 1.9 L 20 mg of furosemide before cisplatin. Both groups: on days 2-4, 1-2 L. |
Renal function estimation: CG equation. Definition of nephrotoxicity: c 2 according to CTCAE v. 5.0. AKI: KDIGO criteria. |
Prevalence eCcr ≥ grade 2: 4% for Mg group and 13% for control group (P = .027) Prevalence of AKI and median SCr levels did not differ significantly between the groups |
| Suppadungsuk et al (2022)55 | To determine the effect of preloading Mg 16 mEq on AKI and AKD in patients with head and neck cancer. Type of intervention: addition of electrolytes |
Prospective randomized, single-blinded, parallel controlled, single-center study (n = 30) | 40 mg/m2 weekly for 7-8 weeks | Intervention group n = 15 500 mL NS + KCL 20 mEq in 4 hours + 10% MgSO4 16 mEq with 500 mL NS + KCL 20 mEq. Control group n = 15 500 mL NS + KCL 20 mEq in 4 hours. |
Renal function estimation: CKD-EPI formula. Definition of nephrotoxicity: AKI: increase SCr ≥0.3 compared to previous week. AKD: increase SCr ≥ 0.3 between the precisplatin baseline last SCr. |
AKI: more in control group than in treatment group but not statistically significant AKD was significantly higher in the control group. SCr was unchanged in the Mg group and increased in the control group. (mean SCr increase 0.36, 95% CI, ¼ 0.19-0.52, P < .001) |
| Tanaka et al (2018)40 | To compare nephrotoxicity between SH and CH regimen with thoracic malignancies. Type of intervention: duration and hydration volume |
Constructive retrospective non-randomized analysis (n = 467) | ≥ 60 mg/m² (1-6 cycles) Time interval per cycles: not described |
KCl in both regimes, MgSO4 not always in the CH group. SH regimen n = 111 0.5 L in 1 hours + 200 mL Mannitol 20 in 30 minutes + cisplatin + 0.5 L in 1 hours. CH regimen n = 356 1 L in 4 hours + cisplatin + 200 mL mannitol in 2 hours + 1 L in 4 hours Total: 3.2-3.6 L of fluid in 12 hours. Each day after for at least 3 days: 1-2 L. |
Renal function estimation: CG equation Definition of nephrotoxicity: serum creatinine level > 1.1 for men and > 0.8 mg/dL women |
SH group: 3.6% creatinine increase ≥grade 1 after the first cycle and 14.4% after the last cycle. 0% creatinine increase ≥grade 2. CH group: 13.5% creatinine increase ≥grade 1 after the first cycle and 33.1% after the last cycle. 4.2% creatinine increase ≥grade 2. More dose reduction or discontinuation |
Abbreviations: AC: glucose-added acetic acid solution; AKD: acute kidney disease; AKI: acute kidney injury; BUN: blood urea nitrogen; Ca: calcium; CCr: creatinine clearance; CG: Cockcroft-Gault; CH: Conventional hydration; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; 51Cr-EDTA: Chrome51-Ethylenediaminetetraacetic acid; CTCAE: Common terminology criteria for adverse events; FUR: furosemide; IV: intravenous; K: potassium; KDIGO: Kidney Disease Improving Global Outcomes; MAN: mannitol; MDRD: modification of diet in renal disease; Mg: magnesium; NS: normal saline; NSCLC: non-small cell lung cancer; OS-1 = Commercially available oral hydration solution, Otsuka Pharmaceutical Factory, Tokushima, Japan; PO: Per os; PTH: parathyroid hormone; PTH-rP: parathyroid hormone-related peptide; RIFLLE: risk, injury, failure, loss and end-stage kidney disease; RS: Ringer’s lactate solution; SCr: serum creatinine; SH: short hydration.
Addition of Electrolytes
Nine articles evaluated the role of electrolytes in hydration schemes.30,42,47-51,54,55 In two studies, weekly schemes with doses of >50 mg or 40 mg/m2 were used. Seven studies dosed cisplatin every 3 weeks in doses ≥50 mg/m2,30,42,47-49,54 and in 2 studies, weekly schemes with doses of >50 mg or 40 mg/m2 were used.50,55 All studies investigated the role of magnesium whether or not in combination with other electrolytes such as sodium chloride (NaCl) (n = 7),30,42,47-50,55 potassium chloride (KCl) (n = 5),42,47,48,50,55 calcium (Ca) (n = 1),48 and calcium gluconate (C12H22CaO14) (n = 1).50 However, the magnesium dose varied between the studies (8-20 mEq). Of the 9 studies, 7 studies consisted of both an intervention and a control group,42,47-50,54,55 one study investigated different doses of magnesium without a control group51 and one study did not have a control group.30 Also, the endpoints used to define a decline in renal function differed between the studies. The applied definitions have been described in Table 2. Seven of the 9 articles found that magnesium supplementation protected against the development of nephrotoxicity.30,47-50,54,55 In contrast, one study did not find a protective effect of magnesium supplementation.42 The only study focusing on the influence of varying doses of magnesium found no differences in effect between doses.51
Method of Administration
Three studies investigated the influence of the administration route of the hydration fluid.45,52,53 One study consisted of two groups, an intravenous (IV)/intravenous (IV) and intravenous/oral (PO) group,52 and one study did not have an IV/IV group as control group.45 In both studies, the prehydration fluid (ie, hydration before administration of cisplatin) was administered intravenously. The type of oral fluid used differed between the two studies: water or an oral fluid containing sodium, chloride, potassium, magnesium sulfate, lactate, and glucose. Both studies found that oral hydration did not result in more nephrotoxicity,45,52 and one study suggested that orally administered posthydration (ie, hydration after administration of cisplatin) of at least 1 L water per day might even be better than intravenously administered hydration.52 The incidence of nephrotoxicity grade ≥1 was 39.4% in the IV before/IV after group and 25.7% in the IV before/PO after group (P = .001).52 In the third study, the type of oral hydration was investigated. In this study, commercially available Oral Rehydration Solution (ORS) such as OS-1 was compared with other beverages.53 ORS intake was not associated with CIN but the ORS group experienced more anorexia. It is unknown if this is related to the ORS intake or is associated with the differences in baseline characteristics at the beginning of the study.
Other Interventions
The influence of acetazolamide, vitamin E, and melatonin are investigated in three studies.43,44,46 All of these studies had a small study population but revealed potentially effective methods for the prevention of CIN and, therefore, topics for further investigation. Oral acetazolamide in a dose of 250 mg before cisplatin administration was found to be effective in the reduction of nephrotoxicity.44 Melatonin administration in a dose of 20 mg daily for 5 days also resulted in less nephrotoxicity.46 The use of vitamin E did has a significant effect on serum creatinine concentrations in the first, second, fourth cycle, and 1 month after chemotherapy.43
Discussion
This systematic review investigated the effect of several hydration schemes with and without additional supplements to prevent cisplatin-related nephrotoxicity. Between January 2018 and March 2023, 27 relevant new articles have been published. Of these, only five studies were randomized controlled trials, and more than half (15/27) of the studies had a retrospective design. The quality of most studies was low; endpoints were poorly described, there was no power calculation conducted upfront, or the definition of nephrotoxicity was not given. Although most studies had a control group with parameters comparable with the intervention group, there was a large heterogeneity among the included studies. For example, the cisplatin doses, intervals between the cisplatin cycles and number of cisplatin cycles being studied were different, but also the applied definitions of nephrotoxicity (when provided) and endpoints, as well as different hydration schemes being used. These differences complicate the comparison between the studies.
The type of hydration fluid, such as normal saline, glucose, a combination, or Ringer’s lactate was not subject of investigation in any of the studies even though different fluids are being used in the included studies. This assumes that the type of hydration fluid does not affect the risk of nephrotoxicity, however, without underlying evidence. Also, the timing of fluid hydration, before or after the cisplatin administration, was has never been investigated, although differences in the moment of fluid administration were observed in the included studies. Therefore, it is not possible to draw conclusions regarding the best moment of fluid administration.
Based on the results in our review, the application of mannitol is useful, which is in contrast with earlier conclusions,21,22,26 but comparable with the recent study from Li et al.27 The positive contrast with earlier results might be due to the different doses of the mannitol in the included studies. Due to over-diuresis, nephrotoxicity could occur. Moreover, the treatment of cisplatin has been optimized due to the use of antiemetic’s over the years resulting in less vomiting and dehydration. The comparison with recent studies is, therefore, more in concordance with the current treatment and real-world situation. Due to the heterogeneity in study design, a general dose of mannitol for all patients could not be given. Dosing should, therefore, be based on the Summary of Product Characteristics (SmPC), which describes a loading dose followed by dosing based on the degree diuresis taking the hydration scheme into account.
Besides the addition of mannitol, a shorter, low volume hydration scheme (<24 hours) should be preferred over a longer and more voluminous hydration scheme. This substantiates the previous conclusions of Crona et al22 but seems in contrast with the ESCP recommendations which recommend to aim for an urine flow of 3 till 4 L per day for 3 days21 (based on expert opinion). Given the homogeneous results of the studies included in this review, which were performed in the presence of different types of malignancies, it should be concluded that a short-hydration scheme should be applied for all patients receiving cisplatin treatment. In addition, a short-hydration scheme is more patient friendly and could reduce healthcare costs because of a shorter in hospital stay.
The third effective preventive measure was the addition of magnesium to the hydration scheme. All studies showed a positive effect of adding magnesium to the hydration scheme except for one article who found a conflicting result.42 However, the quality of this latter study was low. The addition of magnesium to the hydration schemes is aligned by the conclusions of the reviews of Crona et al, Casanova et al, Hamroun et al, Danwilai et al, and the ESCP recommendation.21-24,26 The mechanism of this renal protective effect of magnesium addition is investigated in a single animal study. The effect of magnesium on renal transporters might explain the suppression of cisplatin accumulation in the kidneys.19 In the studies included in our review, doses between 8 and 20 mEq were applied. Only one study actually compared a dose of 8 mEq with a dose of 20 mEq.51 In this study, there was no significant difference in nephrotoxicity. More studies should be conducted to investigate the specific dose of magnesium.
The effect of oral hydration remains unclear, and the type of oral hydration that should be used is also still a subject of investigation. A study evaluating the safety of oral hydration should, therefore, first be repeated with all kind of malignancies before it is possible to draw conclusions for all patients.
Other methods to prevent CIN, like the influence of acetazolamide,44 vitamin E,25,43 or melatonin46 were each investigated in only one study and could, therefore, not be compared with other studies. The populations were small, so the results should be confirmed in larger other studies before solid conclusions regarding their application can be drawn.
This study has some limitations. First, the included studies were heterogeneous. This hampered the comparison between the studies; however, most studies had a comparable control group. Second, many different hydration methods were included, which makes it difficult to draw overall recommendations. Third, some CIN preventive methods were investigated in only a few studies, which makes it impossible to conclude if a hydration method is safe and effective.
An important strength of this study is that it covers the full scope of the subject. This makes it possible to make overall conclusions of the best method to prevent nephrotoxicity. Furthermore, the comparison with previous reviews makes it clear which information gaps should be further investigated.
Conclusion
To conclude, the hydration scheme of cisplatin should be short and should comprise a diuretic and magnesium. The difference between mannitol and furosemide should be investigated in a head-to-head study. The optimal dose of the diuretic and magnesium should be further investigated.
Supplementary Material
Acknowledgments
We thank Information specialists from the Albert Schweitzer hospital and the Erasmus MC Medical Library for developing and updating the search strategies. This study was performed at the Albert Schweitzer Hospital.
Contributor Information
Charlotte Sikking, Department of Clinical Pharmacy, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Kelly L Niggebrugge-Mentink, Department of Clinical Pharmacy, HagaZiekenhuis, Den Haag, The Netherlands.
A S Elise van der Sman, Samenwerkende Apotheken Bollenstreek, Duinrand Pharmacy Noordwijk, Noordwijk, The Netherlands.
Roefke H P Smit, Department of Clinical Pharmacy, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Esther W Bouman-Wammes, Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Marieke M Beex-Oosterhuis, Department of Clinical Pharmacy, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Charlotte van Kesteren, Department of Clinical Pharmacy, Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Funding
The researchers did not receive any funding or grant.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: C.S., K.N.M., M.B.O., C.K. Provision of study material or patients: C.S., K.N.M., E.S., M.B.O., C.K. Collection and/or assembly of data: C.S., E.S. Data analysis and interpretation: C.S., K.N.M., R.S., E.B.W., M.B.O., C.K. Manuscript writing: C.S., M.B.O., C.K. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Dasari S, Tchounwou PB.. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-378. 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan BA, Coward JIG.. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5(Suppl 5):S565-S578. 10.3978/j.issn.2072-1439.2013.07.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vokes EE. Induction chemotherapy for head and neck cancer: recent data. Oncologist. 2010;15(Suppl 3):3-7. 10.1634/theoncologist.2010-S3-03 [DOI] [PubMed] [Google Scholar]
- 4. Ismaili N, Amzerin M, Flechon A.. Chemotherapy in advanced bladder cancer: current status and future. J Hematol Oncol. 2011;4:35. 10.1186/1756-8722-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Treatment of non-small cell lung cancer (NSCLC). J Thorac Dis. 2013;5(Suppl 4):S389-S396. 10.3978/j.issn.2072-1439.2013.07.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown A, Kumar S, Tchounwou PB.. Cisplatin-based chemotherapy of human cancers. J Cancer Sci Ther. 2019;11(4):97. [PMC free article] [PubMed] [Google Scholar]
- 7. Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012;7(10):1713-1721. 10.2215/CJN.02780312 [DOI] [PubMed] [Google Scholar]
- 8. Hanigan MH, Devarajan P.. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47-61. [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta S, Portales-Castillo I, Daher A, Kitchlu A.. Conventional chemotherapy nephrotoxicity. Adv Chronic Kidney Dis. 2021;28(5):402-414.e1. 10.1053/j.ackd.2021.08.001 [DOI] [PubMed] [Google Scholar]
- 10. Burns CV, Edwin SB, Szpunar S, Forman J.. Cisplatin-induced nephrotoxicity in an outpatient setting. Pharmacotherapy. 2021;41(2):184-190. 10.1002/phar.2500 [DOI] [PubMed] [Google Scholar]
- 11. Arunkumar P, Viswanatha G, Radheshyam N, Mukund H, Belliyappa M.. Science behind cisplatin-induced nephrotoxicity in humans: a clinical study. Asian Pac J Trop Biomed. 2012;2(8):640-644. 10.1016/s2221-1691(12)60112-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Volarevic V, Djokovic B, Jankovic MG, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26(1):25. 10.1186/s12929-019-0518-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Zhang T, Song Q, et al. Cisplatin ototoxicity mechanism and antagonistic intervention strategy: a scope review. Front Cell Neurosci. 2023;17:1197051. 10.3389/fncel.2023.1197051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McSweeney KR, Gadanec LK, Qaradakhi T, et al. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers. 2021;13(7):1572. 10.3390/cancers13071572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB.. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490-2518. 10.3390/toxins2112490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh GS, Kim HJ, Shen A, et al. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014;12(2):55-65. 10.5049/EBP.2014.12.2.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Zhou Y, Wang D, et al. Cisplatin-induced ototoxicity: from signaling network to therapeutic targets. Biomed Pharmacother. 2023;157:114045. 10.1016/j.biopha.2022.114045 [DOI] [PubMed] [Google Scholar]
- 18. Hanigan MH, Deng M, Zhang L, Taylor PT, Lapus MG.. Stress response inhibits the nephrotoxicity of cisplatin. Am J Physiol Renal Physiol. 2005;288(1):F125-F132. 10.1152/ajprenal.00041.2003 [DOI] [PubMed] [Google Scholar]
- 19. Saito Y, Okamoto K, Kobayashi M, et al. Magnesium attenuates cisplatin-induced nephrotoxicity by regulating the expression of renal transporters. Eur J Pharmacol. 2017;811:191-198. 10.1016/j.ejphar.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 20. Niggebrugge-Mentink KL, Beex-Oosterhuis MM, ter Horst PGJ, et al. Difference in decline in renal function due to cisplatin after a short or long hydration scheme in non–small-cell lung cancer: a retrospective cohort study (HYCIS-XL). J Clin Pharm Ther. 2020;45(5):1153-1158. 10.1111/jcpt.13200 [DOI] [PubMed] [Google Scholar]
- 21. Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M; European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61(6):903-909. 10.1007/s00280-008-0711-0 [DOI] [PubMed] [Google Scholar]
- 22. Crona DJ, Faso A, Nishijima TF, et al. A systematic review of strategies to prevent cisplatin‐induced nephrotoxicity. Oncologist. 2017;22(5):609-619. 10.1634/theoncologist.2016-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casanova AG, Hernández-Sánchez MT, López-Hernández FJ, et al. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur J Clin Pharmacol. 2020;76(1):23-33. 10.1007/s00228-019-02771-5 [DOI] [PubMed] [Google Scholar]
- 24. Danwilai K, Lohitnavy O, Sakunrag I, Dilokthornsakul P.. The effect of magnesium supplementation on cisplatin induced nephrotoxicity: a systematic review and meta-analysis. Pharm Sci Asia. 2021;48(1):25-36. [Google Scholar]
- 25. Kandhare AD, Mukherjee A, Bodhankar SL.. Efficacy of antioxidant supplements on prevention and amelioration of cisplatin-induced nephrotoxicity: a systematic review and meta-analysis of randomized controlled trials. Jundishapur J Nat Pharm Prod. 2019;14(3):e61527. [Google Scholar]
- 26. Hamroun A, Lenain R, Bigna JJ, et al. Prevention of cisplatin-induced acute kidney injury: a systematic review and meta-analysis. Drugs. 2019;79(14):1567-1582. 10.1007/s40265-019-01182-1 [DOI] [PubMed] [Google Scholar]
- 27. Li S, He X, Ruan L, et al. Protective effect of mannitol on cisplatin-induced nephrotoxicity: a systematic review and meta-analysis. Front Oncol. 2021;11:804685. 10.3389/fonc.2021.804685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hase T, Miyazaki M, Ichikawa K, et al. Short hydration with 20 mEq of magnesium supplementation for lung cancer patients receiving cisplatin-based chemotherapy: a prospective study. Int J Clin Oncol. 2020;25(11):1928-1935. 10.1007/s10147-020-01755-1 [DOI] [PubMed] [Google Scholar]
- 31. Bégin AM, Monfette ML, Boudrias-Dalle E, et al. Effect of mannitol on acute kidney injury induced by cisplatin. Support Care Cancer. 2021;29(4):2083-2091. 10.1007/s00520-020-05703-7 [DOI] [PubMed] [Google Scholar]
- 32. Hägerström E, Lindberg L, Bentzen J, et al. The nephroprotective effect of mannitol in head and neck cancer patients receiving cisplatin therapy. Clin Med Insights Oncol. 2019;13. 10.1177/1179554918821320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andarsari MR, Kusumaningrum YD, Hapsari RN, Susilo DH, Shinta DW.. Effect of mannitol hydration as renoprotective on cisplatin induced nephrotoxicity (CIN) in head and neck cancer patients. Indian J Forensic Med Toxicol. 2021;15(4):1757-1765. [Google Scholar]
- 34. Dhillon P, Amir E, Lo M, et al. A case–control study analyzing mannitol dosing for prevention of cisplatin-induced acute nephrotoxicity. J Oncol Pharm Pract. 2019;25(4):875-883. 10.1177/1078155218771461 [DOI] [PubMed] [Google Scholar]
- 35. Makimoto G, Hotta K, Oze I, et al. Randomized study comparing mannitol with furosemide for the prevention of cisplatin-induced renal toxicity in non-small cell lung cancer: the OLCSG1406 trial. Asia Pac J Clin Oncol. 2021;17(1):101-108. 10.1111/ajco.13423 [DOI] [PubMed] [Google Scholar]
- 36. Rachman A, Wafa S, Nugroho P, Koesnoe S.. The effect of mannitol addition to hydration on acute kidney injury event after high dose cisplatin chemotherapy: an ambispective cohort study. BMC Cancer. 2022;22(1):395. 10.1186/s12885-022-09456-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sainamthip P, Saichaemchan S, Satirapoj B, Prasongsook N.. The effect of intravenous mannitol combined with normal saline in preventing cisplatin-induced nephrotoxicity: a randomized, double-blind, placebo-controlled trial. JCO Glob Oncol. 2022;8:e2100275. 10.1200/GO.21.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naiki T, Sugiyama Y, Tasaki Y, et al. Efficacy of a newly modified short hydration method for gemcitabine and cisplatin combination chemotherapy in patients with urothelial carcinoma. Oncology. 2020;98(9):612-620. 10.1159/000506992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aoyama T, Tsunoda T, Kato H, et al. Comparison of cisplatin-induced nephrotoxicity when using conventional versus short hydration in gastric cancer—a retrospective study. J Chemother. 2020;32(3):144-150. 10.1080/1120009X.2020.1713507 [DOI] [PubMed] [Google Scholar]
- 40. Tanaka M, Horinouchi H, Goto Y, et al. Reduction in nephrotoxicities using short hydration for chemotherapy containing cisplatin: a consecutive analysis of 467 patients with thoracic malignancies. ESMO Open. 2018;3(4):e000342. 10.1136/esmoopen-2018-000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chantharakhit C, Sujaritvanichpong N.. Efficacy of short hydration for intermediate to high-dose cisplatin-based chemotherapy for outpatients: SHORTCIS trial. Asian Pac J Cancer Prev APJCP. 2022;23(10):3323-3330. 10.31557/apjcp.2022.23.10.3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ashrafi F, Erfani M, Mousavi S.. The effect of hydration therapy with and without magnesium sulfate on prevention of cisplatin-induced nephrotoxicity. IJBC. 2019;11(1):13-17. [Google Scholar]
- 43. Ashrafi F, Tabiei MN, Mousavi S, et al. Does vitamin E mitigate cisplatin-induced nephrotoxicity in cancer patients: results from a randomized placebo-controlled clinical trial. Middle East Journal of Cancer. 2020;11(2):174-184. [Google Scholar]
- 44. El Hamamsy M, Kamal N, Bazan NS, El Haddad M.. Evaluation of the effect of acetazolamide versus mannitol on cisplatin-induced nephrotoxicity, a pilot study. Int J Clin Pharm. 2018;40(6):1539-1547. 10.1007/s11096-018-0677-x [DOI] [PubMed] [Google Scholar]
- 45. Horinouchi H, Kubota K, Miyanaga A, et al. Oral rehydration solution (OS-1) as a substitute of intravenous hydration after cisplatin administration in patients with lung cancer: a prospective multicenter trial. ESMO Open. 2018;3(1):e000288. 10.1136/esmoopen-2017-000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karvan S, Sadeghi A, Farrokhi P, et al. Melatonin in the prevention of cisplatin-induced acute nephrotoxicity: a randomized, controlled clinical trial. Res Pharm Sci. 2022;17(2):176-188. 10.4103/1735-5362.335176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kimura T, Ozawa T, Hanai N, et al. Renal protective effect of a hydration supplemented with magnesium in patients receiving cisplatin for head and neck cancer. J Otolaryngol Head Neck Surg. 2018;47(1):10. 10.1186/s40463-018-0261-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Konishi H, Fujiwara H, Itoh H, et al. Influence of magnesium and parathyroid hormone on cisplatin-induced nephrotoxicity in esophageal squamous cell carcinoma. Oncol Lett. 2018;15(1):658-664. 10.3892/ol.2017.7345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kubo Y, Miyata H, Sugimura K, et al. Profylactic effect of premedication of intravenous magnesium. Oncology. 2019;97(6):319-326. [DOI] [PubMed] [Google Scholar]
- 50. Minzi OMS, Lyimo TE, Furia FF, et al. Electrolytes supplementation can decrease the risk of nephrotoxicity in patients with solid tumors undergoing chemotherapy with cisplatin. BMC Pharmacol Toxicol. 2020;21(1):69. 10.1186/s40360-020-00448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miyoshi T, Hayashi T, Uoi M, et al. Preventive effect of 20 mEq and 8 mEq magnesium supplementation on cisplatin-induced nephrotoxicity: a propensity score–matched analysis. Support Care Cancer. 2022;30(4):3345-3351. 10.1007/s00520-021-06790-w [DOI] [PubMed] [Google Scholar]
- 52. Puisset F, Bigay-Game L, Paludetto MN, et al. Safety of oral hydration after cisplatin infusion in an outpatient lung cancer unit. Support Care Cancer. 2019;27(5):1679-1686. 10.1007/s00520-018-4415-7 [DOI] [PubMed] [Google Scholar]
- 53. Saito Y, Takekuma Y, Kobayashi M, et al. Suitability of oral rehydration solution (ORS) for use in the cisplatin short hydration method. Anticancer Res. 2022;42(6):3185-3193. 10.21873/anticanres.15808 [DOI] [PubMed] [Google Scholar]
- 54. Sugisaki T, Aoyama T, Kawakami K, et al. Correlation between magnesium pre-loading and cisplatin-induced nephrotoxicity in 5-fluorouracil/cisplatin combination therapy for esophageal cancer. Pharmazie. 2022;77(2):85-88. 10.1691/ph.2022.11038 [DOI] [PubMed] [Google Scholar]
- 55. Suppadungsuk S, Phitakwatchara W, Reungwetwattana T, et al. Preloading magnesium attenuates cisplatin-associated nephrotoxicity: pilot randomized controlled trial (PRAGMATIC study). ESMO Open. 2022;7(1):100351. 10.1016/j.esmoop.2021.100351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.


