Abstract
Background
In patients with renal cell carcinoma (RCC) enrolled in the phase III KEYNOTE-564 trial (NCT03142334), disease-free survival (DFS) following nephrectomy was prolonged with use of adjuvant pembrolizumab therapy versus placebo. Patient-reported outcomes (PROs) provide an important measure of health-related quality of life (HRQoL) and can complement efficacy and safety results.
Patients and Methods
In KEYNOTE-564, 994 patients were randomly assigned to receive pembrolizumab 200 mg (n = 496) or placebo (n = 498) intravenously every 3 weeks for ≤17 cycles. Patients who received ≥1 dose of treatment and completed ≥1 HRQoL assessment were included in this analysis. HRQoL end points were assessed using the EORTC QLQ-C30, FKSI-DRS, and EQ VAS. Prespecified and exploratory PRO end points were mean change from baseline in EORTC QLQ-C30 GHS/QoL score, EORTC QLQ-C30 physical function subscale score, and FKSI-DRS score.
Results
No clinically meaningful difference in least squares mean scores for pembrolizumab versus placebo were observed at week 52 for EORTC QLQ-C30 GHS/QoL (–2.5; 95% CI –5.2 to 0.1), EORTC QLQ-C30 physical functioning (–0.87; 95% CI –2.7 to 1.0), and FKSI-DRS (–0.7; 95% CI –1.2 to –0.1). Most PRO scores remained stable or improved for the EORTC QLQ-C30 GHS/QoL (pembrolizumab, 54.3%; placebo, 67.5%), EORTC QLQ-C30 physical functioning (pembrolizumab, 64.7%; placebo, 68.8%), and FKSI-DRS (pembrolizumab, 58.2%; placebo, 66.3%).
Conclusions
Adjuvant treatment with pembrolizumab did not result in deterioration of HRQoL. These findings together with the safety and efficacy findings support adjuvant pembrolizumab treatment following nephrectomy.
Trial Registration
Clinicaltrials.gov Identifier: NCT03142334
Keywords: renal cell carcinoma, health-related quality of life, nephrectomy, pembrolizumab
Patient-reported outcomes provide an important measure of health-related quality of life and can complement efficacy and safety results. This article presents analyses of health-related quality of life in patients enrolled in the KEYNOTE-564 trial.
Implications for Practice.
Disease-free survival was prolonged by adjuvant pembrolizumab compared with placebo in patients with renal cell carcinoma. No clinically meaningful differences in HRQoL were observed between the pembrolizumab and placebo groups. Results from KEYNOTE-564 showed improved efficacy and manageable safety with adjuvant pembrolizumab without compromising quality of life.
Introduction
Standard-of-care treatment for locoregional renal cell carcinoma (RCC) consists of radical or partial nephrectomy.1,2 In general, patients are more likely to experience disease recurrence within 5 years after surgery, and patients with higher primary tumor stage, higher tumor nuclear grade, lymph node involvement, and the presence of sarcomatoid features have greater risk of recurrence.3 Studies assessing health-related quality of life (HRQoL) in RCC are scarce, and those that are available are often heterogenous in their methodology. The available data generally suggest that HRQoL improves following nephrectomy but that disease recurrence and fear of recurrence have significant negative effects on HRQoL4,5
Improved disease-free survival (DFS) was found in patients treated with adjuvant sunitinib, a multi-targeted receptor tyrosine kinase inhibitor, compared with placebo in patients with high-risk locoregional RCC in the phase III Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy (S-TRAC) study; however, no difference between adjuvant sunitinib and placebo was observed in a similar population in the phase III ASSURE trial.6,7 In the S-TRAC study, patients in the sunitinib group also had significantly lower HRQoL scores as assessed by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and Euro-QoL 5-dimension visual analog scale (EQ VAS) compared with patients in the placebo group, although only diarrhea and loss of appetite measures showed a clinically meaningful increase.6,8 Owing to concerns of toxicity and the conflicting results between trials, National Comprehensive Cancer Network Guidelines have a category 3 recommendation, which indicates there is a major disagreement on whether the intervention is appropriate.1 Adjuvant sunitinib therapy is not recommended for RCC in other parts of the world, including Western Europe, because of these concerns.2
Recent advances with immunotherapies targeting programmed cell death protein 1/programmed cell death ligand 1 have greatly altered the treatment landscape for RCC.9-12 In the phase III KEYNOTE-564 trial, the efficacy and safety of adjuvant pembrolizumab therapy was compared with placebo following nephrectomy in patients with RCC who had an increased risk of recurrence.13 At the first interim analysis, improvement in DFS was observed with adjuvant pembrolizumab therapy over placebo (hazard ratio [HR] 0.68; 95% confidence interval [CI] 0.53-0.87; P = 0.002). With an additional 6 months of follow-up, improvement in DFS continued to be shown with adjuvant pembrolizumab therapy over placebo (HR 0.63; 95% CI 0.50-0.80).14 Based on the results of KEYNOTE-564, pembrolizumab was approved by the US Food and Drug Administration and European Medicines Agency for the adjuvant treatment of patients with RCC at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.15,16 In the adjuvant setting, patients are often free of active disease until recurrence. An adjuvant treatment that delays disease recurrence should also be evaluated for impact on quality of life. Here, we present the prespecified analyses of HRQoL in patients enrolled in the KEYNOTE-564 trial.
Methods
Study Design and Patients
This randomized, double-blind, phase III study has been reported on previously.13,14 In brief, patients eligible for KEYNOTE-564 were aged ≥ 18 years, had an Eastern Cooperative Oncology Group performance status score of 0 or 1, and had histologically confirmed RCC with a clear cell component. Patients also had to have had an intermediate or high risk of recurrence after nephrectomy, with or without metastasectomy (Supplementary Table S1). Patients could not have received any previous systemic therapy for RCC and must have undergone partial or radical nephrectomy or metastasectomy with negative surgical margins within 12 weeks before randomization.
The study protocol and all amendments were approved by the appropriate ethics committee at each center. The study was conducted in accordance with the protocol, its amendments, and standards of Good Clinical Practice. All patients provided written informed consent.
Treatments
Patients were randomly assigned in a 1:1 ratio to receive either treatment with adjuvant pembrolizumab 200 mg or placebo intravenously once every 3 weeks for a maximum of 17 cycles (approximately 1 year) or until disease recurrence, unacceptable toxic effects, intercurrent illness preventing further administration of pembrolizumab or placebo, decision by the investigator, a new cancer resulting in active treatment, pregnancy, or non-adherence to the protocol.13 Randomization was stratified according to metastatic status on the basis of the investigator’s review (M0 versus M1 with no evidence of disease). Within the M0 subgroup, randomization was further stratified according to the Eastern Cooperative Oncology Group performance status score (0 versus 1) and geographic location (US versus outside the US).
Outcomes
The primary efficacy end point in KEYNOTE-564 (DFS) and safety and tolerability data (adverse events [AEs]) have previously been reported.13,14
The prespecified HRQoL secondary end points were least-squares mean (LSM) change from baseline to week 52 in symptom scores as assessed by the EORTC QLQ-C30 global health status/quality of life (GHS/QoL) and physical functioning scale scores17 and Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index—Disease-Related Symptoms (FKSI-DRS).18 Exploratory end points included mean change from baseline in EORTC QLQ-C30 functioning and symptom scales and the EQ VAS.19 Other exploratory end points were the proportion of patients with improved, stable, or deteriorated symptom scores and the empirical mean change from baseline for the EORTC QLQ-C30 and the FKSI-DRS.
The 3 validated patient-reported outcome (PRO) instruments were administered by trained site personnel and completed electronically by the patient before all other study procedures in the following order: EQ-5D-5L, EORTC QLQ-C30, and FKSI-DRS. PRO assessments for both the pembrolizumab and placebo groups occurred on day 1 of cycles 1, 5, 9, 13, and 17; then at discontinuation and at the 30-day follow-up; and then annually until disease recurrence or the start of a new anticancer therapy.
The EORTC QLQ-C30 was developed to assess the quality of life of patients with cancer and comprises 5 functional subscales (physical, role, emotional, cognitive, and social) and 9 symptom/item subscales (fatigue, nausea and vomiting, pain, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact) scored on a 4-point scale (1: not at all; 2: a little; 3: quite a bit; 4: very much).20 The instrument also includes a GHS/QoL subscale comprising 2 items scored on a 7-point scale (1 [very poor] to 7 [excellent]). Scores were calculated by averaging items within scales and transforming linearly from 0 to 100. The clinically meaningful change threshold was considered a ≥ 10-point increase (improvement) or decrease (deterioration) from baseline in the EORTC QLQ-C30 GHS/QoL and functional subscales at any time during the trial.21 For symptom subscales, a decrease of ≥ 10 points was classified as improved, whereas an increase of ≥ 10 points was classified as deteriorated.
The FKSI-DRS is a patient-reported 9-item instrument that measures tumor-specific symptoms in patients with kidney cancer.18 Patients rate their symptoms on a 5-point scale, with responses ranging from 0 (not at all) to 4 (very much). The FKSI-DRS contains the following domains: lack of energy, fatigue, weight loss, pain, bone pain, shortness of breath, cough, fever, and blood in the urine. The FKSI-DRS total score is based on the sum of all 9 items and ranges from 0 to 36, with higher scores indicating improved (more favorable) symptom status. The clinically meaningful change threshold was defined as a 2- to 3-point change from baseline.18
The EQ-5D-5L is a standardized instrument that measures general health status and includes the following 5 domains: mobility, self-care, usual activities, pain/discomfort, and depression/anxiety.19 The EQ-5D-5L also uses a 100-point EQ VAS, by which patients rate their general health state at the time of the assessment, ranging from 0 (worst health imaginable) to 100 (best health imaginable). The clinically meaningful change threshold for the EQ VAS was defined as a ≥ 7-point change from baseline.22
Statistical Analysis
The PRO analysis population included all randomly assigned patients who received ≥ 1 dose of study treatment and completed ≥ 1 PRO assessment. The primary PRO analysis time point for change from baseline in HRQoL measures was defined as week 52. A constrained longitudinal data analysis model was applied to assess the mean change from baseline up to week 52 in the EORTC QLQ-C30 GHS/QoL and physical functioning subscale and the FKSI-DRS. Proportions of deterioration, stability, and improvement were calculated when there was a defined clinically meaningful change in score from baseline at any time during the study. The PRO completion rate at each time point was calculated as the proportion of patients who completed the PROs at the assessment time point among those in the PRO analysis population. The compliance rate was calculated as the proportion of patients who completed the PROs at the assessment time point among those expected to complete the instruments at that time point (ie, excluding those missing by design: discontinuation due to AEs, death, or disease progression; translations not available; no visit scheduled).
Analyses for the HRQoL outcomes were predefined. No formal hypotheses were tested for the HRQoL outcomes and, therefore, no formal power calculations or multiplicity controls were performed for HRQoL end points. Changes from baseline in LSM scores were evaluated for EORTC QLQ-C30, FKSI-DRS, and EQ VAS, and compared between the pembrolizumab and placebo groups. Descriptive analyses of empirical mean score changes from baseline and 95% CIs were summarized at each time point for the EORTC QLQ-C30 and FKSI-DRS.
The proportion of patients whose scores “deteriorated,” [were] “stable,” or “improved” from baseline was summarized by pembrolizumab and placebo groups. Improvement was defined as a clinically meaningful increase in score from baseline at any time during the study and confirmed by a clinically meaningful improvement at the next consecutive visit. Stability was defined as when the criteria for improvement and deterioration were not met and was confirmed at the next consecutive visit. Deterioration was defined as when the criteria for improvement or stability were not met and when a clinically meaningful worsening from baseline occurred at any time during the study. Completion and compliance rates were summarized by pembrolizumab and placebo groups and by visit.
Results
As previously reported, 994 patients were randomly assigned to treatment in KEYNOTE-564 between June 30, 2017, and September 20, 2019 (Fig. 1).13 The database cutoff date for this analysis was June 14, 2021. The PRO analysis included 484 patients in the pembrolizumab group and 493 patients in the placebo group for the EORTC QLQ-C30 and EQ VAS, and 483 and 493 patients, respectively, for the FKSI-DRS. Completion and compliance rates for all PRO measures were > 90% at baseline (Supplementary Tables S2-S4). At baseline, 438 patients (90.5%) in the pembrolizumab group and 450 patients (91.3%) in the placebo group completed the EORTC QLQ-C30. At week 52, completion rates were 62.8% (compliance, 85.4%) in the pembrolizumab group and 65.9% (compliance, 84.6%) in the placebo group for the EORTC QLQ-C30. For the FKSI-DRS, completion rates decreased from 90.1% at baseline to 62.7% (compliance, 85.1%) at week 52 in the pembrolizumab group and from 90.7% at baseline to 66.5% (compliance, 85.2%) at week 52 in the placebo group. For the EQ VAS, completion rates decreased from 92.1% at baseline to 62.8% (compliance, 85.4%) at week 52 in the pembrolizumab group and from 93.5% at baseline to 66.3% (compliance, 85.2%) at week 52 in the placebo group.
Figure 1.
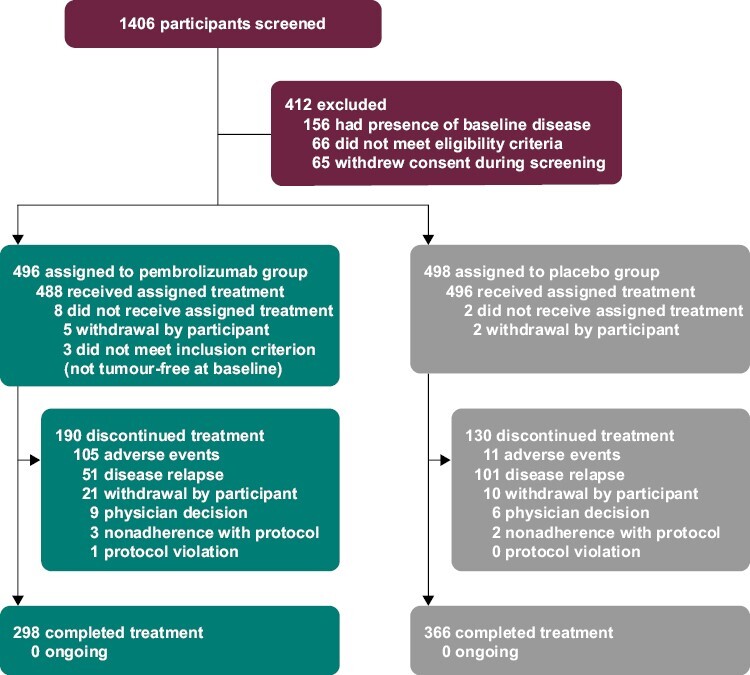
Trial profile.
Baseline EORTC QLQ-C30 GHS/QoL and physical functioning subscale, FKSI-DRS, and EQ VAS scores were well balanced between the pembrolizumab and placebo groups (Table 1). No clinically meaningful between-group differences in LSM scores for pembrolizumab versus placebo were observed at week 52 for the EORTC QLQ-C30 GHS/QoL (difference in LSM –2.5; 95% CI –5.2 to 0.1), EORTC QLQ-C30 physical functioning (difference in LSM –0.9; 95% CI –2.7 to 1.0), and FKSI-DRS (difference in LSM –0.7; 95% CI –1.2 to –0.1) scores. Similar results were observed for the EQ VAS (difference in LSM –1.6; 95% CI –3.6 to 0.4; Table 1) and the EORTC QLQ-C30 functioning and symptom subscales, including the nausea and vomiting symptom scale (difference in LSM –0.1; 95% CI –1.6 to 1.3) and the diarrhea single-item scale (difference in LSM 1.2; 95% CI –1.2 to 3.4) (Fig. 2A,B). Overall improvement rates between pembrolizumab and placebo were similar for the EORTC QLQ-C30 GHS/QoL (difference in percent improved versus placebo –3.0%; 95% CI –7.3 to 1.2), EORTC QLQ-C30 physical functioning scale (difference in percent improved versus placebo –0.4; 95% CI –4.2 to 3.5), and FKSI-DRS (difference in percent improved versus placebo –4.4%; 95% CI –8.3 to –0.7). Most patient scores remained stable or improved in both the pembrolizumab and placebo groups for the EORTC QLQ-C30 GHS/QoL (pembrolizumab, 54.3%; placebo, 67.5%), EORTC QLQ-C30 physical functioning (pembrolizumab, 64.7%; placebo, 68.8%), and FKSI-DRS (pembrolizumab, 58.2%; placebo, 66.3%) (Fig. 3). The empirical mean change from baseline through week 104 in the EORTC QLQ-C30 GHS/QoL, EORTC QLQ-C30 physical functioning, and FKSI-DRS remained stable for both the pembrolizumab and placebo groups (Fig. 4A-C).
Table 1.
Change from baseline to week 52 in the health status, physical functioning, and quality of life measures.
| EORTC QLQ-C30 global health status/quality of life | EORTC QLQ-C30 physical functioning | FKSI-DRS | EQ VAS | |||||
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab | Placebo | Pembrolizumab | Placebo | Pembrolizumab | Placebo | Pembrolizumab | Placebo | |
| No. of patients | 438 | 450 | 438 | 450 | 435 | 447 | 446 | 461 |
| Baseline score, mean (SD) | 79.2 (18.5) | 77.0 (17.6) | 88.6 (15.0) | 88.6 (14.3) | 32.9 (3.5) | 32.8 (3.5) | 84.0 (14.0) | 83.2 (14.6) |
| No. of patients | 304 | 325 | 304 | 325 | 300 | 328 | 304 | 327 |
| Week 52 score, mean (SD) | 75.0 (18.2) | 76.8 (19.6) | 86.7 (17.3) | 89.0 (15.9) | 31.9 (4.7) | 32.5 (4.1) | 80.8 (15.7) | 82.5 (14.9) |
| Change from baseline at week 52, LSM (95% CI) | –4.2 (–6.3 to –2.1) |
–1.7 (–3.7 to 0.3) |
–1.8 (–3.1 to –0.4) |
–0.9 (–2.2 to 0.4) |
–1.1 (–1.5 to –0.7) |
–0.4 (–0.8 to –0.1) |
–3.4 (–4.9 to –1.8) |
–1.8 (–3.3 to –0.3) |
| Difference in LSM (95% CI) | –2.5 (–5.2 to 0.1) | –0.9 (–2.7 to 1.0) | –0.7 (–1.2 to –0.1) | –1.6 (–3.6 to 0.4) | ||||
Abbreviations: CI, confidence interval; EQ VAS, EuroQoL 5-dimension visual analog scale; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FKSI-DRS, Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index—Disease-Related Symptoms; LSM, least-squares mean; No., number.
Figure 2.
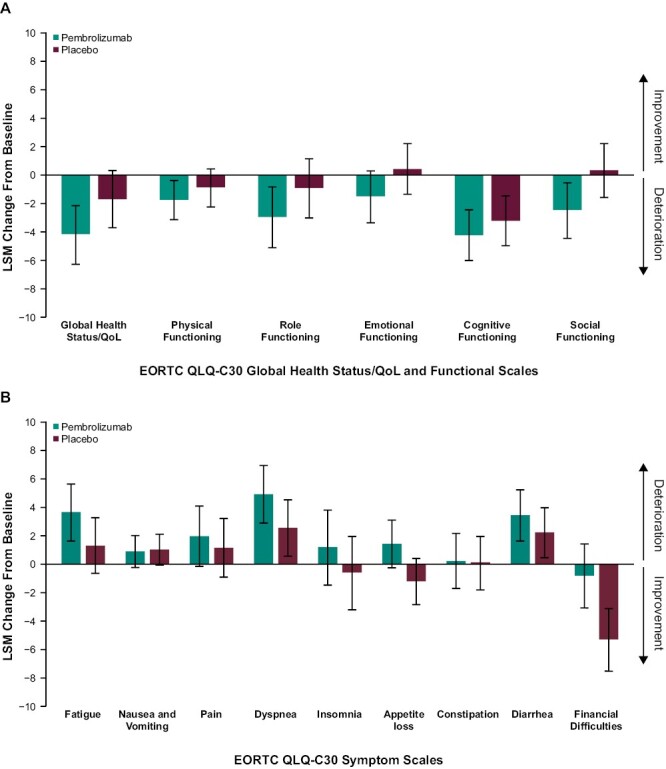
LSM change from baseline to week 52 in EORTC QLQ-C30 global health status/quality-of-life (A) functioning scales and (B) symptom scales. EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; LSM, least-squares mean; QoL, quality of life.
Figure 3.
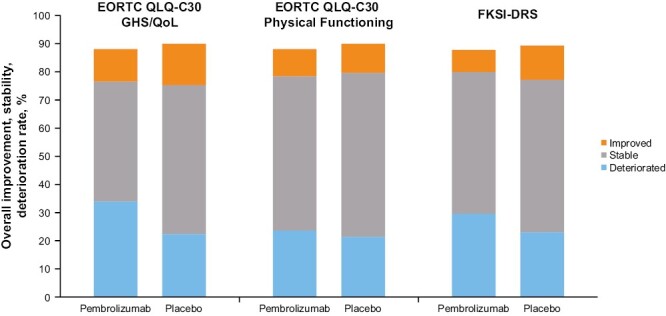
Proportion of patients with improved, stable, and deteriorated EORTC QLQ-C30 and FKSI-DRS scoresa. an = 60 in the pembrolizumab group and n = 53 in the placebo group had no assessment in the FKSI-DRS; n = 58 in the pembrolizumab group and n = 50 in the placebo group had no assessment in the EORTC QLQ-C30. EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FKSI-DRS, Functional Assessment of Cancer Therapy–Kidney Symptom Index Disease-Related Symptoms; GHS, global health status; QoL, quality of life.
Figure 4.
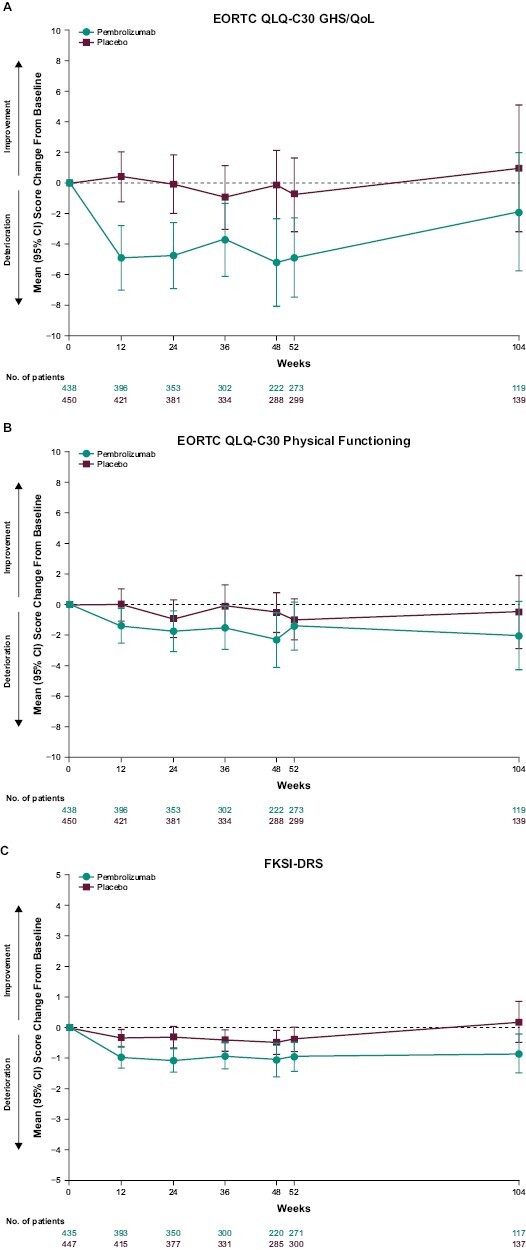
Empirical mean change from baseline to week 104 in (A) EORTC QLQ-C30 GHS/QoL, (B) EORTC QLQ-C30 physical functioning, and (C) FKSI-DRS. CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FKSI-DRS, Functional Assessment of Cancer Therapy–Kidney Symptom Index Disease-Related Symptoms; GHS, global health status; QoL, quality of life.
Discussion
An effective adjuvant therapy for RCC after nephrectomy can decrease the likelihood of disease recurrence and potentially improve overall survival. In the KEYNOTE-564 trial, adjuvant pembrolizumab therapy was associated with significant prolongation of DFS compared with placebo and had a manageable safety profile in patients with RCC.13,14,23 Along with the efficacy and safety findings from KEYNOTE-564, results from this analysis suggest that adjuvant pembrolizumab therapy does not compromise the HRQoL of patients.
In the KEYNOTE-564 study, no clinically meaningful differences between treatment arms were observed in LSM change from baseline to week 52 in EORTC QLQ-C30 GHS/QoL or physical functioning scales, or the FKSI-DRS. No meaningful differences were observed between the pembrolizumab and placebo groups in the other EORTC QLQ-C30 functional or symptom scales or in the EQ-5D VAS. Further, the empirical mean change from baseline in the EORTC QLQ-C30 GHS/QoL and physical functioning scales and in the FKSI-DRS remained stable through week 104 for both the pembrolizumab and placebo groups. Although patients in the placebo group did not receive active treatment, PRO outcomes were comparable between groups. Therefore, pembrolizumab did not have a negative impact on HRQoL.
This study represents one of the few studies of HRQoL in patients with RCC who underwent nephrectomy. The lack of adjuvant therapies for RCC contributes to the paucity of research on HRQoL in this patient population, compared with the metastatic RCC population. In the S-TRAC study of adjuvant sunitinib therapy versus placebo in patients with increased risk of RCC recurrence, EORTC QLQ-C30 GHS/QoL scores favored placebo over sunitinib (–4.76 difference [95% CI –6.82 to –2.71]) but did not reach the clinically meaningful change threshold.8 Results of the other EORTC QLQ-C30 scales, including physical, social, and emotional functioning, fatigue and pain, diarrhea, and decreased appetite, also consistently favored placebo, although only diarrhea and decreased appetite reached the clinically meaningful change threshold (≥10-point change from baseline).8
In addition to demonstrating higher DFS compared with placebo, pembrolizumab therapy was also generally tolerable. In the primary analysis of KEYNOTE-564, incidences of treatment-related AEs were higher in the pembrolizumab group than in the placebo group.13,14 However, no new safety signals with pembrolizumab emerged in this study, and most of the common AEs, including hyperthyroidism, hypothyroidism, and pruritus, were manageable. These results indicate that treatment with pembrolizumab maintained HRQoL while achieving therapeutic efficacy, which is a fundamental goal in oncology.
A limitation of the KEYNOTE-564 PRO analysis is the decrease over time in the number of patients with PRO data, which is a common challenge in HRQoL studies. Patients who discontinued because of disease progression may be more likely to experience HRQoL deterioration. Therefore, a higher discontinuation rate in one group of a study may then result in patients with better HRQoL in the one group over the other. However, compliance rates were similar between both the pembrolizumab and placebo groups and remained higher than 75% for all instruments throughout the study. Although common in oncology studies evaluating HRQoL, this study lacked multiplicity control, and results should be interpreted with caution. Finally, the FKSI-DRS was developed to assess symptoms of advanced kidney cancer, whereas patients in this study had localized RCC; however, many of the symptoms assessed by this instrument are generic, such as fatigue, cough, shortness of breath, or fever. To our knowledge, PROs specifically designed for use in the RCC adjuvant setting do not currently exist and could be the focus of future research.
Conclusion
Taken together, data from KEYNOTE-564 show improved efficacy and manageable safety with adjuvant pembrolizumab therapy without compromising quality of life. Although there was numerically higher deterioration for pembrolizumab compared with placebo, no clinically meaningful differences between treatment arms were observed. These data further support the use of adjuvant pembrolizumab therapy in patients with RCC who have an increased risk of disease recurrence.
Supplementary Material
Acknowledgments
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The authors thank the patients and their families and all investigators and site personnel. Medical writing and/or editorial assistance was provided by Robert Steger, PhD, and Matthew Grzywacz, PhD, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Contributor Information
Toni K Choueiri, Department of Medical Oncology, Lank Center for Genitourinary Oncology at Dana-Farber Cancer Institute, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA, USA.
Piotr Tomczak, Department of Medical Oncology, Poznan University of Medical Sciences, Poznan, Poland.
Se Hoon Park, Department of Hematology and Oncology, Sungkyunkwan University Samsung Medical Center, Seoul, South Korea.
Balaji Venugopal, Department of Medical Oncology, The Beatson West of Scotland Cancer Centre and University of Glasgow, Glasgow, UK.
Stefan Symeonides, Department of Medical Oncology, Edinburgh Cancer Centre, NHS Lothian, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK.
Jaroslav Hajek, Department of Medical Oncology, Fakultní Nemocnice Ostrava, Ostrava, Czech Republic.
Thomas Ferguson, Department of Medical Oncology, Fiona Stanley Hospital, Perth, Western Australia, Australia.
Yen-Hwa Chang, Department of Urology, Taipei Veterans General Hospital, Taipei, Taiwan.
Jae Lyun Lee, Department of Oncology, Asan Medical Center and University of Ulsan College of Medicine, Seoul, South Korea.
Naomi Haas, Division of Hematology and Oncology, Abramson Cancer Center, Philadelphia, PA, USA.
Piotr Sawrycki, Chemotherapy Department, Wojewódzki Szpital Zespolony im. L. Rydygiera, Torun, Poland.
Naveed Sarwar, Department of Surgery and Cancer, Imperial College Healthcare NHS Trust, London, UK.
Marine Gross-Goupil, Department of Medical Oncology, University Hospital Bordeaux–Hôpital Saint-André, Bordeaux, France.
Antoine Thiery-Vuillemin, Department of Medical Oncology, University Hospital Jean Minjoz, Besançon, France.
Mauricio Mahave, Department of Oncology, Fundación Arturo López Pérez FALP, Santiago, Chile.
Go Kimura, Department of Urology, Nippon Medical School Hospital, Tokyo, Japan.
Rodolfo F Perini, Merck & Co., Inc., Rahway, NJ, USA.
Todd L Saretsky, Merck & Co., Inc., Rahway, NJ, USA.
Rituparna Bhattacharya, Merck & Co., Inc., Rahway, NJ, USA.
Lei Xu, Merck & Co., Inc., Rahway, NJ, USA.
Thomas Powles, Department of Oncology, Royal Free Hospital NHS Trust, University College London, London, UK.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Conflict of Interest
Toni K. Choueiri reported institutional and personal paid and unpaid support for research, advisory boards, consultancy, and honoraria from: AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Calithera, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVIA, Infinity Pharmaceuticals, Ipsen, Janssen, Kanaph Therapeutics, Lilly, Merck, Nikang, Precede Bio, Novartis, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest Therapeutics, UpToDate, and CME events (PeerView, OncLive, Michael J. Hennessy Associates, and others) outside the submitted work; intellectual property: Molecular mutations and immunotherapy response and circulating tumor DNA; ownership interest: Tempest Therapeutics, Pionyr, Osel, Precede Bio; Scientific Advisory Boards: National Comprehensive Cancer Network, Genitourinary Steering Committee, American Society of Clinical Oncology/European Society for Medical Oncology, ACCRU, KidneyCAN; and has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components; T.K.C. is supported in part by research funding from the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942-16) and Program 5P30CA006516-56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber Cancer Institute. Piotr Tomczak has received research funding from Merck Sharp & Dohme Oncology. Balaji Venugopal has received honoraria from Bristol Myers Squibb, Eisai, EUSA Pharma, and Ipsen. Stefan Symeonides has received research funding from MSD and Verastem, honoraria from Eisai BioNTech, EUSA, Ipsen, and MSD, has consulting/advisory relationships with Bristol Myers Squibb, Boxer Capital, Duke Street Bio, Eisai, Ellipses, EUSA, Medannex, Merck Serono, Merck Sharp and Dohme, Pfizer, and Vaccitech, and serves on the scientific advisory board for Cancer Drug Development Forum. Thomas Ferguson has consulting/advisory relationships with Merck Sharp and Dohme, Bristol Myers Squibb, and Pfizer. Jae Lyun Lee has received research funding from Pfizer, Ipsen, Bristol Myers Squibb, Merck Sharp and Dohme, Merck, Roche, AstraZeneca, and Seagen, serves on the scientific advisory board for Pfizer Korea, Astella Korea, Bristol Myers Squibb, Merck, Merck Sharp and Dohme, and AstraZeneca, and has ownership interests in Amgen, Johnson and Johnson, Merck, Beigene, and Innovent. Naomi Haas serves on the scientific advisory board for Aveo, Merck, Eisai, Bristol Myers Squibb, Exelixis, and Roche. Marine Gross-Goupil serves on the scientific advisory board and received honoraria from Bristol Myers Squibb, AstraZeneca, Merck Sharp and Dohme, Ipsen, Eisai, Pfizer, Roche, and Novartis, and has a consulting/advisory relationship with Bristol Myers Squibb, AstraZeneca, Merck Sharp and Dohme, Ipsen, Eisai, and Pfizer. Antoine Thiery-Vuillemin receives research funding from Merck Sharp and Dohme, Pfizer, Ipsen, and Bayer, has consulting/advisory relationship with Roche, Merck Sharp and Dohme, Johnson & Johnson, Bristol Myers Squibb, Astellas, Ipsen, AstraZeneca, and Novartis, has received honoraria from Roche, Merck Sharp and Dohme, Johnson & Johnson, Bristol Myers Squibb, Astellas, Ipsen, AstraZeneca, and Novartis, and serves on the scientific advisory board for BIONIKK trial and steering committee of French GETUG academic group. Go Kimura has received honoraria from Ono Pharmaceutical Co, Ltd., Bristol Myers Squibb K.K., Bayer Yakuhin Ltd., Merck Biopharma, Chugai Pharmaceutical Co Ltd., MSD K.K., Eisai Co., Ltd., Takeda Pharmaceutical Company Limited, and Sanofi K.K. Rodolfo F. Perini is an employee of and has ownership interests in Merck & Co., Inc., Rahway, NJ, USA. Todd L. Saretsky is an employee of and has ownership interests in Merck & Co., Inc., Rahway, NJ, USA. Rituparna Bhattacharya is an employee of and has ownership interests in Merck & Co., Inc., Rahway, NJ, USA. Lei Xu is an employee of and has ownership interests in Merck & Co., Inc., Rahway, NJ, USA. Thomas Powles receives research funding from Astellas, Open Health, AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen, Merck, Merck Sharp and Dohme, Novartis, Pfizer, Seattle Genetics, Merck Serono, Johnson & Johnson, and Eisai; has consulting/advisory relationships with AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Incyte, Ipsen, Merck, Merck Sharp and Dohme, Novartis, Pfizer, Seagen, Merck Serono, Astellas, Johnson & Johnson, Eisai, and Mashup Ltd.; and has received honoraria from Roche, Pfizer, Merck Sharp and Dohme, AstraZeneca, and Ipsen. The other authors indicated no financial relationships.
Author Contributions
Conception/design: T.K.C., N.H., R.F.P., T.P. Provision of study material or patients: All authors. Collection and/or assembly of data: P.T., S.H.P., B.V., S.S., T.F., Y.-H.C., J.L.L., P.S., N.S., M.G.-G., A.T.-V., M.M. Data analysis and interpretation: All authors. Manuscript writing: T.K.C. Final approval of manuscript: All authors.
Data Availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at https://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Previous Publication
Data from the study were presented in part at the European Society for Medical Oncology Congress, Virtual, September 16-21, 2021.
References
- 1. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(1):71-90. 10.6004/jnccn.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706-720. 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 3. Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34(8):1081-1086. 10.1007/s00345-016-1773-y [DOI] [PubMed] [Google Scholar]
- 4. Can A, Siregar GP, Sihombing B, Papriska FF, Warli SM.. Five years survival and quality of life after radical nephrectomy: a descriptive single-center study. Indonesian J Urol. 2021;15:5. 10.15562/ijbs.v15i1.306 [DOI] [Google Scholar]
- 5. Rossi SH, Klatte T, Stewart GD.. Quality of life outcomes in patients with localised renal cancer: a literature review. World J Urol. 2018;36(12):1961-1972. 10.1007/s00345-018-2415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246-2254. 10.1056/NEJMoa1611406 [DOI] [PubMed] [Google Scholar]
- 7. Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008-2016. 10.1016/S0140-6736(16)00559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staehler M, Motzer RJ, George DJ, et al. Adjuvant sunitinib in patients with high-risk renal cell carcinoma: safety, therapy management, and patient-reported outcomes in the S-TRAC trial. Ann Oncol. 2018;29(10):2098-2104. 10.1093/annonc/mdy329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choueiri TK, Atkins MB, Bakouny Z, et al. Summary from the First Kidney Cancer Research Summit, September 12-13, 2019: A focus on translational research. J Natl Cancer Inst. 2021;113(3):234-243. 10.1093/jnci/djaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choueiri TK, Motzer RJ.. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354-366. 10.1056/NEJMra1601333 [DOI] [PubMed] [Google Scholar]
- 11. Braun DA, Bakouny Z, Hirsch L, et al. Beyond conventional immune-checkpoint inhibition – novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. 2021;18(4):199-214. 10.1038/s41571-020-00455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravi P, Bakouny Z, Schmidt A, Choueiri TK.. Novel therapeutic approaches and the evolution of drug development in advanced kidney cancer. Cancer J. 2020;26(5):464-470. 10.1097/PPO.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 13. Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683-694. 10.1056/NEJMoa2106391 [DOI] [PubMed] [Google Scholar]
- 14. Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133-1144. 10.1016/S1470-2045(22)00487-9 [DOI] [PubMed] [Google Scholar]
- 15. Keytruda® (pembrolizumab) Injection, for Intravenous Use. 4/2023. Merck Sharp & Dohme LLC: Rahway, NJ; 2023. [Google Scholar]
- 16. KEYTRUDA 25 mg/mL concentrate for solution for infusion (summary of product characteristics). MSD B.V.: Haarlem, The Netherlands; 2023. [Google Scholar]
- 17. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 18. Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health. 2007;10(4):285-293. 10.1111/j.1524-4733.2007.00183.x [DOI] [PubMed] [Google Scholar]
- 19. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bjordal K, de Graeff A, Fayers PM, et al. A 12-country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer. 2000;36(14):1796-1807. 10.1016/s0959-8049(00)00186-6 [DOI] [PubMed] [Google Scholar]
- 21. Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 Reference Values. 2008. Accessed June 5, 2023, https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf
- 22. Pickard AS, Neary MP, Cella D.. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labaki C, Choueiri TK.. Perioperative immunotherapy for renal cell carcinoma: looking beyond the data. Nat Rev Clin Oncol. 2022;20(2):65-66. 10.1038/s41571-022-00710-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at https://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.


