Abstract
An enzyme-linked immunosorbent assay-based attachment model using the human intestinal cell line Caco-2A was developed to study attachment of Cryptosporidium parvum sporozoites in vitro and to assess potential inhibitors of sporozoite binding. In this system, attachment was related to sporozoite dose, incubation time, and host cell differentiation status. Polyclonal antibodies to C. parvum as well as glycoprotein inhibitors of a sporozoite lectin reduced attachment. This model will be a valuable tool in elucidating specific molecules and mechanisms involved in sporozoite-host cell attachment.
The protozoan parasite Cryptosporidium parvum is a causative agent of human gastrointestinal disease worldwide (19). The illness is self-limiting in immunocompetent individuals, but in immunocompromised hosts it may result in severe diarrhea and wasting, for which no effective therapy is currently available. Knowledge of the pathogenesis of cryptosporidiosis is necessary in order to design preventative and interventional strategies against this disease. Initial attachment of the parasite to host cells is a prerequisite for the pathophysiological events in infection. The molecular mechanisms and specific proteins involved in adhesion of C. parvum sporozoites to host epithelium have not yet been elucidated. Earlier studies demonstrated the presence of a galactose–N-acetylgalactosamine (Gal-GalNAc)-specific C. parvum sporozoite surface lectin which may mediate attachment of sporozoites to host cells (9, 23). To investigate the role of this lectin and other putative adhesins in adherence of the parasite to intestinal cells, it was important to establish a relevant and reliable in vitro attachment model. Numerous assays with a variety of cell lines have been reported for use as in vitro models to study the development of C. parvum in host cells (2, 4, 7, 10, 11, 14, 18, 22, 27, 28). These include enzyme-linked immunosorbent assay (ELISA)-based methods of measuring C. parvum infection of human ileocecal adenocarcinoma cells (28) and human intrahepatic biliary epithelial cells (27). The Caco-2 cell line has been widely used to investigate various biological interactions of C. parvum with host cells. Buraud et al. (2) showed complete development (asexual and sexual) of C. parvum in Caco-2 cells. Subsequent reports confirmed that this cell line supports C. parvum infection to an extent comparable to that in other cell lines (14, 25, 27). Caco-2 cells have been used (i) to evaluate the effects of anticryptosporidial drugs and antibodies on infection in vitro (5, 11), (ii) to study C. parvum-induced intestinal epithelial cell secretory and transport defects (6, 7), and (iii) to investigate parasite-induced chemokine secretion by intestinal epithelial cells (12). These studies indicate that Caco-2 cells are well suited to investigations of the mechanisms involved in C. parvum-intestinal cell interactions. In this study we characterized attachment of sporozoites to these cells in vitro using an ELISA-based model.
Parasites and cell culture.
C. parvum oocysts (GCH1 isolate) originally isolated from an AIDS patient were cultivated by serial passages through neonatal calves (24). Oocysts were purified and excysted and sporozoites were isolated as described earlier (1). Caco-2 cells originally obtained from Hans Buller, Academic Medical Center, Amsterdam, The Netherlands (26) were maintained and designated Caco-2A by the GRASP Center Cell Culture Core at New England Medical Center and provided to us as needed. Caco-2A cells have been previously shown to support intracellular growth and development of C. parvum (6, 27). Cells were grown to confluence in Dulbecco’s minimum essential medium (Gibco BRL, Gaithersburg, Md.) supplemented with 10% (vol/vol) fetal calf serum, 25 mM HEPES, penicillin (100 U/ml), and streptomycin (100 μg/ml) for 72 h at 37°C in 5% CO2, in 96-well tissue culture plates (Costar, Cambridge, Mass.) for the ELISA-based method and in collagen-coated 16-well Lab-Tek chamber slides (Nunc, InterMed Corp., Naperville, Ill.) for the immunofluorescence (IF)-based method.
Attachment assays.
In order to develop an ELISA-based assay specific for attachment, Caco-2A monolayers were fixed to prevent sporozoites from invading host cells. Fixation of host cells does not appear to significantly affect sporozoite attachment to erythrocytes or MDCK cells (8, 23). Fixed target cells have been employed to investigate attachment of other protozoan parasites, including Entamoeba histolytica (3), Trypanosoma cruzi (20), and Giardia lamblia (16). For the ELISA-based method, cells were fixed with 1% (vol/vol) glutaraldehyde in Hanks balanced salt solution containing 10 mM HEPES (HBSS-HEPES), pH 7.2, for 10 min at room temperature (RT). For the IF-based method, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 30 min at RT (8). Purified sporozoites in 50 μl of Dulbecco’s minimum essential medium, 25 mM HEPES, penicillin (100 U/ml), streptomycin (100 μg/ml), and 0.1% (wt/vol) bovine serum albumin (adhesion medium) were incubated with fixed monolayers for 1 h at 37°C in 5% CO2. Wells were washed twice with HBSS-HEPES, and adherent sporozoites were fixed with methanol for 10 min at RT. After three washes with Tris-buffered saline (TBS; 20 mM Tris–0.5 M NaCl [pH 7.5]), attached sporozoites were quantified by ELISA- and IF-based methods. For the ELISA, nonspecific binding was blocked with 1% (vol/vol) normal goat serum (NGS; Sigma Chemical Co., St. Louis, Mo.) in TBS for 1 h at RT, followed by incubation with a rabbit polyclonal anti-C. parvum antibody (which recognizes sporozoites, but not oocysts) diluted 1:4,000 in 1% NGS-TBS at 4°C overnight (8). After three washes with TBS, wells were incubated for 1 h at RT with goat anti-rabbit immunoglobulin G (IgG)–biotin conjugate (Vector Laboratories, Burlingame, Calif.) at 2 μg/ml in 1% NGS-TBS, washed three times with TBS, and incubated with an avidin-biotin-alkaline phosphatase complex (ABC reagent; Vector Laboratories) for 1 h at RT. Wells were washed five times with TBS and incubated with p-nitrophenyl phosphate (1 mg/ml in 100 mM Tris–100 mM NaCl–5 mM MgCl2 [pH 9.5]) for 10 min at RT. After termination of the reaction with 0.1 M EDTA, the absorption at 405 nm (A405) was read against blanks in which wells were processed identically except that sporozoites were not added. Attachment was quantified by IF microscopy using a modification of the method described earlier (8). After nonspecific binding was blocked with 1% NGS-TBS for 1 h at RT, slides were incubated with the rabbit antibody described above, diluted 1:6,000 in 1% NGS-TBS, overnight at 4°C. After three washes with TBS, slides were incubated for 1 h at RT with goat anti-rabbit IgG–fluorescein isothiocyanate conjugate (Sigma Chemical Co.) diluted 1:80 in 1% NGS-TBS. Slides were washed three times, mounted with buffered glycerol containing p-phenylenediamine (1 mg/ml), and examined by epifluorescence microscopy under oil immersion. The number of attached sporozoites in 25 high-power fields (HPFs) per well was counted, and the results were expressed as the number of sporozoites per HPF. All assays were performed in triplicate, and the results were expressed as the mean ± 1 standard error of the mean. Unpaired two-tailed Student’s t tests were used to evaluate differences. Differences were considered significant at the level at which P is <0.05.
Dose-response and time course studies.
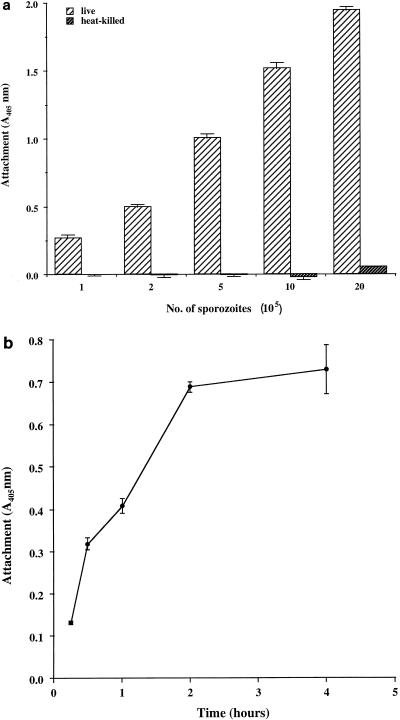
Sporozoite attachment to Caco-2A cells was shown to be dependent on the number of parasites added (Fig. 1a). There was no significant signal obtained with heat-inactivated sporozoites (100°C, 10 min), confirming the specificity of the assay. Attachment was also dependent on incubation time, with maximal levels reached at 2 h (Fig. 1b). For subsequent assays an incubation time of 1 h was chosen to ensure sporozoite viability and to shorten the assay time.
FIG. 1.
Dose-response and time course studies of attachment of sporozoites to Caco-2A monolayers. (a) Live or heat-killed sporozoites (1 × 105 to 20 × 105) were incubated with fixed Caco-2A cells for 1 h, and attachment was quantified by ELISA. (b) Sporozoites (105 per well) were incubated with fixed Caco-2A cells for the specified periods, and attachment was quantified by ELISA. The number of attached parasites is expressed as A405. Values are the means ± standard errors of the means for triplicate samples.
Comparison of IF-based and ELISA-based methods.
In a previous study, we used an immunofluorescent-antibody assay (IFA) to quantify sporozoite attachment to MDCK cells (8). Although the IFA is useful for direct visualization of attachment, it is time-consuming and subject to interobserver variation. In order to compare the ELISA-based and IF-based methods of quantifying attachment, the assays were performed in parallel with a wide range of sporozoite doses. For a given dose, the numbers of sporozoites per HPF obtained by IFA were correlated to the corresponding A405 values obtained by the ELISA, and the correlations were evaluated by linear regression analysis. A significant correlation was found between the numbers of attached sporozoites per HPF and the A405 values (r = 0.956; P < 0.0001). This indicated that while the ELISA was an indirect measure of sporozoite adhesion it nonetheless provided reliable and accurate results. Furthermore, there was less variation in replicate determinations in the ELISA than in the results obtained by IF microscopy.
Effect of differentiation of Caco-2A cells on sporozoite attachment.
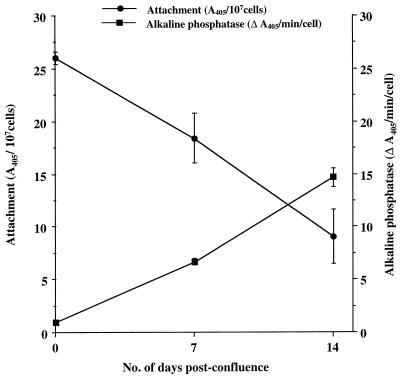
Upon growth in culture, Caco-2A cells differentiate to epithelium resembling that of the small intestine (21), exhibiting changes in cell surface glycoconjugates (29) and increased activities of specific enzymes such as alkaline phosphatase (15). To investigate whether differentiation of Caco-2A cells influences sporozoite attachment, monolayers were grown to confluence and examined at confluence and at 7 and 14 days postconfluence. At each time point the number of cells per well was determined in triplicate after trypsin-EDTA treatment to detach cells. The sporozoite attachment at each time point was assayed by ELISA and expressed as the A405 per 107 cells. As a marker of differentiation, the alkaline phosphatase activity of cells grown in parallel plates was determined in triplicate by incubation with 50 μl of p-nitrophenyl phosphate (1 mg/ml) for up to 10 min at RT. Absorbance at 405 nm was measured, and the enzyme activity was expressed as A405 per minute per cell. Increased differentiation over time was confirmed by an increase in alkaline phosphatase levels (Fig. 2). However, increased differentiation of Caco-2A cells resulted in a decrease in sporozoite attachment (Fig. 2). This suggests that maturation of Caco-2A cells leads to a reduction in the number of host cell receptors recognized by sporozoite adhesion molecules. These results are similar to those obtained with an in vitro model of E. histolytica interaction with Caco-2 cells, in which trophozoite adhesion to differentiated cells was found to be less extensive than that to undifferentiated cells (13). Interestingly, E. histolytica trophozoites also express a surface Gal-GalNAc-specific lectin which mediates attachment to host cells (reviewed in reference 17). Diminished attachment to differentiated Caco-2 cells was postulated to be related to a known decrease in the number of polylactosamine units (which may serve as carbohydrate receptors for the Gal-GalNAc-specific adhesion lectin) in differentiated cells (13, 29). This explanation may also hold true for diminished lectin-mediated adhesion of C. parvum sporozoites to differentiated Caco-2A cells.
FIG. 2.
Effect of differentiation of Caco-2A cells on sporozoite attachment. Caco-2A cells were cultured to confluence, and two sets of microtiter plates were examined at each time indicated. In the first set the cells were assayed for alkaline phosphatase. Monolayers in the second set were glutaraldehyde fixed and assayed for sporozoite attachment (105 parasites added per well). For each set of plates the average number of cells per well was enumerated. Alkaline phosphatase activity was expressed as A405 per minute per cell, and sporozoite attachment was expressed as the A405 per 107 cells. Values are the means ± standard errors of the means for triplicate samples.
Inhibition studies. (i) Polyclonal anti-C. parvum antibodies.
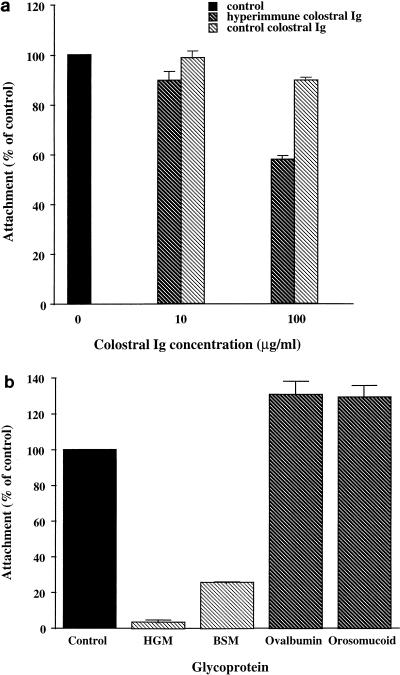
In a previous study we showed that sporozoite attachment to MDCK cells was inhibited by rabbit and mouse polyclonal antibodies to C. parvum (8). In order to determine the effects of polyclonal antibodies on attachment to intestinal cells, we used hyperimmune bovine colostrum (HBC), which has been previously shown to inhibit C. parvum growth in vitro and in vivo and has also been used with some success for passive immunotherapy of C. parvum-infected patients (reviewed in reference 19). HBC Ig as well as bovine colostral Ig from a nonimmunized cow (as a control) was obtained as a gift from Joe Crabb, Immucell Corporation, Portland, Maine, and used at final concentrations of 10 and 100 μg/ml (4). As shown in Fig. 3a, antibodies to C. parvum present in HBC Ig inhibited attachment by 30% (P < 0.001) at a concentration of 100 μg/ml compared to the control. These findings indicate that antibodies which specifically block sporozoite attachment are present in HBC Ig and would likely contribute to reducing parasite infectivity. Interestingly, Doyle et al. showed that the same preparation of HBC Ig inhibited invasion and intracellular development of the parasite in MDCK cells by 45% at a concentration of 100 μg/ml (4).
FIG. 3.
Effects of inhibitors on attachment of sporozoites to Caco-2A cells. Sporozoites were incubated with 10 or 100 μg of HBC Ig or control per ml (a) and 100 μg of glycoprotein per ml (b) for 30 min and then added to fixed Caco-2A cells, and attachment was quantified by ELISA. Attachment is expressed as a percentage of the control, to which no colostral Ig or glycoprotein had been added. Values are the means ± standard errors of the means for triplicate samples. HGM, hog gastric mucin; BSM, bovine submaxillary mucin.
(ii) Lectin-specific glycoproteins.
We have previously described the presence of a Gal-GalNAc-specific lectin in C. parvum sporozoites (9, 23). Consistent with previous studies on lectin-receptor interactions (17), the sporozoite lectin exhibited a preference for complex carbohydrates. Lectin activity was more effectively inhibited by Gal-GalNAc-containing glycoproteins than by Gal or GalNAc. These glycoproteins were also shown to inhibit adhesion of C. parvum sporozoites to MDCK cells (9), suggesting that the lectin may mediate binding of the parasite to host cells. In order to determine whether these inhibitors block parasite attachment to Caco-2A cells, the attachment assay was performed in the presence of glycoproteins (Sigma Chemical Co.). Hog gastric mucin and bovine submaxillary mucin, which were the glycoproteins most inhibitory for lectin activity, also inhibited attachment to intestinal cells by 97% (P < 0.0001) and 75% (P < 0.0001), respectively (Fig. 3b). Orosomucoid and ovalbumin, which were noninhibitory for hemagglutinating activity, did not inhibit attachment. These findings suggest that the lectin may mediate sporozoite attachment to intestinal epithelial cells and warrant further investigation of its role in the host-parasite interaction.
The C. parvum–Caco-2A attachment assay will be a valuable model system for further investigation of the role of the sporozoite lectin and other potential adhesins in mediating adhesion to intestinal cells. From these studies, we hope to gain valuable insights into the molecular basis of a crucial step in C. parvum pathogenesis. The attachment assay also serves as a fast, reproducible, and quantitative means of screening potential inhibitors of sporozoite adhesion. Compounds which block parasite attachment to host cells may prove useful for developing new interventional therapies against cryptosporidiosis.
Acknowledgments
This work was supported by PHS grants AI33384 and AI40344 from NIAID and by the Cell Culture Core of the GRASP Digestive Disease Center, under grant P30 DK34928 from NIDDK. R.V. was supported by a fellowship from the Programme Lavoisier (Ministère des Affaires Etrangères, France).
REFERENCES
- 1.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 2.Buraud M, Forget E, Favennec L, Bizet J, Gobert J-G, Deluol A-M. Sexual stage development of cryptosporidia in the Caco-2 cell line. Infect Immun. 1991;59:4610–4613. doi: 10.1128/iai.59.12.4610-4613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrero J C, Díaz M Y, Viveros M, Espinoza B, Acosta E, Ortiz-Ortiz L. Human secretory immunoglobulin A anti-Entamoeba histolytica antibodies inhibit adherence of amebae to MDCK cells. Infect Immun. 1994;62:764–767. doi: 10.1128/iai.62.2.764-767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle P S, Crabb J, Petersen C. Anti-Cryptosporidium parvum antibodies inhibit infectivity in vitro and in vivo. Infect Immun. 1993;61:4079–4084. doi: 10.1128/iai.61.10.4079-4084.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favennec L, Egraz-Bernard M, Comby E, Lemeteil D, Ballet J J, Brasseur P. Immunofluorescence detection of Cryptosporidium parvum in Caco-2 cells: a new screening method for anticryptosporidial agents. J Eukaryot Microbiol. 1994;41:39S. [PubMed] [Google Scholar]
- 6.Griffiths J K, Moore R, Dooley S, Keusch G T, Tzipori S. Cryptosporidium parvum infection of Caco-2 cell monolayers induces an apical monolayer defect, selectively increases transmonolayer permeability, and causes epithelial cell death. Infect Immun. 1994;62:4506–4514. doi: 10.1128/iai.62.10.4506-4514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarino A, Canani R B, Casola A, Pozio E, Russo R, Bruzzese E, Fontana M, Rubino A. Human intestinal cryptosporidiosis: secretory diarrhea and enterotoxic activity in Caco-2 cells. J Infect Dis. 1995;171:976–983. doi: 10.1093/infdis/171.4.976. [DOI] [PubMed] [Google Scholar]
- 8.Hamer D H, Ward H, Tzipori S, Pereira M E A, Alroy J P, Keusch G T. Attachment of Cryptosporidium parvum sporozoites to MDCK cells in vitro. Infect Immun. 1994;62:2208–2213. doi: 10.1128/iai.62.6.2208-2213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joe A, Hamer D H, Kelley M A, Pereira M E A, Keusch G T, Tzipori S, Ward H D. Role of a Gal/GalNAc-specific sporozoite surface lectin in Cryptosporidium parvum-host cell interaction. J Eukaryot Microbiol. 1994;41:44S. [PubMed] [Google Scholar]
- 10.Kuhls T L, Mosier D A, Crawford D L. Effects of carbohydrates and lectins on cryptosporidial sporozoite penetration of cultured cell monolayers. J Protozool. 1991;38:74S–76S. [PubMed] [Google Scholar]
- 11.Langer R C, Riggs M W. Neutralizing monoclonal antibody protects against Cryptosporidium parvum infection by inhibiting sporozoite attachment and invasion. J Eukaryot Microbiol. 1996;43:76S–77S. doi: 10.1111/j.1550-7408.1996.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 12.Laurent F, Eckmann L, Savidge T C, Morgan G, Theodos C, Naciri M, Kagnoff M F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li E, Stenson W F, Kunz-Jenkins C, Swanson P E, Duncan R, Stanley S L., Jr Entamoeba histolytica interactions with polarized human intestinal Caco-2 epithelial cells. Infect Immun. 1994;62:5112–5119. doi: 10.1128/iai.62.11.5112-5119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillot C, Favennec L, Francois A, Ducrotte P, Brasseur P. Sexual and asexual development of Cryptosporidium parvum in five oocyst- or sporozoite-infected human enterocytic cell lines. J Eukaryot Microbiol. 1997;44:582–585. doi: 10.1111/j.1550-7408.1997.tb05963.x. [DOI] [PubMed] [Google Scholar]
- 15.Matusumoto H, Erickson R H, Gum J R, Yoshioka M, Gum E, Kim Y S. Biosynthesis of alkaline phosphatase during differentiation of the human colon cancer cell line Caco-2. Gastroenterology. 1990;98:1199–1207. doi: 10.1016/0016-5085(90)90334-w. [DOI] [PubMed] [Google Scholar]
- 16.McCabe R E, Yu G S M, Conteas C, Morrill P R, McMorrow B. In vitro model of attachment of Giardia intestinalis trophozoites to IEC-6 cells, an intestinal cell line. Antimicrob Agents Chemother. 1991;35:29–35. doi: 10.1128/aac.35.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy J J, Mann B J, Petri W A., Jr Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect Immun. 1994;62:3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald V, Stables R, Warhurst D C, Barer M R, Blewett D A, Chapman H D, Connolly G M, Chiodini P L, McAdam K P W J. In vitro cultivation of Cryptosporidium parvum and screening for anticryptosporidial drugs. Antimicrob Agents Chemother. 1990;34:1498–1500. doi: 10.1128/aac.34.8.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 20.Ortega-Barria E, Pereira M E A. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 21.Pinto M, Robine-Leon S, Appay M-D, Kedinger M, Triadou E, Dussauix E, Lacroix B, Simon-Assman P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 22.Rasmussen K R, Larsen N C, Healey M C. Complete development of Cryptosporidium parvum in a human endometrial carcinoma cell line. Infect Immun. 1993;61:1482–1485. doi: 10.1128/iai.61.4.1482-1485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thea D M, Pereira M E A, Kotler D, Sterling C R, Keusch G T. Identification and partial purification of a lectin on the surface of the sporozoite Cryptosporidium parvum. J Parasitol. 1992;78:886–893. [PubMed] [Google Scholar]
- 24.Tzipori S, Rand W, Theodos C. Evaluation of a two-phase scid mouse model preconditioned with anti-interferon-gamma monoclonal antibody for drug testing against Cryptosporidium parvum. J Infect Dis. 1995;172:1160–1164. doi: 10.1093/infdis/172.4.1160. [DOI] [PubMed] [Google Scholar]
- 25.Upton S J, Tilley M, Brillhart D B. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–236. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Beers E H, Al R H, Rings E H, Einerhand A W, Dekker J, Buller H A. Lactase and sucrase-isomaltase gene expression during Caco-2 cell differentiation. Biochem J. 1995;308:769–775. doi: 10.1042/bj3080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdon R, Keusch G T, Tzipori S, Grubman S M, Jefferson D M, Ward H D. An in vitro model of infection of human biliary epithelial cells by Cryptosporidium parvum. J Infect Dis. 1997;175:1268–1272. doi: 10.1086/593695. [DOI] [PubMed] [Google Scholar]
- 28.Woods K M, Nesterenko M V, Upton S J. Development of a microtitre ELISA to quantify development of Cryptosporidium parvum in vitro. FEMS Microbiol Lett. 1995;128:89–94. doi: 10.1111/j.1574-6968.1995.tb07505.x. [DOI] [PubMed] [Google Scholar]
- 29.Youakim A, Herscovics A. Differentiation-associated decrease in the proportion of fucosylated polylactosaminoglycans of Caco-2 human colonic adenocarcinoma cells. Biochem J. 1987;247:299–306. doi: 10.1042/bj2470299. [DOI] [PMC free article] [PubMed] [Google Scholar]