Abstract
Decades of research have suggested that stimulation of supraspinal structures, such as the periaqueductal gray (PAG) and rostral ventromedial medulla (RVM), inhibits nocifensive responses to noxious stimulation through a process known as descending modulation. Electrical stimulation and pharmacologic manipulations of the PAG and RVM identified transmitters and neuronal firing patterns that represented distinct cell types. Advances in mouse genetics, in vivo imaging, and circuit tracing methods, in addition to chemogenetic and optogenetic approaches, allowed the characterization of the cells and circuits involved in descending modulation in further detail. Recent work has revealed the importance of PAG and RVM neuronal cell types in the descending modulation of pruriceptive as well as nociceptive behaviors, underscoring their roles in coordinating complex behavioral responses to sensory input. This review summarizes how new technical advances that enable cell type-specific manipulation and recording of neuronal activity have supported, as well as expanded, long-standing views on descending modulation.
Recent progress in our understanding of the descending modulation of nociceptive responses
As early as the 1970s, initial forays to investigate endogenous modulation revealed that stimulation of the ventrolateral PAG (vlPAG) was sufficient to inhibit behavioral responses to noxious stimulation in rats [1,2]. This discovery was further translated and reproduced in humans, whereby neurosurgical electrical stimulation of the vlPAG produced patient-reported relief for pain [3,4]. Since then, a large body of work using a combination of pharmacology and electrical stimulation has identified the roles of key structures, particularly the PAG and a downstream projection field, the RVM, in the descending modulation of nociceptive behaviors [5–15]. These studies were instrumental in identifying the brain regions, neurotransmitters, and neuropeptides involved in descending modulation.
It has been challenging to identify specific cell populations and precise neural circuits that mediate descending modulation because previously available tools have broad effects on the regions targeted. For instance, electrical stimulation and microinjections of pharmacologic agents could act on multiple cell types and axons of passage in the vicinity of the site of stimulation or infusion. Furthermore, many studies were conducted in lightly anesthetized animals, which made it difficult to study neurons in the PAG and RVM in the context of natural behaviors.
Spurred by recent technological advances enabling cell type-specific recording and manipulation of neuronal activity, there has been a renewed interest in characterizing the mechanisms of descending modulation, with a focus on identifying the roles of specific cell types involved in descending modulation, and the organization of their connections (Figure 1). Recent efforts have used combinatorial genetic strategies, viral tools that enable improved connectivity tracing, in vivo imaging approaches, and targeted manipulation of identified cell types in awake, freely behaving mice. This review summarizes recent and foundational models for descending modulation and explores the roles of the descending modulatory systems in coordinating the responses to different types of sensory input, particularly nociception and pruriception.
Figure 1. Identified circuits within the PAG, RVM, and SC.
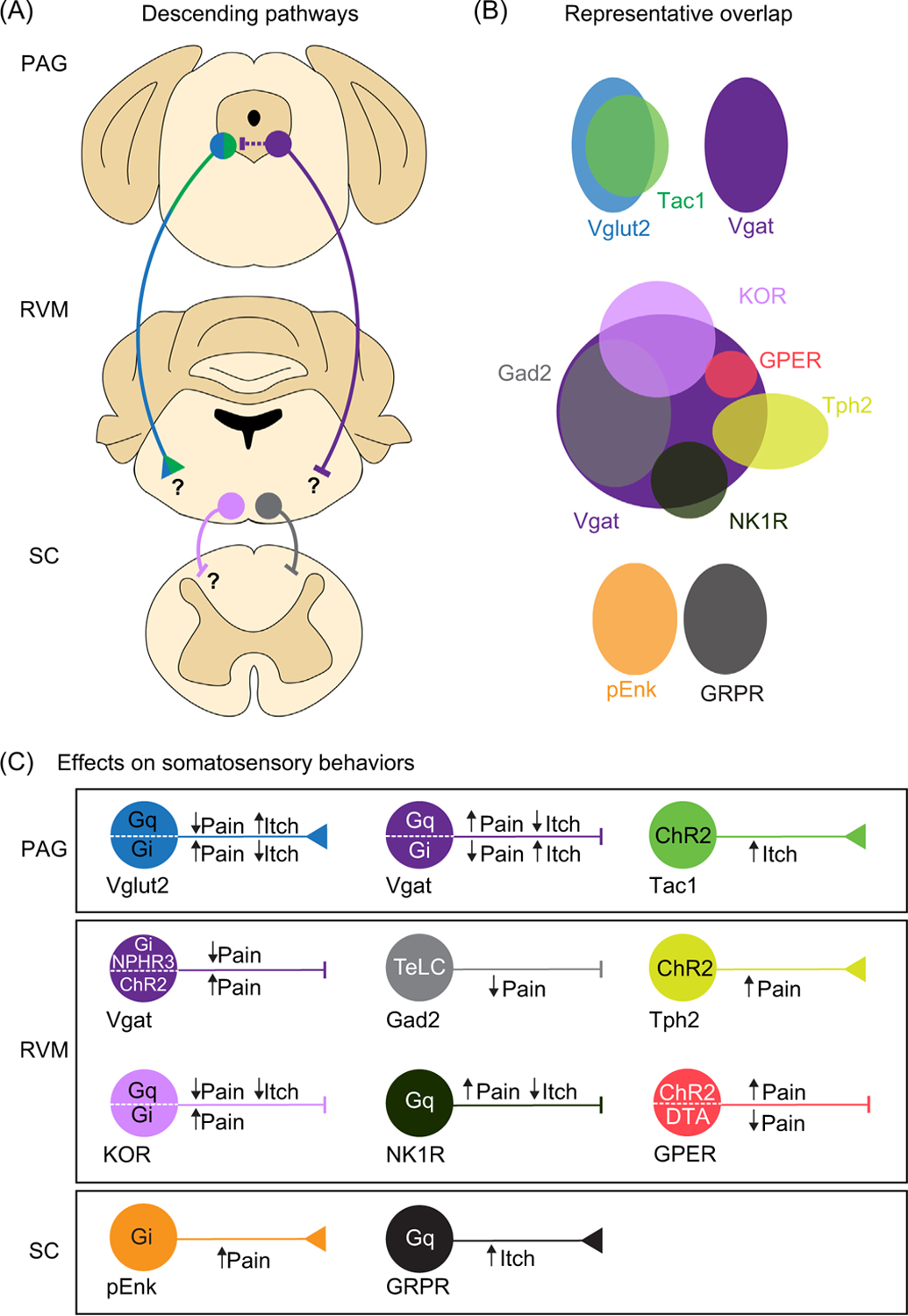
(A) An overview of the major components of descending modulatory pathways for nociception including the PAG, RVM, and SC. (B) Representative genetic overlap of PAG, RVM, and SC neuronal populations in the modulation of nociception and pruriception. Color codes in A are described in further detail. (C) Summary of effects of cell type-specific manipulations in the descending modulatory axis. Abbreviations: ChR2, channelrhodopsin-2; DTA, diphtheria toxin A; Gad2, glutamate decarboxylase 2; Gi, hM4Di, Gi-coupled human M4 muscarinic DREADD; GPER; G protein-coupled estrogen receptor; Gq, hM3Dq, Gq-coupled human M3 muscarinic designer receptor exclusively activated by designer drug (DREADD); GRPR, gastrin-releasing peptide receptor; KOR, kappa-opioid receptor; NK1R, neurokinin 1 receptor; PAG, periaqueductal gray; pENK, proenkephalin; RVM, rostral ventromedial medulla; SC, spinal cord; Tac1, tachykinin 1; TeLC, tetanus toxin light chain; Tph2, tryptophan hydroxylase 2; Vgat, vesicular GABA transporter; Vglut2, vesicular glutamate transporter 2.
Dissection of neuronal populations in the PAG and RVM using transgenic approaches
Recent technological advances enable the targeting of distinct neuronal subsets expressing specific molecular markers. Expression of loci-specific gene recombinases (such as Cre, Flp, and Dre) in mice, via either targeted gene engineering or viral vectors, has led to the targeting of neuronal populations based on their anatomical location, their spatial projections, or genetic profile [16]. Combinatorial use of specific recombinase-dependent vectors further permits visualization, mapping, and direct manipulation of targeted neuronal circuits [17,18].
Overall, recent efforts using Cre-driven approaches have supported the conclusions of previous investigations. For example, activation of all neurons by delivery of glutamate or any agonist of ionotropic glutamate receptors to the vlPAG results in the elevation of sensory thresholds and/or reduced responses to noxious input [5,14,19]. By contrast, injection of GABA and GABA agonists into the vlPAG enhances behavioral response to noxious input [15,20,21], suggesting that pharmacological manipulations that increase PAG excitation facilitate antinociceptive behaviors. Consistent with prior studies, studies using Cre drivers that target Vgat (GABAergic) and Vglut2 (glutamatergic) neurons in the PAG have likewise revealed that these two classes of neurons reciprocally modulate nociceptive behaviors (Figure 1). Chemogenetic and optogenetic studies have shown that activation of Vgat neurons enhances behavioral responses to noxious input, whereas activation of Vglut2 neurons reduces behavioral responses to noxious input [22–24], consistent with previous models established using pharmacology and electrophysiology. However, a new insight was the discovery that GABAergic and glutamatergic PAG neurons exhibit modality specificity and are differentially engaged to modulate nociceptive and pruriceptive behaviors [22]. Targeting of the Vgat- and Vglut2-Cre populations in the PAG revealed that responses to nociceptive and pruriceptive input are divergently modulated: activation of Vglut2-Cre neurons reduces sensitivity to noxious heat but facilitates chloroquine-induced scratching, whereas activation of Vgat-Cre neurons does the reverse [22,23]. Thus, the ability to manipulate specific neuronal populations during complex behaviors has exposed apparent opposing roles of PAG neurons in the modulation of itch and pain behaviors.
The use of Cre drivers has led to the re-examination of prior models as well. For example, it was previously thought that glutamatergic PAG neurons comprised the majority of the descending projections to the RVM [5,25–29], while GABAergic neurons represented a tightly regulated inhibitory local microcircuit [5,11,30,31]. New evidence suggests that this idea is an over-simplification. Targeting of Cre-defined PAG populations has revealed that those projecting to the RVM can be glutamatergic [32–35] as well as GABAergic [36]. PAG GABAergic neurons have been observed to innervate spinally projecting RVM neurons [37]. Although most behavioral studies have tested the role of glutamatergic projections from the PAG to the RVM [32,33,35], the specific roles of local GABAergic interneurons versus neurons in the PAG that project to the RVM remain to be tested directly.
The use of Cre alleles to explore the function of the RVM in descending modulation has also helped to reframe the traditional classification of RVM neurons. In the RVM, single-unit recordings in lightly anesthetized rats have classified neurons into three types: ON, OFF, and NEUTRAL cells, based on their firing activity and responses to noxious stimulation [25,38,39]. ON cells exhibit increased discharge during noxious stimulation, are inhibited by morphine, and have been proposed to facilitate nociceptive responses. By contrast, OFF cells exhibit decreased discharge during noxious stimulation, are excited by morphine, and are thought to inhibit nociceptive responses [25,40–42]. NEUTRAL cells are unaffected by noxious cutaneous stimuli or by exogenous opioids, and their role in nociception is unclear, although they are generally thought to be largely serotonergic [43] and are believed to participate in autonomic and homeostatic functions [39,44–47].
The role of inhibitory neurons in the RVM, as a collective population, has been hotly contested. GABAergic RVM neurons have historically been identified as pro-nociceptive, or ON cells, based on pharmacology and electrophysiology studies [48–50]. However, it has been shown that inhibitory neurons in the RVM also function to suppress the activity of ON cells, suggesting they serve mixed functions [48,49,51]. When GABAergic (Vgat-Cre) RVM neurons were activated using optogenetic or chemogenetic actuators, they were found to facilitate mechanical, but not thermal responses [52]. Facilitation of thermal and mechanical responses was also observed when a subpopulation of GABAergic RVM neurons, marked by G protein-coupled estrogen receptor (GPER-Cre) was activated [53]. However, another study found that chemogenetic activation of Gad2 RVM neurons (which comprise a subpopulation of Vgat neurons) produced inhibition of both thermal and mechanical responses [54]. Activation of descending KOR neurons in the RVM, an exclusively GABAergic population, robustly inhibited nociceptive and pruriceptive behaviors [33,55], a finding consistent with previous literature indicating that KOR neurons correspond to OFF cells [48,49,51]. RVM neurons containing the neurokinin-1 receptor (NK1R), which are also GABAergic, were recently shown to be ON cells that facilitated nociceptive responses yet suppressed pruritogen-induced scratching behavior [56]. Together, recent efforts further refine the ON/OFF model by reinforcing the idea that GABAergic neurons in the RVM likely comprise several discrete cell types, including both ON and OFF cells, which have opposing functions in the behavioral responses related to itch and pain. Furthermore, the manipulations of defined cell types have now delineated the specific roles of the GABAergic subpopulations in specific nociceptive behaviors.
Lastly, the role of serotonergic neurons, classically thought of as NEUTRAL cells [57], in nociception is also controversial. For example, in vivo recordings of serotonergic neurons have shown that they are predominantly NEUTRAL cells [43], but pharmacological blockade of serotonergic signaling between the RVM and spinal cord has also revealed that they can have both pro- and antinociceptive roles [58–60]. A cell type-specific approach has been used to define the role of 5HT neurons in the modulation of nociceptive behaviors in awake mice using targeting of Tph2 expression for transgenic approaches. Tph2 neurons were found to facilitate mechanical and thermal sensitivity upon optogenetic activation [61]. Fiber photometry recordings of RVM Tph2 neurons also demonstrated that they are activated in the presence of noxious mechanical and thermal stimulation [62]. It is interesting to note that a subset of serotonergic neurons has been shown to co-express Oprm1 [63,64], an indication that serotonergic neurons may comprise subsets of ON and NEUTRAL cells. Reconciling recent findings, it is likely that activation of serotonergic neurons gives rise to a pro-nociceptive phenotype due to the specific excitation of 5HT ON cells [61].
Cell type-specific manipulations represent an efficient means to characterize subpopulations of RVM neurons, which exhibit complex molecular identities. New Cre manipulations reveal the challenges in segregating neuronal subpopulations in the RVM across the original ON/OFF/NEUTRAL schema. To harmonize recent findings with past recordings of lightly anesthetized rats, it is clear that while activation of some GABAergic RVM neurons facilitates nociceptive responses (i.e., consistent with ON cells) [52], not all inhibitory neurons in the RVM are pro-nociceptive [54]. The serotonergic neurons that do not contain the mu-opioid receptor may indeed correspond to NEUTRAL cells based on the classical schema.
Overall, recent studies that use cell type-specific manipulations of neuronal activity in the PAG and RVM have generally supported previous models for descending modulation. However, in the case of RVM ON/OFF/NEUTRAL cells, it is clear that manipulation of distinct cellular subtypes does not always produce results in agreement with classifications based on physiology. Furthermore, although recent efforts have not significantly challenged previous frameworks for descending modulation, cell type-specific approaches have contributed new perspectives on the cells and circuits involved in the descending modulation of nociception. In particular, the ability to directly manipulate distinct neuronal populations in rodents has permitted the study of the role of specific populations in complex behaviors in awake animals, revealing that descending modulatory systems are differentially engaged in pruriceptive and nociceptive behaviors.
Neurons in descending modulatory pathways participate in distinct aspects of somatosensation
Exploration of cell types in the PAG and RVM using cell type-specific manipulations has yielded surprising insights into the modulation of pruriception and nociception. Although most work examining the role of endogenous sensory modulation has emphasized reflexive responses to nociceptive assays, recent areas of focus have been broadened to include other sensory modalities, particularly pruriception.
Previously, electric stimulation of the PAG had been shown to reduce the histamine-evoked activity of neurons in the spinal cord dorsal horn in lightly-anesthetized rats [65]. Much like noxious stimuli, pruritogens were thought to facilitate the activity of ON cells and inhibit OFF cells in the RVM [66], suggesting that descending modulatory systems regulate pruriception and nociception similarly. However, more recent cell type-specific manipulation studies of PAG pathways have shown that both glutamatergic and GABAergic neurons differentially modulate pruriceptive and nociceptive behaviors [22]. Activation of PAG GABAergic neurons inhibits scratching but facilitates nociceptive behavior, whereas activation of glutamatergic neurons inhibits responses to noxious input yet facilitates scratching behavior [23]. This divergence illustrates that there are at least two classes of PAG neurons that exert dichotomous effects on nociceptive and pruriceptive signaling pathways.
Thus, a major benefit of manipulating cell types in awake mice is the ability to examine a diverse repertoire of responses to sensory testing. Chloroquine induced conditioned place aversion (CPA) in control animals, but CPA was not observed with activation of GABAergic neurons in the PAG [22], suggesting that the PAG is important for both the expression of the affective component of itch as well as the motor response to pruriception. While the PAG and RVM are important for the reflexive responses to pain and itch assays, additional work is necessary to examine their contributions to the modulation of affective responses, and whether affective responses could be due entirely to descending modulation, or whether ascending projections from the PAG and RVM to other supraspinal structures may also be involved.
Advances in protein engineering permit imaging of neuronal activity during complex behaviors based on changes in intracellular calcium. For example, using fiber photometry recordings, it was found that Tac1-Cre neurons in the PAG are active during pruritogen-induced scratching [32]. Chemogenetic or optogenetic manipulation of Tac1 neurons further demonstrated that they are both sufficient and necessary for eliciting scratching behavior and that the behavioral effects of activating Tac1-Cre neurons in the PAG are proposed to be mediated through glutamatergic signaling in the RVM. Interestingly, Tac1-Cre neurons were not found to be involved in nociception [32], further illustrating a role in modality specificity among PAG neurons.
In the RVM, Cre-dependent manipulations of neuronal populations have also revealed that they have different effects on behaviors that are mediated by distinct nociceptive circuits. Manipulations of RVM GABAergic neurons drive opposing behavioral phenotypes in distinct nociceptive assays. Whereas Vgat-Cre neurons facilitate mechanical withdrawal responses, Gad2-Cre neurons inhibit thermal sensitivity to hotplate testing [52,54]. GABAergic RVM neurons containing NK1R were recently shown to attenuate scratching behaviors yet drive different effects in mechanical sensitivity depending on the Cre allele used [56]. Activation of NK1R neurons in the RVM using the NK1R-CreER allele reduced mechanical thresholds in the von Frey assay, but when NK1R neurons were activated with the NK1R-Cre line, no effect on mechanical thresholds was observed [56], possibly due to the differences in the efficiency of capturing the NK1R population in the different Cre alleles. It is also important to note that it is generally difficult to compare the functions of different neuronal populations across different papers because not all studies included the same assays. Nevertheless, recent observations contrast with those from single-unit recordings of RVM neurons, which previously highlighted that RVM neurons respond to different sensory stimuli – including innocuous and noxious mechanical, thermal, and itch stimuli – similarly [66–68]. The variety of stimuli that can elicit similar responses from individual RVM neurons previously suggested that these cells may not be tuned to the processing of distinct modalities of sensory input. Recent evidence challenges this view, though testing with consistent behavioral assays is necessary to understand the extent to which RVM populations modulate responsivity to distinct types of somatosensation or distinct types of behavioral responses. Additional work using recordings and imaging in freely behaving animals will also help to address these important questions.
The evidence that manipulations of PAG and RVM neurons drive diverse responses to somatosensory input supports the idea that these structures contribute to the state dependence of somatosensation, including nociception [39,45,69–72]. Although the PAG and RVM have been classically thought to be important for the descending modulation of nociception, a view extended more recently to pruriception, new research has also elucidated their roles beyond somatosensation. These other functions include the modulation of anxiety, the activation of defensive responses, and the regulation of the autonomic nervous system [24,37,73–82]. The PAG and RVM are extensively connected with several brain regions involved in other complex functions, including the parabrachial nucleus (PBN), central amygdala (CeA), zona incerta, prefrontal cortex (PFC), locus coeruleus (LC), hypothalamus, and spinal dorsal horn [25,36,83–95]. Recent studies have begun to identify how some complex behaviors are modulated by distinct neuron populations in the PAG and RVM that are defined by projection target as well as neurochemical phenotype.
As an example, dopaminergic (DA) neurons in the PAG are critically important for the modulation of nociception by opioids [96,97]. The antinociceptive effects of PAG DA neurons have been attributed to projections to the RVM [98] as well as to the bed nucleus of the stria terminalis (BSNT) [78]. Recent chemogenetic and optogenetic characterizations of PAG dopaminergic projections to the BNST have unveiled their novel roles in modulating defensive responses in a sex-dependent manner [99]. Activation of PAG dopaminergic projection to the BNST inhibits thermal and mechanical nociception and responses to inflammatory injury in male, but not female, rodents. By contrast, activation of this pathway promotes locomotion in female, but not male, mice [99]. These observations hint at the possibility that the PAG concurrently organizes complex motor responses (including increased locomotion and reflexive withdrawal to noxious testing) in the modulation of nociception.
Extensive neural networks may also explain the mechanisms by which the endogenous modulatory system could coordinate responses to nociception in addition to its other roles in homeostasis and survival. Recordings of neurons in modulatory circuits revealed that these neurons are tuned for diverse functions including sleep, micturition, respiration, sexual arousal, and thermoregulation [39,69,71,100,101]. Optogenetic stimulation of excitatory PAG neurons projecting to the magnocellular nucleus of the medulla induces freezing [35] and a group of excitatory neurons within the gigantocellular nucleus have also been shown to be involved in the modulation of ongoing locomotion through a direct pathway to the ventral horn [102]. Although behavioral results following manipulations of cell types in the PAG and RVM are often attributed to a descending pathway, the connectivity of the PAG and RVM with other brain regions could also account for the observed changes in pruriception and nociception with global manipulation of cell types in these areas. Thus, the diverse sensory, autonomic, and defensive roles of the pain-modulatory pathways underscore their various contributions to integrating and coordinating ascending and descending pathways that are crucial for survival.
Spinal neurons targeted by descending modulation
The spinal cord represents the final target of the descending modulatory system. The necessity of spinal transmission within the context of descending modulation has been well characterized [13,103–106]. Recent efforts have begun to elucidate the precise identities of spinal neurons that receive input from the RVM in the context of somatosensation (Figure 2). Optogenetic and chemogenetic manipulations of Vgat-Cre RVM neurons have suggested that GABAergic RVM neurons descend to pre-synaptically inhibit mechanosensory input onto spinal inhibitory enkephalinergic (Penk) interneurons [52]. Thus, descending GABAergic neurons could facilitate mechanical nociception through the process of disinhibition in the dorsal horn. Alternatively, combined optogenetic and electrophysiological experiments in spinal cord slices have suggested that descending GABAergic RVM neurons inhibit GRPR-expressing neurons in the spinal cord [107], a population that has been implicated in driving scratching behavior [108,109]. Together, these reports suggest that GABAergic RVM neurons could facilitate nociceptive behaviors through the inhibition of Penk spinal neurons and inhibit pruriceptive behaviors through the inhibition of excitatory GRPR neurons (Figure 2). Using similar approaches, studies have also identified cell types in other brain regions including the LC [110], medullary dorsal reticular nucleus [111], and hypothalamus [112] that directly modulate spinal nociceptive circuits (Figure 2). It remains unclear whether these other hubs for descending modulation of nociception intersect with PAG and RVM pathways or represent independent and parallel circuits for the descending modulation of nociception. Together, these different pathways likely represent partially overlapping components of descending modulation.
Figure 2. Genetically defined spinally projecting pathways for nociception and pruriception.
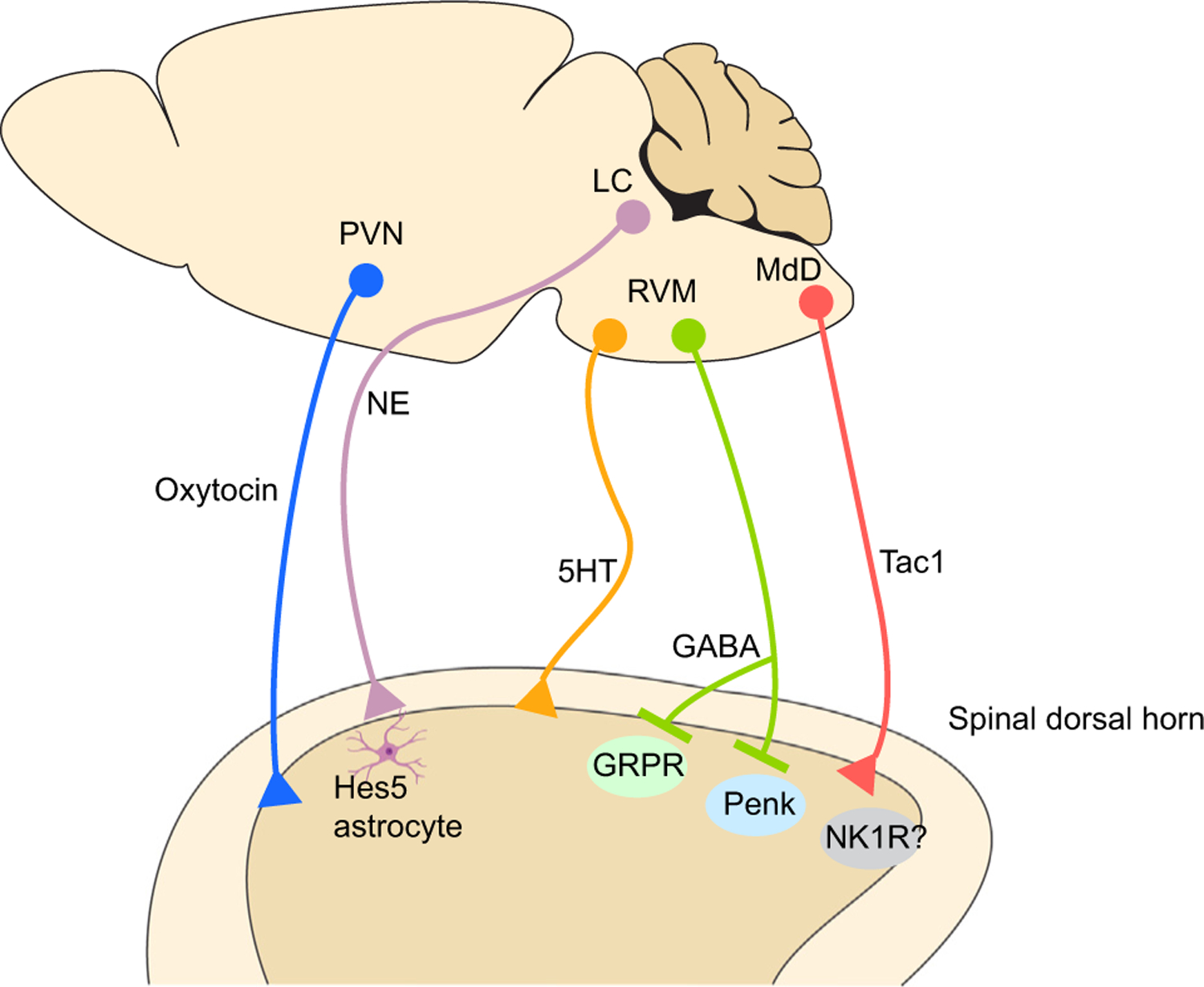
Schematic demonstrating various spinal projection pathways involved in the modulation of nociception and pruriception that have been revealed using cell type-specific measurements and manipulations. Abbreviations: 5HT, 5-hydroxytryptamine; GABA, glutamate decarboxylase; GRPR, gastrin-releasing peptide receptor; LC, locus coeruleus; MdD, medullary reticular nucleus; NE, norepinephrine; NK1R, neurokinin 1 receptor; Penk, proenkephalin; PVN, paraventricular nucleus of hypothalamus; RVM, rostral ventromedial medulla; Tac1, tachykinin 1.
Concluding remarks and future perspectives
Technological advances have allowed for the precise spatial and temporal manipulations of molecularly identified neuronal populations. New investigations into descending modulatory circuits expand upon decades of work involving in vivo pharmacology, electrical stimulation, lesion studies, as well as single-unit recordings conducted in lightly anesthetized animals. Assessment of neuronal activity in awake and freely behaving animals has confirmed and extended the roles of the PAG and RVM in the descending modulation of nociception. Both past and present investigations shed light on the complexity of the heterogeneous populations of neurons involved in descending modulation. The application of novel tools and techniques has largely confirmed previous models, and, by extension, permitted the anatomical and behavioral characterization of discrete neurons in awake, freely behaving rodents.
A more detailed understanding of descending modulatory systems could provide insights into the basis for interesting observations such as the finding that chronic pain disproportionately affects women compared with men [113]. For example, sex differences have been observed in the rat PAG with respect to opioid receptor expression and intrinsic GABA signaling [21,114], which could explain differences in response to opioid medications observed in humans [113]. Furthermore, it was recently shown that dopaminergic activity in the PAG differentially engages motor and sensory behaviors in male and female mice [99]. Additional work in this area is important because the existing models for descending modulation are based on work conducted primarily in male rodents.
It is important to note that most recent studies have been conducted in mice whereas many original discoveries on descending modulation were performed in rats. Despite the use of different models, work from both species has generally yielded congruent results. For example, the functional similarities observed in the PAG across both species are reflected in antinociceptive responses to local opioid administration within the PAG [115,116], the electrophysiological responses of PAG neurons to noxious stimuli [117,118], and the anatomical connection between the PAG and the RVM [37,77,119,120]. However, differences between species have also been observed with respect to opioid receptor expression and signaling, highlighting the limitations of extrapolating findings from single-species studies [119,121–124]. Thus, the lack of parallel comparison obscures the potential and fundamental differences between the two models. Translation of research conducted in both mice and rats to humans is an important consideration for future studies.
Another interesting question surrounds the contributions of the descending modulatory system to chronic pain states. The structural and molecular changes that could occur in identified PAG and RVM circuits during chronic injury states may underlie the development of chronic pain conditions. Plasticity, such as increases in GABA tone within the PAG, has been observed in models of persistent inflammation [21,125]. The use of cell type-specific manipulations, combined with electrophysiological techniques could further facilitate investigations into alterations in specific pathways in the context of chronic pain.
While cell type-specific manipulations represent relatively new developments in the field of neurobiology, one limitation is that behavioral responses elicited by chemogenetic and optogenetic manipulations may not represent physiological states. Furthermore, artificial activation may not necessarily reflect responses to natural settings. Another major limitation of these approaches is that conclusions made from the targeting of individual populations often ignore or oversimplify the heterogeneity within the nervous system. Although it is possible to discern unique properties among neurons within a subset, these neurons may not represent truly unique populations per se because the diversity of cell types within descending modulatory hubs, such as the PAG and RVM, remains only partially characterized. The testing of specific cell types has been limited by the lack of a full understanding of the diversity of cell types in these areas as well as the availability of related mouse lines. A detailed atlas of cell types in the PAG and RVM would tremendously advance research in the field of descending modulation by identifying additional cell types that could then be validated and targeted, for instance, using Cre alleles (see Outstanding questions). The validation of the precise neuronal subpopulations and pathways involved in descending modulation holds promise for the discovery of targets and therapies for disorders of somatosensation including pain and itch.
Outstanding questions.
The PAG and the RVM are involved in the integration of homeostatic processes such as arousal, motor, and defensive behaviors. What are the responsible neuronal ensembles that coordinate these behaviors with somatosensation?
What are the genetic cell types involved in descending modulation? A comprehensive genetic atlas of cell types in the PAG and RVM is presently lacking. To date, the characterization of cell types using Cre drivers has been conducted based on the availability of mouse lines. Single-cell sequencing of these structures would provide a more detailed understanding of the diverse cell types, which could then be targeted through specific Cre alleles.
Sex differences in rodents, as well as humans, have been reported in descending modulatory pathways. How do sex differences affect the endogenous pain modulatory system in both uninjured and injured states?
Acute and chronic stress can elicit analgesia and hyperalgesia, respectively. Structures such as the PAG and its descending circuitry are thought to be involved in mediating these responses to stress. What is the neural basis for stress-induced modulation of pain?
As discussed in this review, the descending modulation of pain, and more recently, itch has been studied in detail. What is the role for descending modulation in the context of other sensory modalities, such as touch, cold, and heat?
Highlights.
The periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) are critically important hubs in the endogenous analgesia pathway.
Technological and conceptual advances have permitted the identification and targeting of neural ensembles in the PAG and RVM during complex behaviors, revealing that they divergently modulate distinct components of somatosensation.
In vivo imaging, viral tracing, and molecular genetic manipulations have afforded a new layer of insight by characterizing the function of genetically defined neuronal populations within the PAG and RVM.
How these identified pathways coordinate other autonomic, motivational, and defensive responses during ongoing nociception remains to be addressed in greater detail.
Further investigations into the PAG and RVM in the descending modulation of nociception, itch, and other complex behaviors are ongoing.
Acknowledgments
We thank Dr Judith P. Golden for editing the manuscript. This work was funded by the NIH/NIAMS AR063772 and NIH/NINDS NS096705 (S.E.R.), NINDS F31 F31NS113371, and NIH/NIGMS T32GM008208 (E.N.), NINDS F31NS103472 and NIH/NIGMS T32GM007200 (J.G.G-R.), and NINDS R01NS106953 (R.W.G.).
Footnotes
Declaration of interests
The authors declare no conflicts of interest.
References
- 1.Mayer DJ and Liebeskind JC (1974) Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 68, 73–93 [DOI] [PubMed] [Google Scholar]
- 2.Mayer DJ et al. (1971) Analgesia from electrical stimulation in the brainstem of the rat. Science 174, 1351–1354 [DOI] [PubMed] [Google Scholar]
- 3.Hosobuchi Y et al. (1977) Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 197, 183–186 [DOI] [PubMed] [Google Scholar]
- 4.Baskin DS et al. (1986) Autopsy analysis of the safety, efficacy and cartography of electrical stimulation of the central gray in humans. Brain Res. 371, 231–236 [DOI] [PubMed] [Google Scholar]
- 5.Behbehani MM and Fields HL (1979) Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 170, 85–93 [DOI] [PubMed] [Google Scholar]
- 6.Fields HL et al. (1983) The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J. Neurosci 3, 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreau JL and Fields HL (1986) Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 397, 37–46 [DOI] [PubMed] [Google Scholar]
- 8.Heinricher MM et al. (1987) Evidence for two classes of nociceptive modulating neurons in the periaqueductal gray. J. Neurosci 7, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan MM et al. (1989) Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 502, 61–66 [DOI] [PubMed] [Google Scholar]
- 10.Cho HJ and Basbaum AI (1991) GABAergic circuitry in the rostral ventral medulla of the rat and its relationship to descending antinociceptive controls. J. Comp. Neurol 303, 316–328 [DOI] [PubMed] [Google Scholar]
- 11.Reichling DB and Basbaum AI (1990) Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: II. Electron microscopic immunocytochemical evidence of GABAergic control over the projection from the periaqueductal gray to the nucleus raphe magnus in the rat. J. Comp. Neurol 302, 378–393 [DOI] [PubMed] [Google Scholar]
- 12.Prieto GJ et al. (1983) N. raphe magnus lesions disrupt stimulation-produced analgesia from ventral but not dorsal midbrain areas in the rat. Brain Res. 261, 53–57 [DOI] [PubMed] [Google Scholar]
- 13.Jensen TS and Yaksh TL (1984) Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 321, 287–297 [DOI] [PubMed] [Google Scholar]
- 14.Aimone LD et al. (1987) Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain 31, 123–136 [DOI] [PubMed] [Google Scholar]
- 15.Behbehani MM et al. (1990) The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain 40, 195–204 [DOI] [PubMed] [Google Scholar]
- 16.Plummer NW et al. (2015) Expanding the power of recombinase-based labeling to uncover cellular diversity. Development 142, 4385–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth BL (2016) DREADDs for neuroscientists. Neuron 89, 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CK et al. (2017) Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci 18, 222–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maione S et al. (1998) Characterisation of mGluRs which modulate nociception in the PAG of the mouse. Neuropharmacology 37, 1475–1483 [DOI] [PubMed] [Google Scholar]
- 20.Lin Q et al. (1994) Glycine and GABAA antagonists reduce the inhibition of primate spinothalamic tract neurons produced by stimulation in periaqueductal gray. Brain Res. 654, 286–302 [DOI] [PubMed] [Google Scholar]
- 21.Tonsfeldt KJ et al. (2016) Sex differences in GABAA signaling in the periaqueductal gray induced by persistent inflammation. J. Neurosci 36, 1669–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samineni VK et al. (2019) Cell type-specific modulation of sensory and affective components of itch in the periaqueductal gray. Nat. Commun 10, 4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samineni VK et al. (2017) Divergent modulation of nociception by glutamatergic and gabaergic neuronal subpopulations in the periaqueductal gray. eNeuro 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor NE et al. (2019) The role of glutamatergic and dopaminergic neurons in the periaqueductal gray/dorsal raphe: separating analgesia and anxiety. eNeuro 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinricher MM et al. (2009) Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev 60, 214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan MJ and Franklin KB (1988) Stimulation-produced analgesia (SPA) from brain-stem and diencephalic sites in the rat: relationships between analgesia, aversion, seizures and catalepsy. Pain 33, 109–121 [DOI] [PubMed] [Google Scholar]
- 27.Fardin V et al. (1984) A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 306, 125–139 [DOI] [PubMed] [Google Scholar]
- 28.Levine R et al. (1991) Stimulation of the periaqueductal gray matter of the rat produces a preferential ipsilateral antinociception. Brain Res. 567, 140–144 [DOI] [PubMed] [Google Scholar]
- 29.Maione S et al. (2009) Functional interaction between TRPV1 and mu-opioid receptors in the descending antinociceptive pathway activates glutamate transmission and induces analgesia. J. Neurophysiol 101, 2411–2422 [DOI] [PubMed] [Google Scholar]
- 30.Basbaum AI and Fields HL (1978) Endogenous pain control mechanisms: review and hypothesis. Ann. Neurol 4, 451–462 [DOI] [PubMed] [Google Scholar]
- 31.Reichling DB and Basbaum AI (1990) Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: I. GABA-immunoreactive projection neurons in the periaqueductal gray and nucleus raphe magnus. J. Comp. Neurol 302, 370–377 [DOI] [PubMed] [Google Scholar]
- 32.Gao Z-R et al. (2019) Tac1-expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron 101, 45–59.e9 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen E et al. (2022) Medullary kappa-opioid receptor neurons inhibit pain and itch through a descending circuit. Brain 145, 2586–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin J-B et al. (2020) dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. J. Clin. Invest 130, 6555–6570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tovote P et al. (2016) Midbrain circuits for defensive behaviour. Nature 534, 206–212 [DOI] [PubMed] [Google Scholar]
- 36.Huang J et al. (2019) A neuronal circuit for activating descending modulation of neuropathic pain. Nat. Neurosci 22, 1659–1668 [DOI] [PubMed] [Google Scholar]
- 37.Morgan MM et al. (2008) Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 140, 376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields HL et al. (1995) Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J. Neurophysiol 74, 1742–1759 [DOI] [PubMed] [Google Scholar]
- 39.Mason P et al. (2007) Serotonergic raphe magnus cell discharge reflects ongoing autonomic and respiratory activities. J. Neurophysiol 98, 1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleary DR et al. (2008) Are opioid-sensitive neurons in the rostral ventromedial medulla inhibitory interneurons? Neuroscience 151, 564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason P (2012) Medullary circuits for nociceptive modulation. Curr. Opin. Neurobiol 22, 640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuo M and Gebhart GF (1997) Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J. Neurophysiol 78, 746–758 [DOI] [PubMed] [Google Scholar]
- 43.Potrebic SB et al. (1994) Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J. Neurosci 14, 1655–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellman KM et al. (2009) Opioid microinjection into raphe magnus modulates cardiorespiratory function in mice and rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 297, R1400–R1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foo H and Mason P (2003) Brainstem modulation of pain during sleep and waking. Sleep Med. Rev 7, 145–154 [DOI] [PubMed] [Google Scholar]
- 46.Foo H et al. (2010) The modulatory effects of rostral ventromedial medulla on air-puff evoked microarousals in rats. Behav. Brain Res 215, 156–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foo H and Mason P (2005) Sensory suppression during feeding. Proc. Natl. Acad. Sci. U. S. A 102, 16865–16869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan ZZ et al. (1990) Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J. Physiol. Lond 427, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan ZZ et al. (1997) Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature 389, 382–385 [DOI] [PubMed] [Google Scholar]
- 50.Fields HL et al. (1991) Neurotransmitters in nociceptive modulatory circuits. Annu. Rev. Neurosci 14, 219–245 [DOI] [PubMed] [Google Scholar]
- 51.Pan Z et al. (2000) A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron 26, 515–522 [DOI] [PubMed] [Google Scholar]
- 52.François A et al. (2017) A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiao Y et al. (2023) Molecular identification of bulbospinal ON neurons by GPER, which drives pain and morphine tolerance. J. Clin. Invest 133, e154588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y et al. (2015) Identifying local and descending inputs for primary sensory neurons. J. Clin. Invest 125, 3782–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otsu Y and Aubrey KR (2022) Kappa opioids inhibit the GABA/glycine terminals of rostral ventromedial medulla projections in the superficial dorsal horn of the spinal cord. J. Physiol. Lond 600, 4187–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Follansbee T et al. (2022) Inhibition of itch by neurokinin 1 receptor (Tacr1)-expressing ON cells in the rostral ventromedial medulla in mice. eLife 11, e69626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potrebic SB et al. (1995) The density and distribution of serotonergic appositions onto identified neurons in the rat rostral ventromedial medulla. J. Neurosci 15, 3273–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond DL and Yaksh TL (1984) Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 298, 329–337 [DOI] [PubMed] [Google Scholar]
- 59.Suzuki R et al. (2004) Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol. Sci 25, 613–617 [DOI] [PubMed] [Google Scholar]
- 60.Wei F et al. (2010) Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci 30, 8624–8636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai Y-Q et al. (2014) Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol. Pain 10, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moriya S et al. (2019) Acute nociceptive stimuli rapidly induce the activity of serotonin and noradrenalin neurons in the brain stem of awake mice. IBRO Rep. 7, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marinelli S et al. (2002) Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes. J. Neurosci 22, 10847–10855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H and Wessendorf MW (1999) Mu- and delta-opioid receptor mRNAs are expressed in spinally projecting serotonergic and nonserotonergic neurons of the rostral ventromedial medulla. J. Comp. Neurol 404, 183–196 [DOI] [PubMed] [Google Scholar]
- 65.Carstens E (1997) Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J. Neurophysiol 77, 2499–2514 [DOI] [PubMed] [Google Scholar]
- 66.Follansbee T et al. (2018) Effects of pruritogens and algogens on rostral ventromedial medullary (RVM) ON and OFF cells. J. Neurophysiol 120, 2156–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young AA and Dawson NJ (1987) Static and dynamic response characteristics, receptive fields, and interaction with noxious input of midline medullary thermoresponsive neurons in the rat. J. Neurophysiol 57, 1925–1936 [DOI] [PubMed] [Google Scholar]
- 68.Fields HL and Anderson SD (1978) Evidence that raphe-spinal neurons mediate opiate and midbrain stimulation-produced analgesias. Pain 5, 333–349 [DOI] [PubMed] [Google Scholar]
- 69.Mason P (2001) Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu. Rev. Neurosci 24, 737–777 [DOI] [PubMed] [Google Scholar]
- 70.Mason P et al. (2001) Nociceptive responsiveness during slow-wave sleep and waking in the rat. Sleep 24, 32–38 [DOI] [PubMed] [Google Scholar]
- 71.Leung CG and Mason P (1999) Physiological properties of raphe magnus neurons during sleep and waking. J. Neurophysiol 81, 584–595 [DOI] [PubMed] [Google Scholar]
- 72.Hellman KM and Mason P (2012) Opioids disrupt pro-nociceptive modulation mediated by raphe magnus. J. Neurosci 32, 13668–13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tovote P et al. (2015) Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16, 317–331 [DOI] [PubMed] [Google Scholar]
- 74.Umana IC et al. (2017) Nicotinic modulation of descending pain control circuitry. Pain 158, 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneeberger M et al. (2019) Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J et al. (2014) Morphological evidence for a neurotensinergic periaqueductal gray-rostral ventromedial medulla-spinal dorsal horn descending pathway in rat. Front. Neuroanat 8, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozawa T et al. (2017) A feedback neural circuit for calibrating aversive memory strength. Nat. Neurosci 20, 90–97 [DOI] [PubMed] [Google Scholar]
- 78.Li C et al. (2016) Mu opioid receptor modulation of dopamine neurons in the periaqueductal gray/dorsal raphe: a role in regulation of pain. Neuropsychopharmacology 41, 2122–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krout KE et al. (1998) Periaqueductal gray matter projection to the parabrachial nucleus in rat. J. Comp. Neurol 401, 437–454 [PubMed] [Google Scholar]
- 80.Omelchenko N and Sesack SR (2010) Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J. Neurosci. Res 88, 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ntamati NR et al. (2018) Periaqueductal efferents to dopamine and GABA neurons of the VTA. PLoS One 13, e0190297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie L et al. (2022) Divergent modulation of pain and anxiety by GABAergic neurons in the ventrolateral periaqueductal gray and dorsal raphe. Neuropsychopharmacology Published online December 16, 2022. 10.1038/s41386-022-01520-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Millan MJ (2002) Descending control of pain. Prog. Neurobiol 66, 355–474 [DOI] [PubMed] [Google Scholar]
- 84.Millan MJ (1999) The induction of pain: an integrative review. Prog. Neurobiol 57, 1–164 [DOI] [PubMed] [Google Scholar]
- 85.Kuner R and Kuner T (2021) Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev 101, 213–258 [DOI] [PubMed] [Google Scholar]
- 86.Kash TL et al. (2015) Neuropeptide regulation of signaling and behavior in the BNST. Mol. Cells 38, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lueptow LM et al. (2018) The contribution of the descending pain modulatory pathway in opioid tolerance. Front. Neurosci 12, 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gauriau C and Bernard J-F (2002) Pain pathways and parabrachial circuits in the rat. Exp. Physiol 87, 251–258 [DOI] [PubMed] [Google Scholar]
- 89.Chiang MC et al. (2020) Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106, 927–939.e5 [DOI] [PubMed] [Google Scholar]
- 90.Chen Q et al. (2017) Optogenetic evidence for a direct circuit linking nociceptive transmission through the parabrachial complex with pain-modulating neurons of the rostral ventromedial medulla (RVM). eNeuro 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J-N and Sheets PL (2018) The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J. Physiol. Lond 596, 6289–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Winters BL et al. (2022) Cannabinoids and opioids differentially target extrinsic and intrinsic GABAergic inputs onto the periaqueductal grey descending pathway. J. Neurosci 42, 7744–7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chou X-L et al. (2018) Inhibitory gain modulation of defense behaviors by zona incerta. Nat. Commun 9, 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh S et al. (2022) An inhibitory circuit from central amygdala to zona incerta drives pain-related behaviors in mice. eLife 11, e68760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu T-T et al. (2019) Activation of the intrinsic pain inhibitory circuit from the midcingulate Cg2 to zona incerta alleviates neuropathic pain. J. Neurosci 39, 9130–9144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C and Kash TL (2019) κ-opioid receptor modulation of GABAergic inputs onto ventrolateral periaqueductal gray dopamine neurons. Mol. Neuropsychiatry 5, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flores JA et al. (2004) Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain 110, 205–214 [DOI] [PubMed] [Google Scholar]
- 98.Ferrari LF et al. (2021) D2 receptors in the periaqueductal gray/dorsal raphe modulate peripheral inflammatory hyperalgesia via the rostral ventral medulla. Neuroscience 463, 159–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu W et al. (2021) Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron 109, 1365–1380.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baez MA et al. (2005) Roles for pain modulatory cells during micturition and continence. J. Neurosci 25, 384–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morrison SF (2016) Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci 196, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bouvier J et al. (2015) Descending command neurons in the brainstem that halt locomotion. Cell 163, 1191–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sandkühler J et al. (1987) Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus raphe magnus are separate in the cat. J. Neurophysiol 58, 327–341 [DOI] [PubMed] [Google Scholar]
- 104.Carstens E et al. (1981) Descending inhibition from medial and lateral midbrain of spinal dorsal horn neuronal responses to noxious and nonnoxious cutaneous stimuli in the cat. J. Neurophysiol 45, 1029–1042 [DOI] [PubMed] [Google Scholar]
- 105.Rydenhag B and Andersson S (1981) Effect of DLF lesions at different spinal levels on morphine induced analgesia. Brain Res. 212, 239–242 [DOI] [PubMed] [Google Scholar]
- 106.Camarata PJ and Yaksh TL (1985) Characterization of the spinal adrenergic receptors mediating the spinal effects produced by the microinjection of morphine into the periaqueductal gray. Brain Res. 336, 133–142 [DOI] [PubMed] [Google Scholar]
- 107.Liu M-Z et al. (2019) Synaptic control of spinal GRPR+ neurons by local and long-range inhibitory inputs. Proc. Natl. Acad. Sci. U. S. A 116, 27011–27017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun Y-G and Chen Z-F (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 [DOI] [PubMed] [Google Scholar]
- 109.Kiguchi N et al. (2021) Chemogenetic activation of central gastrin-releasing peptide-expressing neurons elicits itch-related scratching behavior in male and female mice. Pharmacol. Res. Perspect 9, e00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kohro Y et al. (2020) Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hyper-sensitivity. Nat. Neurosci 23, 1376–1387 [DOI] [PubMed] [Google Scholar]
- 111.Barik A et al. (2018) A brainstem-spinal circuit controlling nocifensive behavior. Neuron 100, 1491–1503.e3 [DOI] [PubMed] [Google Scholar]
- 112.Eliava M et al. (2016) A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89, 1291–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mills SEE et al. (2019) Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth 123, e273–e283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loyd DR et al. (2008) Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J. Neurosci 28, 14007–14017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X-Y et al. (2020) Different neuronal populations mediate inflammatory pain analgesia by exogenous and endogenous opioids. eLife 9, e55289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis VA and Gebhart GF (1977) Evaluation of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation-produced analgesia at coincident PAG loci. Brain Res. 124, 283–303 [DOI] [PubMed] [Google Scholar]
- 117.Tryon VL et al. (2016) Analysis of morphine-induced changes in the activity of periaqueductal gray neurons in the intact rat. Neuroscience 335, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Samineni VK et al. (2017) Neuropathic pain-induced enhancement of spontaneous and pain-evoked neuronal activity in the periaqueductal gray that is attenuated by gabapentin. Pain 158, 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park C et al. (2010) T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray. Proc. Natl. Acad. Sci. U. S. A 107, 14857–14862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin J-B et al. (2014) Neurochemical properties of BDNF-containing neurons projecting to rostral ventromedial medulla in the ventrolateral periaqueductal gray. Front. Neural Circuits 8, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McPherson KB et al. (2021) Physiologically distinct neurons within the ventrolateral periaqueductal gray are not defined by mu-opioid receptor expression but are differentially activated by persistent inflammation. bioRxiv Published online June 17, 2021. 10.1101/2021.06.16.448597 [DOI] [Google Scholar]
- 122.Chieng B and Christie MJ (1994) Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br. J. Pharmacol 113, 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vaughan CW et al. (2003) Cellular actions of opioids on periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br. J. Pharmacol 139, 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McPherson KB and Ingram SL (2022) Cellular and circuit diversity determines the impact of endogenous opioids in the descending pain modulatory pathway. Front. Syst. Neurosci 16, 963812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ho Y-C et al. (2013) Hypofunction of glutamatergic neurotransmission in the periaqueductal gray contributes to nerve-injury-induced neuropathic pain. J. Neurosci 33, 7825–7836 [DOI] [PMC free article] [PubMed] [Google Scholar]


