The 1.6 Å resolution crystal structure of the RNA-recognition motif of D. melanogaster tRNA (uracil-5-)-methyltransferase homolog A is reported.
Keywords: RRMs, TRMT2A, methyltransferases, X-ray crystallography, Drosophila melanogaster, neurodegenerative disease
Abstract
Human tRNA (uracil-5-)-methyltransferase 2 homolog A (TRMT2A) is the dedicated enzyme for the methylation of uridine 54 in transfer RNA (tRNA). Human TRMT2A has also been described as a modifier of polyglutamine (polyQ)-derived neuronal toxicity. The corresponding human polyQ pathologies include Huntington’s disease and constitute a family of devastating neurodegenerative diseases. A polyQ tract in the corresponding disease-linked protein causes neuronal death and symptoms such as impaired motor function, as well as cognitive impairment. In polyQ disease models, silencing of TRMT2A reduced polyQ-associated cell death and polyQ protein aggregation, suggesting this protein as a valid drug target against this class of disorders. In this paper, the 1.6 Å resolution crystal structure of the RNA-recognition motif (RRM) from Drosophila melanogaster, which is a homolog of human TRMT2A, is described and analysed.
1. Introduction
RNAs have been described to be post-transcriptionally modified with more than 170 chemical alterations installed by a multitude of enzymes or enzyme complexes. Amongst different RNA species, tRNA is the most heavily modified class, with tRNA modification and its abrogation being associated with different disease states (Orellana et al., 2022 ▸; Suzuki, 2021 ▸).
Several nucleotides in the T-loop of tRNA are modified during evolution, such as m1A58 or m5U54, suggesting an important role of these modifications. In humans, tRNA methyltransferase 2 homolog A (hsTRMT2A) is the dedicated enzyme for the methylation of uridine at position 54 of cytosolic tRNA, whereas tRNA methyltransferase 2 homolog B (TRMT2B) methylates mitochondrial tRNA (Carter et al., 2019 ▸; Powell & Minczuk, 2020 ▸). The Escherichia coli paralog TrmA has been associated with increased efficiency and fidelity of protein translation in vitro (Davanloo et al., 1979 ▸; Kersten et al., 1981 ▸). However, the biological function of TRMT2A and TRMT2B beyond the methylation of U54 in metazoa has largely been unexplored. A recent observation suggested hsTRMT2A to be involved in modulating translation fidelity (Witzenberger et al., 2023 ▸).
PolyQ diseases constitute a group of diseases that are autosomally dominantly inherited and linked to an expanded cytosine–adenine–guanine (CAG) tract in the respective disease-linked gene. In patients with Huntington’s disease (HD) the disease-linked Huntingtin gene harbours a pathologically long CAG tract that is transcribed and then translated into an uninterrupted polyglutamine (polyQ) tract. Accumulation of the aggregation-prone Huntingtin protein, CAG repeat-containing RNA and the associated toxic gain of function interfere with normal cellular function on various levels (Bates et al., 2015 ▸; Bennett et al., 2007 ▸; Berendzen et al., 2016 ▸). Various approaches to ameliorate disease symptoms or to slow disease progression have included attempts to reduce mutant Huntingtin mRNA or the mutant protein and therefore protein aggregation (Estevez-Fraga et al., 2022 ▸; Tabrizi et al., 2019 ▸). A promising clinical trial using antisense oligonucleotides to degrade mutant Huntingtin mRNA was recently halted (Tabrizi et al., 2019 ▸; Generation HD1, NCT03761849). Therefore, the need for other strategies to lower polyQ-induced aggregation and toxicity is pressing.
A high-throughput RNAi knockdown screen using a Drosophila melanogaster HD disease model identified dCG3808, the homolog of hsTRMT2A, as a novel modifier of polyQ-induced toxicity and aggregation (Vossfeldt et al., 2012 ▸). TRMT2A is predicted to consist of an N-terminal RNA-recognition motif (RRM) and a C-terminal catalytic domain folded into the Rossmann motif typical of methyltransferases (Carter et al., 2019 ▸). Previously, we solved the structure of hsTRMT2A RRM (Margreiter et al., 2022 ▸). This experimental structure, together with structural predictions of the catalytic domain, allowed us to develop in silico predicted inhibitors of hsTRMT2A and a tRNA–protein binding model (Witzenberger et al., 2023 ▸). In cell culture, some of these compounds showed reduced polyQ protein aggregation in a HEK cell polyQ disease model. To further confirm the validity of the results from a fly RNAi screen for a human disease model, we solved the X-ray structure of the RRM from Drosophila TRMT2a (dmTRMT2A). The structure shows the typical fold of an RRM, with high structural similarity to hsTRMT2A RRM, despite low sequence similarity (32%). These findings confirm the high structural and most likely functional similarity of the proteins, but also shed light on the highly versatile yet conserved structural motif class of RNA-binding domains.
2. Materials and methods
2.1. Macromolecule production
Standard molecular-biology procedures were applied for the cloning of SUMO-His-tagged dmTRMT2A RRM (57–137) (Table 1 ▸). The dmTRMT2A RRM DNA sequence was PCR-amplified from a template using Phusion polymerase (Thermo). The correctly sized PCR product was excised for purification with a NucleoSpin Gel and PCR Clean-Up kit (Qiagen). Gibson cloning of dmTRMT2A RRM with pOPINS3C vector was performed using an InFusion HD cloning kit (Takara). For this purpose, pOPINS3C was linearized with the restriction enzymes KpnI and NcoI. Religation was prevented by 5′-dephosphorylation with FastAP thermosensitive alkaline phosphatase (Thermo). Finally, 3–10 µl of InFusion reaction was transformed into Escherichia coli DH5α cells.
Table 1. Macromolecule-production information.
| Source organism | D. melanogaster |
| DNA source | #FI05218, Gold vector containing dGC3808 (dmTRMT2A) from Drosophila Genomics Center |
| Forward primer† | AAGTTCTGTTTCAGGGCCCG ACTTCGGAAATATTCAAA |
| Reverse primer† | ATGGTCTAGAAAGCTTTA ATCGGCCGAGGCCTTGGCA |
| Cloning vector | pOPINS3C |
| Expression vector | pOPINS3C |
| Expression host | E. coli Rosetta (DE3) strain |
| Complete amino-acid sequence of the construct produced‡ | GPTSEIFKVEVKNMGYFGIGEFKKLLRNTLKFDVTKIKAPTRKEFAFVCFRSQEDQQRALEILNGYKWKGKVLKAHVAKASAD |
The primer sequence complementary to the selected fragment of TRMT2A is shown in bold. The stop codon in the reverse primer is underlined. The 3C PreScission protease-cleavage site sequence is shown in italics.
The GP residues (underlined) at the N-terminus of the produced protein fragment are artefacts left after truncation with 3C PreScission protease.
The expression of native SUMO-tagged dmTRMT2A RRM (57–137) in E. coli Rosetta (DE3) cells was induced with 0.5 M isopropyl β-d-1-thiogalactopyranoside at an OD of 0.6. The protein was expressed in ampicillin-supplemented LB at 37°C for 3 h. The bacterial cells were harvested by centrifugation for 20 min at 4°C at 5000g. For cell lysis, the pellet was resuspended in lysis buffer (500 mM NaCl, 50 mM HEPES pH 8.5, 20 mM imidazole, 0.5% Tween, 2% glycerol) containing one Pierce Protease Inhibitor tablet (Roche). Typically, 25 ml lysis buffer was added per 3 l of culture. The resuspended bacterial cell pellet was sonified at 4°C with a Branson sonifier 250 (Emerson) 3–4 times for 6 min at an amplitude of 40%. Insoluble cellular debris was separated from soluble E. coli-expressed proteins by centrifugation for 30 min at 4°C at 20 000g. Before proceeding with further purification steps, the supernatant was filtered with a 2.7 µm filter (Whatman). The clarified and filtered supernatant was loaded onto a 5 ml HisTrap FF column (GE Healthcare) equilibrated with His-A buffer (500 mM NaCl, 50 mM HEPES pH 8.5, 20 mM imidazole). The protein was then washed with 10 column volumes (CV) of His-A buffer (500 mM NaCl, 50 mM HEPES pH 8.5, 20 mM imidazole) followed by washing with 10 CV of high-salt-containing His-B buffer (2000 mM NaCl, 50 mM HEPES pH 8.5, 20 mM imidazole). SUMO-tagged protein was eluted with a 10 CV gradient of His-A buffer and His-C elution buffer (500 mM NaCl, 50 mM HEPES pH 8.5, 500 mM imidazole). For SUMO-tag cleavage, the eluate was supplemented with 100 µg PreScission protease and dialyzed against dialysis buffer (500 mM NaCl, 50 mM HEPES pH 7.5, 1 mM DTT) in 6.0 S tubing (ZelluTrans, Carl Roth) overnight at 4°C. The next day, the cleaved protein was applied onto an equilibrated subtractive HisTrap FF column and the protein-containing flowthrough was collected. This flowthrough was concentrated to 2 ml with a centrifugal filter (Amicon Ultra, Merck, 3K cutoff) and loaded onto a Superdex 75 (10/300 GL) size-exclusion chromatography column equilibrated in SEC buffer (500 mM NaCl, 50 mM HEPES pH 7.5). The protein-containing fractions were combined and concentrated to 8 mg ml−1 (Supplementary Fig. S1). Aliquots were flash-frozen in liquid nitrogen and stored at −80°C.
2.2. Crystallization
Crystallization experiments for the dmTRMT2A RRM domain were performed at the X-ray Crystallography Platform at Helmholtz Zentrum München. dmTRMT2A RRM crystals grew in 2 M ammonium sulfate, 2 M NaCl (Ammonium Sulfate Screen, Hampton Research) at 292 K using a protein concentration of 4–8 mg ml−1 (Table 2 ▸). Heavy-atom-containing compounds were soaked into dmTRMT2A RRM crystals to obtain a data set for phase calculation and hence structure determination of the protein by single-wavelength anomalous dispersion (SAD). For soaking, 10 mM stock solution was added to the drop to obtain a final concentration of 1–3 mM. For screening, Ta6Br12, NaBr and the derivatives Pt2 [(NH4)2PtCl4] and Au3 (NaAuCl4) from the Heavy Atom Screen (Hampton Research) were used. The soaked crystals were cooled either directly or after 24 h equilibration. 30%(v/v) ethylene glycol was used as a cryoprotectant.
Table 2. Crystallization of the dmTRMT2A RRM domain.
| Method | Vapour diffusion, hanging drop |
| Plate type | VDX 24-well plate |
| Temperature (K) | 292 |
| Protein concentration (mg ml−1) | 4–8 |
| Buffer composition of protein solution | 500 mM NaCl, 50 mM HEPES pH 7.5 |
| Composition of reservoir solution | 2 M ammonium sulfate, 2 M NaCl |
| Volume and ratio of drop | 3 µl, 1:1 ratio |
| Volume of reservoir (ml) | 1 |
2.3. Data collection and processing
Diffraction data were collected from crystals cooled to 100 K on the X06DA beamline at Swiss Light Source (SLS), Villigen, Switzerland. Heavy-atom-containing crystals were measured to obtain the structure of dmTRMT2A RRM. A fluorescence scan was performed on the mounted crystal to identify the respective heavy-atom absorption edge and thus determine the X-ray energy needed for data collection. The diffraction data were indexed and integrated using XDS (Kabsch, 2010 ▸) and scaled using SCALA (Evans, 2006 ▸). Intensities were converted to structure-factor amplitudes using TRUNCATE (French & Wilson, 1978 ▸). Data-collection and processing statistics are presented in Table 3 ▸. Only the data set for the crystals soaked with the heavy-atom compound NaAuCl4 contained strong anomalous signal that was useful for further steps.
Table 3. Data-collection and processing statistics for dmTRMT2A RRM.
Values in parentheses are for the outer shell.
| Diffraction source | Beamline X06DA, SLS |
| Wavelength (Å) | 1.0366445 |
| Temperature (K) | 100 |
| Detector | PILATUS 2M-F |
| Crystal-to-detector distance (mm) | 185.057 |
| Rotation range per image (°) | 0.1 |
| Total rotation range (°) | 360 |
| Exposure time per image (s) | 0.1 |
| Space group | C2 |
| a, b, c (Å) | 92.41, 41.89, 48.82 |
| α, β, γ (°) | 90, 110.80, 90 |
| Mosaicity (°) | 0.3 |
| Resolution range (Å) | 50–1.6 (1.64–1.60) |
| Total No. of reflections | 150144 (9039) |
| No. of unique reflections | 23209 (1684) |
| Completeness (%) | 99.7 (99.0) |
| CC1/2 | 99.8 (91.1) |
| Multiplicity | 6.5 (5.6) |
| 〈I/σ(I)〉 | 11.6 (3.5) |
| R r.i.m. (%) | 10.1 (43.3) |
| Overall B factor from Wilson plot (Å2) | 11.9 |
2.4. Structure solution and refinement
The structure of dmTRMT2A RRM (57–137) was solved using the SAD protocol of Auto-Rickshaw, the EMBL Hamburg automated crystal structure-determination platform (Panjikar et al., 2005 ▸, 2009 ▸). The input diffraction data were prepared and converted for use in Auto-Rickshaw using programs from the CCP4 suite (Agirre et al., 2023 ▸). F A values were calculated with SHELXC (Sheldrick, 2010 ▸). On the basis of an initial analysis of the data, the maximum resolution for substructure determination and initial phase calculation was set to 2.3 Å. Eight heavy-atom positions were located with SHELXD (Sheldrick, 2010 ▸). The correct hand of the substructure was determined with ABS (Hao, 2004 ▸) and SHELXE (Sheldrick, 2010 ▸). The occupancies of all substructure atoms were refined with MLPHARE from the CCP4 suite (Agirre et al., 2023 ▸) and the phases were improved by density modification with DM (Cowtan & Zhang, 1999 ▸). The initial model was partially built with ARP/wARP (Morris et al., 2004 ▸; Perrakis et al., 2001 ▸). Further model building and refinement were performed with Coot (Emsley et al., 2010 ▸) and REFMAC5 (Murshudov et al., 2011 ▸), respectively, with the maximum-likelihood target function including anisotropic refinement. The final model was characterized by R work and R free factors of 14.1% and 19.9%, respectively (Table 4 ▸). Stereochemical analysis of the final model with PROCHECK (Laskowski et al., 1993 ▸) showed no residues with generously allowed or unfavourable backbone dihedral angles, whereas 93% of all residues are in the core region of the Ramachandran plot. The final model was deposited in the Protein Data Bank (https://www.rcsb.org) with PDB code 7pv5.
Table 4. Structure solution and refinement.
Values in parentheses are for the outer shell.
| Resolution range (Å) | 45.64–1.60 (1.64–1.60) |
| Completeness (%) | 99.9 |
| σ Cutoff | F > 0.000σ(F) |
| No. of reflections, working set | 22079 (1584) |
| No. of reflections, test set | 1130 (99) |
| Final R cryst | 0.141 (0.173) |
| Final R free | 0.199 (0.323) |
| Cruickshank DPI | 0.082 |
| No. of non-H atoms | |
| Protein | 1337 |
| Solvent | 147 |
| Ions | 68 |
| Total | 1552 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.016 |
| Angles (°) | 1.936 |
| Average B factors (Å2) | |
| Protein | 21.1 |
| Solvent | 40.0 |
| Ions | 38.9 |
| Ramachandran plot | |
| Most favoured (%) | 93 |
| Allowed (%) | 7 |
3. Results and discussion
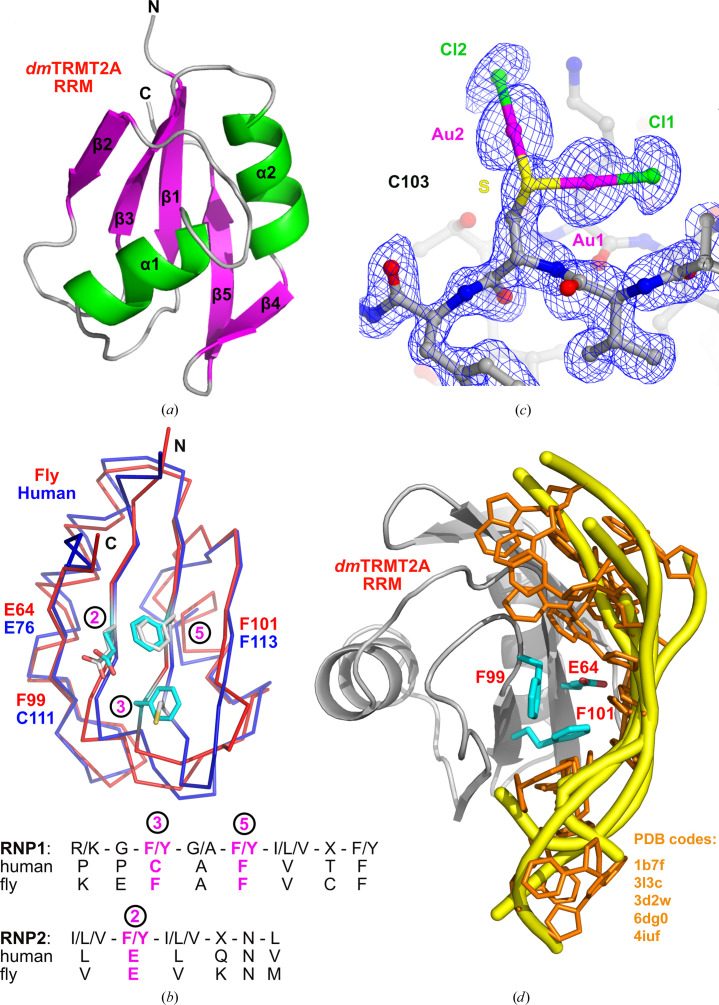
To obtain a three-dimensional experimental model of the RNA-recognition domain (RRM) of D. melanogaster tRNA (uracil-5-)-methyltransferase homolog A, the protein fragment comprising amino acids 57–137 was successfully cloned, expressed, purified to homogeneity and crystallized. To solve the phase problem, the crystals were soaked with different heavy-atom derivatives. However, only one of them, NaAuCl4, allowed us to obtain sufficient anomalous signal. The anomalous diffraction data were collected at the Au L III absorption edge at a wavelength of 1.0366445 Å. The structure was solved using the single-wavelength anomalous dispersion method as implemented in the Auto-Rickshaw pipeline (Panjikar et al., 2005 ▸, 2009 ▸). The dmTRMT2A 57–137 fragment poses the typical domain fold of an RRM, with a five-stranded antiparallel β-sheet and two α-helices (Fig. 1 ▸ a) with the topology β1–α1–β2–β3–α2–β4–β5. It highly resembles the recently published structure of the RRM from human TRMT2A (hsTRMT2A; Margreiter et al., 2022 ▸; Fig. 1 ▸ b). These protein fragments share 32% sequence identity. The crystals of dmTRMT2A RRM contain two molecules in the asymmetric unit, with an r.m.s.d. of 0.39 Å for 74 superimposed Cα atoms (Supplementary Fig. S2). The NaAuCl4 compound used to derivatize the crystals caused chemical modification of the surface Cys103. The S atom of Cys103 has been covalently modified by two AuCl moieties (Fig. 1 ▸ c).
Figure 1.
(a) The crystal structure of D. melanogaster TRMT2A RRM, fragment 57–137, at 1.6 Å resolution. The structure is shown as a ribbon coloured according to the labelled secondary-structure elements. (b) The superposition of dmTRMT2A (PDB entry 7pv5, shown as a red ribbon) and hsTRMT2A RRM (PDB entry 7nto, shown as a navy blue ribbon). The putative and conserved RNA-binding residues are depicted. The sequence alignment below shows the positions of the consensus RNA-binding platforms RNP1 and RNP2 in human and fly TRMT2A. Conserved RNA-binding residues at positions 3 and 5 of RNP1 and position 2 of RNP2 are indicated in magenta. (c) A 2F o − F c electron-density map contoured at 1σ is shown for the modified Cys103 residues. After soaking the crystals with NaAuCl4, the cysteine residue was chemically modified at its S atom with two AuCl moieties. (d) Superposition of the dmTRMT2A RRM domain (PDB entry 7pv5, shown as a grey cartoon) with its homologous RRM structures in complex with nucleic acids as identified by the PDBeFold search. The amino-acid residues in the dmTRMT2A RRM domain indicated in (b) are shown as blue sticks. For clarity, only nucleic acids are shown (as yellow cartoons and orange sticks) in the overlapped homologous structures. The following structures were used for the in silico analysis: human U1 small nuclear ribonucleoprotein A with glmS ribozyme derived from B. anthracis (PDB entry 3l3c; Cochrane et al., 2009 ▸), the Sex-lethal (Sxl) protein of D. melanogaster in complex with ssRNA (PDB entry 1b7f; Handa et al., 1999 ▸), the C-terminal RRM2 domain of mouse TDP-43 in complex with single-stranded DNA (PDB entry 3d2w; Kuo et al., 2009 ▸), human TDP-43 RRM1–DNA complex (PDB entry 4iuf; Kuo et al., 2014 ▸) and Caenorhabditis elegans MEC-8 C-terminal RRM domain bound to AGCACA (PDB entry 6dg0; H. Soufari & C. D. Mackereth, unpublished work).
Previously, conserved consensus regions in the two middle β-sheets have been identified as a canonical RNA-binding platform where RNA nucleotides interact with solvent-exposed aromatic residues. They were termed ribonucleoprotein 1 (RNP1) and ribonucleoprotein 2 (RNP2) (Cléry & Allain, 2011 ▸; Fig. 1 ▸ b). For dmTRMT2A RRM, RNP1 consists of Lys97-Glu98- Phe99 -Ala100- Phe101 -Val102-Cys103-Phe104 and RNP2 consists of Val63-Glu64-Val65-Lys66-Asn67-Met68 (where the conserved dmTRMT2A residues are shown in bold). Positions 3 and 5 of RNP1, as well as position 2 of RNP2 (all three of which are underlined), are the respective RNA-binding residues (Cléry & Allain, 2011 ▸; Fig. 1 ▸ b). The structure of dmTRMT2A RRM shows the expected conserved RNA-binding residues Phe99 and Phe101 in positions 3 and 5 of RNP1, whereas the nonconserved residue Glu64 is found in position 2 of RNP2. A comparison with human TRMT2A showed that Glu76 of its RRM is in the same position 2 in RNP2, as well as Cys111 and Phe113 in positions 3 and 5 in RNP1, respectively (Fig. 1 ▸ b). To further investigate the importance of Glu64, Phe99 and Phe101 for the interaction with nucleic acids, we performed an in silico analysis. Using the PDBeFold protein structure comparison service at the European Bioinformatics Institute (https://www.ebi.ac.uk/msd-srv/ssm; Krissinel & Henrick, 2004 ▸), we identified structurally similar proteins in complex with nucleic acids and superimposed them onto the structural model of dmTRMT2A RRM (Fig. 1 ▸ d). In the analysed co-complexes, the nucleic acids interact with the solvent-exposed parts of the β-sheet where RNP1 and RNP2 are located. Phe101 emerges as being particularly crucial, as it is fully conserved across the analysed homologous structures (Supplementary Table S1). Its involvement in nucleic acid interaction is marked by hydrophobic stacking with the bases (Fig. 1 ▸ d). In contrast, Glu64 and Phe99, which lack conservation, make a minor contribution (Glu64) or no contribution (Phe99) to the binding event. This emphasizes the specificity of Phe101 (Phe113 in the human protein) in mediating the interactions between the protein and nucleic acids and its central role in this molecular-recognition mechanism. While the binding mode of nucleic acids to the analysed RRM domains remains largely consistent, it is important to acknowledge the possibility of variations in the interaction in the case of the Drosophila or human homologs. To better understand such differences and their impact on RNA–protein binding, additional experiments will be required.
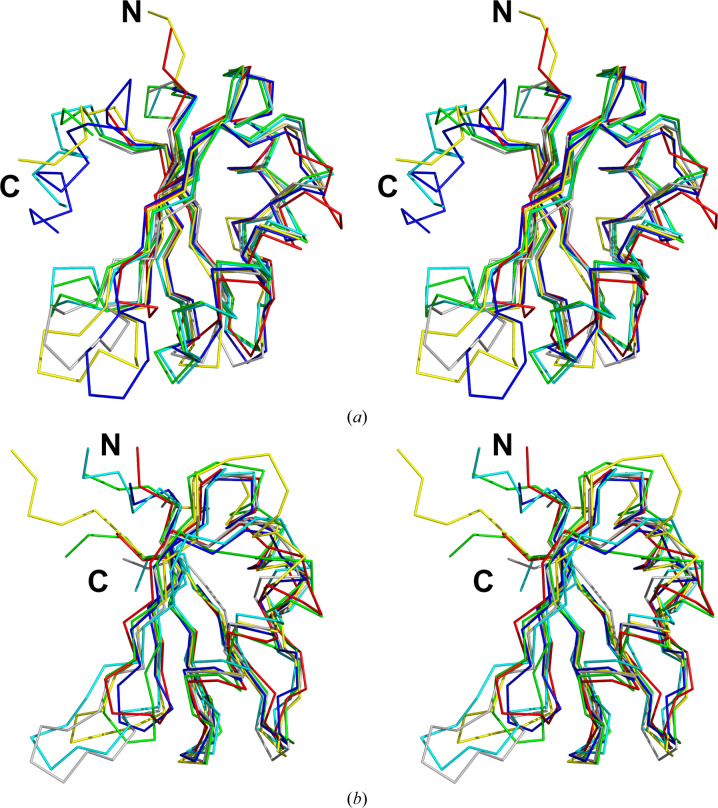
A comparison of the RRM from dmTRMT2A with other known structures using PDBeFold (Krissinel & Henrick, 2004 ▸) yielded high conservation of the fold among different species. Despite very low sequence identity, the Drosophila structure shows very high fold similarity, as measured by the r.m.s.d. to the compared models (Fig. 2 ▸, Tables 5 ▸ and 6 ▸). So far, the most similar structure with regard to sequence identity is the abovementioned hsTRMT2A RRM (Margreiter et al., 2022 ▸; Fig. 1 ▸ b; Table 6 ▸). On the other hand, it is remarkable how different protein sequences can lead to nearly identical folds. The best example is the RRM of the U1 small nuclear ribonucleoprotein A from D. melanogaster (PDB entry 6f4j; Weber et al., 2018 ▸), which shares an r.m.s.d. of 1.14 Å with dmTRMT2A RRM with an insignificant sequence identity of 18% (Fig. 2 ▸ a, Table 5 ▸).
Figure 2.
(a) A stereoview of the crystal structure of dmTRMT2A RRM superimposed with the top five results from the PDBeFold search. The selected structures show the lowest r.m.s.d. compared with dmTRMT2A RRM. The structures are shown as ribbons and coloured as follows: dmTRMT2A, PDB entry 7pv5, red; PDB entry 6f4j, cyan (Weber et al., 2018 ▸); PDB entry 1oia, green (Nagai et al., 1990 ▸); PDB entry 4a8x, yellow (Murachelli et al., 2012 ▸); PDB entry 2x1f, navy blue (Pancevac et al., 2010 ▸); PDB entry 1b7f, grey (Handa et al., 1999 ▸). (b) A stereoview of the superposition of the structures identified by PDBeFold to have the highest sequence identity to dmTRMT2A RRM. The structures are shown as ribbons and coloured as follows: dmTRMT2A RRM, PDB entry 7pv5, red; PDB entry 7nto, green (Margreiter et al., 2022 ▸); PDB entry 5iqq, cyan (Sofos et al., 2016 ▸); PDB entry 1b7f, grey (Handa et al., 1999 ▸); PDB entry 6e4n, navy blue (Travis et al., 2019 ▸); PDB entry 2cpx, yellow (RIKEN Structural Genomics/Proteomics Initiative, unpublished work).
Table 5. The results of the structural similarity search performed with PDBeFold .
The structures are ordered according to their r.m.s.d. to dmTRMT2A RRM. The values in the table include the r.m.s.d. in Å, the number of aligned Cα atoms (N align) and the sequence identity in % (%seq).
| R.m.s.d. | N align | %seq | PDB code/chain | Organism | Protein | Reference |
|---|---|---|---|---|---|---|
| 1.14 | 65 | 18 | 6f4j/D | D. melanogaster | U1 small nuclear ribonucleoprotein A | Weber et al. (2018 ▸) |
| 1.23 | 65 | 18 | 1oia/B | H. sapiens | U1 small nuclear ribonucleoprotein A | Nagai et al. (1990 ▸) |
| 1.24 | 69 | 17 | 4a8x/A | H. sapiens | RNA-binding protein with serine-rich domain 1 | Murachelli et al. (2012 ▸) |
| 1.24 | 69 | 26 | 2x1f/A | S. cerevisiae | mRNA 3′-end-processing protein RNA15 | Pancevac et al. (2010 ▸) |
| 1.27 | 68 | 29 | 1b7f/A | D. melanogaster | Sex-lethal (Sxl) protein | Handa et al. (1999 ▸) |
Table 6. The results of the structural similarity search performed with PDBeFold .
The structures are ordered according to their sequence identity to the dmTRMT2A RRM fragment. The values in the table include the r.m.s.d. in Å, the number of aligned Cα atoms (N align) and the sequence identity in % (%seq).
| R.m.s.d. | N align | %seq | PDB code/chain | Organism | Protein | Reference |
|---|---|---|---|---|---|---|
| 1.65 | 74 | 32 | 7nto/A | H. sapiens | RNA-recognition motif of TRMT2A | Margreiter et al. (2022 ▸) |
| 1.65 | 71 | 30 | 5iqq/E | H. sapiens | RNA-recognition motif of RNA-binding protein 7 | Sofos et al. (2016 ▸) |
| 1.27 | 68 | 29 | 1b7f/A | D. melanogaster | Sex-lethal (Sxl) protein | Handa et al. (1999 ▸) |
| 1.46 | 67 | 28 | 6e4n/A | T. brucei | RNA-recognition motif of the putative tbrgg2 protein | Travis et al. (2019 ▸) |
| 1.61 | 68 | 28 | 2cpx/A | H. sapiens | RNA-binding domain of hypothetical protein FLJ11016 | RIKEN Structural Genomics/Proteomics Initiative (unpublished work) |
Supplementary Material
PDB reference: RNA-recognition motif of TRMT2A, 7pv5
Supplementary Figures and Table. DOI: 10.1107/S2053230X24000645/ek5035sup1.pdf
Acknowledgments
We thank Vera Roman for technical support. The Drosophila Genomics Center kindly provided the vector for dmTRMT2A cloning. The diffraction data were recorded at the SLS X06DA beamline in Villigen. Open access funding enabled and organized by Projekt DEAL.
Funding Statement
DN received funding from the BMBF (PolyQure, 16GW0307).
References
- Agirre, J., Atanasova, M., Bagdonas, H., Ballard, C. B., Baslé, A., Beilsten-Edmands, J., Borges, R. J., Brown, D. G., Burgos-Mármol, J. J., Berrisford, J. M., Bond, P. S., Caballero, I., Catapano, L., Chojnowski, G., Cook, A. G., Cowtan, K. D., Croll, T. I., Debreczeni, J. É., Devenish, N. E., Dodson, E. J., Drevon, T. R., Emsley, P., Evans, G., Evans, P. R., Fando, M., Foadi, J., Fuentes-Montero, L., Garman, E. F., Gerstel, M., Gildea, R. J., Hatti, K., Hekkelman, M. L., Heuser, P., Hoh, S. W., Hough, M. A., Jenkins, H. T., Jiménez, E., Joosten, R. P., Keegan, R. M., Keep, N., Krissinel, E. B., Kolenko, P., Kovalevskiy, O., Lamzin, V. S., Lawson, D. M., Lebedev, A. A., Leslie, A. G. W., Lohkamp, B., Long, F., Malý, M., McCoy, A. J., McNicholas, S. J., Medina, A., Millán, C., Murray, J. W., Murshudov, G. N., Nicholls, R. A., Noble, M. E. M., Oeffner, R., Pannu, N. S., Parkhurst, J. M., Pearce, N., Pereira, J., Perrakis, A., Powell, H. R., Read, R. J., Rigden, D. J., Rochira, W., Sammito, M., Sánchez Rodríguez, F., Sheldrick, G. M., Shelley, K. L., Simkovic, F., Simpkin, A. J., Skubak, P., Sobolev, E., Steiner, R. A., Stevenson, K., Tews, I., Thomas, J. M. H., Thorn, A., Valls, J. T., Uski, V., Usón, I., Vagin, A., Velankar, S., Vollmar, M., Walden, H., Waterman, D., Wilson, K. S., Winn, M. D., Winter, G., Wojdyr, M. & Yamashita, K. (2023). Acta Cryst. D79, 449–461.
- Bates, G. P., Dorsey, R., Gusella, J. F., Hayden, M. R., Kay, C., Leavitt, B. R., Nance, M., Ross, C. A., Scahill, R. I., Wetzel, R., Wild, E. J. & Tabrizi, S. J. (2015). Nat. Rev. Dis. Primers, 1, 15005. [DOI] [PubMed]
- Bennett, E. J., Shaler, T. A., Woodman, B., Ryu, K.-Y., Zaitseva, T. J., Becker, C. H., Bates, G. P., Schulman, H. & Kopito, R. R. (2007). Nature, 448, 704–708. [DOI] [PubMed]
- Berendzen, K. M., Durieux, J., Shao, L.-W., Tian, Y., Kim, H., Wolff, S., Liu, Y. & Dillin, A. (2016). Cell, 166, 1553–1563. [DOI] [PMC free article] [PubMed]
- Carter, J.-M., Emmett, W., Mozos, I. R., Kotter, A., Helm, M., Ule, J. & Hussain, S. (2019). Nucleic Acids Res. 47, e113. [DOI] [PMC free article] [PubMed]
- Cléry, A. & Allain, F. H.-T. (2011). RNA Binding Proteins, edited by Z. J. Lorkovic, pp. 137–158. Austin: Landes Bioscience.
- Cochrane, J. C., Lipchock, S. V., Smith, K. D. & Strobel, S. A. (2009). Biochemistry, 48, 3239–3246. [DOI] [PMC free article] [PubMed]
- Cowtan, K. D. & Zhang, K. Y. J. (1999). Prog. Biophys. Mol. Biol. 72, 245–270. [DOI] [PubMed]
- Davanloo, P., Sprinzl, M., Watanabe, K., Albani, M. & Kersten, H. (1979). Nucleic Acids Res. 6, 1571–1581. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Estevez-Fraga, C., Rodrigues, F. B., Tabrizi, S. J. & Wild, E. J. (2022). J. Huntingtons Dis. 11, 105–118. [DOI] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- French, S. & Wilson, K. (1978). Acta Cryst. A34, 517–525.
- Handa, N., Nureki, O., Kurimoto, K., Kim, I., Sakamoto, H., Shimura, Y., Muto, Y. & Yokoyama, S. (1999). Nature, 398, 579–585. [DOI] [PubMed]
- Hao, Q. (2004). J. Appl. Cryst. 37, 498–499.
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kersten, H., Albani, M., Männlein, E., Praisler, R., Wurmbach, P. & Nierhaus, K. H. (1981). Eur. J. Biochem. 114, 451–456. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2004). Acta Cryst. D60, 2256–2268. [DOI] [PubMed]
- Kuo, P. H., Chiang, C. H., Wang, Y. T., Doudeva, L. G. & Yuan, H. S. (2014). Nucleic Acids Res. 42, 4712–4722. [DOI] [PMC free article] [PubMed]
- Kuo, P. H., Doudeva, L. G., Wang, Y. T., Shen, C. K. & Yuan, H. S. (2009). Nucleic Acids Res. 37, 1799–1808. [DOI] [PMC free article] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Margreiter, M. A., Witzenberger, M., Wasser, Y., Davydova, E., Janowski, R., Metz, J., Habib, P., Sahnoun, S. E. M., Sobisch, C., Poma, B., Palomino-Hernandez, O., Wagner, M., Carell, T., Jon Shah, N., Schulz, J. B., Niessing, D., Voigt, A. & Rossetti, G. (2022). Comput. Struct. Biotechnol. J. 20, 443–458. [DOI] [PMC free article] [PubMed]
- Morris, R. J., Zwart, P. H., Cohen, S., Fernandez, F. J., Kakaris, M., Kirillova, O., Vonrhein, C., Perrakis, A. & Lamzin, V. S. (2004). J. Synchrotron Rad. 11, 56–59. [DOI] [PubMed]
- Murachelli, A. G., Ebert, J., Basquin, C., Le Hir, H. & Conti, E. (2012). Nat. Struct. Mol. Biol. 19, 378–386. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Nagai, K., Oubridge, C., Jessen, T. H., Li, J. & Evans, P. R. (1990). Nature, 348, 515–520. [DOI] [PubMed]
- Orellana, E. A., Siegal, E. & Gregory, R. I. (2022). Nat. Rev. Genet. 23, 651–664. [DOI] [PMC free article] [PubMed]
- Pancevac, C., Goldstone, D. C., Ramos, A. & Taylor, I. A. (2010). Nucleic Acids Res. 38, 3119–3132. [DOI] [PMC free article] [PubMed]
- Panjikar, S., Parthasarathy, V., Lamzin, V. S., Weiss, M. S. & Tucker, P. A. (2005). Acta Cryst. D61, 449–457. [DOI] [PubMed]
- Panjikar, S., Parthasarathy, V., Lamzin, V. S., Weiss, M. S. & Tucker, P. A. (2009). Acta Cryst. D65, 1089–1097. [DOI] [PMC free article] [PubMed]
- Perrakis, A., Harkiolaki, M., Wilson, K. S. & Lamzin, V. S. (2001). Acta Cryst. D57, 1445–1450. [DOI] [PubMed]
- Powell, C. A. & Minczuk, M. (2020). RNA Biol. 17, 451–462. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2010). Acta Cryst. D66, 479–485. [DOI] [PMC free article] [PubMed]
- Sofos, N., Winkler, M. B. L. & Brodersen, D. E. (2016). Acta Cryst. F72, 397–402. [DOI] [PMC free article] [PubMed]
- Suzuki, T. (2021). Nat. Rev. Mol. Cell Biol. 22, 375–392. [DOI] [PubMed]
- Tabrizi, S. J., Ghosh, R. & Leavitt, B. R. (2019). Neuron, 101, 801–819. [DOI] [PubMed]
- Tabrizi, S. J., Leavitt, B. R., Landwehrmeyer, G. B., Wild, E. J., Saft, C., Barker, R. A., Blair, N. F., Craufurd, D., Priller, J., Rickards, H., Rosser, A., Kordasiewicz, H. B., Czech, C., Swayze, E. E., Norris, D. A., Baumann, T., Gerlach, I., Schobel, S. A., Paz, E., Smith, A. V., Bennett, C. F., Lane, R. M. & Phase 1–2a IONIS-HTTRx Study Site Teams (2019). N. Engl. J. Med. 380, 2307–2316.
- Travis, B., Shaw, P. L. R., Liu, B., Ravindra, K., Iliff, H., Al-Hashimi, H. M. & Schumacher, M. A. (2019). Nucleic Acids Res. 47, 2130–2142. [DOI] [PMC free article] [PubMed]
- Vossfeldt, H., Butzlaff, M., Prüssing, K., Ní Chárthaigh, R. A., Karsten, P., Lankes, A., Hamm, S., Simons, M., Adryan, B., Schulz, J. B. & Voigt, A. (2012). PLoS One, 7, e47452. [DOI] [PMC free article] [PubMed]
- Weber, G., DeKoster, G. T., Holton, N., Hall, K. B. & Wahl, M. C. (2018). Nat. Commun. 9, 2220. [DOI] [PMC free article] [PubMed]
- Witzenberger, M., Burczyk, S., Settele, D., Mayer, W., Welp, L. M., Heiss, M., Wagner, M., Monecke, T., Janowski, R., Carell, T., Urlaub, H., Hauck, S. M., Voigt, A. & Niessing, D. (2023). Nucleic Acids Res. 51, 8691–8710. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: RNA-recognition motif of TRMT2A, 7pv5
Supplementary Figures and Table. DOI: 10.1107/S2053230X24000645/ek5035sup1.pdf