ABSTRACT
Aim:
The aim of this study was to examine the association between endogenous hormones and bone mineral density (BMD) in postmenopausal women.
Materials and Methods:
This was a cross-sectional study of 798 postmenopausal women aged 47–85 years. Data were collected on age, age at menopause, years since menopause, smoking status, body mass index, adiposity, BMD, physical activity, and Vitamin D supplementation. Measured hormonal parameters were: follicle-stimulating hormone (FSH), estradiol, testosterone, dehydroepiandrosterone sulfate, ∆4-androstenedione, cortisol, insulin-like growth factor-1, 25-hydroxyvitamin D, and parathormone (PTH) levels. BMD was measured at the lumbar spine, femoral neck, and total hip using dual-energy X-ray absorptiometry. A directed acyclic graph was used to select potential confounding variables.
Results:
Multivariable analysis showed significant associations between cortisol and femoral neck BMD (β: −0.02, 95% confidence interval [CI]: −0.03–−0.00), and PTH with femoral neck BMD (β: −0.01, 95% CI: −0.02–−0.01) and total hip BMD (β: −0.01, 95% CI: −0.01–−0.00). Hormonal factors more likely associated with a higher risk of low BMD (osteopenia or osteoporosis) were FSH (odds ratio [OR]: 1.02, 95% CI: 1.01–1.03) and PTH (OR: 1.02, 95% CI: 1.01–1.04).
Conclusions:
Higher cortisol and PTH levels were inversely associated with BMD. Postmenopausal women with higher FSH or PTH levels were likely to have low BMD.
KEYWORDS: Adrenal hormones, bone mineral density, directed acyclic graph, osteoporosis, ovarian steroids, Vitamin D
INTRODUCTION
Aging is associated with a progressive decline of bone mineral density (BMD), contributing to the development of osteoporosis. The bone loss mass is accompanied by the microarchitectural deterioration of bone tissue, which produces an increase in bone fragility and predisposes to fracture. In addition to aging, other factors such as female gender, low body mass index (BMI), cigarette smoking, alcohol consumption, early menopause, and inadequate physical activity are associated with an increased risk of osteoporosis.[1]
With increasing life expectancy, it is a fact that the incidence of postmenopausal osteoporosis is increasing, causing an important public health and economic burden.[2] For the maintenance of bone properties, bone turnover is necessary, and when there is an imbalance between bone resorption and bone formation, osteoporosis develops. This process is influenced by sex steroids, Vitamin D, Parathormone (PTH), and plasma factors involved in cell growth.[3]
The decrease in plasma sex steroid levels during the menopause transition leads to a series of changes that affect body composition and BMD. In this period, BMD progressively decreases, with an accelerated rate of decline in density over the early postmenopausal years.[4] Whereas plasma estrogen deficiency plays an essential role in postmenopausal osteoporosis,[5] the action of other hormones on the bone mass is still not entirely clear. Some study suggests that high plasma follicle-stimulating hormone (FSH) levels cause hypogonadal bone loss,[6] instead others did not find this association.[7,8] The effect of testosterone on BMD in postmenopausal women is not well clarified.[9,10] In the same way, several studies have reported the relationship between dehydroepiandrosterone sulfate (DHEAS) and osteoporosis but with conflicting results.[11,12] Similarly, the reports regarding insulin-like growth factor-1 (IGF-1) and BMD are controversial.[13,14] The purpose of this study was to analyze the association between endogenous hormones and BMD applying a directed acyclic graph (DAG) to select potential confounding variables.[15]
MATERIALS AND METHODS
Study design
This cross-sectional study was approved by the Institutional Review Board and the University Hospital Ethics Committee (2022/13-GIN-DEX). It was performed from January 2021 to September 2022 at the Department of Obstetrics and Gynecology of the University Hospital. This analysis examines the impact of endogenous hormones on BMD as measured by dual-energy X-ray absorptiometry (DXA). Inclusion criteria: being naturally postmenopausal (amenorrhea of 1 year or more before the initiation of the study) and not physical disability. Women were excluded if (i) were using treatments that are known to influence bone mineralization (menopausal hormone therapy, corticosteroids, anticonvulsants, heparin, thiazide diuretics, and antiresorptive agents) or (ii) had cardiovascular, liver, or renal diseases and history of cancer. Finally, the results from 798 postmenopausal women aged 47–85 years were analyzed.
Bone mineral density measurements and laboratory parameters
BMD was measured at the lumbar spine (L1-L4), femoral neck, and total hip, by DXA using the Lunar iDXA system (GE healthCare. Chicago, IL, USA). Daily quality control of the scanner was performed by calibration with a spine phantom’s density, and the coefficients of variation of DXA measurements ranged from 0.22% to 0.39%. The evaluation of BMD was performed using the raw data generated by the DXA. The values were expressed as grams of mineral content per square centimeters of bone area (g/cm2) and T-scores. Women were categorized according to the WHO classification system on the lowest T-score as follows: normal BMD with a T-score >−1 standard deviation (SD), osteopenia with T-scores ranging from −1 SD to −2.5 SD, and osteoporosis with a T-score ≤−2.5 SD.[16] In our study, women with osteopenia or osteoporosis were categorized as low BMD.
Blood samples were collected after an overnight fast. Hormonal parameters (FSH, estradiol, testosterone, DHEAS, ∆4-androstenedione, cortisol, IGF-1, 25-hydroxyvitamin D, and parathormone plasma levels) were determined by electrochemiluminescence immunoassay by the use of Roche Elecsys reagents and were measured by an automated Cobas® 8000 Modular Analyzer System (Roche Diagnostics. Pleasanton, CA, USA). Laboratory performs daily quality control of each parameter, and the coefficients of variation ranged from 1.3% to 1.8% for FSH, 1.2% to 1.9% for estradiol, 1.3% to 1.9% for testosterone, 1.5% to 2.3% for DHEAS, 1.7% to 2.1% for ∆4-androstenedione, 1.5% to 1.7% for cortisol, 1.1% to 1.7% for IGF-1, 2.3% to 3.1% for 25-hydroxyvitamin D, and 1.4% to 1.7% for PTH. No women had hormonal plasma levels below the level of detection.
Confounding variables and directed acyclic graph
Data were collected on age, age at menopause, years since menopause, BMI, adiposity, current smoking status, physical activity, and Vitamin D supplementation. BMI was calculated as the weight in kilograms divided by the square of the height in meters. Adiposity was assessed by bioelectrical impedance analysis equipment Omron BF306 monitor (Omron Healthcare Co. Ltd, Kyoto, Japan), and the results were expressed as a percentage of fat. Information on smoking status was recorded and classified as never or smoker. Physical activity was assessed using the Spanish version of the International Physical Activity Questionnaire-Short Form (IPAQ-SF). This instrument includes seven items that provide information about the average number of days per week and the average time per day that the individual spent in moderate and vigorous activities, walking, and sitting in the past 7 days. With the final score obtained, the physical activity was classified according to the official IPAQ as low, moderate, and high levels.[17]
A DAG representation [Figure 1] was used to distinguish the appropriate set of confounders for estimating the effect of endogenous hormones as the main exposure and BMD as the outcome.[15] The DAG represents each variable as a node, and the variables with a relationship are connected by an arrow. The arrowhead represents a direct effect of one variable over the other, but not the other way around. The absence of an arrow between two variables represents the assumption of no causal direct effect between those variables.[18] The following variables, age, adiposity, BMI, smoking status, physical activity, and years since menopause, were considered in the regression model to assess the associations between endogenous hormones and BMD.
Figure 1.
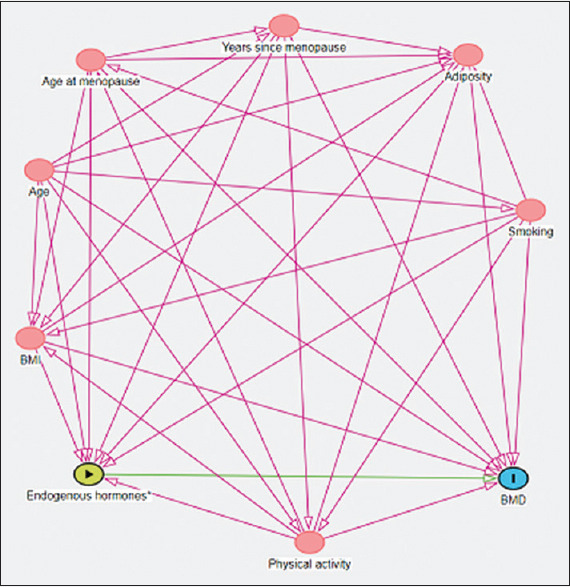
Directed acyclic graph for estimating the effect of endogenous hormones on bone mineral density. The arrowhead represents a direct effect of one variable over the other. BMD: Bone mineral density, BMI: Body mass index
Statistical analysis
Continuous variables were calculated as mean and SD, whereas percentages and numbers were used for categorical variables The Student’s t-test and Chi-square test were applied to compare continuous and categorical outcomes between women with normal BMD and women with low BMD. The Pearson correlation was used to estimate the relation between numeric parameters with the BMD. Multivariable linear regression models, adjusting for age, adiposity, BMI, smoking status, physical activity, and years since menopause according to DAG were applied to determine the associations between endogenous hormones and BMD. Finally, a multivariable logistic regression model was performed to analyze the association between endogenous hormones and the risk of low BMD adjusting for confounding factors. Results are reported as β-coefficients, odds ratios (OR), and 95% confidence interval (CI). All analyses were performed using the R software (R Core Team, 2019). The R package “dagitty” was used to design the DAG.[19] All the analyses were exploratory. No formal a priori sample size calculation was performed.
RESULTS
This study included 798 postmenopausal women with a mean age of 62.8 ± 6.4 years, mean age at menopause of 50.2 ± 2.8 years, and mean years since menopause of 12.6 ± 6.8. The mean whole BMI sample was 24.7 ± 3.7 kg/m2, and the mean adiposity was 40.5% ±4.7%. The mean values of the laboratory parameters, expressed as means ± SDs, are reported in Table 1. In accordance with the lowest T-score in any of the skeletal sites where BMD was calculated, 270 women had osteoporosis (33.8%), 408 had osteopenia (51.1%), and 120 had normal BMD values (15.1%). Regular physical activity and current smoking habits were respectively reported in 61.9% and 19.2% of the sample.
Table 1.
Hormonal parameters reported as mean±standard deviation
| Parameters | Estimate (n=798) | 95% CI | Reference ranges |
|---|---|---|---|
| FSH | 78.1±25.4 | 76.3–79.8 | 40–116 mU/mL |
| Estradiol | 11.6±6.7 | 11.1–12.0 | 5–37 pg/mL |
| Testosterone | 0.32±0.2 | 0.31–0.34 | 0.02–0.40 ng/mL |
| DHEAS | 82.9±53.1 | 79.2–86.6 | 35–430 µg/dL |
| ∆4 androstenedione | 0.86±0.6 | 0.82–0.91 | 0.49–1.31 ng/mL |
| Cortisol | 14.1±4.9 | 13.8–14.4 | 4.3–22.4 µg/dL |
| IGF-1 | 127±41.3 | 124–129 | 44–241 ng/mL |
| Vitamin D | 30.7±8.3 | 30.2–31.3 | 30–100 ng/mL |
| Parathormone | 43.1±14.7 | 42.1–44.1 | 15–65 pg/mL |
DHEAS: Dehydroepiandrosterone sulfate, FSH: Follicle-stimulating hormone, IGF-1: Insulin-like growth factor, CI: Confidence interval
A comparison of the women with normal BMD and low BMD displayed that the low-BMD group was significantly older (OR: 1.10, 95% CI: 1.07–1.14). In women with low BMD, the mean years since menopause was significantly higher (OR: 1.10, 95% CI: 1.06–1.14), the mean BMI was significantly lower (OR: 0.89, 95% CI: 0.85–0.94), and had higher adiposity (OR: 1.14, 95% CI: 1.09–1.19) compared to women with normal BMD. There were no significant associations between BMD with either age at menopause, smoking status, degree of physical activity, and Vitamin D supplementation. Regarding the hormonal parameters, in the low-BMD group was significantly higher the mean plasma levels of FSH (OR: 1.01, 95% CI: 1.00–1.02) and PTH (OR: 1.02, 95% CI: 1.00–1.03) compared with the normal BMD group. Women with low BMD had significantly lower mean plasma levels of DHEAS (OR: 1.00, 95% CI: 0.99–1.00) and ∆ 4-androstenedione (OR: 0.69, 95% CI: 0.54–0.90) with respect to normal BMD women. There was no statistically significant difference among the groups with regard to the other hormonal parameters. The results are summarized in Table 2.
Table 2.
General characteristics of studied parameters reported as mean±standard deviation or n (%) in women with normal bone mineral density and low bone mineral density (osteopenia/osteoporosis)
| Parameters | Normal BMD (n=120) | Low BMD (n=678) | OR (95% CI) | P |
|---|---|---|---|---|
| Age | 59.7±6.2 | 63.4±6.2 | 1.10 (1.07–1.14) | <0.001 |
| Age at menopause | 50.2±2.6 | 50.2±2.7 | 1.01 (0.94–1.08) | 0.835 |
| Years since menopause | 9.4±6.8 | 13.1±6.6 | 1.10 (1.06–1.14) | <0.001 |
| Smoking | ||||
| Smoker | 28 (23.3) | 126 (18.6) | Reference | |
| Never | 92 (76.7) | 552 (81.4) | 1.34 (0.38–2.11) | 0.276 |
| BMI (kg/m2) | 26.2±3.6 | 24.5±3.7 | 0.89 (0.85–0.94) | <0.001 |
| Adiposity (% fat) | 37.9±3.9 | 40.9±4.7 | 1.14 (1.09–1.19) | <0.001 |
| FSH (mU/mL) | 72.4±22.9 | 79.1±25.7 | 1.01 (1.00–1.02) | 0.004 |
| Estradiol (pg/mL) | 11.6±6.9 | 11.6±6.6 | 1.00 (0.97–1.03) | 0.980 |
| Testosterone (ng mL) | 0.35±0.2 | 0.32±0.2 | 0.57 (0.25–1.28) | 0.157 |
| DHEAS (µg/dL) | 95.4±51.9 | 80.7±53.0 | 1.00 (0.99–1.00) | 0.005 |
| ∆4 androstenedione (ng/mL) | 1.02±0.6 | 0.83±0.6 | 0.69 (0.54–0.90) | 0.008 |
| Cortisol (µg/dL) | 13.8±4.7 | 14.2±4.9 | 1.02 (0.98–1.06) | 0.442 |
| IGF-1 (ng/mL) | 133±42.3 | 125±41.1 | 1.00 (0.99–1.00) | 0.083 |
| Vitamin D (ng/mL) | 29.5±10.0 | 31.0±8.0 | 1.02 (1.00–1.04) | 0.145 |
| Parathormone (pg/mL) | 40.4±14.1 | 43.5±14.8 | 1.02 (1.00–1.03) | 0.027 |
| Physical activity | ||||
| Low | 40 (33.4) | 264 (38.9) | Reference | |
| Moderate/high | 80 (66.6) | 414 (61.1) | 0.79 (0.52–1.18) | 0.288 |
| Vitamin D supplementation | 44 (36.6) | 249 (36.7) | 0.99 (0.67–1.49) | 0.998 |
BMD: Bone mineral density, CI: Confidence interval, DHEAS: Dehydroepiandrosterone sulfate, FSH: Follicle-stimulating hormone, IGF-1: Insulin-like growth factor, BMI: Body mass index, OR: Odds ratio
The Pearson correlation coefficients (r) of the parameters pertaining to all the women are shown in Table 3. The present study observed that lumbar spine BMD was correlated to DHEAS (r = 0.10, P < 0.001). The femoral neck BMD was correlated with FSH (r = −0.08, P = 0.020), testosterone (r = 0.1, P = 0.010), DHEAS (r = 0.12, P < 0.001), ∆4-androstenedione (r = 0.09, P = 0.020), cortisol (r = −0.12, P < 0.001), and PTH (r = −0.14, P < 0.001). We also observed that there were correlations between the total hip with FSH (r = −0.08, P = 0.020), testosterone (r = 0.11, P < 0.001), DHEAS (r = 0.12, P < 0.001), ∆4-androstenedione (r = 0.10, P = 0.010), cortisol (r = −0.08, P = 0.030), and PTH (r = −0.07, P = 0.040). Multivariable linear regression analysis was used to determine the association between endogenous hormones and BMD [Table 4]. After adjusting for confounding variables, a significant association was found between cortisol and femoral neck BMD (β: −0.02, 95% CI: −0.03–−0.00), and PTH with femoral neck BMD (β: −0.01, 95% CI: −0.02–−0.01) and total hip BMD (β: −0.01, 95% CI: −0.01–−0.00). Finally, a multivariable logistic regression adjusted for confounding variables was performed to analyze possible hormonal predictors of low BMD and showed that women with higher FSH levels (OR: 1.02, 95% CI: 1.01–1.03) or higher PTH levels (OR: 1.02, 95% CI: 1.01–1.04) were more likely to have low BMD [Table 5].
Table 3.
The Pearson correlation between clinical and hormonal parameters and bone mineral density
| Parameter | Lumbar spine BMD | Femoral neck BMD | Total hip BMD | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| r | P | r | P | r | P | |
| Age | −0.12 | <0.001 | −0.15 | <0.001 | −0.08 | 0.030 |
| Age at menopause | 0.07 | 0.050 | 0.02 | 0.490 | 0.06 | 0.110 |
| Years since menopause | −0.15 | <0.001 | −0.15 | <0.001 | −0.10 | <0.001 |
| BMI | 0.27 | <0.001 | 0.32 | <0.001 | 0.36 | <0.001 |
| Adiposity | −0.20 | <0.001 | −0.06 | 0.080 | −0.02 | 0.580 |
| FSH | −0.03 | 0.430 | −0.08 | 0.020 | −0.08 | 0.020 |
| Estradiol | −0.06 | 0.110 | 0.01 | 0.790 | 0.03 | 0.400 |
| Testosterone | 0.05 | 0.140 | 0.10 | 0.010 | 0.11 | <0.001 |
| DHEAS | 0.10 | <0.001 | 0.12 | <0.001 | 0.12 | <0.001 |
| ∆4 androstenedione | 0.06 | 0.070 | 0.09 | 0.020 | 0.10 | 0.010 |
| Cortisol | −0.03 | 0.380 | −0.12 | <0.001 | −0.08 | 0.030 |
| IGF-1 | 0.05 | 0.180 | 0.05 | 0.200 | 0.04 | 0.290 |
| Vitamin D | −0.04 | 0.240 | −0.02 | 0.500 | −0.05 | 0.180 |
| Parathormona | −0.04 | 0.220 | −0.14 | <0.001 | −0.07 | 0.040 |
BMD: Bone mineral density, DHEAS: Dehydroepiandrosterone sulfate, FSH: Follicle-stimulating hormone, IGF-1: Insulin-like growth factor, r: Pearson correlation, BMI: Body mass index
Table 4.
Multivariable linear regression model to analyze the association between endogenous hormones and bone mineral density (g/cm2) adjusting for age, adiposity, body mass index, smoking status, physical activity, and years since menopause
| β (95% CI) | |||
|---|---|---|---|
|
| |||
| Lumbar spine BMD | Femoral neck BMD | Total hip BMD | |
| FSH | 0.00 (−0.00–0.00) | −0.00 (−0.00–0.00) | −0.00 (−0.00–0.00) |
| Estradiol | −0.01 (−0.02–0.00) | 0.00 (−0.01–0.01) | 0.00 (−0.01–0.01) |
| Testosterone | −0.10 (−0.53–0.32) | 0.17 (−0.15–0.49) | 0.19 (−0.15–0.54) |
| DHEAS | 0.00 (−0.00–0.00) | −0.00 (−0.00–0.00) | 0.00 (−0.00–0.00) |
| ∆4 androstenedione | 0.05 (−0.11–0.20) | 0.09 (−0.02–0.21) | 0.09 (−0.03–0.21) |
| Cortisol | 0.01 (−0.01–0.03) | −0.02 (−0.03–−0.00) | −0.01 (−0.02–0.01) |
| IGF-1 | 0.00 (−0.00–0.00) | 0.00 (−0.00–0.00) | 0.00 (−0.00–0.00) |
| Vitamin D | −0.00 (−0.01–0.01) | −0.00 (−0.01–0.01) | −0.00 (−0.01–0.00) |
| Parathormona | −0.01 (−0.01–0.00) | −0.01 (−0.2–−0.01) | −0.01 (−0.01–−0.00) |
| R 2 | 0.22 | 0.22 | 0.21 |
| Adjusted R2 | 0.20 | 0.21 | 0.19 |
BMD: Bone mineral density, CI: Confidence interval, FSH: Follicle-stimulating hormone, DHEAS: Dehydroepiandrosterone sulfate, IGF-1: Insulin-like growth factor
Table 5.
Multivariable logistic regression to analyze the association between endogenous hormones and risk of presenting low bone mineral density, adjusted by age, adiposity, body mass index, smoking status, physical activity, and years since menopause
| Hormonal parameters | Low-BMD (osteopenia/osteoporosis) | |
|---|---|---|
|
| ||
| OR | 95% CI | |
| FSH | 1.02 | 1.01–1.03 |
| Estradiol | 1.00 | 0.97–1.04 |
| Testosterone | 1.03 | 0.35–3.43 |
| DHEAS | 1.00 | 1.00–1.01 |
| ∆4 androstenedione | 0.71 | 0.48–1.04 |
| Cortisol | 0.99 | 0.94–1.03 |
| IGF-1 | 1.00 | 0.99–1.00 |
| Vitamin D | 1.01 | 0.98–1.04 |
| Parathormona | 1.02 | 1.01–1.04 |
CI: Confidence interval, DHEAS: Dehydroepiandrosterone sulfate, FSH: Follicle-stimulating hormone, IGF-1: Insulin-like growth factor, OR: Odds ratio, BMD: Bone mineral density
DISCUSSION
The present study showed in postmenopausal women that cortisol was associated with femoral neck BMD, and PTH with femoral neck BMD and total hip BMD. Hormonal factors more likely associated with a higher risk of low BMD were FSH and PTH. To the best of our knowledge, DAG has been rarely used in specific clinical issues of postmenopausal women.[20,21,22] We previously studied the association between handgrip strength and endogenous hormones in postmenopausal women using a DAG.[23] The present is the first study that analyzed the association between endogenous hormones and BMD in postmenopausal women using a DAG. This tool is a graphical structure connecting with arrows associated with variables dependence, may identify confounders, and avoids superfluous adjustments.[24,25]
During the menopausal transition, there is a progressive increase in circulating FSH levels, which remain high after menopause. This hormone activates osteoclastogenic pathways; hence, it has been postulated that high plasma FSH levels cause bone loss.[6] However, the possible direct effects of FSH on bone loss have generated controversy. Data from the Study of Women’s Health Across the Nation showed that BMD loss during the menopause transition was related to plasma FSH levels, independent of plasma estradiol levels.[26] The AGES-Reykjavik Study reported a negative correlation between plasma FSH levels and BMD in older postmenopausal women.[27] On the contrary, Drake et al.[7] demonstrated that pharmacological suppression of FSH secretion is not associated with bone resorption markers in postmenopausal women, concluding that FSH does not regulate bone resorption. A cross-sectional study conducted in postmenopausal women aged 50–64 years found no significant association between FSH or bioavailable estradiol and BMD.[8] Our study finds a significant association between FSH and BMD, and higher FSH levels present a higher risk of low BMD, expressing the secondary adjustment of the global hypoestrogenism of postmenopause. Therefore, FSH levels are the expression of low estradiol levels in postmenopausal women in determining BMD.
The decline in estrogen levels in postmenopausal women was associated with low BMD[5,28] and increased risk of osteoporotic fractures.[29,30] However, not all women with estrogen deficiency develop osteoporosis. During the postmenopausal period, the effect of the serum levels of estradiol only explains a small percentage of bone loss. Other factors such as age, age at menopause, years since menopause, BMI, adiposity, smoking status, and physical activity may also affect the BMD.[31,32] Our study results confirm a lack of association between estradiol and BMD, and a possible explanation of these results would be that the low levels of estradiol during postmenopause do not have a relevant effect on BMD.
Previous studies reported that premenopausal women with high androgens levels are associated with increased BMD at the lumbar spine and the femoral neck.[33] In contrast, the effect of androgens on BMD in postmenopausal women is still controversial. The prospective study of Wu et al.[9] showed that testosterone correlates with the rate of change of bone density. Furthermore, endogenous testosterone levels were significantly associated with femoral neck BMD.[12] However, Crandall et al.[34] showed no significant associations between testosterone levels with rates of lumbar spine or femoral neck bone loss. Furthermore, during the menopausal transition, Guthrie et al.[10] demonstrated no significant effect of testosterone on BMD. In postmenopausal women, DHEAS is the dominant circulating androgen secreted by the adrenal glands and converted to estradiol or testosterone in the target organs. Its circulating levels decline with aging.[35] Several studies have examined the relationship between DHEAS and BMD but with a discrepancy in results. The longitudinal study of Ghebre et al.[36] demonstrated that high endogenous DHEAS levels at baseline were associated with reduced bone loss at the lumbar spine and the femoral neck, although this association decreases over time. Bácsi et al.[37] reported that plasma DHEAS levels correlate positively with BMD at the lumbar spine, and Park et al.[38] related a correlation with femur BMD but not at the lumbar spine. However, other studies found no association between DHEAS levels with BMD or with the rate of bone loss.[10,12,39] The positive effects of endogenous ∆ 4-androstenedione on BMD have been reported, showing a positive association between ∆ 4-androstenedione and femoral neck BMD.[12] The Women’s Health in the Lund Area cohort study reported an increased fracture risk during follow-up in postmenopausal women with low serum ∆ 4-androstenedione, suggesting that postmenopausal osteoporosis is influenced by lower levels of androgens.[40] Concerning the relationship between androgens and BMD, our multivariable analysis did not demonstrate the association between BMD and the studied androgens. In postmenopausal women, androgens are lower and possibly have less influence on BMD than in premenopausal women.
Corticosteroids are related to decreased BMD and increased fracture risk due to detrimental effects on osteoblast and osteocyte functions, prolonged osteoclast life span, decreased intestinal absorption of calcium, and stimulated PTH secretion.[41] In recent years, there has been a growing interest in the subtle cortisol excess because it is associated with an increased risk of complications, in particular osteoporosis and fragility fractures. On the other hand, the skeletal effect of cortisol excess may vary by ethnicity due to associating with polymorphism in the enzyme 11β-hydroxysteroid dehydrogenase type 1 and in the glucocorticoid receptor. Cortisol excess has a detrimental effect on the trabecular bone; however, the data on the effect on cortical bone are discordant.[42] Our results support the notion that endogenous plasma cortisol levels are associated with low BMD at the femoral neck, showing the detrimental effect of cortisol excess on the cortical bone.
The role of IGF-1 in the maintenance of BMD in postmenopausal women is controversial. Some studies found that higher plasma IGF-1 levels reduced BMD loss,[13,43] but not in others.[14,44] Some studies suggest gender-specific differences in relation to IGF-1 and BMD due to an association in men but not in women.[45] A recent meta-analysis in the Chinese population found that IGF-1 single-nucleotide polymorphism rs35767 TT genotype was associated with an increased risk of osteoporosis. However, this association needs to be evaluated in other ethnic groups.[46] Our present results were obtained in women of only Spanish ethnicity, and we observed no significant association between IGF-1 and BMD.
Vitamin D status influences calcium-phosphorus homeostasis and bone metabolism. Vitamin D deficiency is a risk factor associated with the development of osteoporosis. Several studies and systematic reviews have reported that more than 50% of the population has Vitamin D deficiency.[47] The threshold for Vitamin D deficiency of <12 ng/ml (30 nmol/L) is compatible with adverse effects on bone health.[48] Although Vitamin D deficiency coexists with low BMD, some studies found no correlation between serum Vitamin D levels and BMD.[49] In the present study, plasma 25-hydroxyvitamin D levels were not associated with BMD. However, this finding might be explained by the fact that in our study, there were no women with very low levels of Vitamin D. Therefore, this issue requires a future evaluation, including women with different Vitamin D levels.
Aging is associated with increased plasma PTH levels independent of Vitamin D, calcium, phosphorus, and renal function.[50] Hyperparathyroidism is usually asymptomatic and closely related to Vitamin D deficiency, particularly in the elderly.[51] High PTH levels cause osteoclasts hyperstimulation, BMD reduction, and increased fracture risk.[52] The results identified that PTH was associated with lower femoral neck and total hip BMD. Likewise, women with higher PTH levels had a higher risk of low BMD. In postmenopausal women, the effect of other endogenous factors on BMD is not completely understood. In this sense, the future use of DAGs to identify other risk factors of osteoporosis might facilitate future investigations.
Limitations and strengths
This study has certain limitations, including its cross-sectional design, and the longitudinal effect of the studied hormones on BMD could not be appropriately assessed. However, our study has several strengths. First, it includes a relatively large sample of postmenopausal women. Second, all BMD and measurements were conducted with the same densitometer and laboratory, with daily quality controls. Third, careful adjustments for confounders were performed in the regression model. Finally, we are not aware of a previous approach using DAGs to study BMD in postmenopausal women.
CONCLUSIONS
The present study showed that in studied postmenopausal women, (i) FSH is a secondary marker of low or nil estrogen ovarian production, rather than the direct cause of bone mineral alteration; (ii) high cortisol levels are associated with lower femoral neck BMD, showing the detrimental effect of cortisol excess on the cortical bone; (iii) higher PTH levels are associated with lower BMD in both the femoral neck and total hip; and (iv) high FSH and PTH levels are more likely associated with the risk of low BMD. Therefore, due to the clinical implications of osteoporosis, FSH, cortisol, and PTH measurements might be used in assessing BMD loss in postmenopausal women.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study has been done under the auspices of the Professorship in Obstetrics and Gynecological Research of the Hospital Universitario Dexeus of the Universidad Autónoma de Barcelona.
REFERENCES
- 1.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: *An executive summary and recommendations – Update 2019-2020. J Midlife Health. 2020;11:96–112. doi: 10.4103/jmh.JMH_143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anupama DS, Noronha JA, Acharya KK, Prabhu MM, Shetty J, Shankar R, et al. Burden of osteopenia and osteoporosis among postmenopausal women in India: A systematic review and meta-analysis. J Midlife Health. 2022;13:107–14. doi: 10.4103/jmh.jmh_207_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–96. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 4.Sowers MR, Zheng H, Jannausch ML, McConnell D, Nan B, Harlow S, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95:2155–62. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riggs BL, Khosla S, Atkinson EJ, Dunstan CR, Melton LJ., 3rd Evidence that type I osteoporosis results from enhanced responsiveness of bone to estrogen deficiency. Osteoporos Int. 2003;14:728–33. doi: 10.1007/s00198-003-1437-9. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006;125:247–60. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 7.Drake MT, McCready LK, Hoey KA, Atkinson EJ, Khosla S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5063–8. doi: 10.1210/jc.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gourlay ML, Preisser JS, Hammett-Stabler CA, Renner JB, Rubin J. Follicle-stimulating hormone and bioavailable estradiol are less important than weight and race in determining bone density in younger postmenopausal women. Osteoporos Int. 2011;22:2699–708. doi: 10.1007/s00198-010-1505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F, Ames R, Clearwater J, Evans MC, Gamble G, Reid IR. Prospective 10-year study of the determinants of bone density and bone loss in normal postmenopausal women, including the effect of hormone replacement therapy. Clin Endocrinol (Oxf) 2002;56:703–11. doi: 10.1046/j.1365-2265.2002.01534.x. [DOI] [PubMed] [Google Scholar]
- 10.Guthrie JR, Lehert P, Dennerstein L, Burger HG, Ebeling PR, Wark JD. The relative effect of endogenous estradiol and androgens on menopausal bone loss: A longitudinal study. Osteoporos Int. 2004;15:881–6. doi: 10.1007/s00198-004-1624-3. [DOI] [PubMed] [Google Scholar]
- 11.Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S. The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas. 2004;48:235–42. doi: 10.1016/j.maturitas.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Lambrinoudaki I, Christodoulakos G, Aravantinos L, Antoniou A, Rizos D, Chondros C, et al. Endogenous sex steroids and bone mineral density in healthy Greek postmenopausal women. J Bone Miner Metab. 2006;24:65–71. doi: 10.1007/s00774-005-0648-x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Kanatani M, Yamauchi M, Kaji H, Sugishita T, Baylink DJ, et al. Serum levels of insulin-like growth factor (IGF);IGF-binding proteins-3, -4, and -5;and their relationships to bone mineral density and the risk of vertebral fractures in postmenopausal women. Calcif Tissue Int. 2006;78:18–24. doi: 10.1007/s00223-005-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001;28:113–7. doi: 10.1016/s8756-3282(00)00408-7. [DOI] [PubMed] [Google Scholar]
- 15.Digitale JC, Martin JN, Glymour MM. Tutorial on directed acyclic graphs. J Clin Epidemiol. 2022;142:264–7. doi: 10.1016/j.jclinepi.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series 843. Geneva, Switzerland: World Health Organization; 1994. [PubMed] [Google Scholar]
- 17.The IPAQ Group. International Physical Activity Questionnaire. 2016. [[Last accessed on 2020 Mar 20]]. Available from: http://www.ipaq.ki.se .
- 18.Shahar E, Shahar DJ. Causal diagrams and change variables. J Eval Clin Pract. 2012;18:143–8. doi: 10.1111/j.1365-2753.2010.01540.x. [DOI] [PubMed] [Google Scholar]
- 19.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: The R package 'dagitty'. Int J Epidemiol. 2016;45:1887–94. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 20.Sung VW. Reducing bias in pelvic floor disorders research: Using directed acyclic graphs as an aid. Neurourol Urodyn. 2012;31:115–20. doi: 10.1002/nau.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung HF, Zhu D, Dobson AJ, Kuh D, Gold EB, Crawford SL, et al. Age at menarche and risk of vasomotor menopausal symptoms: A pooled analysis of six studies. BJOG. 2021;128:603–13. doi: 10.1111/1471-0528.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z, Zhang S, Thio C, Wang Y, Li M, Wu Y, et al. Serum total bilirubin and new-onset hypertension in perimenopausal women: A cross-sectional study. Menopause. 2022;29:944–51. doi: 10.1097/GME.0000000000001999. [DOI] [PubMed] [Google Scholar]
- 23.García-Alfaro P, García S, Rodriguez I, Bergamaschi L, Pérez-López FR. Relationship between handgrip strength and endogenous hormones in postmenopausal women. Menopause. 2023;30:11–7. doi: 10.1097/GME.0000000000002093. [DOI] [PubMed] [Google Scholar]
- 24.Jansen JP, Schmid CH, Salanti G. Directed acyclic graphs can help understand bias in indirect and mixed treatment comparisons. J Clin Epidemiol. 2012;65:798–807. doi: 10.1016/j.jclinepi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Liu HX, Wang HB, Wang N. Application of directed acyclic graphs in identifying and controlling confounding bias. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:585–8. doi: 10.3760/cma.j.cn112338-20190729-00559. [DOI] [PubMed] [Google Scholar]
- 26.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–7. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 27.Veldhuis-Vlug AG, Woods GN, Sigurdsson S, Ewing SK, Le PT, Hue TF, et al. Serum FSH is associated with BMD, bone marrow adiposity, and body composition in the AGES-Reykjavik study of older adults. J Clin Endocrinol Metab. 2021;106:e1156–69. doi: 10.1210/clinem/dgaa922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers A, Saleh G, Hannon RA, Greenfield D, Eastell R. Circulating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women. J Clin Endocrinol Metab. 2002;87:4470–5. doi: 10.1210/jc.2002-020396. [DOI] [PubMed] [Google Scholar]
- 29.Finigan J, Gossiel F, Glüer CC, Felsenberg D, Reid DM, Roux C, et al. Endogenous estradiol and the risk of incident fracture in postmenopausal women: The OPUS study. Calcif Tissue Int. 2012;91:59–68. doi: 10.1007/s00223-012-9611-8. [DOI] [PubMed] [Google Scholar]
- 30.Prince RL, Dick IM, Beilby J, Dhaliwal SS, Devine A. A cohort study of the effect of endogenous estrogen on spine fracture risk and bone structure in elderly women and an assessment of its diagnostic usefulness. Bone. 2007;41:33–8. doi: 10.1016/j.bone.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Akdeniz N, Akpolat V, Kale A, Erdemoglu M, Kuyumcuoglu U, Celik Y. Risk factors for postmenopausal osteoporosis: Anthropometric measurements, age, age at menopause and the time elapsed after menopause onset. Gynecol Endocrinol. 2009;25:125–9. doi: 10.1080/09513590802549817. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan SD, Lehman A, Nathan NK, Thomson CA, Howard BV. Age of menopause and fracture risk in postmenopausal women randomized to calcium+Vitamin D, hormone therapy, or the combination: Results from the women's health initiative clinical trials. Menopause. 2017;24:371–8. doi: 10.1097/GME.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karadağ C, Yoldemir T, Gogas Yavuz D. Determinants of low bone mineral density in premenopausal polycystic ovary syndrome patients. Gynecol Endocrinol. 2017;33:234–7. doi: 10.1080/09513590.2016.1250256. [DOI] [PubMed] [Google Scholar]
- 34.Crandall CJ, Tseng CH, Karlamangla AS, Finkelstein JS, Randolph JF, Jr, Thurston RC, et al. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. J Clin Endocrinol Metab. 2013;98:E654–63. doi: 10.1210/jc.2012-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labrie F, Martel C, Bélanger A, Pelletier G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J Steroid Biochem Mol Biol. 2017;168:9–18. doi: 10.1016/j.jsbmb.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Ghebre MA, Hart DJ, Hakim AJ, Kato BS, Thompson V, Arden NK, et al. Association between DHEAS and bone loss in postmenopausal women: A 15-year longitudinal population-based study. Calcif Tissue Int. 2011;89:295–302. doi: 10.1007/s00223-011-9518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bácsi K, Kósa JP, Borgulya G, Balla B, Lazáry A, Nagy Z, et al. CYP3A7 *1C polymorphism, serum dehydroepiandrosterone sulfate level, and bone mineral density in postmenopausal women. Calcif Tissue Int. 2007;80:154–9. doi: 10.1007/s00223-006-0227-8. [DOI] [PubMed] [Google Scholar]
- 38.Park SG, Hwang S, Kim JS, Park KC, Kwon Y, Kim KC. The association between dehydroepiandrosterone sulfate (DHEA-S) and bone mineral density in Korean men and women. J Bone Metab. 2017;24:31–6. doi: 10.11005/jbm.2017.24.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zofková I, Bahbouh R, Hill M. The pathophysiological implications of circulating androgens on bone mineral density in a normal female population. Steroids. 2000;65:857–61. doi: 10.1016/s0039-128x(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 40.Moberg L, Nilsson PM, Samsioe G, Borgfeldt C. Low androstenedione/sex hormone binding globulin ratio increases fracture risk in postmenopausal women. The women's health in the Lund area study. Maturitas. 2013;75:270–5. doi: 10.1016/j.maturitas.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 42.Ahn SH, Kim JH, Cho YY, Suh S, Kim BJ, Hong S, et al. The effects of cortisol and adrenal androgen on bone mass in Asians with and without subclinical hypercortisolism. Osteoporos Int. 2019;30:1059–69. doi: 10.1007/s00198-019-04871-5. [DOI] [PubMed] [Google Scholar]
- 43.Rosen CJ. Insulin-like growth factor I and bone mineral density: Experience from animal models and human observational studies. Best Pract Res Clin Endocrinol Metab. 2004;18:423–35. doi: 10.1016/j.beem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd ME, Hart DJ, Nandra D, McAlindon TE, Wheeler M, Doyle DV, et al. Relation between insulin-like growth factor-I concentrations, osteoarthritis, bone density, and fractures in the general population: The Chingford study. Ann Rheum Dis. 1996;55:870–4. doi: 10.1136/ard.55.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssen JA, Burger H, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW, et al. Gender-specific relationship between serum free and total IGF-I and bone mineral density in elderly men and women. Eur J Endocrinol. 1998;138:627–32. doi: 10.1530/eje.0.1380627. [DOI] [PubMed] [Google Scholar]
- 46.Chen YC, Zhang L, Li EN, Ding LX, Zhang GA, Hou Y, et al. Association of the insulin-like growth factor-1 single nucleotide polymorphisms rs35767, rs2288377, and rs5742612 with osteoporosis risk: A meta-analysis. Medicine (Baltimore) 2017;96:e9231. doi: 10.1097/MD.0000000000009231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-López FR, Chedraui P, Pilz S. Vitamin D supplementation after the menopause. Ther Adv Endocrinol Metab. 2020;11:1–13. doi: 10.1177/2042018820931291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 49.Kamineni V, Latha AP, Ramathulasi K. Association between serum 25-hydroxyvitamin D levels and bone mineral density in normal postmenopausal women. J Midlife Health. 2016;7:163–8. doi: 10.4103/0976-7800.195694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrivick SJ, Walsh JP, Brown SJ, Wardrop R, Hadlow NC. Brief report: Does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function, and ionized calcium? J Clin Endocrinol Metab. 2015;100:2131–4. doi: 10.1210/jc.2014-4370. [DOI] [PubMed] [Google Scholar]
- 51.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–58. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 52.Bruce DG, St John A, Nicklason F, Goldswain PR. Secondary hyperparathyroidism in patients from Western Australia with hip fracture: Relationship to type of hip fracture, renal function, and vitamin D deficiency. J Am Geriatr Soc. 1999;47:354–9. doi: 10.1111/j.1532-5415.1999.tb03001.x. [DOI] [PubMed] [Google Scholar]


