ABSTRACT
Female genital tuberculosis (FGTB) is a significant health concern that can lead to infertility in women. FGTB is a common form of tuberculosis that affects the female reproductive organs. In India, around 27.5 million individuals are facing infertility issues due to female factors, and FGTB might be one of the leading causes. A systematic review and proportion meta-analysis of six studies was conducted using MedCalc 20.116 to examine the association between FGTB and infertility. The studies were identified through an electronic search of PubMed, MEDLINE, Elsevier, and the Cochrane Library from 2010 to 2023. The results showed that FGTB is significantly associated with infertility, with a prevalence of approximately 34.86%. These findings underscore the need for effective interventions to improve reproductive health in women with FGTB. Based on pathway analysis, we conclude that more clinical trials should be conducted to explore the potential utilization of interferon gamma and nuclear receptors as therapeutic drug targets and biomarkers for the prevention of FGTB. The findings of this review will contribute to raising awareness, facilitating accurate diagnosis, and improving the management of FGTB-related infertility.
KEYWORDS: Biomarkers, female genital tuberculosis, interferon gamma, infertility, key pathway mechanism, meta-analysis, meta-analysis, nuclear receptors, reproductive health, reproductive health, systematic review
INTRODUCTION
Tuberculosis (TB) is a global health threat that can cause significant morbidity in women’s reproductive health.[1] Female genital TB (FGTB) is a form of TB that specifically targets the female reproductive organs, leading to menstrual disorders and infertility.[2] FGTB is a significant cause of infertility in women, and it is often underdiagnosed due to poor clinical awareness and nonspecific symptoms.[3] Early recognition and appropriate management of FGTB are essential to preserving women’s reproductive functions and preventing long-term complications.[4,5,6]
A systematic review and meta-analysis of clinical studies has found that FGTB is associated with a high prevalence of infertility in women. As depicted in Figure 1, a study[7] found that FGTB can affect various organs in the female reproductive system, including the fallopian tubes, ovaries, cervix, and uterus. The study’s findings highlight the importance of early diagnosis and treatment of FGTB in order to prevent infertility and other long-term complications. The study also provides valuable insights into the mechanisms by which FGTB can cause infertility.[8,9]
Figure 1.
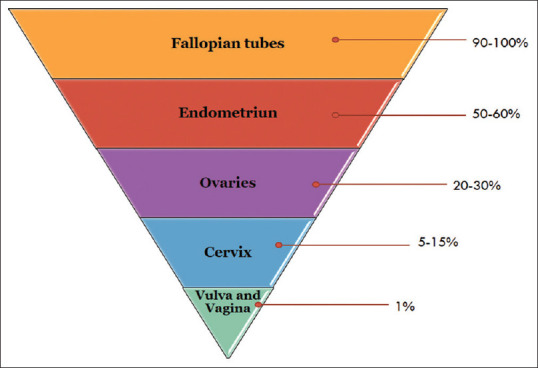
Frequency of Bacterial Infection in female genital organs during female genital tuberculosis[7]
Why is this review undertaken?
This review aims to elucidate the correlation between FGTB and female infertility, providing valuable insights into the specific pathways involved. By understanding these pathways, we can develop targeted strategies to enhance reproductive health and outcomes for women affected by FGTB. The ultimate goal is to address the challenges posed by FGTB and improve the reproductive well-being of affected individuals.
METHODOLOGY
Literature search and database
We conducted an extensive literature search to investigate the association between FGTB and infertility, along with a pathway analysis of infertility causes. A combination of keywords “female infertility,” tubal factor infertility,” “Mycobacterium tuberculosis,” “TB pathway,” “FGTB pathway,” and “infertility Pathway in females,” were entered into the Google Scholar; PubMed/MEDLINE; CINAHL (EBSCO); Scopus; Web of Science; Elsevier Bio Base; EMBASE; and Science Direct search engines. The search was limited to publications in the English language. The purpose of this systematic review was to gather original and review articles that provide insights into the relationship between FGTB and infertility, while also exploring the pathways involved in causing infertility in females.
Inclusion and exclusion criteria
For this systematic review, we applied inclusion and exclusion criteria to select relevant articles. We prioritized highly reviewed articles and abstracts that focused on the impact of FGTB on infertility. Our inclusion criteria encompassed: (a) studies dedicated to female infertility; (b) data suitable for clinical analysis of FGTB; (c) studies investigating the correlation between FGTB and infertility; (e) studies exploring the pathways involved in female infertility attributed to the Tubercle bacterium; and (f) preference for recent or high-quality publications when multiple sources covered the same or overlapping data. Our exclusion criteria encompassed: (a) studies exclusively focusing on male infertility; (b) studies centered on urogenital TB; (c) studies on extrapulmonary TB not specifically related to female genital organs; and (d) studies centered on assisted reproductive treatment.
Figure 2 illustrates the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) process that was used to analyze the correlation between FGTB and infertility. The PRISMA guidelines are used to enhance research transparency, completeness, and the reliability of findings in systematic reviews and meta-analyses.[10] They ensure comprehensive reporting and adherence to standardized methods, ultimately improving the quality of the research.
Figure 2.
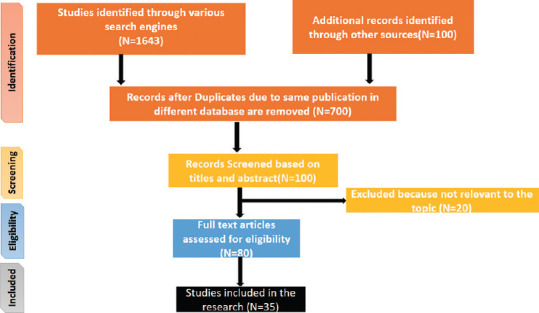
Preferred reporting items for systematic reviews and meta-analyses flowchart for the systematic review and meta-analysis of the correlation between female genital tuberculosis and infertility
Statistical analysis
Meta-analysis is a widely employed technique for aggregating quantitative data from distinct yet comparable studies included within a systematic review.[11] Proportion meta-analysis is commonly used in systematic reviews to address questions concerning the prevalence or incidence of a particular ailment in a population.[12] It is a statistical tool for combining data from multiple studies that report disease prevalence or percentages. This approach yields a pooled proportion, which is a weighted average of individual proportions from all studies, alongside a confidence interval that underscores the estimate’s precision. This method facilitates the understanding of disease prevalence across diverse populations, thus offering evidence-based insights for potential interventions.
In this study, we conducted a proportionate meta-analysis with a specific focus on the impact of FGTB on infertility. Our analysis included six studies, as detailed in Table 1. We used the freely accessible MedCalc-20.116 software by MedCalc Software Ltd. in Belgium which is used to conduct the meta-analysis of biomedical research. This evaluation entailed the assessment of combined effects between total cases of infertility and positive instances of FGTB, aimed at determining their statistical significance.
Table 1.
Studies included in the meta-analysis of prevalence of female genital tuberculosis in infertile women
| Study | Positive FGTB cases | Total infertility cases |
|---|---|---|
| Agarwal et al., 2016[13] | 19 | 54 |
| Sharma et al., 2022[14] | 38 | 70 |
| Al eryani et al., 2015[15] | 47 | 151 |
| Thangappah et al., 2022[16] | 51 | 153 |
| Saxena et al., 2022[17] | 6 | 43 |
| Khanna and Agrawal, 2011[18] | 26 | 63 |
| Total | 187 | 534 |
FGTB: Female genital tuberculosis
RESULTS
In this section, we discuss the findings from the meta-analysis of six studies, focusing on heterogeneity and publication bias. The proportion meta-analysis provided a pooled proportion estimate of infertility cases and the associated FGTB cases, along with corresponding confidence intervals.
Table 2 reveals substantial heterogeneity among the included studies, indicating variations in effect sizes beyond random chance. On the other hand, Table 2 indicates no significant publication bias in the selected studies. These results shed light on the variability and reliability of the data and contribute to a comprehensive understanding of the impact of FGTB on female infertility.
Table 2.
Test for heterogeneity and publication bias in studies assessing the impact of female genital tuberculosis on infertility
| Heterogeneity | |
|---|---|
| Q | 23.0438 |
| Df | 5 |
| Significance level (P) | 0.0003 |
| I2 (inconsistency) (%) | 78.30 |
| 95% CI for I2 | 52.17–90.16 |
|
| |
| Publication bias | |
|
| |
| Egger’s test | |
| Intercept | 0.02710 |
| 95% CI | −11.1661–11.2204 |
| Significance level | 0.9950 |
| Begg’s test | |
| Kendall’s Tau | −0.2000 |
| Significance level (P) | 0.5730 |
CI: Confidence interval
According to Figure 3, it evaluates the potential impact of publication bias and the reliability of the included studies’ results. It can be seen from this plot that the spread of the data points is uniform, and the observed differences in effect estimates are likely due to random variation, which indicates that there is likely no publication bias or heterogeneity among the included studies.
Figure 3.
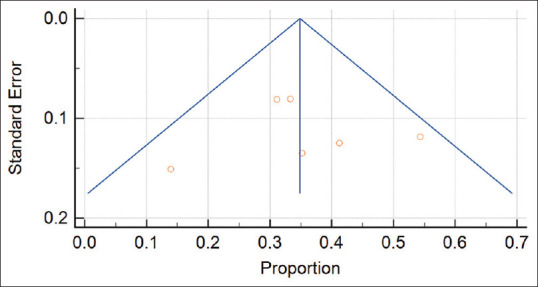
Funnel plot of pooled effect estimates for correlation of female genital tuberculosis and infertility
Figure 4 illustrates the forest plot containing data from six studies, encompassing a total of 534 cases. The meta-analysis provides compelling evidence supporting the association between FGTB and infertility. Both the fixed effects and random effects models show a significant correlation between FGTB and infertility, with a prevalence of approximately 34.86%. The results emphasize the significant impact of FGTB on infertility, highlighting the need for effective interventions to improve reproductive health in affected women.
Figure 4.
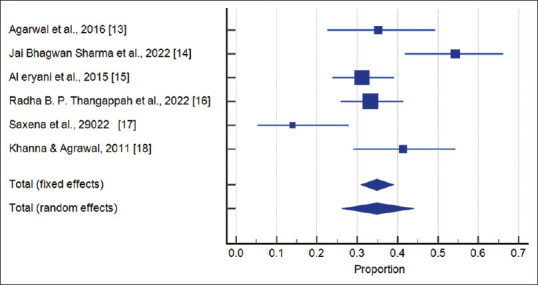
Forest plot of the association between genital tuberculosis and infertility in women
The systematic review and meta-analysis conducted have highlighted the significant impact of FGTB on female infertility. To effectively address this issue, it is imperative to delve into the underlying pathways through which TB bacteria contribute to infertility in women. In the upcoming section, we will explore the specific FGTB pathways that play a role in causing female infertility. Understanding these pathways will help us identify potential targets for intervention and develop strategies to enhance reproductive outcomes for women affected by FGTB.
Mechanism of female genital tuberculosis mediated female infertility
FGTB-mediated female infertility involves complex mechanisms that disrupt the reproductive system, primarily affecting the fallopian tubes, endometrium, and ovaries. The major mechanisms involved in FGTB-induced toxicity are immune dysregulation, altered secretion of growth factors and cytokines, hormonal signaling disruption, reduced expression of cell adhesion molecules, and disrupted gene expression and signaling pathways. These mechanisms play significant roles in implantation failure and infertility outcomes. Table 3 explores the intricate pathways through which FGTB exerts its adverse effects on female fertility.
Table 3.
Key pathway mechanisms involved in female genital tuberculosis-induced female infertility
| Mechanism | Prominent/causal genes | Altered function | Reference |
|---|---|---|---|
| Immune dysregulation | IFN-γ CXCL9 |
MTB and its virulent proteins function as antigens, initiating an inflammatory response and triggering IFN-γ production IFN-γ further induces the secretion of CXCL9 by inflammatory cells CXCL9 binds to its receptor CXCR3, forming a complex that activates the PI3K pathway and downstream molecule AKT Elevated levels of IFN-γ and IL-2, along with increased CXCL9 expression, contribute to implantation failure | [6,19,20,21,22,23] |
| Modulation of nuclear receptors | Nurr 77 Nurr1 RORA PXR FXR PPARa LXR Rev-erba PPARs |
MTB lipids and mycolic acid modulate orphan nuclear receptors This modulation suppresses steroid signaling and follicle maturation Cell wall lipids of MTB interact with nuclear receptors This interaction promotes survival and forms lipid-enriched foamy macrophages These macrophages weaken the immune response by inhibiting cytokine secretion Apoptosis is abated due to this weakening of the immune response | [6] |
| Reduced expression of cell adhesion molecules | CDH1 MUC1 MECA79 ITGAVB3 |
Reduced level of cell adhesion molecules leads to recurrent implantation failure | [24] |
| Growth factors | LIF VEGF |
LIF activates STAT3 pathways, which induce VEGF transcription Lowered LIF and VEGF lead to reduced uterine receptivity Insufficient levels result in unsuccessful placentation Imbalanced levels cause improper embryonic implantation | [25,26,27] |
| Endocrine dysfunction | LRH1 VDR LXR TR4 PPARg PXR |
Reduces anti-Mullerian hormone Modulates estrogen, human chorionic gonadotropin, progesterone, and steroidogenesis Lowers granulosa cell differentiation, folliculogenesis, oocyte yield, and ovarian reserve Disturbs ovarian steroidogenesis and ovulation | [28,29,30,31] |
MTB: Mycobacterium tuberculosis, VEGF: Vascular endothelial growth factor, IFN-γ: Interferon gamma, CXCL9: Chemokine ligand 9, RORA: RAR related orphan receptor A gene, PXR: Pregnane X receptor gene, LIF: Leukemia inhibitory factor, LRH1: Liver receptor homolog 1, VDR: Vitamin D receptor, LXR: Liver X receptor, TR4: Testicular receptor, PPARg: Peroxisome proliferator activated receptor, PXR: Pregnane X receptor
CONCLUSION
This systematic review and meta-analysis provide compelling evidence of the significant association between FGTB and female infertility. The findings underscore the urgent need for heightened awareness and understanding of FGTB’s impact on reproductive health. Pathway analysis highlights potential therapeutic targets to enhance uterine receptivity, immune modulation, and FGTB treatment while combating bacterial resistance. By delving deeper into the underlying mechanisms, we have identified promising interventions, including the utilization of interferon gamma and nuclear receptors, which could revolutionize FGTB management and amplify women’s prospects for successful conception and pregnancy.
This study serves as a clarion call to address FGTB as a critical health concern, advocating for increased clinical trials and targeted strategies to enhance global reproductive health and empower women. We can pave the way toward a future where infertility caused by FGTB becomes a relic of the past, allowing every woman to experience the profound joy of motherhood.
Contribution
All authors contributed to the study conception and design. Material preparation and data collection were performed by AV. The first draft of the manuscript was written by AV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Tuberculosis. World Health Organization; 2023. [[Last accessed on 2023 Jun 06]]. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis . [Google Scholar]
- 2.Wang Y, Shao R, He C, Chen L. Emerging progress on diagnosis and treatment of female genital tuberculosis. J Int Med Res. 2021;49:1–8. doi: 10.1177/03000605211014999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munne KR, Tandon D, Chauhan SL, Patil AD. Female genital tuberculosis in light of newer laboratory tests: A narrative review. Indian J Tuberc. 2020;67:112–20. doi: 10.1016/j.ijtb.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Djuwantono T, Permadi W, Septiani L, Faried A, Halim D, Parwati I. Female genital tuberculosis and infertility: Serial cases report in Bandung, Indonesia and literature review. BMC Res Notes. 2017;10:683. doi: 10.1186/s13104-017-3057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace GA, Devaleenal DB, Natrajan M. Genital tuberculosis in females. Indian J Med Res. 2017;145:425–36. doi: 10.4103/ijmr.IJMR_1550_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Gupta P. Etiopathogenesis, Challenges and Remedies Associated With Female Genital Tuberculosis: Potential Role of Nuclear Receptors. Front Immunol. 2020;11:02161. doi: 10.3389/fimmu.2020.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer G. Tuberculosis of the Female Genital Tract. Clin Obstet Gynecol. 1970;13:965–98. doi: 10.1097/00003081-197012000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Cornell JE, Liao JM, Stack CB, Mulrow CD. Annals understanding clinical research: Evaluating the meaning of a summary estimate in a meta-analysis. Ann Intern Med. 2017;167:275–7. doi: 10.7326/M17-1454. [DOI] [PubMed] [Google Scholar]
- 9.Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, et al. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21:189. doi: 10.1186/s12874-021-01381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PRISMA. PRISMA Flow Diagram. 2020. [[Last accessed on 2023 Aug 05]]. Available from: https://www.prisma statement.org/PRISMAStatement/FlowDiagram .
- 11.Tzelios C, Neuhausser WM, Ryley D, Vo N, Hurtado RM, Nathavitharana RR. Female Genital Tuberculosis. Open Forum Infect Dis. 2022;9:ofac543. doi: 10.1093/ofid/ofac543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh FR, Nadkarni SG, Kamat SA, Naik N, Soonawala SB, Parikh RM. Genital tuberculosis –A major pelvic factor causing infertility in Indian women. Fertil Steril. 1997;67:497–500. doi: 10.1016/s0015-0282(97)80076-3. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Chauhan J, Vacchani A, Ahir KK. Study on co-relation of infertility and female genital tuberculosis. [[Last accessed on 2023 Aug 10]];Natl J Med Res. 2016 6:151–4. Available from: https://www.njmr.in/index.php/file/article/view/254 . [Google Scholar]
- 14.Sharma JB, Kumari S, Jaiswal P, Dharmendra S, Hari S, Singh UB. Hysterosalpingography observations in female genital tuberculosis with infertility. J Hum Reprod Sci. 2022;15:362–9. doi: 10.4103/jhrs.jhrs_111_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Eryani AA, Abdelrub AS, Al Harazi AH. Genital tuberculosis is common among females with tubal factor infertility: Observational study. Alex J Med. 2015;51:321–4. [Google Scholar]
- 16.Thangappah RB, Madhava T, Sundaravadivelu P, Sowparnika AK, Ravichandran MR. Hysterosalpingographic findings in infertile women diagnosed with genital tuberculosis. Int J Reprod Contracept Obstet Gynecol. 2022;11:1140. [Google Scholar]
- 17.Saxena R, Shrinet K, Rai SN, Singh K, Jain S, Jain S, et al. Diagnosis of genital tuberculosis in infertile women by using the composite reference standard. Kosmas I, editor. Dis Markers. 2022;2022:1–10. doi: 10.1155/2022/8078639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna A, Agrawal A. Markers of genital tuberculosis in infertility. Singapore Med J. 2011;52:864–7. [PubMed] [Google Scholar]
- 19.Bhanothu V, Lakshmi V, Theophilus JP, Rozati R, Badhini P, Vijayalaxmi B. Investigation of Toll-Like receptor-2 (2258G/A) and interferon gamma (+874T/A) gene polymorphisms among infertile women with female genital tuberculosis. PLoS One. 2015;10:e0130273. doi: 10.1371/journal.pone.0130273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkan ZS, Deveci D, Kumbak B, Simsek M, Ilhan F, Sekercioglu S, et al. What is the impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility? J Reprod Immunol. 2014;103:53–8. doi: 10.1016/j.jri.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Sharma JB, Sharma S, Sharma E, Dharmendra S, Singh S. Immune disturbances in female genital tuberculosis and latent genital tuberculosis. Am J Reprod Immunol. 2023;89:e13632. doi: 10.1111/aji.13632. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Fan J, Shang X, Tuohetaerbaike B, Li Y, Lv J, et al. Study on the relationship between CXCR3 and its ligands and tubal tuberculosis. Life Sci. 2021;272:119047. doi: 10.1016/j.lfs.2021.119047. [DOI] [PubMed] [Google Scholar]
- 23.Parikh FR, Panpalia M, Mehta T, Agarwal S, Khandeparker M, Chettiar SS, et al. Dysfunctional regulation of pivotal and key inflammatory pathways in infertile Indian women with genital tuberculosis. Am J Reprod Immunol. 2022;88:e13624. doi: 10.1111/aji.13624. [DOI] [PubMed] [Google Scholar]
- 24.Casals G, Ordi J, Creus M, Fábregues F, Carmona F, Casamitjana R, et al. Osteopontin and alphavbeta3 integrin as markers of endometrial receptivity: The effect of different hormone therapies. Reprod Biomed Online. 2010;21:349–59. doi: 10.1016/j.rbmo.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Margioula-Siarkou C, Prapas Y, Petousis S, Milias S, Ravanos K, Dagklis T, et al. LIF endometrial expression is impaired in women with unexplained infertility while LIF-R expression in all infertility sub-groups. Cytokine. 2017;96:166–72. doi: 10.1016/j.cyto.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Keltz MD, Attar E, Buradagunta S, Olive DL, Kliman HJ, Arici A. Modulation of leukemia inhibitory factor gene expression and protein biosynthesis in the human fallopian tube. Am J Obstet Gynecol. 1996;175:1611–9. doi: 10.1016/s0002-9378(96)70114-x. [DOI] [PubMed] [Google Scholar]
- 27.Subramani E, Madogwe E, Ray CD, Dutta SK, Chakravarty B, Bordignon V, et al. Dysregulated leukemia inhibitory factor and its receptor regulated signal transducers and activators of transcription 3 pathway: A possible cause for repeated implantation failure in women with dormant genital tuberculosis? Fertil Steril. 2016;105:1076–84.e5. doi: 10.1016/j.fertnstert.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra N, Sharma V, Bahadur A, Sharma JB, Roy KK, Kumar S. The effect of tuberculosis on ovarian reserve among women undergoing IVF in India. Int J Gynaecol Obstet. 2012;117:40–4. doi: 10.1016/j.ijgo.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Danforth DR, Arbogast LK, Mroueh J, Kim MH, Kennard EA, Seifer DB, et al. Dimeric inhibin: A direct marker of ovarian aging. Fertil Steril. 1998;70:119–23. doi: 10.1016/s0015-0282(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 30.Jirge PR, Chougule SM, Keni A, Kumar S, Modi D. Latent genital tuberculosis adversely affects the ovarian reserve in infertile women. Hum Reprod. 2018;33:1262–9. doi: 10.1093/humrep/dey117. [DOI] [PubMed] [Google Scholar]
- 31.Datta A, Chaudhuri AR, Chatterjee S, Chowdhury R, Bhattacharya B. Role of endometrial cytokines of the female genital tract tuberculosis in the context of infertility. BLDE Univ J Health Sci. 2018;3:24. [Google Scholar]


