Abstract
Interactions between human neutrophils and Borrelia burgdorferi, the Lyme disease spirochete, were studied by dark-field microscopy combined with video technology. A previously unrecognized mechanism for neutrophils to phagocytize the spirochete was discovered. During phagocytosis, the spirochete attaches to the neutrophil head-on, the neutrophil forms a thin tubelike protrusion around the bacterium, and the fully covered spirochete is drawn into the cell.
Borrelia burgdorferi is the spirochete responsible for Lyme disease. Previous studies have shown that B. burgdorferi can be ingested by human phagocytes (1, 5, 6, 8). Because of the length and mobility of the spirochete, this ingestion is a formidable task for the cells. Coiling phagocytosis, in which the spirochete is wrapped in a pseudopod coil of the cell membrane, has been suggested as the principal mechanism of B. burgdorferi ingestion (7). However, this concept is based on the study of fixed preparations, which cannot provide detailed information on ingestion dynamics. Such dynamics can be studied only by means of living cells. A recent study by Nomarski microscopy and video recordings indicated that mouse macrophages ingest the spirochete predominantly by conventional phagocytosis, with B. burgdorferi entering macrophages head-on (4). We have found that dark-field microscopy combined with modern video technology is a highly useful method for observing and recording interactions between phagocytes and the spirochete. Using this approach, we have discovered a previously unrecognized mechanism for neutrophils to phagocytize B. burgdorferi, which we have designated tube phagocytosis.
Human neutrophils were isolated from the heparin-anticoagulated venous blood of five healthy donors. Dextran T-500 (Pharmacia Biotech, Uppsala, Sweden) sedimentation and Percoll (Pharmacia Biotech) gradient centrifugation were used, remaining erythrocytes were lysed with 0.83% ammonium chloride, and the neutrophils were washed with phosphate-buffered saline as previously described (3). The neutrophils were suspended in Hanks’ balanced salt solution supplemented with 0.25% bovine serum albumin (Wilfrid Smith, Edgware, Middlesex, United Kingdom) at 20.0 × 106 cells/ml. A high-passage prototype strain, B. burgdorferi sensu stricto B31 (ATCC 35210), which was maintained in liquid Barbour-Stoenner-Kelly II medium at 30°C, was used. Borreliae were counted in a Neubauer counting chamber and were diluted in Barbour-Stoenner-Kelly II medium at 10.0 × 107 borreliae/ml.
A 200-μl volume of the neutrophil suspension (4.0 × 106 cells) and 200 μl of the borrelial suspension (2.0 × 107 organisms) were mixed for the phagocytosis study in a polystyrene tube (Falcon 2054; Beckton Dickinson, Lincoln Park, N.J.). A 50-μl volume of borrelia antibody-negative human serum was added as a source of complement. Of the mixture, 20 μl was applied to a standard microscopic glass slide and covered with a thin glass slip. The temperature of the slide was kept at approximately 32°C by using a thermostat-controlled heating plate (Linkam CO 102 Warm Stage Controller and Linkam TH 60; Linkam Scientific Instruments, Tadworth, Surrey, United Kingdom). Phagocytosis was observed under a dark-field microscope (Leitz Orthoplan) equipped with a video camera (Hitachi VK-C220E) connected to a monitor (Sony Trinitron) and to a video recorder (Sony SVT S3000P). To study the effect of cytochalasin B on tube formation, the neutrophils were incubated in Hanks’ balanced salt solution supplemented with 0.25% bovine serum albumin and with 5 μg of cytochalasin B (Sigma, St. Louis, Mo.) per ml for 5 min at 37°C before being mixed with the borreliae and the serum; the following steps of this experiment were performed in a way similar to that for the phagocytosis study described above.
In each of the five separate experiments carried out (each with neutrophils from a different donor), we observed neutrophils successfully ingesting the spirochete in a manner which, to our knowledge, has not been described previously. During this tube phagocytosis, the bacterium attached to the neutrophil head-on and the neutrophil started to form a thin tubelike protrusion surrounding the borrelia (Fig. 1). The protrusion gradually extended over the long and often vigorously moving spirochete (Fig. 2A through E). Once the spirochete was fully covered, the tube was retracted and the spirochete was drawn within it into the cell (Fig. 2E through G). After a few minutes, the neutrophil returned to a resting shape (Fig. 2H). In the experiment in which cytochalasin B was used, no tube formation or any other type of phagocytic activity was detected, although borreliae attached avidly to the neutrophils. This suggests that polymerization of the actin filaments of the neutrophil cytoskeleton is necessary for tube formation.
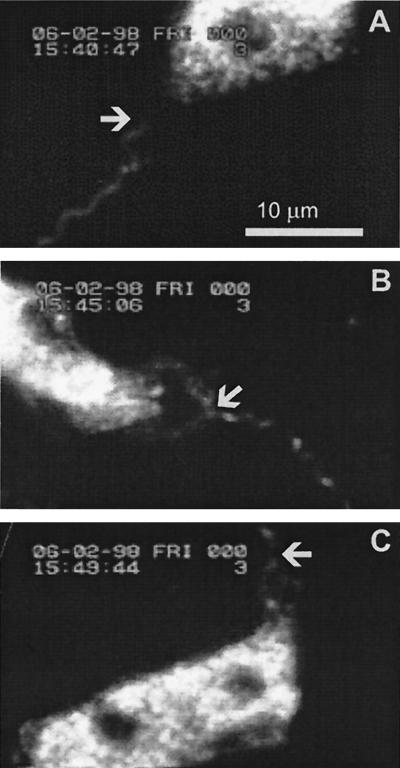
FIG. 1.
Still pictures of a video recording were captured by using a custom-made, Windows-based, frame grabber program in a PC. The pictures were further processed for printing by using Adobe Photoshop 4.0 and Corel DRAW 7. They represent a video recording, in which the attachment of the spirochete and the early events of phagocytosis can be seen. (A) A borrelia spirochete approaches a neutrophil. The arrow indicates the neutrophil-approaching end of the spirochete (time, 15:40:47). (B) The spirochete has attached to the neutrophil. The neutrophil is activated, as indicated by its elongated form. The arrow indicates the site of the attachment (time, 15:45:06). (C) The neutrophil has started to form a tubelike protrusion along the spirochete. The arrow indicates the distal end of the protrusion (time, 15:49:44).
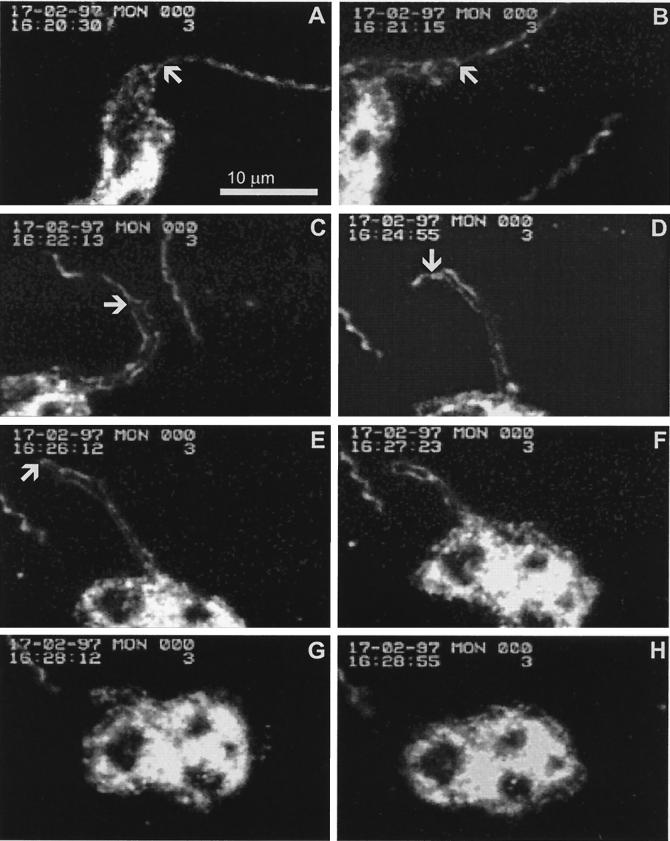
FIG. 2.
Video recording in which the neutrophil and the whole spirochete are simultaneously in focus during tube formation. The arrows point out the progression of the tube. (A) A borrelia spirochete has attached to a neutrophil (time, 16:20:30). (B) The tube has started to form (time, 16:21:15). (C through E) The tube gradually extends over the whole spirochete. The spirochete is continuously moving. Another spirochete is passing by the cell during phagocytosis (time, 16:22:13 to 16:26:12). (F through G) The tube is retracted, and the spirochete is drawn into the neutrophil (time, 16:27:23 to 16:28:12). (H) The neutrophil returns to a resting shape (time, 16:28:55). A digitized version of the original video, from which the pictures have been selected, can be accessed at the following homepage: http://www.utu.fi/research/tic/projects/viljanen.html.
Tube phagocytosis may represent an extreme type of the classical zipper mechanism (2). Constructing the long projection needed in the process is an instance of excellent cooperation between the cytoskeleton and the membrane of the neutrophil. Additional studies are needed to define the borrelial ligands and the neutrophil receptors involved. The significance of this phenomenon in the first line of host defenses against B. burgdorferi remains to be shown. Furthermore, tube phagocytosis might also play a role in the ingestion of other excessively long organisms.
Acknowledgments
This study was supported by a grant from the Emil Aaltonen Foundation.
We thank Erkki Eerola and Sami Tohka for help in processing the imaging data.
REFERENCES
- 1.Benach J L, Fleit H B, Habicht G S, Coleman J L, Bosler E M, Lane B P. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J Infect Dis. 1984;150:497–507. doi: 10.1093/infdis/150.4.497. [DOI] [PubMed] [Google Scholar]
- 2.Griffin F M, Jr, Griffin J A, Leider J E, Silverstein S C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartiala K T, Langlois L, Goldstein I M, Rosenbaum J T. Endotoxin-induced selective dysfunction of rabbit polymorphonuclear leukocytes in response to endogenous chemotactic factors. Infect Immun. 1985;50:527–533. doi: 10.1128/iai.50.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery R R, Malawista S E. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect Immun. 1996;64:2867–2872. doi: 10.1128/iai.64.7.2867-2872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery R R, Nathanson M H, Malawista S E. Fc- and non-Fc-mediated phagocytosis of Borrelia burgdorferi by macrophages. J Infect Dis. 1994;170:890–893. doi: 10.1093/infdis/170.4.890. [DOI] [PubMed] [Google Scholar]
- 6.Peterson P K, Clawson C C, Lee D A, Garlich D J, Quie P G, Johnson R C. Human phagocyte interactions with the Lyme disease spirochete. Infect Immun. 1984;46:608–611. doi: 10.1128/iai.46.2.608-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittig M G, Krause A, Häupl T, Schaible U E, Modolell M, Kramer M D, Lütjen-Drecoll E, Simon M M, Burmester G R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szczepanski A, Fleit H B. Interaction between Borrelia burgdorferi and polymorphonuclear leukocytes: phagocytosis and the induction of the respiratory burst. Ann N Y Acad Sci. 1988;539:425–428. [Google Scholar]