Abstract
In dairy goat kids, weaning is often associated with poor growth leading to a decline in welfare and performance; however, little is known about optimal weaning practices. This study aimed to determine the optimal weaning age for dairy goat kids to maximize outcome measures of welfare related to growth, feed intake, and behavior. Thirty-six newborn female Alpine kids were blocked by weight and birth date, paired with a similar male companion and randomly allocated to one of the three weaning age treatments: 6 (6W), 8 (8W), and 10 wk (10W). Kids had ad libitum access to acidified milk replacer refilled twice daily, concentrates, hay, and water. Milk consumption was measured daily, and concentrate consumption, weekly. Ten behaviors were live observed on days −8, −4, 0, 6, and 12 relative to weaning (i.e., weaning day = 0). Kruskal–Wallis tests were used to assess differences from baseline between the 6W, 8W, and 10W treatments. Post hoc analysis using the Dwass, Steel, Critchlow-Fligner (DSCF) multiple comparison analysis was used to evaluate pairwise treatment differences based on two-sample Wilcoxon comparisons. Kids weaned at 10 wk had the greatest increase compared to baseline in concentrate consumption (P = 0.0160), and greatest decrease compared to baseline in vocalization (P = 0.0008) while both 8- and 10- wk kid’s groups had the greatest increase compared to baseline in self-grooming time (P < 0.0001), and cross-sucking time (P = 0.0006). Kids weaned at 6 wk of age were found to have the smallest increase compared to baseline in concentrate consumption (P = 0.0160) and self-grooming time (P < 0.0001), and the greatest increase compared to baseline in allogrooming time (P = 0.0032) and in redirected behaviors aimed towards the environment (biting and licking time [P = 0.0173]; displacement at the nipple frequency [P = 0.0236]). No negative impact of weaning on growth of either group was identified. Overall, our results tend towards a higher degree of discomfort behaviors (allogrooming, biting/licking, displacement, and vocalizations) in kids weaned earlier compared to later weaning, while kids weaned later showed higher levels of positive behaviors (lying time and self-grooming).
Keywords: behavior, dairy goat kid, weaning, welfare
Age at weaning seems to have an impact on the behavior of dairy goat kids and their ability to cope with the transition to solid feed. In this study, kids weaned at later ages seemed to have adapted better than kids weaned at an early age.
Introduction
The dairy goat industry has shown tremendous global growth within the past two decades, with the majority of intensive dairy goat production systems being present in Europe and North America (Miller and Lu, 2019). In these systems, preventing negative effects on the growth and health of dairy goat kids during the weaning period is essential for optimizing future herd productivity (Gökdal et al., 2017). The weaning period occurs as the kid loses access to milk and transitions to solid feed, a process that requires rapid changes in endocrine and metabolic function that often coincides with heightened stress responses and reduced welfare (Magistrelli et al., 2010).
Current management practice guidelines are to wean kids at 6 to 8 wk of age using a progressive weaning method, by reducing milk access over a 5- to 7-d period (Bélanger-Naud and Vasseur, 2021b). Unfortunately, limited scientific research has been performed on dairy goat kids to refine and validate these practices (Bélanger-Naud and Vasseur, 2021b). The negative effects associated with stress during the weaning period may be minimized by adhering to weaning protocols that are supported by scientific literature. Early weaning (5 wk of age) has been associated with lower weight gains and higher mortality rates postweaning in male kids from dual utility breeds (milk and meat) weaned at 8 wk (Luparia et al., 2009) while Panzuti et al. did not report an effect of early weaning on growth or milk yield in Alpine goats. Male and female kids of Sirohi, Kutchi, and Marwari breeds weaned by 8 wk of age showed no long-term deficits in growth compared to kids weaned late at 12 wk of age (Nagpal et al., 1995). In addition, a study that compared growth parameters, behavioral traits, and variables of lying time between goat kids weaned at 6 and 8 wk found no differences between the two groups, and favored that kids weaned at 6 wk had more experience with roughage consumption by 8 wk due to earlier exposure (Ugur et al., 2004).
While there is support to indicate that weaning at 8 wk of age (industry practice; Bélanger-Naud et al., 2021a) may have no negative impact on weight gain or mortality, other indicators of welfare (e.g., incidence of abnormal behavior, lying time) still require investigation. It is also unclear whether weaning at earlier age than 8 wk is optimal. Furthermore, weaning at 10 wk of age could represent a compromise between early (6 wk) and late (12 wk) weaning ages. Our rational was to expand the knowledge on dairy goat weaning practices in the existing literature supporting current on-farm weaning practices. The objective of the current study was to determine the optimal weaning age for dairy goat kids by comparing weaning at 6-, 8-, and 10-wk of age in animal outcome measures related to growth, feed intake, and behavior around the weaning period.
Materials and Methods
Ethics statement
This experiment was conducted at the Centre de Recherche en Sciences Animales de Deschambault (CRSAD) between March and June 2019. The use of animals and all procedures were approved by the Animal Care Committee of CRSAD (#1819CL362).
Study design
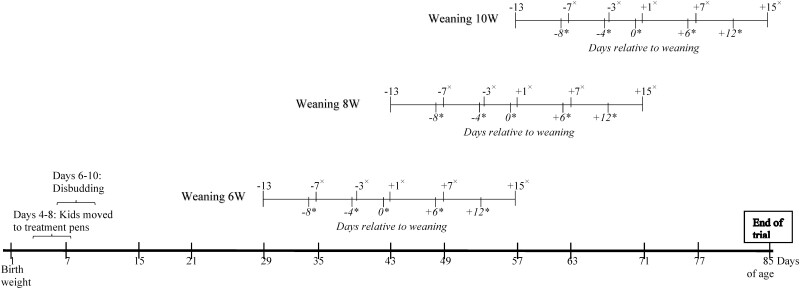
Thirty-six purebred Alpine female kids, born between March 4 and April 4, 2019, were enrolled in this study. Kids were blocked into 12 groups of three females, based on their birth date and birth weight (mean ± SD; 4.34 ± 0.33 kg). Each block was completed within 4 to 6 d after birth (5.08 ± 1.08 d), each female within a block was randomly assigned to one of the three weaning treatments: weaning at 6 wk of age (6W), weaning at 8 wk of age (8W), and weaning at 10 wk of age (10W) and to 1 of the 36 pens (1.2 × 2.1 m). Each female within a pen was paired with a companion male of similar age (i.e., ≤ 2 d difference, except for one pen where the male was 7 d younger; mean difference = 0.78 d) and weight (i.e., ≤ 1 kg difference; mean difference = 0.37 kg). The mean difference in the average birth weight per pen (one male and one female) was 0.15 kg. The start date of each pen was defined as the average birth date of the two kids (Figure 1).
Figure 1.
Timeline depicting the events of each weaning period across treatments 6- (6W), 8- (8W), and 10-wk (10W) over the course of the study period. Visual behavior measures and lying behaviors were obtained on all of the marked (*) days over the weaning period for the following events: day before the start of weaning (baseline; day −8), middle of the weaning process (day −4), weaning day (day 0), and occasions in the 2 wk following weaning (days 6 and 12). Body weights were obtained on all denoted (×) days over the weaning period for the following event: start of weaning process (day −7), day −3 relative to weaning day, day after weaning was completed (day 1), and 7 and 15 d postweaning.
Kid care and feeding
Kids were separated from their dams in the first few hours after birth, then weighed, identified, and injected with 0.25 mL of a selenium (3 mg/mL) and vitamin E (136 IU/mL) supplement (Selon-E, Vetoquinol, Lavaltrie, QC) to prevent white muscle disease. Kids were fed artificial colostrum replacer (LambGro/KidGroTM Bovine Dried Colostrum, Grober Nutrition, ON) within 2 h after birth (mean ± SD; 1.15 ± 0.57 h) and had a minimum of three meals in their first 24 h of life (932.78 ± 147.64 mL). Passive transfer of immunity was measured in the blood at 24 h of life using a refractometer (Palm Abbe Refractometer, Misco, Solon, OH; 5.71 ± 0.53 g/dL), and did not differ across treatments (P = 0.89; tested using the method of least squares to fit a general linear model). After the first 24 h, kids were fed ad libitum with milk replacer (CapriLait Milk Replacer, Grober Nutrition, ON) acidified with citric acid (Citric Acid Anhydrous, Weifang Ensign Industry Co., Ltd., China; 4.4 g per 100 g of dry powder) and delivered twice daily (0900 and 1600 hours) in a 9-L bucket outside their pen until the start of the weaning process. Two teats were present per pen to allow both kids to feed at the same time and prevent competition. Kids had access to fresh pelleted concentrates (Goliath VO-21 Deccox, La Coop Novago, Saint-Casimir, QC; 86.24 DM; 19.80 CP; ADF 9.28; NDF 24.40; fat 3.89%; and ME 2.35 Mcal/kg), chopped dry hay (83.16 DM, 10.95 CP, ADF 33.10, NDF 55.25, fat 2.01%, and ME 1.26 Mcal/kg), and clean water at all times. Milk buckets were washed with an acid soap (AcidiShine, DeLaval, Peterborough, ON), every day, and tubes and nipples were washed every 2 d. Bedding (wood shavings) was changed as needed every 1 to 2 wk to keep kids in a clean and dry environment. Disbudding was performed by a veterinarian, between 6 and 10 d of age, and was done at the same age for both animals in the same pen. Weaning was performed progressively, by reducing the milk quantity by 12.5% each day for 7 d (based on common practices found on commercial Canadian dairy goat farms; Bélanger-Naud et al., 2021a). Weaning quantity was calculated from the average milk consumption of each pen in the 4 d prior to the start of weaning. Kids had completed weaning by days 42 (6W), 56 (8W), and 70 (10W), on the 8th d of the weaning phase (days −7 to −1: weaning process, day 0: weaning completed).
Outcome measures
To monitor adequate conditions for development and performance outcomes, kids’ feed intake, growth (body weight), health, and environmental conditions were monitored through the trial, but only the data around weaning will be reported. To evaluate behavioral outcomes around weaning, lying, grooming, redirected, and other behaviors were measured. A timeline of the outcome measures taken around weaning is presented in Figure 1. All outcome measures are detailed in the following sections.
Feed intake
Feed consumption measures were recorded and calculated electronically, using FileMaker Pro (version 16, Claris, Santa Clara, CA). Milk consumption was measured daily for each pen with a generic scale based on the difference in weight of milk offered and refused and averaged weekly. Consumption of concentrates was measured for each pen weekly based on the difference in weight of the quantity offered and refused.
Growth
Kids were weighed on a scale (ALU 36X48, Carga, Drummondville, QC) that was calibrated before each use to ensure accurate measurements. Growth measures were assessed on days −7 (baseline), −3, 1 (day after weaning was completed), and days 7 and 15 postweaning (see timeline presented in Figure 1).
Health
Detailed health monitoring of kids has been conducted across the study and no kid was treated during the duration of the trial. As such, the data on health occurrences has not been reported or included in the analysis due to a lack of occurrence. Visual health measures were recorded weekly, according to an adapted version of respiratory disease scoring system for preweaned dairy calves (Young, 2019) and a diarrhea scoring chart was established based on the study by Bath and van Wyk (2009), Love et al. (2014, 2016), and Aly et al. (2014) and revised by S. Buczinski (personal communication). The respiratory disease system assigned a score based on the presence of 6 clinical signs including cough (=2), eye discharge (=2), fever (=2), abnormal respiration (=2), nasal discharge (=4), and ear droop/head tilt (=5). A total score of 5 or higher resulted in a classification of respiratory disease system. The diarrhea scoring chart was based on a visual evaluation of the kids rear-end cleanliness, with scores ranging from 0 (i.e., clean) to 3 (i.e., severe liquid diarrhea extending below the hocks). No cases of respiratory disease or diarrhea necessitating treatment were observed over the course of the study.
Environmental conditions
Bedding quantity and cleanliness in each pen were monitored daily to ensure good bedding conditions. Temperature and humidity were automatically recorded using three data loggers (HOBO MX2301A, Onset Computer Corp., Bourne, MA) placed in different locations around the building, at the height of the kids. Measures were recorded every 10 min for the duration of the study. Across the study, average temperature was 18.57 ± 1.52 °C (range = 13.50 to 25.84 °C) and humidity was 55.82% ± 10.03% (range = 28.31% to 93.65%).
Lying behavior
Accelerometers (HOBO pendant G data loggers; Onset Computer Corp., Bourne, MA) were used to monitor lying time (see timeline in Figure 1). Data loggers were attached using veterinary bandaging (Vet-Rap, CoFlex, Andover Coated Products Inc., Salisbury, MA, USA) to one of the hind legs, above the metatarsophalangeal joint. Lying time, number of lying bouts, and lying bouts duration were continuously recorded for five periods of 24 h on days −8 (baseline), −4, 0, 6, and 12 relative to weaning (i.e., weaning day = 0) at 1-min intervals (Hempstead et al., 2017). Data were downloaded using a Onset HOBOware Pro software (Onset Computer Corp., version 3.4.1) and electronically converted to hourly measures of lying time (UBC AWP, 2013) in SAS version 9.4 (SAS Institute Inc., Cary, NC).
Grooming, redirected, and other behaviors
All behaviors were assessed using visual observation. An ethogram has been presented in Table 1 for the behaviors reported in the text and in Supplementary Table S1 for the behaviors not reported in the text to outline the specific behaviors observed, the method of observation, the type of measurement, a description, and adapted literature. The categories of behaviors observed were related to grooming, redirection, playing, feeding, standing/lying, and others. Behaviors were recorded before, during, and after the morning milk feeding (i.e., when fresh acidified milk was delivered). Observations were carried out on the day before the start of weaning (day −8; baseline), in the middle of the weaning process (day −4), on weaning day (day 0), and twice in the 2 wk following weaning (days 6 and 12). The female kid in each pen was observed continuously for 1 min, every 6 min, for a total of 12 nonconsecutive minutes; each pen was observed non-simultaneously, and the observer rotated between pens. Five nonconsecutive minutes of observation occurred prior to the morning milk feeding (i.e., fresh acidified milk delivery was done twice a day to ensure milk was available ad lib), two occurred during milk feeding, and five occurred after milk feeding. While social and redirected behaviors were observed continuously in this manner, feeding and lying behaviors had two scan observations per minute, at 30 and 60 s. Observations for each pen were performed by a total of four trained observers with each observer in charge of a maximum of six pens per observation period. To help the kids distinguish between activities, white coveralls were worn by observers, while blue coveralls were worn by staff for feeding and all other interactions. Inter-observer repeatability (Kw = 0.75) was evaluated at the beginning and end of the project using an average Cohen’s kappa coefficient for each behavior. Intra-observer repeatability was not assessed due to the limitations of live observation.
Table 1.
Ethogram of behavioral observation for dairy goat kids (n = 36), including the methods and unit of observation
| Behaviors1 | Observation method2 | Unit(s)3 | Description | Adapted from |
|---|---|---|---|---|
| Grooming behaviors | ||||
| Allogrooming | Continuous | Frequency and duration | The kid grooms or licks another kid. Includes rubbing head or body against another kid. A separate grooming event is considered to occur after a break of > 1 s. | Hempstead et al. (2017), Wormsbecher et al. (2017) |
| Self-grooming | Continuous | Frequency and duration | The kid’s mouth comes in contact with any part of the body or legs (excluding biting on their HOBO). A separate self-grooming event is considered to occur after a pause of > 1 s and/or the kid is biting, or attempting to bite, objects on themselves (i.e., their own collar or HOBO on their leg). A separate biting event is considered to occur after a break of > 1 s and/or the kid tilts their head so the rear foot scratches (or attempts to scratch) any part of their head or neck. The rear fetlock must reach the shoulder. Head scratches separated by > 1 s are considered separate events and/or the kid’s head is tilted and comes in contact with any surface of the pen (e.g., wall, bar), while moving back and forth (lasting > 1 s). Head rubs separated by > 3 s are considered separate events. | Hempstead et al. (2017) |
| Redirected behaviors | ||||
| Cross-sucking | Continuous | Frequency and duration | The kid’s nose and mouth are close to any part of the other kid’s body and the kid is performing sucking movements, not including licking. Includes sucking on ear, tail, or other parts of the other kid. Bout is recorded as finished when the kid stops and turns head away and/or the kid is biting, or attempting to bite, objects on their conspecific (i.e., other kid’s collar). A separate biting event is considered to occur after a break of > 1 s. | De Paula Vieira et al. (2008), Wormsbecher et al. (2017) |
| Biting or licking | Continuous | Frequency and duration | The kid’s mouth comes in contact with any part of the pen (e.g., bars, buckets, and tubes) and the kid opens its mouth to lick or bite, or attempt to lick or bite the object. A separate biting/licking event is considered to occur after a break of > 1 s. | — |
| Nipple sucking | Continuous | Frequency and duration | The kid is sucking or chewing on the milk nipple but is not drinking milk. | — |
| Digging or pawing | Continuous | Frequency | The kid is digging down in the shavings, or pawing, with one of its forelegs. | — |
| Other behaviors | ||||
| Vocalization | Continuous | Frequency | The kid vocalizes. Each vocalization is recorded as one separate event. | De Paula Vieira et al. (2008) |
| Displacement at the nipple |
Continuous | Frequency | The kid displaces another kid at the nipple, and the other kid leaves the feeder within 5 s of a contact. | De Paula Vieira et al. (2008), Wormsbecher et al. (2017) |
1Behaviors were observed over 12 nonconsecutive minutes per day of observation.
2Continuous observations were taken throughout the observation period.
3Frequency was observed continuously as the total number of events per observation period. Duration was recorded in seconds when a behavioral event lasted over 5 s. For events that lasted 5 s or less, the duration was assigned to be 2 s.
Statistical analysis
Across the three treatment groups, behavior and lying data as well as growth data were summarized at the kid level (female only) while consumption data were summarized at the pen level (averaged). Data for the outcome measures of behavior and lying behaviors were analyzed at the day level and compiled for days −8 (baseline), −4 (mid-weaning), 0 (weaning day), 6, and 12 (postweaning). Data for growth (females only) were analyzed at day level and compiled for days −7 (baseline), −3 (relative to weaning day), 1 (day after weaning), 7, and 15 (postweaning). Data for feed intake were analyzed at the week level and compiled for weeks −1 (baseline), 0 (weaning process), 1, and 2 (postweaning). Differences from baseline were calculated for the remaining occasions via substraction. Analyses were conducted on the differences with baseline data and statistical tests chosen were non-parametric to accommodate for the nature of the data.
Kruskal–Wallis tests were performed for each variable of behavior, lying time, growth, and feed intake to determine whether significant differences existed overall (all days or weeks combined), by days or weeks between the 6W, 8W, and 10W weaning treatments. The tests were performed using the differences from baseline for each variable. Post hoc analysis using the Dwass, Steel, Critchlow-Fligner (DSCF) multiple comparison analysis was used to evaluate pairwise treatment differences based on two-sample Wilcoxon comparisons (Dwass 1960; Steel 1960; Critchlow and Fligner 1991). Only treatment differences with a P-value of <0.05 were considered significant.
Results
Baseline (mean ± SD) data are presented in Table 2. Overall treatment differences, all days or weeks combined, for the outcome measures of behavior, growth, and feed intake are presented in Table 3. Treatment differences are presented by week for feed intake, and by day for weight variables (Figure 2) and behavior variables (Table 4).
Table 2.
Treatment groups baseline mean ± SD for feed intake, growth measure, and behavior variables.
| Variables2 | Baseline1 ± SD | ||
|---|---|---|---|
| 6W | 8W | 10W | |
| Feed intake | |||
| Milk consumption, mL | 2,476.5 ± 216.09 | 2,628.8 ± 350.27 | 3,300.0 ± 391.26 |
| Concentrate consumption, g | 3.9 ± 3.00 | 11.0 ± 7.93 | 49.1 ± 79.32 |
| Growth measure | |||
| Weight, kg | 12.4 ± 0.94 | 15.6 ± 1.45 | 20.1 ± 2.42 |
| Lying behaviors | |||
| Lying time, min | 893.8 ± 62.41 | 849.5 ± 51.39 | 854.6 ± 76.00 |
| Lying bouts, # | 44.0 ± 9.93 | 44.8 ± 10.43 | 38.5 ± 12.60 |
| Lying bouts duration, min | 21.4 ± 5.91 | 19.9 ± 5.25 | 24.3 ± 9.56 |
| Grooming behaviors | |||
| Allogrooming frequency | 1.3 ± 1.82 | 1.7 ± 1.37 | 1.5 ± 2.32 |
| Allogrooming time, s | 3.8 ± 7.88 | 3.3 ± 2.74 | 3.7 ± 6.08 |
| Self-grooming frequency | 5.3 ± 5.77 | 3.3 ± 2.90 | 3.1 ± 2.68 |
| Self-grooming time, s | 20.6 ± 22.97 | 8.7 ± 9.96 | 7.3 ± 7.05 |
| Redirected behaviors | |||
| Cross-sucking frequency | 2.4 ± 2.02 | 0.8 ± 1.14 | 0.6 ± 0.90 |
| Cross-sucking time, s | 5.8 ± 5.36 | 1.5 ± 2.28 | 1.6 ± 2.47 |
| Biting and licking frequency | 10.8 ± 7.19 | 11.5 ± 8.50 | 7.7 ± 6.11 |
| Biting and licking time, s | 30.3 ± 21.57 | 39.3 ± 33.49 | 31.1 ± 27.81 |
| Nipple sucking frequency | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.5 ± 1.17 |
| Nipple sucking time, s | 0.0 ± 0.00 | 0.0 ± 0.00 | 5.5 ± 17.81 |
| Digging or pawing frequency | 0.6 ± 1.73 | 0.1 ± 0.29 | 0.9 ± 1.78 |
| Displacement at the nipple frequency | 0.0 ± 0.00 | 1.1 ± 3.45 | 0.3 ± 0.89 |
| Other behaviors | |||
| Vocalization frequency | 2.5 ± 4.91 | 2.7 ± 3.42 | 4.7 ± 9.19 |
1Baseline relative to weaning (day 0 or week 0) = week −1 for feed intake, day −7 for growth measure, and day −8 for behavior variables.
2Feed intakes were averaged at the pen level, while growth and all behavior measures were averaged at the kid level (female only).
Table 3.
Overall mean (days or weeks averages) differences relative to baseline ± SD for daily feed intake and behavior variables, across the 6W, 8W, and 10W treatment groups
| Variable | Mean difference from baseline ± SD | P-value3 | ||
|---|---|---|---|---|
| 6W | 8W | 10W | ||
| Feed intake1 | ||||
| Daily milk consumption, mL | −1,315.0 ± 228.52c | −1,682.5 ± 212.86b | −2,163.6 ± 324.45a | < 0.0001 |
| Daily concentrate consumption, g | 215.7 ± 201.11a | 286.9 ± 240.87a,b | 421.7 ± 334.03b | 0.0160 |
| Growth measure | ||||
| Body weight, kg | 1.2 ± 1.65 | 1.5 ± 1.56 | 1.5 ± 2.06 | 0.4119 |
| Lying behaviors | ||||
| Lying time, min | −34.7 ± 71.70 | −10.6 ± 80.94 | −34.6 ± 89.46 | 0.5851 |
| Lying bouts, # | −11.6 ± 16.05a,b | −12.7 ± 13.82b | −6.0 ± 13.08a | 0.0395 |
| Lying bouts duration, min | 9.2 ± 13.01 | 8.6 ± 8.92 | 4.1 ± 12.69 | 0.2270 |
| Grooming behaviors2 | ||||
| Self-grooming frequency | −2.9 ± 6.16 | −0.9 ± 3.29 | −0.6 ± 3.06 | 0.0787 |
| Self-grooming time, s | −14.9 ± 23.39a | 1.2 ± 14.25b | 0.7 ± 9.51b | < 0.0001 |
| Allogrooming frequency | 0.1 ± 2.81a | −1.4 ± 1.39b | −1.0 ± 2.22a,b | 0.0022 |
| Allogrooming time, s | −0.7 ± 10.20a | −2.8 ± 2.79b | −2.1 ± 5.91a,b | 0.0032 |
| Redirected behaviors2 | ||||
| Cross-sucking frequency | −1.3 ± 2.44a | −0.04 ± 1.78b | 0.4 ± 1.20b | 0.0001 |
| Cross-sucking time, s | −2.2 ± 8.64a | 1.2 ± 8.81b | 2.0 ± 6.60b | 0.0006 |
| Biting and licking frequency | −2.9 ± 9.49a,b | −6.3 ± 9.06b | −1.5 ± 7.35a | 0.0256 |
| Biting and licking time, s | 1.5 ± 40.14a,b | −21.0 ± 34.03b | −0.3 ± 39.53a | 0.0173 |
| Nipple sucking frequency | 2.6 ± 3.61 | 2.0 ± 4.19 | 1.2 ± 3.65 | 0.0506 |
| Nipple sucking time, s | 11.9 ± 21.76 | 13.4 ± 44.05 | 11.1 ± 51.54 | 0.1651 |
| Digging or pawing frequency | −0.3 ± 1.72 | −0.04 ± 0.36 | −0.8 ± 1.75 | 0.1016 |
| Displacement at the nipple frequency | 0.2 ± 0.52a | −0.9 ± 3.47a,b | −0.3 ± 0.87b | 0.0236 |
| Other behaviors2 | ||||
| Vocalization frequency | 7.2 ± 18.07a | 4.3 ± 9.27a | −1.3 ± 8.95b | 0.0008 |
1Feed intake was measured daily for milk consumption (averaged weekly) and weekly for concentrate consumption based on the difference in weight of feed offered and refused in each pen.
2Behaviors were observed over 12 nonconsecutive minutes per day of observation.
3Evaluated using a Kruskal–Wallis test.
a,bValues in a row with different superscripts had significant differences between the treatment groups (P < 0.05; DSCF multiple comparison analysis).
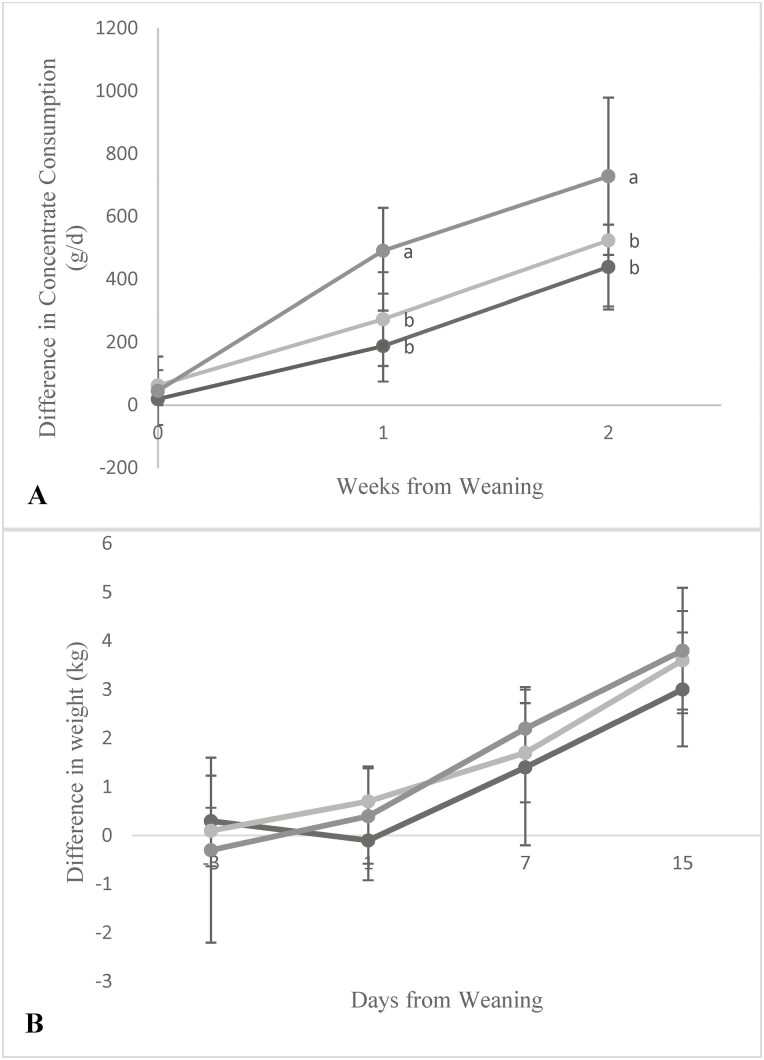
Figure 2.
The difference from baseline in weekly concentrate consumption (A) for weeks 0, 1, and 2, and in daily body weight (B) for days −3, 1, 7, and 15 relative to weaning across treatment groups (6W: black, 8W: light gray, and 10W: medium gray). Feed intake was measured based on the difference in weight of feed offered and refused in each pen. Standard deviation bars are shown. Values for each week with different superscripts (a and b) had significant differences between the treatment groups (P < 0.05; DSCF multiple comparison analysis).
Table 4.
Days mean differences relative to baseline ± SD for behavior variables, across the 6W, 8W, and 10W treatment groups
| Variable | Day | Mean difference from baseline ± SD | P-value2 | ||
|---|---|---|---|---|---|
| 6W | 8W | 10W | |||
| Grooming behaviors1 | |||||
| Self-grooming frequency | −4 | −1.7 ± 7.89 | −1.3 ± 2.63 | −0.4 ± 3.15 | 0.6690 |
| 0 | −4.1 ± 5.73 | −1.2 ± 3.82 | −1.3 ± 2.06 | 0.3038 | |
| 6 | −3.2 ± 6.01 | −0.6 ± 3.70 | −1.4 ± 3.29 | 0.6967 | |
| 12 | −2.5 ± 5.25 | −0.7 ± 3.31 | 0.6 ± 3.48 | 0.3099 | |
| Self-grooming time, s | −4 | −12.1 ± 25.33 | 3.2 ± 12.36 | 0.2 ± 9.21 | 0.1376 |
| 0 | −18.1 ± 23.27 | −3.0 ± 12.16 | −1.7 ± 6.77 | 0.0546 | |
| 6 | −15.6 ± 24.57 | 3.8 ± 16.02 | −2.3 ± 10.02 | 0.0973 | |
| 12 | −13.8 ± 23.02b | 0.6 ± 16.59a,b | 6.6 ± 10.05a | 0.0176 | |
| Allogrooming frequency | −4 | 0.9 ± 2.43 | −1.3 ± 1.48 | −0.6 ± 2.43 | 0.0734 |
| 0 | 0.7 ± 3.89 | −1.5 ± 1.57 | −1.1 ± 2.15 | 0.2216 | |
| 6 | −0.3 ± 2.56 | −1.6 ± 1.38 | −1.2 ± 2.21 | 0.1481 | |
| 12 | −1.0 ± 1.91 | −1.3 ± 1.30 | −1.3 ± 2.31 | 0.5320 | |
| Allogrooming time, s | −4 | 0.5 ± 8.23 | −2.5 ± 2.97 | −1.1 ± 7.20 | 0.1075 |
| 0 | 1.5 ± 14.37 | −2.9 ± 3.14 | −2.8 ± 5.15 | 0.2517 | |
| 6 | −1.4 ± 9.50 | −3.2 ± 2.76 | −1.5 ± 5.25 | 0.1374 | |
| 12 | −3.3 ± 8.06 | −2.7 ± 2.61 | −3.1 ± 6.32 | 0.5022 | |
| Redirected behaviors1 | |||||
| Cross-sucking frequency | −4 | −0.8 ± 1.59 | −0.1 ± 1.93 | 0.4 ± 1.08 | 0.1000 |
| 0 | −0.3 ± 2.86 | 0.1 ± 2.34 | 0.8 ± 1.64 | 0.4387 | |
| 6 | −2.0 ± 2.41 | 0.2 ± 1.53 | −0.2 ± 0.58 | 0.0358 | |
| 12 | −2.2 ± 2.48b | 0.0 ± 1.48a | 0.5 ± 1.17a | 0.0032 | |
| Cross-sucking time, s | −4 | 0.6 ± 9.17 | 3.9 ± 16.43 | 2.8 ± 9.73 | 0.4686 |
| 0 | 1.0 ± 10.97 | 0.2 ± 4.69 | 2.5 ± 5.78 | 0.5359 | |
| 6 | −5.0 ± 6.12 | 0.8 ± 3.84 | −0.8 ± 1.76 | 0.0409 | |
| 12 | −5.3 ± 6.17b | 0.0 ± 2.95a | 3.6 ± 6.54a | 0.0010 | |
| Biting and licking frequency | −4 | 2.1 ± 8.82 | −2.4 ± 10.54 | 1.5 ± 7.08 | 0.6497 |
| 0 | −2.5 ± 11.17 | −6.5 ± 10.06 | −0.5 ± 9.25 | 0.4138 | |
| 6 | −6.6 ± 7.51 | −7.8 ± 7.47 | −4.3 ± 5.72 | 0.4264 | |
| 12 | −4.5 ± 9.00 | −8.6 ± 7.74 | −2.7 ± 6.47 | 0.1348 | |
| Biting and licking time, s | −4 | 19.3 ± 41.76 | −10.3 ± 40.14 | 22.3 ± 43.42 | 0.2187 |
| 0 | 2.5 ± 46.70 | −18.9 ± 39.02 | 2.3 ± 47.24 | 0.5901 | |
| 6 | −12.8 ± 35.44 | −25.7 ± 25.96 | −15.0 ± 26.83 | 0.4250 | |
| 12 | −3.1 ± 33.30 | −29.1 ± 30.80 | −10.8 ± 30.30 | 0.0980 | |
| Nipple sucking frequency | −4 | 4.0 ± 2.59 | 3.3 ± 5.51 | 1.3 ± 2.53 | 0.0543 |
| 0 | 5.9 ± 4.76 | 4.1 ± 5.72 | 3.5 ± 6.17 | 0.8144 | |
| 6 | 0.3 ± 0.65 | 0.3 ± 0.89 | −0.1 ± 1.56 | 0.6463 | |
| 12 | 0.3 ± 0.45 | 0.4 ± 0.90 | 0.0 ± 0.95 | 0.7935 | |
| Nipple sucking time, s | −4 | 14.0 ± 16.73 | 14.8 ± 30.80 | 6.3 ± 34.00 | 0.2151 |
| 0 | 27.2 ± 31.40 | 37.5 ± 82.84 | 39.6 ± 89.52 | 0.4393 | |
| 6 | 5.5 ± 17.81 | 1.3 ± 3.23 | 0.5 ± 25.56 | 0.6459 | |
| 12 | 0.8 ± 1.80 | 1.8 ± 5.15 | −1.8 ± 17.70 | 0.8866 | |
| Digging or pawing frequency | −4 | 0.2 ± 2.44 | −0.1 ± 0.29 | −0.8 ± 1.75 | 0.1211 |
| 0 | −0.6 ± 1.73 | −0.1 ± 0.30 | −0.8 ± 1.96 | 0.8130 | |
| 6 | −0.6 ± 1.73 | 0.0 ± 0.43 | −0.8 ± 1.71 | 0.5402 | |
| 12 | −0.1 ± 0.51 | 0.0 ± 0.43 | −0.9 ± 1.78 | 0.3194 | |
| Displacement at the nipple frequency | −4 | 0.6 ± 0.90 | −0.6 ± 3.75 | −0.3 ± 0.97 | 0.1916 |
| 0 | 0.1 ± 0.29 | −1.0 ± 3.69 | −0.3 ± 0.87 | 0.6240 | |
| 6 | — | — | — | — | |
| 12 | — | — | — | — | |
| Other behaviors1 | |||||
| Vocalization frequency | −4 | 12.6 ± 30.66 | 7.8 ± 13.47 | −0.4 ± 10.89 | 0.2429 |
| 0 | 13.9 ± 14.23a | 7.3 ± 7.79b | 2.1 ± 4.68b | 0.0113 | |
| 6 | 1.6 ± 7.34 | 0.1 ± 3.68 | −3.8 ± 8.99 | 0.0607 | |
| 12 | 0.7 ± 6.05 | 2.4 ± 8.01 | −2.8 ± 9.92 | 0.3179 | |
1Behaviors were observed over 12 nonconsecutive minutes per day of observation.
2Evaluated using a Kruskal–Wallis test.
a,b,cValues in a row with different superscripts had significant differences between the treatment groups (P < 0.05; DSCF multiple comparison analysis).
Feed intake and growth
The monitoring measures of feed intake were investigated to quantify ad lib consumption at the start and across the weaning process. Overall treatment differences were observed for milk and concentrate consumption across the three treatment groups (Table 3; P < 0.05). Relative to baseline, the 10W group had the greatest reduction in milk intake compared to the 8W group, followed by the 6W group (P < 0.05). For concentrate consumption, the 10W group had the greatest increase in concentrate consumption relative to baseline, compared to the 6W group (P < 0.05) but no difference in consumption difference was found between the 8W group and the 6W or 10W groups (P ˃ 0.05). Overall treatment differences for the monitoring measure of growth are reported in Table 3. No treatment differences were found for the outcome measure of body weight relative to baseline (P ˃ 0.05).
The impact of the weaning on feed intake postweaning was documented and reported in Figure 2a. Differences in concentrate intake between treatment groups were observed in weeks 0 (weaning week), 1, and 2 independently across the three treatment groups (P < 0.05). In week 0 (weaning week), no difference was found in concentrate consumption relative to baseline (week −1) across the three treatment groups (P ˃ 0.05). However, in week 1, the 10W treatment had the greatest increase in concentrate consumption relative to baseline (mean ± SD, 491.0 ± 136.59 g/d) compared to the 8W (273.6 ± 148.86 g/d) and 6W groups (188.2 ± 113.18 g/d) (P < 0.05; Figure 2a). In week 2, the increase in concentrate consumption relative to baseline remained different among the three treatments, with the 10W group again showing the greatest increase (728.3 ± 250.6 g/d), followed by the 8W (524.0 ± 209.4 g/d) and 6W groups (439.4 ± 134.6 g/d), respectively (P ≤ 0.05; Figure 2a). As for documenting the impact of weaning on growth around and postweaning, differences in weight between treatment groups were reported in Figure 2b for days −3, 1, 7, and 15 relative to weaning (weaning day = 0). There were no treatment differences for weight relative to baseline (P ˃ 0.05).
Lying behaviors
No treatment differences were found around weaning for the outcome measures of lying time and lying bouts duration (P ˃ 0.05; Table 3). However, the 10W treatment had the smallest reduction in lying bouts duration relative to baseline compared to the 8W (P < 0.05) while no differences between the 6W treatment group and the 8W or 10W treatment groups were found (P ˃ 0.05; Table 3).
Grooming, redirected, and other behaviors
For the outcome measures of behavior, only behaviors in the grooming, redirected, and other categories are presented for overall and day analyses. Behavior categories not presented in text can be found in Supplementary Table S2; overall only.
Grooming behaviors
Overall treatment differences were observed for the variables of self-grooming and allogrooming across the three treatment groups (P < 0.05; Table 3). The 8W treatment group had the greatest increase in self-grooming time over the weaning period relative to baseline, followed by the 10W group (P < 0.05). The 6W treatment was the only group to have a reduction in self-grooming time over the weaning period relative to baseline (P < 0.05). There were no treatment differences for self-grooming frequency (P ˃ 0.05). Over the weaning period, the 6W group had the smallest reduction in allogrooming time and an increase in frequency compared to 8W group (P < 0.05) but no differences in allogrooming time or frequency were found between the 10W group and the 6W or 8W groups (P ˃ 0.05). Treatment differences were also observed on day 12 across the three treatment groups for the variable of self-grooming time (P < 0.05; Table 4). On day 12, the difference in self-grooming time from baseline for the 10W treatment was 20.4 s greater than the 6W treatment per 12-min observation period (P < 0.05). While the 10W and 8W treatments had an increase in self-grooming time relative to baseline on day 12, the 6W treatment had a decrease relative to baseline (P < 0.05) but no difference from baseline was found between 8W treatment and the 6W or 10W treatment groups (P ˃ 0.05).
Redirected behaviors
Overall treatment differences were observed for the variables of cross-sucking, biting and licking, and displacement at the nipple across the three treatment groups (P < 0.05; Table 3). The 6W treatment group had the greatest reduction in cross-sucking frequency and time over the weaning period relative to baseline compared to the 8W and 10W groups (P < 0.05). As for biting and licking frequency and time, the 10W treatment group had the smallest reduction over the weaning period relative to baseline compared to the 8W group (P < 0.05) but no treatment differences were found between the 6W group and the 8W or 10W treatment groups (P ˃ 0.05). Relative to baseline, the 6W group had an increase in displacement at the nipple frequency compared to the 10W group (P < 0.05) but there were no differences found between the 8W group and the 6W or 10W treatment group (P ˃ 0.05). There were also no differences relative to baseline for both nipple sucking frequency and time and digging or pawing frequency (P ˃ 0.05). Treatment differences were also observed independently on day 12 across the three treatment groups for the variables of cross-sucking frequency and time (P < 0.05; Table 4).
On day 12, the 10W treatment group had the greatest increase in cross-sucking frequency and time relative to baseline, followed by the 8W group (P < 0.05). The 6W treatment was the only group to have a reduction in cross-sucking frequency and time over the weaning period relative to baseline (P < 0.05). The 6W treatment had 2.2 fewer occurrences of cross-sucking per 12-min observation period relative to baseline than kids weaned at 8-wk, and 2.7 fewer occurrences than kids weaned at 10-wk. On day 12, the 6W treatment spent 5.3 s shorter engaging in cross-crossing per 12-min observation period relative to baseline than kids weaned at 8 wk and 8.9 s shorter than kids weaned at 10 wk (P < 0.05). No other day differences from baseline were found for the behaviors in the redirected behavior category (P ˃ 0.05).
Other behaviors
Overall treatment differences were observed for the variable of vocalization (P < 0.05; Table 3). The 6W treatment had the greatest increase in vocalization frequency over the weaning period relative to baseline, followed by the 8W group (P < 0.05). The 10W treatment was the only group to have an overall reduction in vocalization frequency over the weaning period relative to baseline (P < 0.05). Treatment differences were also observed on day 0 across the three treatment groups for the variable of vocalization frequency (P < 0.05; Table 4). On day 0, the 6W treatment had 6.6 more vocalizations occurrence per 12-min observation period relative to baseline than kids weaned at 8 wk, and 11.8 more vocalizations occurrence than kids weaned at 10 wk.
Discussion
Feed intake and growth measures
Kids weaned at 10 wk were found to have the greatest reduction from baseline in milk consumption, which was expected given that the 10-wk kids had a higher preweaning milk allowance than the 8-wk followed by the 6-wk kids. Regardless of treatment, all kids continued to drink all the milk offered until the final day of weaning, which seems to indicate that some kids might have continued to drink milk if given the choice and may not all have been ready to voluntarily wean themselves even at 10 wk of age. These results echo what was found in a study investigating voluntary weaning based on calves’ individual ability to and willingness to consume solid feed, where some calves continued to consume milk up to 87 d (de Passillé and Rushen, 2016). However, practical and economical considerations play an important role in the producer’s decisions in implementation of management practices, such that the choice of weaning age may not be established considering solely the willingness of kids to wean themselves; a cost–benefit analysis should be conducted to further support weaning kids at a later age and favor adoption of this practice by producers (Bélanger-Naud et al., 2021a).
The higher preweaning concentrate intake and greater increase from baseline in concentrate consumption of the 10-wk kids compared to the 6-wk kids could be explained by the experience the older kids acquired with consuming solid feed, specifically concentrates, before weaning. This experience prior to weaning for older kids might have acted to facilitate the transition to exclusively solid feed. This has been demonstrated in dairy calves, wherein delayed weaning at 89 d of age compared to 48 d of age reduced the negative effect of weaning on energy intake during and after weaning (de Passillé et al., 2011). This effect could be linked to the fact that ruminants have a nonfunctional rumen at birth and require solid feed intake to trigger rumen growth and maturation (Khan et al., 2011), a process that requires time. This trend was also observed at the week level, during the weaning period in week 0, there was no difference amongst the treatment groups but in the 2 wk after weaning, kids weaned at 10 wk had the highest increase in concentrate consumption relative to baseline. This could further support the idea that kids weaned at an older age have more experience with consuming concentrate due to a higher baseline intake.
In prior literature, the weaning period has been associated with growth stasis (Greenwood and Cafe, 2007; Magistrelli and Rosi, 2009). The extent of growth stasis over the weaning period has been particularly supported for kids weaned earlier (by 36 d of age or at 10 kg) as opposed to later (by 60 d of age or at 15 kg; Palma and Galina, 1995; Luparia et al., 2009). However, this was not reported in the present study as no difference from baseline was found for growth parameters. Comparably, a study by Magistrelli et al. (2013) evaluated the impact of a progressive weaning over 17 d on growth, behavior, and physiological stress indicators of kids at 48 d of age, against unweaned kids of the same age. The results showed no difference between the weaned and unweaned kids on growth parameters, expression of abnormal behavior, or other physiological stress levels (Magistrelli et al., 2011, 2013). Management practices such as ad libitum access to hay, concentrates, and water at all times are likely to minimize the negative impact of stress during the weaning period on growth and on other parameters (Magistrelli et al., 2013). Such management practices were implemented in our study, which could explain the absence of growth stasis we observed in newly weaned kids.
Lying behaviors
Mean lying time across all treatment groups over the weaning period represented 53.9% to 62.6% of daily activity which is similar to that reported by Zobel et al. (2020; 63.2% of daily activity spend lying down prior to weaning, 63.0% postweaning), but much higher than what was reported by Zobel et al. (2015; 8.5 ± 3.2 h/d lying down, or 35.4% of daily activity). In our study, the mean number of lying bouts around the weaning period ranged from 21.4 to 43.3 bouts/d across all treatment groups. Results reported by Zobel et al. (2015) show a much lower number of lying bouts, averaging 8 ± 4 bouts/d. While lying behavior is often used in welfare research as an indicator of comfort (Zobel et al. 2015), it has also been shown to vary with age (Lickliter, 1987); individual variation in dairy goat behavior could also account for the variability observed between studies. Nonetheless, our results corroborate previous findings and may suggest that the different treatments we subjected the goat kids to in our study did not yield negative outcomes on lying behavior.
Grooming, redirected, and other behaviors
Grooming behaviors
Grooming serves many adaptive functions, mainly to clean and condition the hair coat (Hart and Pryor 2004), but also to build social relationships (Val-Laillet et al. 2009). The most apparent purpose for self-grooming relates to coat hygiene, more specifically targeted towards removing ectoparasites, a role which has been long demonstrated in goats (Koch 1988). Higher self-grooming rates are observed in young kids rather than adults due to increased vulnerability to parasites (Hart and Pryor, 2004). Despite coat hygiene being identified as the main role of self-grooming, reductions in time allocated to this behavior have been established as an indicator of pain or discomfort (Hempstead et al., 2018). A study that evaluated posttreatment pain in dairy goat kids across alternative disbudding techniques found that decreases in self-grooming time were present at 1-h posttreatment when plasma cortisol levels were most elevated, with the greatest reduction in self-grooming time observed with the most painful disbudding method (Hempstead et al., 2018). Allogrooming, another grooming behavior, consists in grooming another individual’s coat. Aside from hygiene purposes, this behavior is also considered to play a role in the development and maintenance of social relationships and is thus considered a positive welfare indicator (Val-Laillet et al., 2009). On the other hand, allogrooming is also thought to serve as a mechanism to release stress (Sato et al., 1991). A study observing calves in different environmental conditions found that allogrooming tended to increase when feed was restricted or when the barn was not cleaned (Sato et al., 1991). Moreover, higher rates of allogrooming were observed in tethered cattle compared to loose-housed cattle, perhaps due to habituation to imposed neighbors or boredom (Krohn, 1994). Although data from studies on goats is lacking with regard to allogrooming, it appears that both grooming behaviors can serve as indicators of welfare. In the present study, kids weaned at 6-wk were the only treatment group for which self-grooming time during the weaning period was reduced relative to the baseline, as the 8- and 10-wk weaning groups exhibited increases in self-grooming time after weaning. The reduction relative to baseline that was observed in the present study (−14.9 ± 23.39 seconds per 12-min observation period = −1.24 ± 1.95 min/h) was proportionally smaller than the reduction in self-grooming time observed with painful disbudding method (−8.92 ± 0.0 min/h; Hempstead et al., 2018). However, based on the associations found in prior literature, this reduction in grooming time could nonetheless indicate ineffective coping in early weaned kids, resulting in the reduced presentation of adaptive behaviors we observed. Yet, the results of this study also showed 6-wk weaning kids as having the smallest reduction relative to baseline in allogrooming time after weaning, compared to the 8-wk weaning treatment. Given the role allogrooming could play in releasing stress, our results make it partly unclear whether the differences we observed between the 6- and 8-wk groups are representative of a slightly different mechanism for coping with the stress of weaning in 6-wk treatment kids compared to other groups. Further investigations focusing more closely on grooming behaviors in kids could yield more information on the matter.
Redirected behaviors
The redirected behaviors we observed encompassed many behaviors that are usually directed towards a conspecific (cross-sucking) or towards the environment (biting and licking, nipple sucking, digging or pawing, or displacement at the nipple). Cross-sucking is a non-nutritive sucking behavior that may cause hair loss or inflammation to the afflicted area of a conspecific if prolonged (Sambraus, 1980; observed in calves: Rushen and de Passillé, 1995). Fortunately, cross-sucking can be easily redirected by providing sufficient access to dry teats to fulfill sucking motivation without negative outcomes (Veissier et al., 2002). In a study investigating behavioral indicators of hunger in dairy calves, no difference in cross-sucking was observed between calves fed restricted milk quantities and calves fed ad libitum (De Paula Vieira et al., 2008). In that study, cross-sucking was not an effective indicator of hunger between the treatment groups, with only three cross-sucking events occurring in the entire study period due to the presence of effective outlets for sucking motivation. In the current study, two milk teats were present in each pen to allow both kids to access milk at the same time. In addition, the milk teats were physically separated within the pens to further limit competition, and were kept in place at all times during the weaning process. Yet, this was not sufficient to completely redirect this behavior as occurrences were nonetheless observed in all groups. In the present study, 8- and 10-wk kids have engaged in more cross-sucking relative to baseline than the 6-wk kids, regardless of dry teat availability. It is possible that the older kids were hungrier as a result of a greater decrease in milk allowance during the weaning period; their baseline consumption was greater than the 6-wk kids, which possibly led them to find another source to fulfill their sucking motivation due to unsuccessful sucking at the teat. Another hypothesis is that these kids could have habituated, over the course of the weeks, to sucking for longer; these groups initially drank more milk, which would require sucking for longer to be ingested. They may have needed a longer adaptation period postweaning as a result. However, the 6 wk kids were the only group for which the biting and licking time numerically increased (nonsignificant) compared to their baseline, with the other two groups decreasing the time allocated to these behaviors. Moreover, the displacement at the nipple for the kids weaned at 6 wk increased from their baseline whereas it decreased for the kids weaned at 8 and 10 wk. As such, it is possible that younger kids redirected their need to suckle more frequently towards their environment rather than towards conspecifics, as the kids weaned at an older age did, thus coping with weaning in a slightly different manner. The results we obtained do not allow us to draw any specific conclusion with regard to how these redirected behaviors and if their causes are linked with weaning. Nevertheless, mean cross-sucking time across all treatment groups over the study period occurred only for 1.39 ± 3.42 s per 12-min (=0.12 ± 0.29 min/h) observation period. Proportional to the length of the observation period, this cross-sucking time is much shorter than that observed in previous studies and is unlikely to be associated with negative outcomes (Veissier et al., 2002; Babu et al., 2004).
Other behaviors
Vocalizations, while varied and part of the regular behavioral repertoire of dairy goats, are widely considered an indicator of hunger in dairy cattle when included as part of a weaning study, wherein calves that are fed less milk usually vocalize more than calves fed ad libitum (Watts and Stookey, 2000; Thomas et al., 2001; De Paula Vieira et al., 2008). Increased vocalizations have also been associated with other stress-inducing events not linked to hunger, including social isolation and disbudding procedures (Jensen, 2002; Alvarez and Gutiérrez, 2010; Miranda-de la Lama and Mattiello, 2010). Magistrelli et al. (2013) found that vocalizations doubled in male kids in the immediate postweaning period for kids weaned at 48 d of age compared to unweaned kids and remained elevated until 50 d of age at the end of the study period. These authors interpreted the increased vocalizations after weaning to be a request for milk in response to hunger cues. Based on prior literature, it is also possible that the vocalizations could be in response to ineffective coping with the stress of transitioning to solid feed. While it is also possible that these vocalization differences were due to other factors (time of day, age, etc.), in conjunction with our other findings, the differences observed in vocalizations between groups seem to support the hypothesis that they were performed as a result of discomfort associated with weaning. During weaning in the present study, an increase in vocalizations was observed in kids weaned at 6- and 8-wk while a decrease was observed for the 10-wk kids. More specifically, although the 10 wk group vocalization was highest at baseline, the 6 wk and the 8 wk kids vocalized 2.5× and 1.5× more than the 10 wk kids, respectively, on the day of weaning. Based on prior literature, the higher vocalization observed in the youngest group could indicate stress or hunger over the weaning period. In contrast, the decreased vocalizations observed in kids weaned at 10 wk could indicate that the stress or hunger commonly associated with weaning could be more tolerable when initiated later on in age after an exposure to solid feed was more established.
Limitations
In terms of study limitations, behaviors were only recorded for 12 nonconsecutive minutes per day of observation. While the timing of observation was likely to coincide with a high prevalence of grooming, redirected, or other behaviors (before, during, and after morning feeding), the results can only represent a snapshot of the entire day. Furthermore, due to the real-time nature of the observations, it was only possible to conduct inter-reliability assessments. Our limited sampling schedule combined with live observation could have led to missing behavior events or incorrect coding of behavior events, limiting the extrapolation of the results. The design of the experiment and statistical analysis were chosen based on our target variables, that is; discomfort-related outcome measures based on live behavioral observations around weaning as opposed to continuous measures through video recording across the entire trial period. The latter would have allowed us to apply validated automated technologies to compare differences in behavior more precisely and continuously over the course of the trial (e.g., through the application of deep learning methods: Jiang et al., 2020).
Conclusion
Kids weaned at 10 wk of age had the greatest increase in concentrate consumption, and greatest decrease in vocalizations while both 8- and 10-wk kid’s groups had the greatest increase in self-grooming and cross-sucking. Kids weaned at 6 wk of age were found to have the smallest increase in concentrate consumption and the greatest decrease in self-grooming, and the greatest increase in allogrooming and in redirected behaviors aimed toward the environment. No negative impact of weaning on growth of either group was identified. Overall, while not all results were in line, they tend towards a higher degree of discomfort behaviors (allogrooming, biting/licking, displacement, and vocalizations) in kids weaned earlier (6 wk) compared to later weaning at 8 or 10 wk, while kids weaned later showed higher levels of positive behaviors (lying time, self-grooming). Among the kids weaned at later ages, while the degree of most discomfort behaviors was similar, the difference from baseline in vocalizations for kids weaned at 8 wk of age was greatest. Although the limited amount of relevant literature pertaining to goat kids complicates the interpretation of our results, they seem to tend towards a better capacity of kids weaned at later ages (8 or 10 wk) to cope with the stress of this nutritional transition compared to kids weaned at an early age (6 wk). Further research supporting the development of goat-specific recommendations with regard to the management of weaning would be needed.
Supplementary Material
Acknowledgments
We would like to extend their gratitude to all the students who assisted in the data collection process, notably Claudia Marcela Perdomo Rincon (Master of Veterinary Science, Université de Montréal), Sonia Yacini (Summer Intern at CRSAD), and Cannelle Boyer (Intern from Université de Strasbourg, France). We thank the farm staff who provided animal care during the kidding period and assisted with collection of outcome measures, including Luc Gignac, Marc-André Corriveau, Sébastien Coursol, and Hélène Lavallée. We also thank Dany Cinq-Mars (Université Laval, QC), and Julie Arsenault and Sébastien Buczinski (Université de Montréal, QC) for assisting with the experimental design of the study, and to Véronique Boyer (McGill University, QC) for editing. Funding was partly provided through the Mitacs Accelerate program (PI/co-PI: Vasseur, McGill and Julien, CRSAD, IT12264, IT16238) and through the Canada Research Continuity Emergency Fund (PI: Vasseur, McGill). A grant from Agriculture and and Agri-Food Canada through the AgriScience program also assisted to fund this study (PI: Julien, CRSAD, ID# ASP-018). Funding support was provided as follows: stipend for SBN by Natural Sciences and Engineering Research Council of Canada (NSERC) Canada Graduate Scholarships-Master’s Program and Mitacs Accelerate, grant for animal trial by Agriculture et Agroalimentaire Canada Programme Agri-Science (Principal Investigator: CJ), and grant for manuscript edit work and publication charge by NSERC Discovery Grants—Established Researcher (Principal Investigator: EV).
Glossary
Abbreviations
- ADF
acid detergent fiber
- BRP
bovine respiratory disease
- CP
crude protein
- DM
dry matter
- Kw
weighted Kappa
- ME
metabolizable energy
- NDF
neutral detergent fiber
Contributor Information
Stéphanie Bélanger-Naud, Department of Animal Science, McGill University, Sainte-Anne-de-Bellevue, QC, Canada H9X 3V9.
Tania Wolfe, Department of Animal Science, McGill University, Sainte-Anne-de-Bellevue, QC, Canada H9X 3V9.
Athena Zambelis, Department of Animal Science, McGill University, Sainte-Anne-de-Bellevue, QC, Canada H9X 3V9.
Janie Lévesque, Centre de recherche en sciences animales de Deschambault, Deschambault, QC, Canada G0A 1S0.
Carl Julien, Centre de recherche en sciences animales de Deschambault, Deschambault, QC, Canada G0A 1S0.
Elsa Vasseur, Department of Animal Science, McGill University, Sainte-Anne-de-Bellevue, QC, Canada H9X 3V9.
Conflict of interest statement
The authors declare no conflict of interest.
Literature Cited
- Alvarez, L., and Gutiérrez J... 2010. A first description of the physiological and behavioural responses to disbudding in goat kids. Anim. Welf. 19:55–59. doi: 10.1017/s0962728600001160 [DOI] [Google Scholar]
- Aly, S. S., Love W. J., Williams D. R., Lehenbauer T. W., Van Eenennaam A., Drake C., Kass P. H., and Farver T. B... 2014. Agreement between bovine respiratory disease scoring systems for pre-weaned dairy calves. Anim. Health Res. Rev. 15:148–150. doi: 10.1017/S1466252314000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu, L. K., Pandey H. N., and Sahoo A... 2004. Effect of individual versus group rearing on ethological and physiological responses of crossbred calves. Appl. Anim. Behav. Sci. 87:177–191. doi: 10.1016/j.applanim.2004.01.006 [DOI] [Google Scholar]
- Bath, G. F., and van Wyk J.A... 2009. The five point check© for targeted selective treatment of internal parasites in small ruminants. Small Rumin. Res. 86:6–13. doi: 10.1016/j.smallrumres.2009.09.009 [DOI] [Google Scholar]
- Bélanger-Naud, S., Cinq-Mars D., Julien C., Arsenault J., Buczinski S., Lévesque J., and Vasseur E... 2021a. A survey of dairy goat kid-rearing practices on Canadian farms and their associations with self-reported farm performance. J. Dairy Sci. 104:9999–10009. doi: 10.3168/jds.2020-18663 [DOI] [PubMed] [Google Scholar]
- Bélanger-Naud, S., and Vasseur E... 2021b. Graduate student literature review: current recommendations and scientific knowledge on dairy goat kid rearing practices in intensive production systems in Canada, the United States, and France. J. Dairy Sci. 104:7323–7333. doi: 10.3168/jds.2020-18859 [DOI] [PubMed] [Google Scholar]
- Critchlow, D. E., and Fligner M. A... 1991. On distribution-free multiple comparisons in the one-way analysis of variance. Commun. Stat. Theory Methods 20:127–139. doi: 10.1080/03610929108830487 [DOI] [Google Scholar]
- De Passillé, A. M., and Rushen J... 2016. Using automated feeders to wean calves fed large amounts of milk according to their ability to eat solid feed. J. Dairy Sci. 99:3578–3583. doi: 10.3168/jds.2015-10259 [DOI] [PubMed] [Google Scholar]
- De Passillé, A. M., Borderas T. F., and Rushen J... 2011. Weaning age of calves fed a high milk allowance by automated feeders: effects on feed, water, and energy intake, behavioral signs of hunger, and weight gains. J. Dairy Sci. 94:1401–1408. doi: 10.3168/jds.2010-3441 [DOI] [PubMed] [Google Scholar]
- De Paula Vieira, A., Guesdon V., de Passillé A. M., von Keyserlingk M. A. G., and Weary D. M... 2008. Behavioural indicators of hunger in dairy calves. Appl. Anim. Behav. Sci. 109:180–189. doi: 10.1016/j.applanim.2007.03.006 [DOI] [Google Scholar]
- Dwass, M. 1960. Some k-sample rank-order tests. In: Olkin, I., Ghurye S. G., Hoeffding W., Madow W. G., and Mann H. B., editors. Contributions to probability and statistics. Redwood City, CA, USA: Stanford University Press; p. 198–202. [Google Scholar]
- Gökdal, O., Özuğur A. K., Atay O., and Eren V... 2017. The effects of individual weaning based on birth weight on growth performance and milk yield in dairy goats Turkish. J. Vet. Anim. Sci. 41:672–678. doi: 10.3906/vet-1611-71 [DOI] [Google Scholar]
- Greenwood, P. L., and Cafe L. M... 2007. Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production. Animal. 1:1283–1296. doi: 10.1017/S175173110700050X [DOI] [PubMed] [Google Scholar]
- Hart, B. L., and Pryor P. A... 2004. Developmental and hair-coat determinants of grooming behaviour in goats and sheep. Anim. Behav. 67:11–19. doi: 10.1016/j.anbehav.2003.01.002 [DOI] [Google Scholar]
- Hempstead, M. N., Waas J. R., Stewart M., Cave V. M., and Sutherland M. A... 2017. Behavioural response of dairy goat kids to cautery disbudding. Appl. Anim. Behav. Sci. 194:42–47. doi: 10.1016/j.applanim.2017.04.001 [DOI] [Google Scholar]
- Hempstead, M. N., Waas J. R., Stewart M., Cave V. M., and Sutherland M. A... 2018. Evaluation of alternatives to cautery disbudding of dairy goat kids using behavioural measures of post-treatment pain. Appl. Anim. Behav. Sci. 206:32–38. doi: 10.1016/j.applanim.2018.05.035 [DOI] [PubMed] [Google Scholar]
- Jensen, P. 2002. The etiology of domestic animals: an introductory text. Wallingford, UK: CAB International. [Google Scholar]
- Jiang, M., Rao Y., Zhang J., and Shen Y... 2020. Automatic behavior recognition of group-housed goats using deep learning. Comput. Electron. Agric. 177:105706. doi: 10.1016/j.compag.2020.105706 [DOI] [Google Scholar]
- Khan, M. A., Weary D. M., and von Keyserlingk M. A. G... 2011. Hay intake improves performance and rumen development of calves fed higher quantities of milk. J. Dairy Sci. 94:3547–3553. doi: 10.3168/jds.2010-3871 [DOI] [PubMed] [Google Scholar]
- Koch, H. G. 1988. Suitability of white-tailed deer, cattle, and goats as hosts for the lone star tick, Amblyomma Americanum (Acari: Ixodidae). J. Kans. Entomol. Soc. 61:251–257. http://www.jstor.org/stable/25085000. [Google Scholar]
- Krohn, C. C. 1994. Behaviour of dairy cows kept in extensive (loose housing/pasture) or intensive (tie stall) environments III Grooming, exploration and abnormal behaviour. Appl. Anim. Behav. Sci. 42:73–86. doi: 10.1016/0168-1591(94)90148-1 [DOI] [Google Scholar]
- Lickliter, R. E. 1987. Activity patterns and companion preferences of domestic goat kids. Appl. Anim. Behav. Sci. 19:137–145. doi: 10.1016/0168-1591(87)90210-3 [DOI] [Google Scholar]
- Love, W. J., Lehenbauer T. W., Kass P. H., Van Eenennaam A. L., and Aly S. S... 2014. Development of a novel clinical scoring system for on-farm diagnosis of bovine respiratory disease in pre-weaned dairy calves. PeerJ 2:e238. doi: 10.7717/peerj.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, W. J., Lehenbauer T. W., Van Eenennaam A. L., Drake C. M., Kass P. H., Farver T. B., and Aly S. S... 2016. Sensitivity and specificity of on-farm scoring systems and nasal culture to detect bovine respiratory disease complex in preweaned dairy calves. J. Vet. Diagn. Invest. 28:119–128. doi: 10.1177/1040638715626204 [DOI] [PubMed] [Google Scholar]
- Luparia, F., Martínez M., and Candotti J. J... 2009. Goat kids rearing: solid diets for early weaning. Revista Argentina de Producción Animal. 29(2):89–97. [Google Scholar]
- Magistrelli, D., Aufy A. A., Pinotti L., and Rosi F... 2013. Analysis of weaning‐induced stress in Saanen goat kids. J Anim Physiol. Anim. Nutr. (Berl). 97:732–739. doi: 10.1111/j.1439-0396.2012.01315.x [DOI] [PubMed] [Google Scholar]
- Magistrelli, D., Pinotti L., Rapetti L., and Rosi F... 2011. Ghrelin, insulin and pancreatic activity in the peri‐weaning period of goat kids. J Anim Physiol. Anim. Nutr. (Berl). 95:40–46. doi: 10.1111/j.1439-0396.2009.00980.x [DOI] [PubMed] [Google Scholar]
- Magistrelli, D., Polo Dimel G., and Rosi F... 2010. Endocrine and metabolic traits in goat kids around weaning Ital. J. Anim. Sci. 6:625–627. doi: 10.4081/ijas.2007.1s.625 [DOI] [Google Scholar]
- Magistrelli, D., and Rosi F... 2009. Plasma insulin and IGF-1 and hepatic activity in Saanen goat kids, around weaning. Trop. Subtrop. Agroecosyst. 11:205–208. https://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/77. [Google Scholar]
- Miller, B. A., and Lu C. D... 2019. Current status of global dairy goat production: an overview. Asian-Australas. J. Anim. Sci. 32:1219–1232. doi: 10.5713/ajas.19.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-de la Lama, G. C., and Mattiello S... 2010. The importance of social behaviour for goat welfare in livestock farming. Small Ruminant Res. 90:1–10. doi: 10.1016/j.smallrumres.2010.01.006 [DOI] [Google Scholar]
- Nagpal, A. K., Sigh D., Prasad V. S. S., and Jain P. C... 1995. Effect of weaning age and feeding system on growth performance and carcass traits of male kids in three breeds in India. Small Rumin. Res. 17:45–50. doi: 10.1016/0921-4488(95)00649-6 [DOI] [Google Scholar]
- Palma, J. M., and Galina M. A... 1995. Effect of early and late weaning on the growth of female kids. Small Ruminant Res. 18:33–38. doi: 10.1016/0921-4488(95)00681-a [DOI] [Google Scholar]
- Rushen, J., and de Passillé A. M... 1995. The motivation of non-nutritive sucking in calves, Bos taurus. Anim. Behav. 49:1503–1510. doi: 10.1016/0003-3472(95)90071-3 [DOI] [Google Scholar]
- Sambraus, H. H. 1980. Social rank and milk yield of cows. Milchpraxis. 18:14–16. [Google Scholar]
- Sato, S., Sako S., and Maeda A... 1991. Social licking patterns in cattle (Bos taurus): influence of environmental and social factors. Appl. Anim. Behav. Sci. 32:3–12. doi: 10.1016/s0168-1591(05)80158-3 [DOI] [Google Scholar]
- Steel, R. G. D. 1960. A rank sum test for comparing all pairs of treatments. Technometrics 2:197–207. doi: 10.1080/00401706.1960.10489894 [DOI] [Google Scholar]
- Thomas, T. J., Weary D. M., and Appleby M. C... 2001. Newborn and 5-week-old calves vocalize in response to milk deprivation. Appl. Anim. Behav. Sci. 74:165–173. doi: 10.1016/s0168-1591(01)00164-2 [DOI] [Google Scholar]
- Ugur, F., Savas T., Dosay M., Karabayir A., and Atasoglu C... 2004. Growth and behavioral traits of Turkish Saanen kids weaned at 45 and 60 days. Small Ruminant Res. 52:179–184. doi: 10.1016/s0921-4488(03)00253-0 [DOI] [Google Scholar]
- University of British Columbia Animal Welfare Program (UBC AWP). 2013. UBC Animal Welfare Program: SOP-HOBO data loggers University of British Columbia, Canada: Vancouver, BC; p. 1–23. [Google Scholar]
- Val-Laillet, D., Guesdon V., von Keyserlingk M. A. G., de Passillé A. M., and Rushen J... 2009. Allogrooming in cattle: Relationships between social preferences, feeding displacements and social dominance. Appl. Anim. Behav. Sci. 116:141–149. doi: 10.1016/j.applanim.2008.08.005 [DOI] [Google Scholar]
- Veissier, I., de Passillé A. M., Després G., Rushen J., Charpentier I., Ramirez de la Fe A. R., and Pradel P... 2002. Does nutritive and non-nutritive sucking reduce other oral behaviors and stimulate rest in calves? J. Anim. Sci. 80:2574–2587. doi: 10.2527/2002.80102574x [DOI] [PubMed] [Google Scholar]
- Watts, J. M., and Stookey J. M... 2000. Vocal behaviour in cattle: the animal’s commentary on its biological processes and welfare. Appl. Anim. Behav. Sci. 67:15–33. doi: 10.1016/s0168-1591(99)00108-2 [DOI] [PubMed] [Google Scholar]
- Wormsbecher, L., Bergeron R., Haley D., de Passillé A. M., Rushen J., and Vasseur E... 2017. A method of outdoor housing dairy calves in pairs using individual calf hutches. J. Dairy Sci. 100:7493–7506. doi: 10.3168/jds.2017-12559 [DOI] [PubMed] [Google Scholar]
- Young, A. 2019. Simplified scoring system to identify respiratory disease in dairy calves. Dairy- Cattle Extension. https://dairy-cattle.extension.org/simplified-scoring-system-to-identify-respiratory-disease-in-dairy-calves/. Accessed May 6, 2020.
- Zobel, G., Weary D. M., Leslie K., Chapinal N., and von Keyserlingk M. A. G... 2015. Technical note: Validation of data loggers for recording lying behavior in dairy goats. J. Dairy Sci. 98:1082–1089. doi: 10.3168/jds.2014-8635 [DOI] [PubMed] [Google Scholar]
- Zobel, G., Freeman H., Watson T., Cameron C., and Sutherland M... 2020. Effect of different milk-removal strategies at weaning on feed intake and behavior of goat kids. J. Vet. Behav. 35:62–68. doi: 10.1016/j.jveb.2019.10.004 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.