Abstract
Diabetes mellitus (DM) poses a significant challenge to public health. Effective diabetes self-management education (DSME) interventions may play a pivotal role in the care of people with type 2 diabetes mellitus (T2DM) in low- and middle-income countries (LMICs). A specific up-to-date systematic review is needed to assess the effect of DSME interventions on glycaemic control, cardiometabolic risk, self-management behaviours, and psychosocial well-being among T2DM across LMICs. The MEDLINE, Embase, CINAHL, Global Health, and Cochrane databases were searched on 02 August 2022 and then updated on 10 November 2023 for published randomised controlled trials (RCTs) and quasi-experimental studies. The quality of the studies was assessed, and a random-effect model was used to estimate the pooled effect of diabetes DSME intervention. Heterogeneity (I2) was tested, and subgroup analyses were performed. Egger’s regression test and funnel plots were used to examine publication bias. The risk of bias of the included studies was assessed using the Cochrane risk-of-bias tool for randomized trial (RoB 2). The overall assessment of the evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation approach. A total of 5893 articles were retrieved, and 44 studies (n = 11838) from 21 LMICs met the inclusion criteria. Compared with standard care, pooled analysis showed that DSME effectively reduced the HbA1c level by 0.64% (95% CI: 0.45% to 0.83%) and 1.27% (95% CI: -0.63% to 3.17%) for RCTs and quasi-experimental design studies, respectively. Further, the findings showed an improvement in cardiometabolic risk reduction, diabetes self-management behaviours, and psychosocial well-being. This review suggests that ongoing support alongside individualised face-to-face intervention delivery is favourable for improving overall T2DM management in LMICs, with a special emphasis on countries in the lowest income group.
Introduction
Diabetes mellitus (DM) is a prevalent public health concern [1], with an estimated 537 million (10.5%) adults aged between 20 to 79 affected globally in 2021 [2]. Among those adults, approximately 90% had type 2 diabetes (T2DM) [2, 3]. T2DM is the primary cause of major micro- and macro-vascular complications contributing to significant adverse clinical sequelae, including premature death [4]. In recent decades, the prevalence of T2DM has escalated more rapidly in low- and middle-income countries (LMICs) compared with high-income countries (HICs), with an estimated 79.4% of the global T2DM population residing in LMICs [2]. In 2021, the estimated global annual cost of diabetes treatment was 966 billion USD [2], imposing a substantial health and economic burden on individuals, their families, and healthcare systems [5–10].
The cornerstone of T2DM management is controlling glycosylated haemoglobin (HbA1c) and optimising cardiometabolic risk factors [11]. Self-management of healthy lifestyle strategies, typically involving optimisation of diet, increasing physical activity, and weight loss in those who are overweight and obese, are recommended as first-line interventions; however, these are highly dependent on individual health literacy, self-efficacy, and motivation [12]. For this reason, diabetes education is crucial in optimising self-management strategies by enhancing knowledge as well as by encouraging and consolidating behaviour-change skills [13, 14]. All of these can be addressed using diabetes self-management education (DSME) intervention [15–17]. DSME intervention includes educating patients through the application of self-care strategies (facilitating with the knowledge, skill and ability) in a cost-effective manner to enhance treatment adherence, diabetes self-management (diabetes knowledge and self-efficacy), lifestyle change (diet, physical activity and weight management where appropriate) and psychological well-being (health-related quality of life [HrQoL]) [15, 18, 19].
Previous systematic reviews and meta-analyses conducted in HICs demonstrate that DSME intervention is associated with improved glycaemic control, diabetes knowledge, self-efficacy, HrQoL [20–22], and reduction in all-cause mortality [23]. This includes a 0.4% reduction in HbA1c, a more than 5 mg/dl reduction in total cholesterol (TC) and a more than 1 mmol/L reduction in fasting blood glucose (FBG) when compared to standard care [24–29]. In addition, DSME intervention in HICs showed positive changes in diabetes-specific knowledge and lifestyle [30]. However, generalising evidence from HICs to LMICs needs to be interpreted with caution given cultural, ethnic, and economic disparities, as well as the variations among study populations [30, 31]. Recent reviews conducted in LMICs demonstrated that DSME intervention, short-term nutrition education and/or lifestyle modification intervention may enhance glycaemic control [30, 32–35] and anthropometric measures [33]. However, to our knowledge, limited attempts have been made in the literature to assess the effectiveness of DSME interventions on a comprehensive outcome measures in LMICs [36–39], which include the effectiveness in the change in diabetes control and cardiometabolic risk, diabetes self-management behaviours and psychosocial well-being. Thus, the aim of the present review is to comprehensively assess the effectiveness of DSME intervention on glycaemic control (eg. HbA1c/FBG), cardiometabolic risk factors (eg. WC, BMI, LDL, HDL, TC, TG, SBP, and DBP), diabetes self-management behaviours (eg. diabetes knowledge and self-care) and psychosocial well-being (eg. health-related quality of life) among people with T2DM living in LMICs and to explore intervention characteristics, as well as their mode of delivery, frequency, intensity and duration in relation to the improvement in outcomes.
Methods
This systematic review and meta-analysis was registered with PROSPERO (CRD: 42022364447) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [40] (S1 Table).
Selection criteria
Inclusion criteria
The Participant, Intervention, Comparison, Outcome and Study type (PICOS) framework (S2 Table) informed the inclusion and exclusion criteria. Participants included adults with T2DM residing in LMICs. Any form of educational intervention (e.g. self-management intervention with a variety of educational/behavioural components and/or lifestyle modification to diet and exercise) delivered in an LMIC to people with T2DM and targeting diabetes care management compared with standard care/usual care. Outcomes included any one or combination of the following: glycaemic control (HbA1c/fasting blood glucose [FBG]), cardiometabolic risk body mass index (BMI), waist circumference (WC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), total cholesterol (TC), systolic blood pressure (SBP), diastolic blood pressure (DBP), diabetes knowledge, self-efficacy and health-related quality of life (HrQoL). The study types included either RCT or quasi-experimental designs without language or time restrictions.
Exclusion criteria
Studies reporting on type 1 diabetes and gestational diabetes were excluded. Qualitative studies, editorials, commentary, reviews and case reports were excluded.
Search strategy
Five electronic databases (MEDLINE, Embase, CINAHL, Global Health and Cochrane) were searched from their dates of inception through 02 August 2022 and updated on 10 November 2023 (S3 Table) by two authors (HAC and BNS) in consultation with a senior librarian at Monash University. A range of keywords relating to T2DM including educational intervention and model/tools of diabetes care were used, and the list of LMICs was based on the current World Bank Database [41].
Study selection process
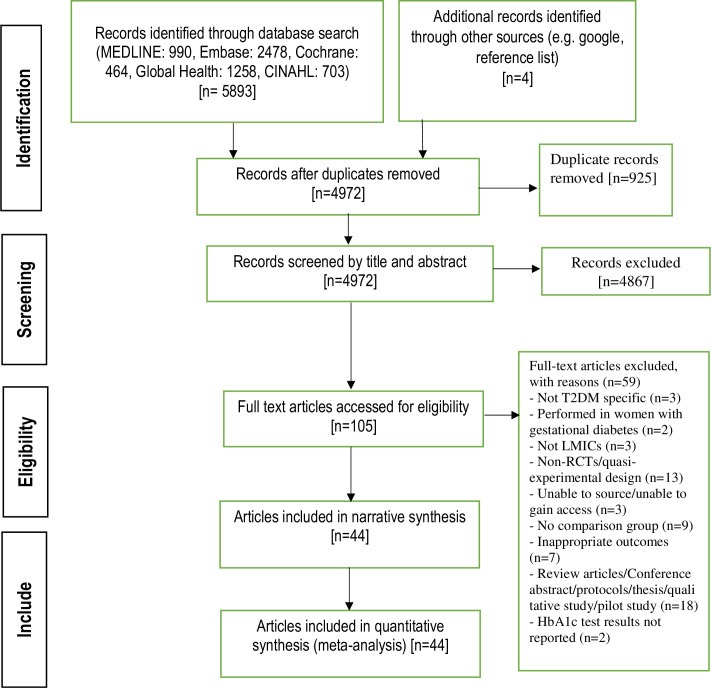
Retrieved articles were stored and managed using the citation software EndNote X20. Following the searches, two authors (HAC and BNS) independently screened all titles as well as abstracts and excluded studies that did not meet the inclusion criteria. A total of 105 articles were selected for a comprehensive full-text review. Following a review for accuracy, two authors (HAC, and BNS) independently reviewed the full text of these 105 articles, and any discrepancy was discussed with a third author (ST) with the supervision of senior author (BB). Finally, a set of 44 articles were selected to determine final article eligibility (Fig 1). A manual search of reference lists of included studies was also performed.
Fig 1. PRISMA flow diagram.
Study outcomes
The primary outcome of this study was to assess any changes in glycaemic control (i.e. HbA1c or fasting blood glucose [FBG]) after intervention. Secondary outcomes were cardiometabolic risk factors (i.e. BMI, WC, HDL, LDL, TG, TC, SBP or DBP), HrQoL and changes in behavioural outcomes (i.e. diabetes knowledge and self-efficacy [S4 Table]).
Data extraction
Data from the included articles were extracted independently by two authors (HAC and BNS) using Microsoft Excel. The following information was extracted: publication details (author/s, year of publication and journal), study characteristics (country, study design, setting, population and sample size), demographics (age of the participants), details of the intervention (type, frequency, intensity, intervention format, duration, number of educational sessions, intervention provider and mode of delivery of the intervention) as well as primary and secondary outcomes (i.e. HbA1c/FBG, BMI, WC, LDL, TG, TC, SBP, DBP, diabetes knowledge, self-efficacy and HrQoL). Discrepancies were discussed and resolved through consensus or arbitration between reviewers.
Quality assessment
Study quality was appraised independently by two authors (HAC and BNS) using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2) [42, 43] for randomised controlled trials, and the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for quasi-experimental studies (non-randomised experimental studies) [44]. The Cochrane’s RoB 2 tool evaluates randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result [42]. For this review, the overall risk of bias was rated as high/low/some concerns, in agreement with the RoB 2 tool. Senior author (BB) was consulted to resolve instances of disagreement. A detailed description of the quality assessment has been provided as supporting information (S5 Fig and S6 Table).
Assessment of certainty of the evidence
Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) was used to evaluate the quality of the evidence [45]. GRADE pro-GDT was employed to summarise the quality of evidence [46]. The certainty of the evidence encompasses consideration of the within-study risk of bias which comprises methodological worth, indirectness of evidence, unexplained heterogeneity, imprecision and, probability of publication bias. The GRADE approach has following four levels of quality such as high-quality evidence that recommends that additional study is very unlikely to change our confidence in the estimate of effect size; moderate quality reflects further research as likely to have a vital impact on the estimate of effect size and may alter the estimate; low quality reveals that further research is very unlikely to have a significant influence on the current estimate of effect size and is likely to change the estimate; and very low quality suggests one is precise indeterminate about the estimate.
Data analysis
All statistical analyses were performed using Stata V.16 (StataCorp, College Station, Texas, USA). A random-effects model was used to estimate pooled mean differences (MD) for HbA1c or FBG and other relevant quantitative data with a 95% confidence interval (CI). Heterogeneity was tested using the ꭓ2-test on Cochran’s Q statistic, which was calculated by means of H and I2 indices. I2 values of over 75% were considered to represent substantial heterogeneity [47]. Subgroup analyses were also performed with the covariates of income level of the country, intervention type, mode of delivery of the intervention and study quality to identify possible sources of heterogeneity. Egger’s regression test and funnel plots were used to examine publication bias [48]. As standard deviation of the mean change from baseline is defined as a common missing outcome data [49], and difficulties in running a meta-analysis without missing standard deviations (SDs). The following formula was used to calculate missing SDschange [50]:
SDchange = . If the SDbaseline and SDfinal values were known, the SDchange value was calculated by assigning a value of 0.7 to the r in the formula, to provide a conservative estimate as undertaken by previous systematic reviews [50]. All data are reported as a mean difference (95% confidence limits). Characteristics of the included studies are reported as mean (±SD) or number percentages as appropriate. In order to readability of the results, all p-values (where applicable) generated in the tables and forest plots have been approximated to three decimal places while reported in the results section. Statistical tests were considered significant at p-values ≤5% (≤0.05)
Results
Selection of studies
A total of 58974 articles were retrieved from the five databases (MEDLINE, Embase, Cochrane, global health and CINAHL) and manual searches. After removing duplicates through title and abstract screening, 105 articles were included for full-text review. Of those, 44 studies (n = 41 RCTs and n = 3 quasi-experimental studies) conducted in 21 LMICs that included 11,838 participants (5,887 in the intervention arm and 5,951 in the comparator arm) (Fig 1).
Characteristics of the included studies
The characteristics of the included studies are reported in Table 1. Of the 44 studies, 21 were conducted in upper-middle-income countries [51–71], 21 in lower-middle-income countries [1, 38, 72–90], and two were conducted in low-income countries [91, 92], as grouped by the World Bank criteria [41]. The studies were conducted in diabetes clinics or hospitals (n = 15 [34%]), public or private hospitals/clinics (n = 21[48%]) and community settings/home-based locations (n = 8 [18%]). All community settings/home-based studies were conducted in the upper-middle-income countries except one from a low-income country [91]. No community-based studies were conducted in the Southeast Asian region. The HbA1c was reported most frequently (n = 42 [95%] studies), followed by FBG (n = 19 [43%]), BMI (n = 23 [52%]), WC (n = 10 [23%]), LDL (n = 18 [41%]), HDL (n = 17 [39%]), TC (n = 17 [39%]), TG (n = 12 [27%]), SBP (n = 20 [45%]), DBP (n = 17 [39%]), diabetes knowledge (n = 10 [23%]), self-efficacy (n = 7 [16%]), and HrQoL (n = 6 [14%]).
Table 1. Summary characteristics of the included studies.
| Sl No | First author (year) | Study design | Country | Country by income | Sample size | Study duration (in weeks) | Age in years Mean (SD) | Mode of delivery of the intervention | Intervention format | Model/theory used | Intervention duration; number of sessions (min/session) | Type of intervention | Intervention provider | Settings | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Askari et al (2018) [72] | Randomised clinical trial | Iran | Lower middle income | 108 (I: 54, C: 54) | 12 | I: 66.45 (3.40); C: 67.11 (3.25) | Face to face and telephone follow up | Group session | BASNEF model | 12 weeks; 8 (70) | Lifestyle modification (focus on diet and exercise) | Researcher | Diabetes centre | HbA1c, FBS, TG, LDL, HDL |
| 2 | Azami et al (2018) [93] | Randomised control trial | Malaysia | Upper middle income | 142 (I: 71, C: 71) | 39 | 54.2 (11.8) | Face to face and telephone follow up | Group session | Nurse led DSME (diabetes self-management education) | 12 weeks; 4 (120) | DSME intervention | Nurse | Urban primary and secondary outpatient endocrine clinic within a teaching hospital | HbA1c, TG, HDL, LDL, SBP, DBP, BMI, quality of life, self-efficacy |
| 3 | Baviskar et al (2021), [1] | Randomised control trial | India | Lower middle income | 80 (I: 40, C: 40) | 26 | NR | Face to face | Group session | Self-care and diabetes realted educational intervention | 24 weeks; NR | DSME intervention | Investigator and Medical Social worker | Malavni Urbran Health Training Centre | HbA1c, FBG, BMI, Quality of Life |
| 4 | Chow et al (2016) [51] | Non-clinical randomised controlled trial | Malaysia | Upper middle income | 150 (I:75, C:75) | 26 | NR | Face to face and telephone reminder | Individual session | Home-based educational intervention | 24 weeks; 2 (62) | DSME intervention | Pharmacist | Home based | HbA1c, diabetes knowledge |
| 5 | Debussche et al (2018) [91] | Randomised control trial | Mali | Low income | 151 (I: 76 C: 75) | 52 | I: 53.9 (9.8); C: 51.1 (9.6) | Face to face | Group and individual session | Self-management educational intervention | 52 weeks; 4 (120) | DSME intervention | Peer educators | Community | HbA1c, BMI, SBP, DBP, WC, diabetes Knowledge |
| 6 | Didarloo et al (2016) [73] | Randomised control trial | Iran | Lower middle income | 90 (I: 45, C:45) | 12 | NR | Face to face | Group session | Collaborative and interactive teaching methods | 12 weeks; 4 (60) | DSME intervention | Nurse | Diabetes clinic | HbA1c, Quality of Life |
| 7 | Ebrahimi et al (2016) [74] | Double blind Randomised clinical trial | Iran | Lower middle income | 106 (I:53, C:53) | 8 | I: 46.97 (5.54); C:48.15 (6.52) | Face to face | Group session | Empowerment approach training | 8 weeks; 5 to 7 (60 to 90) | DSME intervention | Nurse, endocrinologist and nutritionist | Diabetes center | HbA1c |
| 8 | Essien et al (2017) [75] | Individually-randomised controlled trial | Nigeria | Lower middle income | 158 (I: 59, C:59) | 26 | All: 52.7; I:52.6; C: 52.8 | Face to face and Mobile phone messages | Group session | Diet, nutrition and medication related education | 24 weeks; 12 (120) | DSME intervention | Physician and nurse | Endocrinology clinic, Teaching Hospital | HbA1c |
| 9 | Gathu et al (2018) [76] | Non-blinded randomised clinical trial | Kenya | Lower middle income | 140 (I:70, C:70) | 26 | All: 48.8 (9.8); (I: 50.2 (9.93); C: 47.5 (9.54) | Face to face and telephone reminders | Group session | Diabetes self-management education and support (DSMES): an empowerment and interactive teaching model | 24 weeks; 6 (60) | DSME intervention | Family physician and diabetes educator | Family medicine clinic (private, urban-based) of a university hospital | HbA1c, SBP, DBP, BMI |
| 10 | Goldhaber-Fiebert et al (2003) [52] | Randomised conrol trial | Vietnam | Upper middle income | 75 (I:40, C:35) | 12 | I: 60 (10); C: 57 (9) | Face to face | Group session | Community-based nutrition and exercise intervention | 12 weeks; 11 (90) | Lifestyle modification (focus on diet and exercise) | Physician | Community centres | HbA1c, FBG, BMI, SBP, DBP, TC, HDLc, LDLc, TG |
| 11 | Goodarzi et al (2012) [77] | Randomised conrol trial | Iran | Lower middle income | 100 (I:50, C:50) | 12 | I: 50.98 (10.32); C: 56.71 (9.77) | Text message | Individual session | Distance education via mobile phone text messaging | 12 weeks; 48 (messages) | DSME intervention | Researcher | Hospital | HBA1c, TC, HDL, LDLc, TG, Knowledge, self-efficacy |
| 12 | Grillo et al (2016) [53] | Single-center, parallel-group, randomised study | Brazil | Upper middle income | 131 (I: 69, C:62) | 54 | I: 61.7 (9.9); C: 63.2 (9.7) | Face to face | Group session | Education on diabetes care | 7 weeks; 7 (120) | DSME intervention | Nurse | Primary care unit | HbA1c, BMI, WC, SBP, DBP, TC, LDL, HDL, TG |
| 13 | Hosseini et al (2017) [78] | Randomised control trial | Iran | Lower middle income | 106 (I:53, C: 53) | 26 | I: 51.55 (8.3); C: 58.09 (1.6) | Face to face | Group session | PRECEDE model | 4 weeks; 4 (120) | DSME intervention | General physician and specialist in health education and promotion | Diabetes clinic | HbA1c, BMI |
| 14 | Huo et al (2019) [55] | Randomised clinical trial | China | Upper middle income | 502 (I: 251, C: 251) | 26 | All: 59.5 | Text message | Individual session | A text messaging–based secondary prevention program with the regular automatic delivery of text messages. | 26 weeks; 156 (text messages) | DSME intervention | Text messages | Hospital | HbA1c, FBG, SBP, LDL, BMI |
| 15 | Jain et al (2018) [79] | Open-label randomised controlled trial | India | Lower middle income | 299 (I: 153, C:146) | 24 | I: 55.69 (10.94); C:57.42 (10.95) | Face to face and telephone reminder | Individual session | Combining face-to-face interaction with telephonic reminders by community health workers | 24 weeks; 4 (home visits) | DSME intervention | Community health workers | Tertiary teaching institute | HbA1c, FBS, SBP, DBP, BMI, WC, TC, TG, LDLC, HDL |
| 16 | Jayasuria et al, (2015) [80] | Randomised control trial | Sri Lanka | Lower middle income | 87 (I: 43, C: 42) | 26 | All: 51.4 (7.2) | Face to face | Group and individual session | Diabetes Self-Management-Sri Lanka (DSM-SL) model | 26 weeks; 9 (60) | Lifestyle modification (diet and exercise) | Physician and nurse | Colombo North Teaching Hospital | HbA1c, SBP, TC, LDL, HDL, BMI, self-efficacy |
| 17 | Jiang et al (2019) [56] | Multicentre randomised controlled trial | China | Upper middle income | 265 (I: 133, C: 132) | 26 | All: 56.91 (10.05) | Face to face | Group session | Structured education programme Self-Efficacy for Diabetes (C-SED) Diabetes Distress Scale (C-DDS) Summary of Diabetes Self Care Activities (C-SDSCA) |
26 weeks; 4 (60 to 90) | DSME intervention | Physician and nurse | Multicentre at Bejing, Fujiam, Jiangxi | HbA1c, WC, BMI, blood pressure, TC, TG, LDL, HDL, diabetes knowledge, self-efficacy |
| 18 | Ju et al (2018) [57] | Cluster randomised control trial | China | Upper middle income | 400 (I:200, C:200) | 52 | I: 67.8 (7.4); C: 68.8 (8) | Face to face | Group session | A community based peer support programe | 52 weeks; 12 (120) | DSME intervention | Peer support/Leaders | Eight community health centres | HBA1c, FBG |
| 19 | Kong et al (2019) [58] | Group Randomized Experimental Study | China | Upper middle income | 278 (I: 142, C: 136) | 39 | I: 69.12 (10.54); C: 71.48 (8.79) | Face to face | Group session | Chronic Care Model | 39 weeks; 9 (NR) | DSME intervention | Physician, health manager and public health assistant | Community health service center | HbA1c, SBP, DBP, BMI, TC, LDL, HDL |
| 20 | Lamptey et al (2023) [38] | Single-blind randomised parallel comparator controlled multi-centre trial | Ghana | Lower middle income | 206 (I:103; C:103) | 13 | I: 59; C: 57 | Face to face | Group session | DESMOND: EXTENDing availability of self-management structured education programmes | 13 weeks; 1 (720) | DSME intervention | Educator | Hospitals | HbA1c, WC, SBP, DBP, PAID |
| 21 | Li et al (2016) [59] | Randomized controlled trial | China | Upper middle income | 196 (I: 98, C: 98) | 4 | I: 59.1 (4.6); C: 58.3 (4.1) | Face to face | Group session | Structured diet and/or exercise program (SDEP) | 4 weeks; NR (NR) | DSME intervention | Health educators, doctors, and nutritionists | Hospital | HbA1c, FPG, BMI, TG, TC, HDL, LDL |
| 22 | Lou et al (2020) [60] | Randomised control trial | China | Upper middle income | 1095 (I: 563, C: 532) | 104 | 66.5 (8.7) | Face to face | Group session | Clinic-based intensified diabetes management model (C-IDM) | GPs and nurses: 24 weeks; NR (NR) Patients with diabetes: 78 weeks; 18 (NR) | DSME intervention | Not stated | Disease control centers, general hospitals and local clinics | HbA1c, FBG, SBP, DBP, BMI, TG, TC, HDL, LDL |
| 23 | Mohammadi et al (2018) [81] | A matched-pair design randomized controlled trial | Iran | Lower middle income | 240 (I: 120, C: 120) | 48 | I: 51.2 (6.2); C: 51.4 (6.1) | Face to face | Group session | Health Belief Model (HBM) | 12 weeks; 8 (120) | DSME intervention | Not stated | Golestan Hospital outpatient diabetes clinic | HbA1c, FBS, BMI, TC, TG, LDL, HDL, nutrition knowledge, quality of life, self-efficacy |
| 24 | Muchiri et al (2016) [61] | Randomised control trial | South Africa | Upper middle income | 82 (I: 41, C: 41) | 52 | I: 59·4 (6.9); C: 58·2 (8.0) | Face to face | Group session | Nutrition education | 52 weeks; 8 (120 to 180) and follow-up 6 (90) | DSME intervention | Health professionals | Community health centres | HbA1c, FBS, BMI, TC, TG, LDL, HDL |
| 25 | Myers et al (2017) [82] | Cluster randomised control trial | India | Lower middle income | 239 (I: 85, C: 154) | 52 | 46.3 (9.5) | Face to face | Group session | Nutrition practice guidelines | 24 weeks; NR (NR) | Lifestyle modification (focus on diet) | Dietitian | Diabetes centres hospitals | HbA1c, BMI, TC, LDL, HDL, TG |
| 26 | Mash et al (2014) [62] | Pragmatic clustered randomized controlled trial | South Africa | Upper middle income | 1570 (I: 710, C: 860) | 52 | I: 55.8 (11.5); C: 56.4 (11.6) | Face to face | Group session | Diabetes education programme | 30 weeks; 4 (60) | DSME intervention | Educator | Community health centres | HbA1c, SBP, DBP, WC, TC, self-efficacy |
| 27 | Ojieabu et al (2017) [83] | Randomised control trial | Nigeria | Lower middle income | 150 (I:75, C:75) | 17 | Face to face | Group session | Intervention of medication and treatment adherence | 17 weeks; 4 (NR) | DSME intervention | Pharmacist | Endocrinology Clinic, Teaching Hospital | FBS, BMI, SBP, DBP | |
| 28 | Ramadas et al (2018) [63] | Multi-centre randomised control trial | Malaysia | Upper middle income | 128 (I: 66, C: 62) | 104 | I:49.6 (10.7); C:51.5 (10.3) | Web based | Web session | Malaysian Dietary Intervention for People with Type 2 Diabetes: An e-Approach (myDIDeA) | 26 weeks; 12 (12) | Lifestyle modification (focus on diet) | Nutritionist | Public hospital | HbA1c, FBG, diabetes knowledge |
| 29 | Ramli et al (2016) [64] | Pragmatic cluster randomised controlled trial | Malaysia | Upper middle income | 888 (I: 471, C:417) | 104 | I: 58 (0.48); C: 57 (0.5) | Face to face | Group session | EMPOWER-PAR (Participatory action research) interventions | 52 weeks; 2 (NR) | DSME intervention | Physician, nurse, pharmacist and dietitian/nutritionist | Public primary care clinics | HbA1c, BMI, SBP, DBP, WC, TC, TG, LDL, HDL |
| 30 | Samtia et al (2013) [84] | Randomized study | Pakistan | Lower middle income | 344 (I: 174, C: 170) | 20 | I: 46.1; C: 42.3 | Face to face | Group session | Intervention regarding disease knowledge and self-care | 20 weeks; NR (NR) | DSME intervention | Physician and pharmacist | Diabetes clinic at hospital | HbA1c, FBS, BMI |
| 31 | Sanaeinasab et al (2021) [85] | Randomised controlled trial | Iran | Lower middle income | 80 (I: 40, C: 40) | 30 | All: 50.7 (5.9) | Face to face | Group session | Comprehensive systematic health education and promotion (SHEP) model | 7 weeks; 6 (90) | DSME intervention | Not stated | Diabetic clinics | HBA1c, FBG, BMI, SBP, DBP, TC, HDL, LDL, TG |
| 32 | Salahshouri (2018) [86] | Randomised control trial | Iran | Lower middle income | 145 (I: 73; C: 72) | 26 | I: 55.93 (12.4); C: 54.53 (9.43) | Face to face | Group session | Intervention based on psychological factors and nutrition | NR weeks; 8 (60) | Lifestyle modification (focus on diet) | Internal specialists, dietitians, diabetes experts, a psychologist, as well as a religious expert | Diabetic clinics and healthcare centres | HbA1c, FBS, self-efficacy |
| 33 | Tan et al (2011) [65] | Single-blind randomised control trial | Malaysia | Upper middle income | 164 (I:82, C:82) | 12 | I: 54 (9.94); C:54 (10.74) | Face to face and telephone follow up | Group session | Self-efficacy theory | 12 weeks; 3 (45) | DSME intervention | Not stated | Govt state hospital | HbA1c, diabetes knowledge, self-efficacy |
| 34 | Thanh et al (2021) [87] | Randomized controlled single-center trial | Vietnam | Lower middle income | 364 (I: 182, C: 182) | 52 | All: 62.2 (9.3) | Face to face | Group session | Education on diet, exercise, drug therapy and adherence | 12 weeks; 3 (45) | DSME intervention | Medical staff educators | Diabetes clinic | HbA1c, FBG, SBP |
| 35 | Wattana et al (2007) [66] | Randomised controlled trial | Thailand | Upper middle income | 147 (I:75, C:72) | 26 | I: 58.40 (10.05); C: 55.14 (10.22) | Face to face | Group and individual session | Diabetes self-efficacy and diabetes self-management program | 24 weeks; 5 (90 to 120) and one-off 2 home visits (45) | DSME intervention | Physician and researcher | Diabetic clinics | HbA1c, HrQol |
| 36 | Whittemore et al (2020) [67] | Randomised control trial | Mexico | Upper middle income | 47 (I: 26, C: 21) | 52 | 55.35 (8.75) | Face to face and follow up by phone calls | Group session | Si Yo Puedo DSME program | 52 weeks; 7 (NR) and phone call every 2 weeks and text/picture messages sent daily for 6 months | DSME intervention | Nurse and social worker | Seguro Popular clinics | HbA1c, BMI, SBP, DBP, self-efficacy |
| 37 | Wichit et al (2017) [68] | Randomised controlled trial | Thailand | Upper middle income | 140 (I:70, C:70) | 13 | I: 61.3 (11.6); C: 55.5 (10.5) | Face to face, home visit and telephone follow up | Group session | Self-efficacy theory | 9 weeks; 3 (120) | DSME intervention | Nurse | Hospital | HbA1c, diabetes knowledge, HrQoL |
| 38 | Yan et al (2014) [92] | Randomised study | Mozambique | Low income | 41(I: 31, C: 10) | 12 | I: 53 (2); C: 55 (3) | Face to face | Group session | Exercise training intervention | 12 weeks; 36 to 60 (45) | Lifestyle modification (focus on exercise) | Not stated | Diabetes clinic | HbA1c, BMI, WC, SBP, DBP |
| 39 | Zhang et al (2018) [69] | Randomised study | China | Upper middle income | 998(I:498, C: 500) | 348 | I: 50.8 (14.3); C: 52.6 (13.2) | Face to face | Group and individual session | Intervention on nutrition therapy, individualized exercise program, screening of complications | 104 weeks; 24 (NR) | DSME intervention | Physician | Hospital | HbA1c, BMI, SBP, DBP, TC, HDL, LDL |
| 40 | Zheng et al (2019) [70] | Randomised controlled trial | China | Upper middle income | 60 (I: 30, C:30) | 104 | 52.22 (11.32) | Face to face | Group session | Diabetes self-management education programme | 104 weeks; 2 (45) | DSME intervention | Therapist guidance | Hospital | HbA1c, FBG |
| 41 | Zhong et al (2015) [71] | Randomised study | China | Upper middle income | 726 (I: 365; C: 361) | 64 | Face to face | Group session | Peer leader–support program for diabetes management | 24 weeks; 12 (120) | DSME intervention | Peer leaders and staff of Community Health Service Centers (CHSCs) | Community | FBS, BMI, SBP, DBP, diabetes knowledge, self-efficacy | |
| 42 | Al-Halaweh et al (2019) [88] | Quasi-experimental study | Palestine | Lower middle income | 200 (I: 100; C: 100) | 52 | I: 56.58 (8.76); C: 57.9 (7.79) | Face to face | Group and individual session | Diabetes comprehensive care model (DCCM) | 52 weeks; 4 (NR) | DSME intervention | Team of internal specialists, dietitians, diabetes experts, psychologist, and religious expert | Mobile diabetes clinic | Wt, Ht, BP, HbA1c, TC, Creatinine, Microalbuminuria |
| 43 | Pamungkas et al (2020) [89] | Quasi-experimental research | Indonesia | Lower middle income | 60 (I: 30; C:30) | 12 | I: 56.5 (7.63); C: 54.2 (9.20) | Face to face | Group session | The diabetes mellitus self-management (DMSM) based coaching program | 12 weeks; 3 (NR) and 1 (home visit) | DSME intervention | Researcher | Public health centers | HbA1c, SBP, DBP, BMI, TC, HDL, LDL |
| 44 | Kumari et al (2018) [90] | Quasi-experimental prospetive trial | India | Lower middle income | 202 (I:102; C: 100) | 65 | I: 51.9 (9.3); C: 54 (8.6) | Face to face | Group and individual session | Lifestyle intervention holistic model (LIHM) | 52 weeks; 6 (10 to 15) | Lifestyle modification (focus on diet) | Dietician, diabetes educator, physical trainer and diabetologist | Delhi Diabetes Research Centre | HbA1c, blood sugar fasting, blood sugar postprandial |
The sample size in the studies ranged from 41 [92] to 1,570 [62], and the average age of the participants was 55 (SD: 6, range 42 to 71 years). The intervention durations ranged from four [59] to 348 weeks [69], with two-thirds (66.6%) of the studies lasting six months in duration. Standard care/usual care comprised the current standard of care as defined by the local programme or setting.
Intervention characteristics
Overall, the majority of interventions utilised a behaviour-change approach focused on building knowledge, self-efficacy and self-management skills through counselling, coaching, brainstorming or supporting the control of T2DM and its related complications [S5 Table]. Five trials used DM self-management-based coaching programmes [54, 67, 80, 89, 91], four trials used the empowerment approach and interactive teaching model [63, 64, 74, 76], and three used the theory of self-efficacy as a theory or model to underpin intervention content [65, 66, 68]. Each of the following models was used by one trial only: the beliefs, attitudes, subjective norms and enabling factors (BASNEF) model [72]; the predisposing, reinforcing and enabling constructs in educational diagnosis and evaluation (PRECEDE) model [78]; the chronic care model [58]; clinic-based intensified diabetes management model (C-IDM) [60]; the health-belief model [81]; the comprehensive systematic health education and promotion (SHEP) model [85]; the diabetes comprehensive care model (DCCM) [88]; the structured DSME model [38] and the lifestyle intervention holistic model (LIHM) [90]. The remaining 23 trials [1, 51–53, 55–57, 59, 61, 62, 69–71, 73, 75, 77, 79, 82–84, 86, 87, 92] cited no theoretical framework or model used to inform the intervention designs.
Approximately 73% (n = 32) of the interventions were delivered using a face-to-face format, 20% (n = 9) utilising face-to-face intervention with telephone follow-up and 7% (n = 3) using a remotely delivered text message/web-based intervention. Intervention was delivered by healthcare professionals (e.g. physician, nurse, pharmacist, health educator, dietitian or nutritionist) in 32 trials [1, 38, 51–54, 56, 58, 59, 61–64, 66–70, 73–76, 78–80, 82–84, 86–88, 90], by the research team in three trials [72, 77, 89], by peer leaders or lay facilitators in three trials [57, 71, 91] and by trained educators in one trial [62]. Five trials did not report the type of intervention facilitator [60, 65, 81, 85, 92]. The intervention formats included groups (n = 33 [75%]), individuals (n = 4 [9%]), a combination of groups and individuals (n = 6 [14%]) and web-based (n = 1 [2%]) intervention strategies.
Effect of DSME intervention on HbA1c and FBG control
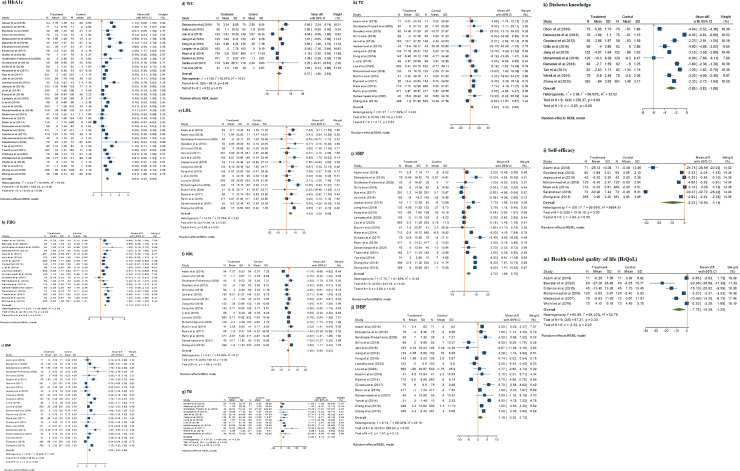
Of 41 RCT studies, 39 reported HbA1c (n = 10,500 participants). Upon meta-analysis, intervention significantly lowered HbA1c levels compared to the control, with a MD of 0.64% (95% CI: 0.64% to 0.83%; p = 0.001). Heterogeneity was very high between the studies (I2 = 94%) with no publication bias (Egger’s regression test, p = 0.068) (Fig 2 and Table 2).
Fig 2.
Meta-analysis results showing the effect of DSME interventions on clinical outcomes (a) HBA1c (b) FBG (c) BMI (d) WC (e) LDL (f) HDL (g) TG (h) TC (i) SBP (j) DBP, (k) diabetes knowledge, (l) self-efficacy, and (m) health-related quality of life of RCTs studies [Data are reported as mean difference (95% confidence limits)].
Table 2. Summary results.
| Study design | Outcome types | Measures | n | Mean change difference (with 95% CI), p-value | Effect of intervention | Heterogeneity (I2 in %) | Publication bias (Egger’s regression test p) |
|---|---|---|---|---|---|---|---|
| RCTs | Clinical | HbA1c | 39 | 0.64 (0.45, 0.83), 0.001 | Effective | 94 | 0.0680 |
| FBG | 19 | 0.74 (0.57, 0.91), 0.001 | Effective | 5996 | 0.5927 | ||
| Metabolic risk factors | BMI | 23 | 0.60 (0.32, 0.88), 0.001 | Effective | 75 | 0.1738 | |
| WC | 10 | 0.37 (-1.89, 2.63), 0.001 | Effective | 93.01 | 0.6884 | ||
| LDL | 18 | 4.33 (2.33–6.65), 0.001 | Effective | 71 | 0.0758 | ||
| HDL* | 17 | -1.35 (-2.69, 0.02), 0.05 | Effective | 84.06 | 0.2715 | ||
| TC | 17 | 4.50 (0.32, 8.68), 0.03 | Effective | 779 | 0.5804 | ||
| TG | 12 | 14.80 (8.18, 21.43), 0.001 | Effective | 69 | 0.0535 | ||
| SBP | 20 | 3.72 (1.69, 5.75), 0.001 | Effective | 92 | 0.8676 | ||
| DBP | 17 | 1.19 (-0.35, 2.73), 0.13 | Effective | 96 | 0.5148 | ||
| Diabetes self-managemnt behaviours | Diabetes knowledge* | 10 | -2.85 (-3.83, -1.79), 0.001 | Effective | 97 | 0.0070 | |
| Self-efficacy* | 7 | -9.23 (-18.60, 0.14), 0.001 | Effective | 99 | 0.0001 | ||
| Psychosocial | HrQoL* | 6 | -7.78 (-14.36, -1.20), 0.02 | Effective | 98 | 0.0005 | |
| Quasi-experimental design study | Clinical | HbA1c | 3 | 1.27 (-0.63, 3.17), 0.19 | Effective | 97 | 0.4515 |
*Negative results consider the positive effect of the intervention
Among 19 studies (n = 5,370 patients) that reported FBG, an overall decrease by 0.74 mmol/L (95% CI: 0.57% to 0.91%; p < 0.001) was observed in the intervention as compared with the control, with moderate heterogeneity (I2 = 59%) and no publication bias (Egger’s regression test, p = 0.592) (Table 2).
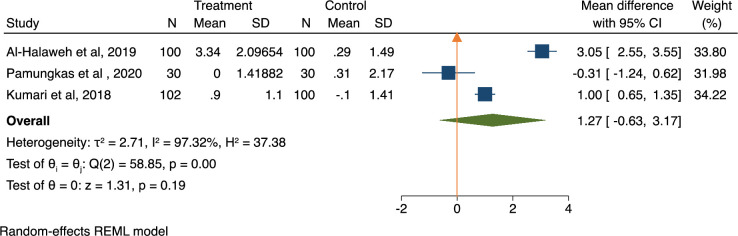
In trials with quasi-experimental designs, the findings showed a mean reduction in HbA1c of 1.27% (95% CI: -0.63% to 3.17%; p = 0.19) in the intervention as compared to the control (Fig 3). The I2 indicator was 97%, indicating a high heterogeneity with no publication bias (Egger’s regression test, p = 0.451) (Table 2). These studies did not report FBG levels.
Fig 3. Meta-analysis results showing the effect of DSME interventions on glycaemic control (HbA1c) of quasi-experimental studies.
Effect of DSME interventions on cardiometabolic risk factors
DSME intervention reduced BMI by 0.60 kg/m2 (95% CI: 0.32% to 0.88%; p = 0.001, I2 = 75.33%) in 23 studies comprising 7,253 participants (Fig 2). Similarly, the results presented in Table 2 and forest plots showed a positive intervention effect on all cardiometabolic risk factors: WC (n = 4,173, MD 0.37, 95% CI: -1.89% to 2.63%; p = 0.001, I2 = 93%), LDL (n = 5803, MD 4.33, 95% CI: 2.33% to 6.65%; p = 0.001, I2 = 71%), HDL (n = 5301, MD -1.35, 95% CI: -2.69% to -0.02%; p = 0.05, I2 = 84.06%), TG (n = 6763, MD 14.80. 95% CI: 8.18% to 21.43%; p < 0.001, I2 = 69%), TC (n = 6,763, MD 4.50, 95% CI: 0.32% to 8.68%; p = 0.03, I2 = 779%), SBP (n = 8,128 MD 3.93, 95% CI: 1.83% to 6.04%; p <0.001, I2 = 926%) and DBP (n = 7,177, MD 1.19, 95% CI: -0.35% to 2.73%; p = 0.13, I2 = 96%). Moderate-to-high heterogeneity was observed across all forest-plot analyses of cardiometabolic risk factors.
The effect of DSME intervention on diabetes knowledge, self-efficacy and HrQoL
Ten studies (n = 2,195) that evaluated knowledge of diabetes showed an improvement by MD of -2.85 (95% CI: -3.83% to -1.86%; p<0.001, I2 = 97%) with presence of publication bias (Egger’s regression test, p = 0. 0.007) (Fig 2). Impact on self-efficacy was addressed in seven studies (n = 1,588), showing an increase by 9.23 (95% CI: -18.60% to 0.14%; p = 0.05, I2 = 99%) with presence of publication bias (Egger’s regression test, p = 0.0070) (Fig 2). Six trials (n = 839) that reported HrQoL showed improvement by -7.78 (95% CI: -14.36% to –1.20%; p = 0·02, I2 = 98%). Publication bias was present in these studies (Egger’s regression test, p = 0.0005) (Fig 2).
Subgroup/Sensitivity analysis
Moderate-to-high heterogeneity was observed across the studies regarding primary as well as secondary outcomes. In order to identify the sources of heterogeneity, subgroup/sensitivity analysis was conducted for the DSME intervention by the income level of the country, intervention type, mode of delivery of intervention and quality of the studies. As outlined in S1 Fig, DSME intervention showed that lower-middle-income countries had improvement in HbA1c with a MD of 0.75% (95% CI: 0.45% to 1.06%; p<0.001, I2 = 92%). Further, lifestyle modification (i.e. diet and/or exercise) intervention showed a greater effect on HbA1c reduction (MD: 0.69%, 95% 0.22% to 1.16%; p<0.001, I2 = 78%) than DSME interventions (MD: 0.63%, 95% CI: 0.42 to 0.86; p<0.001, I2 = 95%) (Table 3 and S2 Fig). In addition, subgroup analysis by mode of delivery of intervention showed that face-to-face intervention with periodic telephone follow-up had the highest efficacy on HbA1c reduction (MD: 1.02%, 95% CI: 0.63% to 1.40%; p<0.001, I2 = 86%) followed by face-to-face intervention alone (MD: 0.56%, 95% CI:0.32% to 0.80%; p<0.001, I2 = 95%) and text message or web-based intervention (MD: 0.33%, 95% CI: 0.17% to 0.49%; p = 0.35, I2 = 0.00) (Table 3 and S3 Fig). The quality of the trials with some concerns showed (S4 Fig) reduction in HbA1c with a MD of 0.66% (95% CI: 0.41% to 0.90%, p<0.001, I2 = 93%) compared with trails rated as high or weak. The S1–S4 Figs present subgroup analyses for BMI and lipid profiles (LDL, HDL, TG and TC) by the income level of the country, intervention type, mode of delivery of the intervention and quality of the study. In studies from low-income countries (MD: 0.87, 95% CI: -0.48% to 2.22%; p = 0.05, I2 = 75%), DSME intervention (MD: 0.63, 95% CI: 0.31% to 0.94%; p<0.001, I2 = 78%), face-to-face intervention (MD: 0.71, 95% CI: 0.41% to 1.01%; p<0.001, I2 = 74%) and trials evaluated as high risk (MD: 0.68, 95% CI: 0.18% to 1.18%, p<0.001; I2 = 82%) showed a better BMI reduction. Further, studies conducted in lower-middle income countries presented an improvement in LDL (MD: 7.32%, CI: 3.50% to 11.15%; p = 0.05, I2 = 56%), HDL (MD: -3.12, 95% CI: -5.62% to -0.62%; p<0.001, I2 = 89%), TC (MD:8.72, 95% CI: 0.88% to 18.32%; p<0.001, I2 = 83%) and TG (MD: 21.73, 95% CI: 15.26% to 28.19%; p<0.19, I2 = 10.66%).
Table 3. Subgroup analysis, based on the income level of the country, intervention type, mode of delivery of the intervention, and quality of the studies.
| Subgroup | HbA1c | BMI | LDL | HDL | TG | TC |
|---|---|---|---|---|---|---|
| Income level of the country | ||||||
| Low income | MD: 0.62 (0.13–1.11), I2 67% | MD: 0.87 (-0.48–2.22), I2 75% | N/A | N/A | N/A | N/A |
| Lower middle income | MD: 0.75 (0.45–1.06), I2 92% | MD: 0.69 (0.32–1.06), I2 46% | MD: 7.32 (3.50–11.15), I2 56% | MD: -3.12 (-5.62 –-0.62), I2 88% | MD: 21.73 (15.26–28.19), I2 10% | MD: 8.72 (-0.88–18.32), I2 83% |
| Upper middle income | MD: 0.55 (0.28–0.83), I2 94% | MD: 0.53 (0.10–0.96), I2 83% | MD: 2.78 (0.20–6.65), I2 71% | MD: -0.34 (-1.69–1.00), I2 69 | MD: 8.85 (8.21–9.48), I2 0.00% | MD: 2.05 (-1.99–6.09), I2 660% |
| Intervention type | ||||||
| Lifestyle modifications (diet and/or exercise) | MD: 0.69 (0.22–1.16), I2 78% | MD:0.35 (-0.03–0.74), I2 0.00% | MD:1.63 (-5.58–8.84), I2 716% | MD: -1.77 (-6.75–3.22), I2 91% | MD:42.24 (-4.21–88.70), I2 70% | MD: 0.11 (-17.99–18.22), I2 78% |
| Self-management | MD: 0.63 (0.42–0.85), I2 95% | MD:0.63 (0.31–0.94), I2 78% | MD: 4.33 (2.00–6.65), I2 71% | MD: -1.14 (-2.38–0.11), I2 74% | MD:13.64 (6.52–20.77), I2 69% | MD: 4.86 (0.38–9.35), I2 77% |
| Mode of delivery of the intervention | ||||||
| Face-to-face | MD: 0.55 (0.32–0.78), I2 94% | MD: 0.71 (0.41–1.01), I2 74% | MD: 3.77 (0.77–6.77), I2 75% | MD: -0.50 (-1.68–0.68), I2 76% | MD: 16.93 (8.19–25.68), I2 74% | MD: 3.15 (-1.08–7.39), I2 754% |
| Face-to-face and telephone follow up | MD: 1.02 (0.63–1.40), I2 86% | MD: 0.03 (0.56–0.62), I2 0.00% | MD: 6.79 (3.58–10.01), I2 0.00% | MD: -4.18 (-7.46 - -0.89), I2 70% | MD: 11.30 (-1.79–24.39), I2 62% | MD: 5.44 (-1.62–12.51), I2 0.00% |
| Text messages or web-based | MD: 0.33 (0.17–0.49), I2 0.00% | MD: -0.20 (-0.65–0.25), I2 N/A* | MD: 3.87 (-5.51–13.25). I2 708% | MD: -3.32 (-6.63–0.0.01), I2.%N/A* | MD: 15.22 (-15.33–45.77), I2.% N/A* | MD: 25.30 (13.73–36.87), I2.% NA* |
| Quality of the studies | ||||||
| High | MD: 0.60 (0.30–0.91), I2 94% | MD: 0.68 (0.18–1.18), I2 82% | MD: 5.40 (-2.26–8.55), I2 60% | MD: -1.87 (-5.09–1.34), I2 92% | MD:-2.36 (-10.13–5.42), I2 71%* | MD: -2.36 (-10.13–5.42), I2 71% |
| Some concerns | MD: 0.66 (0.41–0.90), I2 94% | MD: 0.49 (0.19–0.78), I2 75% | MD: 3.94 (0.79–7.09), I2 71% | MD: -0.69(-1.31–0.07), I2 84% | MD: 7.26 (3.00–11.52), I2 70% | MD: 7.26 (-3.00–11.52), I2 70% |
*N/A = not applicable, as ≤ one study in analysis.
In addition, intervention focused on DSME intervention demonstrated the highest MDs in LDL and TC (LDL: MD 4.33, 95% CI: 2.00% to 6.65%; p<0.001, I2 71; and TC: MD 4.86 95% CI: 0.38% to 9.35%; p<0.001, I2 77%) (Table 3). Lifestyle modification intervention alone showed better efficacy in reducing HDL (MD: -1.77, 95% CI: -6.75% to 3.22%; p<0.001, I2 = 91%) and TG (MD 42.24, 95% CI: -4.21 to 88.70; p<0.001, I2 70%) (Table 3). Furthermore, face-to-face intervention with periodic telephone follow-up showed the highest MDs in LDL (MD 6.79, 95% CI: 3.58% to 10.01%; p = 0.52, I2 = 0.00%) and HDL (MD -4.18, 95% CI: -7.46% to -0.89%; p = 0.03, I2 = 0.03%) (Table 3). However, face-to-face intervention alone was more effective at reducing TG (MD 16.93, 95% CI:8.19% to 25.68%; p<0.001, I2 = 73.96%) (Table 3). Trials classified as high risk of bias showed improvement in the lipid profile of LDL (MD 5.40, 95% CI: -2.26% to 8.55%; p<0.010, I2 = 59.58%), HDL (MD -1.87, 95% CI: -5.09% to 1.34%; p = 0.001, I2 = 92%) and TG (MD 7.26, 95% CI: 3.00% to 11.52%; p = 0.001, I2 = 77% (Table 3).
Risk of bias in the included studies
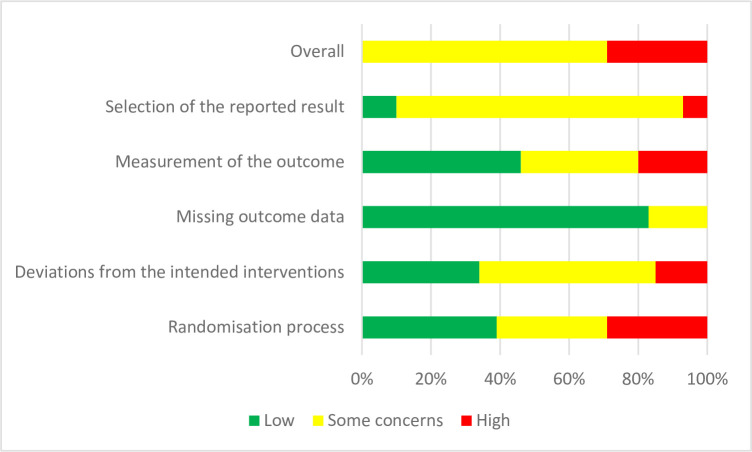
The randomisation process for allocation was evaluated as low risk of bias in 16 studies [1, 30, 52–56, 61, 62, 65, 67, 68, 70, 73, 77, 85], and 13 studies measured as having some concerns of bias [51, 58–60, 63, 64, 75, 79–81, 84, 86, 87]. No trials were rated as low in all five components of the assessment tool. Deviations from the intended interventions were rated as high risk of bias in six studies [57, 69, 72, 82–84]. The risk of bias was rated as some concerns due to missing outcome data in seven studies [51, 59, 71, 76, 77, 85, 93]. Regarding measurement of the outcome reporting, eight studies [54, 69–72, 80, 81, 85, 92] were apparent as high risk of bias. However, for the selection of the reported results, four studies were evaluated as low risk of bias [53, 74, 86, 91], and three studies were assessed as high risk of bias [58, 75, 93]. The overall risk of bias for studies is summarised in Fig 4 and the risk of bias in individual study is reported in S5 Fig.
Fig 4. Risk of bias graph: Review authors’ judgements about each risk of bias item presented as percentages across all included studies.
A quality assessment was carried out for each of the quasi-experimental studies using the JBI Critical Appraisal Checklist [44, 89, 90]. However, the assessment was a subjective measure that was dependent on the author carrying out the assessment. As per the appraisal checklist, three studies [88–90] were considered and included in the meta-analysis. The details are shown in S6 Table.
Publication bias
The presence of publication bias for RCTs was visually assessed using a funnel plot for the primary outcome (HbA1c), which showed that there was no publication bias (Table 2). This was supported by the Egger’s test (p = 0.0680). Publication bias was also assessed for the secondary outcomes and presented in the Table 2, which showed that there was no publication bias for FBG (p = 0.5927), BMI (p = 0.1738), WC (p = 0. 6884), LDL (p = 0.0758), HDL (p = 0.2715), TC (p = 0.5804), TG (p = 0.0535), SBP (p = 0.8676) and DBP (p = 0.5148). Publication bias, however, was present for HrQoL (p = 0.0005), self-efficacy (p < 0.001) and diabetes knowledge (p = 0.0070). Regarding quasi-experimental studies, no publication bias was observed for HbA1c (p = 0.4515) (Table 2).
Overall quality of the evidence
The GRADE approach was employed to assess the overall quality of evidence, and the results are summarized in the main comparison’s findings. Findings showed that the overall certainty of evidence for HDL and WC were moderate, which suggests further studies will increase our confidence in the estimate of effect size. The quality of the evidence for HbA1c, FBG, and BMI were low, which reflects that the effect size is limited and the true effect may be substantially different from the estimate of the effect size. The quality of evidence for LDL, TC and TG were very low, which showed that the true effect is probably markedly different from the estimated effect (S7 Table).
Discussion
This systematic review and meta-analysis aimed to systematically examine the efficacy of DSME interventions on overall T2DM management and cardiometabolic outcomes. Pooled data were used covering 11,838 participants across 44 studies conducted in 21 LMICs. Comprehensive assessment was conducted to evaluate the effectiveness of DSME intervention on 13 outcomes measures including HbA1c control, cardiometabolic risk factors, self-efficacy, diabetes knowledge and psychosocial well-being factors among people with T2DM in LMICs. The outcomes were compared with those generated by standard care across both RCT and quasi-experimental trials. Consequently, a greater number of studies than the earlier reviews were included. This review and meta-analysis demonstrated that DSME intervention leads to better glycaemic control as compared to lifestyle modification intervention alone. Further, it also shows that face-to-face interventions followed by periodic phone calls results in better glycaemic control compared with only face-to-face or remote delivery strategies. The findings suggest that ongoing support is important in optimising intervention efficacy.
Compared with the standard care, this review showed that DSME intervention reduced HbA1c by 0.64% (95% CI: 0.45% to 0.83%) and 1.27% (95% CI: -0.63% to 3.17%) in RCTs and quasi-experimental design studies, respectively. This finding is consistent with previous reviews [20, 21, 93, 94] that reported a reduction in HbA1c levels by 0.83% (95% CI: 1.17% to 0.49%, n = 18 studies) [94] and 0.26% (95% CI: 0.05 to 0.48 n = 31 studies) [25] after DSME interventions. A decrease in HbA1c levels is known to reduce micro- and macro-vascular complications of people with T2DM in long-term follow-up [95–97]. Thus, DSME intervention should be a priority for optimising glycaemic control among people with T2DM in LMICs.
This review demonstrated that DSME intervention leads to significant improvement in FBG and other cardiometabolic risk factors (i.e. BMI, WC, SBP, DBP, LDL, HDL, TG and TC). The findings are in line with those of the previous review that showed the positive effects of group-based self-management education interventions on HbA1c, FBG, body weight, WC, TG and diabetes knowledge [98]. Another review, however, showed that there was no effect of community-based educational interventions on SBP and DBP [99]. Overall, these findings support the potential clinical, behavioural and psychological efficacy of DSME intervention in patients with T2DM.
Adults with diabetes or other metabolic diseases are more likely to have lower self-efficacy, knowledge about their illness and HrQoL [100] as compared with individuals without diabetes and metabolic syndrome. This meta-analysis showed that DSME intervention effectively increased self-efficacy, which is supported by a previous systematic review [101]. Additionally, in a tailored web-based intervention, patients with the highest self-efficacy had better outcomes; therefore, self-efficacy may play a moderating role in intervention outcomes and thus should be considered in tailoring DSME intervention for people with diabetes [102]. Peyrot and Rubin [103] found that those who had the worst self-care, improved the most following DSME intervention and that those with higher self-efficacy had a higher level of self-care behaviours. Self-efficacy provides the confidence necessary to overcoming disease barriers [104] and it receives the most consistent support as a strong determinant of diabetes self-care behaviours [105]. Further, in the present review, diabetes knowledge was significantly improved in the intervention group compared to controls (MD -2.85; 95% CI: -3.83% to -1.79%, p<0.001). Several meta-analyses have similarly shown that DSME interventions are associated with significant improvements in knowledge of T2DM [94, 106, 107]. Our results also showed that DSME intervention leads to improvement in HrQoL, as reported previously [108]. Other reviews have also demonstrated that DSME and behavioural modification improve HrQoL, which in turn impacts self-care and patients’ perceptions about diabetes care [109–112].
Subgroup analyses were performed by the income levels of the countries, intervention types, modes of delivery of the intervention, and quality of the studies. The analysis showed an overall improvement in HbA1c, BMI, LDL, HDL, TG and TC in the LMICs; however, low-income countries had a higher improvement in BMI (MD: 0.87, 95% CI: -0.48 to 2.22). It is possible that health-educational attainment has a direct impact on BMI. In addition, individuals with T2DM in low-income countries may be more physically active due to their need to secure income and also due to limited access to private transportation, leading to a less sedentary lifestyle as compared to those living in lower-middle-income countries [113]. In relation to intervention types, a noteworthy finding in this review is that people with T2DM who received DSME intervention had better BMI, LDL and TC reduction than those who received lifestyle (diet and physical activity) modification alone. This finding is similar to some [33, 34, 114] but not all [10] previous reviews reporting DSME intervention having a better effect on HbA1c control and BMI reduction. In addition to HbA1c and BMI, this current review demonstrated the efficacy of DSME interventions and lifestyle modification intervention in LDL, HDL, TG and TC. Another notable finding of this review is that the face-to-face interventions with periodic telephone follow-up results in better effects on glycaemic control and cardiometabolic risk than face-to-face or text message/web-based interventions alone, which is in line with the National Services Scheme by Diabetes Australia [115]. Periodic phone calls encouraging and reminding patients to practice self-management behaviours consistently over time improves their adherence to overall diabetes control [116]. Thus, face-to-face interventions with periodic telephone follow-up should be prioritised in future DSME intervention programmes for better T2DM management.
This systematic review and meta-analysis is noteworthy in terms of its synthesis of the evidence of outcomes through inclusion of trials using both RCTs and quasi-experimental intervention designs. Overall, it comprehensively summarises the potential clinical, behavioural and psychosocial efficacies of DSME interventions among people with T2DM in LMICs. In addition, five electronic databases were meticulously searched by the authors. As a result, a larger number of trials were identified leading to an impressive sample size of 11,838 participants. This review, however, has a few limitations. First, only a small number of studies were found from low-income countries. Second, the majority of the studies reported outcomes from less than one year follow-up, therefore the long-term effectiveness of DSME intervention in the management of T2DM population cannot be demonstrated. Third, high heterogeneity was observed in the meta-analyses for most of the outcome measures, which is likely due to variation in intervention programme design across the studies [99] as typically noted in intervention programmes of this nature. Fourth, no trial was categorised as low risk in all five components of the ROB 2 assessment tool. Particularly, randomisation process, deviations from the intended interventions, and measurement of the outcome were the most common risks of bias among the RCTs; hence, a prudent approach is warranted when interpreting the results of this present review. It is therefore recommended to follow the CONSORT statement [117] for parallel-group randomised trials to reduce the risk of biases when designing the methodology of the future RCTs. Further, the assessment of outcomes data was measured in heterogeneous ways in the included studies of this review and the certainty of evidence is not sufficient to assert the effectiveness of interventions among patients with T2DM. Hence, to enhance the certainty of evidence regarding the efficacy of these interventions, future RCTs should address the limitations observed in existing research in the literature.
Conclusion
In conclusion, this systematic review and meta-analysis may have found a positive effect of DSME on the clinical and cardiometabolic risk factors, diabetes self-management behaviours and psychosocial well-being of people with T2DM in LMICs. Therefore, DSME interventions may enhance disease management and support to improve self-care strategies for people with T2DM. Further, interventions utilising a face-to-face delivery coupled with periodic ongoing support may be useful in improving glycaemic and lipid control as well as anthropometric measures. This study suggests that ongoing support alongside individualised face-to-face intervention delivery needs to be prioritised in order to improve overall T2DM management in LMICs, with a special emphasis on countries in the lowest income groups.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Subgroup meta-analysis results showing the effect of interventions on (A) HbA1c, (B) BMI, (C) LDL, (D) HDL, (E) TG, and (F) TC based on the income level of the country.
(TIF)
Subgroup meta-analysis results showing the effect of interventions on (A) HbA1c, (B) BMI, (C) LDL, (D) HDL, (E) TG, and (F) TC based on intervention type.
(TIF)
Subgroup meta-analysis results showing the effect of interventions on (A) HbA1c, (B) BMI, (C) LDL, (D) HDL, (E) TG, and (F) TC based on the mode of delivery of intervention.
(TIF)
Subgroup meta-analysis results showing the effect of interventions on (A) HbA1c, (B) BMI, (C) LDL, (D) HDL, (E) TG, and (F) TC based on the quality of study.
(TIF)
(TIF)
Acknowledgments
We would like to acknowledge Mohammad Rocky Khan Chowdhury (PhD Fellow, Department of Epidemiology and Preventive Medicine, Monash University) for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Baviskar MP, Rangari S, Mishra S, Mohanta BS. Assessment of a group-based comprehensive diabetes management program to improve glycemic control, quality of life and self-care behavior in patients with type 2 diabetes mellitus in a primary healthcare setting of a metropolitan city in India: CDMP MUM Trial. International Journal of Diabetes in Developing Countries. 2021;41:156–63. [Google Scholar]
- 2.IDF. Diabetes Atlas. International Diabetes Federation. 10th Edition. 2021;ISBN: 978-2-930229-98-0. https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf [Google Scholar]
- 3.WHO. Diabetes, The World Health Organization. https://www.who.int/news-room/fact-sheets/detail/diabetes. 2022. [Google Scholar]
- 4.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Physical therapy. 2008;88:1322–35. doi: 10.2522/ptj.20080008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. 2015;33:811–31. doi: 10.1007/s40273-015-0268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22:149–64. doi: 10.2165/00019053-200422030-00002 [DOI] [PubMed] [Google Scholar]
- 7.Atun R, Davies JI, Gale EA, Bärnighausen T, Beran D, Kengne AP, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. The lancet Diabetes & endocrinology. 2017;5:622–67. doi: 10.1016/S2213-8587(17)30181-X [DOI] [PubMed] [Google Scholar]
- 8.Xu K, Evans DB, Kawabata K, Zeramdini R, Klavus J, Murray CJ. Household catastrophic health expenditure: a multicountry analysis. The lancet. 2003;362:111–7. doi: 10.1016/S0140-6736(03)13861-5 [DOI] [PubMed] [Google Scholar]
- 9.Bloom DE, Chatterji S, Kowal P, Lloyd-Sherlock P, McKee M, Rechel B, et al. Macroeconomic implications of population ageing and selected policy responses. The Lancet. 2015;385:649–57. doi: 10.1016/S0140-6736(14)61464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moucheraud C, Lenz C, Latkovic M, Wirtz VJ. The costs of diabetes treatment in low-and middle-income countries: a systematic review. BMJ global health. 2019;4:e001258. doi: 10.1136/bmjgh-2018-001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellman CW. Achieving glycemic control: cornerstone in the treatment of patients with multiple metabolic risk factors. Journal of Osteopathic Medicine. 2009;109:8–13. [PubMed] [Google Scholar]
- 12.Siddiquea BN, Afroz A, Chowdhury MRK, Savira F, Alif SM, Bhattacharya O, et al. Knowledge, attitudes and practices of COVID-19 in rural Bangladesh: a cross-sectional study. BMJ open. 2023;13:e064754. doi: 10.1136/bmjopen-2022-064754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosiek A, Kornatowski T, Frąckowiak-Maciejewska N, Rosiek-Kryszewska A, Wyżgowski P, Leksowski K. Health behaviors of patients diagnosed with type 2 diabetes mellitus and their influence on the patients’ satisfaction with life. Therapeutics and clinical risk management. 2016:1783–92. doi: 10.2147/TCRM.S118014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamptey R, Amoakoh-Coleman M, Djobalar B, Grobbee DE, Adjei GO, Klipstein-Grobusch K. Diabetes self-management education interventions and self-management in low-resource settings; a mixed methods study. Plos one. 2023;18:e0286974. doi: 10.1371/journal.pone.0286974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan L, Sidani S. Effectiveness of diabetes self-management education intervention elements: a meta-analysis. Canadian journal of diabetes. 2009;33:18–26. [Google Scholar]
- 16.Kumah E, Otchere G, Ankomah SE, Fusheini A, Kokuro C, Aduo-Adjei K, et al. Diabetes self-management education interventions in the WHO African Region: A scoping review. PloS one. 2021;16:e0256123. doi: 10.1371/journal.pone.0256123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almutairi N, Hosseinzadeh H, Gopaldasani V. The effectiveness of patient activation intervention on type 2 diabetes mellitus glycemic control and self-management behaviors: a systematic review of RCTs. Primary care diabetes. 2020;14:12–20. doi: 10.1016/j.pcd.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. Journal of diabetes & Metabolic disorders. 2013;12:1–5. doi: 10.1186/2251-6581-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clinical diabetes. 2004;22:123–8. [Google Scholar]
- 20.Aquino JA, Baldoni NR, Flôr CR, Sanches C, Oliveira CDL, Alves GCS, et al. Effectiveness of individual strategies for the empowerment of patients with diabetes mellitus: a systematic review with meta-analysis. Primary care diabetes. 2018;12:97–110. doi: 10.1016/j.pcd.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Hildebrand JA, Billimek J, Lee J-A, Sorkin DH, Olshansky EF, Clancy SL, et al. Effect of diabetes self-management education on glycemic control in Latino adults with type 2 diabetes: a systematic review and meta-analysis. Patient education and counseling. 2020;103:266–75. doi: 10.1016/j.pec.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. Bmj. 2011;343. doi: 10.1136/bmj.d6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X, Li J, Wang B, Yao Q, Li L, Song R, et al. Diabetes self-management education reduces risk of all-cause mortality in type 2 diabetes patients: a systematic review and meta-analysis. Endocrine. 2017;55:712–31. doi: 10.1007/s12020-016-1168-2 [DOI] [PubMed] [Google Scholar]
- 24.Welch G, Garb J, Zagarins S, Lendel I, Gabbay RA. Nurse diabetes case management interventions and blood glucose control: results of a meta-analysis. Diabetes research and clinical practice. 2010;88:1–6. doi: 10.1016/j.diabres.2009.12.026 [DOI] [PubMed] [Google Scholar]
- 25.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes care. 2002;25:1159–71. doi: 10.2337/diacare.25.7.1159 [DOI] [PubMed] [Google Scholar]
- 26.Pillay J, Armstrong MJ, Butalia S, Donovan LE, Sigal RJ, Vandermeer B, et al. Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta-analysis. Annals of internal medicine. 2015;163:848–60. doi: 10.7326/M15-1400 [DOI] [PubMed] [Google Scholar]
- 27.Sherifali D, Bai JW, Kenny M, Warren R, Ali M. Diabetes self‐management programmes in older adults: a systematic review and meta‐analysis. Diabetic Medicine. 2015;32:1404–14. doi: 10.1111/dme.12780 [DOI] [PubMed] [Google Scholar]
- 28.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient education and counseling. 2016;99:926–43. doi: 10.1016/j.pec.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Afable A, Karingula NS. Evidence based review of type 2 diabetes prevention and management in low and middle income countries. World journal of diabetes. 2016;7:209. doi: 10.4239/wjd.v7.i10.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamptey R, Robben MP, Amoakoh‐Coleman M, Boateng D, Grobbee DE, Davies MJ, et al. Structured diabetes self‐management education and glycaemic control in low‐and middle‐income countries: a systematic review. Diabetic Medicine. 2022;39:e14812. doi: 10.1111/dme.14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagisetty PA, Priyadarshini S, Terrell S, Hamati M, Landgraf J, Chopra V, et al. Culturally targeted strategies for diabetes prevention in minority population: a systematic review and framework. The Diabetes Educator. 2017;43:54–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guilbert E, Perry R, Whitmarsh A, Sauchelli S. Short-term effectiveness of nutrition therapy to treat type 2 diabetes in low-income and middle-income countries: systematic review and meta-analysis of randomised controlled trials. BMJ open. 2022;12:e056108. doi: 10.1136/bmjopen-2021-056108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donoghue G, O’Sullivan C, Corridan I, Daly J, Finn R, Melvin K, et al. Lifestyle interventions to improve glycemic control in adults with type 2 diabetes living in low-and-middle income countries: A systematic review and meta-analysis of randomized controlled trials (RCTs). International journal of environmental research and public health. 2021;18:6273. doi: 10.3390/ijerph18126273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed A, Staite E, Ismail K, Winkley K. A systematic review of diabetes self‐management education interventions for people with type 2 diabetes mellitus in the Asian Western Pacific (AWP) region. Nursing Open. 2019;6:1424–37. doi: 10.1002/nop2.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamptey R, Amoakoh-Coleman M, Barker MM, Iddi S, Hadjiconstantinou M, Davies M, et al. Change in glycaemic control with structured diabetes self-management education in low-resource settings: randomized trial. Structured diabetes self-management education and glycaemic control in low-resource urban primary care settings. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim LL, Lau ES, Kong AP, Davies MJ, Levitt NS, Eliasson B, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care. 2018;41:1312–20. doi: 10.2337/dc17-2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diriba DC, Leung DY, Suen LK. The effects of diabetes self‐management interventions on physiological outcomes in people living with diabetes in Africa: a systematic review and meta‐analysis. Diabetic Medicine. 2021;38:e14501. doi: 10.1111/dme.14501 [DOI] [PubMed] [Google Scholar]
- 38.Lamptey R, Amoakoh-Coleman M, Barker MM, Iddi S, Hadjiconstantinou M, Davies M, et al. Change in glycaemic control with structured diabetes self-management education in urban low-resource settings: multicentre randomised trial of effectiveness. BMC Health Services Research. 2023;23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirinzadeh M, Afshin-Pour B, Angeles R, Gaber J, Agarwal G. The effect of community-based programs on diabetes prevention in low-and middle-income countries: a systematic review and meta-analysis. Globalization and health. 2019;15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International journal of surgery. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 41.The World Bank. World bank country classification by income level. World bank country classification by income level, https://data.worldbank.org/country. 2021. [Google Scholar]
- 42.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions. 2019:205–28. [Google Scholar]
- 43.Armijo‐Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. Journal of evaluation in clinical practice. 2012;18:12–8. doi: 10.1111/j.1365-2753.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 44.Institute JB. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews: checklist for quasi-experimental studies (non-randomized experimental studies), 2017. JBI_Quasi-Experimental_Appraisal_Tool2017 pdf. 2017. [Google Scholar]
- 45.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? Bmj. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.GRADEpro G. GRADEpro guideline development tool [software]. McMaster University. 2015;435. [Google Scholar]
- 47.Deeks JJ, Higgins JP, Altman DG, Group CSM. Analysing data and undertaking meta‐analyses. Cochrane handbook for systematic reviews of interventions. 2019:241–84. [Google Scholar]
- 48.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2. The Cochrane Collaboration. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yagiz G, Akaras E, Kubis H-P, Owen JA. The effects of resistance training on architecture and volume of the upper extremity muscles: A systematic review of randomised controlled trials and meta-analyses. Applied Sciences. 2022;12:1593. [Google Scholar]
- 51.Chow EP, Hassali MA, Saleem F, Aljadhey H. Effects of pharmacist-led patient education on diabetes-related knowledge and medication adherence: A home-based study. Health Education Journal. 2016;75:421–33. [Google Scholar]
- 52.Goldhaber-Fiebert JD, Goldhaber-Fiebert SN, Tristán ML, Nathan DM. Randomized controlled community-based nutrition and exercise intervention improves glycemia and cardiovascular risk factors in type 2 diabetic patients in rural Costa Rica. Diabetes care. 2003;26:24–9. doi: 10.2337/diacare.26.1.24 [DOI] [PubMed] [Google Scholar]
- 53.Grillo MdFF, Neumann CR, Scain SF, Rozeno RF, Beloli L, Perinetto T, et al. Diabetes education in primary care: a randomized clinical trial. Cadernos de Saude Publica. 2016;32:e00097115. doi: 10.1590/0102-311X00097115 [DOI] [PubMed] [Google Scholar]
- 54.Azami G, Soh KL, Sazlina SG, Salmiah M, Aazami S, Mozafari M, et al. Effect of a nurse-led diabetes self-management education program on glycosylated hemoglobin among adults with type 2 diabetes. Journal of diabetes research. 2018;2018. doi: 10.1155/2018/4930157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huo X, Krumholz HM, Bai X, Spatz ES, Ding Q, Horak P, et al. Effects of mobile text messaging on glycemic control in patients with coronary heart disease and diabetes mellitus: a randomized clinical trial. Circulation: Cardiovascular Quality and Outcomes. 2019;12:e005805. doi: 10.1161/CIRCOUTCOMES.119.005805 [DOI] [PubMed] [Google Scholar]
- 56.Jiang XJ, Jiang H, Lu YH, Liu SL, Wang JP, Tang RS, et al. The effectiveness of a self‐efficacy‐focused structured education programme on adults with type 2 diabetes: A multicentre randomised controlled trial. Journal of Clinical Nursing. 2019;28:3299–309. doi: 10.1111/jocn.14908 [DOI] [PubMed] [Google Scholar]
- 57.Ju C, Shi R, Yao L, Ye X, Jia M, Han J, et al. Effect of peer support on diabetes distress: a cluster randomized controlled trial. Diabetic Medicine. 2018;35:770–5. doi: 10.1111/dme.13625 [DOI] [PubMed] [Google Scholar]
- 58.Kong J-X, Zhu L, Wang H-M, Li Y, Guo A-Y, Gao C, et al. Effectiveness of the chronic care model in type 2 diabetes management in a community health service center in China: a group randomized experimental study. Journal of diabetes research. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Xu M, Fan R, Ma X, Gu J, Cai X, et al. The effects of intensive nutrition education on late middle-aged adults with type 2 diabetes. International Journal of Environmental Research and Public Health. 2016;13:897. doi: 10.3390/ijerph13090897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lou Q, Ye Q, Wu H, Wang Z, Ware RS, Xiong Y, et al. Effectiveness of a clinic-based randomized controlled intervention for type 2 diabetes management: an innovative model of intensified diabetes management in Mainland China (C-IDM study). BMJ Open Diabetes Research and Care. 2020;8:e001030. doi: 10.1136/bmjdrc-2019-001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muchiri JW, Gericke GJ, Rheeder P. Effect of a nutrition education programme on clinical status and dietary behaviours of adults with type 2 diabetes in a resource-limited setting in South Africa: a randomised controlled trial. Public health nutrition. 2016;19:142–55. doi: 10.1017/S1368980015000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mash RJ, Rhode H, Zwarenstein M, Rollnick S, Lombard C, Steyn K, et al. Effectiveness of a group diabetes education programme in under‐served communities in South Africa: a pragmatic cluster randomized controlled trial. Diabetic Medicine. 2014;31:987–93. doi: 10.1111/dme.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramadas A, Chan CKY, Oldenburg B, Hussein Z, Quek KF. Randomised-controlled trial of a web-based dietary intervention for patients with type 2 diabetes: changes in health cognitions and glycemic control. BMC Public Health. 2018;18:1–13. doi: 10.1186/s12889-018-5640-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramli AS, Selvarajah S, Daud MH, Haniff J, Abdul-Razak S, Tg-Abu-Bakar-Sidik TMI, et al. Effectiveness of the EMPOWER-PAR intervention in improving clinical outcomes of type 2 diabetes mellitus in primary care: a pragmatic cluster randomised controlled trial. BMC family practice. 2016;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan M, Magarey J, Chee S, Lee L, Tan M. A brief structured education programme enhances self-care practices and improves glycaemic control in Malaysians with poorly controlled diabetes. Health education research. 2011;26:896–907. doi: 10.1093/her/cyr047 [DOI] [PubMed] [Google Scholar]
- 66.Wattana C, Srisuphan W, Pothiban L, Upchurch SL. Effects of a diabetes self‐management program on glycemic control, coronary heart disease risk, and quality of life among Thai patients with type 2 diabetes. Nursing & health sciences. 2007;9:135–41. doi: 10.1111/j.1442-2018.2007.00315.x [DOI] [PubMed] [Google Scholar]
- 67.Whittemore R, Vilar-Compte M, De La Cerda S, Delvy R, Jeon S, Burrola-Méndez S, et al. ¡ Sí, Yo Puedo Vivir Sano Con Diabetes! A self-management randomized controlled pilot trial for low-income adults with type 2 diabetes in Mexico City. Current Developments in Nutrition. 2020;4:nzaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wichit N, Mnatzaganian G, Courtney M, Schulz P, Johnson M. Randomized controlled trial of a family-oriented self-management program to improve self-efficacy, glycemic control and quality of life among Thai individuals with Type 2 diabetes. Diabetes research and clinical practice. 2017;123:37–48. doi: 10.1016/j.diabres.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Chu L. Effectiveness of systematic health education model for type 2 diabetes patients. International journal of endocrinology. 2018;2018. doi: 10.1155/2018/6530607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng F, Liu S, Liu Y, Deng L. Effects of an outpatient diabetes self-management education on patients with type 2 diabetes in China: a randomized controlled trial. Journal of diabetes research. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong X, Wang Z, Fisher EB, Tanasugarn C. Peer support for diabetes management in primary care and community settings in Anhui Province, China. The Annals of Family Medicine. 2015;13:S50–S8. doi: 10.1370/afm.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Askari A, Jeihooni AK, Kashfi SM, Marzban A, Khiyali Z. The effect of educational program based on belief, attitude, subjective norm, and enabling factors model on changing the metabolic indices in elderly patients with type II diabetes. International journal of preventive medicine. 2018;9. doi: 10.4103/ijpvm.IJPVM_308_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Didarloo A, Shojaeizadeh D, Alizadeh M. Impact of educational intervention based on interactive approaches on beliefs, behavior, hemoglobin A1c, and quality of life in diabetic women. International journal of preventive medicine. 2016;7. doi: 10.4103/2008-7802.176004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ebrahimi H, Sadeghi M, Amanpour F, Vahedi H. Evaluation of empowerment model on indicators of metabolic control in patients with type 2 diabetes, a randomized clinical trial study. Primary care diabetes. 2016;10:129–35. doi: 10.1016/j.pcd.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 75.Essien O, Otu A, Umoh V, Enang O, Hicks JP, Walley J. Intensive patient education improves glycaemic control in diabetes compared to conventional education: a randomised controlled trial in a Nigerian tertiary care hospital. PloS one. 2017;12:e0168835. doi: 10.1371/journal.pone.0168835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gathu CW, Shabani J, Kunyiha N, Ratansi R. Effect of diabetes self-management education on glycaemic control among type 2 diabetic patients at a family medicine clinic in Kenya: A randomised controlled trial. African Journal of Primary Health Care & Family Medicine. 2018;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodarzi M, Ebrahimzadeh I, Rabi A, Saedipoor B, Jafarabadi MA. Impact of distance education via mobile phone text messaging on knowledge, attitude, practice and self efficacy of patients with type 2 diabetes mellitus in Iran. Journal of Diabetes & Metabolic Disorders. 2012;11:1–8. doi: 10.1186/2251-6581-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosseini S, Shojaeizadeh D, Sanagu A, Vakili M, Mirkarimi K, Jahanshahi R. Effect of educational intervention on self-care behaviors among patients with diabetes: An application of PRECEDE model. Annals of Tropical Medicine and Public Health. 2017;10. [Google Scholar]
- 79.Jain V, Joshi R, Idiculla J, Xavier D. Community health worker interventions in type 2 diabetes mellitus patients: Assessing the feasibility and effectiveness in Rural Central India. Journal of Cardiovascular Disease Research. 2018;9. [Google Scholar]
- 80.Jayasuriya R, Pinidiyapathirage MJ, Jayawardena R, Kasturiratne A, de Zoysa P, Godamunne P, et al. Translational research for diabetes self-management in Sri Lanka: a randomized controlled trial. Primary Care Diabetes. 2015;9:338–45. doi: 10.1016/j.pcd.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 81.Mohammadi S, Karim NA, Talib RA, Amani R. The impact of self-efficacy education based on the health belief model in Iranian patients with type 2 diabetes: a randomised controlled intervention study. Asia Pacific journal of clinical nutrition. 2018;27:546–55. doi: 10.6133/apjcn.072017.07 [DOI] [PubMed] [Google Scholar]
- 82.Myers EF, Trostler N, Varsha V, Voet H. Insights from the diabetes in india nutrition guidelines study: adopting innovations using a knowledge transfer model. Topics in Clinical Nutrition. 2017;32:69. doi: 10.1097/TIN.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ojieabu WA, Bello SI, Arute JE. Evaluation of pharmacists’ educational and counselling impact on patients’ clinical outcomes in a diabetic setting. Journal of Diabetology. 2017;8:7. [Google Scholar]
- 84.Samtia AM, Rasool MF, Ranjha NM, Usman F, Javed I. A multifactorial intervention to enhance adherence to medications and disease-related knowledge in type 2 diabetic patients in Southern Punjab, Pakistan. Tropical Journal of Pharmaceutical Research. 2013;12:851–6. [Google Scholar]
- 85.Sanaeinasab H, Saffari M, Yazdanparast D, Zarchi AK, Al-Zaben F, Koenig HG, et al. Effects of a health education program to promote healthy lifestyle and glycemic control in patients with type 2 diabetes: A randomized controlled trial. Primary Care Diabetes. 2021;15:275–82. doi: 10.1016/j.pcd.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 86.Salahshouri A, Zamani Alavijeh F, Mahaki B, Mostafavi F. Effectiveness of educational intervention based on psychological factors on achieving health outcomes in patients with type 2 diabetes. Diabetology & metabolic syndrome. 2018;10:1–12. doi: 10.1186/s13098-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thanh HTK, Tien TM. Effect of Group Patient Education on Glycemic Control Among People Living with Type 2 Diabetes in Vietnam: A Randomized Controlled Single-Center Trial. Diabetes Therapy. 2021;12:1503–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Halaweh AA, Almdal T, O’Rourke N, Davidovitch N. Mobile care teams improve metabolic control for adults with Type II diabetes in the Southern West Bank, Palestine. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13:782–5. doi: 10.1016/j.dsx.2018.11.066 [DOI] [PubMed] [Google Scholar]
- 89.Pamungkas RA, Chamroonsawasdi K. Self-management based coaching program to improve diabetes mellitus self-management practice and metabolic markers among uncontrolled type 2 diabetes mellitus in Indonesia: A quasi-experimental study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14:53–61. doi: 10.1016/j.dsx.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 90.Kumari G, Singh V, Jhingan AK, Chhajer B, Dahiya S. Effectiveness of lifestyle modification counseling on glycemic control in type 2 diabetes mellitus patients. Current Research in Nutrition and Food Science. 2018;6:70. [Google Scholar]
- 91.Debussche X, Besançon S, Balcou-Debussche M, Ferdynus C, Delisle H, Huiart L, et al. Structured peer-led diabetes self-management and support in a low-income country: The ST2EP randomised controlled trial in Mali. PLoS One. 2018;13:e0191262. doi: 10.1371/journal.pone.0191262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan H, Prista A, Ranadive SM, Damasceno A, Caupers P, Kanaley JA, et al. Effect of aerobic training on glucose control and blood pressure in T2DDM East African males. International Scholarly Research Notices. 2014;2014. doi: 10.1155/2014/864897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Azami G, Soh KL, Sazlina S-G, Salmiah MS, Aazami S. Behavioral interventions to improve self-management in Iranian adults with type 2 diabetes: a systematic review and meta-analysis. Journal of Diabetes & Metabolic Disorders. 2018;17:365–80. doi: 10.1007/s40200-018-0376-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiferaw WS, Akalu TY, Desta M, Kassie AM, Petrucka PM, Aynalem YA. Effect of educational interventions on knowledge of the disease and glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. BMJ open. 2021;11:e049806. doi: 10.1136/bmjopen-2021-049806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Group UPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). The Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 96.Group AC. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England journal of medicine. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 97.Group UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 98.Odgers‐Jewell K, Ball L, Kelly J, Isenring E, Reidlinger D, Thomas R. Effectiveness of group‐based self‐management education for individuals with Type 2 diabetes: a systematic review with meta‐analyses and meta‐regression. Diabetic Medicine. 2017;34:1027–39. doi: 10.1111/dme.13340 [DOI] [PubMed] [Google Scholar]
- 99.Shirvani T, Javadivala Z, Azimi S, Shaghaghi A, Fathifar Z, Devender Bhalla H, et al. Community-based educational interventions for prevention of type II diabetes: a global systematic review and meta-analysis. Systematic reviews. 2021;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen C-N, Chuang L-M, Korivi M, Wu Y-T. Home-based exercise may not decrease the insulin resistance in individuals with metabolic syndrome. Journal of Physical Activity and Health. 2015;12:74–9. doi: 10.1123/jpah.2013-0284 [DOI] [PubMed] [Google Scholar]
- 101.Bilgin A, Muz G, Yuce GE. The Effect of Motivational Interviewing on Metabolic Control and Psychosocial Variables in Individuals Diagnosed with Diabetes: Systematic Review and Meta-Analysis. Patient Education and Counseling. 2022. doi: 10.1016/j.pec.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 102.Wangberg SC. An Internet-based diabetes self-care intervention tailored to self-efficacy. Health education research. 2008;23:170–9. doi: 10.1093/her/cym014 [DOI] [PubMed] [Google Scholar]
- 103.Peyrot M, Rubin RR. Modeling the effect of diabetes education on glycemic control. The Diabetes Educator. 1994;20:143–8. doi: 10.1177/014572179402000210 [DOI] [PubMed] [Google Scholar]
- 104.Bandura A, Watts RE. Self-efficacy in changing societies. Springer; 1996. [Google Scholar]
- 105.Walker RJ, Smalls BL, Hernandez-Tejada MA, Campbell JA, Egede LE. Effect of diabetes self-efficacy on glycemic control, medication adherence, self-care behaviors, and quality of life in a predominantly low-income, minority population. Ethnicity & disease. 2014;24:349. [PMC free article] [PubMed] [Google Scholar]
- 106.Creamer J, Attridge M, Ramsden M, Cannings‐John R, Hawthorne K. Culturally appropriate health education for Type 2 diabetes in ethnic minority groups: an updated Cochrane Review of randomized controlled trials. Diabetic Medicine. 2016;33:169–83. doi: 10.1111/dme.12865 [DOI] [PubMed] [Google Scholar]
- 107.Cheng L, Sit JW, Choi Kc, Chair Sy, Li X, He Xl. Effectiveness of interactive self‐management interventions in individuals with poorly controlled type 2 diabetes: A meta‐analysis of randomized controlled trials. Worldviews on Evidence‐Based Nursing. 2017;14:65–73. doi: 10.1111/wvn.12191 [DOI] [PubMed] [Google Scholar]
- 108.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes/metabolism research and reviews. 1999;15:205–18. doi: [DOI] [PubMed] [Google Scholar]
- 109.Keers JC, Bouma J, Links TP, ter Maaten JC, Gans RO, Wolffenbuttel BH, et al. One-year follow-up effects of diabetes rehabilitation for patients with prolonged self-management difficulties. Patient education and counseling. 2006;60:16–23. doi: 10.1016/j.pec.2004.10.013 [DOI] [PubMed] [Google Scholar]
- 110.Forlani G, Zannoni C, Tarrini G, Melchionda N, Marchesini G. An empowerment-based educational program improves psychological well-being and health-related quality of life in Type 1 diabetes. Journal of endocrinological investigation. 2006;29:405–12. doi: 10.1007/BF03344123 [DOI] [PubMed] [Google Scholar]
- 111.Wolf AM, Conaway MR, Crowther JQ, Hazen KY, L. Nadler J, Oneida B, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes care. 2004;27:1570–6. doi: 10.2337/diacare.27.7.1570 [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Norris SL, Chowdhury FM, Gregg EW, Zhang P. The effects of interventions on health-related quality of life among persons with diabetes: a systematic review. Medical care. 2007:820–34. doi: 10.1097/MLR.0b013e3180618b55 [DOI] [PubMed] [Google Scholar]
- 113.Siefken K, Varela AR, Waqanivalu T, Schulenkorf N. Physical activity in low-and middle-income countries: Routledge; 2021. [DOI] [PubMed] [Google Scholar]
- 114.Robson N, Hosseinzadeh H. Impact of telehealth care among adults living with type 2 diabetes in primary care: a systematic review and meta-analysis of randomised controlled trials. International journal of environmental research and public health. 2021;18:12171. doi: 10.3390/ijerph182212171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Association ADE. The International Centre for Allied Health Evidence (2014). Rapid review of literature for consumer centred care in people with diabetes. Technical Report. Prepared for the Australian Diabetes Educators Association. 2014. [Google Scholar]
- 116.Brown‐Deacon C, Brown T, Creech C, McFarland M, Nair A, Whitlow K. Can follow‐up phone calls improve patients self‐monitoring of blood glucose? Journal of clinical nursing. 2017;26:61–7. doi: 10.1111/jocn.13367 [DOI] [PubMed] [Google Scholar]
- 117.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Journal of Pharmacology and pharmacotherapeutics. 2010;1:100–7. doi: 10.4103/0976-500X.72352 [DOI] [PMC free article] [PubMed] [Google Scholar]