Abstract
Normal cell polarity protects epithelial cells against Pseudomonas aeruginosa invasion and cytotoxicity. Using epithelial cell clones with selective defects in sorting of membrane constituents, and using hepatocyte growth factor pretreatment, we found that polarized susceptibility to P. aeruginosa can be altered without disrupting tight junctions. The results also showed that cellular susceptibility factors for invasion and cytotoxicity are not the same, although both are localized to the basolateral cell surface in polarized epithelial cells.
Pseudomonas aeruginosa clinical isolates can be broadly classified into two types, those that invade epithelial cells (invasive) (6, 7) and others that are cytotoxic toward epithelial cells (1, 8). These two phenotypes are associated with genetic differences in the ExsA-regulated pathway of genes on the bacterial chromosome (9). Healthy tissues are normally resistant to infection with both types of P. aeruginosa isolates (23).
Epithelial cells throughout the body are polarized; that is, they have distinct apical and basolateral membranes that are separated by tight junctions. In intact healthy tissues, only the apical membrane is exposed to the environment. Using methods that disrupt the integrity of tight junctions, we have shown that the basolateral membranes of epithelial cells are more susceptible to both P. aeruginosa invasion and cytotoxicity than is the apical cell membrane (10).
Injury can expose basolateral cell surfaces, and this may, in part, explain why tissues become susceptible to infection with P. aeruginosa after overt injury (23). However, P. aeruginosa infection can occur without overt injury; e.g., during contact lens wear, in cystic fibrosis, and in immunocompromised patients.
Polarity of epithelial cells is determined by sorting of membrane constituents to, from, and between apical and basolateral cell membrane compartments. Tight junctions function to maintain polarity subsequent to sorting (12). The aim of this study was to determine whether or not the susceptibility of apically exposed cell membranes to P. aeruginosa invasion and cytotoxicity could be altered without disrupting tight junctions between epithelial cells.
Hepatocyte growth factor (HGF) is a polypeptide growth factor with multiple functions on epithelial cells and tissues, including mitogenesis, cell motility, and the development and regeneration of organs (13, 14, 16, 18). Recently, HGF has also been found to alter normal polarized sorting of apical and basolateral membrane components in Madin-Darby canine kidney II (MDCK) cells without disrupting tight junctions when applied to the basolateral cell membrane (2).
Effect of HGF pretreatment.
The basolateral surface of filter-grown MDCK cells was pretreated with HGF by adding 0.1-μg/ml HGF to the basolateral growth medium and incubating the cells for 72 h. Susceptibility of HGF-treated and control MDCK cells to P. aeruginosa 6294 adherence and invasion and to the cytotoxicity of strain 6206 was measured.
MDCK cells were grown as previously described (10). Transepithelial resistance (TER) of cells grown on filters was monitored by using an EVOM meter (World Precision Instruments Inc., Sarasota, Fla.). TER was measured to ensure that tight junctions were intact and that cells were morphologically polarized. The baseline TER, determined by measuring resistance across filters treated in the same manner without cells, was approximately 100 Ω/cm2; TER increased when cells of all types became confluent, indicating that tight junctions had formed. Bacterial viable counts, gentamicin survival assays, and trypan blue exclusion assays were used to measure bacterial adherence, invasion, and cytotoxic activity as previously described (8, 10). The t test was used for all statistical analyses.
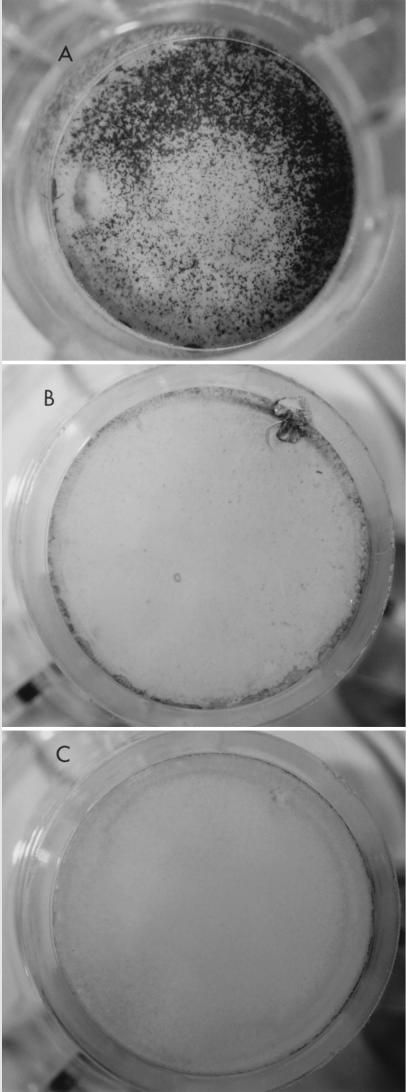
The results of HGF treatment mirrored the effect of disrupting tight junctions; there was enhanced susceptibility of MDCK cells to adherence (P = 0.003), invasion (P = 0.0001), and cytotoxicity by P. aeruginosa (Table 1 and Fig. 1). The TER of the cells was not reduced by HGF; in fact, the treatment increased TER slightly (P = 0.04), confirming that HGF treatment did not disrupt tight junctions (Table 1).
TABLE 1.
Effect of HGF pretreatment on adherence to, and invasion of, MDCK cells by invasive P. aeruginosa 6294
| Treatment | Avg no. of CFU ± SD
|
TER (Ω/cm2) | |
|---|---|---|---|
| Adherence | Invasion | ||
| None | (5.3 ± 0.7) × 105 | (4.5 ± 0.8) × 103 | 188 ± 5 |
| HGF at 0.1 μg/ml | (9.5 ± 0.9) × 105 | (3.0 ± 0.2) × 104 | 200 ± 1 |
FIG. 1.
Effect of HGF (0.1 μg/ml) pretreatment on cell susceptibility to P. aeruginosa cytotoxicity. A, HGF pretreatment; B, no HGF pretreatment; C, HGF pretreatment with no bacterial exposure.
Role of E-cadherin in HGF-induced invasion.
The role of E-cadherin in HGF-induced bacterial invasion was explored, since it has been found to be a receptor for basolateral internalization of Listeria monocytogenes (20) and because it is sent to the apical surface of HGF-treated MDCK cells (2). The apically exposed surface of HGF-treated MDCK epithelial cells was preincubated with DECMA-1 (anti-uvomorulin [E-cadherin] rat immunoglobulin G1 in ascites; Sigma) for 30 min prior to addition of bacteria in the presence of the antibody and incubation for 3 h. Control HGF-treated cells were incubated with rat ascites immunoglobulin G against ZO1 (Chemicon International) instead of DECMA-1. Antibodies against E-cadherin (DECMA-1) were found to inhibit cell invasion by L. monocytogenes 10403S but not by P. aeruginosa 6294 after HGF pretreatment (data not shown). Similarly, antibodies to E-cadherin did not block P. aeruginosa 6206 cytotoxicity (data not shown).
Effect of sorting defects.
Since HGF might induce effects on cells that alter susceptibility to bacteria other than the apical exposure of basolateral membrane factors, bacterial interactions with two MDCK cell clones with different sorting defects were explored.
MDCK II/J cells are likely to have a defect in sphingolipid synthesis and missort Na/K ATPase but not E-cadherin (19).
MDCK clone C13 cells were obtained after transfection of MDCK cells obtained from the American Type Culture Collection with a plasmid expressing a mutant influenza virus hemagglutinin that contains a basolateral sorting signal (3). C13 cells have a selective sorting defect (4) that appears to be quite different from the defect in the clone II/J cells described above. After infection with influenza virus, the hemagglutinin protein is sent to the apical membrane as in control C15 cells; however, transferrin and methionine receptors are not restricted to the basolateral surface. Vesicular stomatitis virus infects exclusively through the basolateral surface of C15 cells but preferentially from the apical surface of C13 cells. For C15 cells, 80% of an endogenously secretory glycoprotein, gp80, is secreted apically, but for C13 cells, apical and basolateral cell membranes secrete equal amounts of gp80.
Cells from each of these lines were found to produce TERs across monolayers that were at least as high as those of their respective control clone cells (Table 2). C13 clone cells produced TERs similar to those of C15 control clone cells (P = 0.6), and II/J cells produced a higher TER than their control II/G cells (P = 0.0001). These results showed that all of the cell types studied were able to form functional tight junctions when grown as described.
TABLE 2.
Invasion of MDCK clone cells by invasive P. aeruginosa 6294
| MDCK clone | Cell type | Inoculum size (no. of CFU) | Invasion (avg no. of CFU ± SD) | TER (Ω/cm2) |
|---|---|---|---|---|
| C13 | Sorting defective | 1.5 × 106 | (3.5 ± 0.2) × 104 | 190 ± 7 |
| C15 | Control for C13 | 1.5 × 106 | (4.0 ± 0.6) × 104 | 193 ± 5 |
| II/J | Sorting defective | 1.5 × 105 | (2.9 ± 1.1) × 103 | 256 ± 5 |
| II/G | Control for II/J | 1.5 × 105 | (7.3 ± 1.4) × 103 | 178 ± 3 |
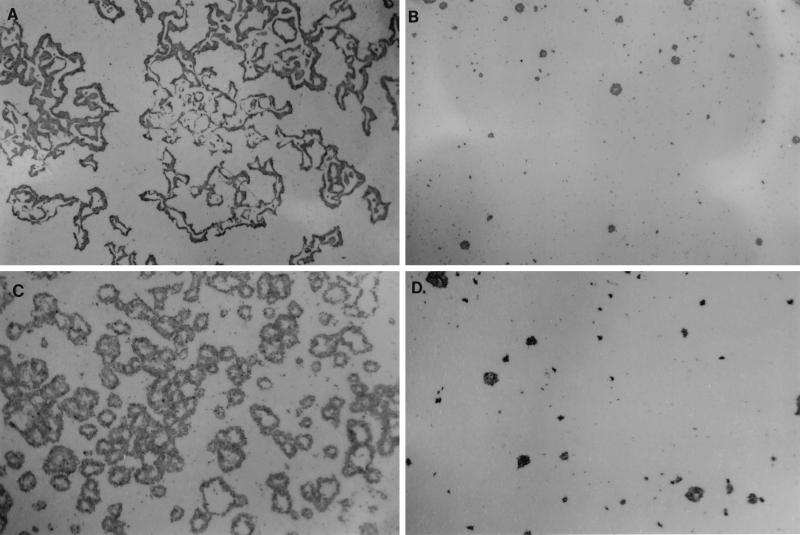
Both of the sorting-defective cell types (C13 and clone II/J cells) were more susceptible to the cytotoxicity of P. aeruginosa 6206 than were their respective control clone cells (C15 and clone II/G) (Fig. 2), providing further evidence that factors involved in susceptibility to P. aeruginosa cytotoxicity are associated with the basolateral membrane in polarized epithelial cells.
FIG. 2.
Susceptibility of cells with partial sorting defects to P. aeruginosa cytotoxicity. A, sorting-defective clone II/J cells; B, clone II/G cells (control for clone II/J cells); C, sorting-defective C13 cells; D, C15 cells (control for C13 cells). Clear areas in the centers of the foci are areas where cells have been completely destroyed.
The effects of these sorting defects on invasion by invasive strain 6294 were entirely different from their effects on cytotoxicity. C13 cells were equally susceptible to P. aeruginosa invasion as the control C15 cells (P = 0.16), suggesting that factors involved in invasion were not missorted by the C13 cells. Clone II/J cells were even less susceptible to P. aeruginosa invasion than were the control clone II/G cells (P = 0.003) (Table 2). The most likely explanation for this result is that there may also be apical factors on correctly polarized MDCK cells that are involved in invasion; i.e., some apical receptors for invasion may be sent basolaterally in II/J cells (based on the assumption that, in fact, II/G cells sort all membrane constituents “correctly”). In this regard, CFTR has recently been shown to be a receptor for P. aeruginosa invasion of certain epithelial cell types (22); when present, CFTR is usually an apically expressed protein. The low-level presence of apical receptors on MDCK cells might explain why P. aeruginosa can invade confluent heterogeneous MDCK cells, albeit to a lesser extent than MDCK cells with disrupted tight junctions. This is clearly not the case for some epithelial cell types that appear to be more efficiently polarized for susceptibility; i.e., the apical surfaces of human nasal epithelia do not permit P. aeruginosa invasion at all, while their basolateral surfaces are susceptible (10).
When cytotoxic P. aeruginosa kills MDCK or corneal epithelial cells, it begins by killing only occasional single cells. Once the first cell is killed, bacteria gain access to the basolateral sides of adjacent cells in the monolayer and spread outwards to form foci of dead cells (10). This might be explained by some form of random activity that is related to the bacteria, since cytotoxicity is regulated in cytotoxic P. aeruginosa by ExsA and is contact dependent (11, 25). Alternatively, the pattern might reflect heterogeneity in the efficiency of polarity among a population of cells growing as a monolayer. In this regard, the “sorting-defective” C13 and II/J clone cells that were used in this study were originally isolated from heterogeneous populations of MDCK cells. Interestingly, cytotoxicity still occurred as isolated foci with the C13 and II/J cell clones, although there were many more foci. In this case, differences in cell maturity or development of individual cells might account for the pattern of killing.
These results, which showed that susceptibility factors for invasion and cytotoxicity are not the same, support the conclusions of other studies in this laboratory that P. aeruginosa-induced cytotoxicity occurs from outside of the target epithelial cell (5). Whether this indicates that different receptors are involved has yet to be determined.
The effect of HGF on cell polarity could help explain why tissue injury and inflammation are risk factors for P. aeruginosa infection. HGF production increases during various pathological states of various organs with epithelial cell layers, such as the eye, kidney, liver, lung, and pancreas (15, 17, 18, 21, 24, 26). The results presented here suggest the potential for HGF, and therefore possibly other factors, to alter susceptibility to bacterial virulence factors in the absence of direct injury to the affected cells. They also raise the possibility that injury at one site could lead to enhanced susceptibility of cells distant from the site of the injurious stimuli.
Acknowledgments
This work was supported by NIH grant RO1 EY11221 and a UC Berkeley Faculty Research Grant to S.M.J.F.; by grant RO1 HL55980 to K.E.M., who is an established investigator of the American Heart Association; and by grant RO1 GM3754 to M.G.R.
We thank James Nelson of Stanford University for the II/J and II/G MDCK cell clones and Ralph Schwall at Genentech for generous gifts of recombinant human hepatocyte growth factor.
REFERENCES
- 1.Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K E, Wiener-Kronish J. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkovetz D F, Pollack A L, Mostov K E. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem. 1997;272:3471–3477. doi: 10.1074/jbc.272.6.3471. [DOI] [PubMed] [Google Scholar]
- 3.Brewer C B, Roth M G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of the influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer, C. B., and M. G. Roth. Unpublished data.
- 5.Evans D J, Frank D W, Finck-Barbançon V, Wu C, Fleiszig S M J. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect Immun. 1998;66:1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleiszig S M J, Zaidi T S, Fletcher E L, Preston M J, Pier G B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62:3485–3492. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleiszig S M J, Zaidi T S, Pier G B. Pseudomonas aeruginosa survival and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63:4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleiszig S M J, Zaidi T S, Preston M J, Grout M, Evans D J, Pier G B. The relationship between cytotoxicity and epithelial cell invasion by corneal isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanda D, Sawa T, Yen T S B, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate to distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig S M J, Evans D J, Do N, Shin S, Mostov K E. Epithelial cell polarity affects Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank D W, Nair G, Schweizer H P. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect Immun. 1994;62:554–563. doi: 10.1128/iai.62.2.554-563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK) J Membr Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- 13.Igawa T, Matsumoto K, Kanda S, Saito Y, Nakamura T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol. 1993;265:F61–F69. doi: 10.1152/ajprenal.1993.265.1.F61. [DOI] [PubMed] [Google Scholar]
- 14.Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Weng J, Mohan R R, Bennett G L, Schwall R, Wang Z, Tabor K, Kim J, Hargrave S, Cuevas K H, Wilson S E. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest Ophthalmol Vis Sci. 1996;37:727–739. [PubMed] [Google Scholar]
- 16.Mason R J, Leslie C C, McCormick-Shannon K, Deterding R R, Nakamura T, Rubin J S, Shannon J M. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Resp Cell Mol Biol. 1994;11:561–567. doi: 10.1165/ajrcmb.11.5.7524567. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Tajima H, Hamanoue M, Kohno S, Kinoshita T, Nakamura T. Identification and characterization of “injurin,” an inducer of expression of the gene for hepatocyte growth factor. Proc Natl Acad Sci USA. 1992;89:3800–3804. doi: 10.1073/pnas.89.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, Nakamura T. Pleiotropic roles of HGF in mitogenesis, morphogenesis, and organ regeneration. Gann Monogr Cancer Res. 1994;42:91–112. [Google Scholar]
- 19.Mays R W, Siemers K A, Fritz B A, Lowe A W, Van M G, Nelson W J. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengaud J, Ohayon H, Gounon P, Mége R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa K, Shimomura T, Naka D, Kitamura N. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem. 1994;269:8966–8970. [PubMed] [Google Scholar]
- 22.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramphal R, McNiece M T, Polack F D. Adherence of Pseudomonas aeruginosa to the injured cornea. A step in the pathogenesis of corneal infections. Ann Ophthalmol. 1981;100:1956–1958. [PubMed] [Google Scholar]
- 24.Ueda T, Takeyama Y, Toyokawa A, Kishida S, Yamamoto M, Saitoh Y. Significant elevation of serum human hepatocyte growth factor levels in patients with acute pancreatitis. Pancreas. 1996;12:76–83. doi: 10.1097/00006676-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Yahr T L, Goranson J, Frank D W. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III secretion pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 26.Yanagita K, Matsumoto K, Sekiguchi K, Ishibashi H, Niho Y, Nakamura T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem. 1993;268:21212–21217. [PubMed] [Google Scholar]