Abstract
Background
Standardized exercise protocols have been shown to improve overall cardiovascular fitness, but direct effects on left ventricular (LV) function, particularly diastolic function and relation to post-transcriptional molecular pathways (microRNAs (miRs)) are poorly understood. This project tested the central hypothesis that adaptive LV remodeling resulting from a large animal exercise training protocol, would be directly associated with specific miRs responsible for regulating pathways relevant to LV myocardial stiffness and geometry.
Methods and results
Pigs (n = 9; 25 Kg) underwent a 4 week exercise training protocol (10 degrees elevation, 2.5 mph, 10 min, 5 days/week) whereby LV chamber stiffness (KC) and regional myocardial stiffness (rKm) were measured by Doppler/speckle tracking echocardiography. Age and weight matched non-exercise pigs (n = 6) served as controls. LV KC fell by approximately 50% and rKm by 30% following exercise (both p < 0.05). Using an 84 miR array, 34 (40%) miRs changed with exercise, whereby 8 of the changed miRs (miR-19a, miR-22, miR-30e, miR-99a, miR-142, miR-144, miR-199a, and miR-497) were correlated to the change in KC (r ≥ 0.5 p < 0.05) and mapped to matrix and calcium handling processes. Additionally, miR-22 and miR-30e decreased with exercise and mapped to a localized inflammatory process, the inflammasome (NLRP-3, whereby a 2-fold decrease in NLRP-3 mRNA occurred with exercise (p < 0.05).
Conclusion
Chronic exercise reduced LV chamber and myocardial stiffness and was correlated to miRs that map to myocardial relaxation processes as well as local inflammatory pathways. These unique findings set the stage for utilization of myocardial miR profiling to identify underlying mechanisms by which exercise causes changes in LV myocardial structure and function.
Introduction
Exercise training has been shown to reduce cardiovascular risk [1] and improve left ventricular (LV) function [2]. Specifically, exercise training can increase LV ejection fraction (EF) and reduce resting and exercise heart rate [3, 4]. These effects would imply that improved LV filling (i.e. diastolic function) as well as LV ejection performance have improved with exercise training. LV diastolic function is highly dependent upon LV chamber stiffness [5, 6], however, the direct effects of an exercise training protocol on LV stiffness properties are not well understood and may be due in part to confounding factors in clinical studies such as comorbidities, differences in the exercise protocols, and the complexity of measurements. Pigs have been successfully utilized for exercise training studies with respect to cardiovascular function [7, 8]. Accordingly, the first goal of the present study was to examine LV function, in particular LV stiffness properties, in a pig model before and following an exercise training protocol.
MicroRNAs (miRs) constitute an important post-transcriptional regulatory pathway primarily by binding to mRNA transcripts and inhibiting gene expression [9, 10]. Changes in specific miRs have been reported with exercise training and LV remodeling, but are usually focused upon a specific miR target or pathway or rely on miR profiles extracted from plasma or skeletal muscle [11–13]. However, the relationship between changes in LV function and miR profiles, particularly to that of LV stiffness properties, with exercise training is not well established. Therefore, the second goal of this study was to utilize a previously established cardiovascular focused miR array [14] and determine whether and to what degree specific shifts in myocardial miR profiles occur with exercise training in pigs. This project tested the central hypothesis that a reduction in LV stiffness properties occur with exercise training and is associated with a shift in myocardial miRs that regulate pathways and components which contribute to changes in LV stiffness.
Methods
Exercise protocol
Yorkshire pigs (3 months, n = 9; Palmetto Research Swine, Reeseville, South Carolina) utilized were castrated males and for the purposes of this initial study, sex dependent differences in response to exercise were not considered. Pigs acclimated to the treadmill for seven days prior to initiating the exercise protocol. Baseline LV echocardiograms were acquired within 4 hours prior to the initiation of the exercise protocol. Final LV echocardiograms were obtained 4 days after the completion of the exercise protocol. An additional group of Yorkshire pigs (male, castrated, n = 6; Palmetto Research Swine, Reeseville, South Carolina) of matched age and weight were included to obtain referent control echocardiographic images as well as LV myocardium for comparative analysis. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Eighth Edition. Washington, DC: 2011), and all protocols were approved by the University of South Carolina School of Medicine and WJB Dorn VA Institutional Animal Care and Use Committee. To minimize pain and distress the following steps were taken. First, for the non-invasive echocardiographic studies, the potential for stress/anxiety was minimized through administration of oral diazepam (200mg) one hour prior to the imaging procedure and supplemental midazolam (0.5–0.6mg/kg) administered intramuscularly at the time of the echocardiogram. Second, the treadmill protocol was performed under the direct supervision of the Attending Veterinarian (SB, University of South Carolina). Thirdly, the terminal procedures were performed under a full surgical plane of anesthesia using 5% isoflurane.
LV structure and function
All LV function and subsequent analyses was performed in a blinded analysis and the code was not broken until full study completion. LV echocardiograms were performed on exercised pigs during the study (GE Vivid E9 with XDclear Ultrasound System: M5S [1.5 to 4.6 Hz] transducer probe; GE, Boston, MA). LV dimensions and functions were assessed by 2-dimensional and M-mode echocardiographic studies [15]. From the obtained LV measurements, LV end-diastolic volume (EDV), end-systolic volume (ESV), and EF were calculated using the biplane method of disks. LV wall thickness was determined and LV mass computed using conventional formulae [16]. Pulmonary capillary wedge pressure was computed using conventional Doppler methods [17]. Aortic valve pressure gradient was determined by Doppler ultrasound [18]. Speckle tracking echocardiography (STE) was also performed on the acquired ultrasound images using established approaches [19]. LV mass and EDV were indexed to body surface area (BSA) whereby the BSA was determined using a standard approach [20].
LV regional myocardial and chamber stiffness
Using STE on the short-axis view, LV posterior region circumferential myocardial strain (ε) was computed as:
| (1) |
where LED and LES refer to the lengths of a circumferentially-oriented segment (within the posterior wall) length at end diastole (ED) and end systole (ES), respectively. Thus, this segmental measure considers ES as the reference configuration, and reflects a linearized strain measure to characterize the regional deformation at ED. The mean regional circumferential wall stress (σϴ) at ED was then computed as:
| (2) |
where P is the LV pressure, r is the LV inner radius, and t is the posterior wall thickness. We assumed LV pressure at ES was zero and at ED was equivalent to pulmonary capillary wedge pressure and computed the associated strain and stress values for both LV states. The slope of the line between these two points (corresponding to ES and ED) in the circumferential stress-strain plane, developed in posterior region, was used to compute regional myocardial stiffness (rKm) [21] as:
| (3) |
LV chamber stiffness (KC) was computed as:
| (4) |
in which volume strain was defined as the difference between EDV and ESV divided by the ESV.
LV sampling
After completion of the protocol, and under a full surgical plane of anesthesia (5% isoflurane), the LV was harvested and the transmyocardial sections of the posterior region placed in RNAlater (Qiagen, Valencia, CA) and stored at -80⁰C until testing was performed.
miR extraction & profiling
LV samples (30 mg) underwent miR extraction (miRNeasy Mini cat # 217084, Qiagen, Valencia, CA) and the miR pool checked for quality (Agilent RNA 6000 Nano Kit Santa Clara, CA). The miR pool was then reverse transcribed (miScript II RT HiSpec Kit cat # 218161, Qiagen, Valencia, CA). This study utilized a custom pig cardiovascular miR array (cat # 331221, MIHS-113Z, Qiagen, Valencia, CA), which contained 84 individual miRs. The resulting cDNA from miRs was used for SYBR Green PCR (miScript SYBR Green PCR Kit cat # 218073, Qiagen, Valencia, CA). Quantitative RT-PCR was performed (Bio-Rad CFX96 Touch) according to the vendor protocol. The maximum threshold cycle (CT) for detection was set at 35 CTs. CT values of the mean for SNORD42B, SNORD69, SNORD61, SNORD68, SNORD96A, and RNU6-6P were used as reference values for normalization.
Data analysis
LV function and geometry at the beginning and completion of the exercise protocol was first examined by paired, two-tailed t-tests. Next, LV function and geometry was compared between age/weight matched referent controls using unpaired, two-tailed t-tests. In the first level of miR analysis, individual miRs with a greater than or equal to two fold-change values as a result of exercise and statistically significant miRs were identified (GeneGlobe Data Analysis, Qiagen, Valencia, CA). The differential expression of individual miRs relative to referent control was analyzed using the comparative fold-change method [22]. In addition, individual miRs were clustered with respect to predominant functional pathways structured by a literature review (S1 and S3 Tables). The association between changes in LV regional and chamber stiffness to changes in individual miRs was performed using Pearson correlation. All statistical procedures were performed using SPSS (SPSS Software, Version 27, IBM SPSS) and P values of < 0.05 were considered statistically significant. Unless otherwise indicated, data are expressed as means ± SEM.
Results
LV geometry and function
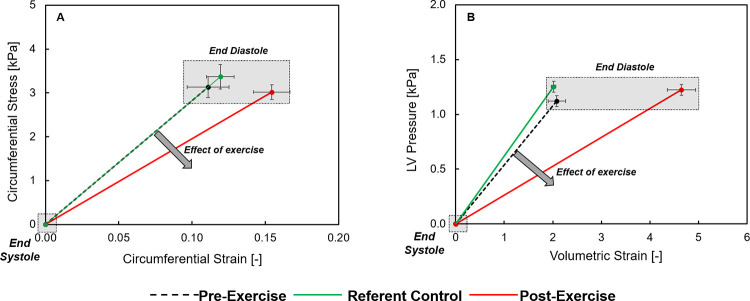
All pigs successfully completed the exercise protocol and a summary of LV function and geometry is shown in Table 1. The values presented include pre-exercise and post-exercise values. Over the four week exercise protocol, normal weight gain occurred and accordingly, weight matched untrained referent control pigs were also included in this analysis as shown in Table 1. Resting heart rate decreased from pre-exercise values post-exercise but was similar to referent control values. The mean aortic pressure gradient, an index of aortic pressure [23], was unchanged between referent control and post-exercise values (3.15 ± 0.31 vs 4.02 ± 0.53 mmHg respectively, p = 0.273). LV mass increased following the exercise protocol, with post-exercise LV mass being higher than referent control values, and was associated with an increase in LV EDV. When indexed to BSA, the directional changes in LV mass and LV EDV remained the same. The LV posterior wall thickness (end-diastolic) to EDV ratio was similar between referent control and post exercise (0.13 ± 0.01 vs 0.12 ± 0.01 mm/mL respectively, p = 0.461). The likely reason for this index remaining within referent control values is that the LV wall thickness and LV volumes increased with the exercise protocol, consistent with concentric remodeling [24]. LV EF values were higher post-exercise from both pre-exercise and referent control values. LV posterior wall thickness increased post-exercise, as did regional ED strain. Composite plots of LV regional and chamber stiffness are shown in Fig 1, and demonstrated that both were reduced following the exercise protocol. LV rKm was reduced by approximately 30% following exercise and LV KC decreased by approximately 50% (Table 1).
Table 1. LV function and geometry: Exercise cohort.
| Pre-Exercise | Referent Control | Post-Exercise | |
|---|---|---|---|
| Body Weight [Kg] | 16.0 ± 0.5 | 25.6 ± 0.8* | 24.9 ± 0.8* |
| HR [beats/min] | 124 ± 5.2 | 107 ± 5.5 | 101 ± 2.8* |
| LV | |||
| LV Mass [g] | 69.4 ± 3.8 | 110.5 ± 5.5* | 143.2 ± 6.5*,# |
| LV Mass-i | 154.3 ± 8.9 | 179.1 ± 6.4 | 238.2 ± 12.3*,# |
| LV EDV [mL] | 40.9 ± 2.8 | 54.8 ± 3.1* | 67.4 ± 3.3*,# |
| LV EDV-i [mL/m2] | 90.4 ± 5.3 | 89.2 ± 5.4 | 111.7 ± 5.1*,# |
| LV Mass/LV EDV | 1.74 ± 0.12 | 2.05 ± 0.15 | 2.13 ± 0.06* |
| LV Wall Thickness/LV EDV [mm/mL] | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01* |
| LV EF [%] | 66.7 ± 1.9 | 66.4 ± 1.8 | 82.0 ± 0.7*,# |
| LV Chamber Stiffness [kPa] | 0.56 ± 0.04 | 0.60 ± 0.06 | 0.27 ± 0.02*,# |
| Posterior LV Region | |||
| ED Wall Thickness [mm] | 6.3 ± 0.3 | 7.2 ± 0.3 | 8.3 ± 0.3*,# |
| ED Circumferential Strain [%] | 11.1 ± 1.4 | 11.9 ± 0.9 | 15.5 ± 1.3*,# |
| ED Wall Stress [kPa] | 3.1 ± 0.2 | 3.4 ± 0.3 | 3.0 ± 0.2 |
| Regional Myocardial Stiffness [kPa] | 31.4 ± 3.8 | 29.1 ± 3.0 | 20.8 ± 2.3*,# |
Sample sizes: Pre-exercise (n = 9); Post-Exercise (n = 9); Referent Control (n = 6).
HR = Heart Rate; BSA = Body Surface Area; EDV = End-Diastolic Volume; EDVi = End-Diastolic Volume Index; EF = ejection fraction; ED = End-Diastolic; i = indexed to BSA
All values reported as mean ± SEM
*p≤0.05 vs pre-exercise
#p≤0.05 vs referent control.
Fig 1.
(A) LV regional circumferential stress and strain was measured by speckle tracking echocardiography (STE) in which values were calculated at the onset of diastole (end-systole) and at end diastole. A rightward and downward shift in this relation, indicative of a reduction in myocardial stiffness, occurred following the exercise protocol. (B) LV volumetric strain and pressure during diastole was computed and this relation, which is indicative of LV chamber stiffness, fell downward and to the right following the exercise protocol. Values shown are composite mean values and SEM. Summary values for LV regional myocardial stiffness and chamber stiffness are shown in Table 1.
miR profiles following exercise
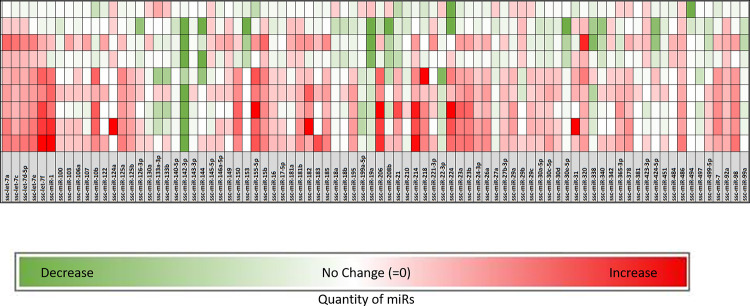
A heat map was generated as a function of referent control values for all of the included miRs (Fig 2). Several miRs changed following the exercise protocol, and those with statistically significant changes or a 2-fold or higher change were selected for further analysis (Table 2). While it must be recognized that post-transcriptional regulation by individual miRs encompasses multiple targets, predominant functional domains relevant to myocardial remodeling and function can be identified [25, 26]. This approach, and a literature review, was utilized to generate Table 2 and thus demonstrated shifts in miRs following exercise from several functional domains. A total of 25 miRs were identified as significantly (p<0.05) upregulated (56%) or downregulated (44%) following the exercise protocol. A cluster of miRs relative to myocardial growth were altered with exercise. For example, miR-199a decreased with exercise and has been identified in past studies to be altered with LV hypertrophy [27]. Although there was an even split between positive and negative fold change values within the miRs assigned to the functional domain for myocardial growth, two miRs in particular, miR-19a and miR-144, had large, significant fold decreases in expression following exercise (Table 2). Another cluster of miRs increased with exercise and have been associated with changes in LV myocardial extracellular matrix (ECM) growth and accumulation. Specifically, miR-214 and miR-155 had 3-fold increases in expression and have been previously identified as regulators of cardiac fibrosis [28, 29]. In addition, a number of miRs associated with inflammation were altered with exercise including miR-155 and miR-206, both of which were identified for their potential roles as circulatory biomarkers in inflammatory cardiomyopathy [30].
Fig 2. A heat map was generated for all the miRs utilized in the array, as a function of a fold change from referent control values.
The rows represent individual pig values following the exercise protocol. A number of miRs changed as a function of exercise within the LV myocardium and were selected for further analysis as indicated in Table 2.
Table 2. Distribution of miRs with a greater than two fold-change.
| miR | Fold-Change | p-value | Differentiation and Development | Disease and Dysfunction | Growth | Injury |
|---|---|---|---|---|---|---|
| ssc-let-7a | 2.86 ± 0.68 | 0.005 | x | x | ||
| ssc-let-7c | 2.36 ± 0.66 | 0.022 | x | x | x | |
| ssc-let-7d | 2.61 ± 0.66 | 0.008 | x | x | ||
| ssc-let-7e | 2.68 ± 0.88 | 0.019 | x | |||
| ssc-let-7f | 3.95 ± 1.37 | 0.008 | x | x | ||
| ssc-miR-1 | 3.59 ± 1.64 | 0.034 | x | x | x | x |
| ssc-miR-15b | 2.98 ± 0.75 | 0.011 | x | x | ||
| ssc-miR-19a | -7.63 ± 3.18 | 0.026 | x | x | x | |
| ssc-miR-22 | -3.07 ± 0.92 | 0.023 | x | x | x | |
| ssc-miR-23a | 2.02 ± 1.13 | 0.106 | x | x | ||
| ssc-miR-23b | 2.36 ± 0.99 | 0.030 | x | x | ||
| ssc-miR-30e | -3.28 ± 1.08 | 0.018 | x | x | ||
| ssc-miR-31 | 6.96 ± 6.47 | 0.177 | x | x | x | |
| ssc-miR-98 | 2.16 ± 0.92 | 0.032 | x | x | x | x |
| ssc-miR-99a | -2.43 ± 0.46 | 0.002 | x | x | ||
| ssc-miR-124a | 17.81 ± 17.18 | 0.175 | x | x | x | x |
| ssc-miR-133a | -2.02 ± 1.00 | 0.079 | x | x | x | x |
| ssc-miR-142 | -23.39 ± 9.91 | 0.022 | x | x | x | |
| ssc-miR-144 | -6.20 ± 2.90 | 0.031 | x | x | x | |
| ssc-miR-153 | -9.49 ± 5.01 | 0.077 | x | |||
| ssc-miR-155 | 3.45 ± 1.69 | 0.034 | x | x | x | |
| ssc-miR-181b | 2.14 ± 0.56 | 0.021 | x | x | ||
| ssc-miR-182 | 3.69 ± 2.29 | 0.110 | x | x | ||
| ssc-miR-199a | -2.44 ± 0.67 | 0.020 | x | x | x | |
| ssc-miR-206 | 3.36 ± 1.80 | 0.054 | x | x | x | |
| ssc-miR-208b | -3.93 ± 1.71 | 0.047 | x | x | x | |
| ssc-miR-214 | 3.71 ± 1.35 | 0.028 | x | x | x | |
| ssc-miR-320 | 2.90 ± 0.87 | 0.022 | x | x | ||
| ssc-miR-338 | -3.55 ± 0.99 | 0.022 | x | x | ||
| ssc-miR-340 | -2.13 ± 0.96 | 0.038 | x | x | ||
| ssc-miR-424 | -2.37 ± 1.14 | 0.094 | x | x | x | x |
| ssc-miR-486 | 2.59 ± 0.38 | 0.001 | x | x | ||
| ssc-miR-494 | -5.83 ± 3.53 | 0.063 | x | |||
| ssc-miR-497 | -2.19 ± 0.35 | 0.001 | x | x | x |
Distribution of miRs with a greater than two fold change organized by functional domain from exercised pigs (n = 9). All values reported as mean fold change ± SEM.
In order to examine the relationship between the miRs that changed with exercise (Table 2) with changes in LV regional myocardial stiffness and chamber stiffness, a correlation analysis was performed (S2 Table). A total of 8 miRs were significantly and positively correlated to either LV regional or chamber stiffness (Table 3). All 8 miRs decreased with exercise, which was associated with concomitant reductions in LV stiffness properties. The strongest correlation was observed between miR-142 and LV chamber stiffness. It is noteworthy to recognize that miR-142 is the only miR identified as having a significant association that does not belong to the myocardial growth functional group. Finally, miR-30e mapped to several metallopeptidases which would also potentially influence ECM structure and function [31]. Specifically, miR-30e likely affects post-transcriptional regulation of the metallopeptidase. Both miR-99a and miR-497 mapped to heparin sulfate proteoglycans, which have been implicated in myocardial healing and protection [32]. A literature review (S3) was utilized to place the 8 miRs that correlated to LV stiffness indices and demonstrated regulation of processes involved in adaptive remodeling such as inflammation, ECM remodeling and calcium handling (Table 4). This study mapped the specific changes in myocardial miR levels to potential functional domains, identified a relationship to LV diastolic function and, as outlined in the following paragraph, identified relevance to localized myocardial inflammation.
Table 3. Correlation between myocardial miR levels and stiffness indices.
| miR | Regional Myocardial Stiffness | LV Chamber Stiffness | ||
|---|---|---|---|---|
| Correlation Coefficient | p-value | Correlation Coefficient | p-value | |
| ssc-let-7a | -0.303 | 0.273 | -0.468 | 0.078 |
| ssc-let-7c | -0.422 | 0.118 | -0.492 | 0.062 |
| ssc-let-7d-5p | -0.282 | 0.309 | -0.496 | 0.060 |
| ssc-let-7e | -0.218 | 0.434 | -0.462 | 0.083 |
| ssc-miR-15b | -0.282 | 0.308 | -0.508 | 0.053 |
| ssc-miR-19a | 0.263 | 0.344 | 0.543 | 0.037 |
| ssc-miR-22 | 0.283 | 0.307 | 0.646 | 0.009 |
| ssc-miR-30e | 0.579 | 0.024 | 0.671 | 0.006 |
| ssc-miR-99a | 0.515 | 0.050 | 0.638 | 0.010 |
| ssc-miR-142 | 0.388 | 0.153 | 0.814 | 0.000 |
| ssc-miR-144 | 0.256 | 0.399 | 0.613 | 0.026 |
| ssc-miR-199a | 0.078 | 0.782 | 0.575 | 0.025 |
| ssc-miR-208b | 0.156 | 0.579 | 0.452 | 0.091 |
| ssc-miR-320 | -0.445 | 0.096 | -0.441 | 0.100 |
| ssc-miR-486 | -0.287 | 0.300 | -0.496 | 0.060 |
| ssc-miR-497 | 0.391 | 0.149 | 0.726 | 0.002 |
Table 4. miRs with association to LV stiffness indices and functional domain.
| miR | Inflammation | ECM | Calcium Handling |
|---|---|---|---|
| ssc-miR-19a | x | x | x |
| ssc-miR-22 | x | x | x |
| ssc-miR-30e | x | x | |
| ssc-miR-99a | x | ||
| ssc-miR-142 | x | x | x |
| ssc-miR-144 | x | x | x |
| ssc-miR-199a | x | x | x |
| ssc-miR-497 | x |
miR profiles and localized inflammation
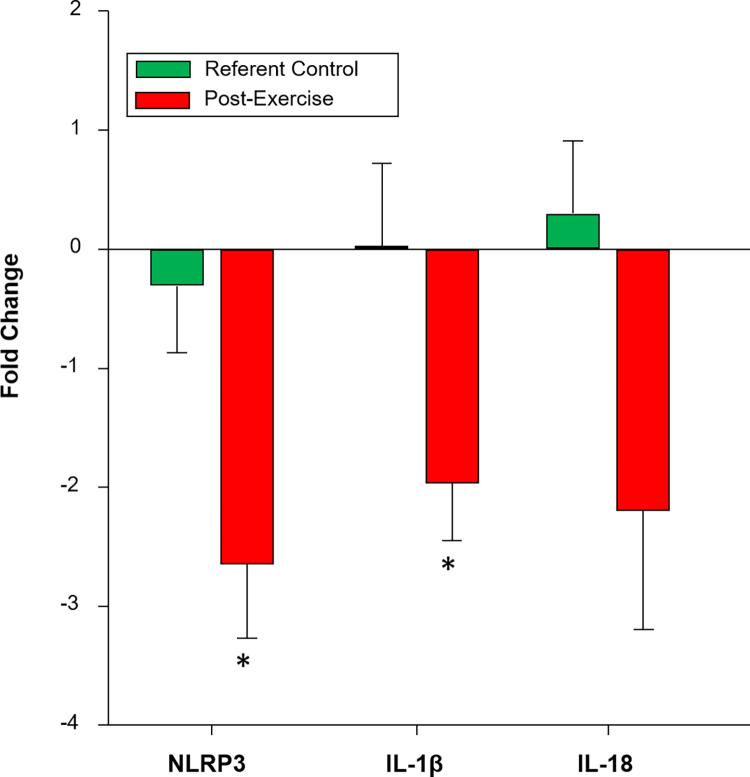
Several miRs mapping to local myocardial inflammatory pathways changed with exercise, which included miR-22 and miR-30e. Past studies have identified shifts in local inflammation with LV remodeling and exercise training [33–36]. Specifically, studies have identified the intracellular formation of the inflammasome, notably the NOD-LRR pyrin containing protein 3 (NLRP3), which in turn causes release of cytokine signaling molecules such as interleukin-1beta (IL1-β) and interleukin-18 (IL-18) [37, 38]. Using miR mapping algorithms (TargetScan-http://www.targetscan.org/vert_72/), several of the miRs which changed with exercise mapped to the NLRP3 inflammasome and its downstream effector proteins IL-1β and IL-18, including miR-22 and miR-30e. Myocardial mRNA analysis for these localized inflammatory components was performed using extraction approaches described previously [15]. Briefly, targeted qPCR was performed using individual primers for NLRP3 (TaQMan, Ss04953519_m1, ThermoFisher, Waltham, MA), IL-18 (TaQMan, Ss03391203_m1, ThermoFisher), and IL-1β (TaQMan, Ss03393804_m1, ThermoFisher). The maximum threshold cycle (CT) for detection was set at 35 CTs. The CT value of GAPDH (TaQMan, Ss03375629_u1, ThermoFisher) was used as the reference gene value for normalization. An approximately 2-fold down regulation of mRNA levels for NLRP3, IL-1β, and IL-18 was observed following the exercise protocol (Fig 3). Furthermore, a strong positive correlation was observed between mRNA levels and LV chamber stiffness for NLRP3 (Spearman’s rho = 0.701, p<0.05), and IL-1β (Spearman’s rho = 0.643, p<0.05).
Fig 3. Fold change with steady state myocardial mRNA levels for components of the inflammasome.
NLRP3 = NOD-LRR pyrin containing protein 3; IL-18 = interleukin 18; IL-1β = interleukin 1 beta. Values are presented as fold change mean ± SEM. *p < 0.05 vs referent control. IL-18 p = 0.083.
Discussion
The overarching goal of the present study was to utilize a pig model of exercise training to examine LV function, particularly stiffness properties and the relation to changes in myocardial miRs that may be relevant to the regulation of LV structure and function. The new and unique findings from this study were three-fold. First, a standardized exercise training protocol in pigs induced LV hypertrophy as evidenced by increased LV mass, increased LV pump function as assessed by LV EF, and caused a reduction in both LV myocardial and chamber stiffness. Second, the exercise regimen caused a shift in the myocardial miR profile, in which a subset of miRs were moderately-to-strongly associated with LV stiffness properties. These miRs mapped to regulation of active relaxation (calcium handling) or to the ECM, both of which contribute to LV stiffness. Third, a subset of miRs which mapped to local inflammation were identified to change with exercise, and in particular the local inflammasome (NLRP3) pathway. The exercise protocol reduced myocardial NLRP3 expression. Taken together, these findings identified key miRs which influence LV form and function following an exercise protocol and may serve as a biomarker signature for evaluation of exercise efficacy.
LV stiffness properties and exercise
While past studies have identified the potential benefits of a structured exercise regimen, particularly in patients with pre-existing cardiovascular conditions [39–45], the mechanistic underpinnings with respect to changes in LV structure and function remain unclear. The HF-ACTION clinical trial established that in patients with heart failure, a reduction in all cause-mortality could be achieved with exercise training [45]. In another clinical study, chronic heart failure patients underwent 2 weeks of in-hospital exercise followed by six months of home-based exercise [39]. In this study, resting HR was significantly reduced, LV mass increased, and resting LV EF and EDV were significantly improved in the exercise group- all similar findings to our present study. In a large clinical study of patients without a diagnosis of heart failure, increased physical activity was associated with reduced cardiovascular outcomes, suggestive of improved LV function [46]. However, extrapolation of the findings from the present study to a clinical context may be problematic. Specifically, the present study utilized a training protocol in young pigs in the absence of cardiovascular disease. Nevertheless, the unique findings of the present study provided a relationship betweeen a functional (LV stiffness properties) outcome and a molecular response (shifts in miRs) with an exercise protocol.
One of the underlying factors for exercise intolerance, an indication of heart failure, particularly in the absence of significant coronary artery disease, is impaired LV diastolic function due to increased LV stiffness properties [43]. However, clinical studies which have examined the effects of exercise and LV stiffness properties, both at the chamber and myocardial level, are limited. This is due to in part to problematic issues of outpatient based exercise programs and imaging modalities. In a small clinical study, patients with LV hypertrophy and evidence of increased LV filling pressures were randomized to a high-intensity exercise protocol and LV chamber stiffness was computed using LV EDV and pressure estimates [44]. At the conclusion of the year-long study LV chamber stiffness was reduced by approximately 50% in the exercise group, but was unchanged in sedentary controls. In another clinical study [47], the same group demonstrated that an exercise program could attenuate the progressive increase in LV chamber stiffness- an all too common consequence of aging and a sedentary lifestyle. While these clinical studies demonstrated a positive impact on LV chamber stiffness with a structured exercise program, the underlying contributory mechanisms for these effects remained unclear. LV chamber stiffness is determined by a summation of active and passive processes during diastole [48]. Specifically, impairment in active relaxation processes (i.e. calcium handling and myofilament interactions) will increase LV chamber stiffness. Changes in myocardial structure (such as ECM) influence passive stiffness properties, most commonly measured by LV myocardial stiffness. In the present large animal study a chronic exercise protocol reduced LV chamber stiffness as well as myocardial stiffness. These results imply that a contributory mechanism for the reduction in LV chamber stiffness observed in past clinical reports in an exercise protocol is due, at least in part, to a reduction in LV myocardial stiffness. The present study also established that a post-transcriptional mechanism for this reduction in myocardial stiffness was a reduction in specific miRs which regulate ECM and inflammation.
Several previous studies used large animal models of exercise, but were primarily focused upon mechanisms contributing to LV pump function and contractility [7, 8, 49, 50]. In one study, progressive treadmill training in pigs resulted in increased myofilament force production and computed myocardial power [7]. In another study, exercise training in pigs was shown to shift calcium myofilament sensitivity and also change active relaxation kinetics [51]. The relevance of these past studies to the present study are three-fold. First, these past studies identified increased myocyte/myofilament contractility and thus a likely contributing factor for the increased LV pump function observed in the present study following a treadmill exercise protocol. Second, these past studies identified shifts in calcium myofilament sensitivity and improved active relaxation and are likely contributing factors for the reduction in LV myocardial stiffness observed in the present study following the exercise protocol. Thirdly, as detailed in the subsequent section, the present study identified shifts in miR profiles that map to key factors in calcium handling and sensitivity and represent a potential molecular mechanism contributing to improved myocyte and myofilament function reported in past studies following an exercise protocol.
LV myocardial miRs and exercise
Previous human studies evaluating miR levels following exercise training primarily include miR levels taken from peripheral blood samples of elite athletes with years of training experience [12, 52] or populations with pre-existing conditions such as heart failure or cancer [14, 53, 54]. There are limited studies of healthy populations that have quantified miR levels following exercise training focused upon a pre-selected, restricted number of miRs taken from peripheral blood samples [11, 55–57]. In a study of sedentary but otherwise healthy adults (HERITAGE Family Study) [58], we examined plasma miR profiles before and after 20 weeks of a cycle ergometer based exercise training protocol. There were discordant findings between our past study and the present study with respect to certain miRs. Specifically, in the 20 week exercise protocol, no changes in let-7c, miR-181b, miR-23b, miR-320, and miR-98 were observed, whereas the present study identified increased LV myocardial levels of these miRs. In another study, plasma levels of miR-1 and miR-486 were increased in endurance athletes compared to healthy controls [59]. These observations are consistent with the findings from the present study in which increased myocardial levels of miR-1 and miR-486 were observed following a treadmill-based exercise training protocol in pigs. However, as stated previously, direct comparisons between this large animal exercise study to that of past exercise studies in humans may be problematic.
To our knowledge this is the first study to quantify myocardial miR profiles in a pig model of treadmill-based exercise. Previously, pigs have been utilized to quantify miR profiles in other organs [60, 61] and as a large animal model of exercise [62], but studies implementing both miR profiling and exercise are lacking. Studies utilizing other large animal models such as horses or dogs to quantify miR profiles following exercise included either training for the purposes of weight loss in overweight animals [63] or single bouts of exercise [64]. However, several studies have utilized rodents to examine changes in miRs with respect to a defined exercise training protocol [65–68]. For example, an 8 week treadmill protocol caused an increase in myocardial levels of miR-1 and miR-206 in mice [67]. Using a swimming training protocol in rats, decreased myocardial levels of miR-208b and miR-133a were reported [65, 68]. The directional changes in myocardial miRs reported in these past studies are similar to those observed in the present study in which a large, cardiac-focused miR array was utilized.
The present study identified a cluster of 8 miRs which were correlated with LV stiffness properties, and while this does not imply a cause-effect, these miRs did map to relevant pathways which could affect LV stiffness (Table 4). For example, miR-144 and miR-142 exhibit post-transcriptional control of calcium/calmodulin serine protein kinases, which in turn would affect calcium handling within the cardiomyocyte [69]. This in turn would affect LV active relaxation, an important component which contributes to overall LV chamber stiffness. In addition, miR-144 mapped to a critical pathway which affects ECM structure and composition: TGF-β [70]. Changes in TGF- β signaling, particularly through the SMAD signaling cascade, can directly alter collagen synthesis. Specifically, SMAD-7 has been shown to exert an inhibitory effect on the TGF intracellular signaling cascade [71, 72]. Since miR-144 levels decreased with the exercise training protocol, then this may have altered TGF- β activation, and thus in turn reduced LV myocardial collagen accumulation, a key component of LV myocardial stiffness.
LV myocardial inflammation and exercise
A localized inflammatory cascade, defined as the inflammasome, has received recent attention in the context of LV myocardial structure and function [33, 34]. Specifically, the NLRP3 inflammasome has been shown to be activated in the context of ischemia and related LV dysfunction [73]. In the present study, several miRs (miR-22, miR-30e, miR-140, miR-199a, miR-210 and miR-497) which either map to or correlate with the NLRP3 inflammasome were altered within the LV myocardium following the treadmill exercise protocol. In fact, a strong correlation was observed between NLRP3 and the downstream effector, IL-1β, with LV stiffness. In a past study miR-146a was shown to be mechanistically linked to NLRP3 in the context of spinal cord injury [74]. In hepatic fibrosis miR-21 negatively regulated a key transcription factor necessary for NLRP3 expression [75]. Finally, in dendritic cells, miR-155 has been identified to negatively regulate components of the inflammasome cascade [76]. Taken together, results from previous studies and the current investigation suggest that a treadmill exercise protocol directly affects key myocardial miRs which in turn regulate the myocardial inflammasome. However, it must be recognized that the post-transcriptional regulation of the inflammasome is complex and different miRs may interfere with this process at multiple intersections.
In the present study, a specific pattern of miRs associated with localized inflammation such as the inflammasome, were altered with exercise training in pigs. Exercise-induced inhibition of the activation of the NLRP3 inflammasome and downstream effectors IL-1β and IL-18 has been reported in mice [77, 78]. In a limited number of clinical studies, a reduction in indices of inflammasome activation have been reported following a chronic exercise protocol [79, 80]. However, the underlying mechanisms by which a reduction in the inflammasome, such as NLRP3, occurs as a function of exercise is poorly understood. The present study identified that specific miRs, such as miR-30e, may be important in the post-transcriptional regulation of the inflammasome. Moreover, the present study suggests that profiling a specific cassette of miRs may provide a basis for identifying a beneficial effect of a prescribed exercise training program on localized inflammation. Past studies have examined the effects of exercise training on plasma miR profiles and our group has associated these changes with myocardial structure-function [11–14, 30, 52–59, 81]. However, the present study quantified myocardial miR levels following a specific exercise protocol and whether and to what degree these observations can be translated to plasma, and more importantly to patients following an exercise training protocol, will require future study.
Study limitations and summary
While the present study utilized a large animal model to examine LV function, particularly LV stiffness properties, and the relationship to myocardial miR profiles, there are several study limitations which must be recognized. First, the utilized training protocol, while standardized, did not perform oxygen saturation and consumption measurements, and therefore the relative periods of aerobic and anaerobic exercise was not determined. Second, the exercise protocol was performed in normal pigs and whether and to what degree this protocol can be utilized in the context of ischemia and pressure overload cardiac pathologies remains to be determined. Third, the pigs utilized in this study were 3 months of age and would be considered young since the age for sexual maturity is 5 months of age [82]. In addition, the pigs utilized in this study were castrated, which removes sex as a covariate in these studies. Nevertheless, this large animal model of chronic exercise did allow for assessing relevant measures of LV function, such as LV stiffness properties, which can be problematic in rodent models. Fourth, the present study quantified myocardial miRs using a large array using pre-specified normalization algorithms in terms of the total miR pool and referent normal values. This makes direct comparisons to past studies which utilized plasma samples problematic. Nevertheless, this is the first study to demonstrate a direct relationship between LV stiffness properties (both at the chamber and myocardial level) to shifts in the abundance of myocardial miRs, as well as to localized myocardial inflammation following a standardized exercise regimen. These findings may set the stage for translational studies which utilize a specific miR signature to identify exercise efficacy.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by National Institutes of Health grant R01HL130972-01A1 (F.G.S.) & R01HL5949 (F.G.S.) as well as a Merit Award from the Veterans Health Administration, BX000168-10A1 (F.G.S.) & BX005320 (F.G.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Whellan DJ, Nigam A, Arnold M, et al. Benefit of exercise therapy for systolic heart failure in relation to disease severity and etiology-findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training study. Am Heart J. 2011;162(6):1003–1010. doi: 10.1016/j.ahj.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58(17):1780–1791. doi: 10.1016/j.jacc.2011.06.054 [DOI] [PubMed] [Google Scholar]
- 3.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens. 2010;24(3):175–182. doi: 10.1038/jhh.2009.51 [DOI] [PubMed] [Google Scholar]
- 4.Unnithan VB, Rowland TW, George K, Lord R, Oxborough D. Left ventricular function during exercise in trained pre-adolescent soccer players. Scand J Med Sci Sports. 2018;28(11):2330–2338. doi: 10.1111/sms.13258 [DOI] [PubMed] [Google Scholar]
- 5.Zile M. R., & Brutsaert D. L. (2002). New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation, 105(11), 1387–1393. doi: 10.1161/hc1102.105289 [DOI] [PubMed] [Google Scholar]
- 6.Trifunović-Zamaklar D., Jovanović I., Vratonjić J., Petrović O., Paunović I., Tešić M., et al. (2022). The basic heart anatomy and physiology from the cardiologist’s perspective: Toward a better understanding of left ventricular mechanics, systolic, and diastolic function. Journal of clinical ultrasound: JCU, 50(8), 1026–1040. doi: 10.1002/jcu.23316 [DOI] [PubMed] [Google Scholar]
- 7.Hinken AC, Korte FS, McDonald KS. Porcine cardiac myocyte power output is increased after chronic exercise training. J Appl Physiol (1985). 2006;101(1):40–46. doi: 10.1152/japplphysiol.00798.2005 [DOI] [PubMed] [Google Scholar]
- 8.Kuster DW, Merkus D, Blonden LA, et al. Gene reprogramming in exercise-induced cardiac hypertrophy in swine: A transcriptional genomics approach. J Mol Cell Cardiol. 2014;77:168–174. doi: 10.1016/j.yjmcc.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 9.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315 [DOI] [PubMed] [Google Scholar]
- 10.Bronze-da-Rocha E. MicroRNAs expression profiles in cardiovascular diseases. Biomed Res Int. 2014;2014:985408. doi: 10.1155/2014/985408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoi W, Ichikawa H, Mune K, et al. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol. 2013;4:80. Published 2013 Apr 11. doi: 10.3389/fphys.2013.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baggish AL, Hale A, Weiner RB, et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589(Pt 16):3983–3994. doi: 10.1113/jphysiol.2011.213363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q., Shi C., Lv Y., Zhao C., Jiao Z., & Wang T. (2020). Circulating microRNAs in Response to Exercise Training in Healthy Adults. Frontiers in genetics, 11, 256. doi: 10.3389/fgene.2020.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oatmen KE, Toro-Salazar OH, Hauser K, et al. Identification of a novel microRNA profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am J Physiol Heart Circ Physiol. 2018;315(5):H1443–H1452. doi: 10.1152/ajpheart.00252.2018 [DOI] [PubMed] [Google Scholar]
- 15.Torres WM, Jacobs J, Doviak H, et al. Changes in myocardial microstructure and mechanics with progressive left ventricular pressure overload. JACC Basic Transl Sci, in press [DOI] [PMC free article] [PubMed]
- 16.Devereux R B et al. “Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings.” The American journal of cardiology vol. 57,6 (1986): 450–8. doi: 10.1016/0002-9149(86)90771-x [DOI] [PubMed] [Google Scholar]
- 17.Batson G A et al. “Comparison of pulmonary wedge pressure measured by the flow directed Swan-Ganz catheter with left atrial pressure.” British heart journal vol. 33,4 (1971): 616. [PubMed] [Google Scholar]
- 18.Hatle L., Angelsen B. A., & Tromsdal A. (1980). Non-invasive assessment of aortic stenosis by Doppler ultrasound. British heart journal, 43(3), 284–292. doi: 10.1136/hrt.43.3.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging [published correction appears in Eur Heart J Cardiovasc Imaging. 2018 Jul 1;19(7):830–833]. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600. [DOI] [PubMed] [Google Scholar]
- 20.Kelley KW, Curtis SE, Marzan GT, Karara HM, Anderson CR. Body surface area of female swine. J Anim Sci. 1973;36(5):927–930. doi: 10.2527/jas1973.365927x [DOI] [PubMed] [Google Scholar]
- 21.Torres WM, Jacobs J, Doviak H, et al. Regional and temporal changes in left ventricular strain and stiffness in a porcine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2018;315(4):H958–H967. doi: 10.1152/ajpheart.00279.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 23.Nishimura R. A., & Tajik A. J. (1988). Determination of left-sided pressure gradients by utilizing Doppler aortic and mitral regurgitant signals: validation by simultaneous dual catheter and Doppler studies. Journal of the American College of Cardiology, 11(2), 317–321. doi: 10.1016/0735-1097(88)90096-4 [DOI] [PubMed] [Google Scholar]
- 24.Martinez M. W., Kim J. H., Shah A. B., Phelan D., Emery M. S., Wasfy M. M., et al. (2021). Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology, 78(14), 1453–1470. doi: 10.1016/j.jacc.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 25.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–530. doi: 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L, Zhou G, Soufan O, Xia J. miRNet 2.0: network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020;48(W1):W244–W251. doi: 10.1093/nar/gkaa467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Song Y, Liu L, et al. miR-199a impairs autophagy and induces cardiac hypertrophy through mTOR activation. Cell Death Differ. 2017;24(7):1205–1213 doi: 10.1038/cdd.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang K, Shi J, Hu Z, Hu X. The deficiency of miR-214-3p exacerbates cardiac fibrosis via miR-214-3p/NLRC5 axis. Clin Sci (Lond). 2019;133(17):1845–1856. Published 2019 Sep 10. doi: 10.1042/CS20190203 [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Yan X, Yan L, et al. Inhibition of microRNA‑155 ameliorates cardiac fibrosis in the process of angiotensin II‑induced cardiac remodeling. Mol Med Rep. 2017;16(5):7287–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obradovic D, Rommel KP, Blazek S, et al. The potential role of plasma miR-155 and miR-206 as circulatory biomarkers in inflammatory cardiomyopathy. ESC Heart Fail. 2021;8(3):1850–1860. doi: 10.1002/ehf2.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konttinen Y. T., Ainola M., Valleala H., Ma J., Ida H., Mandelin J., et al. (1999). Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Annals of the rheumatic diseases, 58(11), 691–697. doi: 10.1136/ard.58.11.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korf-Klingebiel M, Reboll MR, Grote K, et al. Heparan Sulfate-Editing Extracellular Sulfatases Enhance VEGF Bioavailability for Ischemic Heart Repair [published correction appears in Circ Res. 2020 Mar 27;126(7):e36]. Circ Res. 2019;125(9):787–801. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi M. Role of NLRP3 Inflammasome in Cardiac Inflammation and Remodeling after Myocardial Infarction. Biol Pharm Bull. 2019;42(4):518–523. doi: 10.1248/bpb.b18-00369 [DOI] [PubMed] [Google Scholar]
- 34.Suetomi T, Willeford A, Brand CS, et al. Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca2+/Calmodulin-Dependent Protein Kinase II δ Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation. 2018;138(22):2530–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- 36.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res. 2012;110(1):126–144. doi: 10.1161/CIRCRESAHA.111.243170 [DOI] [PubMed] [Google Scholar]
- 37.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Zhang S, Xiao Y, et al. NLRP3 Inflammasome and Inflammatory Diseases. Oxid Med Cell Longev. 2020;2020:4063562. Published 2020 Feb 17. doi: 10.1155/2020/4063562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095 [DOI] [PubMed] [Google Scholar]
- 40.Woolstenhulme JG, Guccione AA, Herrick JE, et al. Left Ventricular Function Before and After Aerobic Exercise Training in Women With Pulmonary Arterial Hypertension. J Cardiopulm Rehabil Prev. 2019;39(2):118–126. doi: 10.1097/HCR.0000000000000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fontes-Carvalho R, Sampaio F, Teixeira M, Gama V, Leite-Moreira AF. The role of a structured exercise training program on cardiac structure and function after acute myocardial infarction: study protocol for a randomized controlled trial. Trials. 2015;16:90. Published 2015 Mar 12. doi: 10.1186/s13063-015-0612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson GA, Wilkins GT, Cotter JD, Lamberts RR, Lal S, Baldi JC. HIIT Improves Left Ventricular Exercise Response in Adults with Type 2 Diabetes. Med Sci Sports Exerc. 2019;51(6):1099–1105. doi: 10.1249/MSS.0000000000001897 [DOI] [PubMed] [Google Scholar]
- 43.Del Buono MG, Arena R, Borlaug BA, et al. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(17):2209–2225. doi: 10.1016/j.jacc.2019.01.072 [DOI] [PubMed] [Google Scholar]
- 44.Hieda M, Sarma S, Hearon CM Jr, et al. One-Year Committed Exercise Training Reverses Abnormal Left Ventricular Myocardial Stiffness in Patients With Stage B Heart Failure With Preserved Ejection Fraction. Circulation. 2021;144(12):934–946. doi: 10.1161/CIRCULATIONAHA.121.054117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J Am Coll Cardiol. 2019. Apr 30;73(16):2062–2072. doi: 10.1016/j.jacc.2019.02.031 Erratum in: J Am Coll Cardiol. 2019 Jun 4;73(21):2789. . [DOI] [PubMed] [Google Scholar]
- 47.Howden EJ, Sarma S, Lawley JS, et al. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation. 2018;137(15):1549–1560. doi: 10.1161/CIRCULATIONAHA.117.030617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction. J Cardiovasc Transl Res. 2013;6(4):501–515. doi: 10.1007/s12265-013-9472-1 [DOI] [PubMed] [Google Scholar]
- 49.Emter CA, Baines CP. Low-intensity aerobic interval training attenuates pathological left ventricular remodeling and mitochondrial dysfunction in aortic-banded miniature swine. Am J Physiol Heart Circ Physiol. 2010;299(5):H1348–H1356. doi: 10.1152/ajpheart.00578.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK. Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca2⁺-sensitive K⁺ current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol. 2011;301(4):H1687–H1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarin V, Muthuchamy M, Heaps CL. Ca2⁺ sensitization of cardiac myofilament proteins contributes to exercise training-enhanced myocardial function in a porcine model of chronic occlusion. Am J Physiol Heart Circ Physiol. 2011;301(4):H1579–H1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F, Bai M, Xu J, Zhu L, Liu C, Duan R. Long-Term Exercise Alters the Profiles of Circulating Micro-RNAs in the Plasma of Young Women. Front Physiol. 2020;11:372. Published 2020 May 8. doi: 10.3389/fphys.2020.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witvrouwen I, Gevaert AB, Possemiers N, et al. Circulating microRNA as predictors for exercise response in heart failure with reduced ejection fraction. Eur J Prev Cardiol. 2021;28(15):1673–1681. doi: 10.1093/eurjpc/zwaa142 [DOI] [PubMed] [Google Scholar]
- 54.Pulliero A, You M, Chaluvally-Raghavan P, et al. Anticancer effect of physical activity is mediated by modulation of extracellular microRNA in blood. Oncotarget. 2020;11(22):2106–2119. Published 2020 Jun 2. doi: 10.18632/oncotarget.27609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wardle SL, Bailey ME, Kilikevicius A, et al. Plasma microRNA levels differ between endurance and strength athletes. PLoS One. 2015;10(4):e0122107. Published 2015 Apr 16. doi: 10.1371/journal.pone.0122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos AE, Lo C, Estephan LE, et al. Specific circulating microRNAs display dose-dependent responses to variable intensity and duration of endurance exercise. Am J Physiol Heart Circ Physiol. 2018;315(2):H273–H283. doi: 10.1152/ajpheart.00741.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baggish AL, Park J, Min PK, et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol (1985). 2014;116(5):522–531. doi: 10.1152/japplphysiol.01141.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barber JL, Zellars KN, Barringhaus KG, Bouchard C, Spinale FG, Sarzynski MA. The Effects of Regular Exercise on Circulating Cardiovascular-related MicroRNAs. Sci Rep. 2019;9(1):7527. Published 2019 May 17 doi: 10.1038/s41598-019-43978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denham J, Prestes PR. Muscle-Enriched MicroRNAs Isolated from Whole Blood Are Regulated by Exercise and Are Potential Biomarkers of Cardiorespiratory Fitness. Front Genet. 2016;7:196. Published 2016 Nov 15. doi: 10.3389/fgene.2016.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zichan H, Linfei J, Jinliang W, Zhiqiang S, Yimei C, Shu L. MicroRNA-294 Regulates Apoptosis of the Porcine Cerebellum Caused by Selenium Deficiency via Targeting iNOS. Biol Trace Elem Res. 2021;199(12):4593–4603. doi: 10.1007/s12011-021-02583-8 [DOI] [PubMed] [Google Scholar]
- 61.Qiu J, Zhang J, Zhou Y, et al. MicroRNA-7 inhibits melatonin synthesis by acting as a linking molecule between leptin and norepinephrine signaling pathways in pig pineal gland. J Pineal Res. 2019;66(3):e12552. doi: 10.1111/jpi.12552 [DOI] [PubMed] [Google Scholar]
- 62.Stam K, van Duin RWB, Uitterdijk A, Cai Z, Duncker DJ, Merkus D. Exercise facilitates early recognition of cardiac and vascular remodeling in chronic thromboembolic pulmonary hypertension in swine. Am J Physiol Heart Circ Physiol. 2018;314(3):H627–H642. doi: 10.1152/ajpheart.00380.2017 [DOI] [PubMed] [Google Scholar]
- 63.Herrera Uribe J, Vitger AD, Ritz C, Fredholm M, Bjørnvad CR, Cirera S. Physical training and weight loss in dogs lead to transcriptional changes in genes involved in the glucose-transport pathway in muscle and adipose tissues. Vet J. 2016;208:22–27. doi: 10.1016/j.tvjl.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 64.de Oliveira GP Jr, Porto WF, Palu CC, et al. Effects of endurance racing on horse plasma extracellular particle miRNA. Equine Vet J. 2021;53(3):618–627. doi: 10.1111/evj.13300 [DOI] [PubMed] [Google Scholar]
- 65.Soci U. P., Fernandes T., Hashimoto N. Y., Mota G. F., Amadeu M. A., Rosa K. T., et al. (2011). MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiological genomics, 43(11), 665–673. doi: 10.1152/physiolgenomics.00145.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dastah S, Tofighi A, Bonab SB. The effect of aerobic exercise on the expression of mir-126 and related target genes in the endothelial tissue of the cardiac muscle of diabetic rats. Microvasc Res. 2021;138:104212. doi: 10.1016/j.mvr.2021.104212 [DOI] [PubMed] [Google Scholar]
- 67.Yang J, Xu L, Yin X, et al. Excessive Treadmill Training Produces different Cardiac-related MicroRNA Profiles in the Left and Right Ventricles in Mice. Int J Sports Med. 2022;43(3):219–229. doi: 10.1055/a-1539-6702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soci UPR, Fernandes T, Barauna VG, et al. Epigenetic control of exercise training-induced cardiac hypertrophy by miR-208. Clin Sci (Lond). 2016;130(22):2005–2015. doi: 10.1042/CS20160480 [DOI] [PubMed] [Google Scholar]
- 69.Nafzger S., & Rougier J. S. (2017). Calcium/calmodulin-dependent serine protein kinase CASK modulates the L-type calcium current. Cell calcium, 61, 10–21. doi: 10.1016/j.ceca.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Yi J, Ye R, et al. miR-144 regulates transforming growth factor-β1 iduced hepatic stellate cell activation in human fibrotic liver. Int J Clin Exp Pathol. 2015;8(4):3994–4000. Published 2015 Apr 1. [PMC free article] [PubMed] [Google Scholar]
- 71.Kamiya Y, Miyazono K, Miyazawa K. Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction. J Biol Chem. 2010;285(40):30804–30813. doi: 10.1074/jbc.M110.166140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J Cell Biol. 2001;155(6):1017–1027. doi: 10.1083/jcb.200106023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westman PC, Lipinski MJ, Luger D, et al. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J Am Coll Cardiol. 2016;67(17):2050–2060. [DOI] [PubMed] [Google Scholar]
- 74.Tan Y., Yu L., Zhang C., Chen K., Lu J., & Tan L. (2018). miRNA-146a attenuates inflammation in an in vitro spinal cord injury model via inhibition of TLR4 signaling. Experimental and therapeutic medicine, 16(4), 3703–3709. doi: 10.3892/etm.2018.6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ning ZW, Luo XY, Wang GZ, et al. MicroRNA-21 Mediates Angiotensin II-Induced Liver Fibrosis by Activating NLRP3 Inflammasome/IL-1β Axis via Targeting Smad7 and Spry1. Antioxid Redox Signal. 2017;27(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ceppi M, Pereira PM, Dunand-Sauthier I, et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A. 2009;106(8):2735–2740. doi: 10.1073/pnas.0811073106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Javaid HMA, Sahar NE, ZhuGe DL, Huh JY. Exercise Inhibits NLRP3 Inflammasome Activation in Obese Mice via the Anti-Inflammatory Effect of Meteorin-like. Cells. 2021;10(12):3480. Published 2021 Dec 9. doi: 10.3390/cells10123480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang W, Liu L, Wei Y, et al. Exercise suppresses NLRP3 inflammasome activation in mice with diet-induced NASH: a plausible role of adropin. Lab Invest. 2021;101(3):369–380. doi: 10.1038/s41374-020-00508-y [DOI] [PubMed] [Google Scholar]
- 79.Khakroo Abkenar I, Rahmani-Nia F, Lombardi G. The Effects of Acute and Chronic Aerobic Activity on the Signaling Pathway of the Inflammasome NLRP3 Complex in Young Men. Medicina (Kaunas). 2019;55(4):105. Published 2019 Apr 15. doi: 10.3390/medicina55040105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barrón-Cabrera E, González-Becerra K, Rosales-Chávez G, Mora-Jiménez A, Hernández-Cañaveral I, Martínez-López E. Low-grade chronic inflammation is attenuated by exercise training in obese adults through down-regulation of ASC gene in peripheral blood: a pilot study. Genes Nutr. 2020;15(1):15. Published 2020 Aug 27. doi: 10.1186/s12263-020-00674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szelenberger R, Kacprzak M, Saluk-Bijak J, Zielinska M, Bijak M. Plasma MicroRNA as a novel diagnostic. Clin Chim Acta. 2019;499:98–107. doi: 10.1016/j.cca.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 82.Fox J. G., Anderson L. C., Otto G. M., Pritchett-Corning K. R., & Whary M. T. (2015). Laboratory animal medicine. Elsevier/Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.