Abstract
β-TrCP, the F-box protein of the SCFβ-TrCP ubiquitin ligase (SCF, Skp1/Cul1/F-box protein), recognizes the doubly phosphorylated DSG motif (DpSGΦXpS) in various SCFβ-TrCP target proteins. The Cdc25A phosphatase, a key cell-cycle regulator in vertebrate cells, undergoes a rapid ubiquitin-dependent degradation in response to genotoxic stress. β-TrCP binds to the DSG motif of human Cdc25A in a manner dependent on Chk1 and other unknown kinases. However, Xenopus Cdc25A does not have a DSG motif at the corresponding site of human Cdc25A. Here, we report that both Xenopus Cdc25A and human Cdc25A have a previously undescribed nonphosphorylated DDG motif (DDGΦXD) for recognition by β-TrCP. When analyzed by using Xenopus eggs, the binding of β-TrCP to the DDG motif is essential for the Chk1-induced ubiquitination and degradation of Xenopus Cdc25A and also plays a role in the degradation of human Cdc25A. The DDG motif also exists in human Cdc25B phosphatase (another key cell-cycle regulator), binds β-TrCP strongly, and is essential for the ubiquitination and degradation of the (labile) phosphatase in normal conditions. We provide strong evidence that, in both Cdc25A and Cdc25B, the binding (efficiency) of β-TrCP to the DDG motif is regulated by nearby residues, while ubiquitination is regulated by other events in addition to the β-TrCP binding. Finally, our additional data suggest that β-TrCP may recognize nonphosphorylated DDG-like motifs in many other proteins, including X11L (a putative suppressor of β-amyloid production) and hnRNP-U (a pseudosubstrate of SCFβ-TrCP).
The SCF (Skp1/Cul1/F-box protein) complex E3 ubiquitin ligases target many proteins for proteolysis in diverse cellular processes (reviewed in ref. 1). There are numerous F-box proteins that serve as the substrate recognition subunits for the SCF complexes (2). β-TrCP, a WD40 repeat-containing F-box protein of SCFβ-TrCP (3, 4), recognizes the doubly phosphorylated DSG motif (DpSGΦXpS, where Φ represents a hydrophobic and X represents any amino acid) in various SCFβ-TrCP target proteins, including IκB, β-catenin, and Emi1 (5-14). The phosphoserine residues within the DSG motif are essential for the target proteins to bind β-TrCP (5, 10, 15). Consistently, a recent crystal structure analysis shows that both of the phosphoserines within the DSG motif (of β-catenin) form specific hydrogen bonds and electrostatic interactions with residues in the WD40 domains of β-TrCP (16). From these studies, it is generally believed that β-TrCP recognizes only phosphorylated destruction motifs in SCFβ-TrCP target proteins (see refs. 1, 4, and 16).
The Cdc25A phosphatase, a key positive regulator of the cell cycle in vertebrates (reviewed in ref. 17), is relatively unstable during interphase of the normal cell cycle, due mainly to the activity of the SCF complex (18, 19). In response to DNA damage or stalled replication, activated Chk1 and Chk2 kinases hyperphosphorylate Cdc25A and target it more rapidly for (SCF-dependent) degradation (refs. 20-25; reviewed in ref. 26). Interestingly, recent studies indicate that β-TrCP binds to the DSG motif of human Cdc25A in a manner dependent on Chk1 and other unknown kinases, thereby ubiquitinating the phosphatase for degradation (refs. 27 and 28; reviewed in ref. 29). Curiously, however, Xenopus Cdc25A does not have a DSG motif at the corresponding site of human Cdc25A (30, 31), yet it does undergo a similar Chk1-dependent degradation in response to stalled replication (23, 32). Therefore, it will be of great interest to determine how Xenopus Cdc25A is degraded in response to Chk1 activation.
Cdc25B, another phosphatase that plays a key role in the G2/M transition of the mammalian cell cycle (33, 34), also undergoes a rapid ubiquitin-dependent degradation during the normal cell cycle (35). However, the degradation of Cdc25B, unlike that of Cdc25A, cannot be accelerated by Chk1 phosphorylation (20, 32), nor is its E3 ubiquitin ligase(s) known (17). Therefore, it will also be interesting to know how this phosphatase is degraded under normal conditions.
In this study, we identify an entirely nonphosphorylated DDG motif (DDGΦXD) as the destruction motif of Xenopus Cdc25A. Intriguingly, this previously undescribed motif is a unique β-TrCP binding site in Xenopus Cdc25A and is also present and functions in human Cdc25A. Furthermore, this motif exists in human Cdc25B and is a unique functional β-TrCP binding site in it. Our data provide important mechanistic insights into how β-TrCP binding and ubiquitination are regulated in Cdc25A/Cdc25B phosphatases and also suggest that β-TrCP may target many other proteins that have nonphosphorylated DDG-like motifs.
Materials and Methods
Eggs and Egg Extracts. Routinely, unfertilized eggs were treated with the calcium ionophore A23187 (1 μg/ml) (to induce egg activation, which mimics fertilization), cultured, and microinjected as described in ref. 23. In some experiments, activated eggs were treated with cycloheximide (500 μg/ml). Activated eggs expressing exogenous proteins were homogenized in 10-20 μl per egg of an extraction buffer (20 mM sodium phosphate, pH 8.0/80 mM β-glycerophosphate/0.2% Triton X-100/5 mM EGTA) in the presence of phosphatase inhibitors (5 mM NaF, 1 mM sodium orthovanadate, 5 μM okadaic acid, 1 μM microcystin LR, and 3 μM tautomycin) and protease inhibitors [one tablet of inhibitor mixture (Roche) per 25 ml] at 4°C. The homogenates were centrifuged briefly, and the resulting supernatants (egg extracts) were subjected to either immunoblotting or pull-down assays.
cDNAs and in Vitro Transcription. cDNAs encoding Xenopus Cdc25A, human Cdc25A, human Cdc25B, or Xenopus Δ60-Chk1 are described in ref. 32. A cDNA encoding Xenopus β-TrCP1 was isolated from an oocyte cDNA library. A cDNA encoding β-TrCP lacking the F-box domain (ΔF) was constructed as described in ref. 7. All of the cDNA constructs were subcloned into either the N-terminally Myc-tagged or GST-tagged pT7-G(UKII+) transcription vectors (23). In vitro mutagenesis and transcription of the cDNAs were performed as described in ref. 23.
Antibodies and Immunoblotting. Routinely, proteins from half an egg were analyzed by immunoblotting with anti-Myc antibody (A-14, Santa Cruz Biotechnology), anti-GST antibody (Z-5, Santa Cruz Biotechnology), or anti-ubiquitin antibody (FL-76, Santa Cruz Biotechnology), essentially as described in ref. 23.
GST-Pull-Down Assays. GST-fusion proteins were pulled down from egg extracts by using glutathione-Sepharose beads (Amersham Pharmacia), as described in ref. 32. Co-pulled-down proteins (routinely from 10 eggs) were then analyzed by immunoblotting with appropriate antibodies.
Cdc25A Peptide-β-TrCP Binding Assays. Synthetic peptides (CEGSDDGFLDMLD, CEGSDDGFLAMLD, CAASTSTEGSDDGFLDMLD, and CAApSTSpTEGSDDGFLDMLD) were immobilized to SulfoLink coupling beads (Pierce). The beads (coupled with 10 μg of peptides) were incubated with 200 μl of egg extracts (equivalent to 10 eggs and containing overexpressed β-TrCP) for 30 min at 4°C and pulled down for immunoblotting, as described in ref. 32.
Results
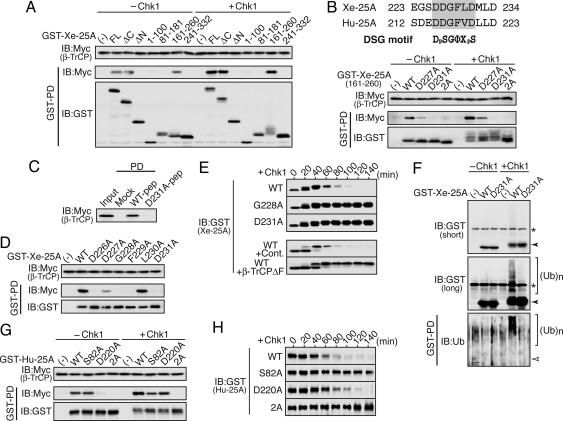
Binding of β-TrCP to Xe-Cdc25A. A recent study identified the DSG motif (in 76SSESTDSGFCLDS88) as the β-TrCP binding site of human (Hu) Cdc25A (27). Phosphorylation of Ser-76 by Chk1 is required for the essential phosphorylation of Ser-82 (within the DSG motif) by an as-yet-unidentified kinase and, hence, for β-TrCP binding in Hu-Cdc25A (28). Interestingly, Xenopus (Xe) Cdc25A does not have a DSG motif but instead has a “naturally mutated” DAG motif, at the corresponding site of Hu-Cdc25A (see refs. 30 and 31). Both the Xenopus DAG and human DSG motifs are embedded in the so-called PEST-like sequence [seen in many short-lived proteins (36)] (Fig. 1A). First, we determined whether the individual residues of the PEST-like sequence would be required for the Chk1-induced degradation of Xe-Cdc25A. For this, we expressed Xe-Cdc25A mutants (with substituted alanine at the individual residues of the PEST-like sequence) in Xenopus eggs and, after expression of a constitutively active form of Xe-Chk1 [Δ60-Chk1 (37)], monitored the levels of the mutants (23, 32). These analyses revealed that most of the residues, including Ser-73 (corresponding to human Ser-76), Asp-78 (Asp-81), Gly-80 (Gly-83), and Ser-85 (Ser-88), were important for Xe-Cdc25A degradation, whereas others, including Ser-76 (Ser-79) and Leu-81 (Leu-84), were less important (Fig. 1B). As was expected from these results, an additional mutant lacking the entire PEST-like sequence (ΔPEST) was very stable even after Δ60-Chk1 expression (Fig. 1C). Thus, although the PEST-like sequence of Xe-Cdc25A lacked the DSG motif (instead it had a DAG motif), it was required for the Chk1-induced degradation of the phosphatase.
Fig. 1.
Requirement of the PEST-like sequence for degradation, but not β-TrCP binding, of Xe-Cdc25A. (A) A schematic representation of Hu/Xe-Cdc25A protein. The PEST-like sequences of Xe-Cdc25A and Hu-Cdc25A are shown and aligned. Within its PEST-like sequence, Xe-Cdc25A has a DAG motif instead of the DSG motif. A previously undescribed DDG motif (DDGΦXD) conserved between Xe-Cdc25A and Hu-Cdc25A is also shown (see also Fig. 2B). RD, regulatory domain; CD, catalytic domain; CT, C-terminal tail. (B and C) Chk1-induced degradation of PEST-like sequence mutants of Xe-Cdc25A. Artificially activated eggs (which mimic fertilized eggs) were injected with 2 ng of mRNA encoding Myc-tagged WT Xe-Cdc25A or indicated Myc-tagged Xe-Cdc25A mutants, reinjected 2.5 h later with 2 ng of Δ60-Chk1 mRNA, and analyzed at 20-min intervals by immunoblotting with anti-Myc antibody. For WT Cdc25A, eggs expressing no Δ60-Chk1 (-Chk1) were also analyzed. Like WT Cdc25A, all of the mutants used were essentially stable in the absence of Δ60-Chk1 (not shown). (D) Binding of β-TrCP to Xe-Cdc25A constructs. Activated eggs were coinjected with 2 ng of mRNA encoding Myc-tagged β-TrCP and 4 ng of mRNA encoding GST alone (-) or indicated GST-Xe-Cdc25A constructs [WT, ΔPEST (lacking residues 73-86), or PEST (residues 38-116)], incubated for 2.5 h, reinjected or not with 2 ng of Δ60-Chk1 mRNA, and further incubated for 45 min (or until the time about the onset of WT Xe-Cdc25A degradation; see B). Egg extracts (Input; equivalent to half an egg) and GST-pulled-down proteins (GST-PD; equivalent to 10 eggs) were then immunoblotted (IB) for GST-Xe-Cdc25A constructs and Myc-β-TrCP.
We then tested whether the PEST-like sequence was required for β-TrCP binding (if any), by coexpressing in eggs GST-fused Xe-Cdc25A constructs (WT or ΔPEST) and Myc-tagged β-TrCP (Xe-β-TrCP1) and then by expressing Δ60-Chk1. [Samples were collected at the time of about the onset of degradation of WT Xe-Cdc25A (see legend of Fig. 1D).] Very interestingly, GST-pull-down assays showed that not only WT Xe-Cdc25A but also the ΔPEST mutant could efficiently bind β-TrCP after expression of Δ60-Chk1 (Fig. 1D). Somewhat surprisingly, even in the absence of Δ60-Chk1, both forms of Xe-Cdc25A could bind β-TrCP, albeit considerably less efficiently than in the presence of Δ60-Chk1. In contrast, an N-terminal fragment (residues 38-116) containing the PEST-like sequence could not bind β-TrCP at all in either condition (Fig. 1D). Thus, although the PEST-like sequence was required for Xe-Cdc25A degradation (Fig. 1C), some other sequence(s) was required for β-TrCP binding in Xe-Cdc25A.
Identification of a DDG Motif as the β-TrCP Binding Site in Xe-Cdc25A. To determine the sequence(s) responsible for β-TrCP binding in Xe-Cdc25A, we coexpressed various (GST-fused) fragments of Xe-Cdc25A and (Myc-tagged) β-TrCP in eggs and then performed GST-pull-down assays. These analyses revealed that β-TrCP could bind specifically to one N-terminal fragment (residues 161-260) of Xe-Cdc25A (Fig. 2A, -Chk1). After Δ60-Chk1 expression, this N-terminal fragment showed a considerably increased β-TrCP binding as well as prominent mobility up-shifts (due to phosphorylation; see Fig. 3C) (Fig. 2 A, +Chk1). Interestingly, in this fragment was present a sequence 226DDGFLD231 (hereafter called a DDG motif) that is also conserved in Hu-Cdc25A (Fig. 2B; see also Fig. 1 A). Because this acidic DDG motif resembles the conventional, doubly phosphorylated DSG motif (DpSGΦXpS), it might function to bind β-TrCP. Indeed, the single D227A and D231A mutations within the DDG motif were able to inhibit the N-terminal fragment from binding β-TrCP largely and completely, respectively, both in the presence and absence of Δ60-Chk1 (Fig. 2B). Moreover, and importantly, a short synthetic peptide (residues 223-234) spanning the DDG motif, but not a peptide with a mutated motif (D231A), was able to specifically bind β-TrCP in egg extracts (Fig. 2C). Thus, the DDG motif was the only site in the N-terminal fragment that could bind β-TrCP.
Fig. 2.
Requirement of a previously undescribed DDG motif for β-TrCP binding, ubiquitination, and degradation of Xe-Cdc25A (and Hu-Cdc25A). (A) Identification of a β-TrCP binding region of Xe-Cdc25A. Binding of β-TrCP to the indicated Xe-Cdc25A fragments was examined as in Fig. 1D. FL, full-length; ΔC, residues 1-332; ΔN, residues 333-521. (B) Conserved DDG motifs of Xe-Cdc25A and Hu-Cdc25A are shown together with the conventional DSG motif (Upper). Binding of β-TrCP to the indicated DDG motif mutants of the Xe-Cdc25A fragment (residues 161-260) was analyzed as in A (Lower). 2A, double alanine mutations of D227 and D231. (C) Binding of β-TrCP to the DDG motif peptides. Control beads (Mock) or Xe-Cdc25A peptide-bound (residues 223-234) beads (WT-pep or D231A-pep) were incubated with egg extracts (equivalent to 10 eggs) containing overexpressed Myc-β-TrCP protein, and co-pulled-down proteins (PD) were analyzed by immunoblotting with anti-Myc antibody. Input, β-TrCP equivalent to half an egg. (D) Binding of β-TrCP to the indicated DDG motif mutants of full-length Xe-Cdc25A was analyzed as in A but without Δ60-Chk1 expression. (E) Chk1-induced degradation of the indicated DDG motif mutants was analyzed as in Fig. 1B (Upper). Either GST (Cont.) or dominant-negative β-TrCPΔF was overexpressed in activated eggs before expressions of WT Xe-Cdc25A and Δ60-Chk1 (Lower). (F) Chk1-induced ubiquitination of Xe-Cdc25A in vivo. Immunoblot of the indicated GST-Cdc25A constructs (from one egg) was either short-exposed (Top) or long-exposed (Middle); GST-pulled-down proteins (from 10 eggs) were immunoblotted with anti-ubiquitin antibody (Bottom). Filled and open arrowheads indicate the positions of nonubiquitinated Xe-Cdc25A protein. An asterisk indicates a nonspecific background protein. (Ub)n indicates polyubiquitinated Xe-Cdc25A proteins. (G) Binding of β-TrCP to the indicated DSG or DDG motif mutants of Hu-Cdc25A was analyzed as in A. 2A, double Ala-mutations of S82 and D220. (H) Chk1-induced degradation of the indicated DSG or DDG motif mutants of Hu-Cdc25A was analyzed as in E.
Fig. 3.
Identification of regions and phosphorylation sites influencing the efficiencies of β-TrCP binding and ubiquitination of Xe-Cdc25A. (A) A sequence spanning the DDG motif (half-tone) of Xe-Cdc25A is shown with mutated residues being dotted (Top). Binding of Myc-β-TrCP to the indicated Ser/Thr → Ala mutants of GST-Xe-Cdc25A was analyzed by GST-pull-down assays as in Fig. 1D (Middle). Because of the different stabilities of the respective Xe-Cdc25A mutants (in +Chk1), the levels of co-pulled-down Myc-β-TrCP were normalized to the levels of (GST-pulled-down) Xe-Cdc25A proteins; the value obtained for WT Xe-Cdc25A (-Chk1) was set at 1.0 (Bottom; results from three independent experiments with standard deviations). (B) Chk1-induced degradation of the indicated mutants of Xe-Cdc25A was analyzed as in Fig. 1B.(C) Chk1-induced phosphorylation of Ser-219 and Thr-222. Activated eggs were injected with 2 ng of mRNA encoding GST-fused Xe-Cdc25A fragments (residues 161-260) (WT or S219A/T222A), cultured for 2 h, reinjected or not with Δ60-Chk1 mRNA, and further incubated for 1 h. Egg extracts were then treated or not with bacterial alkaline phosphatase (BAP) and analyzed by immunoblotting. (D) Binding of β-TrCP to the phosphorylated (pS219/pT222) or nonphosphorylated (S219/T222) peptides (residues 217-234) of Xe-Cdc25A was analyzed as in Fig. 2C. (E) β-TrCP binding and ubiquitination of the indicated mutants of Xe-Cdc25A (see text for the naming of the mutants) were analyzed as in A and Fig. 2F.
We then tested whether the DDG motif was essential for β-TrCP binding and ubiquitination/degradation of full-length Xe-Cdc25A, by using mutants in which the individual residues of the DDG motif were replaced by alanine. As shown in Fig. 2D, D226, G228, F229, and D231 were all essential, D227 was important, and L230 (a nonconsensus residue, according to the DSG motif) was nonessential for β-TrCP binding in full-length Xe-Cdc25A expressed in eggs (in the absence of Δ60-Chk1). We then compared stabilities of WT Xe-Cdc25A and two DDG motif mutants (G228A and D231A) after expression of Δ60-Chk1. These two mutants were both extremely stable even after Δ60-Chk1 expression, whereas WT Xe-Cdc25A was readily degraded (Fig. 2E Upper). Obviously, the degradation of WT Xe-Cdc25A depended on endogenous β-TrCP because it was largely inhibited by overexpression of dominant-negative β-TrCPΔF [which cannot bind to the Skp1 component of SCF (7)] (Fig. 2E Lower). Moreover, after expression of Δ60-Chk1, WT Xe-Cdc25A, but not the D231A mutant, did show high-molecular-weight bands (on longer exposure of the immunoblot) (Fig. 2F Top and Middle), which were due to polyubiquitination as shown by their cross-reactivity to anti-ubiquitin antibody (Fig. 2F Bottom). Thus, these results, together with the above results (Fig. 2 A-C), indicate that the DDG motif is a unique β-TrCP binding site of Xe-Cdc25A and is essential for the Chk1-induced ubiquitination and degradation of the phosphatase. The DDG motif was also essential for the degradation of Xe-Cdc25A at the aphidicolin-induced replication checkpoint in early embryos (data not shown), which is known to be caused by activation of (endogenous) Chk1 (23).
Occurrence of a Functional DDG Motif in Hu-Cdc25A. Intriguingly, Hu-Cdc25A also has a DDG motif at the corresponding site of Xe-Cdc25A (Fig. 2B Upper), in addition to the conventional DSG motif (Fig. 1 A). This finding prompted us to examine whether the DDG motif of Hu-Cdc25A would be functional. When expressed in eggs in the absence of Δ60-Chk1, both WT Hu-Cdc25A and its DSG motif mutant (S82A) could appreciably bind β-TrCP, whereas the DDG motif mutant (D220A) could not (Fig. 2G, -Chk1). After Δ60-Chk1 expression, however, the DDG mutant as well as WT Hu-Cdc25A showed a considerably increased β-TrCP binding, whereas the DSG mutant did not (Fig. 2G, +Chk1). In contrast to these, the DDG/DSG double mutant (2A) did not show any β-TrCP binding in either condition (Fig. 2G). Thus, these results indicate that the DDG motif can bind β-TrCP equally both in the presence and absence of Chk1 activation, whereas the DSG motif can do so only after Chk1 activation (and more strongly than the DDG motif). Consistent with it being capable of binding β-TrCP, the DDG motif contributed to the Chk1-induced degradation of Hu-Cdc25A, albeit to a significantly lesser degree than the DSG motif (Fig. 2H). Thus, it seems that even Hu-Cdc25A has a functional DDG motif.
Regulation of β-TrCP Binding and Ubiquitination of Xe-Cdc25A. In Xe-Cdc25A, the binding of β-TrCP to the (nonphosphorylatable) DDG motif considerably increased after expression of Δ60-Chk1 not only in full-length protein but also in the small N-terminal fragment (residues 161-260) (Figs. 1D and 2 A and B). This finding could imply that some modification, perhaps phosphorylation, of a nearby residues(s) increases the binding of the DDG motif to β-TrCP. To test this possibility, we mutated five serine/threonine residues (just upstream of the DDG motif) individually to alanine (see Fig. 3A Top). Although none of these mutations appreciably affected the (relative) binding of β-TrCP to the full-length Xe-Cdc25A in the absence of Δ60-Chk1, most of them, particularly the S219A and T222A mutations, significantly inhibited the increase of the relative binding after Δ60-Chk1 expression (Fig. 3A Middle and Bottom). Indeed, both the S219A and T222A mutations appreciably affected the degradation kinetics of Xe-Cdc25A after Δ60-Chk1 expression (Fig. 3B). Importantly, an N-terminal fragment having the S219A/T222A double mutations exhibited only a small mobility up-shift after Δ60-Chk1 expression, whereas the WT fragment showed a large phosphatase-sensitive mobility up-shift (Fig. 3C). Moreover, a synthetic peptide containing phosphorylated Ser-219/Thr-222 residues (plus the DDG motif) could reproducibly bind β-TrCP 1.5- to 2-fold more efficiently than the nonphosphorylated (as well as the Ala-substituted; data not shown) peptide in egg extracts (containing no Δ60-Chk1) (Fig. 3D). Thus, these results strongly suggest that at least Ser-219 and Thr-222 are phosphorylated after expression of activated Chk1, thereby contributing largely to the Chk1-induced increase of β-TrCP binding to Xe-Cdc25A. At present, however, it is questionable whether Chk1 phosphorylates Ser-219 and/or Thr-222 directly, because neither residue (in GST-fused peptides) could be phosphorylated by Chk1 in vitro (data not shown).
Somewhat surprisingly, both the S219A and T222A mutants still underwent a (rather) rapid degradation after Δ60-Chk1 expression, if not comparably to the WT form (Fig. 3B). This finding might imply that some other event(s) than the increase of β-TrCP binding also contributes to promoting ubiquitination (and hence degradation) of Xe-Cdc25A after Chk1 activation. The PEST-like sequence, Ser-73 (phosphorylated by an unknown kinase), and four other serine residues (Ser-120, -137, -190, and -295, each phosphorylated by Chk1) are all required for the efficient degradation of Xe-Cdc25A after Chk1 activation (Fig. 1 B and C) (23, 32). Therefore, we examined β-TrCP binding and ubiquitination of their mutants (ΔPEST, S73A, and 4A, respectively, which are all stable, albeit not perfectly, after expression of Δ60-Chk1; see above figures and references). Very interestingly, all of these mutants showed an essentially normal increase in β-TrCP binding after Δ60-Chk1 expression yet showed substantially less ubiquitination than WT Xe-Cdc25A (the perfectly stable DDG mutant, D231A, showed no ubiquitination) (Fig. 3E). Thus, it seems that the ubiquitination efficiency in Xe-Cdc25A is determined not only by the efficiency of β-TrCP binding but also by other factors, such as the PEST-like sequence, its phosphorylation on Ser-73, and Chk1 phosphorylation of other dispersed sites.
Occurrence of a DDG Motif in Cdc25B and Regulation of Its Binding to β-TrCP. Cdc25B phosphatase, another key cell-cycle regulator in mammals, undergoes a rapid ubiquitin-dependent (and Chk1-independent) degradation during the normal cell cycle (20, 23, 33, 35); however, its E3 ubiquitin ligase has not been identified (17). Intriguingly, we noticed that human Cdc25B (as well as other mammalian homologs) contains a DDG motif (254DDG-FVD259) approximately at the equivalent site of Xe-Cdc25A (Fig. 4A). We therefore tested whether the DDG motif would be involved in the degradation of Hu-Cdc25B under normal conditions. When expressed in normal eggs, WT Hu-Cdc25B showed efficient β-TrCP binding and even ubiquitination, whereas its DDG motif mutant (Mu) (G256A/D259A) did not (Fig. 4B). Moreover, when chased after treatment of the eggs with the protein synthesis inhibitor cycloheximide, WT Hu-Cdc25B disappeared very rapidly (indicating its rapid turnover), whereas the DDG motif mutant persisted (Fig. 4C). [Consistent with its higher stability, the DDG motif mutant was already present at significantly higher levels than the WT form at the time of cycloheximide addition (Fig. 4C; see also Fig. 4B).] Thus, the DDG motif of Hu-Cdc25B was able to bind β-TrCP and was essential for the ubiquitination and degradation of the (labile) phosphatase under normal conditions. Under these (normal) conditions, Xe-Cdc25A (as well as Hu-Cdc25A; data not shown) underwent only weak ubiquitination (Fig. 4B) and only slow degradation in a manner dependent on the DDG motif [presumably due to the very low basal activity of endogenous Chk1 in eggs (23)] (Fig. 4C). These results indicate that the DDG motif is a unique β-TrCP-binding destruction motif of Hu-Cdc25B under normal conditions.
Fig. 4.
Occurrence of a functional DDG motif in human Cdc25B. (A) Existence of a DDG-like motif (half-tone) in Hu-Cdc25B. (B) β-TrCP binding and ubiquitination of Xe-Cdc25A and Hu-Cdc25B were analyzed as in Fig. 3E but without Δ60-Chk1 expression. The arrowhead indicates the position of nonubiquitinated Xe-Cdc25A or Hu-Cdc25B proteins (Bottom; long exposure). Mu, a DDG motif mutant (both the third position Gly and the sixth position Asp in the DDG motif of Xe-Cdc25A or Hu-Cdc25B were replaced by Ala). (C) Activated eggs were injected with mRNA encoding GST-fused Xe-Cdc25A or Hu-Cdc25B constructs (WT or Mu; see above), treated 2.5 h later with cycloheximide (CHX), and analyzed at 30-min intervals by immunoblotting with anti-GST antibody. (D) Binding of β-TrCP to the indicated Asp/Glu → Ala mutants of Hu-Cdc25B (A, D250A; 2A, D250A/E252A; 3A, E248A/D250A/E252A) was analyzed as in B.
Hu-Cdc25B was readily ubiquitinated and was degraded much more rapidly than Xe-Cdc25A in normal eggs (Fig. 4 B and C). This result may well be due, at least in part, to the considerably (≈3-fold) stronger β-TrCP binding in Hu-Cdc25B than in Xe-Cdc25A (see Fig. 4B). If so, how can Hu-Cdc25B bind β-TrCP more strongly than Xe-Cdc25A in normal conditions, despite the strong resemblance of their DDG motifs (see Fig. 4A)? In Xe-Cdc25A, phosphorylation of the Ser/Thr residues just upstream of the DDG motif markedly increased β-TrCP binding after Chk1 activation (Fig. 3 A and C-E). Interestingly, in Hu-Cdc25B, there exist multiple acidic residues just upstream of the DDG motif (see Fig. 4A); therefore, these acidic residues might be responsible for the strong β-TrCP binding of Hu-Cdc25B even under normal conditions. Consistent with this idea, in normal eggs, combined mutations of the acidic residues to alanine (2A or 3A) decreased the levels of Hu-Cdc25B binding to β-TrCP down to the levels of Xe-Cdc25A binding (and, consequently, increased the Hu-Cdc25B protein levels) (Fig. 4D). Thus, it seems that negative charges (whether signal-induced or constitutive) near the DDG motif function to upregulate β-TrCP binding in both Xe-Cdc25A and Hu-Cdc25B phosphatases.
Discussion
It is generally believed that β-TrCP recognizes only phosphorylated destruction motifs in SCFβ-TrCP target proteins (1), as exemplified by more than a dozen known target proteins (ref. 4 and references therein, and refs. 5-14). Indeed, none of previous papers have ever described or even suggested that β-TrCP recognizes nonphosphorylated destruction motifs in target proteins (see refs. 1, 3, and 4). Clearly, as shown here, however, β-TrCP can bind to the entirely nonphosphorylated DDG motif not only in Xe-Cdc25A but also in Hu-Cdc25A (expressed in Xenopus eggs). The binding of β-TrCP to the DDG motif is essential for the Chk1-induced ubiquitination and degradation of Xe-Cdc25A (Fig. 2 E and F) and also contributes, albeit weakly, to the Chk1-induced degradation of Hu-Cdc25A (Fig. 2H). [In the case of Hu-Cdc25A degradation, the conventional DSG motif played a major role (Fig. 2 G and H), as shown recently in human cells (27, 28).] Importantly, the DDG motif also exists in human Cdc25B [whose E3 ubiquitin ligase has remained unclear (17)], binds β-TrCP strongly, and is essential for the ubiquitination and degradation of the (labile) phosphatase in normal conditions (Fig. 4 A-C). Thus, the DDG motif seems to serve as a bona fide β-TrCP-binding site for both Cdc25A and Cdc25B phosphatases, which play similar but distinct roles in progression through the cell cycle (17). The DDG motif (D-D-G-Φ-X-D) resembles the conventional doubly phosphorylated DSG motif (D-pS-G-Φ-X-pS) both in the primary sequence and the electric charge. Therefore, the DDG motif, like the DSG motif (16), could bind to the WD40 domains of β-TrCP via multiple contacts.
The binding of β-TrCP to the DDG motifs seems to be strongly enhanced by either phosphorylation (for Xe-Cdc25A) or preexisting negative charges (for Hu-Cdc25B) of the motif-surrounding residues (Figs. 3 A-D and 4D). [In Hu-Cdc25A, however, only the conventional DSG motif showed an enhanced β-TrCP binding after Chk1 activation, presumably because of its stronger affinity to β-TrCP than that of the DDG motif (Fig. 2G).] Such negative charges (whether signal-induced or constitutive) might function to expose the “core” DDG motif or to stabilize its binding to β-TrCP, possibly aided by the Skp1 adaptor protein (16, 38). On the other hand, the ubiquitination efficiency at least in Xe-Cdc25A seems to be determined not only by the efficiency of β-TrCP binding to the DDG motif (Fig. 2F) but also by other events, such as the presence of the PEST sequence and Chk1 phosphorylation of other dispersed sites (Fig. 3E). These additional events might affect the (global) structure of Xe-Cdc25A, thereby making a distantly located ubiquitination site(s) accessible to the bound SCFβ-TrCP. Consistent with this idea, none of the lysine residues near the DDG motif apparently acted as a ubiquitination site(s) of Xe-Cdc25A (data not shown), contrasting with the presence of ubiquitinated lysine residues near the DSG motif in β-catenin (16). Ubiquitination of Hu-Cdc25B could also require similar (but constitutive) phosphorylation by some kinase (39). Similar regulations of β-TrCP binding and ubiquitination might occur, at least in part, even in proteins bearing the conventional DSG motif, although, in this case, phosphorylation of the motif itself is essential for β-TrCP binding (refs. 1 and 4; see refs. 27 and 28 for Hu-Cdc25A).
Finally, the structural and functional similarities between the DDG and DSG motifs would seem to suggest that other nonphosphorylated, DDG-related motifs (such as DEGΦXE) might also function as β-TrCP-binding sites in some protein(s). Indeed, our preliminary results show that β-TrCP can bind to the DDG-related motifs in several proteins tested, including X11L [a putative suppressor of β-amyloid production (40)] and hnRNP-U [a known pseudosubstrate of β-TrCP (41)] (see Fig. 5, which is published as supporting information on the PNAS web site). Thus, given the nonphosphorylated (DDG and DDG-related) as well as the phosphorylated (DSG) motifs and also their potential chimeric motifs [Wee1 may be an example (42)], there would be a very large number of proteins targeted by β-TrCP.
Supplementary Material
Acknowledgments
We thank K. Shimuta for technical advice, members of the N.S. laboratory for discussions, and K. Gotoh for typing the manuscript. This work was supported by scientific grants from the Ministry of Education, Science, and Culture of Japan and the Core Research for Evolutional Science and Technology Research Project of Japan Science and Technology Agency (to N.S.).
Author contributions: Y.K. and N.S. designed research; Y.K. and K.U. performed research; Y.K. analyzed data; and N.S. wrote the paper.
Abbreviations: Hu, human; SCF, Skp1/Cul1/F-box protein; Xe, Xenopus.
References
- 1.Cardozo, T. & Pagano, M. (2004) Nat. Rev. Mol. Cell Biol. 5, 739-751. [DOI] [PubMed] [Google Scholar]
- 2.Deshaies, R. J. (1999) Annu. Rev. Cell Dev. Biol. 15, 435-467. [DOI] [PubMed] [Google Scholar]
- 3.Maniatis, T. (1999) Genes Dev. 13, 505-510. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs, S. Y., Spiegelman, V. S. & Kumar, K. G. (2004) Oncogene 23, 2028-2036. [DOI] [PubMed] [Google Scholar]
- 5.Yaron, A., Hatzubai, A., Davis, M., Lavon, I., Amit, S., Manning, A. M., Andersen, J. S., Mann, M., Mercurio, F. & Ben-Neriah, Y. (1998) Nature 396, 590-594. [DOI] [PubMed] [Google Scholar]
- 6.Winston, J. T., Strack, P., Beer-Romero, P., Chu, C. Y., Elledge, S. J. & Harper, J. W. (1999) Genes Dev. 13, 270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitagawa, M., Hatakeyama, S., Shirane, M., Matsumoto, M., Ishida, N., Hattori, K., Nakamichi, I., Kikuchi, A., Nakayama, K. & Nakayama, K. (1999) EMBO J. 18, 2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki, H., Chiba, T., Kobayashi, M., Takeuchi, M., Suzuki, T., Ichiyama, A., Ikenoue, T., Omata, M., Furuichi, K. & Tanaka, K. (1999) Biochem. Biophys. Res. Commun. 256, 127-132. [DOI] [PubMed] [Google Scholar]
- 9.Orian, A., Gonen, H., Bercovich, B., Fajerman, I., Eytan, E., Israel, A., Mercurio, F., Iwai, K., Schwartz, A. L. & Ciechanover, A. (2000) EMBO J. 19, 2580-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, C., Li, Y., Semenov, M., Han, C., Baeg, G. H., Tan, Y., Zhang, Z., Lin, X. & He, X. (2002) Cell 108, 837-847. [DOI] [PubMed] [Google Scholar]
- 11.Margottin-Goguet, F., Hsu, J. Y., Loktev, A., Hsieh, H. M., Reimann, J. D. & Jackson, P. K. (2003) Dev. Cell 4, 813-826. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani, F. & Banks, L. (2003) J. Biol. Chem. 278, 42477-42486. [DOI] [PubMed] [Google Scholar]
- 13.Besnard-Guerin, C. Belaïdouni, N., Lassot, I., Segeral, E., Jobart, A., Marchal, C. & Benarous, R. (2004) J. Biol. Chem. 279, 788-795. [DOI] [PubMed] [Google Scholar]
- 14.Zhou, B. P., Deng, J., Xia, W., Xu, J., Li, Y. M., Gunduz, M. & Hung, M. C. (2004) Nat. Cell Biol. 6, 931-940. [DOI] [PubMed] [Google Scholar]
- 15.Moshe, Y., Boulaire, J., Pagano, M. & Hershko, A. (2004) Proc. Natl. Acad. Sci. USA 101, 7937-7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu, G., Xu, G., Schulman, B. A., Jeffrey, P. D., Harper, J. W. & Pavletich, N. P. (2003) Mol. Cell 11, 1445-1556. [DOI] [PubMed] [Google Scholar]
- 17.Donzelli, M. & Draetta, G. F. (2003) EMBO Rep. 4, 671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donzelli, M., Squatrito, M., Ganoth, D., Hershko, A., Pagano, M. & Draetta, G. F. (2002) EMBO J. 21, 4875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailand, N., Podtelejnikov, A. V., Groth, A., Mann, M., Bartek, J. & Lukas, J. (2002) EMBO J. 21, 5911-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mailand, N., Falck, J., Lukas, C., Syljuasen, R. G., Welcker, M., Bartek, J. & Lukas, J. (2000) Science 288, 1425-1429. [DOI] [PubMed] [Google Scholar]
- 21.Molinari, M., Mercurio, C., Dominguez, J., Goubin, F. & Draetta, G. F. (2000) EMBO Rep. 1, 71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falck, J., Mailand, N., Syljuasen, R. G., Bartek, J. & Lukas, J. (2001) Nature 410, 842-847. [DOI] [PubMed] [Google Scholar]
- 23.Shimuta, K., Nakajo, N., Uto, K., Hayano, Y., Okazaki, K. & Sagata, N. (2002) EMBO J. 21, 3694-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, H., Watkins, J. L. & Piwnica-Worms, H. (2002) Proc. Natl. Acad. Sci. USA 99, 14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen, C. S., Syljuasen, R. G., Falck, J., Schroeder, T., Ronnstrand, L., Khanna, K. K., Zhou, B. B., Bartek, J. & Lukas, J. (2003) Cancer Cell 3, 247-258. [DOI] [PubMed] [Google Scholar]
- 26.Sagata, N. (2002) Science 298, 1905-1907. [DOI] [PubMed] [Google Scholar]
- 27.Busino, L., Donzelli, M., Chiesa, M., Guardavaccaro, D., Ganoth, D., Dorrello, N. V., Hershko, A., Pagano, M. & Draetta, G. F. (2003) Nature 426, 87-91. [DOI] [PubMed] [Google Scholar]
- 28.Jin, J., Shirogane, T., Xu, L., Nalepa, G., Qin, J., Elledge, S. J. & Harper, J. W. (2003) Genes Dev. 17, 3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busino, L., Chiesa, M., Draetta, G. F. & Donzelli, M. (2004) Oncogene 23, 2050-2056. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki, K., Hayashida, K., Iwashita, J., Harano, M., Furuno, N. & Sagata, N. (1996) Gene 178, 111-114. [DOI] [PubMed] [Google Scholar]
- 31.Kim, S. H., Li, C. & Maller, J. L. (1999) Dev. Biol. 212, 381-391. [DOI] [PubMed] [Google Scholar]
- 32.Uto, K., Inoue, D., Shimuta, K., Nakajo, N. & Sagata, N. (2004) EMBO J. 23, 3386-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrielli, B. G., Clark, J. M., McCormack, A. K. & Ellem, K. A. O. (1997) J. Biol. Chem. 272, 28607-28614. [DOI] [PubMed] [Google Scholar]
- 34.Lammer, C., Wagerer, S., Saffrich, R., Mertens, D., Ansorge, W. & Hoffmann, I. (1998) J. Cell Sci. 111, 2445-2453. [DOI] [PubMed] [Google Scholar]
- 35.Nishijima, H., Nishitani, H., Seki, T. & Nishimoto, T. (1997) J. Cell Biol. 138, 1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rechsteiner, M. & Rogers, S. W. (1996) Trends Biochem. Sci. 21, 267-271. [PubMed] [Google Scholar]
- 37.Oe, T., Nakajo, N., Katsuragi, Y., Okazaki, K. & Sagata, N. (2001) Dev. Biol. 229, 250-261. [DOI] [PubMed] [Google Scholar]
- 38.Schulman, B. A., Carrano, A. C., Jeffrey, P. D., Bowen, Z., Kinnucan, E. R. E., Finnin, M. S., Elledge, S. J., Harper, J. W., Pagano, M. & Pavletich, N. P. (2000) Nature 408, 381-386. [DOI] [PubMed] [Google Scholar]
- 39.Baldin, V., Cans, C., Knibieler, M. & Ducommn, B. (1997) J. Biol. Chem. 272, 32731-32734. [DOI] [PubMed] [Google Scholar]
- 40.Taru, H. & Suzuki, T. (2004) J. Biol. Chem. 279, 21628-21636. [DOI] [PubMed] [Google Scholar]
- 41.Davis, M., Hatzubai, A., Andersen, J. S., Ben-Shushan, E., Fisher, G. Z., Yaron, A., Bauskin, A., Mercurio, F., Mann, M. & Ben-Neriah, Y. (2002) Genes Dev. 16, 439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe, N., Arai, H., Nishihara, Y., Taniguchi, M., Watanabe, N., Hunter, T. & Osada, H. (2004) Proc. Natl. Acad. Sci. USA 101, 4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.