Abstract
Background
Comprehensive analysis of brain tumor incidence and survival in the Veteran population has been lacking.
Methods
Veteran data were obtained from the Veterans Health Administration (VHA) Medical Centers via VHA Corporate Data Warehouse. Brain tumor statistics on the overall US population were generated from the Central Brain Tumor Registry of the US data. Cases were individuals (≥18 years) with a primary brain tumor, diagnosed between 2004 and 2018. The average annual age-adjusted incidence rates (AAIR) and 95% confidence intervals were estimated per 100 000 population and Kaplan–Meier survival curves evaluated overall survival outcomes among Veterans.
Results
The Veteran population was primarily white (78%), male (93%), and between 60 and 64 years old (18%). Individuals with a primary brain tumor in the general US population were mainly female (59%) and between 18 and 49 years old (28%). The overall AAIR of primary brain tumors from 2004 to 2018 within the Veterans Affairs cancer registry was 11.6. Nonmalignant tumors were more common than malignant tumors (AAIR:7.19 vs 4.42). The most diagnosed tumors in Veterans were nonmalignant pituitary tumors (AAIR:2.96), nonmalignant meningioma (AAIR:2.62), and glioblastoma (AAIR:1.96). In the Veteran population, survival outcomes became worse with age and were lowest among individuals diagnosed with glioblastoma.
Conclusions
Differences between Veteran and US populations can be broadly attributed to demographic composition differences of these groups. Prior to this, there have been no reports on national-level incidence rates and survival outcomes for Veterans. These data provide vital information that can drive efforts to understand disease burden and improve outcomes for individuals with primary brain tumors.
Keywords: brain tumors, incidence, survival, veterans
Key Points.
In the VA cancer registry, from 2004 to 2018 the overall AAIR of primary brain tumors was 11.6; the most diagnosed tumors were nonmalignant pituitary tumors, nonmalignant meningioma, and glioblastoma.
Survival outcomes were worse with age and those with glioblastoma.
Importance of the Study.
Veterans account for ~7% of the adult US population, with half seeking care through the Veterans Health Administration (VHA). A comprehensive analysis of incidence and survival for brain tumors in the Veteran population has been lacking. Differences between the Veteran and US population can be broadly attributed to the differences in the demographic composition of these groups. Prior to this study, there have been no reports on national-level incidence rates and survival outcomes for Veterans. Statistics such as these provide vital information that can help drive efforts to understand disease burden and improve outcomes for individuals with primary brain tumors.
Veterans account for approximately 7% of the adult US population, with half seeking care through the Veterans Health Administration (VHA).1,2 As with the general population, brain tumors are an important health concern for Veterans due to the significant morbidity and mortality. Veterans receiving care at the Veterans Affairs (VA) are reported using the Veterans Affairs Cancer Registry System (VACRS). Due to VHA policy changes implemented in 2007, these data are no longer systematically included in state and national cancer registries3,4 and a significant knowledge gap remains regarding diagnosis patterns, treatment trajectories, and clinical outcomes of Veterans with primary brain tumors compared to non-Veterans.
Exposure to chemical and environmental hazards during military service may increase the risk of several diseases, including an array of cancers.5–7 These exposures include ionizing radiation, nerve agents, chemicals from weapons’ demolitions and open-pit fires (ie, burn pits), and oil well fires,8–10 however definitive causative agents have not yet been identified. Previous research has suggested that military exposure may be associated with brain tumors, and in the US Veteran population brain tumor frequency and mortality may be associated with specific periods/types of deployment, though this remains to be conclusively confirmed.9–11 Recently, we compared the distribution of brain tumor histopathologies in Veteran and non-Veteran populations in Ohio. The results showed that 25% of Ohio Veterans with brain tumors were not represented in Ohio Cancer Incidence Surveillance System, and that there were differences in the distribution of various tumor histologic diagnoses (eg, a greater proportion of primary brain tumors were noted to be glioblastomas in Veterans vs. non-Veterans), possibly reflective of approaches to clinical treatment and care, or other factors, such as demographic differences between the veteran and general population.12 That study focused on the population in Ohio; while Ohio contains 4 VA medical centers and is the seventh most populous state, the findings found there may not be representative of the entire US Veteran population.
Despite being the subject of several studies9–12 and an important area of concern for US Veterans, a comprehensive analysis of the incidence and survival of brain tumors in the Veteran population has been lacking. While it is important to determine if deployment exposure impacts incidence and mortality, here we set out to perform a comprehensive study on incidence and survival of primary malignant and nonmalignant brain tumors in the US Veteran population, as it can provide valuable insights into the burden of disease and outcomes, as well as inform the development of effective prevention and treatment strategies. The intent of the current study was to provide valuable insights into the incidence and survival of primary malignant and nonmalignant primary brain tumors using information currently available in the Veteran population.
Methods
This study was conducted under Institutional Review Board approval from Duke University.
Data Sources
Data from VACRS was obtained from the 132 VA Medical Centers that diagnose and treat cancer via the VA Corporate Data Warehouse. Data collected include cancer diagnosis and treatment, which is compiled and submitted by local cancer registry staff.13,14 For brain tumor statistics on the overall US population, data were obtained from the Central Brain Tumor Registry of the United States (CBTRUS), which receives data in collaboration with the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR) and the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).15 The CBTRUS dataset is the largest population-based registry focused exclusively on primary brain and other central nervous system (CNS) tumors in the US, covering the entire US population.15 Additional survival analyses were performed using the CDC’s NPCR survival database, which consists of 42 US registries.16
Study Population
Inclusion criteria were individuals aged ≥ 18 years who were diagnosed with a primary brain or CNS tumor between 2004 and 2018, as collection of nonmalignant CNS tumors did not begin until the passage of Public Law 107-260 in 2002 (Benign Brain Tumor Cancer Registries Amendment Act) which mandated collection to begin January 1, 2004.17 Cases were defined as individuals with a primary brain or CNS tumor, identified using the administrative criteria based on International Classification of Diseases for Oncology, Third Edition (ICD-O-3) anatomic site, histopathology, and behavior codes as defined by the 2021 Annual CBTRUS Report.15 In VACRS, any non-Veterans (including spouses and dependents) were removed. Demographic data contained in the datasets included: Age, sex, race, and ethnicity (Supplementary Table 1). Data on Veterans included in the analysis is based on the time period in which the individual sought care at a VA health center and is not reflective of the time the individual was in active military service.
Histopathology
Cases were selected based on ICD-O-3 topography codes for the primary site of brain and other CNS (Supplementary Table 2). In addition, using ICD-O-3 histopathology codes, brain tumors were classified as either Malignant (ICD-O-3 behavior code of /3) or Nonmalignant (ICD-O-3 behavior code /0,/1). Select histopathological categories were generated using ICD-O-3 morphological codes. Malignant tumors were classified into three groups: Glioblastoma, other Gliomas, and other malignant tumors. nonmalignant tumors were classified into four groups: Meningioma, Pituitary tumors, Vestibular Schwannoma, and other nonmalignant tumors (Supplementary Table 3). These categories are focused exclusively on the behaviors listed. For example, malignant classifications for meningioma and pituitary tumors are grouped as “other malignant tumors” and not included in their respective nonmalignant groupings. All morphological and behavior codes for each category are listed in Supplementary Table 3.
Statistical Analysis
To calculate incidence rates in the VACRS dataset, the number of Veterans using VA services was defined as the number having at least one visit reported in the Outpatient Workload table. This adjustment was made since using the full Veteran population would understate incidence rates, as only individuals who use VA services will have tumors included in the VACRS. For each demographic category, unadjusted incidence rates and average annual age-adjusted incidence rates (AAIR), and 95% confidence intervals were estimated per 100 000 population18 and were directly adjusted to the US 2000 standard population19 using 5-year age buckets. Similarly, CBTRUS incidence rates are also age-adjusted to the US 2000 standard population. However, as mentioned previously, there is evidence that shows that US Veterans with primary brain tumors may seek care at heath centers outside of the VA for treatment, where these cancer diagnoses are reported to the central cancer registry within that state.12 Additionally, some VA facilities do report to their state central cancer registry, and those cases would appear in the data provided to CBTRUS. Due to this, VACRS and CBTRUS data are not independent, and therefore statistical comparisons between demographic and clinical distributions and AAIR cannot be performed.
Survival analyses were performed to evaluate differences in survival across age and histopathology groups. Survival was calculated as the number of days between diagnosis and death. Individuals who were alive, or did not have a death recorded, as of December 31, 2019 were censored, one year after the end of the study period. Univariate Kaplan–Meier analyses were performed using the survival R package20 and median survival estimates and survival curves are reported for individuals with malignant tumors. Log-rank tests were performed to assess differences in survival curves. Median survival estimates for the general US population were calculated using the NPCR survival database.
Results
VACRS contains 1 398 661 distinct individuals, of which 22 097 individuals had a tumor diagnosis with a primary site of brain or other CNS sites (ICD-O-3 C70.0-C72.9, C75.1-C75.2, and C30.0). There were 297 non-veterans (eg, spouses, dependents) excluded from the study population. The dataset was further refined to those Veterans diagnosed between January 1, 2004 and December 31, 2018 (n = 12 888). Finally, the dataset was restricted to individuals diagnosed with an ICD-O-3 histopathology code for malignant or nonmalignant primary brain tumors (Supplementary Table 2). In the final brain tumor dataset of Veterans receiving care, 12 237 individuals remained with primary brain tumors of the brain, meninges, spinal cord, olfactory, pineal gland, or pituitary, diagnosed in Veterans 18 years and older between 2004 and 2018 (Figure 1).
Figure 1.
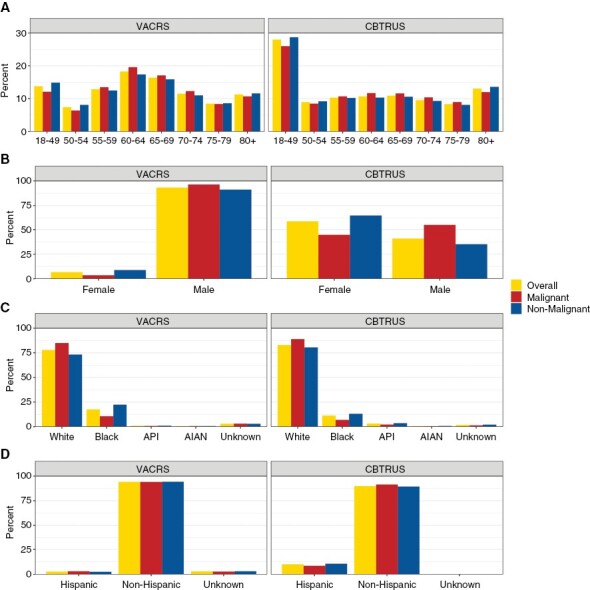
Demographic characteristics of individuals with primary brain tumors in VACRS and CBTRUS by (A) age at diagnosis, (B) sex, (C) race*, and (D) ethnicity (VACRS, 2004–2018; CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004–2018).
Demographic characteristics of the primary brain tumor Veteran dataset are summarized in Table 1. Overall, 93% of tumors occurred in males, and about 79% of Veterans with primary brain tumors were aged 55 years old or older. When compared to the demographics in the US (CBTRUS), the Veteran dataset has a larger proportion of individuals between 50 and 70 years old (Figure 1). The racial and ethnic distribution of individuals with primary brain tumors in the VA cancer registry was comparable to CBTRUS, however, there was a higher proportion of Veterans with primary brain tumors who were Black (18%) compared to CBTRUS (11%) (Figure 1). Primary brain tumors in males occurred at a much higher proportion in VACRS (93%) than in the CBTRUS dataset over the same time period (41%) (Figure 1).
Table 1.
Demographics and Incidence Rates (Per 100 000) of Primary Malignant and Nonmalignant Brain Tumors, Veterans Affairs Cancer Registry System 2004–2018
| Overall | Malignant | NonMalignant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Counts | Incidence Rate (per 100k) | Age-Adjusted IR (95% CI) | Total Counts | Incidence Rate (per 100k) | Age-Adjusted IR (95% CI) | Total Counts | Incidence Rate (per 100k) | Age-Adjusted IR (95% CI) | |
| Total | 12 237 | 14.5 | 11.4 (11.1–11.8) | 4924 | 5.8 | 4.4 (4.2–4.6) | 7313 | 8.7 | 7.1 (6.8–7.3) |
| Female | 818 (6.7%) | 10.3 | 9.8 (9.0–10.6) | 175 (3.6%) | 2.2 | 2.0 (1.7–2.4) | 643 (8.8%) | 8.1 | 7.8 (7.1–8.5) |
| Male | 11419 (93.3%) | 14.9 | 11.9 (11.4–12.3) | 4749 (96.4%) | 6.2 | 4.9 (4.6–5.2) | 6670 (91.2%) | 8.7 | 7.0 (6.6–7.3) |
| Age | |||||||||
| 18–49 years | 1684 (13.8%) | 9.3 | 8.7 (8.2–9.2) | 597 (12.1%) | 3.3 | 3.1 (2.8–3.5) | 1087 (14.9%) | 6.0 | 5.6 (5.2–6.0) |
| 50–54 years | 909 (7.4%) | 14.8 | 14.8 (13.8–15.7) | 315 (6.4%) | 5.1 | 5.1 (4.6–5.7) | 594 (8.1%) | 9.7 | 9.7 (8.9–10.4 |
| 54–59 years | 1578 (12.9%) | 18.2 | 18.2 (17.3–19.1) | 663 (13.5%) | 7.6 | 7.6 (7.1–8.2) | 915 (12.5%) | 10.5 | 10.5 (9.9–11.2) |
| 60–64 years | 2235 (18.3%) | 20.4 | 20.4 (19.5–21.2) | 964 (19.6%) | 8.8 | 8.8 (8.2–9.3) | 1271 (17.4%) | 11.6 | 11.6 (10.9 -12.2) |
| 65–69 years | 2001 (16.4%) | 17.5 | 17.5 (16.7–18.3) | 840 (17.1%) | 7.3 | 7.3 (6.9–7.8) | 1161 (15.9%) | 10.1 | 10.1 (9.6–10.7) |
| 70–75 years | 1410 (11.5%) | 15.7 | 15.7 (14.9–16.5) | 605 (12.3%) | 6.7 | 6.7 (6.2–7.3) | 805 (11%) | 8.9 | 8.9 (8.3–9.6) |
| 75–80 years | 1043 (8.5%) | 14.2 | 14.2 (13.3–15.0) | 412 (8.4%) | 5.6 | 5.6 (5.1–6.2) | 631 (8.6%) | 8.6 | 8.6 (7.9–9.3) |
| 80 + years | 1377 (11.3%) | 10.9 | 10.9 (10.3–11.5) | 528 (10.7%) | 4.2 | 4.2 (3.8–4.6) | 849 (11.6%) | 6.7 | 6.7 (6.3–7.2) |
| Race | |||||||||
| American Indian | 73 (0.6%) | 14.2 | 11.3 (7.9–15.3) | 28 (0.6%) | 5.5 | 4.6 (2.3–7.7) | 45 (0.6%) | 8.8 | 6.7 (4.4–9.4) |
| Asian or Pacific Islander | 108 (0.9%) | 8.3 | 6.4 (5.0 – 7.8) | 34 (0.7%) | 2.6 | 2.0 (1.3–2.9) | 74 (1.0%) | 5.7 | 4.3 (3.2–5.6) |
| Black | 2157 (17.6%) | 17.3 | 13.2 (12.4–14.0) | 523 (10.6%) | 4.2 | 3.1 (2.7–3.5) | 1634 (22.3%) | 13.1 | 10.1 (9.4–10.8) |
| White | 9536 (77.9%) | 16.2 | 13.5 (13.0–14.1) | 4186 (85%) | 7.1 | 5.6 (5.3 – 6.0) | 5350 (73.2%) | 9.1 | 7.9 (7.5–8.3) |
| Unknown | 363 (3.0%) | 3.2 | -- | 153 (3.1%) | 1.4 | -- | 210 (2.9%) | 1.8 | -- |
| Ethnicity | |||||||||
| Hispanic | 344 (2.8%) | 8.1 | 6.4 (5.6–7.3) | 155 (3.1%) | 3.7 | 2.6 (2.2–3.1) | 189 (2.6%) | 4.5 | 3.8 (3.1–4.5) |
| Non-Hispanic | 11527 (94.2%) | 16.1 | 13.4 (12.7–14.2) | 4631 (94.0%) | 6.5 | 5.2 (4.8–5.7) | 6896 (94.3%) | 9.6 | 8.2 (7.7–8.7) |
| Unknown | 366 (3.0%) | 4.3 | -- | 138 (2.8%) | 1.6 | -- | 228 (3.1%) | 2.7 | -- |
| Histopathology group | |||||||||
| Glioblastoma | -- | -- | -- | 2824 (23.1%) | 3.4 | 1.9 (1.8 – 2.0) | -- | -- | -- |
| Glioma (excluding glioblastoma) | -- | -- | -- | 1230 (10.1%) | 1.5 | 1.8 (1.6–1.9) | -- | -- | -- |
| Other (malignant) | -- | -- | -- | 870 (7.1%) | 1.0 | 0.7 (0.6–0.8) | -- | -- | -- |
| Meningioma | -- | -- | -- | -- | -- | -- | 3340 (27.3%) | 4.0 | 2.5 (2.4–2.6) |
| Pituitary tumors | -- | -- | -- | -- | -- | -- | 2556 (20.9%) | 3.0 | 2.9 (2.7–3.1) |
| Vestibular schwannoma | -- | -- | -- | -- | -- | -- | 845 (6.9%) | 1.0 | 0.8 (0.7–0.9) |
| Other (nonmalignant) | -- | -- | -- | -- | -- | -- | 572 (4.7%) | 0.7 | 0.8 (0.7–1.0) |
The primary brain tumor incidence rate in the Veteran dataset was 14.5 per 100 000 Veterans (Table 1). Male Veterans were found to have a higher tumor incidence rate (14.9 per 100 000) compared to female Veterans (10.3 per 100 000), which persisted after adjusting for age (11.9 per 100 000 (95% CI: 11.4–12.3) for male Veterans versus 9.8 per 100 000 (95% CI: 9.0–10.6) for female Veterans). Veterans who were Black also experienced a higher incidence rate (17.3 per 100 000) than Veterans who were White (16.2 per 100 000), although after adjusting for age the incidence rate is slightly lower for Veterans who were Black (13.2 per 100 000, 95% CI: 12.4–14.0) than for Veterans who were White (13.5 per 100 000, 95% CI: 13.0–14.1). In contrast, Veterans who were Asian, Native Hawaiian (HI), or Other Pacific Islander had a lower tumor incidence rate (8.3 per 100 000) than Veterans who were White or Black, including a lower age-adjusted incidence rate (6.4 per 100 000, 95% CI: 5.0–7.8).
When analyzing malignant and nonmalignant tumor incidence, Veterans who were male had twice the incidence rate for malignant tumors compared to Veterans who were female even after adjusting for age (4.9 per 100 000 (95% CI: 4.6–5.2), versus 2.0 per 100 000 (95% CI: 1.7 – 2.4)) (Table 1). In contrast, female Veterans had a higher nonmalignant tumor incidence after adjusting for age. The number of primary brain tumors for each histopathologic category, as well as the incidence rate per 100 000 VA individuals, is also reported. Glioblastoma accounted for just over half of all malignant tumors among Veterans, as 2824 out of 4924 malignant tumors (57.4%) were classified as glioblastoma (a rate of 188.3 tumors per year, or 3.4 per 100 000) (Table 1). When adjusted for age, the incidence rate of glioblastoma among Veterans (1.9 per 100 000, 95% CI: 1.8–2.0) was similar to the age-adjusted incidence rate of other gliomas excluding glioblastoma (1.8 per 100 000, 95% CI: 1.6–1.9). The most common nonmalignant tumors in the Veteran dataset after adjusting for age were pituitary tumors, with an incidence rate of 2.9 per 100 000 (95% CI: 2.7–3.1), followed by meningioma at 2.5 per 100 000 (95% CI: 2.4–2.6).
The distribution of specific histopathological subtypes for primary brain tumors in VACRS and CBTRUS is shown in Figure 2. Overall, the most common tumor in CBTRUS was meningioma, followed by pituitary tumors, and then glioblastoma. In VACRS, meningioma was the most common tumor, followed by glioblastoma and then pituitary tumors. While meningioma was the most common tumor in both data sources, the overall proportion was much higher in CBTRUS (37%) than in VACRS (27%). A large difference was also seen in glioblastoma, with glioblastoma comprising 23% of all primary brain tumor diagnoses in VACRS, and 14% in CBTRUS. Among only malignant primary brain tumors, glioblastoma was highest in both data sources (VACRS: 57%, CBTRUS: 46%). Among only nonmalignant primary brain tumors, meningioma had largest percentage of cases (VACRS: 46%, CBTRUS: 57%), followed by pituitary tumors (VACRS: 35%, CBTRUS: 24%).
Figure 2.
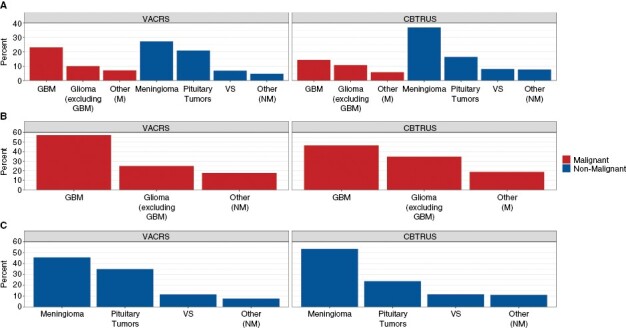
Histopathological subtype distribution of (A) all primary BT, (B) malignant brain tumors only, and (C) nonmalignant* brain tumors only, in VACRS and CBTRUS (VACRS, 2004-2018; CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004–2018).
Within the veteran population, there are noteworthy differences in incidence rates for male and female Veterans (Supplemental Table 4). Male Veterans have an incidence rate of glioblastoma (3.6 per 100 000) over three times as high as female Veterans (1.0 per 100 000), even after adjusting for age (2.1 per 100 000 (95% CI: 1.9–2.2) for male Veterans, versus 0.9 per 100 000 (95% CI: 0.7–1.1) for female Veterans). Among nonmalignant tumors, we see a higher incidence rate of meningioma for female Veterans (4.5 per 100 000) than for male Veterans (3.9 per 100 000). The difference in this rate for male and female Veterans is further emphasized after adjusting for age, given that the male Veteran population skews much older than the female Veteran population. Among female Veterans, the AAIR for meningioma (4.0 per 100 000, 95% CI: 3.6–4.5) is much higher than the AAIR for male Veterans (2.3 per 100 000, 95% CI: 2.2–2.4). When assessing differences in distribution across age groups and sex, the proportion of histopathologic was comparable between VACRS and CBTRUS (Figure 3). Among individuals ages 18–49, pituitary tumors were the most diagnosed tumors in both males (VACRS: 28%, CBTRUS: 23%) and females (VACRS: 45%, CBTRUS: 32%). Among individuals ages 50–69, meningioma was most diagnosed tumor in females (VACRS: 51%, CBTRUS: 52%) and GBM in males (VACRS: 26%, CBTRUS: 26%). While meningioma remained as the most diagnosed tumor in females aged 70 or older (VACRS: 53%, CBTRUS: 61%), meningioma was also the most diagnosed tumor in males ages 70+ (VACRS: 34%, CBTRUS: 37%).
Figure 3.
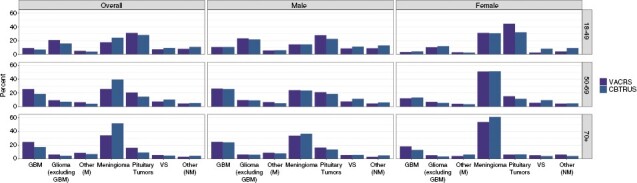
Sex distribution of histopathological subtype distribution among individuals (A) ages 18–49, (B) ages 50–69, and (C) ages 70+, in VACRS and CBTRUS (VACRS, 2004–2018; CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004–2018).
Across histopathologic types and age buckets, Veterans with glioblastoma had the lowest median survival time (Table 2). Survival times varied by age for those with glioblastoma, ranging from 19 months (95% CI: 17–25) for Veterans aged 50 years and younger to 4.1 months (95% CI: 3.9–4.5) for veterans 70 years and older. For a given age bracket, Veterans with other gliomas, excluding glioblastoma, had a shorter median survival time compared to Veterans with non-glioma tumors. However, the overall (all ages) median survival time for Veterans with non-gliomas was shorter (26 months, 95% CI: 21–34) than the median survival time for Veterans with non-glioblastoma gliomas (30 months, 95% CI: 25–36), which is a logical observation as a larger portion of non-glioblastoma gliomas occurs in individuals below 50 years old. Overall, the median follow-up time for all individuals with malignant tumors was approximately 10 months. Similar trends were seen in the NPCR survival data, with median survival being lowest in glioblastoma, and lowest in individuals over 70 years of age.
Table 2.
Median Survival Time (In Months) of Individuals With a Primary Malignant Brain Tumor in VACRS and NPCR (VACRS, 2004–2018; NPCR Survival Data: Data Provided by CDC’s National Program of Cancer Registries SEER*Stat Database: NPCR Survival Analytic File, 2004–2018)
| VACRS | NPCR | |||||||
|---|---|---|---|---|---|---|---|---|
| Histopathology Categorization | 18–49 Years Old | 50–69 Years Old | 70+ Years Old | All Ages | 18–49 Years Old | 50–69 Years Old | 70+ Years Old | All Ages |
| Glioblastoma | 19 (17–25) | 9 (8–9) | 4 (4–4) | 7 (7–8) | 19 (18–19) | 11 (11–11) | 4 (3–4) | 9 (9–9) |
| Glioma (excluding glioblastoma) | 135 (111–**) | 21 (18–26) | 5 (4–6) | 30 (25–36) | 168 (162–176) | 27 (26–28) | 4 (4–5) | 68 (66–69) |
| Other (Malignant) | 180 (126–**) | 39 (32–53) | 7 (5–13) | 26 (21–34) | **(**) | 47 (44–50) | 4 (4–5) | 29 (28–31) |
**Median survival not reached.
The 3-year survival probability of malignant tumors for Veterans was evaluated in the following age brackets: <50 years old, 50–69 years old, and > 69 years old. Across all age groups, Veterans with glioblastoma display the shortest survival times (Figure 4). For Veterans < 50 years old, Veterans with non-glioblastoma gliomas had a higher survival probability for each time period compared to those with other malignant tumors, while Veterans at least 50 years old with other malignant tumors have the highest survival probabilities starting 6–9 months after diagnosis.
Figure 4.
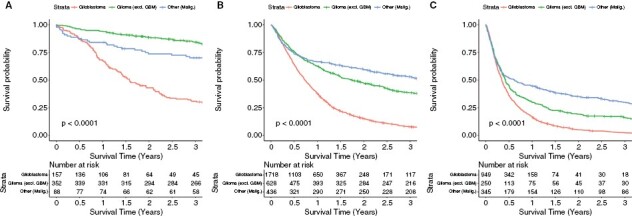
Overall survival for (A) 18–49 years old, (B) 50–69 years old, and (C) 70 + years old individuals with primary malignant brain tumors in VACRS (2004–2018).
Discussion
To the best of our knowledge, this is the first large-scale study aimed at examining primary malignant and nonmalignant primary brain tumor incidence and survival among Veteran Affairs users across the entire US. While there has been a previous study assessing the distribution of brain tumors among the Veteran population,12 that study was performed at the state level and did not assess the entire VA population. The study here includes the largest and most current assessment of the disease burden of brain tumors among Veterans, a population that is largely under-reported in US and VA central cancer registries. In addition, this is one of the first studies aimed at developing a methodology to calculate cancer incidence rates within the VA Cancer Registry.
In addition to investigating VA-wide trends, this study also utilized incidence rates for the US population, generated from CBTRUS data, to serve as a general reference of the total disease burden of primary brain tumors in the US. While data from the VA cancer registry are not systemically reported to all central cancer registries, it is still plausible that Veterans diagnosed with primary brain tumors may seek treatment and care, such as surgical resection, outside of their local VA health center. A 2017 survey found that three-fourths of Veterans received some aspect of healthcare outside of the VA system.21 Thus, it is likely that individuals with primary brain tumors may be reported by the facility to the state central cancer registry and thus be present in both registries. Due to confidentially issues, Veterans are not identified in CBTRUS data. Therefore, one cannot assume that VACRS data and CBTRUS data are truly independent, with previous analysis at the state level indicating the crossover could be 25%.12 Therefore, statistical assessment of differences between the 2 sources cannot be performed with the current datasets. Nonetheless, Veterans represent a small proportion of the total adult US population and should not be contributing a significant bias in the CBTRUS results reported in this manuscript.
The population demographics of Veterans with a primary brain tumor differed from the general US population. Individuals who were Veterans were primarily white (78%), male (93%), and between 60 and 64 years old (18%). In contrast, individuals with a primary brain tumor in the general US population were mainly female (59%) and between 18 and 49 years old (28%). These differences are not unexpected, given the fact that the incidence of a number of different brain tumor types is different between males and females, and the distribution of primary brain tumors in the VACRS is reflective only of the US Veteran population available in the study dataset, which are largely male and over 60.22 Since the Veteran population is not generally representative of the US population, it is critical to calculate these statistics within this group to truly understand the overall disease burden in the Veteran population.
The overall AAIR of primary brain tumors from 2004 to 2018 within the VA cancer registry was 11.6. nonmalignant tumors were more common than malignant tumors, occurring 1.63 times more often. The most commonly diagnosed tumors within the VA cancer registry were nonmalignant pituitary tumors (AAIR 2.96), followed by nonmalignant meningioma (AAIR: 2.62), and glioblastoma (AAIR: 1.96). When comparing the distribution of histopathological tumor types between VACRS and CBTRUS, the three most common tumors were the same: Meningioma, pituitary tumors, and glioblastoma. However, the relative distributions of these tumor types had large differences. Meningioma was the most common primary brain tumor in both data sources but had a much higher frequency in CBTRUS (37%) than VACRS (27%). This was notable among nonmalignant primary brain tumors, as the difference in the frequency of meningioma and pituitary tumors was 30% (54% vs. 24%, respectively), but only 11% in VACRS (46% vs. 35%, respectively). This disparity can largely be contributed to the sex distribution within the Veteran population, as meningiomas are much more common in females.23 When assessing the histopathology distribution by age and sex, the proportion of meningioma diagnoses was similar between VACRS and CBTRUS. There were also large differences seen in the frequency of glioblastoma between data sources, with VACRS having a higher proportion of individuals with glioblastoma than CBTRUS (14% vs. 23%, respectively). Among only malignant primary brain tumors, the difference in the frequency of glioblastoma and other gliomas was much higher in VACRS (32%, 57% vs. 25%, respectively), than in CBTRUS (11%, 46% vs. 35%, respectively). Like the differences seen with meningioma, much of this can be attributed to the differences in demographic distribution. Glioblastoma is most common in males and adults over 55 years old23, which largely represents the VA population. Similarly, when evaluating distribution of histopathologies across age and sex, comparable proportions of GBM were seen between VACRS and CBTRUS. While these differences between VACRS and CBTRUS can be broadly attributed to the demographic composition of US Veterans, it is still an important distinction to note, as this drives the types of primary brain tumor cases that appear within VA health centers. Statistics such as these inform VA physicians and clinical staff of the disease burden of these tumors and optimize preparation for treatments and referrals.
Differences in overall survival for malignant primary brain tumors were observed across age groups (18–9 years, 50–69 years, and 70 + years old at diagnosis). In general, survival outcomes became worse with age and were lowest among individuals diagnosed with glioblastoma. However, individuals diagnosed with “other” gliomas (excluding glioblastoma) had better survival outcomes than those diagnosed with non-glioma malignant tumors in the 18–49 years-old age group, unlike the other age groups, which saw the best survival outcomes in individuals with non-glioma primary brain tumors. This difference in survival outcomes may be due to a difference in the distribution of specific histopathologies contained in these grouped categories across age groups. However, this analysis is out of the scope of the current manuscript and further investigation will be required to assess these differences in greater detail. When examining median survival estimates there was overall longer median survival in the NPCR data, particularly in glioma (excluding GBM). These differences can be attributed to the differences in age distribution between the VA and the general US population, with the vast majority of gliomas (excluding GBM) having been diagnosed in the 18–49-year-old age group in the NPCR data. Unsurprisingly, survival for male Veterans (Supplemental Figure 1) was similar to survival for the overall veteran population, given that 93% of Veterans with brain tumors were male. When comparing survival across histopathologies in female Veterans (Supplemental Figure 2) it is similar to the survival observed for male Veterans with the exception of Veterans 70+ years old. However, the small number of female Veterans diagnosed at age 70 or older limits definitive conclusions to be drawn from these survival curves.
There are a limited number of known risk factors for primary brain tumors, with ionizing radiation and hereditary disorders being identified.24,25 As with other cancers, increased age is also a risk factor.24–26 Some studies have suggested that environmental exposures to pesticides and other environmental toxins may contribute to primary brain tumor development,6,7 though this remains inconclusive. Subsequently, there has been much discussion and research concerning an increased primary brain tumor incidence and mortality among US Veterans.9–11 However, the 21-year follow-up of Veterans deployed in the Gulf War showed that rates of primary brain tumor mortality did not differ from those Veterans who were not deployed, although those deployed may have had a higher risk in the time immediately following the Gulf War.11 Although it is important to determine if deployment exposure impacts incidence and mortality, the intent of the current study was to provide valuable insights into the incidence and survival of primary malignant and nonmalignant primary brain tumors in the Veteran population, which can be utilized to inform the development of effective prevention and treatment strategies. While information on exposure, such as branch of military, time served, and occupation, is of interest, ascertainment, and analysis of this data are out of the scope of this current study. Future study in these areas are warranted and has the potential to provide important information regarding the Veteran population. The CBTRUS statistics provided here are solely to provide a known reference of the national disease burden of primary brain tumors.
This study has some limitations. There is currently no mechanism that exists for central pathology review of cases within the US registry system, and histopathology code assignment at case registration is based on histopathology information contained in the individual’s medical record. In addition, this analysis is limited to Veterans who access a VA medical center for at least some aspect of their care. The VHA does not account for Veterans who receive care only through non-VHA settings and there may not be completeness of case ascertainment. Furthermore, the incidence rates calculated are based on the annual number of VA users, and thus may underestimate individuals who generally receive care from the VA but did not access a VA medical center for a given year. Information regarding occupation and potential exposure of the US Veterans was not available. Lastly, this study was limited to individuals diagnosed from 2004 to 2018, prior to 2004 the collection of central cancer data for nonmalignant tumors was not mandated.
This study provides a comprehensive overview of the incidence and survival of Veterans diagnosed with primary malignant and nonmalignant primary brain tumors, who seek care within the VA health system and are included in the available dataset. Prior to this study, there have been no reports on national-level incidence rates and survival outcomes within the Veteran population. Statistics such as these provide vital information to VA clinicians, staff, and researchers that can help drive efforts to understand disease burden and improve outcomes for individuals with primary brain tumors.
Supplementary Material
Contributor Information
John R Bihn, VA Boston Healthcare System, Boston, Massachusetts, USA.
Gino Cioffi, Trans Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Kristin A Waite, Trans Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Corey Neff, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Mackenzie Price, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Quinn T Ostrom, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA.
Kaitlin N Swinnerton, VA Boston Healthcare System, Boston, Massachusetts, USA.
Danne C Elbers, VA Boston Healthcare System, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Michael A Mooney, VA Boston Healthcare System, Boston, Massachusetts, USA; Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Jacob Rachlin, VA Boston Healthcare System, Boston, Massachusetts, USA.
Thor D Stein, VA Boston Healthcare System, Boston, Massachusetts, USA; Boston University, Chobanian and Avedisian School of Medicine, Boston, Massachusetts, USA.
Mary T Brophy, VA Boston Healthcare System, Boston, Massachusetts, USA; Boston University, Chobanian and Avedisian School of Medicine, Boston, Massachusetts, USA.
Nhan V Do, VA Boston Healthcare System, Boston, Massachusetts, USA; Boston University, Chobanian and Avedisian School of Medicine, Boston, Massachusetts, USA.
Ryan E Ferguson, VA Boston Healthcare System, Boston, Massachusetts, USA; Boston University, Chobanian and Avedisian School of Medicine, Boston, Massachusetts, USA.
David S Priemer, Department of Pathology, Uniformed Services University School of Medicine, Bethesda, Maryland, USA; Henry M. Jackson Foundation for The Advancement of Military Medicine, Bethesda, Maryland, USA.
Daniel P Perl, Department of Pathology, Uniformed Services University School of Medicine, Bethesda, Maryland, USA.
Richard A Hickman, Henry M. Jackson Foundation for The Advancement of Military Medicine, Bethesda, Maryland, USA; Human Oncology and Pathogenesis Program, Sloan Kettering Institute, New York, New York, USA; Murtha Cancer Center Research Program, Department of Surgery, Uniformed Services University of Health Sciences, Bethesda, Maryland, USA.
Burt Nabors, Department of Neurology, Heersink School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Jennifer Rusiecki, Department of Preventive Medicine and Biostatistics, Uniformed Services University School of Medicine, Bethesda, Maryland, USA.
Jill S Barnholtz-Sloan, Trans Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Center for Biomedical Informatics and Information Technology, National Cancer Institute, Bethesda, Maryland, USA.
Nathanael R Fillmore, VA Boston Healthcare System, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Conflict of interest statement
The authors have no conflict of interest to declare. Jill S. Barnholtz-Sloan is a full-time paid employee of the NIH/NCI. Gino Cioffi and Kristin A. Waite are full-time contractors of the NIH/NCI.
Funding
This work was supported by the VA Cooperative Studies Program and the Department of Defense/Uniformed Services University of the Health Sciences (HU00012220063). Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No.75D30119C06056/Amendment 0003, the American Brain Tumor Association, Novocure, Inc., the Musella Foundation for Brain Tumor Research & Information, Inc., National Brain Tumor Society, the Pediatric Brain Tumor Foundation, The Sontag Foundation, the Uncle Kory Foundation, National Cancer Institute (NCI), Neuro-Oncology Branch under Contract No.75N91022P00827, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. The research services of Jill S. Barnholtz-Sloan, Kristin A. Waite, and Gino Cioffi were provided by the Division of Cancer Epidemiology and Genetics (DCEG) of the National Cancer Institute (NCI).
Authorship statement
John R. Bihn: investigation, data curation, methodology, formal analysis and interpretation, visualization, writing—original draft and editing. Gino Cioffi: investigation, data curation methodology, formal analysis and interpretation, visualization, writing—original draft and editing. Kristin A. Waite: investigation, visualization, interpretation, funding acquisition, project administration, supervision, writing—original draft and editing. Carol Kruchko: funding acquisition, investigation, resources, interpretation, writing—review and editing. Corey Neff: formal analysis and interpretation, visualization, writing–original draft and editing. Mackenzie Price: formal analysis and interpretation, visualization, writing–original draft and editing. Quinn T. Ostrom: investigation, methodology, interpretation, visualization, writing—review and editing. Kaitlin N. Swinnerton: methodology, interpretation, writing—review and editing. Danne C. Elbers: methodology, interpretation, writing—review and editing. Michael Mooney: methodology, interpretation, writing—review and editing. Jacob Rachlin: methodology, interpretation, writing—review and editing. Thor D. Stein: methodology, interpretation, writing—review and editing. Mary T. Brophy: methodology, interpretation, writing—review and editing. Nhan V. Do: methodology, interpretation, writing—review and editing. Ryan E. Ferguson: methodology, interpretation, writing—review and editing. David S. Priemer: methodology, interpretation, writing—review and editing. Daniel P. Perl: methodology, interpretation, writing—review and editing. Richard Hickman: methodology, interpretation, writing—review and editing. Burt Nabors: methodology, interpretation, writing—review and editing. Jennifer Rusiecki: methodology, interpretation, writing—review and editing. Jill S. Barnholtz-Sloan: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—review and editing. Nathanael R. Fillmore: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—review and editing.
Data Availability
The data in this study are governed by the Central Brain Tumor Registry of the United States (CBTRUS) and the Veterans Affairs Cancer Registry (VACRS). Requests for collaboration with CBTRUS for access to the data can be made at: https://cbtrus.org/contact-us-request-database/.
References
- 1. United States Census Bureau. https://www.census.gov/en.html.
- 2. Veterans Health Administration: About VHA. Veterans Health Administration. https://www.va.gov/health/aboutvha.asp. Accessed June 1, 2023.
- 3. Savage L. Unreported VA data may affect SEER research, cancer surveillance, and statistics gathering. J Natl Cancer Inst. 2007;99(23):1744–1752. [DOI] [PubMed] [Google Scholar]
- 4. Kolata G. States and V.A. at Odds on Cancer Data. New York, NY: The New York Times; 2007. [Google Scholar]
- 5. Department of Veteran Affairs. Environmental Health Registry Evaluations for Veterans. Washington, DC: Department of Veterans Affairs; 2023. [Google Scholar]
- 6. Vienne-Jumeau A, Tafani C, Ricard D.. Environmental risk factors of primary brain tumors: A review. Rev Neurol (Paris). 2019;175(10):664–678. [DOI] [PubMed] [Google Scholar]
- 7. Pagano C, Navarra G, Coppola L, et al. Impacts of environmental pollution on brain tumorigenesis. Int J Mol Sci. 2023;24(5):5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohgaki H, Kleihues P.. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 9. Bullman TA, Mahan CM, Kang HK, Page WF.. Mortality in US Army Gulf War veterans exposed to 1991 Khamisiyah chemical munitions destruction. Am J Public Health. 2005;95(8):1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barth SK, Kang HK, Bullman TA, Wallin MT.. Neurological mortality among U.S. veterans of the Persian Gulf War: 13-year follow-up. Am J Ind Med. 2009;52(9):663–670. [DOI] [PubMed] [Google Scholar]
- 11. Barth SK, Dursa EK, Bossarte RM, Schneiderman AI.. Trends in brain cancer mortality among U.S. Gulf War veterans: 21 year follow-up. Cancer Epidemiol. 2017;50(Pt A):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo C, Cioffi GN, Bej TA, et al. Data matching to support analysis of cancer epidemiology among veterans compared with non-veteran populations-an exemplar in brain tumors. JCO Clin Cancer Inform. 2021;5(Sep):985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein M, Scaria G, Ganti AK.. Utilization of the Veterans Affairs Central Cancer Registry to evaluate lung cancer outcomes. Semin Oncol. 2019;46(4–5):321–326. [DOI] [PubMed] [Google Scholar]
- 14. Zullig LL, Jazowski SA, Chawla N, et al. Summary of veterans health administration cancer data sources. J Registry Manag. 2019;46(3):76–83. [PubMed] [Google Scholar]
- 15. Ostrom Q, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: Primary brain and other central nervous (CNS) tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021;6(24):iii1–iii38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Program of Cancer Registries SEER*Stat Database: NPCR Survival Analytic file. United States Department of Health and Human Services, Centers for Disease Control and Prevention. Released June 2020, based on the 2019 submission; 2001–2016. [Google Scholar]
- 17. Benign Brain Tumor Cancer Registries Amendment Act, 107th Cong. § 260 2002. Accessed June 28, 2023. http://www.gpo.gov/fdsys/pkg/PLAW-107publ260/pdf/PLAW-107publ260.pdf
- 18. Fay MP, Feuer EJ.. Confidence intervals for directly standardized rates: A method based on the gamma distribution. Stat Med. 1997;16(7):791–801. [DOI] [PubMed] [Google Scholar]
- 19. Use of the 2000 U.S. Standard Population for Age-Adjustment National Cancer Institute, Surveillance, Epidemiology, and End Results Progream. https://seer.cancer.gov/stdpopulations/2000stdpop-use.html.
- 20. Therneau T. A Package for Surivival Analysis in R. R package version 3.5-5 ed2023. https://cran.r-project.org/web/packages/survival/index.html [Google Scholar]
- 21. Huff C. Caring for Veterans Outside the VA System. ACP Internist and American College of Physicians; 2017. July/August. Accessed June 1, 2023. [Google Scholar]
- 22. National Center for Veterans Analysis and Statistics US Department of Veterans Affairs. Available from: https://www.va.gov/vetdata/veteran_population.asp. Accessed June 1, 2023.
- 23. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ostrom QT, Adel Fahmideh M, Cote DJ, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019;21(11):1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNeill KA. Epidemiology of brain tumors. Neurol Clin. 2016;34(4):981–998. [DOI] [PubMed] [Google Scholar]
- 26. White MC, Holman DM, Boehm JE, et al. Age and cancer risk: A potentially modifiable relationship. Am J Prev Med. 2014;46(3 Suppl 1):S7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are governed by the Central Brain Tumor Registry of the United States (CBTRUS) and the Veterans Affairs Cancer Registry (VACRS). Requests for collaboration with CBTRUS for access to the data can be made at: https://cbtrus.org/contact-us-request-database/.


