Abstract
Background
Central nervous system (CNS) WHO grade 2 low-grade glioma (LGG) patients are at high risk for recurrence and with unfavorable long-term prognosis due to the treatment resistance and malignant transformation to high-grade glioma. Considering the relatively intact systemic immunity and slow-growing nature, immunotherapy may offer an effective treatment option for LGG patients.
Methods
We conducted a prospective, randomized pilot study to evaluate the safety and immunological response of the multipeptide IMA950 vaccine with agonistic anti-CD27 antibody, varlilumab, in CNS WHO grade 2 LGG patients. Patients were randomized to receive combination therapy with IMA950 + poly-ICLC and varlilumab (Arm 1) or IMA950 + poly-ICLC (Arm 2) before surgery, followed by adjuvant vaccines.
Results
A total of 14 eligible patients were enrolled in the study. Four patients received pre-surgery vaccines but were excluded from postsurgery vaccines due to the high-grade diagnosis of the resected tumor. No regimen-limiting toxicity was observed. All patients demonstrated a significant increase of anti-IMA950 CD8+ T-cell response postvaccine in the peripheral blood, but no IMA950-reactive CD8+ T cells were detected in the resected tumor. Mass cytometry analyses revealed that adding varlilumab promoted T helper type 1 effector memory CD4+ and effector memory CD8+ T-cell differentiation in the PBMC but not in the tumor microenvironment.
Conclusion
The combinational immunotherapy, including varlilumab, was well-tolerated and induced vaccine-reactive T-cell expansion in the peripheral blood but without a detectable response in the tumor. Further developments of strategies to overcome the blood-tumor barrier are warranted to improve the efficacy of immunotherapy for LGG patients.
Keywords: immunotherapy, IMA950, low-grade glioma, poly-ICLC, varlilumab
Key Points.
The combination of IMA950, poly-ICLC, and varlilumab is well-tolerated in LGG patients.
The regimen induces vaccine-reactive CD8+ T cells in the peripheral blood.
Varlilumab promotes effector memory T-cell differentiation in the peripheral blood.
Importance of the Study.
We evaluated an innovative combinatory vaccine regimen using IMA950, poly-ICLC, and varlilumab in patients with CNS WHO grade 2 LGG as a randomized study, including the presurgical vaccine administrations. We hypothesized that the regimen would be safe and that varlilumab would enhance the induction of vaccine-reactive T cells in the patients. While the regimen was well-tolerated and induced vaccine-reactive T-cell responses in the peripheral blood, varlilumab did not enhance the induction of vaccine-reactive T-cell response. In addition, while varlilumab promoted the effector memory phenotype of T cells in the peripheral blood, tumor-infiltrating leukocytes did not show vaccine-reactive T cells or the effects of varlilumab. These data suggest that the tumor microenvironment (TME) of LGG may not be permissive to the peripherally induced immune response. Further modulations of the LGG TME, such as disruption of the blood–brain barrier, may need to be considered.
Gliomas are the most common primary malignant central nervous system (CNS) tumors and are classified according to histology and molecular characteristics as grades 1–4 by the WHO.1 Among these clinically and molecularly diverse tumors, CNS WHO grade 2 low-grade gliomas (LGGs), which include diffuse astrocytomas and oligodendrogliomas, are common in young adults during the third and fourth decades of life.2 LGGs are at risk of undergoing malignant transformation into more aggressive and lethal WHO grade 3 or 4 high-grade gliomas (HGGs).3 Even with a combination of available therapeutic modalities (ie, surgery, radiation therapy [RT], chemotherapy), their invasive growth and resistance to treatment result in recurrence and death in most patients.3 Furthermore, the majority of LGG harbor mutations in isocitrate dehydrogenase (IDH) 1 or 2, resulting in the oncometabolite D-2-hydroxyglutarate (2-HG), which promotes gliomagenesis and induces immunosuppression in the tumor microenvironment (TME).4,5 Taken together, LGGs are considered a premalignant condition for HGGs, such that novel interventions to prevent malignant transformation for patients with LGGs need to be developed.
Immunotherapeutic modalities, such as vaccines, may offer a safe and effective option for these patients due to the slower growth rate of LGGs in contrast with HGGs, which should allow sufficient time for multiple immunizations and hence high levels of anti-glioma immunity. In addition, the systemic immunity of patients with LGGs may not be as immunocompromised as that of patients with HGGs, as a prior study showed that they exhibit excellent immunological responses to a peptide-based vaccine.6 Furthermore, the generally mild toxicity of vaccines may improve the quality of life compared with chemotherapy or RT.
The IMA950 vaccine consists of nine human leukocyte antigen (HLA)-A2 class I-restricted epitopes, 2 class II-restricted epitopes, and the synthetic Hepatitis B virus marker epitope.7 The HLA class I-restricted epitopes were identified on ex vivo (ie, surgically resected but not cultured) HLA-A*02+ glioblastoma (GBM) tissue using the peptidome approach.7 These antigens are highly expressed in the GBM tissue with very low or no expression in healthy tissues and are immunogenic. Furthermore, CD8+ T cells recognizing IMA950 antigens are present in GBM tissue.7 We identified IMA950 antigens expressed in LGG tissues and IMA950 epitope-specific CD8+ T cells in peripheral blood mononuclear cells (PBMCs) of LGG patients with a higher frequency compared with GBM patients.8 These data suggest that IMA950 may provide therapeutic and prophylactic immunities by targeting existing LGG cells and preventing transformation to HGG cells.
Varlilumab (CDX-1127) is a fully-human monoclonal antibody against CD27.9 HLA-binding peptide-based vaccines activate peptide-reactive T cells by engaging the T-cell receptor (TCR); however, these T cells require costimulation for optimal activation, clonal expansion, and effector differentiation.10 Varlilumab enhances the CD27-mediated T-cell costimulatory pathway in the presence of concomitant TCR signaling. Thus, combining varlilumab with IMA950 can stimulate IMA950-specific T cells with reduced risks of autoimmune toxicity compared to blocking antibodies against coinhibitory receptors that primarily reinvigorate exhausted T cells.11 In preclinical models, varlilumab was shown to mediate antitumor effects and may be particularly effective in combination with other immunotherapies, such as vaccines.12
Poly-ICLC is a synthetic double-stranded RNA molecule that binds to endosomal Toll-like receptor-3 and the cytoplasmic receptors, including retinoic acid-inducible gene I and melanoma differentiation-associated gene.13 Poly-ICLC consequently elicits the secretion of type I IFN and proinflammatory cytokines, resulting in the induction of an immunostimulatory response.13 We found poly-ICLC effectively induces expression of the chemokine CXCL10 in glioma and very late activation antigen (VLA)-4 on vaccine-activated CTLs, thereby promoting their migration to gliomas.14,15 We have subsequently demonstrated that poly-ICLC can be safely combined with peptide vaccines in patients with gliomas.6,16–22 These data provide a strong rationale for combining varlilumab with IMA950 and poly-ICLC to stimulate IMA950 epitope-specific T cells.
We conducted a prospective, randomized pilot study to evaluate the safety and immunological response of the multipeptide IMA950 vaccine with varlilumab in CNS WHO grade 2 LGG patients. Ultimately, the effects of the vaccine need to be prospectively evaluated in the human glioma TME.19 To this end, we randomized patients to receive combination therapy with IMA950 + poly-ICLC and varlilumab (Arm 1) or IMA950 + poly-ICLC (Arm 2) before clinically indicated surgery, followed by adjuvant vaccines.
Materials and Methods
Study Design and Patients
This pilot clinical trial (NCT02924038) was a randomized trial evaluating the safety and immunological activity of agonistic anti-CD27 antibody varlilumab (Celldex) in addition to the IMA950 vaccine (Immatics) plus poly-ICLC (Hiltonol, Oncovir) in patients 18 years and older with both newly diagnosed and recurrent CNS WHO grade 2 LGG for which surgical resection of the tumor was clinically indicated. Coprimary objectives were to determine: the safety of the novel combination of subcutaneously administered IMA950 and poly-ICLC and intravenously administered varlilumab in the neoadjuvant approach; and whether the addition of varlilumab increased the response rate (quantified as the number of patients who responded to IMA950-epitopes) and degree of T-cell responses against the IMA950 peptides (quantified as the mean percentage of total IMA950 tetramer-positive CD8+ T cells among total CD8+ T cells per available postvaccine time point) in postvaccine PBMC and surgically resected tumor samples obtained from participating patients.
The study design is shown in Figure 1. In brief, after consent and eligibility confirmation, patients were randomized 1:1 between the two arms (Arm 1: IMA950 vaccine + poly-ICLC + varlilumab; Arm 2 IMA950 vaccine + poly-ICLC) with the stratification based on whether the patient was newly diagnosed vs recurrent. IMA950 and poly-ICLC were administered subcutaneously at subinguinal sites as a single formulation. Varlilumab was administrated intravenously. Key inclusion criteria included being a candidate for surgical resection; prior histological confirmation with grade 2 astrocytoma, oligoastrocytoma, or oligodendroglioma through biopsy or surgical resection; positivity for HLA-A2 based on flow cytometry or genotyping; nonenhancing T2-FLAIR lesion; surgical resection of at least 0.5 grams of tumor; KPS of ≥70%; off or low-dose (≤4 mg/day of dexamethasone) corticosteroid therapy at least 2 weeks before the first presurgical vaccine; and adequate organ function. Prior RT after the initial diagnosis was allowed, but there must have been at least 6 months from the completion of RT (or radiosurgery) to sign informed consent. Prior chemotherapy and any systemic molecularly targeted antitumor therapy were allowed, but patients must have recovered from any prior treatment. The study was approved by the UCSF Institutional Review Board and was conducted according to the Declaration of Helsinki. All patients signed an informed consent document indicating that they are aware of the investigational nature of this study.
Figure 1.
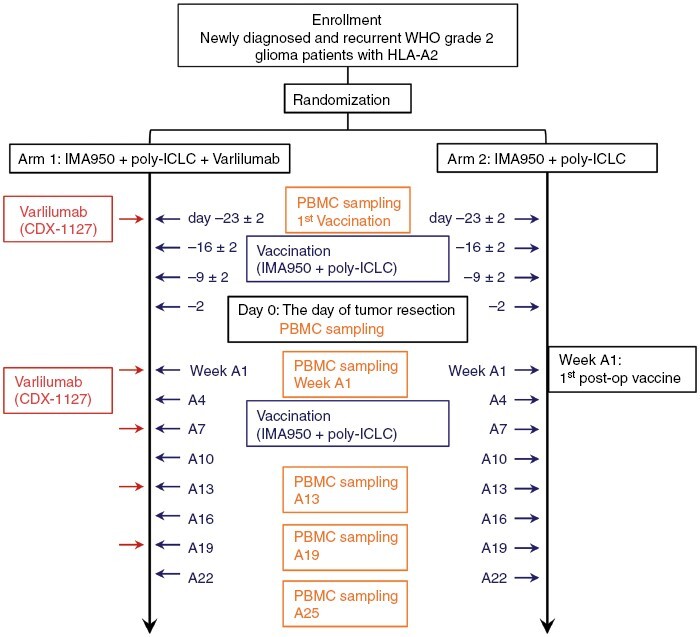
Schematic diagram of the study. Patients in Arm 1 received IMA950 + poly-ICLC and varlilumab. Patients in Arm 2 received IMA950 + poly-ICLC. All patients received subcutaneous injections of IMA950 + poly-ICLC vaccine on days −23 ± 2, −16 ± 2, −9 ± 2, and −2 relative to the scheduled surgery. IMA950 + poly-ICLC vaccines were resumed within 10 weeks post-surgery and repeated every 3 weeks for up to 8 doses (weeks A1, A4, A7, A10, A13, A16, A19, and A22; defining Week A1 as the week of the first post-surgery vaccine dose). For arm 1 patients, varlilumab was administered intravenously on the day of the first vaccine, weeks A1, A7, A13, and A19. PBMC samples were obtained at the time of the first vaccination and on the day of surgery, weeks A1, A13, A19, and A25.
A detailed description of patient registration and randomization is available in the Supplementary Materials.
Follow-Up
All patients were followed for response and toxicity assessments until disease progression, the start of a new therapy, or a maximum of 24 months from study registration (whichever occurred earlier). Toxicity was determined using the revised NCI Common Toxicity Criteria version 5.0 for Toxicity and Adverse Event Reporting (CTCAE). Regimen-limiting toxicities (RLTs) were defined as follows: grade 2 or more infusion reaction except for grade 2 pain; grade 2 or more cytokine release syndrome; grade 2 or more bronchospasm or generalized urticaria; grade 2 or more allergic reaction, such as exfoliative erythroderma, anaphylaxis, or vascular collapse; grade 2 or more autoimmune disease; any grade 3 or more toxicity related to the vaccine or varlilumab with particular attention to the following events; dosing delays for longer than 6 weeks in the administration of study regimen; and delay of the scheduled surgical resection for longer than 2 weeks related to the toxicity of the study regimen.
Processing of Human Samples
A detailed description of the procedure for human sample processing is provided in the Supplementary Materials.
Assessment of T-Cell Responses
The sequences and source protein of IMA950 are listed previously.23 T-cell responses to IMA950 peptides were identified by HLA tetramer staining after in vitro stimulation of PBMCs with IMA950 peptides, as shown previously.24 A detailed description of the flow cytometry procedure is provided in the Supplementary Materials.
CD8+ T-cell response to the IMA950-epitope was defined to be positive when the percentage of tetramer-positive CD8+ T cells showed a four-fold or higher increase within at least one-time point in the postvaccine phase relative to the corresponding percentage at the prevaccine.23,25 The expansion of IMA950-reactive CD8+ T cell frequencies over postvaccine periods was assessed by calculating the mean percentage of total IMA950 tetramer-positive CD8+ T cell per available postvaccine time point.
Immunohistochemistry Assessment of Tumor Samples
A detailed description of the immunohistochemistry procedure is provided in the Supplementary Materials.
Mass Cytometry Staining and Data Acquisition
A detailed description of the mass cytometry procedure is provided in the Supplementary Materials.
Mass Cytometry Data Analysis
A detailed description is available in the Supplementary Materials.
Evaluation of Blocking Effect of Varlilumab on Anti-CD27 Antibody
A detailed description is provided in the Supplementary Materials.
Statistics
We assumed the expansion of IMA950-reactive CD8+ T-cell frequencies over postvaccine periods was greater in Arm 1 than in Arm 2. The sample size was determined to be 15 per arm to provide 80% power for detecting the difference of 0.8, with a standard deviation for both arms of 1.0 with a significant level of 0.10. The statistical differences in the proportion of each cluster among samples within the same arm were calculated using a paired t-test or Wilcoxon signed rank test, as appropriate. A nonpaired Student’s t-test with Welch’s correction or Wilcoxon rank test was used to compare Arm 1 and Arm 2 samples. Multiple testing was adjusted using the Benjamini–Hochberg method. Progression-free survival (PFS) was defined as the time from the start of treatment to the time of progression or death. Overall survival (OS) was defined as the time from the start of treatment to the time of death. Patients who did not progress or die during the follow-up period were censored at the time of their last follow-up. The survival time was estimated using the Kaplan–Meier method, and the log-rank test was used to assess differences in the survival distributions between the patients. Statistical analyses and data visualization were performed using the ggplot2 R package (R version 4.2.0), and P-values less than .05 were regarded as statistically significant.
Results
Patient Characteristics
From January 11, 2017 until July 1, 2021, 14 patients were enrolled and randomized—eight into Arm 1 and six into Arm 2—and underwent resection. The study was designed to enroll 30 (15 patients/arm) eligible patients. However, the enrollment was terminated earlier than expected due to Celldex’s prioritization of other programs, terminating Varliumab development, and COVID-19-related challenges. Four patients received presurgery vaccines but were excluded from postsurgery vaccines due to the high-grade diagnosis of the resected tumor. As the study defined that patients must receive at least four post-surgery vaccines for the safety evaluation, 10 patients (median age, 41 years; 30% female, 70% male) were evaluable (Table 1); six patients on Arm 1 and four on Arm 2. One patient on Arm 1 had oligodendroglioma, while the remaining five were astrocytoma. Three of four patients on Arm 2 had oligodendroglioma with one astrocytoma. All patients harbored an IDH1 mutation, although this was not required for eligibility. One patient on Arm 1 received radiation prior to study participation (Table 1). We analyzed our clinical data in the current study (1) with all eligible patients and (2) per arm. There were no significant differences between Arms 1 and 2 regarding race, ethnicity, and the median time between surgical resection and first adjuvant vaccination (Table 1).
Table 1.
Patients’ baseline characteristics
| Characteristics | Arm 1, n = 6 | Arm 2, n = 4 | Total |
|---|---|---|---|
| Median age, years (range) | 41 (22–50) | 38 (27–49) | 41 (22–50) |
| Gender, no. (%) | |||
| Male | 5 (83.3) | (50.0) | 7 (70.0) |
| Female | 1 (16.7) | 2 (50.0) | 3 (30.0) |
| Reccurent, no. (%) | 6 (100) | 2 (50.0) | 8 (80.0) |
| Histology, no. (%) | |||
| Oligodendroglioma | 1 (16.7) | 3 (75.0) | 4 (40.0) |
| Astrocytoma | 5 (83.3) | 1 (25.0) | 6 (60.0) |
| IDH1 mutation, no. (%) | 6 (100) | 4 (100) | 10 (100) |
| Race | |||
| White | 5 (83.3) | 3 (75.0) | 8 (80.0) |
| African American | 0 (0) | 0 (0) | 0 (0) |
| Asian | 0 (0) | 0 (0) | 0 (0) |
| Other | 1 (16.7) | 1 (25.0) | 2 (20.0) |
| Median no. of days from presurgury vaccine to surgery (range) | 24 (22–30) | 24.5 (23–25) | 24 (22–30) |
| Median no. of days from surgery to first postsurgical vaccine (range) | 29.5 (21–37) | 33.5 (29–58) | 33 (21–58) |
| Median no. of adjuvant vaccines (range) | 12 (11–12) | 12 (12–12) | 12 (11–12) |
| Median time from original diagnosis to surgery on trial, years (range) | 4.3 (2.1–13.0) | 7.9 (0.4–14.1) | 4.8 (0.4–14.1) |
| Prior radiotherapy, no. (%) | 1 (16.7) | 0 (0) | 1 (10.0) |
| Prior systemic therapy, no. (%) | 3 (50.0) | 4 (100) | 7 (70.0) |
| Median lymphocyte count, x109/L (range) | 2.26 (1.55–3.04) | 1.69 (1.35–2.45) | 1.82 (1.35–3.04) |
Treatment and Safety
Treatment was well-tolerated, with four subjects experiencing five grade 3 and no grade 4 or 5 treatment-related adverse events (TRAEs) (Table 2). The most common TRAE was a headache. Of the five grade 3 TRAEs, there were three counts of lymphopenia, one count of leukopenia, and one headache. All grade 3 TRAEs occurred in Arm 1 patients aside from the headache TRAE. No patients experienced any RLT.
Table 2.
Adverse events
| Arm 1 | Arm 2 | |
|---|---|---|
| Adverse event | (N = 202) | (N = 103) |
| General disorders | ||
| Injection site reaction | 91 (45.0%) | 44 (42.7%) |
| Chills | 0 (0%) | 1 (1.0%) |
| Fatigue | 6 (3.0%) | 17 (16.5%) |
| Fever | 3 (1.5%) | 1 (1.0%) |
| Flu-like symptoms | 26 (12.9%) | 1 (1.0%) |
| Pain | 1 (0.5%) | 0 (0%) |
| Nervous system related | ||
| Headache | 7 (3.5%) | 19a (18.4%) |
| Seizure | 0 (0%) | 2 (1.9%) |
| Dizziness | 2 (1.0%) | 0 (0%) |
| Insomnia | 4 (2.0%) | 0 (0%) |
| Gastrointestinal disorders | ||
| Nausea | 12 (5.9%) | 7 (6.8%) |
| Upset stomach | 1 (0.5%) | 0 (0%) |
| Vomiting | 3 (1.5%) | 7 (6.8%) |
| Dyspepsia | 1 (0.5%) | 0 (0%) |
| Skin disorder | ||
| Pruritus | 2 (1.0%) | 1 (1.0%) |
| Rash Maculo-Papular | 0 (0%) | 0 (0%) |
| Itching | 0 (0%) | 0 (0%) |
| Blood disorders | ||
| Leukopenia | 12a (5.9%) | 0 (0%) |
| Neutropenia | 2 (1.0%) | 0 (0%) |
| Lymphopenia | 12a (5.9%) | 0 (0%) |
| Laboratory results | ||
| ALT increase | 1 (0.5%) | 0 (0%) |
| AST increase | 0 (0%) | 0 (0%) |
| Respiratory disorder | ||
| Laryngeal inflammation | 0 (0%) | 0 (0%) |
| Cough | 0 (0%) | 0 (0%) |
| Wheezing | 0 (0%) | 0 (0%) |
| Sore throat | 1 (0.5%) | 0 (0%) |
| Musculoskeletal and connective tissue disorders | ||
| Pain in extremity | 4 (2.0%) | 0 (0%) |
| Arthralgia | 1 (0.5%) | 0 (0%) |
| Body aches | 1 (0.5%) | 0 (0%) |
| Renal disorders | ||
| Urinary frequency | 1 (0.5%) | 0 (0%) |
| Metabolism and nutrition disorders | ||
| Anorexia | 0 (0%) | 2 (1.9%) |
aGrade 3 adverse event
Vaccination With IMA950/Poly-ICLC Induces Epitope-Specific CD8+ T-Cell Response in the Peripheral Blood
To determine the CD8+ T-cell response to the IMA950 vaccine, we evaluated the frequency of epitope-specific CD8+ T cells in PBMCs from 10 eligible patients (six in Arm 1 and four in Arm 2). To maximize the ability to detect the epitope-specific T cells, we stimulated PBMCs with the corresponding peptides before evaluation. The gating strategy and representative dot plots are shown in Supplementary Figure S1A and S1B. The frequencies of IMA950-reactive cells among total CD8+ T cells over time are summarized in Figure 2A (CSP-001, IGF2BP3-001, NLGN4X-001, and PTP-003) and Supplementary Figure S2 (BCA-002, FABP7-001, NRCAM-001, PTP-005, and TNC-001). Because prevaccine PBMCs were unavailable from one patient in Arm 1 (108-013), CD8+ T-cell response to IMA950-epitopes was evaluated in nine patients. All patients responded to at least a single IMA950-epitope, and four of five Arm 1 patients and all Arm 2 patients responded to multiple IMA950-epitopes (Figure 2B). There was no statistically significant difference between the two arms in the number of IMA950-epitopes that induced CD8+ T-cell response per patient (P = .14; Wilcoxon rank test; Figure 2B). The mean percentage of IMA950-reactive CD8+ T cells among total CD8+ T cells was evaluated in available postsurgery PBMC samples during the study. No statistically significant difference was observed between the two arms (P = .30, Student’s t-test with Welch’s correction, Figure 2C). Given the imbalance in the newly diagnosed and recurrent cases distribution between Arm 1 and Arm 2 (Table 1), we compared the mean percentage of IMA950-reactive CD8+ T cells between newly diagnosed and recurrent LGG. We observed no statistically significant difference between newly diagnosed and recurrent LGG. (P = .62, Wilcoxon rank test, data not shown).
Figure 2.
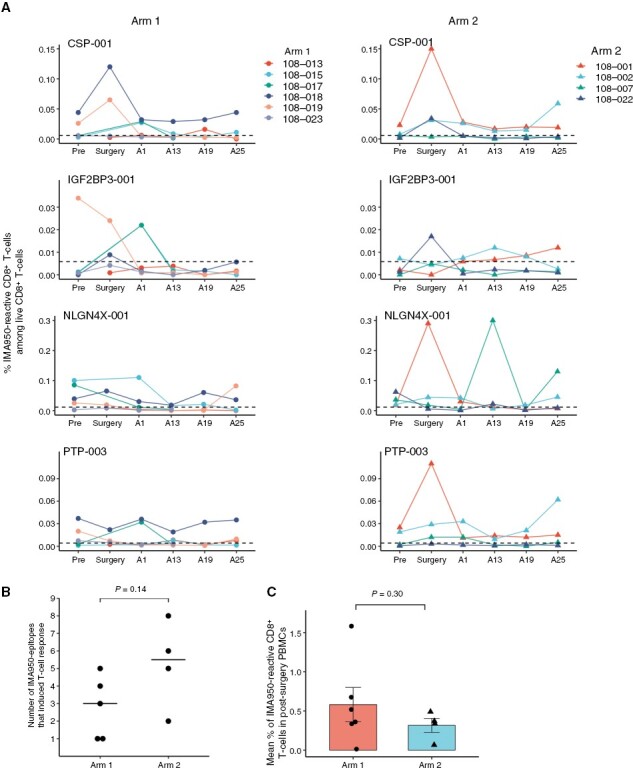
Vaccination with IMA950 and poly-ICLC induces IMA950-specific CD8+ T-cell response. (A) The longitudinal changes in the frequency of CD8+ T cells reactive to IMA950 antigens CSP-001, IGF2BP3-001, NLGN4X-001, and PTP-003. Horizontal dashed lines indicate the threshold for the background tetramer reactivity as determined by the nonspecific reactivity of HLA-A*02-negative CD8+ T cells to each tetramer. (B) The number of IMA950-epitopes that induced CD8+ T-cell response. All patients showed CD8+ T-cell response to at least a single IMA950- epitope, defined as the percentage of tetramer+ CD8+ T cells showing a four-fold or higher increase in at least one postvaccine time point compared to prevaccine. Comparison calculated with the Wilcoxon rank test. (C) The expansion of IMA950-reactive CD8+ T cells over postvaccine periods is evaluated by calculating the mean percentage of total IMA950-reactive CD8+ T cells among CD8+ T cells in available postsurgery PBMC samples during the study. Comparison calculated with nonpaired Student’s t-test with Welch’s correction.
Expression of BCAN and PTPRZ1, Two of the IMA950 Antigens, in the Tumor Tissue
To assess the expression of IMA950 antigens in the tumors of patients, we conducted an immunohistochemical evaluation on the expression of brevican (BCAN) and receptor-type tyrosine-protein phosphatase zeta (PTPRZ1), two of the IMA950 antigens, in tissues collected at distinct time points: before (six samples, at the initial surgery/prevaccination), during (11 samples, at the protocol-defined surgery; post four cycles of the vaccination), and after the study (two samples, after the study treatment and recurrence) (representative images in Supplementary Figure S3A). BCAN and PTPRZ1 were expressed in all evaluated tissue samples (Supplementary Figure S3B). However, no association was observed between the expression of the antigens, either before or during the study, and the vaccine-reactive CD8+ T-cell response (data not shown). Interestingly, an immunohistochemistry score (H-score) for BCAN exhibited a decline from an average of 245.9 (the protocol-defined surgery) to 152.5 (postrecurrence) in two available cases (patients 108-013 and 108-018, both in Arm 1) (Supplementary Figure S3C). However, unfortunately, none of these patients showed a robust CD8+ T-cell response to BCAN (Supplementary Figure S2). On the other hand, the H-score for PTPRZ demonstrated a slight increase from an average of 199.1 to 245.0.
Varlilumab Induces an Effector Memory Subset of T Cells
We conducted mass cytometric immune analyses to evaluate the effect of varlilumab on the development of epitope-specific T cells in PBMCs. As T-cell responses induced by the vaccine and the proinflammatory responses elicited by varlilumab are more robust following the initial dose in comparison to subsequent cycles,23,26 we evaluated PBMCs at both pretreatment and surgery (post four cycles of IMA950 + poly-ICLC with or without one cycle of varlilumab) from 11 patients, including 4 patients who were found to have HGG (6 and 5 patients in Arms 1 and 2, respectively). CD8+ and CD4+ T cells were identified using a conventional gating strategy (Supplementary Figure S4). We initially attempted to identify IMA950- and viral epitope-specific CD8+ T cells by ex vivo metal-labeled tetramer stainings in PBMCs from patients.27 However, epitope-specific CD8+ T cells, except for viral epitopes, were not observed in any patients (data not shown). Therefore, we first compared the expression of T-cell phenotypic markers in bulk CD8+ T cells between both treatment arms at pre- and postvaccine. Although the expression of CD27 on CD8+ T cells appeared to be decreased after the treatment with varlilumab, CD27 was not included in our unsupervised analysis of bulk CD8+ T-cell clustering due to the interference of the evaluation by competitive binding of varlilumab and anti-CD27 antibody clone L128 to human CD27 (Supplementary Figure S5A and S5B).
CD8+ T cells were clustered using the ClusterX algorithm and visualized on the t-distributed stochastic neighbor embedding (t-SNE) plot. Based on the expression levels of T-cell phenotypic markers—CD45RA, CD45RO, CCR7, CD28, CD127, CXCR3, T-bet, TIM-3, and PD-1, 21 subpopulations (clusters) of CD8+ T cells were identified (Figure 3A and 3B). The proportion of CD45RA+/−, CD45RO−, Tbethi, and PD-1+ effector CD8+ T cell (cluster 3) and CD45RO+, Tbethi, and PD-1+ effector memory CD8+ T cell (cluster 8) was significantly upregulated in postvaccine samples compared to prevaccine samples in Arm 1 patients (Figure 3A and 3C). Notably, these effector and effector memory CD8+ T cells exhibited a low-expression level of CXCR3, which is a crucial homing receptor required for T-cell infiltration into tumors.28 In contrast, the upregulation was not observed in any effector or effector memory CD8+ T-cell subpopulation in Arm 2 patients (Figure 3C and Supplementary Figure S6). CD4+ T cells were also clustered into nine subpopulations based on the expression levels of CD45RA, CD45RO, CCR7, CD127, CD25, CXCR3, HLA-DR, FoxP3, CTLA-4, and PD-1 using the same algorithm for CD8+ T-cell clustering (Figure 4A and 4B). The addition of varlilumab to the IMA950 vaccine (Arm 1), but not the vaccine alone (Arm 2), significantly upregulated the proportion of CD45RO+, CCR7−, CXCR3+, and Tbetmid-hi effector memory T helper type 1 (Th1) CD4+ T-cell subpopulations (clusters 1 and 6, Figure 4A and 4C). The addition of varlilumab did not affect the proportion of regulatory T cells (Figure 4D). These results indicate that the varlilumab-mediated CD70–CD27 costimulation induced T-cell activation and effector memory differentiation in circulating bulk T cells in LGG patients.
Figure 3.
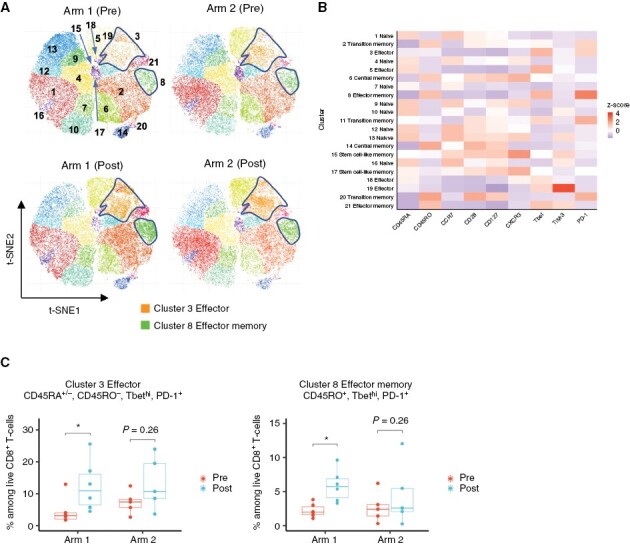
Varlilumab induces an increase of effector and effector memory CD8+ T cells in the peripheral blood. (A) t-SNE plot of pooled CD8+ T cells from patients in each treatment arm at pre- and postvaccine (at surgery; patients received four cycles of IMA950 vaccine + poly-ICLC with or without one cycle of varlilumab). CD8+ T cells from Arm 1 (six cases including two ineligible cases with high-grade diagnosis) and Arm 2 (five cases including two ineligible cases with high-grade diagnosis) were subjected to dimension reductional algorithm t-SNE for visualization in 2D space and clustered by ClusterX algorithm based on the expression level of T-cell phenotypic markers (CD45RA, CD45RO, CCR7, CD28, CD127, CXCR3, T-bet, TIM-3, and PD-1). The highlighted areas represent the cell density of Cluster 3 and Cluster 8. (B) The heatmap visualizes each T-cell phenotypic marker’s relative expression score (z score) in each subpopulation. Each cluster was annotated based on the expression status of T-cell phenotypic markers as listed above. C. Box plots show the change in percentages of Cluster 3 and Cluster 8 in both treatment arms at pre- and post-vaccine (at surgery). *P < 0.05 (paired t-test); multiple testing was adjusted using the Benjamini-Hochberg method.
Figure 4.
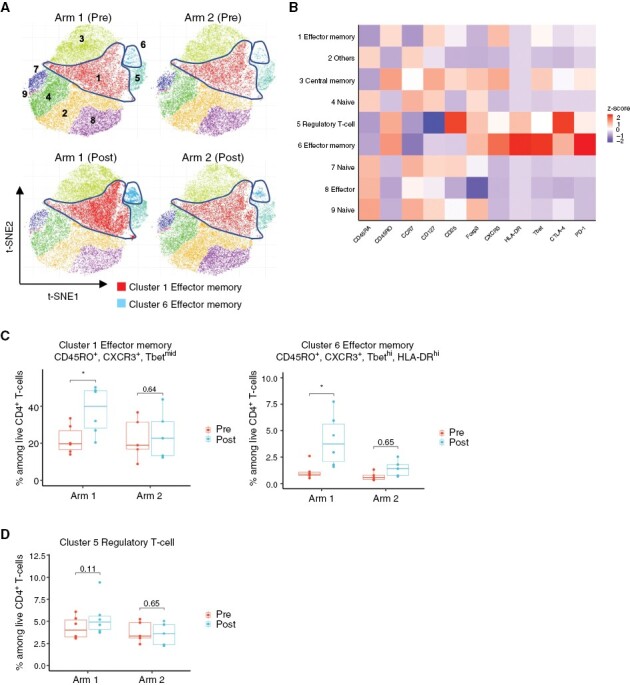
Varlilumab induces effector memory Th1 CD4+ T cells. (A) t-SNE plot of pooled CD4+ T cells from patients in each treatment arm at pre- and postvaccine (at surgery; patients received four cycles of IMA950 vaccine + poly-ICLC with or without one cycle of varlilumab). CD4+ T cells in Arm 1 (six cases including two ineligible cases with high-grade diagnosis) and Arm 2 (five cases including two ineligible cases with high-grade diagnosis) were subjected to dimension reductional algorithm t-SNE for visualization in 2D space and clustered by ClusterX algorithm based on the expression level of CD4+ T-cell phenotypic markers (CD45RA, CD45RO, CCR7, CD127, CD25, HLA-DR, FoxP3, CTLA-4, and PD-1). The highlighted areas represent the cell density of Cluster 1 or Cluster 6. (B) Heatmap visualizes each T-cell phenotypic marker’s relative expression score (z score) in each subpopulation. Each cluster was annotated based on the expression status of CD4+ T-cell phenotypic markers as listed above. (C) Box plots show the change in percentages of clusters 1 and 6 in both treatment arms at pre- and postvaccine (at surgery). *P < .05 (paired t-test); multiple testing was adjusted using the Benjamini–Hochberg method. (D) Box plot shows the change in the percentage of regulatory T cells in both treatment arms at pre- and postvaccine (at surgery). P value was calculated by paired t-test. Multiple testing was adjusted using the Benjamini–Hochberg method.
Varlilumab Does Not Induce Immune Responses in Tumor-Infiltrating T Cells
To evaluate the effect of varlilumab on tumor-infiltrating immune cells, we performed mass cytometric immune analyses in eight evaluable tumor samples (three in Arm 1 and five in Arm 2). Ex vivo metal-labeled tetramer stainings did not detect IMA950 epitope-specific CD8+ T cells in the tumor samples from any patients (data not shown). Live single CD45+ cells were gated from resected tumor tissues and clustered into 18 immune cell populations (Supplementary Figure S7A and S7B). The addition of varlilumab (Arm 1) did not induce effector or effector memory T-cell population in the tumor in contrast to our observations in the PBMC samples (Supplementary Figure S7C). These findings suggest that the vaccine-induced T-cell response in the periphery did not penetrate the blood-tumor barrier.
Clinical Efficacy
At the cutoff date (January 2023), two patients in Arm 1 and one patient in Arm 2 survived without progression, and one patient in Arm 1 died (Supplementary Figure S8A). Although this study was not powered for detecting significant differences in the survival between the two arms, the median PFS was 24.4 months (95% confidence interval [CI], 20.0 months–not reached) for Arm 1 and 22.5 months (95% CI, 7.5 months–not reached) for Arm 2, and the median OS was not reached in either group (Supplementary Figure S8B).
Discussion
The current study evaluated the safety and immunological response of the novel combination regimen with the IMA950 vaccine, poly-ICLC, and varlilumab in HLA-A*02:01-positive patients with CNS WHO grade 2 LGGs. The study also integrated presurgical therapy and prospective evaluation of the resected tumor. Ultimately, the study demonstrated the safety of the combination regimen; moreover, the regimen including varlilumab induced IMA950-reactive effector memory Th1 CD4+ and CD8+ T cells in PBMC. However, IMA950-reactive T cells were not detected in resected tumor samples.
Regarding the safety evaluation as one of the primary objectives, three of six patients in Arm 1 experienced grade 3 lymphopenia, which has been reported as the most prevalent laboratory abnormality observed in a phase I study of varlilumab monotherapy.26 Subsequently, all patients recovered from lymphopenia without specific treatment or interruption of the study regimen. In addition, no patients presented RLTs; thus, the combination regimen was safe and well tolerated in patients with LGGs.
The other primary objective of the study was to determine whether varlilumab would increase the response rate and degree of IMA950-reactive T-cell responses in postvaccine PBMC samples. Arm 1 patients (ie, with varlilumab) did not demonstrate a significant augmentation of IMA950-reactive CD8+ T cells compared to Arm 2 patients (Figure 2C). This result could be due to a transient effect of varlilumab on antigen-specific CD8+ T-cell expansion and exhaustion of IMA950-reactive CD8+ T cells. IL2 is a crucial CD27 target gene, and CD27/CD70 costimulation promotes clonal expansion of primed CD8+ T cells through autocrine IL-2-dependent survival signaling.29 The pharmacodynamic study of varlilumab indicates that a single dose of varlilumab saturates CD27 on nearly all T cells for at least 1 month. However, the upregulation of the serum proinflammatory cytokines, including IL-2, is transient, with a peak of a few hours after the administration.26 Based on our mass cytometric analysis of PBMCs, the expression level of IL2-receptor alpha chain, CD25, on CD8+ T cells was not augmented at 3 weeks after the initial administration of varlilumab (data not shown). These observations suggest the transient effect of varlilumab on IL-2 induction underlay the lack of enhanced IMA950-reactive CD8+ T-cell expansion over the postvaccine time points compared to the control (Arm 2) patients. The dose escalation of varlilumab with a shorter administration interval may enhance the IMA950-reactive CD8+ T-cell expansion, as the serum concentration of inflammatory chemokines and cytokines during the weekly dosing phase was significantly upregulated from baseline values, compared to the single dosing phase.30
Moreover, our mass cytometric analysis of PBMCs revealed the induction of PD-1+ effector and effector memory CD8+ T cells at 3 weeks after the administration of varlilumab (Figure 3C). As transient upregulation of PD-1 on antigen-specific CD8+ T cells by constitutive antigenic stimulation and CD27/CD70 costimulation negatively regulates T-cell expansion through T-cell exhaustion,10 the exhaustion of IMA950-reactive CD8+ T cells may have contributed to the underwhelming T-cell expansion observed in Arm 1 patients over the postvaccine time points. Reinvigoration of vaccine-reactive CD8+ T cells through an immune checkpoint inhibitor appears to be an effective strategy to improve the combinational therapy with peptide-based vaccine and anti-CD27 agonist. However, despite the promising preclinical results of dual PD-1 blockade and CD27 agonism in terms of T-cell activation and antitumor activity, combination therapy with varlilumab and an anti-PD-1 antibody, nivolumab, in patients with advanced solid tumors, including GBM, has shown limited clinical activity comparable to that observed with nivolumab monotherapy.31,32 Further research is warranted to improve the immunological response and clinical outcomes of peptide-based vaccines in combination with the costimulatory agonist and immune checkpoint inhibition.
The CD27/CD70 costimulation promotes the differentiation of Th1 CD4+ T cells by facilitating the expression of the master transcription factor T-bet, which IL-12 induces.10 While dendritic cells activated by pattern recognition receptor signals can induce effector CD8+ T-cell differentiation in the absence of Th1 CD4+ T cells, Th1 CD4+ T cells help is essential for CD8+ memory differentiation.10 Based on these observations, MHC class I-restricted peptide vaccines generally incorporate at least one MHC class II-restricted peptide.6,16 In the current study, we demonstrated for the first time to our knowledge that varlilumab promoted the induction of effector memory Th1 CD4+ and effector memory CD8+ T cells in the PBMCs in Arm 1 patients. These findings support the rationale for combining the multipeptides vaccine with varlilumab.
The neoadjuvant treatment design allows for an evaluation of the effects of IMA950 and varlilumab in the human glioma TME. However, our analyses failed to show the presence of IMA950-reactive CD8+ T cells or the induction of effector memory T cells in the TME. In contrast to the current study, our prior tumor-cell lysate vaccine study, in combination with poly-ICLC, detected the presence of vaccine-reactive CD8+ T-cell clones in the TME of LGGs using single-cell RNA sequencing and TCR sequencing.19 Our ex vivo tetramer assay failed to detect IMA950-reactive CD8+ T cells even in PBMCs. Therefore, the frequencies of IMA950-reactive CD8+ T cells in PBMCs and the TME are below the detection threshold of current ex vivo multimer assays.24 High-resolution single-cell RNA/TCR sequencing analyses might allow us to detect vaccine-reactive T-cell responses in the TME of LGGs.19 The neoadjuvant design of the study also allows for evaluating the possible change in the expression levels of vaccine target antigens in the tumor as a potential mechanism employed by tumor cells to evade T-cell immunosurveillance.33 While the limited sample size precluded definitive conclusions, it is intriguing to observe the decline in BCAN expression subsequent to disease progression. This observation may support the underlying concept of our multipeptide vaccination, which attempts to address the challenge of antigen loss and mitigate the risk of immune escape by simultaneously targeting 11 different antigens.23
The absence of the effector memory response following the varlilumab-including regimen in the TME could be attributed to factors, including (1) the intact blood–brain barrier in LGG patients, which limits the entry of immune cells and immune mediators into the CNS,34 (2) high incidence of CD70 expression on LGG cells which induce CD8+ specific T-cell death through the epiregulin–epidermal growth factor receptor pathway,35 (3) multifactorial immunosuppression in the TME of IDH-mutant LGGs,4,5 and (4) the absence of CNS-homing receptor expression on vaccine-reactive T cells. We have reported that the accumulation of oncometabolite 2-HG derived from the mutant IDH enzyme reduces the expression of IFN-γ–inducible chemokines, including CXCL9 and CXCL10 suppressing the T-cell accumulation in TME of LGG.4 We also found the low expression of CXCR3, the cognate receptor for CXCL9 and CXCR10, on effecter and effecter memory CD8+ T cells (Figure 3B). Notably, a panoply of homing receptors, including CXCR3, on T cells, as well as increased expression of homing receptor ligands in TME, are crucial factors for T-cell infiltration in tumors.36 Thus, the novel strategies combining an inhibitor of mutant IDH activity, multipeptide vaccine, and adjuvants that effectively induce homing receptors, such as CXCR3, on T cells may improve the efficacy of immunotherapy in patients with LGGs.5,37
Our study includes several limitations. First, this is a pilot study with a small sample size, early termination before the accrual of initially determined 30 patients, and short follow-up periods to evaluate the clinical benefit for patients with CNS WHO grade 2 LGGs. Second, the quantity of resected tumors available for immunological analyses was limited. Only 8 tumor samples, including 3 samples from ineligible patients with high-grade diagnosis, were evaluable for mass cytometric assay. No tumor sample was available for tetramer-based flow cytometry analyses to identify IMA950-reactive CD8+ T cells after in vitro stimulation. Third, no patients exhibited ex vivo-measurable IMA950-reactive CD8+ T-cell responses by state-of-the-art mass cytometric assay combined with metal-labeled tetramer stainings, which was expected to allow for the evaluation of the multiple phenotypes of antigen-specific T cells.18 The use of higher order multimer, an antibody against pMHC, and protein kinase inhibitors that inhibit TCR internalization, may improve the sensitivity of tetramer staining for T cells with low-affinity TCR, such as nonmutated tumor antigen-specific T cells.18,38
In conclusion, our study demonstrated the regimen’s safety and varlilumab’s ability to promote the differentiation of effector memory Th1 CD4+ T cells and effector memory CD8+ T cells in the peripheral blood of CNS WHO grade 2 LGG patients. However, no discernible effects on T-cell differentiation were observed within the TME. Therefore, to achieve better clinical outcomes in patients with LGGs, further refinement of combinatorial therapy of multipeptide vaccine with immunotherapies and more active disruption of the blood–brain barrier are warranted.
Supplementary Material
Acknowledgments
The authors thank the following individuals and organizations: the study participants and their families; all staff of the Department of Neurological Surgery and Division of Neuro-Oncology at UCSF for their assistance, advice, and helpful discussions; Lawrence Fong and the Cancer Immunotherapy Laboratory at UCSF for the processing and banking of patient-derived peripheral blood samples; and the UCSF Parnassus Flow Cytometry Core for mass cytometry services and use of the CyTOF2 Charmander.
Contributor Information
Atsuro Saijo, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Internal Medicine, Tokushima Prefecture Naruto Hospital, Tokushima, Japan.
Hirokazu Ogino, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Respiratory Medicine & Rheumatology, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan.
Nicholas A Butowski, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Meghan R Tedesco, Department of Neurology, University of California, San Francisco, CA, USA.
David Gibson, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Payal B Watchmaker, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Kaori Okada, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Albert S Wang, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Pathology, University of California, San Francisco, CA, USA.
Anny Shai, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Pathology, University of California, San Francisco, CA, USA.
Andres M Salazar, Oncovir Inc., Washington, DC, USA.
Annette M Molinaro, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA; Immatics Biotechnologies GmbH, Tuebingen, Germany.
Jane E Rabbitt, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Maryam Shahin, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Arie Perry, Department of Pathology, University of California, San Francisco, CA, USA.
Jennifer L Clarke, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Neurology, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Jennie W Taylor, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Neurology, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Mariza Daras, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Nancy Ann Oberheim Bush, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Department of Neurology, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Shawn L Hervey-Jumper, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Joanna J Phillips, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA; Department of Pathology, University of California, San Francisco, CA, USA.
Susan M Chang, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Norbert Hilf, Immatics Biotechnologies GmbH, Tuebingen, Germany.
Andrea Mayer-Mokler, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Tibor Keler, Celldex Theraepeutics, Inc., Hampton, NJ, USA.
Mitchel S Berger, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA.
Hideho Okada, Department of Neurological Surgery, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA; Parker Institute for Cancer Immunotherapy, San Francisco, CA, USA.
Conflict of interest statement
A.M.S. is the chairman, CEO, scientific director, and cofounder of Oncovir, Inc. N.H. and A.M.-M. are employees of Immatics Biotechnologies, GmbH. T.K. is an employee of Celldex Therapeutics, Inc.
Funding
National Institutes of Health (1R35NS105068 and 1R21CA233856 to H. Okada); loglio Foundation; Parker Institute for Cancer Immunotherapy.
Data availability
All data supporting the findings of this study will be made available upon reasonable request: hideho.okada@ucsf.edu
Author contributions
Concept and design: H. Okada, N.H., A.M.-M., A.M.S, T.K. and N.A.B. Methodology: A. Saijo, H. Ogino, N.A.B., P.B.W., K.O., A.S.W., A.Shai, J.J.P., and H. Okada. Data acquisition: A. Saijo, H. Ogino, N.A.B., M.R.T., D.G., K.O., J.E.R., M.S., A.P., J.L.C., J.W.T., M.D., N.A.O.B., S.L.H-J., S.M.C., and M.S.B. Analysis and data interpretation: A. Saijo, H. Ogino, N.A.B., M.R.T., D.G., A.M.M., and H. Okada. Manuscript preparation: A. Saijo, H. Ogino, N.A.B., M.R.T., D.G., P.B.W., K.O., A.S.W., A.Shai, A.M.S., A.M.M., J.E.R., M.S., A.P., J.L.C., J.W.T., M.D., N.A.O.B., S.L.H-J., J.J.P., S.M.C., N.H., A.M.-M., T.K., M.S.B., and H. Okada. Study supervision: N.A.B and H. Okada.
References
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Price M, Neff C, et al. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lombardi G, Barresi V, Castellano A, et al. Clinical management of diffuse low-grade gliomas. Cancers (Basel). 2020;12(10):3008–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127(4):1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chuntova P, Yamamichi A, Chen T, et al. Inhibition of D-2HG leads to upregulation of a proinflammatory gene signature in a novel HLA-A2/HLA-DR1 transgenic mouse model of IDH1R132H-expressing glioma. J ImmunoTher Cancer. 2022;10(5):e004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okada H, Butterfield LH, Hamilton RL, et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 Low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. Clin Cancer Res. 2015;21(2):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dutoit V, Herold-Mende C, Hilf N, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135(Pt 4):1042–1054. [DOI] [PubMed] [Google Scholar]
- 8. Dutoit V, Migliorini D, Ranzanici G, et al. Antigenic expression and spontaneous immune responses support the use of a selected peptide set from the IMA950 glioblastoma vaccine for immunotherapy of grade II and III glioma. Oncoimmunology. 2018;7(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vitale LA, He LZ, Thomas LJ, et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 2012;18(14):3812–3821. [DOI] [PubMed] [Google Scholar]
- 10. Van De Ven K, Borst J.. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: Rationale and potential. Immunotherapy. 2015;7(6):655–667. [DOI] [PubMed] [Google Scholar]
- 11. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Starzer AM, Berghoff AS.. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open. 2020;4(Suppl 3):e000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Waele J, Verhezen T, van der Heijden S, et al. A systematic review on poly(I:C) and poly-ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy. J Exp Clin Cancer Res. 2021;40(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu X, Nishimura F, Sasaki K, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu X, Fallert-Junecko BA, Fujita M, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-α and IFN-γ dependent manners. Cancer Immunol Immunother. 2010;59(9):1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. [DOI] [PubMed] [Google Scholar]
- 18. Mueller S, Taitt JM, Villanueva-Meyer JE, et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Invest. 2020;130(12):6325–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogino H, Taylor JW, Nejo T, et al. Randomized trial of neoadjuvant vaccination with tumor-cell lysate induces T cell response in low-grade gliomas. J Clin Investig. 2022;132(3):e151239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pollack IFl, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and n. J Clin Oncol. 2014;32(19):2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollack IF, Jakacki RI, Butterfield LH, et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol. 2016;18(8):1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study. J Neurooncol. 2016;130(3):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rampling R, Peoples S, Mulholland PJ, et al. A cancer research UK first time in human phase i trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. [DOI] [PubMed] [Google Scholar]
- 25. Migliorini D, Dutoit V, Allard M, et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro Oncol. 2019;21(7):923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burris HA, Infante JR, Ansell SM, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, in patients with advanced solid tumors. J Clin Oncol. 2017;35(18):2028–2036. [DOI] [PubMed] [Google Scholar]
- 27. Newell EW, Sigal N, Nair N, et al. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat Biotechnol. 2013;31(7):623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peske JD, Woods AB, Engelhard VH.. Control of CD8 T-cell infiltration into tumors by vasculature and microenvironment. Adv Cancer Res. 2015;128:263–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peperzak V, Xiao Y, Veraar EAM, Borst J.. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120(1):168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ansell SM, Flinn I, Taylor MH, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 2020;4(9):1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buchan SL, Fallatah M, Thirdborough SM, et al. PD-1 Blockade and CD27 Stimulation activate distinct transcriptional programs that synergize for CD8+ T-cell-driven antitumor immunity. Clin Cancer Res. 2018;24(10):2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanborn RE, Pishvaian MJ, Callahan MK, et al. Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J ImmunoTher Cancer. 2022;10(8):e005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu MW, Quail DF.. Immunotherapy for glioblastoma: Current progress and challenge. Front Immunol. 2021;12:676301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muldoon LL, Alvarez JI, Begley DJ, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J. Cereb. Blood Flow Metab. 2013;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin L, Ge H, Long Y, et al. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol. 2018;20(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brinkman CC, Peske JD, Engelhard VH.. Peripheral tissue homing receptor control of naïve, effector, and memory CD8 T cell localization in lymphoid and non-lymphoid tissues. Front Immunol. 2013;4(AUG):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han X, Wang Y, Sun J, et al. Role of CXCR3 signaling in response to anti-PD-1 therapy. EBioMedicine. 2019;48:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dolton G, Tungatt K, Lloyd A, et al. More tricks with tetramers: a practical guide to staining T cells with peptide–MHC multimers. Immunology. 2015;146(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study will be made available upon reasonable request: hideho.okada@ucsf.edu


