Abstract
Background/Objective
The aim of this study was to assess the impact of sinonasal morbidity on quality of life (QoL) in antineutrophil cytoplasmic antibody–associated vasculitis (AAV).
Methods
This cross-sectional case-control study enrolled 71 patients—44 AAV cases with (ear, nose, and throat [ENT]–AAV) or without ENT involvement (non–ENT-AAV) undergoing multidisciplinary evaluations and 27 chronic rhinosinusitis (CRS) cases. Three validated QoL questionnaires (Sino-Nasal Outcomes Test-22 [SNOT-22], Nasal Obstruction Symptom Evaluation [NOSE], and Short-Form 36) were administered, and the 3 groups were compared.
Results
The ENT-AAV patients were significantly younger (p = 0.01), with less antineutrophil cytoplasmic antibody positivity frequency (p = 0.035) and lower renal involvement (p = 0.003) than the non–ENT-AAV patients.
The SNOT-22 questionnaire demonstrated significantly greater sinonasal morbidity in ENT-AAV patients compared with CRS patients (p < 0.001). The NOSE score of ENT-AAV patients was comparable to those of CRS patients, but higher than that of non–ENT-AAV patients (p < 0.001). The SNOT-22 and NOSE scores positively correlated with disease activity (p = 0.037; p = 0.004, respectively). Short-Form 36 domain-by-domain analysis revealed a significantly poorer QoL in ENT-AAV patients, especially with physical functioning being progressively impaired in CRS, non–ENT-AAV, and ENT-AAV patients (p < 0.001). No significant differences in QoL came to light when AAV patients were stratified according to current systemic o local treatments.
Conclusions
The QoL in AAV patients is significantly reduced, especially in the presence of ENT involvement. The AAV-related nasal morbidity is consistent and comparable to that reported by CRS patients. It significantly affects patients' QoL and in particular social functioning, leading to limitation in daily/work activities. Organ-focused questionnaires and multidisciplinary management are warranted to pursue a treat-to-target approach in these patients.
Key Words: ANCA-associated vasculitis, granulomatosis with polyangiitis, microscopic polyangiitis, quality of life, SNOT-22
Granulomatosis with polyangiitis (GPA), formerly known as Wegener granulomatosis, and microscopic polyangiitis (MPA) are rare systemic diseases characterized by autoimmune necrotizing vasculitis of small and medium vessels that are classified among the antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAVs).1
Antineutrophil cytoplasmic antibody–associated vasculitides present with systemic involvement, classically affecting the upper and lower respiratory tract,2 kidneys, joints, and nerves3 accompanied by fever, weight loss, and fatigue.4 Granulomatosis with polyangiitis and MPA share clinical and histopathological features, a tight link with positive ANCA serology, and similar treatment modalities.5,6 Therefore, they may represent a continuum of disease phenotypes with a predominantly granulomatous or vasculitic disease pattern; risk of relapse is linked to the granulomatous component, whereas mortality is associated with vasculitis.7 Newer treatment regimens have modified the clinical course of AVV, from an acute form, characterized by high mortality rates, to a chronic condition.8,9 Despite treatment, the risk of relapse is 30% to 50% over 5 years.10,11 Many patients experience persistent disease activity, long-term exposure to toxic therapies, and almost one third of patients presents irreversible damage at diagnosis.12
Impaired health-related quality of life (QoL) has been reported in patients with AAV, especially as a consequence of fatigue and pain.4,13–19 Moreover, it is reported that one fourth of patients experience depression and more than 40% present anxiety.17 Work disability is also high with approximately 25% unemployed20 and 50% reporting that their career is hampered because of AAV.18
Ear, nose, and throat (ENT) involvement in AAV, especially GPA, represents one of the most frequent symptoms.21 Although patients with ENT symptoms have better survival2,22 and less renal involvement,2,23 they typically present a relapsing disease.24,25 The consequently impaired health-related QoL caused by ENT symptoms is considered to be even worse than other severe conditions, such as dialysis, seizures, and oxygen dependence.13,26,27
The aim of the present study is to evaluate the sinonasal morbidity and its impact on global QoL in patients with AVV.
PATIENTS AND METHODS
The present study was carried out at the University of Padua in accordance with the National Health Research Ethics guidelines, and thus formal ethical approval was not required. However, informed consent was obtained from each subject before starting any study-related procedure.
A cross-sectional case-control study was performed through collaboration between the Otorhinolaryngology and Rheumatology Units. We enrolled patients with an established diagnosis of GPA or MPA with a minimum disease duration of 6 months, regularly followed at the Rheumatology Unit, and patients with chronic rhinosinusitis (CRS) matched by age and sex, followed at the Otorhinolaryngology Unit. Patients with AAV at disease onset or with relapsing disease were excluded to avoid possible confoundment.
Granulomatosis with polyangiitis or MPA and CRS were classified according to the European Medicines Agency algorithm28,29 (including biopsies when deemed necessary) and the European Position Paper on Rhinosinusitis and Nasal Polyps guidelines,30 respectively.
All AAV patients were clinically evaluated by 3 expert rheumatologists (R.P., M.F., and F.S.) and assessed for disease activity and damage, using the Birmingham Vasculitis Activity Score (BVAS) version 331 and the Vasculitis Damage Index (VDI),32 respectively. Data on demographics and clinical features, as well as organ involvement and laboratory findings, were collected. Patients' treatment details including immunosuppressive agents, glucocorticoids, topical nasal treatments, and history of nasal surgery were also noted. The ENT evaluation consisted in nasal endoscopic examination with high-definition rigid endoscopes to ascertain the presence of nasal inflammation, nasal crusting, nasal discharge, septal perforation, or bony erosion. Three validated questionnaires were administered to each patient. To widen the spectrum of sinonasal symptoms assessment, the Sino-Nasal Outcomes Test-22 (SNOT-22)33 and Nasal Obstruction Symptom Evaluation (NOSE)34 were applied. Sino-Nasal Outcomes Test-22 results were analyzed as total score and domains score.35 Nasal Obstruction Symptom Evaluation total scores were multiplied by 5 to reach a score of 0 to 100. A modified sinonasal outcome test score (SNOT-25), which included 3 additional sinonasal symptoms (nasal crusting, bleeding, and external nasal deformity) specific for AAV patients, was also calculated.26 Moreover, the Medical Outcome Study Short-Form 36 (SF-36),36 which is commonly used for the measurement of QoL and consists of 8 domains investigating physical function, physical role, bodily pain, general health, vitality, social functioning, emotional role, and mental health, was also administered. Responses were scored for their perceived current disease activity.
Because the questionnaires investigate not only the sinonasal manifestations but also extranasal and psychological involvement, they were administered to all the patients, irrespective of ENT involvement. For further analysis, AAV patients were divided into 2 groups (ENT-AAV and non–ENT-AAV) depending on the presence of active sinonasal involvement at the time of the survey. The non–ENT-AAV group and the CRS group served as double controls to the ENT-AAV group.
Statistical Analysis
All data were entered into a computerized anonymized database. Statistical analysis was carried out with SPSS 24.0 and GraphPad Prism 7.0 software. The hypothesis of normality was tested with d'Agostino-Pearson test. Categorical variables were expressed with absolute frequencies and percentages, continuous variables as mean average and standard deviation, and median and interquartile range (IQR) or median with maximum and minimum based on the distribution of the variable. Dichotomous or ordinal categorical variables were compared with the χ2 test or the Fisher exact test. Continuous variables were studied with t tests for dependent and independent samples or with the nonparametric Mann-Whitney U test for independent samples based on data distribution. Spearman correlation was also used to identify associations between continuous variables. The level of significance was α = 0.05.
RESULTS
The study included 71 patients—44 (62.0%) with AAV and 27 (38.0%) with CRS. Within the AAV group, 20 GPA patients (45.5%) presented ENT involvement, whereas 24 patients (54.5%) without ENT symptoms were classified as GPA (n = 17, 70.8%) and MPA (n = 7, 29.2%).
Population Demographics and Characteristics of Disease and Treatment
Clinical and demographic data on AVV patients at evaluation are summarized in Table 1. Comparing non–ENT-AAV and ENT-AAV patients, the former were older (63.5 years [52.2–71.0 years] vs 53 years [26.7–61.0 years], p = 0.021). Patients with ENT involvement presented a slightly higher disease activity index (BVAS 0 [0–3.7] vs 0 [0–0], p = 0.037) and a higher VDI for the ENT region (90.0% vs 30.0%, p < 0.001). No difference was noted in the inflammation indexes and total VDI score. At diagnosis, patients with ENT involvement were significantly younger (44 years [31–54 years] vs 56 years [46–56 years], p = 0.01), with less ANCA positivity frequency (80.0% vs 100%, p = 0.035) and lower renal involvement (20.0% vs 66.7%, p = 0.003), and with a history of acute kidney injury, rapidly progressive glomerulonephritis, or subacute/chronic nephritis. No significant differences were observed between patients with ENT involvement and those without in terms of systemic treatment regimens (oral glucocorticoids and immunosuppressive agents) and disease duration. Additional details on patients' characteristics are reported in Table 1.
TABLE 1.
Demographic, Clinical, and Laboratory Characteristics of Patients With ENT-AAV, Non–ENT-AAV, and CRS
| ENT-AAV (n = 20) | Non–ENT-AAV (n = 24) | p value | CRS (n = 27) | p value | |
|---|---|---|---|---|---|
| Age, y | 53.0 (26.7–61.0) | 63.5 (52.2–71.0) | 0.0214 | 56.0 (38.0–68.0) | NS |
| Female, n (%) | 11 (55.0) | 13 (54.2) | NS | 11 (40.7) | NS |
| Disease duration (IQR), mo | 49.5 (6–196) | 68.0 (6–248) | NS | — | — |
| Organ involvement, n (%) | — | — | |||
| Systemic | 12 (60.0) | 17 (70.8) | NS | ||
| Lung | 11 (55.0) | 15 (62.5) | NS | ||
| Skin | 4 (20.0) | 8 (33.3) | NS | ||
| Eye | 3 (15.0) | 3 (12.5) | NS | ||
| Cardiovascular | 0 (0) | 1 (4.2) | NS | ||
| Abdominal | 4 (20.0) | 2 (8.3) | NS | ||
| Renal | 4 (20.0) | 16 (66.7) | 0.0027 | ||
| Nervous | 6 (30.0) | 12 (50.0) | NS | ||
| ANCA positivity, n (%) | 16 (80.0) | 24 (100) | — | — | |
| ANCA PR3 | 15 (93.7) | 13 (54.2) | 0.0357 | ||
| ANCA MPO | 1 (6.3) | 11 (45.8) | |||
| BVAS v3 | 0 (0–10) | 0 (0–7) | 0.0374 | — | — |
| BVAS v3 > 0, n (%) | 7 (35.0) | 2 (8.3) | 0.028 | — | — |
| VDI | 3 (2–4) | 3 (2–4) | NS | — | — |
| ESR, mm/h | 21 (9–35) | 22.5 (9–55) | NS | — | — |
| CRP, mg/L | 1.8 (0.3–7.8) | 1.9 (0.5–24.5) | NS | — | — |
| Immunosuppressive agents, n (%) | 11 (55.0) | 13 (54.2) | NS | — | — |
| DMARDs, n (%) | 11 (100) | 12 (92.3) | NS | ||
| Rituximab, n (%) | 0 (0) | 1 (7.7) | NS | ||
| Oral glucocorticoids, n (%) | 10 (50.0) | 13 (54.2) | NS | — | — |
| Oral glucocorticoids, mg/d | 1.25 (0–7.5) | 3.25 (0–5) | NS | ||
| Oral antibiotic (cotrimoxazole), n (%) | 2 (10.0) | 0 (0) | NS | — | — |
| Nasal steroid, n (%) | 6 (30.0) | 1 (4.2) | 0.0353 | 16 (59.2) | 0.047 |
| Local antibiotic, n (%) | 4 (20.0) | — | — | — | — |
| Nasal douche, n (%) | 11 (55.0) | — | — | 4 (14.8) | 0.0035 |
| Moisturizing ointments, n (%) | 6 (30.0) | 1 (4.2) | 0.0353 | 3 (11.1) | NS |
Values in bold mean that they are statistically significant (p < 0.05).
All data are presented as median and IQR, if not otherwise specified.
BVAS v3, Birmingham Vasculitis Activity Score version 3; ESR, erythrocytes sedimentation rate; CRP, C-reactive protein; DMARDs, disease-modifying antirheumatic drugs; MPO, myeloperoxidase; NS, nonsignificant; PR3, proteinase 3.
Score Analysis
SNOT-22 Score
The SNOT-22 score was significantly different in the 3 groups of patients. Patients with AAV-ENT had a significantly higher score compared with non–ENT-AAV patients (33.5 [24.0–60.3] vs 14.5 [4.0–26.8], p < 0.001) and CRS patients (26.0 [17.0–38.0], p < 0.001). Among all ENT-AAV patients, 20.0% reported decreased sense of smell/taste, 15.0% ear fullness, 15.0% fatigue, 15.0% frustrated/restless/irritable, and 20.0% sad as 1 of their 5 most troublesome symptoms. The SNOT-22 symptoms domain analysis revealed significantly higher scores for the rhinologic, extranasal rhinologic, ear/facial, and psychological dysfunction domains in the ENT-AAV cohort, whereas no differences were observed for the sleep dysfunction domain. Similar results were also seen with the SNOT-25 questionnaire, where patients with ENT-AAV scored higher than patients with non–ENT-AAV (43.5 [27.8–63.8] vs 14.5 [4.0–26.8], p < 0.001) and CRS patients (28.0 [18.0–42.0], p < 0.001).
NOSE Score
The NOSE score was significantly different between ENT-AAV and non–ENT-AAV patients (30 [25–51] vs 5 [0–15], p < 0.001). Similar results were obtained comparing patients with CRS to those with non–ENT-AAV 30 [10–45] vs 5 [0–15], p < 0.001). The comparison between ENT-AAV and CRS was not significant in terms of medians and total NOSE scores. Considering comparison of single items between the 3 groups (Fig. 1), ENT-AAV scores were comparable to those of the CRS cohort, but significantly higher than non–ENT-AAV patients, whereas trouble in sleeping showed no difference. In addition, among all ENT-AAV patients, sleep dysfunction was rated as the least troublesome symptom (5.0%).
FIGURE 1.
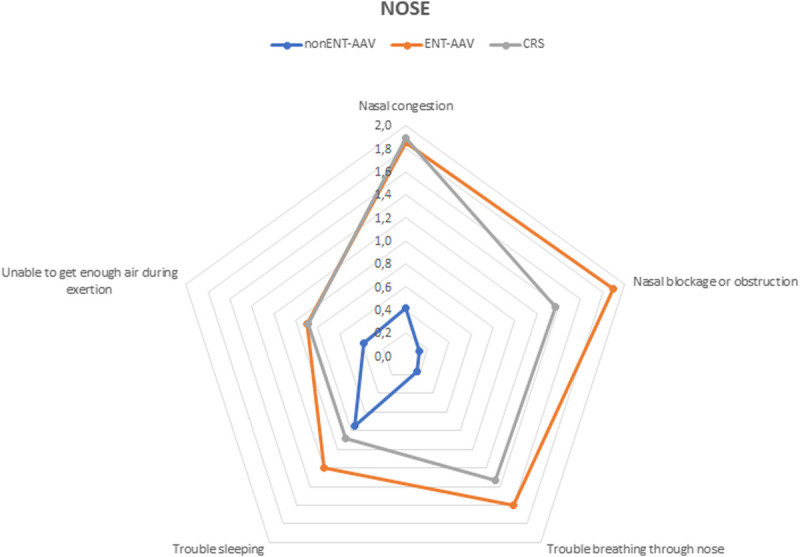
Radar chart displaying NOSE items comparison between the 3 groups of patients: non–ENT-AAV, ENT-AAV, and CRS.
SF-36 Score
Considering the SF-36 score (Fig. 2), physical functioning domains were significantly different among the 3 groups of patients, being progressively impaired in CRS (95.0%), non–ENT-AAV (90.0%), and ENT-AAV (75.0%) patients (Table 2). Antineutrophil cytoplasmic antibody–associated vasculitis patients demonstrated significant impairment of the role limitation because of physical health item in comparison to CRS patients (85.0%, 60.0%, and 45.0%). Furthermore, comparing ENT-AAV patients with non–ENT-AAV and CRS cohorts, significant impairment was observed in the role limitation because of emotional problems, energy/fatigue, and social functioning, whereas a statistical trend emerged for emotional well-being.
FIGURE 2.
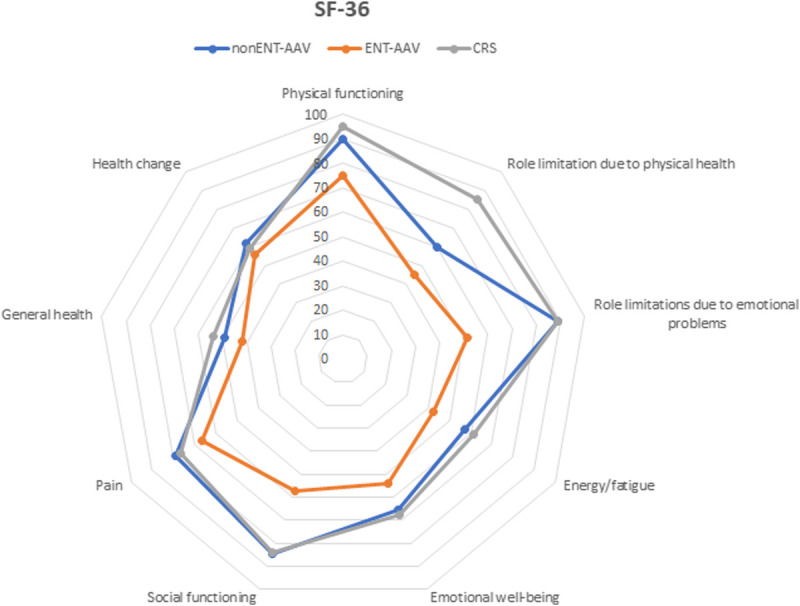
Radar chart displaying SF-36 domains comparison between the 3 groups of patients: non–ENT-AAV, ENT-AAV, and CRS.
TABLE 2.
Statistical Analysis of SF-36 Domain Scores in the 3 Cohorts of Patients
| SF-36 Domains | CRS (n = 27) | p value | ENT-AAV (n = 20) | p value | non–ENT-AAV (n = 24) |
|---|---|---|---|---|---|
| Physical functioning | 95.0 (95.0–100) | <0.0001 | 75.0 (66.3–90.0) | 0.0017 | 90.0 (90.0–95.0) |
| Role limitations because of physical health | 100 (75.0–100) | 0.0009 | 50.0 (0–93.8) | 0.0257 | 75.0 (6.3–100) |
| Role limitations because of emotional problems | 100 (100–100) | 0.0012 | 50.0 (0–100) | 0.0019 | 100 (100–100) |
| Energy/fatigue | 65.0 (50–70) | 0.005 | 42.5 (20.0–67.5) | 0.0276 | 60.0 (50.0–68.7) |
| Emotional well-being | 68.0 (56.0–80.0) | NS | 52.0 (37.0–77.0) | NS | 68.0 (53.0–72) |
| Social functioning | 87.5 (75.0–100) | 0.0007 | 50.0 (40.6–87.5) | 0.0009 | 100 (65.6–100) |
| Pain | 80.0 (67.5–100) | NS | 65.0 (45.0–100) | NS | 90.0 (57.5–100) |
| General health | 55.0 (35.0–70.0) | 0.0443 | 42.5 (20.0–58.8) | NS | 45.0 (36.3–63.8) |
| Health change | 50.0 (50.0–75.0) | NS | 50.0 (50.0–75.0) | NS | 50.0 (50.0–75.0) |
Values in bold mean that they are statistically significant (p < 0.05).
NS, nonsignificant.
Pain, general health, and health change was impaired in all the 3 groups but without any between-group differences (Fig. 2).
Correlation Between ENT Scores and Disease Activity
Focusing on disease activity, there was a significant correlation between BVAS and SNOT-25 (p = 0.037; r = 0.314). The correlation resulted even stronger with the 3 ENT-specific items of the SNOT-25 (p = 0.006; r = 0.405), whereas no correlation came to light between BVAS and SNOT-22. Similarly, a positive correlation between BVAS and NOSE (p = 0.004; r = 0.511) was also observed (Fig. 3). No further correlations were evident between ENT scores and age, disease duration, or damage accrual.
FIGURE 3.
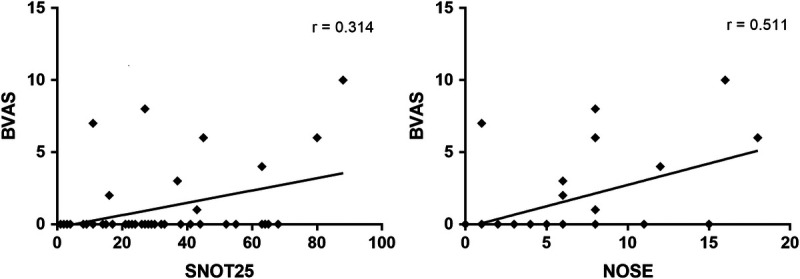
Correlations between BVAS and SNOT-25 (left), and BVAS and NOSE (right) in AAV patients' cohort.
Influence of the Therapy
In the ENT-AAV group, there were no differences in SNOT-22, SNOT-25, and SF-36 scores between users and nonusers of topical douches or topical nasal steroids. No differences were detected in all the scores considering current immunosuppressive treatment or glucocorticoids.
DISCUSSION
In the last decade, AAV has become a chronic condition,8,9 and many patients experience persistent disease activity or a relapsing disease together with long-term exposure to therapies leading to irreversible damage.12 Such clinical course significantly impact on the general QoL of patients who are prone to develop depression, anxiety,17 and work disability.20
Primary outcomes are usually rated using the physician-based BVAS score,31 which assesses disease activity, even if AAV patients often report different perceptions of the disease. The Outcome Measures in Rheumatology core set of outcome measurements for use in clinical trials in AAV included the generic SF-36 patient-reported outcome to evaluate QoL.36 However, generic patient-reported outcome can lack specificity,37 especially regarding the features of vasculitis and its specific organ involvement; thus, focused questionnaires have been used in the present study.26,33,34
Ear, nose, and throat and renal involvement are prominent organ conditions found in 50% to 80% of patients with AAV and reflect the granulomatous and vasculitic components, respectively.7 However, recent observations indicate a clinical interaction between ENT and renal disease. When compared with patients with no ENT involvement, those with ENT involvement showed less frequent renal disease, better renal function,2,23 and a younger age at diagnosis.2 This is consistent with our results.
Chronic rhinosinusitis has been previously demonstrated to have a significant impact on the QoL, comparable to other common chronic diseases such as rheumatoid arthritis, chronic obstructive pulmonary disease, and insulin-dependent diabetes.38
In the present study, the burden of sinonasal morbidity (assessed with SNOT score) in AAV patients with ENT involvement is at least as significant as that of CRS, confirming the results of Srouji and colleagues.26,39 This is also important considering that AAV-related sinonasal morbidity is regarded by many physicians to be of less impact in the setting of multisystem disease. The same results were observed when the NOSE score was applied.
Among the reported nasal symptoms in the sinonasal AAV group, “hearing loss,” “loss of olfactory/taste sensation,” “tiredness/fatigue,” “feeling frustrated/fatigued/irritable,” and “feeling sad” were commonly rated as one of the most troublesome symptoms from a list of 25, confirming the relevance of such symptoms in this condition. Moreover, considering SNOT in its 4 domains (rhinological, facial/audiological, sleep/wake rhythm, and emotional/physical disorders), the physical and emotional disorders domain was significantly impaired in AAV patients compared with those with CRS, confirming a poorer psychological-emotional well-being in vasculitic subjects.
Most importantly, we found that patients with AAV who specifically have active sinonasal disease report poorer general QoL scores (SF-36) compared with the unaffected counterparts. This is consistent with the results of Srouji et al,26 who reported an impairment especially of social functioning, perhaps as a result of the stigma of constant purulent rhinorrhea, embarrassing epistaxis, or nasal deformity from cartilage destruction. Similar conclusions were obtained with asthenia, limitations in daily/work activities because of emotional state (such as feeling depressed and anxious), and physical health. Interestingly, the psychological-emotional domain was statistically comparable between patients with ENT and non-ENT involvement, but significantly poorer when compared with the CRS group, suggesting a general impairment in AAV patients, possibly disease or treatment related.
We also found a positive correlation between both SNOT and NOSE scores and disease activity, measured by means of BVAS version 3. This result is consistent with the findings of Piccirillo et al,33 who reported that SNOT score has a moderate responsiveness to the modification of disease activity in patients with CRS.
The high impact of ENT symptoms on QoL detected in this study confirms the importance of their early treatment through specific local approach. However, even if similar conclusions have been already reported more than 15 years ago,26 to date no randomized, prospective studies evaluating the efficacy of local treatments are available. Interestingly, no significant differences in QoL were noted when considering systemic therapy, including both glucocorticoids and immunosuppressants. It has to be pointed out that, in our cohort, at the time of assessment, only one AAV patient was currently treated with rituximab, which is nowadays recommended for induction and maintenance of remission in relapsing and severe AAV.
Although dealing with a rare autoimmune disease, the limitations of the present study include the relatively small number of patients and the lack of information on their work/employment condition. In addition, the study design does not allow performing a risk factor analysis, and prospective, longitudinal studies are warranted. However, the present findings confirm the results of previous studies, and for the first time, patient-reported outcomes were assessed through multiple specific questionnaires. Further studies are warranted to confirm results on larger cohorts and on other conditions as eosinophilic GPA.
CONCLUSIONS
The QoL in patients with AAV is significantly reduced, especially in the presence of ENT involvement. The AAV-related nasal morbidity is consistent and comparable to the high levels reported by patients with CRS. This involvement seems to affect more the social functioning, leading to limitation in daily/work activities. Although the role of systemic therapy in inducing and maintaining clinical remission has been largely confirmed in the literature, the efficacy of topical therapy and the influence of such treatments on the QoL of patients remains still unclear.
Footnotes
D.C. and R.P. contributed equally to the article.
The authors declare no conflict of interest.
Contributor Information
Diego Cazzador, Email: diego.cazzador@unipd.it.
Roberta Colangeli, Email: roberta.colangeli@gmail.com.
Alfonso Luca Pendolino, Email: alucapendolino@gmail.com.
Mara Felicetti, Email: mara.felicetti@gmail.com.
Elisabetta Zanoletti, Email: elisabetta.zanoletti@tiscali.it.
Enzo Emanuelli, Email: enzoemanuelli@libero.it.
Alessandro Martini, Email: alessandromartini@unipd.it.
Andrea Doria, Email: adoria@unipd.it.
Piero Nicolai, Email: piero.nicolai@unipd.it.
Franco Schiavon, Email: f.schiavon@unipd.it.
REFERENCES
- 1.Jennette JC Falk RJ Bacon PA, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Felicetti M Cazzador D Padoan R, et al. Ear, nose and throat involvement in granulomatosis with polyangiitis: how it presents and how it determines disease severity and long-term outcomes. Clin Rheumatol. 2018;37:1075–1083. [DOI] [PubMed] [Google Scholar]
- 3.Yates M, Watts R. ANCA-associated vasculitis. Clin Med. 2017;17:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu N Jones GT Fluck N, et al. Fatigue: a principal contributor to impaired quality of life in ANCA-associated vasculitis. Rheumatology (Oxford). 2010;49:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahr A Katsahian S Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72:1003–1010. [DOI] [PubMed] [Google Scholar]
- 6.Lionaki S Blyth ER Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahr A, Specks U, Jayne D. Subclassifying ANCA-associated vasculitis: a unifying view of disease spectrum. Rheumatology. 2019;58:1707–1709. [DOI] [PubMed] [Google Scholar]
- 8.Hilhorst M Wilde B van Paassen P, et al. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant. 2013;28:373–379. [DOI] [PubMed] [Google Scholar]
- 9.Rhee RL Hogan SL Poulton CJ, et al. Trends in long-term outcomes among patients with antineutrophil cytoplasmic antibody–associated vasculitis with renal disease. Arthritis Rheum. 2016;68:1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh M Flossmann O Berden A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum. 2012;64:542–548. [DOI] [PubMed] [Google Scholar]
- 11.Terrier B Pagnoux C Perrodeau É, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis. 2018;77:1150–1156. [DOI] [PubMed] [Google Scholar]
- 12.Robson J Doll H Suppiah R, et al. Damage in the ANCA-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74:177–184. [DOI] [PubMed] [Google Scholar]
- 13.Herlyn K Hellmich B Seo P, et al. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken). 2010;62:1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh M Mukhtyar C Mahr A, et al. Health-related quality of life in patients with newly diagnosed antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Care Res (Hoboken). 2011;63:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter DM Thorpe CT Lewis M, et al. Health-related quality of life for patients with vasculitis and their spouses. Arthritis Rheum. 2009;61:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robson JC Dawson J Cronholm PF, et al. Health-related quality of life in ANCA-associated vasculitis and item generation for a disease-specific patient-reported outcome measure. Patient Relat Outcome Meas. 2018;9:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutantji M Harrold E Lane SE, et al. Investigation of quality of life, mood, pain, disability, and disease status in primary systemic vasculitis. Arthritis Rheum. 2003;49:826–837. [DOI] [PubMed] [Google Scholar]
- 18.Benarous L Terrier B Laborde-Casterot H, et al. Employment, work disability and quality of life in patients with ANCA-associated vasculitides. The EXPOVAS study. Clin Exp Rheumatol. 2017;35 Suppl 103:40–46. [PubMed] [Google Scholar]
- 19.Boomsma MM Bijl M Stegeman CA, et al. Patients' perceptions of the effects of systemic lupus erythematosus on health, function, income, and interpersonal relationships: a comparison with Wegener's granulomatosis. Arthritis Rheum. 2002;47:196–201. [DOI] [PubMed] [Google Scholar]
- 20.Basu N McClean A Harper L, et al. Markers for work disability in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatology (Oxford). 2014;53:953–956. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman GS Kerr GS Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. [DOI] [PubMed] [Google Scholar]
- 22.Bligny D Mahr A Le Toumelin P, et al. Predicting mortality in systemic Wegener's granulomatosis: a survival analysis based on 93 patients. Arthritis Rheum. 2004;51:83–91. [DOI] [PubMed] [Google Scholar]
- 23.Rahmattulla C de Lind van Wijngaarden RAF Berden AE, et al. Renal function and ear, nose, throat involvement in anti-neutrophil cytoplasmic antibody-associated vasculitis: prospective data from the European Vasculitis Society clinical trials. Rheumatology (Oxford). 2015;54:899–907. [DOI] [PubMed] [Google Scholar]
- 24.Hogan SL Falk RJ Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody–associated small-vessel vasculitis. Ann Intern Med. 2005;143:621. [DOI] [PubMed] [Google Scholar]
- 25.Sproson EL Jones NS Al-Deiri M, et al. Lessons learnt in the management of Wegener's granulomatosis: long-term follow-up of 60 patients. Rhinology. 2007;45:63–67. [PubMed] [Google Scholar]
- 26.Srouji IA Andrews P Edwards C, et al. General and rhinosinusitis-related quality of life in patients with Wegener's granulomatosis. Laryngoscope. 2006;116:1621–1625. [DOI] [PubMed] [Google Scholar]
- 27.Hofauer B Bas M Straßen U, et al. Liposomal local therapy of sinunasal symptoms in ANCA associated vasculitis [in German]. Laryngorhinootologie. 2014;93:461–466. [DOI] [PubMed] [Google Scholar]
- 28.Hunder GG Bloch DA Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. [DOI] [PubMed] [Google Scholar]
- 29.Watts R Lane S Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2006;66:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fokkens WJ Lund VJ Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. [DOI] [PubMed] [Google Scholar]
- 31.Mukhtyar C Lee R Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68:1827–1832. [DOI] [PubMed] [Google Scholar]
- 32.Exley AR Bacon PA Luqmani RA, et al. Development and initial validation of the vasculitis damage index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–380. [DOI] [PubMed] [Google Scholar]
- 33.Piccirillo JF, Merritt MG, Richards ML. Psychometric and clinimetric validity of the 20-item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg. 2002;126:41–47. [DOI] [PubMed] [Google Scholar]
- 34.Stewart MG Witsell DL Smith TL, et al. Development and validation of the nasal obstruction symptom evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157–163. [DOI] [PubMed] [Google Scholar]
- 35.DeConde AS Mace JC Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merkel PA Aydin SZ Boers M, et al. The OMERACT Core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol. 2011;38:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzpatrick R Davey C Buxton MJ, et al. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess. 1998;2:1–74. [PubMed] [Google Scholar]
- 38.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–109. [DOI] [PubMed] [Google Scholar]
- 39.Srouji I Lund V Andrews P, et al. Rhinologic symptoms and quality-of-life in patients with Churg-Strauss syndrome vasculitis. Am J Rhinol. 2008;22:406–409. [DOI] [PubMed] [Google Scholar]


