Abstract
Purpose:
Distortion product otoacoustic emissions (DPOAEs) and audiometric thresholds have been used to account for the impacts of subclinical outer hair cell (OHC) dysfunction on auditory perception and measures of auditory physiology. However, the relationship between DPOAEs and the audiogram is unclear. This study investigated this relationship by determining how well DPOAE levels can predict the audiogram among individuals with clinically normal hearing. Additionally, the impacts of age, noise exposure, and the perception of tinnitus on the ability of DPOAE levels to predict the audiogram were evaluated.
Method:
Suprathreshold DPOAE levels from 1-10 kHz and pure tone thresholds from 0.25-16 kHz were measured in 366 ears from 194 young adults (19-35 years) with clinically normal audiograms and middle ear function. The measured DPOAE levels at all frequencies were used to predict pure tone thresholds at each frequency. Participants were grouped by age, self-reported noise exposure/Veteran status, and self-report of tinnitus.
Results:
Including DPOAE levels in the pure tone threshold prediction model improved threshold predictions at all frequencies from 0.25-16 kHz compared with a model based only on sample mean pure tone thresholds, but these improvements were modest. DPOAE levels for f2 frequencies of 4 and 5 kHz were particularly influential in predicting pure tone thresholds above 4 kHz. However, prediction accuracy varied based on participant characteristics. On average, predicted pure tone thresholds were better than measured thresholds among Veterans, individuals with tinnitus, and the oldest age group.
Conclusions:
These results indicate a complex relationship between DPOAE levels and the audiogram. Underestimation of pure tone thresholds for some groups suggests that additional factors other than OHC damage may impact thresholds among individuals within these categories. These findings suggest that DPOAE levels and pure tone thresholds may differ in terms of how well they reflect subclinical OHC dysfunction.
Introduction
Among individuals with clinically normal audiograms, pure tone thresholds can vary considerably (from −10 to 20 dB HL). This variation is thought to be driven in large part by outer hair cell (OHC) damage (Wu et al., 2020). Otoacoustic emissions (OAEs) are often used as an indicator of OHC function, because they are reduced when OHCs are damaged or missing due to the reduction in cochlear gain (Brownell, 1990). Given the known relationships between OHC damage and both OAEs and the audiogram, one might expect that it would be relatively easy to predict the audiogram using distortion product otoacoustic emissions (DPOAEs). However, previous attempts at predicting pure tone thresholds in this manner have had limited success (e.g., Kimberley et al., 1994). These results suggest either that there are factors other than OHC function that impact the audiogram or that DPOAEs may not accurately represent OHC damage. Clarification of the relationship between DPOAEs and the audiogram is important because studies that assess physiological measures of “hidden” auditory damage and associated perceptual impacts often use only a single measure (i.e. DPOAEs or the audiogram) to distinguish subclinical OHC dysfunction from synaptic/neuronal loss. If factors other than OHC function, such as synaptic/neuronal loss, impact the audiogram, it may be preferable to use DPOAEs to assess OHC function in these types of studies.
In the past, many researchers were interested in using DPOAEs to predict pure tone thresholds so that hearing loss could be identified in difficult to test populations, such as children or patients who were unable to provide a behavioral response. These studies included ears with both normal and abnormal pure tone thresholds. The techniques used in these investigations varied. Some studies used DPOAE thresholds to predict the behavioral hearing threshold at the corresponding frequency (Boege & Janssen, 2002; de Paula Campos & Carvallo, 2011; Gorga et al., 2003; Rogers et al., 2010), while others used suprathreshold DPOAE levels to predict hearing thresholds (de Waal et al., 2002; Wagner & Plinkert, 1999). One approach used the DPOAE level at f2 as well as adjacent frequencies to predict the matching pure tone threshold (Harris et al., 1989). Kimberley et al. (1994) used a computer-based model to predict pure tone thresholds using DPOAE levels and age as inputs. They found weak correlations between DPOAE levels and behavioral hearing thresholds. However, they were able to predict whether an individual had clinically normal or impaired hearing based on their DPOAEs and age with approximately 85% accuracy. Overall, the ability of DPOAEs to predict pure tone thresholds in these studies was modest at best.
From a theoretical perspective, it is unclear why DPOAEs, which are accepted in the field as a relatively accurate measure of OHC function, would perform so poorly at predicting the audiogram, a measurement which is thought to primarily reflect the extent of OHC damage (Wu et al., 2020). There are several possible explanations: 1) an incorrectly specified statistical model, 2) DPOAEs do not reflect OHC function as accurately as assumed, and 3) functional variation of aspects of the auditory system, other than OHCs, are impacting pure tone thresholds. Provided that the statistical model is reasonable and that DPOAEs are an accurate measure of OHC function, consistent discrepancies between observed and predicted pure tone thresholds suggest that damage to other parts of the auditory system (e.g., inner hair cells (IHCs), cochlear synapses, auditory nerve fibers, auditory brainstem, auditory cortex) are contributing to the audiogram.
The goal of this study was to evaluate how well a suprathreshold DPOAE primary sweep (DP-gram) from 1-10 kHz can predict the pure tone audiogram, including the extended high frequencies from 9-16 kHz, among individuals with clinically normal hearing and varying noise exposure histories. This differs from previous studies that have focused on the ability of DPOAEs to predict abnormal pure tone thresholds because one objective of this study was to determine whether factors other than OHC loss may impact the audiograms of individuals with clinically normal pure tone thresholds. In addition, in contrast to historical studies that attempted to predict the audiogram using DPOAEs, this study modeled the effect of the entire DP-gram on the entire audiogram, including the extended high frequencies, in a single coherent analytical framework. A suprathreshold DP-gram was used because it can be obtained more quickly than DPOAE thresholds at multiple frequencies and it is the OAE measure most commonly used in human studies of synaptopathy (Bramhall et al., 2018; Bramhall et al., 2017; Fulbright et al., 2017; Grinn et al., 2017; Grose et al., 2017; Liberman et al., 2016). The hypotheses were that 1) DPOAE prediction of pure tone thresholds would be poorer at low frequencies due to the lack of DPOAE data below 1 kHz and measurement error introduced by biological noise, 2) DPOAEs at a particular f2 frequency would contribute to the prediction of audiometric thresholds at and above that frequency because both the primary tones used to evoke DPOAEs and the backwards traveling wave must pass through the cochlear region basal to the region being stimulated, and 3) DPOAEs would underestimate audiometric thresholds among individuals at increased risk for cochlear synaptopathy, as indicated by Veteran status, older age, and/or the perception of tinnitus. This hypothesis is based on the observation that although the threshold shifts are small, animal studies suggest that deafferentation can impact behavioral thresholds (Lobarinas et al., 2013).
Methods
Participants
This analysis was conducted using data collected from two larger studies investigating noise-induced hidden hearing loss in young people with normal audiograms. These data sets have been described in previous publications (Bramhall et al., 2018; Bramhall et al., 2017; Bramhall et al., 2019; Bramhall et al., 2020), although some of the participants included in this analysis did not meet the DPOAE criteria to be included in previous reports. A total of 194 participants were included in the analysis, consisting of Military Veterans and non-Veterans, aged 19 to 35 years. Inclusion criteria included: normal air conduction thresholds (≤ 20 dB HL from 0.25 to 8 kHz) with no evidence of a noise notch, no more than one 15 dB air-bone gap with no air-bone gaps greater than 15 dB, and a normal tympanogram (226 Hz tympanogram, compliance 0.3-1.9 ml, and peak pressure between ±50 dPa). The participants were also required to have no history of concussion or significant otologic symptoms. Some participants met these criteria only in a single ear, resulting in a total of 366 ears for the analysis.
Procedures
All study procedures were approved by the Institutional Review Board of the Veterans Administration (VA) Portland Health Care System.
Audiometric Testing:
Pure tone thresholds for the standard audiometric frequencies (0.25 to 8 kHz) were assessed in all participants in 5 dB steps. In addition, audiometric thresholds from 9 to 16 kHz were measured using Sennheiser HDA 200 headphones (Old Lyme, CT) in all but 19 participants (32 ears). Pure tone thresholds at all frequencies were measured in dB HL with a Grason-Stadler GSI 61 clinical audiometer (Eden Prairie, MN) that was professionally calibrated according to American National Standards Institute (ANSI) reference standards (2018). No pure tone thresholds were beyond the limits of the audiometer at any frequency.
Otoacoustic Emissions Testing:
DPOAE testing was conducted using a custom system that includes an ER-10 B+ probe microphone and EMAV software from Boys Town National Research Hospital (Neely & Liu, 1993). The primary frequencies were separately digitized, converted to analog voltages, passed through an amplifier to separate Etymotic Research (ER-2, Elk Grove Village, IL) tubephones, and delivered to the sealed ear canal through separate ports in the probe assembly. Using in the ear calibration, the voltage applied to the tubephones was adjusted to set the SPL of f1 and f2 to the desired levels. DPOAEs were obtained in all participants using a DP-gram from f2 = 1 to 10 kHz in 1/6-octave increments at stimulus frequency levels of L1 = 65 and L2 = 55 dB SPL and an f2/f1 ratio of 1.2. If the DPOAE level was less than −20 dB SPL or the signal-to-noise ratio was less than 6 dB, the DPOAE level was set at −20 dB SPL.
Assessment of Noise Exposure and Tinnitus:
All non-Veteran participants either completed a questionnaire that asked about their lifetime occupational and recreational noise exposure, including firearm use (the Lifetime Exposure to Noise and Solvents Questionnaire (LENS-Q, Bramhall et al., 2017; Gordon et al., 2017) or were screened with questions about previous occupational or recreational noise exposure and asked if they had ever used a firearm. Most Veteran participants also completed the LENS-Q, including a section on military noise exposure. Veterans reported a variety of noise exposure histories, but almost all reported firearm use at some point during their military service. Non-Veterans all reported minimal occupational and recreational noise exposure and were divided into two groups based on any self-reported use of firearms: Non-Veteran Controls and Non-Veteran Firearms. To assess tinnitus, all study participants answered the following question on a questionnaire: “Do you have constant or frequent ringing in the ears?”. Those reporting ringing in the test ear were marked as having tinnitus.
Statistical Analysis
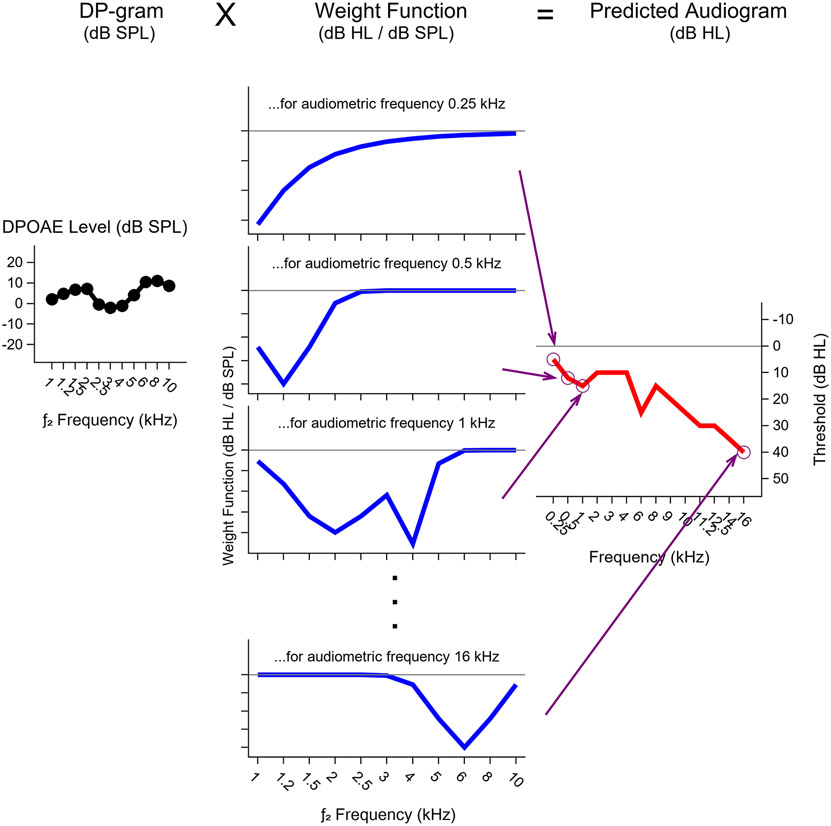
The modeling approach consists of using the entire DP-gram to predict the pure tone threshold at each audiometric frequency. This is accomplished using a regression analysis where the predictors are the measured DPOAE levels at each f2 frequency and the dependent variables are pure tone threshold at each audiometric frequency. Specifically, a function-on-function regression model (see Scheipl et al., 2015) is used because both the DP-gram and the audiogram can be expressed as functions. The outputs of the model are regression coefficients, or weight functions, for each audiometric frequency that can be multiplied by the DP-gram for an individual ear to generate a predicted audiogram for that ear. A schematic illustrating this concept is shown in Figure 1. The DP-gram (left) is multiplied by the weight function for each audiometric frequency (middle) to predict the pure tone threshold at that audiometric frequency (right). Although this type of regression model can be implemented using classical statistics, a Bayesian approach offers certain advantages. In Bayesian analysis, all unknown quantities (such as how the DP-gram is related to the pure tone threshold at 4 kHz) are assigned a probability distribution. Prior knowledge about a quantity, which can be gleaned from publications or previously collected data is combined with newly collected data to generate a posterior probability distribution about that quantity from which inferences such as the effect size and Bayesian confidence intervals, called credible intervals, can be drawn. Complex models such as the function-on-function regression model described here are computationally easier to fit using a Bayesian approach than with classical statistical methods. See McMillan and Cannon (2019)) for a detailed description of the use of Bayesian analysis in auditory research.
Figure 1. Schematic illustrating how pure tone threshold predictions are generated from DP-grams.
The DP-gram (shown in black in the left panel) is multiplied by the weight function for each audiometric frequency (fPTT, blue lines in the middle panel) to predict the pure tone threshold at that fPTT (follow arrows to circles on the red line in the right panel). DPOAE levels were measured in dB SPL, while pure tone thresholds were measured in dB HL, therefore the weight functions are expressed in dB HL/dB SPL to convert the DP-gram to a predicted audiogram. Note that this figure is solely for illustrative purposes and is not based on actual numerical data.
For the purpose of comparison, two different statistical models were used to predict audiograms. In the first model, the entire DP-gram was used to predict the entire audiogram as described in the previous paragraph. This is referred to as the “full model”. This is the approach that is illustrated in Figure 1 and all presented model results are for this full model unless otherwise noted. The second model simply predicts the audiogram to be equal to the sample mean audiogram. This results in a predicted audiogram that is the same for all ears and DPOAE weight functions that are equal to zero across all frequencies because the DPOAE levels do not influence the audiogram predictions. This model is referred to as the “mean audio model”. Additional details for both statistical models can be found in the Supplemental Data.
A random intercept is often included as a modeling parameter to account for subject-to-subject variation that is otherwise not accounted for in the model. In this analysis, a random intercept could be used to account for subject-to-subject variation in the audiogram that is not accounted for by DPOAEs. This approach is often motivated by the notion that left- and right-ear audiograms within subjects are correlated. A random intercept was not included in the current analysis because one of the goals of this analysis was to qualitatively investigate subject-level features such as age, noise exposure, or perception of tinnitus that might affect the ability to predict the audiogram from the DP-gram. Therefore, it would be counterproductive to adjust for subject-level variation.
The mean audio model and the full model were fit in SAS software v9.4M5 with PROC BGLIMM assuming flat priors on all of the model parameters. Three chains were run using the No-U-Turn sampler for the variance components and conjugate sampling for the remaining effects with random starting values in each chain. Convergence was achieved after 10,000 posterior samples.
Results
Sample and group characteristics
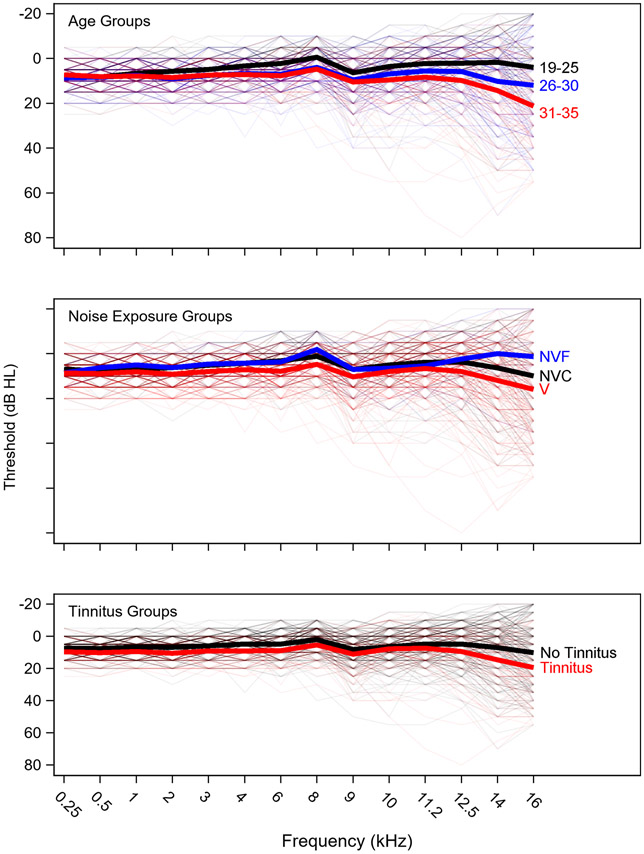
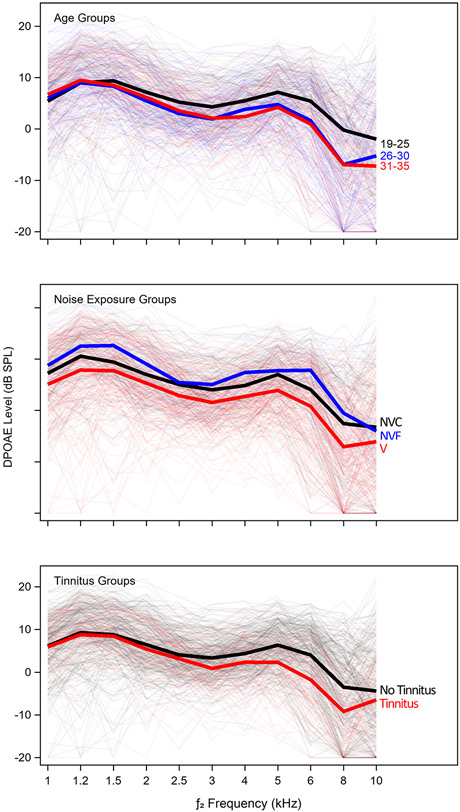
An overview of the characteristics of the sample, including the breakdown by age and noise exposure group, number of males/females, and number of individuals reporting tinnitus are shown in Table 1. Participants were not specifically recruited based on group membership, resulting in unequal numbers across groups. Table 1 shows that there are 114 Veterans, 65 non-Veteran controls, and 15 non-Veterans with firearm use in this sample. Fifty-four participants reported tinnitus, most of them Veterans. The participants are fairly evenly split into three groups based on age (19-25, 26-30, and 31-35 years) and these groups are relatively balanced in terms of sex. Audiograms and DP-grams for the age, noise exposure, and tinnitus groups are plotted in Figures 2 and 3, respectively. Average pure tone thresholds decrease with age, Veteran status, and report of tinnitus. Audiometric differences are particularly noticeable at 12.5 kHz and higher. Surprisingly, average audiometric thresholds at 14-16 kHz are better for the Non-Veteran Firearms group than for the Non-Veteran Control or Veteran groups, but this should be interpreted with caution given the small size of the Non-Veteran Firearms group. DP-grams show lower average DPOAE levels from 2-10 kHz for the two older age groups (26-30 and 31-35 years) compared to the youngest age group (19-25 years), although the DPOAEs for the two older groups are similar. On average, Veterans have lower DPOAE levels across fDPOAE compared to the other noise exposure groups, while the non-Veterans with firearm use have the largest DPOAE levels, except at fDPOAE = 10 kHz. The perception of tinnitus is associated with lower average DPOAE levels beginning at fDPOAE = 3 kHz.
Table 1.
Participant Characteristics
| Non-Veteran Firearms |
Non-Veteran Controls |
Veterans | |||
|---|---|---|---|---|---|
| Sex | Age (yrs) | Tinnitus | |||
| Male | 19-25 | No | 3 | 12 | 4 |
| Yes | 0 | 0 | 3 | ||
| 26-30 | No | 1 | 4 | 15 | |
| Yes | 0 | 0 | 22 | ||
| 31-35 | No | 2 | 5 | 16 | |
| Yes | 0 | 0 | 14 | ||
| Female | 19-25 | No | 4 | 20 | 6 |
| Yes | 0 | 1 | 1 | ||
| 26-30 | No | 4 | 13 | 9 | |
| Yes | 0 | 0 | 7 | ||
| 31-35 | No | 1 | 8 | 13 | |
| Yes | 0 | 2 | 4 |
Figure 2. Audiometric thresholds by group.
Audiograms are plotted by age, noise exposure, or tinnitus group. Groups are color-coded and mean data is shown with a thick line. Thin lines indicate data for individual ears. Note that the same ears are plotted in each subplot, but grouped differently. NVF = Non-Veteran Firearms, NVC = Non-Veteran Controls, V = Veterans
Figure 3. DPOAE levels by group.
DP-grams are plotted by age, noise exposure, and tinnitus group. Mean data is shown with a thick line. Thin lines indicate data for individual ears. Note that the same ears are plotted in each subplot, but grouped differently. If the measured DPOAE level was less than −20 dB SPL or the signal-to-noise ratio was less than 6 dB, the DPOAE level was set at −20 dB SPL. NVF = Non-Veteran Firearms, NVC = Non-Veteran Controls, V = Veterans
Correlations between DPOAE levels and pure tone thresholds
Pearson correlation coefficients between the pure tone thresholds at each fPTT and the DPOAE levels at each fDPOAE are shown in Table 2. Scatterplots illustrating these relationships can be found in the Supplemental Data. The poorest correlation between DPOAE levels and pure tone thresholds was at fDPOAE = fPTT = 1kHz , where the correlation coefficient was −0.15. The strongest correlations were observed between the DPOAE levels at fDPOAE = 4 and 5 kHz and the pure tone threshold at fPTT = 4 kHz (−0.42 to −0.45) and the DPOAE levels at fDPOAE = 5, 6, and 8 kHz and the pure tone threshold at fPTT = 6 kHz (−0.44 to −0.49). Interestingly, the DPOAE levels at fDPOAE = 4 and 5 kHz had correlations with pure tone thresholds in the extended high frequencies (fPTT = 9-16 kHz) that were as strong or stronger (−0.21 to −.031) than the correlations between the DPOAE levels at fDPOAE = 8 and 10 kHz and the extended high frequency (fPTT = 9-16 kHz) pure tone thresholds (−0.10 to −0.25).
Table 2.
Correlations between DPOAE levels and pure tone thresholds
| DPOAE f2 Frequency (kHz) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 1.2 | 1.5 | 2.0 | 2.5 | 3.0 | 4.0 | 5.0 | 6.0 | 8.0 | 10.0 | ||
| Pure Tone Threshold Frequency (kHz) | 0.25 | −0.15 361 |
−0.15 363 |
−0.23 364 |
−0.28 365 |
−0.23 366 |
−0.15 366 |
−0.18 366 |
−0.19 366 |
−0.12 361 |
−0.17 332 |
−0.03 330 |
| 0.50 | −0.22 361 |
−0.24 363 |
−0.33 364 |
−0.30 365 |
−0.25 366 |
−0.21 366 |
−0.20 366 |
−0.20 366 |
−0.17 361 |
−0.21 332 |
0.01 330 |
|
| 1.0 | −0.15 361 |
−0.13 363 |
−0.28 364 |
−0.28 365 |
−0.21 366 |
−0.25 366 |
−0.25 366 |
−0.22 366 |
−0.16 361 |
−0.19 332 |
0.00 330 |
|
| 2.0 | −0.06 361 |
−0.05 363 |
−0.21 364 |
−0.33 365 |
−0.32 366 |
−0.36 366 |
−0.29 366 |
−0.27 366 |
−0.20 361 |
−0.22 332 |
−0.03 330 |
|
| 3.0 | 0.01 361 |
0.03 363 |
−0.07 364 |
−0.12 365 |
−0.17 366 |
−0.34 366 |
−0.36 366 |
−0.36 366 |
−0.22 361 |
−0.23 332 |
−0.08 330 |
|
| 4.0 | 0.04 361 |
0.05 363 |
−0.05 364 |
−0.11 365 |
−0.13 366 |
−0.27 366 |
−0.45 366 |
−0.42 366 |
−0.33 361 |
−0.31 332 |
−0.20 330 |
|
| 6.0 | −0.11 361 |
−0.12 363 |
−0.22 364 |
−0.19 365 |
−0.20 366 |
−0.30 366 |
−0.37 366 |
−0.49 366 |
−0.44 361 |
−0.46 332 |
−0.35 330 |
|
| 8.0 | −0.04 361 |
−0.08 363 |
−0.14 364 |
−0.16 365 |
−0.12 366 |
−0.22 366 |
−0.34 366 |
−0.34 366 |
−0.35 361 |
−0.33 332 |
−0.32 330 |
|
| 9.0 | 0.01 329 |
−0.03 331 |
−0.08 332 |
−0.20 333 |
−0.11 334 |
−0.12 334 |
−0.27 334 |
−0.28 334 |
−0.21 330 |
−0.20 302 |
−0.22 298 |
|
| 10.0 | −0.03 329 |
−0.05 331 |
−0.06 332 |
−0.13 333 |
−0.06 334 |
−0.07 334 |
−0.22 334 |
−0.23 334 |
−0.18 330 |
−0.14 302 |
−0.16 298 |
|
| 11.2 | 0.02 329 |
0.03 331 |
−0.04 332 |
−0.11 333 |
−0.07 334 |
−0.12 334 |
−0.25 334 |
−0.22 334 |
−0.15 330 |
−0.09 302 |
−0.23 298 |
|
| 12.5 | 0.00 329 |
0.00 331 |
−0.04 332 |
−0.14 333 |
−0.12 334 |
−0.15 334 |
−0.31 334 |
−0.31 334 |
−0.27 330 |
−0.09 302 |
−0.25 298 |
|
| 14.0 | 0.05 329 |
0.01 331 |
−0.02 332 |
−0.13 333 |
−0.11 334 |
−0.15 334 |
−0.30 334 |
−0.28 334 |
−0.24 330 |
−0.10 302 |
−0.24 298 |
|
| 16.0 | 0.06 329 |
0.01 331 |
−0.01 332 |
−0.13 333 |
−0.12 334 |
−0.10 334 |
−0.21 334 |
−0.21 334 |
−0.25 330 |
−0.10 302 |
−0.18 298 |
|
Pearson correlation coefficients and number of observations. Black boxes show correlations between DPOAE level and pure tone threshold at the corresponding frequency.
Impact of DPOAE levels on pure tone threshold predictions
Root mean square prediction errors (RMSPEs) for the full model are shown for each pure tone threshold frequency in Table 3. RMSPEs increase as fPTT increases, ranging from 5 dB at 0.25 kHz to 18.9 dB at 16 kHz. Prediction accuracy is poorest in the extended high frequencies (fPTT = 9-16 kHz). RMSPEs for the mean audio model are also shown in Table 3, along with the difference in RMSPEs between the two models. Prediction accuracy is better (by 0.4 to 2.1 dB) for the full model, even for fPTT where DPOAEs were not measured (0.25, 0.5, 9, 11.2, 12.5, 14, and 16 kHz).
Table 3.
RMSPEs by model and pure tone threshold frequency.
| Full Model |
Mean Audio Model |
Difference (Mean Audio – Full) |
|
|---|---|---|---|
|
Freq
(kHz) |
RMSPE
(dB) |
RMSPE
(dB) |
|
| 0.25 | 5.0 | 5.4 | 0.4 |
| 0.5 | 5.0 | 6.0 | 1.0 |
| 1 | 5.3 | 5.8 | 0.5 |
| 2 | 5.5 | 7.6 | 2.1 |
| 3 | 5.5 | 6.4 | 0.9 |
| 4 | 5.5 | 6.8 | 1.3 |
| 6 | 6.0 | 7.4 | 1.4 |
| 8 | 6.5 | 8.3 | 1.8 |
| 9 | 9.2 | 9.8 | 0.6 |
| 10 | 10.2 | 12.0 | 1.8 |
| 11.2 | 10.7 | 11.5 | 0.8 |
| 12.5 | 11.9 | 13.0 | 1.1 |
| 14 | 16.5 | 17.5 | 1.0 |
| 16 | 18.9 | 20.1 | 1.2 |
Freq = Frequency, RMSPE = Root mean squared prediction error
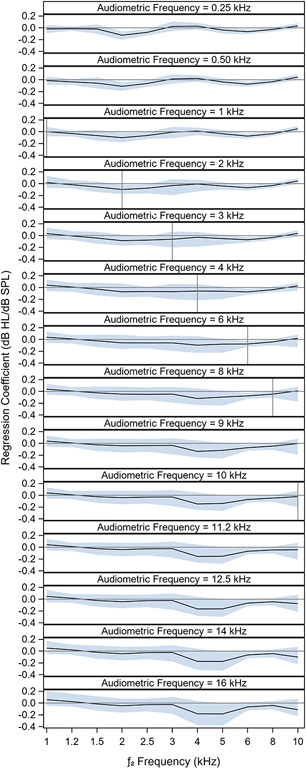
The regression coefficient (or weight) applied to each DPOAE level when predicting the pure tone audiogram is plotted in Figure 4. Overall, the DPOAE level at the fDPOAE corresponding to the fPTT does not have a greater weight than other fDPOAE. In fact, for pure tone thresholds at fPTT ≤ 3 kHz, the DPOAE level at fDPOAE = 2 kHz has the largest weight and for pure tone thresholds at fPTT ≥ 6 kHz, the DPOAE levels at fDPOAE = 4 and 5 kHz have the largest weights.
Figure 4. DPOAE weight functions.
DPOAE levels for the f2 frequency (fDPOAE) corresponding to the frequency of the predicted audiometric threshold (fPTT) do not have the biggest regression coefficients (weights). Regression coefficients are plotted for each combination of fPTT and fDPOAE. This indicates the relative weight that the DPOAE level at the indicated fDPOAE contributes to the predicted audiometric threshold at fPTT. The black line shows the median of the posterior distribution and the blue shaded region indicates the 90% confidence interval. Vertical gray lines show instances where fDPOAE corresponds to fPTT.
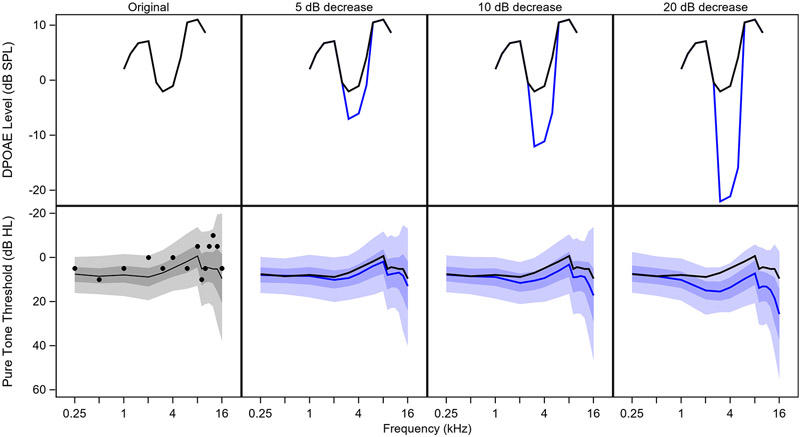
Figure 5 illustrates the impact of DPOAE level on the predicted audiogram by decreasing the DPOAE levels at fDPOAE = 3, 4, and 5 kHz. It is clear from this plot that a dramatic notch in the DPOAE levels does not have a proportional impact on predicted pure tone thresholds at the corresponding fPTT. Interestingly, decreasing the DPOAE level at fDPOAE = 3, 4, and 5 kHz results in poorer predicted pure tone thresholds not only at fPTT = 3, 4, and 5 kHz, but also a similar change in all pure tone thresholds where fPTT ≥ 2 kHz.
Figure 5. Impact of decreased DPOAE levels on pure tone thresholds.
The shape of the audiogram is relatively insensitive to the shape of the DP-gram. DPOAE levels are plotted for an example ear from an actual participant in the top left, labeled “Original”. Moving from left to right, the DPOAE levels at fDPOAE = 3, 4, and 5 kHz are decreased by 5, 10, or 20 dB (shown with the blue line). In the bottom row, black circles indicate the participant’s measured pure tone thresholds. The black line shows the predicted thresholds before changing the DPOAE levels. The predicted pure tone thresholds after decreasing the DPOAE levels are shown with the blue line. Dark shaded regions show the 50th percentile and light shaded regions show the 90th percentile.
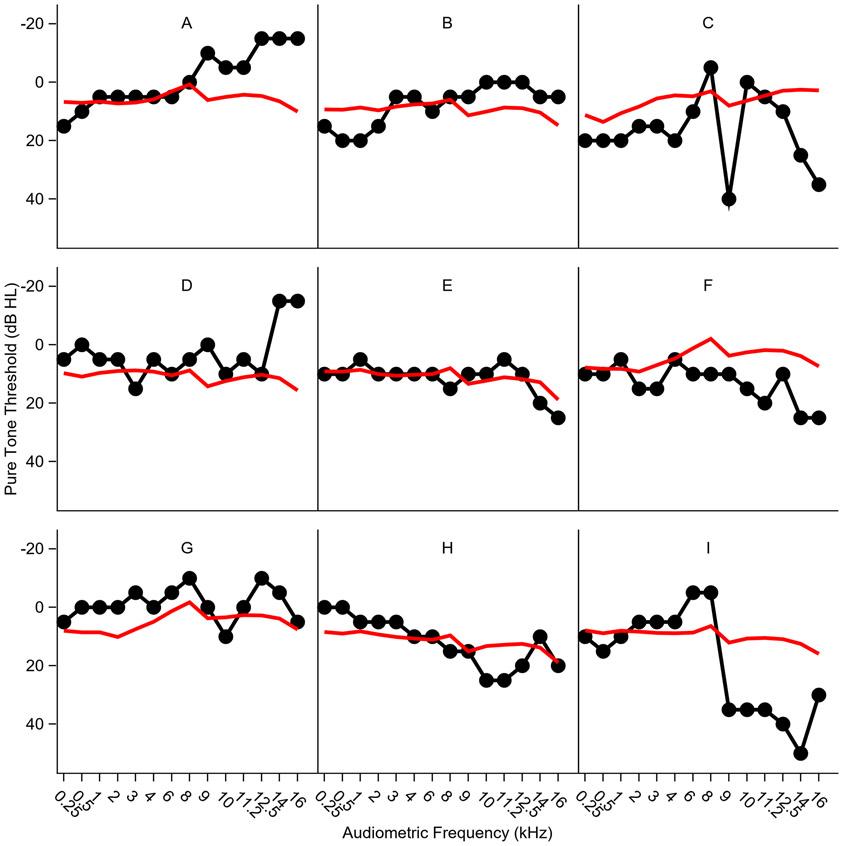
Examples of predicted and observed audiograms are shown for nine ears in Figure 6. These examples were chosen to demonstrate the range of prediction error patterns observed in the sample. In the ideal case, the predicted audiogram closely approximates the measured audiogram (Figure 6E). However, in some ears, predicted thresholds are smaller (better) than observed thresholds (Figure 6F), or are larger (poorer) than observed thresholds (Figure 6D). In other ears, the pattern is less straightforward, with predicted thresholds that are better than observed for low fPTT and poorer than observed for high fPTT (Figure 6B) or vice versa (Figure 6H).
Figure 6. Examples of predicted versus observed audiograms.
A variety of examples of predicted versus observed audiograms are plotted for individual ears to illustrate the various patterns that were observed. Observed audiograms are shown in black, predicted audiograms are shown in red.
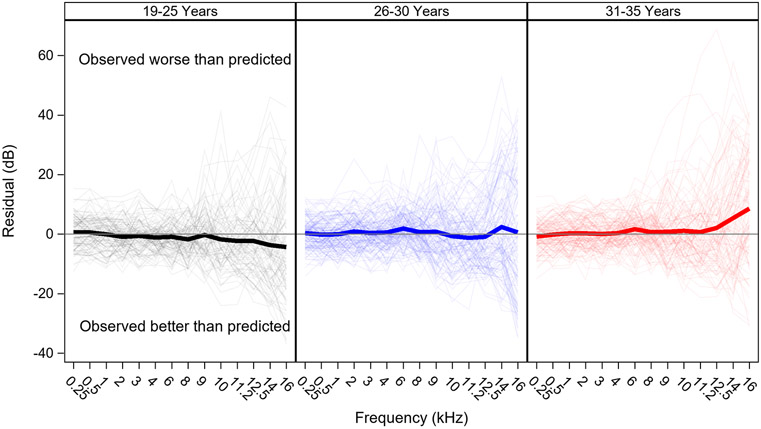
Audiograms are poorer than predicted among the oldest group, but better than predicted in the youngest group
Average residuals at each fPTT (the predicted threshold minus the observed threshold) for each age group are plotted in Figure 7. On average, predicted audiograms for the oldest age group (31-35 years) are better than the actual measured audiograms, particularly for fPTT ≥ 6 kHz (0.7 to 8.6 dB better). In contrast, the opposite pattern is observed for the youngest age group (19-25 years), where predicted audiograms are poorer than measured audiograms for fPTT ≥ 2 kHz (−0.3 to −4.3 dB poorer). Average prediction errors are minimal for the middle group (26-30 years). Mean residuals for each age group are shown by frequency in Table 4.
Figure 7. Residuals by age group.
Pure tone thresholds are poorer (bigger) than predicted among the oldest age group, but better than predicted among the youngest age group. Residuals (observed thresholds minus predicted thresholds) are plotted for each fPTT by age group. Thick lines show mean residuals, thin lines show residuals for individual ears. Actual values for the group mean residuals by frequency are shown in Table 4.
Table 4.
Mean group residuals by pure tone threshold frequency
| Pure Tone Threshold Frequency (kHz) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | 8 | 9 | 10 | 11.2 | 12.5 | 14 | 16 | |
| Noise Exposure Group | ||||||||||||||
| Non-Veteran Firearms | 1.7 | −0.5 | −0.9 | −0.1 | −0.7 | −0.2 | 0.7 | −2.9 | 0.8 | 1.4 | 1.0 | −2.0 | −5.5 | −7.3 |
| Non-Veteran Controls | −0.7 | 0.0 | −0.1 | −1.0 | −1.0 | −0.9 | −0.8 | −0.8 | −0.4 | −1.2 | −1.6 | −1.7 | −0.1 | 0.1 |
| Veterans | 0.2 | 0.1 | 0.2 | 0.8 | 0.6 | 0.6 | 2.1 | 0.8 | 1.0 | −0.1 | −0.7 | 0.7 | 3.6 | 3.9 |
| Age Group | ||||||||||||||
| 19-25 years | 0.5 | 0.6 | −0.1 | −0.9 | −0.6 | −1.1 | −1.0 | −1.8 | −0.3 | −1.8 | −2.3 | −2.3 | −3.7 | −4.3 |
| 26-30 years | 0.4 | −0.2 | −0.1 | 0.9 | 0.4 | 0.5 | 1.9 | 0.7 | 0.8 | −0.7 | −1.2 | −0.9 | 2.4 | 0.5 |
| 31-35 years | −0.9 | −0.2 | 0.3 | 0.2 | 0.0 | 0.4 | 1.6 | 0.7 | 0.8 | 1.1 | 0.7 | 2.1 | 5.4 | 8.6 |
| Tinnitus Group | ||||||||||||||
| No | −0.4 | −0.5 | −0.5 | −0.5 | −0.4 | −0.5 | 0.5 | −0.3 | 0.2 | −0.2 | −1.1 | −1.0 | 0.3 | 0.2 |
| Yes | 1.1 | 1.3 | 1.4 | 1.8 | 1.0 | 1.5 | 2.3 | 0.7 | 1.2 | −0.9 | −0.4 | 1.4 | 5.4 | 6.3 |
Residuals are shown in dB.
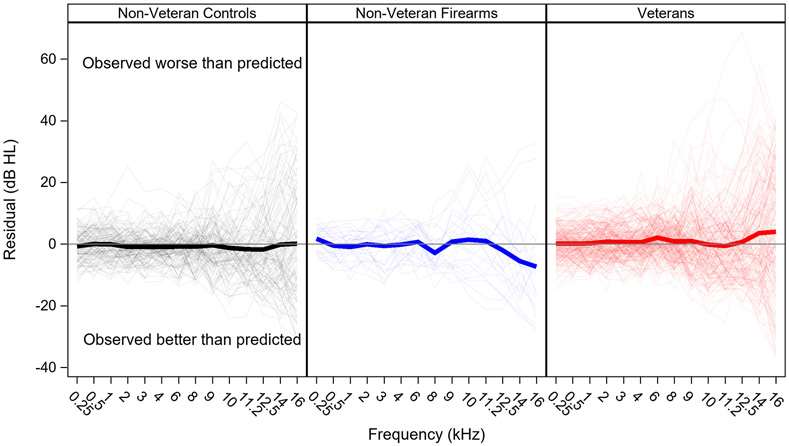
Audiograms are poorer than predicted among Veterans, but better than predicted in non-Veterans
Average residuals are plotted by fPTT according to noise exposure group in Figure 8. Predicted audiograms for Veterans show better thresholds than the measured audiograms on average. This is true across fPTT, with positive residuals (ranging from 0.1 to 3.9 dB) at all fPTT except 10 and 11.2 kHz, where the residuals are −0.1 and −0.7 dB respectively. The reverse is observed for non-Veterans, where the predicted audiograms are poorer than the actual audiograms. Among non-Veterans reporting any history of firearm use, predicted audiograms are worse than measured audiograms for fPTT ≥ 12.5 kHz (residuals range from −2.0 to −7.3 dB). Among non-Veteran controls, residuals are negative at all fPTT except 0.5 and 16 kHz, ranging from −0.1 dB to −1.7 dB. Mean residuals for each noise exposure group are shown by fPTT in Table 4.
Figure 8. Residuals by noise exposure group.
Pure tone thresholds are poorer than predicted among Veterans, but are better than predicted among non-Veterans. Residuals (observed thresholds minus predicted thresholds) are plotted for each fPTT by noise exposure group (Non-Veteran Firearms, Non-Veteran Controls, and Veterans). Thick lines show mean residuals, thin lines show residuals for individual ears. Actual values for the group mean residuals by frequency are shown in Table 4.
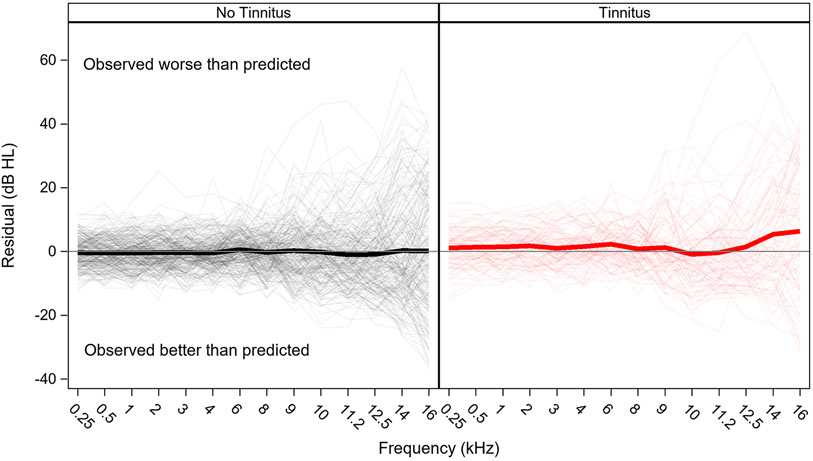
Audiograms are poorer than predicted among individuals with tinnitus, but not individuals without tinnitus
Average residuals for each fPTT are plotted by tinnitus group in Figure 9. On average, predicted audiograms for individuals with tinnitus are better than observed at all fPTT, except 10 and 11.2 kHz (residuals of −0.9 and −0.4 dB), with residuals ranging from 0.7 to 6.3 dB. Mean residuals are close to zero across fPTT for individuals without tinnitus. Mean residuals for each tinnitus group are shown by fPTT in Table 4.
Figure 9. Residuals by tinnitus group.
Pure tone thresholds are poorer than predicted among individuals reporting tinnitus, but not among individuals without tinnitus. Residuals (observed thresholds minus predicted thresholds) are plotted for each fPTT by noise exposure group. Thick lines show mean residuals, thin lines show residuals for individual ears. Actual values for the group mean residuals by frequency are shown in Table 4.
Discussion
Ability of DPOAE levels to predict individual pure tone thresholds
Across fPTT, prediction errors for the full statistical model that included DPOAE levels were smaller than for than the model based only on average pure tone thresholds. However, the magnitude of this improvement was quite small. Adding DPOAEs to the statistical model only improved prediction errors by 0.4 to 2.1 dB.
Prediction accuracy in the standard audiometric range (fPTT = 0.25-8 kHz) was close to the test-retest reliability of clinical audiometric thresholds (± 5 dB). In the extended high frequencies (fPTT = 9-16 kHz), prediction accuracy was considerably poorer (9-19 dB).
DPOAE levels at fDPOAE = 4 and 5 kHz had greater weights than DPOAEs at other frequencies in terms of predicting pure tone thresholds for fPTT ≥ 6 kHz. This may be a consequence of the primary tones at f1 and f2 and the backwards traveling wave passing through more basal regions of the cochlea and therefore increasing sensitivity to basal cochlear damage. Additionally, given the relatively young age of all participants in this study, we would expect most OHC damage in the sample to be related to noise exposure and therefore expect a high correlation with the DPOAE levels in the 4 kHz region.
DPOAE levels at fDPOAE = 4 and 5 kHz were weighted higher in terms of predicting the pure tone thresholds for fPTT = 9-16 kHz than the DPOAE levels at fDPOAE = 8 and 10 kHz. One explanation for this finding is that noise exposure-related OHC damage in the 4 kHz cochlear region is highly correlated with OHC damage at the extreme base of the cochlea. This is consistent with animal studies that have demonstrated two damage foci in response to noise exposure, one at the tonotopic region with maximal noise-induced vibration (equivalent to the 4 kHz region in humans) and one in the basal hook region (Fried et al., 1976; Wang et al., 2002).
Three possible explanations for why predicted audiograms might not correspond with measured audiograms were described earlier: 1) inadequacy of the statistical model, 2) DPOAEs are not a sufficiently accurate measure of OHC function, and 3) pure tone thresholds vary as a function of auditory dysfunction other than OHC damage. In the following paragraphs, each of these explanations will be discussed in the context of the present analysis.
Sufficiency of the statistical model
In this analysis, a function-on-function regression model of the effects of the entire DP-gram on the entire audiogram was developed. Though rooted in concepts that are at least 30 years old (Hastie & Tibshirani, 1990), this type of analysis involves a relatively new methodology that is undergoing refinement at both the theoretical and computational levels. Though no model is inherently correct, we believe that this model offers the most flexible and coherent approach, to date, for addressing the complex functional data that is routinely collected in auditory research.
Relationship between DPOAE levels and OHC function
Harding et al. (2002) and Harding and Bohne (2004) present some of the most comprehensive animal data on the relationship between DPOAE levels and OHC function. They investigated the relationship between DPOAE level shifts and histopathology in noise exposed chinchillas. They observed that permanent DPOAE level shifts were associated with moderate to substantial OHC loss. However, they also found that small focal lesions with 100% loss of OHCs did not result in DPOAE level shifts at the corresponding frequency, potentially indicating that DPOAEs can be generated or supplemented by the activity of OHCs in other cochlear regions, particularly those basal to the damaged region. They also showed that DPOAE levels are sensitive not only to OHC loss, but also the condition of the supporting cells. They concluded that DPOAE levels are useful for detecting broad OHC losses of greater than 10% and large focal OHC lesions greater than 0.6 mm. Although these findings illustrate that DPOAEs generally reflect OHC integrity, they also show that there are limitations in terms of the accuracy with which DPOAEs can represent OHC damage.
Audiograms may be impacted by factors other than OHC loss
DPOAE levels underpredicted audiometric thresholds (i.e. predicted them to be better than measured) in Veterans, individuals with tinnitus, and the oldest age group (31-35 years). Both noise exposure and older age are associated with cochlear synapse loss in animal models (Kujawa & Liberman, 2009; Sergeyenko et al., 2013). Age-related cochlear synaptopathy has been confirmed in humans through temporal bone studies (Viana et al., 2015; Wu et al., 2019) and military noise exposure has been associated with reduced auditory brainstem response (ABR) wave I amplitude (Bramhall et al., 2017), a physiological indicator of synaptopathy in animal models (Kujawa & Liberman, 2009). Tinnitus is one predicted perceptual consequence of synaptopathy (Kujawa & Liberman, 2015). Several human studies have shown relationships between physiological indicators of synaptopathy and the perception of tinnitus (Bramhall et al., 2018; Bramhall et al., 2019; Gu et al., 2012; Paul et al., 2017; Schaette & McAlpine, 2011; Wojtczak et al., 2017). As a measure of peripheral auditory function, specifically OHC function, DPOAEs should not be impacted by synaptopathy. In contrast, because audiometric thresholds require a behavioral response, they can theoretically be impacted by damage at any point in the auditory system. Synaptopathy is generally assumed not to have an effect on auditory thresholds because animal models suggest that low spontaneous rate/high threshold auditory nerve fibers are the most vulnerable to synaptic loss (Furman et al., 2013; Schmiedt et al., 1996). Given that the detection of sound at threshold is coded by high spontaneous rate/low threshold fibers, the impact of synaptopathy on behavioral thresholds is expected to be minimal. In addition, a study of chinchillas with extensive carboplatin-induced IHC loss showed that up to 80% of IHCs could be lost before resulting in behavioral threshold shifts, suggesting that thresholds can be coded by a very small proportion of IHCs forming synapses with auditory nerve fibers (Lobarinas et al., 2013). Similarly, Chambers et al. (2016) found that in a mouse model with approximately 95% loss of spiral ganglion neurons, behavioral pure tone thresholds were relatively unchanged from controls. This suggests that only a small percentage of functional auditory nerve fibers are necessary for tone detection. However, it is perhaps an oversimplification to assume that synaptopathy has no effect on audiometric thresholds. If one looks more closely at the data from Lobaranis et al. (see their Figure 7), there is variability across animals in terms of auditory thresholds after damage, with some animals showing threshold shifts of up to 10 dB at any particular frequency, even with as little as 35% IHC loss. Therefore, although large changes in audiometric thresholds are not an expected result of synaptopathy, threshold shifts of up to 10 dB would not be inconsistent with the animal data. The underpredictions shown here for Veterans, individuals with tinnitus, and the oldest age group are on the order of 1-8 dB and could therefore be explained by synaptopathy-related threshold shifts.
Alternatively, the underpredictions in these groups could be due to the lack of DPOAE data for fDPOAE > 10 kHz in this sample. Noise exposure, tinnitus, and older age may lead to greater OHC loss in the extended high frequencies. This could result in an underestimate of OHC damage in these groups when using DPOAE measurements only out to fDPOAE = 10 kHz. However, this would only be expected to impact pure tone threshold predictions above fPTT = 10 kHz and does not explain why in the Tinnitus and Veteran groups, thresholds are underpredicted across the frequency range.
Limitations
This study only included DPOAE measurements out to fDPOAE = 10 kHz. Ideally, DPOAE levels would have been obtained out to 16 kHz so that the ability of DPOAE levels to predict audiometric thresholds in the extended high frequencies could be better assessed. The higher RMSPEs for pure tone threshold predictions in the extended high frequencies (fPTT = 9-16 kHz) compared to the standard audiometric frequencies (fPTT = 0.25-8 kHz) may be due in part to the limited DPOAE data available for these fDPOAE.
In this sample there is overlap between the groups that is not uniform. For example, more participants in the Veteran group fall into the two older age groups than the youngest age group. The non-Veterans disproportionately fall into the youngest age group. Also, Veterans are more likely to have tinnitus than the non-Veterans. This means that the observed age, Veteran, and tinnitus effects are not necessarily independent from each other. This is not unexpected as older age, Veteran status, and tinnitus are all associated with increased noise exposure and are difficult to separate.
The Non-Veteran Firearms group in this study is quite small, with only 15 subjects (compared to 114 Veterans and 65 Non-Veteran Controls), so it’s difficult to form conclusions about this particular noise exposure group based on the limited amount of data.
This study only investigated the relationship between suprathreshold DPOAE levels (where L1/L2 = 65/55 dB SPL) and the audiogram. It is possible that performing this analysis using DPOAE thresholds or DPOAE levels measured in response to other primary tone levels would yield different results. In particular, compressive non-linearity decreases with age and pure tone thresholds (Ortmann & Abdala, 2016), changing the shape of the DPOAE growth function. If the shape of the growth function differs between experimental groups, comparisons between groups may produce different results depending on the DPOAE measurement used. However, based on the data from Ortmann and Abdala, the suprathreshold DPOAE measurements used here are expected to fall within the compression range for individuals that are young and have clinically normal hearing thresholds, such as the participants described in this report. This would limit the influence that the specific DPOAE measurement has on the model results. Another possibility is that separating the DPOAE measurements into their source components, non-linear distortion and linear reflection (see Shera & Guinan, 1999 for a review of DPOAE source components), and using these components in the model rather than the measured DPOAE levels might lead to better predictions of the audiogram. This is something that could be explored in the future.
Although the ears in this sample had clinically normal middle ear function, as indicated by tympanometry and bone conduction thresholds, subclinical variability in middle ear function could increase the measurement error associated with the DPOAE levels (Kreitmayer et al., 2019). This would reduce the overall accuracy of the predictive model. Incorporation of measurements of middle ear function, such as acoustic admittance (e.g., Hunter et al., 2018), in future models may improve the predictive accuracy. However, variability in middle ear function should not introduce bias into the predictions. Specifically, middle ear dysfunction would be expected to negatively impact DPOAE levels more than the audiogram due to the negative impact on both the forward and backward transmission through the middle ear, resulting in predicted audiograms that are poorer than measured audiograms. This is the opposite of what was observed in the oldest age group, Veterans, and individuals with tinnitus, where the observed audiogram was poorer than what was predicted by DPOAE levels. Therefore, it is unlikely that differences in middle ear function could explain those results.
Conclusions
Overall, these findings indicate a somewhat complicated relationship between DPOAE levels and the audiogram, where pure tone thresholds may be better predicted by off frequency DPOAE levels and impacted by factors other than OHC function. These results should be considered when choosing test measures for subclinical OHC damage. If the goal is to specifically assess OHC function, measuring DPOAEs may be a better option than pure tone thresholds.
Supplementary Material
Supplemental Data 1
A detailed description of the statistical models that were used in the analysis.
Supplemental Data 2
Scatterplots show the relationship between the pure tone thresholds at each fPTT and the DPOAE levels at each fDPOAE across all study participants. The red lines are LOESS smooth lines.
Acknowledgements
The research described here was supported by the the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service - Awards #C1484-M (to NFB), #C2104-W (to NFB), and #C9230-C (to NCRAR) and the Veterans Health Administration Office of Academic Affiliations (to ANM). Research audiologist support was also provided by the Department of Defense Hearing Center of Excellence and zCore Business Solutions, Inc.. The opinions and assertions presented are private views of the authors and are not to be construed as official or as necessarily reflecting the views of the VA or the Department of Defense.
Funding Statement:
This work was supported by VA RR&D award #C1484-M (to NFB), #C2104-W (to NFB), and #C9230-C (to NCRAR) and the Veterans Health Administration Office of Academic Affiliations.
Footnotes
Conflict of Interest Statement: There are no relevant conflicts of interest
References
- American National Standard Institute (ANSI). (2018). American National Standard Specification for Audiometers S3.6 subsection 9.2.1.
- Boege P, & Janssen T (2002). Pure-tone threshold estimation from extrapolated distortion product otoacoustic emission I/O-functions in normal and cochlear hearing loss ears. J Acoust Soc Am, 111(4), 1810–1818. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, & McMillan GP (2018). Tinnitus and Auditory Perception After a History of Noise Exposure: Relationship to Auditory Brainstem Response Measures. Ear Hear, 39(5), 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP, & Griest SE (2017). Auditory Brainstem Response Altered in Humans With Noise Exposure Despite Normal Outer Hair Cell Function. Ear Hear, 38(1), e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, McMillan GP, Gallun FJ, & Konrad-Martin D (2019). Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self-report of tinnitus. J Acoust Soc Am, 146(5), 3849. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Niemczak CE, Kampel SD, Billings CJ, & McMillan GP (2020). Evoked Potentials Reveal Noise Exposure-Related Central Auditory Changes Despite Normal Audiograms. Am J Audiol, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE (1990). Outer hair cell electromotility and otoacoustic emissions. Ear Hear, 11(2), 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, & Polley DB (2016). Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron, 89(4), 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Campos U, & Carvallo R (2011). Correlation between DPOAE I/O functions and pure-tone thresholds. Braz J Otorhinolaryngol, 77(6), 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal R, Hugo R, Soer M, & Kruger JJ (2002). Predicting hearing loss from otoacoustic emissions using an artificial neural network. S Afr J Commun Disord, 49, 28–39. [PubMed] [Google Scholar]
- Fried MP, Dudek SE, & Bohne BA (1976). Basal turn cochlear lesions following exposure to low-frequency noise. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol, 82(3 Pt 1), 285–298. [PubMed] [Google Scholar]
- Fulbright ANC, Le Prell CG, Griffiths SK, & Lobarinas E (2017). Effects of Recreational Noise on Threshold and Suprathreshold Measures of Auditory Function. Semin Hear, 38(4), 298–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, & Liberman MC (2013). Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol, 110(3), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Griest SE, Thielman EJ, Carlson KF, Helt WJ, Lewis MS, … Henry JA (2017). Audiologic characteristics in a sample of recently-separated military Veterans: The Noise Outcomes in Servicemembers Epidemiology Study (NOISE Study). Hear Res, 349, 21–30. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Dorn PA, & Hoover BM (2003). Further efforts to predict pure-tone thresholds from distortion product otoacoustic emission input/output functions. J Acoust Soc Am, 113(6), 3275–3284. [DOI] [PubMed] [Google Scholar]
- Grinn SK, Wiseman KB, Baker JA, & Le Prell CG (2017). Hidden Hearing Loss? No Effect of Common Recreational Noise Exposure on Cochlear Nerve Response Amplitude in Humans. Front Neurosci, 11, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Buss E, & Hall JW 3rd. (2017). Loud Music Exposure and Cochlear Synaptopathy in Young Adults: Isolated Auditory Brainstem Response Effects but No Perceptual Consequences. Trends Hear, 21, 2331216517737417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, & Melcher JR (2012). Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol, 13(6), 819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding GW, & Bohne BA (2004). Temporary DPOAE level shifts, ABR threshold shifts and histopathological damage following below-critical-level noise exposures. Hear Res, 196(1-2), 94–108. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, & Ahmad M (2002). DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res, 174(1-2), 158–171. [DOI] [PubMed] [Google Scholar]
- Harris FP, Lonsbury-Martin BL, Stagner BB, Coats AC, & Martin GK (1989). Acoustic distortion products in humans: systematic changes in amplitudes as a function of f2/f1 ratio. J Acoust Soc Am, 85(1), 220–229. [DOI] [PubMed] [Google Scholar]
- Hastie T, & Tibshirani R (1990). Generalized Additive Models. New York: Chapman and Hall. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Blankenship CM, Keefe DH, Feeney MP, Brown DK, McCune A, … Lin L (2018). Longitudinal Development of Distortion Product Otoacoustic Emissions in Infants With Normal Hearing. Ear Hear, 39(5), 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley BP, Hernadi I, Lee AM, & Brown DK (1994). Predicting pure tone thresholds in normal and hearing-impaired ears with distortion product emission and age. Ear Hear, 15(3), 199–209. [DOI] [PubMed] [Google Scholar]
- Kreitmayer C, Marcrum SC, Picou EM, Steffens T, & Kummer P (2019). Subclinical conductive hearing loss significantly reduces otoacoustic emission amplitude: Implications for test performance. Int J Pediatr Otorhinolaryngol, 123, 195–201. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, & Liberman MC (2009). Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci, 29(45), 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, & Liberman MC (2015). Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res, 330(Pt B), 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, & Maison SF (2016). Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PLoS One, 11(9), e0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, & Ding D (2013). Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res, 302, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan GP, & Cannon JB (2019). Bayesian Applications in Auditory Research. J Speech Lang Hear Res, 62(3), 577–586. [DOI] [PubMed] [Google Scholar]
- Neely S, & Liu Z (1993). EMAV: Otoacoustic emission averager. In Tech Memo No. 17: Boys Town National Research Hospital Omaha. [Google Scholar]
- Ortmann AJ, & Abdala C (2016). Changes in the Compressive Nonlinearity of the Cochlea During Early Aging: Estimates From Distortion OAE Input/Output Functions. Ear Hear, 37(5), 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BT, Bruce IC, & Roberts LE (2017). Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear Res, 344, 170–182. [DOI] [PubMed] [Google Scholar]
- Rogers A, Burke S, Kopun J, Hongyang T, Neely S, & Gorga M (2010). Influence of Calibration Method on DPOAE Measurements: II. Threshold Prediction. Ear Hear, 31(4), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, & McAlpine D (2011). Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci, 31(38), 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheipl F, Staicu A-M, & Greven S (2015). Functional Additive Mixed Models. J Comput Graph Stat, 24(2), 477–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, & Boettcher FA (1996). Age-related loss of activity of auditory-nerve fibers. J Neurophysiol, 76(4), 2799–2803. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, & Kujawa SG (2013). Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci, 33(34), 13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, & Guinan JJ Jr. (1999). Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am, 105(2 Pt 1), 782–798. [DOI] [PubMed] [Google Scholar]
- Viana LM, O'Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, … Liberman MC (2015). Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res, 327, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, & Plinkert PK (1999). The relationship between auditory threshold and evoked otoacoustic emissions. Eur Arch Otorhinolaryngol, 256(4), 177–188. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, & Liberman MC (2002). Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol, 3(3), 248–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak M, Beim JA, & Oxenham AJ (2017). Weak Middle-Ear-Muscle Reflex in Humans with Noise-Induced Tinnitus and Normal Hearing May Reflect Cochlear Synaptopathy. eNeuro, 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Scheipl F, & Faraway J (2012). Straightforward intermediate rank tensor product smoothing in mixed models. Stat and Comput, 23(3), 341–360. [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O'Malley JT, & Liberman MC (2019). Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neurosci, 407, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, Wen WP, O'Malley JT, & Liberman MC (2020). Assessing fractional hair cell survival in archival human temporal bones. Laryngoscope, 130(2), 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data 1
A detailed description of the statistical models that were used in the analysis.
Supplemental Data 2
Scatterplots show the relationship between the pure tone thresholds at each fPTT and the DPOAE levels at each fDPOAE across all study participants. The red lines are LOESS smooth lines.