Abstract
Objective:
To evaluate the benefits of probe-based near infrared autofluorescence (NIRAF) parathyroid identification during parathyroidectomy.
Summary Background Data:
Intraoperative parathyroid gland identification during parathyroidectomy can be challenging, while additionally requiring costly frozen sections. Earlier studies have established NIRAF detection as a reliable intraoperative adjunct for parathyroid identification.
Methods:
Patients undergoing parathyroidectomy for primary hyperparathyroidism were prospectively enrolled by a senior surgeon (>20 years’ experience) and a junior surgeon (<5 years’ experience), while being randomly allocated to the probe-based NIRAF or control group. Data collected included procedure type, number of parathyroids identified with high confidence by the surgeon and the resident, number of frozen sections performed, parathyroidectomy duration, and number of patients with persistent disease at the first post-operative visit.
Results:
One hundred and sixty patients were randomly enrolled under both surgeons to the probe group (n=80) vs. control (n=80). In the probe group, parathyroid identification rate of the senior surgeon improved significantly from 3.2 to 3.6 parathyroids per patient (p<0.001), while that of the junior surgeon also rose significantly from 2.2 to 2.5 parathyroids per patient (p=0.001). Parathyroid identification was even more prominent for residents increasing significantly from 0.9 to 2.9 parathyroids per patient (p<0.001). Furthermore, there was a significant reduction in frozen sections utilized in the probe group vs. control (17 vs 47, p=0.005).
Conclusions:
Probe-based NIRAF detection can be a valuable intraoperative adjunct and educational tool for improving confidence in parathyroid gland identification, while potentially reducing the number of frozen sections required.
Mini Abstract:
Intraoperative parathyroid gland identification during parathyroidectomy can be complex. Prior reports demonstrate near-infrared autofluorescence (NIRAF) detection to be useful for parathyroid identification. The results of this first randomized clinical trial with probe-based NIRAF detection suggest that this technology could improve parathyroid gland identification, while reducing frozen sections needed during parathyroidectomy.
Introduction
Primary hyperparathyroidism (pHPT) is a common endocrine disorder. It has an estimated prevalence of 1 in 500 women and 1 in 2000 men1, 2 and there are over 100,000 parathyroidectomies performed annually in the United States.3 While parathyroidectomy is successful most of the time, experienced surgeons may fail to localize all diseased/hyperfunctioning parathyroid glands (PGs) 2–10% of the time.4–9 The most common cause of failure is the surgeon’s inability to accurately identify or localize the diseased/hyperfunctioning PGs. Failure rates are high especially in low-volume practices and lead to persistent hyperparathyroidism and unnecessary reoperations.4–9 Reoperative procedures are associated with increased technical difficulty and operative duration, risk of hypoparathyroidism, and recurrent laryngeal nerve damage.8 The problem is that PGs have variable anatomic locations, small size, and appear similar to surrounding tissues like thyroid nodules, fat, or lymph nodes.
Preoperative imaging with ultrasound (US), 99mtecnetium-sestamibi scintigraphy and computed tomography (CT) are often used preoperatively to localize enlarged or abnormal PGs and the gamma probe can be used intraoperatively to locate diseased PGs with some success; however, these modalities are not able to localize normal PGs and often miss abnormal glands.10–14 Furthermore, preoperative localization is not always concordant with what is seen by the surgeon intraoperatively. Thus, surgeons rely on their own accrued surgical experience to identify both normal and diseased PGs. When a surgeon is unsure of PG identification, frozen section (FS) or tissue aspirate parathyroid hormone (PTH) analysis can be performed to confirm PG tissue is, in fact, present. However, FS or tissue aspirate PTH analysis is costly, not readily available, can be injurious to normal PGs, and requires at least 20–30 minutes per sample of analysis time. Further improvements in real-time intraoperative PG identification are sorely needed.
In 2011, strong near-infrared autofluorescence (NIRAF) was reported in PGs when compared to surrounding tissue in the neck.15–17 Since that time, several studies have demonstrated that NIRAF allows for detection of both normal and abnormal PGs.18–22 NIRAF technology is currently available in image- and probe-based platforms, which offer the surgeon a noninvasive, label-free (does not require indocyanine green (ICG) or isotope injection) modality for real-time PG identification. There is currently only one FDA-cleared probe-based NIRAF system, the PTeye (Medtronic, Dublin, Ireland).
There has never been a randomized controlled trial utilizing probe-based NIRAF during parathyroid surgery. This study was designed to determine the impact of this probe in clinical use in a high-volume endocrine surgery practice. We sought to determine if the use of probe-based NIRAF during parathyroidectomy impacted the PG identification rates, number of FS analyses, operative time, rate of surgical failure, and permanent hypoparathyroidism.
Methods:
Study Design
A randomized clinical trial was conducted at a single high-volume surgical center, involving two endocrine surgeons from March 2020 to August 2022. The participant surgeons included a senior surgeon with >20 years of independent surgical experience and a junior surgeon with <5 years of independent surgical experience. Adult patients (aged ≥ 18 years) undergoing parathyroid surgery for pHPT or persistent/recurrent pHPT after a failed prior parathyroidectomy, were prospectively enrolled for this study. pHPT was diagnosed based on (i) elevated serum calcium alongside increased PTH levels, (ii) elevated PTH levels with normocalcemia, or (iii) elevated calcium with inappropriately suppressed PTH levels. Patients with secondary or tertiary hyperparathyroidism or concurrent thyroid procedures were excluded from this study. Recruited patients underwent preoperative localization studies with at least an ultrasound, with some patients also obtaining 99mtecnetium-sestamibi scintigraphy or 4D CT scan (parathyroid protocol CT). Patients with radiology localized disease were candidates for focused parathyroidectomy at the discretion of the surgeon, while non-localized/discordant cases underwent bilateral neck exploration (BNE). The senior surgeon in this study routinely performs BNE. Intraoperative PTH (IOPTH) was used in all cases. Appropriate IOPTH drop was defined as >50% drop of IOPTH from baseline at ten minutes after resection of the presumed diseased PG(s) preferably falling into the normal range.
Informed written consent was obtained from all patients at the Endocrine Surgery Clinic or on the day of surgical procedure. Patients were enrolled in equal numbers for the senior and the junior surgeon. The clinical study was first approved by the Institutional Review Board (IRB) at the study site. Subsequently, the study was registered with ClinicalTrials.gov (NCT04299425) prior to the initiation of the clinical trial. Random audits of case report forms were performed at least quarterly by the IRB to ensure
Randomization
Prior to parathyroidectomy, the patient was assigned a unique ID, which was utilized for randomly allocating the patient to the experimental arm (probe-based NIRAF) or a control arm (no probe-based NIRAF). The allocation sequence was generated with the ‘Random Allocation Software’ available online (http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html). For each surgeon, 80 patients were equally distributed into either arm using simple randomization method. The patients remained blinded to the intervention throughout the entire duration of the study, while the operative surgeon and surgical assists were aware of the intervention. The participant surgeons remained blinded to analysis of the outcome parameters measured from the parathyroidectomy procedures between the experimental and control cohorts.
Study Procedure
For patients assigned to the experimental arm, the surgeon(s) first looked for PG tissue as usual and then used probe-based NIRAF detection as an intraoperative tool to identify/confirm if a target tissue was a parathyroid or not, as described in earlier reports. 19, 23–25 As per system design (see Figure 1), the FDA cleared probe-based NIRAF system named PTeye indicates that a tissue is parathyroid in real-time if the ‘Parathyroid Detection Ratio’ is displayed to be > 1.2 without the use of ICG or other injectables. The rest of the surgical procedure will follow according to standard protocol. For patients assigned to the control arm, the surgeons did not use probe-based NIRAF and proceeded with the parathyroidectomy as routine, while relying solely on their surgical experience in identifying the PGs intraoperatively. While diseased PGs were excised and sent for FS analysis (as necessary) and routine histopathology, healthy PGs that were visualized were left in situ, unless they were accidentally devascularized/excised and needed to be histologically validated before auto-transplantation. Tissue aspirate PTH for confirmation of parathyroid tissue was not used in this study. For each case, the confidence levels for the attending surgeon and the resident in identifying PGs were graded and recorded as low (<50%), moderate (50–75%) and high (>75%) in both the probe-based (before and after using the probe) and control groups.
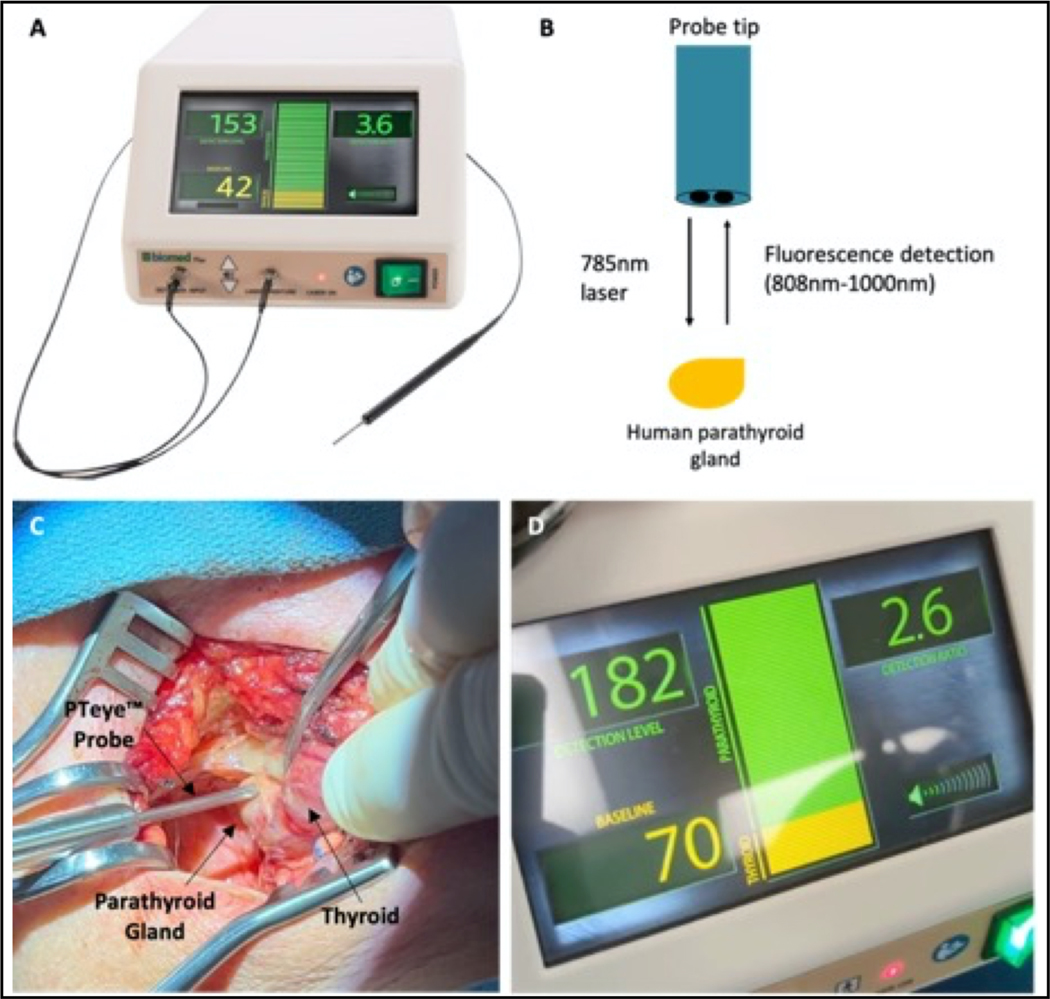
Figure 1.
Probe-based parathyroid near-infrared (NIRAF) autofluorescence detection system (PTeye) (A) PTeye is a probe-based device that consists of a display console containing near-infrared (NIR) light source and detector, a disposable sterile fiber-optic probe, and a foot pedal (not shown). Figure obtained with permission from www.medtronic.com. (B) PTeye probe tip schematic. PTeye™ fiber optic probe emits a 785 nm light when activated by the foot pedal. It then detects the subsequent autofluorescence from the parathyroid tissue, which is interpreted and quantified by the device. No need for indocyanine green injection. (C) The surgeon places the PTeye probe in contact with target tissue for NIR autofluorescence (NIRAF) detection. (D) Tissue measurements are displayed on the console as both a detection level, which is the absolute NIRAF signal intensity from the tissue, and as a detection ratio, which is the absolute intensity of the target tissue over the patient’s baseline autofluorescence (a baseline is obtained before the start of the procedure by averaging five different measurements on the thyroid gland). An auditory beep and visible green bar accompany any tissue measurements with a detection ratio ≥1.2, which indicates possible parathyroid tissue. (Used with permission from St. Amour et al. Annals of Surgical Oncology. 2023)35
Measurement of Outcomes
The outcomes assessed in this trial included (i) the rate of persistent/failure pHPT in each arm, which is characterized by elevated blood calcium (total blood calcium level > 10.5 mg/dL or 2.6 mmol/L) at last follow-up, (ii) number of PGs identified with high confidence by the attending and the resident (before and after PTeye use in the experimental group), (iii) operative time of parathyroidectomy (duration measured from time of incision to end of anesthesia administration), (iv) number of FS sent for analysis, and (v) rate of permanent hypoparathyroidism and were compiled in a de-identified manner for all enrolled patients.
Data Analysis & Statistical Methods
For determining sample size, we relied on an earlier trial where the impact of image-based NIRAF in parathyroid identification was assessed during total thyroidectomy.26 That study reported mean PGs identified by surgeon with a NIRAF camera was significantly higher at 3.1 ± 0.9, while that of the same surgeon without the camera was 2.6 ± 0.1 (p=0.0001). Based on that data (observed mean difference: 0.5 and observed standard deviation: 1.0), it was determined that to observe a statistically significant difference (i.e., for an expected mean difference: 0.7 and expected standard deviation:1.0), 33 patients would be required per group (for a 95% powered study). Since this study may involve patient follow-up for data up to 6 months after surgery, we assumed an approximate data attrition rate of 20%, thus requiring an approximate enrollment of 40 patients per cohort. Therefore, a total of 80 patients were recruited per surgeon, leading to an overall accrual of 160 patients at this study site.
Descriptive outcomes and baseline characteristics were summarized either as continuous (mean ± standard deviation) or categorical (frequency and percentage) variables. For determining significant differences in outcomes between the experimental and control cohort, either 2-tailed t-test or Wilcoxon’s test was applied for continuous variables, paired t-test was applied for variables assessed before and after NIRAF intervention (solely in the NIRAF-based cohort), while chi-square analysis was utilized for categorical variables. In addition, hierarchical cluster analysis was performed, with Spearman correlation coefficients being calculated to examine the collinearity among variables. The association between use of probe-based NIRAF and surgical outcomes was estimated in both univariable and multivariable analyses. Logistic regression model was fitted for binary outcome (appropriate IOPTH drop) and linear regression models were fitted for continuous outcome (operative time). Ordinal logistic regression was used for ordered factor outcomes (final number of PGs seen with high confidence attending and number of frozen sections). The multi-level regression analysis yielded adjusted odds ratio and 95% confidence levels for each study design parameter considered, where a variable with p <0.05 was considered to have significant effect on. All statistical analyses were performed in R software – version 4.1.2 (Vienna, Austria). Furthermore, performance of probe-based NIRAF was evaluated solely within the NIRAF group using (i) histology for excised/biopsied tissues and (ii) the surgeon’s visual confirmation – with high/moderate confidence – for in-situ tissues. Tissues identified with low confidence, which had no corroborative histology were excluded from performance analysis.
Results
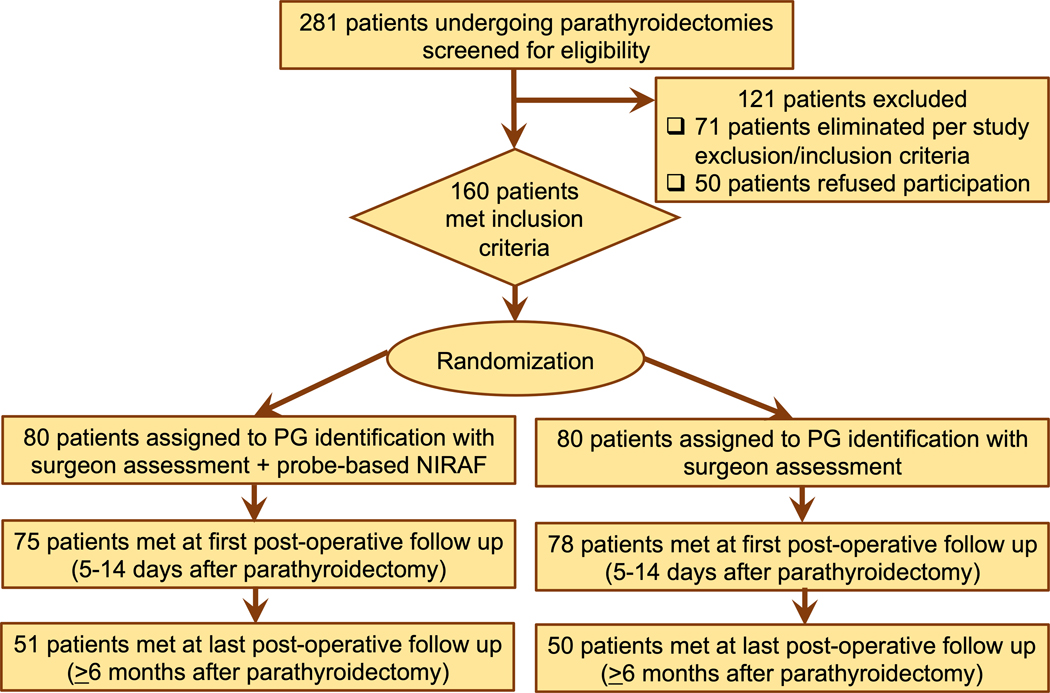
Over the duration of this trial, 281 patients were screened for eligibility to participate (Figure 2), out of which 160 patients were selected and randomly assigned – 80 in the probe-based NIRAF cohort and 80 in the control cohort. Baseline patient characteristics which included demographic as well was preoperative variables demonstrate comparable NIRAF and control cohorts (Table 1). The intraoperative and postoperative variables collected are shown in Table 2. Most patients (98%) had at least one set of follow-up labs with the mean duration of follow-up for the entire cohort and in each arm of ≥6 months. However, follow-up ≥ 6 months to determine ultimate surgical outcome was only accomplished in 63% patients (101/160 patients), with a loss of 59 patients over the follow-up period (29 for NIRAF and 30 for control arm).
Figure 2:
Consort Flowchart for patients screened, enrolled, randomized and followed in the clinical trial for the intervention and control cohort. (NIRAF – near infrared autofluorescence, PG – parathyroid gland)
Table 1:
Baseline characteristics for patients enrolled in the randomized clinical trial evaluating benefit of probe-based near infrared autofluorescence (NIRAF) detection during parathyroidectomy
| Overall | NIRAF (Intervention) | No NIRAF (Control) | |
|---|---|---|---|
|
| |||
| Preoperative patient variables | |||
|
| |||
| Age (years) | 58.3 ± 14.5 | 58.6 ± 14.6 | 59.8 ± 14.4 |
|
| |||
| Sex | |||
| Male | 35 (22) | 18 (22) | 17 (21) |
| Female | 125 (78) | 62 (78) | 63 (79) |
|
| |||
| Race | |||
| White | 147 (92) | 73 (91.25) | 74 (92.5) |
| Black | 11 (7) | 5 (6.25) | 6 (7.5) |
| Asian | 1 (0.5) | 1 (1.25) | 0 (0) |
| Other | 1 (0.5) | 1 (1.25) | 0 (0) |
|
| |||
| Ethnicity | |||
| Non-Hispanic | 159 (99) | 79 (99) | 80 (100) |
| Hispanic | 1 (1) | 1 (1) | 0 (0) |
| BMI | 30.85 ± 7.59 | 30.77 ± 7.52 | 30.94 ± 7.72 |
|
| |||
| Diagnosis | |||
| Primary Sporadic HPT | 143 (89) | 72 (90) | 71 (89) |
| Persistent HPT | 12 (7) | 3 (4) | 9 (11) |
| Recurrent HPT | 1 (1) | 1 (1) | 0 (0) |
| MEN1 | 2 (1) | 2 (3) | 0 (0) |
| Parathyroid carcinoma | 1 (1) | 1 (1) | 0 (0) |
| Lithium induced HPT | 1 (1) | 1 (1) | 0 (0) |
|
| |||
| Preoperative Calcium (mg/dL) | 11.112 ± 0.712 | 11.115 ± 0.689 | 11.112 ± 0.712 |
|
| |||
| Preoperative PTH (pg/mL) | 157.9 ± 112.7 | 152.3 ± 79.8 | 163.6 ± 139.0 |
|
| |||
| Preoperative Vitamin D | 38.1 ± 18.2 | 36.7 ± 16.0 | 39.6 ± 20.5 |
|
| |||
| Procedure by Intention | |||
| Focused | 51 (32) | 27 (34) | 24 (30) |
| Bilateral | 109 (68) | 53 (66) | 56 (70) |
Continuous variables presented as mean ± standard deviation. Categorical or ordinal variables presented as number (percent of population); HPT, hyperparathyroidism; PTH, parathyroid hormone
Table 2:
Intraoperative and postoperative variables evaluating benefit of probe-based NIRAF detection during parathyroidectomy
| Overall | NIRAF (Intervention) | No NIRAF (Control) | p-value | |
|---|---|---|---|---|
|
| ||||
| Intraoperative Variables | ||||
|
| ||||
| Procedure by Protocol | 0.29 | |||
| Focused | 44 (28) | 25 (31) | 19 (24) | |
| Bilateral | 116 (72) | 55 (69) | 61 (76) | |
|
| ||||
| Re-operative surgery | 15 (9) | 6 (7) | 9 (11) | 0.42 |
|
| ||||
| Localized | 89 (56) | 47 (59) | 42 (52) | 0.43 |
|
| ||||
| Appropriate IOPTH Drop | 150 (94) | 76 (95) | 74 (92) | 0.51 |
|
| ||||
| Frozen Sections per case | 0.03 | |||
| 0 | 122 (76) | 67 (84) | 55 (69) | |
| 1 | 22 (14) | 11 (14) | 11 (14) | |
| 2 | 10 (6) | 1 (1) | 9 (11) | |
| 3 | 2 (1) | 0 (0) | 2 (3) | |
| 4 | 4 (3) | 1 (1) | 3 (4) | |
|
| ||||
| Postoperative variables | ||||
|
| ||||
| Length of Stay | 0.36 | |||
| Discharged Same Day | 130 (81) | 64 (80) | 66 (82) | |
| Overnight | 28 (17) | 14 (17) | 14 (17) | |
| Admitted > 23h | 2 (1) | 2 (3) | 0 (0) | |
|
| ||||
| Follow up (months) | 7.54 ± 6.89 | 7.63 ± 7.06 | 7.47 ± 6.77 | 0.86 |
|
| ||||
| >6 month follow up* | 101 (63) | 51 (64) | 50 (62) | 0.63 |
|
| ||||
| Initial hypoparathyroidism | 2 (1) | 0 (0) | 2 (3) | 0.16 |
Continuous variables presented as mean ± standard deviation. Categorical or ordinal variables presented as number (percent of population)
missing data – 4 from NIRAF group and 2 from control group. NIRAF, near infrared autofluorescence
Significantly more PGs were identified with high confidence in the NIRAF cohort (244 PGs, 3.1 PGs per patient) as compared to the control cohort (214 PGs, 2.7 PGs per patient) at p=0.04 as indicated in Table 3. As seen in Table 4, there was no significant difference between the number of PGs identified purely based on visual assessment by either surgeon between both cohorts. With just visual inspection, the senior surgeon was able to only identify 3.2 PGs per patient in NIRAF cohort vs 3.1 PGs in control cohort with high confidence (p=0.67), while the junior surgeon was able to identify 2.2 PGs per patient in NIRAF cohort vs 2.3 PGs per patient in the control cohort (p=0.84). However, after utilizing probe-based NIRAF, the final number of PGs visualized with high confidence rose significantly to 3.6 PGs per patient (p<0.001) for the senior surgeon and 2.5 PGs per patient (p=0.001) for the junior surgeon in the NIRAF cohort. In an additional subgroup analysis, we examined the confidence of PG identification among surgical residents before and after using probe-based NIRAF detection. The numbers of PGs identified with high confidence rose notably from 0.9 PGs per patient before NIRAF to 2.9 PGs per patient after NIRAF (p<0.001) in the NIRAF cohort (Table 4), while the PG identification rate for residents remained at 0.6 PGs per patient in the control cohort (Table 3).
Table 3:
Comparison of outcomes between the intervention (near infrared autofluorescence – NIRAF) and control (No NIRAF) cohorts.
| NIRAF (Intervention) (n=80 patients) |
No NIRAF (Control) (n=80 patients) |
p-value | |
|---|---|---|---|
| Final no. of PGs identified with high confidence overall | 3.1 PGs/patient | 2.7 PGs/patient | 0.04b |
| Final no. of PGs identified with high confidence by senior surgeon | 3.6 PGs/patient | 3.1 PGs/patient | 0.01b |
| Final no. of PGs identified with high confidence by junior surgeon | 2.5 PGs/patient | 2.3 PGs/patient | 0.40b |
| Final no. of PGs identified with high confidence by surgical resident | 2.9 PGs/patient | 0.6 PGs/patient | <0.001b |
| No. of frozen sections sent for PG identification overall | 17 | 47 | 0.005b |
| No. of frozen sections sent for PG identification by senior surgeon | 7 | 21 | 0.07b |
| No. of frozen sections sent for PG identification by junior surgeon | 10 | 26 | 0.04b |
| Operative time (overall) | 89.3 ± 32.6 min | 95.5 ± 39.7 min | 0.55c |
| Operative time (senior surgeon) | 90.9 ± 37.8 min | 86.8 ± 37.4 min | 0.50c |
| Operative time (junior surgeon) | 87.7 ± 26.8 min | 104.2 ± 40.6 min | 0.12c |
| Rate of parathyroidectomy failure: Persistent hyperparathyroidism at the last postoperative visit | 0 | 1 | 1.0a |
Chi-squared analysis,
2-tailed t-test of unequal variance,
Wilcoxon test; PG, parathyroid gland(s)
Table 4:
Comparison of parathyroid glands (PGs) identified with high confidence before and after using NIRAF-probe in the intervention (NIRAF) cohort of 80 patients itself.
| No. of PGs identified with high confidence BEFORE using probe in NIRAF cohort | No. of PGs identified with high confidence AFTER using probe in NIRAF cohort | p-value | |
|---|---|---|---|
| Overall | 2.7 PGs/patient | 3.1 PGs/patient | <0.001a |
| Senior Surgeon | 3.2 PGs/patient | 3.6 PGs/patient | <0.001a |
| Junior Surgeon | 2.2 PGs/patient | 2.5 PGs/patient | 0.001a |
| Resident | 0.9 PGs/patient | 2.9 PGs/patient | <0.001a |
paired t-test analysis. PG, parathyroid gland(s)
No statistical difference was observed with respect to persistent hyperparathyroidism at the last follow-up between the NIRAF and control group (Table 3). The parathyroidectomy failure rate was observed to be 0% (0/80 patients) in the NIRAF group and 1.25% in the control group (1/80 patients) (p=1.0).
The performance accuracy of the probe was validated across 244 PGs and 56 non-parathyroid tissues – thyroid nodules, fatty tissues, lymph nodes, thymic tissues – solely in the experimental NIRAF cohort of 80 patients. With the assigned probe threshold of 1.2 for parathyroid identification,27 the device yielded 98.8% sensitivity, 67.9% specificity and an overall 93.0% accuracy (kappa = 0.74), with a positive predictive value of 93.1% and negative predictive value of 92.7%. False negative measurements were observed in 1 parathyroid carcinoma and 2 adenomatous PGs, for a false negative rate of 1.2% (3/244 PGs). The sources for false positive measurements in non-parathyroid tissues were predominantly brown fat, thymic tissues and thyroid nodules occurring in 18 out of 56 non-parathyroid tissues, leading to a false positive rate of 32.1%.
A notable reduction in FS sent for parathyroid confirmation was observed in the NIRAF cohort compared to the control (17 vs 47, p=0.005). (Table 3) This difference remained significant for the junior surgeon (10 vs 26, p=0.04) between the two groups; however, although the senior surgeon did send less FS for analysis (7 in NIRAF vs 21 in control, p=0.07), it was not statistically significant. There was no significant difference noted in operative time needed for parathyroidectomy between the NIRAF (89.3 ± 32.6 min) and control cohort (95.5 ± 39.7 min) overall. Similar trend was recorded for the operative time needed by the senior surgeon with no difference for NIRAF vs control group (90.9 ± 37.8 min vs 86.8 ± 37.4 min, p=0.50). The operative time needed by the junior surgeon was numerically lower in the NIRAF group at 87.7 ± 26.8 min, compared to 104.2 ± 40.6 min for the control group; however, the measured difference was not statistically significant (p=0.12).
Upon performing multivariate analysis to account for inter-surgeon variation, procedure variability and other demographic parameters, the final number of PGs identified with high confidence was improved with the use of probe-based NIRAF (adjusted odds ratio, 4.25; 2.5–97.5% CI, 2.21–8.52, p<0.001). (Table 4). On multivariate analysis, the use of probe-based NIRAF was associated with reduced number of FS sent for PG identification (adjusted odds ratio, 0.38; 2.5–97.5% CI, 0.17 – 0.87, p=0.02). In addition, seniority of surgeon (p=0.01) and the type of parathyroidectomy procedure (p=0.04) also affected the eventual number of FS sent (see Table 5). Operative time was found to be affected mostly by surgeon seniority (p<0.001) and type of procedure – BNE vs focused parathyroidectomy (p<0.001), with no significant effect with the use of probe-based NIRAF (p=0.58) as depicted in Table 5.
Table 5:
Multivariate effect of specific variables on (α) the final number of parathyroid glands seen, (β) number of frozen sections sent during parathyroidectomy and (Ω) the operative time of parathyroidectomy
| Odds Ratio | Confidence Interval (2.5% – 97.5%) | p-value | |
|---|---|---|---|
| Impact on Number of Parathyroid Glands Seen α | |||
| Use of probe-based NIRAF | 4.25 | 2.12–8.52 | <0.001* |
| Senior surgeon | 1.75 | 0.80–3.82 | 0.15 |
| Bilateral exploration | 70.13 | 20.29–242.43 | <0.001* |
| Re-operative surgery | 0.28 | 0.03–2.37 | 0.24 |
| Localized | 0.65 | 0.29–1.46 | 0.30 |
| Final diagnosis | 3.67 | 0.48–27.62 | 0.20 |
| Age | 0.94 | 0.97–1.01 | 0.62 |
| Sex | 1.55 | 0.69–3.48 | 0.28 |
| BMI | 1.00 | 0.96–1.05 | 0.78 |
| Impact on Number of Frozen Sections Sent β | |||
| Odds Ratio | Confidence Interval (2.5% – 97.5%) |
p-value | |
| Use of probe-based NIRAF | 0.38 | 0.17 – 0.87 | 0.02* |
| Senior surgeon | 0.34 | 0.14 – 0.81 | 0.01* |
| Bilateral exploration | 3.41 | 1.06 – 11.04 | 0.04* |
| Re-operative surgery | 1.69 | 0.12 – 24.31 | 0.67 |
| Localized | 0.44 | 0.18 – 1.09 | 0.08 |
| Final diagnosis | 0.25 | 0.02 – 3.03 | 0.28 |
| Age | 1.00 | 0.97 – 1.03 | 0.81 |
| Sex | 2.21 | 0.76 – 6.45 | 0.15 |
| BMI | 1.01 | 0.96 – 1.06 | 0.58 |
| Impact on Operative Time Ω | |||
| Estimate | Std. Error | p-value | |
| Use of probe-based NIRAF | −3.04 | 5.46 | 0.58 |
| Senior surgeon | −23.27 | 6.41 | <0.001* |
| Bilateral exploration | 28.84 | 7.87 | <0.001* |
| Re-operative surgery | 11.10 | 19.30 | 0.57 |
| Localized | −7.36 | 6.57 | 0.26 |
| Final diagnosis | −14.68 | 18.23 | 0.42 |
| Age | −0.03 | 0.20 | 0.88 |
| Sex | −3.12 | 6.75 | 0.64 |
| BMI | 0.24 | 0.38 | 0.53 |
The effects of these study design variables were analyzed with ordinal logistic regression.
p-value < 0.05 suggests that particular variable has a significant effect on the final number of parathyroid glands seen with high confidence during parathyroidectomy.
The effects of these study design variables were analyzed with ordinal logistic regression.
p-value < 0.05 suggests that a particular variable has a significant effect on the number of frozen sections sent during parathyroidectomy.
The effects of these study design variables were analyzed using linear regression.
p-value < 0.05 suggests that a particular variable has a significant effect on the operative time for parathyroidectomy. NIRAF, near infrared autofluorescence; BMI, body mass index
Discussion
NIRAF detection with either probe- or image-based technologies has been proven to be accurate for real-time label-free (no injectables) intraoperative PG identification during thyroid and parathyroid procedures.23, 28 The PTeye is currently the only FDA-cleared probe-based NIRAF detection device.23, 24, 29 In this first randomized clinical trial evaluating probe-based NIRAF utilization during parathyroid surgery we found that probe-based NIRAF provided real-time intraoperative improvement in PG identification and significantly reduced the use of FS analysis. The use of probe-based NIRAF appeared to reduce operative time although this was not statistically significant when adjusted for multiple covariates. Probe-based NIRAF did not change the short-term outcome of parathyroidectomy.
In this study the probe-based NIRAF has 93% accuracy in PG identification, which concurs with other studies using the device or its prototype version which reported an accuracy of 92–98%.19, 25, 27, 30 A recent three-center prospective nonrandomized study, with a senior and junior surgeon at each center (blinded to PTeye results), demonstrated that PTeye was as accurate as experienced surgeons in PG identification. Furthermore, PTeye proved to be more accurate than junior surgeons who had a per specimen error of 0.25 compared to 0.06 for PTeye.25 The false negative rate remains low 1.2% with a high sensitivity 98.8% also concordant with previously reported data.19, 25, 27, 30 However, the specificity for the device was much lower in this study (67.9%) than what has been reported previously (87.2–93.9%).25 These prior specificities were reported in a study where the six surgeons were blinded and they were asked to place the probe on both PG and non-PG tissues to obtain measurements. Thus, the sample size was much larger on non-PG tissues in prior studies resulting in a lower rate of false positive measurements and thus a higher specificity. In this trial, surgeons were only placing the probe on tissues that were highly suspicious for PG and thus the sample size for non-PG tissues was much lower resulting in a higher false positive rate and thus lower specificity. This trial was designed to reflect the realities of using probe-based NIRAF in clinical practice and these findings highlight the importance of knowing the common etiologies of false positive measurements (brown fat, thymic tissue and thyroid nodules). The surgeon should have a high index of suspicion for a false positive output if the surgeon’s individual confidence level is low that the target tissue is in fact PG.19 In the instance that a surgeon is questioning their own judgement and/or the probe-based NIRAF output, FS analysis or tissue aspirate PTH can be helpful to further evaluate and use previously published strategies to mitigate false positive and negative and pitfalls with the use of this new technology.23–24
Probe-based NIRAF increased the absolute number of PGs identified with high confidence in the NIRAF group when compared to the control group (Table 3). Within the NIRAF cohort itself, it additionally improved the surgeon’s ability to state they saw a PG with high confidence significantly, both for the senior and junior surgeon (Table 4). This positive impact on surgeon confidence has been previously described using both the image-based NIRAF 31 and probe-based NIRAF modalities.19 The most profound effect on surgeon confidence was seen amongst our surgical residents. In the NIRAF cohort, residents identified 0.9 PGs per patient prior to using probe-based NIRAF and 2.9 PGs per case after using the probe-based NIRAF. Although the study was not designed to adequately power this subgroup analysis, our findings suggest that probe-based NIRAF may be a valuable adjunct in teaching surgical residents how to identify both normal and abnormal PGs.
This is the first prospective randomized trial to demonstrate a significant decrease in the number of FS performed when using probe-based NIRAF detection. Previous studies using image-based NIRAF detection demonstrated that FS was avoided in 29% of cases due to improved surgeon confidence with NIRAF detection.31 In the current study, there was a decrease in both the absolute number of FS performed (17 FS in NIRAF arm, 47 FS in control arm) and also a decrease in the proportion of cases that used FS (16% of cases using FS in NIRAF arm, 31% of cases using FS in control arm). While some institutions will use tissue aspirate PTH instead of FS for intraoperative confirmation of PG tissue present, in this institution tissue aspirate PTH was not available. In this study, FS was used during difficult cases when the surgeon was uncertain if a target was PG tissue. Scenarios in which FS was most commonly used included, (i) when the IOPTH did not fall appropriately to ensure that the tissue visualized or removed was in fact PG tissue, (ii) during BNE and particularly in patients with prominent central neck lymph nodes, (iii) when there was uncertainty that the tissue was parathyroid vs. other, (iv) in cases when the patient had a large volume of brown fat, and (v) when the PG was intrathyroidal or with nodular thyroid disease. Both FS and tissue aspirate PTH add time to the procedure whereas an advantage of probe-based NIRAF is that it provides real-time feedback because, as soon as the probe makes contact with the tissue, the device output is immediate (visible and audible). The probe can be used to interrogate tissues readily and allows the surgeon to progress with confidence. Additionally, reduction of FS can lead to cost reduction in parathyroidectomy in centers where the cost of FS is higher than the cost of the NIRAF detection technology. Every institution’s costs and care bundles are different, therefore individual centers will have to determine the cost saving benefits of NIRAF technologies in reducing FS or tissue aspirate PTH. In the authors institution, FS has both technical and professional charges, the charges are based upon the number of blocks analyzed. The charges for a FS on a single specimen can range from $451-$902. In addition, FS takes approximately 20–30 minutes from the time it leaves the operating room to the time results are called. The charge for the use of the NIRAF probe is approximately $400 and the results are immediate. Lastly, probe-based NIRAF could be useful as an intraoperative adjunct to confirm parathyroid tissue in locations were FS or IOPTH may not be available.
Operative time in parathyroidectomy is driven by numerous factors including but not limited to previous radiology localization, anatomic location of gland, presence of ectopic or supernumerary PGs, size of gland, number of PGs excised, duration and number of IOPTH samples, use of FS or tissue aspirate PTH, duration of time for FS or tissue aspirate PTH and surgeon experience/skill. As such it is difficult to demonstrate the true effect of probe-based NIRAF on operative time given we were unable to adjust for all confounding variables. However, on both univariate and multivariate analysis there was no statistically significant difference in operative time between the NIRAF and control arms. This remained true when stratified by surgeon experience. Although there appeared to be clinically significant reduction in OR time for the junior surgeon from a mean operative time of 104.2 ± 40.6 min in the control arm to 87.7 ± 26.8 min in the probe arm, this was not statistically significant.
In this study, the use of probe-based NIRAF detection had no impact on the clinical outcomes of failure or success in parathyroid surgery. The rates of immediate failure were low in both groups (0% in NIRAF group, 1.2% in control) and given this low incidence in the cohort it is likely the study was underpowered to detect a true difference. The rates of temporary hypoparathyroidism (0% in NIRAF group, 3% in control) were also low. There was 1 patient in the study with hypoparathyroidism >6 months after surgery. This occurred after a second parathyroid operation was performed to remove a supernumerary ectopic PG with autotransplantation after the first operation (control group), a subtotal parathyroidectomy, resulted in failure due to a missed ectopic and supernumerary PG. To obtain real-time intraoperative feedback and confidence, many thyroid and parathyroid surgeons have adopted once disruptive technologies such as the nerve monitor and IOPTH, despite lack of level I evidence that such adjuncts always impact outcomes.32–34 Although there may never be a study that demonstrates a clinically significant impact from probe-based NIRAF on the surgical outcomes of parathyroidectomy, surgeons and surgical residents may derive confidence in PG identification by the use of this new technology.
There were several limitations in this study. Most notably we are unable to access our true long-term operative success and failure rate given only 63% of our cohort had ≥ 6-month follow-up. As such, we chose to report our failure rate at last follow-up and this has the potential to underestimate our failure rate. The missing follow-up data is evenly distributed between the NIRAF and control arm, so it is unlikely that it would change the conclusion that there is no difference in outcome in failure rates between the control and test arm; however, it may. By definition, at least 6 month follow up is required to determine cure. Additionally, as previously mentioned although we based our sample size on prior studies, it is possible that our sample size is too small to detect a difference in a low-frequency event such as operative failure in parathyroidectomy. The operative approach was not standardized between surgeons and thus the number of PGs seen per case needs to be interpreted with caution. The senior surgeon routinely performed BNE whereas the junior surgeon performed focused parathyroidectomy when possible. This results in a lower number of glands seen by the junior surgeon both with and without NIRAF as in the focused approach the surgeon was only attempting to find one abnormal gland. Finally, this study was performed in a high-volume center by two high volume endocrine surgeons and therefore may not be generalizable to all surgeons performing parathyroid surgery.
In conclusion, probe-based NIRAF detection can be a valuable intraoperative adjunct and educational tool for improving confidence in PG identification. It can also reduce the number of FS required thus potentially reducing cost.
Acknowledgements
We would like to thank the OR staff and surgical residents for their assistance in data collection. Dr. Dr. G. Thomas, Mr. P. A. Wilmon and Dr. C.C. Solόrzano were supported by the National Institute of Health under Grant No. R01CA212147.
Funding:
Dr. G. Thomas, Mr. P. A. Willmon and Dr. C. C. Solόrzano were supported by the National Institute of Health under Grant No. R01CA212147.
Disclosures:
Authors are affiliated with Vanderbilt University who has a licensing agreement for PTeye with AiBiomed (now officially acquired by Medtronic) but receive no royalties.
Dr. Nancy Perrier (Houston, TX): Congratulations, Drs. Kiernan, Solorzano, and others from the Vanderbilt Biophotonics Center. Thank you for sharing your work this morning. This morning before I came down, I asked ChatGPT can a probe based autofluorescence technology improve parathyroid surgery. The answer was this: These combined modalities can be helpful. It listed CT, US, MRI, IOPTH, nerve monitoring, gamma probe amongst those modalities.
Dr. Kiernan, I have a few comments and three questions.
can the fluorescence quantify gland function?
How do you plan to use this to educate residents but not to set them up to have false confidence that would preclude judgment?
Can we further define who is the right patient for this at the right time for this to be of maximum value to improve cure but not increase cost?
Your study reveals that this novel modality is safe in adults, and you acknowledge that there is no benefit for cure. You demonstrated excellent results; both senior surgeon and you as a junior surgeon had results that were excellent even at baseline but not better with the probe. Your study had 46 and 48 patients in each group at that were cured- with an established definition of normocalcemia six months after parathyroidectomy.
Surgical technique and judgment beyond gland identification are necessary in parathyroid disease. The ability to systematically approach the dissection and be comfortable with maneuvers to expose the glands, know atypical locations, and use of tactile sensation with the tip of the finger to see the parathyroid gland before you actually see the parathyroid gland with your eyes are of paramount importance. Dissection to release the thyroid capsule or expose the TE groove affords the gland delivery. All of this is necessary, even with the autofluorescence because the probe has to be placed within millimeters of the parathyroid tissue.
I believe the most interesting opportunity, which is probably of the most relevance, is the intraoperative ability to distinguish whether tissue is merely enlarged or hyperfunctional. That color, shape, consistency, texture, all those are important to help us define the extent of resection, and IO PTH is one of those tools over the last two decades that has given us that informed information. Frozen section can inform us of this by providing information about cellularity, normal or less amount of fat. These are cues to determine whether multigland disease exists.
Can you take that ratio of autofluorescence that you’ve shown us with regard to background and further explore that ratio to inform whether the tissue is normocellular, hyperfunctional or suppressed? This would be a quantification tool that will provide possibility to be lasting contribution.
As for teaching this augmented reality is upon us. Your study suggests that resident and junior surgeons’ confidence increases with affirmation of fluorescence. How do we set them up to be confident and to learn to not use tools to replace good judgment? My mentor and a friend of many of us was Orlo Clark. He stood and famously stated, “A fool with a tool is still a fool.” How do we train people to use the modality wisely and selectively?
The last comment pertains to value-defined as outcome over cost. Do we know the cost of reusable supplies, disposables, maintenance of operating room equipment, setup time, personnel? Healthcare can’t support any more modalities that don’t improve outcome. We’ve seen the era of blue dye and gamma probes come and go in endocrine surgery. We see the embellishments of recurrent laryngeal nerve monitoring but they haven’t changed the cure rate which has been stable for three decades. Industry will put itself between a good surgeon and a patient as many times as we let it. This has a definite role, and I am hopeful that come back to us and tell us exactly what it is. Is it in that pediatric patient with copious brown fat? Is it in that patient with concomitant thyroid cancer that has nodal involvement? Is it in that patient with thyroiditis and reactive lymph nodes?
Congratulations on this innovative technology. You have shown us a new tool. We can’t claim it’s better for cure, but we can say that the opportunity is here for it to benefit.
Response From Colleen Kiernan: Yes, I got them.
Dr. Nancy Perrier (Houston, TX): Great.
Response From Colleen Kiernan: Thank you very much for your time reviewing our manuscript and also those very thoughtful comments.
So to your first question about the function of parathyroid glands and can we use these ratios to determine function, we have some theories and hypotheses, but we do not have solid data on this yet. What we do know is that the parathyroid glands are incredibly heterogenous in terms of their fluorescence. Because we don’t know what the intrinsic fluorophore is, it’s hard to know why that heterogeneity exists. However, what we know is in a large parathyroid adenoma, the ratios are actually quite a bit lower than in a normal, healthy parathyroid gland. This is not universal, and so we can’t say, “Okay, if you have a large parathyroid gland with a ratio of 1.8, you know that that is an adenoma versus a normal parathyroid gland with a ratio of 6.2,” but I think with further data collection and analysis we will be able to provide a range. I think that particularly if we are able to figure out what the fluorophore is and if that is related to the hypercellularity that we will be able to use this device in that way, and there are pathologists that are interested in this at Medical College of Wisconsin, and it’s something of interest in our group as well.
To the question of resident education and training and how we use the device, I think that whenever we introduce disruptive technology or a new device, the first thing we have to do is learn to use the device, and so I don’t think that initially when people start using this device they should use it selectively. I think just like when we started using the nerve monitor, we have to use it every time to figure out how it’s useful, and I do think our results show this. When you give an inexperienced trainee the device, their confidence improves greatly, and whether that improved confidence is true confidence versus, “Well, the device said it’s a parathyroid, therefore it must be parathyroid,” we don’t know, so you cannot replace the surgical experience, the surgical training, and the expertise that comes as a parathyroid surgeon. I do think that initially we need to use it in every case. I think we need to challenge the trainees to identify the parathyroid glands before they use the device, not, “There’s some fat here. I’m going to interrogate it with the probe.” It’s, “There’s some fat here. I’m going to interrogate this fat and see if I can find a parathyroid gland, and then I’m going to use the probe to confirm that that’s parathyroid tissue,” for that pattern recognition and that identification that comes with experience.
And then to answer your third question about cost and value, every center, as you know, is going to have different agreements with companies and products as to cost. In our institution, the probe is a disposable. Interestingly, it can be sterilized, but that’s not the current use, so the probe is disposable. It’s $400 when you open the probe. That’s the charge. For us, a frozen section, depending on how many sections are sent, the cost is around $1100, and so if you use the probe in place of a frozen section, it is actually a cost-saving device. If you open the probe in every case, it is not a cost-saving device, and you’re right, I think that ultimately the way that we will use this probe will be selectively, and it will be selectively in a way that it makes sense to either shorten the duration of the procedure, to reduce frozen sections or tissue aspirate PTH in places that use that, or in these challenging cases where we cannot find a gland or we think we found all four glands, but our intraoperative PTH is not dropping, and therefore we are asking ourselves, “Was what I thought was a parathyroid, really a parathyroid?” I think as we use this device more, we are going to identify these cases in which it’s most beneficial, and I have my own biases about that but don’t quite have the data to support it yet.
Dr. Larry Kim (Chapel Hill, NC): Larry Kim, Chapel Hill, North Carolina. I think this is a really cool device. I’ve used it selectively, and I want to talk a little bit about cost, which you’ve alluded to and Nancy alluded to also. In our institution, it’s $530, and yes, I work at an ambulatory surgery center where frozen section is not easily available, and so it can be a major help. But the hospital can bill for a frozen section. The hospital eats the cost of the probe. There’s no reason that the probe can’t be reused, as you’ve mentioned. The only motive to make this probe disposable is to maximize profitability.So consider this an open criticism of Medtronic that they have done this and I think priced themselves out of the market. So I guess this is mostly a comment rather than a question, but I’d like for you to comment on the cost and especially the cost to the hospital because this is not recouped cost.
Response From Colleen Kiernan: Yeah, thank you, Dr. Kim, for your comments. I can only agree with them that yes, it is not a recouped cost. We cannot bill for it. I think that’s something that we could consider, right? Is that something we need to advocate for, or do we need to advocate that we need to be able to resterilize these probes, and I think that’s a much larger topic to tackle that I’m not certain that I am prepared to do at the American Surgical, but I do think that your point is well taken.
Dr. Quan-Yang Duh (San Francisco, CA): I have a disclosure. UCSF is one of the sites for the study. Colleen, great talk, and I’m so glad that you brought this to the American Surgical. Regarding the cost, the probe is more than $600 in San Francisco.. One comment and two quick questions.
My comment is that the probe works best for normal parathyroid glands. If the ratio of autofluorescence to background is high, let’s says 7, that’s a normal parathyroid. The abnormal parathyroid tends to be less fluorescent in general, so I do use it to tell me if a parathyroid gland is likely to be normal.
My two questions. You don’t always do bilateral exploration. I believe when you have to do bilateral exploration is when the probe speeds up the operation. Would you agree with that, do you have data to support it?
I also find the probe to be useful for reoperations. Do you agree?
Response From Colleen Kiernan: Yes, thank you very much. So you’re absolutely right. The study was not powered to look at focused versus bilateral, but looking at my own data in focused operations, it plays really no role for me. It doesn’t improve my confidence. It doesn’t improve my time. It doesn’t change the number of frozen sections I send, and in bilateral explorations, it certainly does. I see more glands more quickly. The operative time, while not statistically significant when we look at just those patients, is shorter. Again, it’s underpowered to detect that statistical significance, and yes, I wholeheartedly agree with you that in reoperative settings, it is incredibly helpful.
One of the things that Dr. Perrier brought up was the depth of penetration, the probe needs to be about 4 mm in contact, but that 4 mm can be incredibly helpful in a reoperative field to know that you are heading in the right direction with your exploration.
Dr. Diana Farmer (Sacramento, CA): Thank you, and I think in the interest of time, we’ll take your question, Dr. Schwaitzberg, and the answer we’ll take after the session.
Dr. Steven Schwaitzberg (Buffalo, NY): Steve Schwaitzberg, Buffalo, New York. Nobody wants to follow Nancy Perrier, but she opened with technology, so my first question is have you ever had GPS take you to the wrong place? So ground truth is when you’re staring at a building and you go, “Hmm, that’s not what my GPS says,” so your paper does not include really very much ground truth.
Personally, I’ve been on this journey with ICG, and the problem with all of these is that once I found a parathyroid, I don’t need a piece of technology, (particularly when you’re dealing with an adenoma), to tell me that I’m looking at an adenoma, so cost is a problem for parathyroid surgery., Personally I find the ground truth is when your hormone levels go down, and six months later is your calcium is still normal.
I think I wouldn’t use this for primary hyperparathyroidism. Redos, complicated cases, yes. Where I’m really excited about this, however, is in total thyroidectomy, minimizing the time to identify the parathyroids. I think that’s a spectacular opportunity to reduce hypocalcemia after surgery.
For parathyroids I’m do selective exploreration since I don’t want to stun the glands on the side where there’s no disease, I personally find this to be too expensive.
Dr. Diana Farmer (Sacramento, CA): Thank you for your comments, and thank you for this provocative paper.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT04299425
Data Accessibility Statement:
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- 1.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg 2016; 151(10):959–968. [DOI] [PubMed] [Google Scholar]
- 2.Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 2013; 98(3):1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SM, Shu AD, Long J, et al. Declining Rates of Inpatient Parathyroidectomy for Primary Hyperparathyroidism in the US. PLoS One 2016; 11(8):e0161192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Wang TS, Yen TW, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg 2010; 252(4):691–5. [DOI] [PubMed] [Google Scholar]
- 5.Cron DC, Kapeles SR, Andraska EA, et al. Predictors of operative failure in parathyroidectomy for primary hyperparathyroidism. Am J Surg 2017; 214(3):509–514. [DOI] [PubMed] [Google Scholar]
- 6.Lew JI, Rivera M, Irvin GL 3rd, et al. Operative failure in the era of focused parathyroidectomy: a contemporary series of 845 patients. Arch Surg 2010; 145(7):628–33. [DOI] [PubMed] [Google Scholar]
- 7.Mazotas IG, Yen TWF, Doffek K, et al. Persistent/Recurrent Primary Hyperparathyroidism: Does the Number of Abnormal Glands Play a Role? J Surg Res 2020; 246:335–341. [DOI] [PubMed] [Google Scholar]
- 8.Simental A, Ferris RL. Reoperative parathyroidectomy. Otolaryngol Clin North Am 2008; 41(6):1269–74, xii. [DOI] [PubMed] [Google Scholar]
- 9.Udelsman R, Donovan P, Shaw C. Cure predictability during parathyroidectomy. World J Surg 2014; 38(3):525–33. [DOI] [PubMed] [Google Scholar]
- 10.Grubbs EG, Mittendorf EA, Perrier ND, et al. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am J Surg 2008; 196(6):931–5; discussion 935–6. [DOI] [PubMed] [Google Scholar]
- 11.Norman J, Chheda H. Minimally invasive parathyroidectomy facilitated by intraoperative nuclear mapping. Surgery 1997; 122(6):998–1003; discussion 1003–4. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers SE, Hunter GJ, Hamberg LM, et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006; 140(6):932–40; discussion 940–1. [DOI] [PubMed] [Google Scholar]
- 13.Siperstein A, Berber E, Barbosa GF, et al. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg 2008; 248(3):420–8. [DOI] [PubMed] [Google Scholar]
- 14.Solorzano CC, Carneiro-Pla DM, Irvin GL 3rd. Surgeon-performed ultrasonography as the initial and only localizing study in sporadic primary hyperparathyroidism. J Am Coll Surg 2006; 202(1):18–24. [DOI] [PubMed] [Google Scholar]
- 15.McWade MA, Paras C, White LM, et al. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013; 154(6):1371–7; discussion 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011; 16(6):067012. [DOI] [PubMed] [Google Scholar]
- 17.McWade MA. Development of an intraoperative tool to detect parathyroid gland autofluorescence: Vanderbilt University, 2016. [Google Scholar]
- 18.DiMarco A, Chotalia R, Bloxham R, et al. Autofluorescence in Parathyroidectomy: Signal Intensity Correlates with Serum Calcium and Parathyroid Hormone but Routine Clinical Use is Not Justified. World J Surg 2019; 43(6):1532–1537. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan CM, Thomas G, Baregamian N, et al. Initial clinical experiences using the intraoperative probe-based parathyroid autofluorescence identification system-PTeye™ during thyroid and parathyroid procedures. J Surg Oncol 2021; 124(3):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladurner R, Sommerey S, Arabi NA, et al. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 2017; 31(8):3140–3145. [DOI] [PubMed] [Google Scholar]
- 21.McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 2014; 99(12):4574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWade MA, Sanders ME, Broome JT, et al. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016; 159(1):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver Karcioglu AL, Triponez F, Solórzano CC, et al. Emerging Imaging Technologies for Parathyroid Gland Identification and Vascular Assessment in Thyroid Surgery: A Review From the American Head and Neck Society Endocrine Surgery Section. JAMA Otolaryngol Head Neck Surg 2023; 149(3):253–260. [DOI] [PubMed] [Google Scholar]
- 24.Solórzano CC, Thomas G, Berber E, et al. Current state of intraoperative use of near infrared fluorescence for parathyroid identification and preservation. Surgery 2021; 169(4):868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas G, Solórzano CC, Baregamian N, et al. Comparing intraoperative parathyroid identification based on surgeon experience versus near infrared autofluorescence detection - A surgeon-blinded multi-centric study. Am J Surg 2021; 222(5):944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benmiloud F, Godiris-Petit G, Gras R, et al. Association of Autofluorescence-Based Detection of the Parathyroid Glands During Total Thyroidectomy With Postoperative Hypocalcemia Risk: Results of the PARAFLUO Multicenter Randomized Clinical Trial. JAMA Surg 2020; 155(2):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas G, McWade MA, Paras C, et al. Developing a Clinical Prototype to Guide Surgeons for Intraoperative Label-Free Identification of Parathyroid Glands in Real Time. Thyroid 2018; 28(11):1517–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Zhu CR, Liu H, et al. The Ability of Near-Infrared Autofluorescence to Protect Parathyroid Gland Function During Thyroid Surgery: A Meta-Analysis. Front Endocrinol (Lausanne) 2021; 12:714691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solórzano CC, Thomas G, Baregamian N, et al. Detecting the Near Infrared Autofluorescence of the Human Parathyroid: Hype or Opportunity? Ann Surg 2020; 272(6):973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas G, McWade MA, Nguyen JQ, et al. Innovative surgical guidance for label-free real-time parathyroid identification. Surgery 2019; 165(1):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squires MH, Jarvis R, Shirley LA, et al. Intraoperative Parathyroid Autofluorescence Detection in Patients with Primary Hyperparathyroidism. Ann Surg Oncol 2019; 26(4):1142–1148. [DOI] [PubMed] [Google Scholar]
- 32.Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009; 96(3):240–6. [DOI] [PubMed] [Google Scholar]
- 33.Pisanu A, Porceddu G, Podda M, et al. Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 2014; 188(1):152–61. [DOI] [PubMed] [Google Scholar]
- 34.Irvin GL 3rd, Solorzano CC, Carneiro DM. Quick intraoperative parathyroid hormone assay: surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg 2004; 28(12):1287–92. [DOI] [PubMed] [Google Scholar]
- 35.St Amour TC, Demarchi MS, Thomas G, et al. Educational Review: Intraoperative Parathyroid Fluorescence Detection Technology in Thyroid and Parathyroid Surgery. Ann Surg Oncol 2023; 30(2):973–993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.