Abstract
Although a number of genes that are involved in the establishment of left–right asymmetry have been identified, earlier events in the molecular pathway developing left–right asymmetry remain to be elucidated. Here we present evidence suggesting that the transforming growth factor-β family member derrière is involved in the development of left–right asymmetry in Xenopus embryos. Ectopic expression of derrière on the right side can fully invert cardiac and visceral left–right orientation and nodal expression, and expression of a dominant-negative form of derrière on the left side can partially randomize the left–right orientation and nodal expression. Moreover, while expression of the dominant-negative derrière does not inhibit the activity of Vg1 directly, it can rescue the altered left–right orientation induced by Vg1. Vg1 can induce derrière in animal cap explants. These results suggest that derrière is involved in earlier molecular pathways developing the left–right asymmetry.
INTRODUCTION
In the animal body plan, the left–right axis is specified in accord with the development of anterior–posterior and dorsal–ventral axes (Brown and Wolpert, 1990). Recently, a number of molecules that are involved in the promotion and establishment of left–right asymmetry have been identified (Harvey, 1998; Ramsdell and Yost, 1998; Beddington and Robertson, 1999; Capdevila et al., 2000). Of such molecules, members of the transforming growth factor-β (TGF-β) superfamily are conserved factors implicated in the pathway establishing left–right asymmetry. The expression of nodal and lefty2 in the left lateral plate mesoderm (LPM) is strictly correlated with the development of normal organ situs (Harvey, 1998; Ramsdell and Yost, 1998; Beddington and Robertson, 1999). nodal is antagonized by lefty2 (Meno et al., 1999) and induces Pitx2, a transcription factor that directs subsequent morphological decisions (Harvey, 1998; Ramsdell and Yost, 1998; Beddington and Robertson, 1999). Misexpression of upstream laterality genes disrupts the normal expression pattern of nodal and lefty2, and randomizes heart looping (Lohr et al., 1997; Sampath et al., 1997; Meno et al., 1998; Pagan-Westphal and Tabin, 1998).
Although progress has been made in understanding the molecular mechanism underlying vertebrate left–right patterning, very little is known about the processes that are involved in first developing left–right asymmmetry in the embryo. Recent studies using mice lacking the kinesin superfamily member KIF3A or KIF3B suggest that rotation of nodal cilia is required for the initial breaking of symmetry at the node (Nonaka et al., 1998; Okada et al., 1999; Takeda et al., 1999), although whether this mechanism is universal or not is unknown. Studies in chick and Xenopus suggest that the left–right axis is initiated prior to gastrulation. In the chick, left–right asymmetry of the node is inductively patterned by signals from lateral tissues. Once induced for left–right identity, the node then directs left–right development in adjacent tissues (Pagan-Westphal and Tabin, 1998). In Xenopus, it has been suggested that the activity that confers left–right identity to the organizer/node is mediated by Vg1, a member of the TGF-β superfamily. One of the key observations was that while various experimental manipulations lead to randomization, only Vg1 can fully invert cardiac and visceral left–right orientation and expression of a downstream laterality gene, nodal (Hyatt et al., 1996; Hyatt and Yost, 1998). Here we present evidence suggesting that derrière, a member of the TGF-β superfamily, is also involved in establishing left–right asymmetry. derrière is a recently identified Vg1-related factor and is required for normal mesodermal patterning, paticularly in posterior regions of the Xenopus embryo (Sun et al., 1999). Our results show that ectopic expression of derrière on the right side can also fully invert cardiac and visceral left–right orientation and nodal expression. More importantly, expression of a dominant-negative form of derrière on the left side can randomize the left–right orientation. In Xenopus, the tissues lateral to the organizer that carry left–right identity and are capable of inducing left–right specification of the organizer have been termed the ‘left–right coordinator’ (LRC) (Hyatt and Yost, 1998). Our results suggest that, like Vg1, derrière may act as one of the LRC factors in the left lateral vegetal cells in Xenopus embryos.
RESULTS AND DISCUSSION
derrière proprotein is efficiently processed to a functional, secreted form in embryos
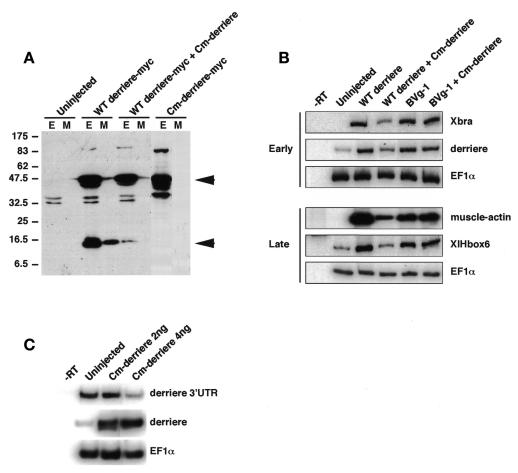
To find out whether derrière is processed to a mature form in embryos, we constructed a C-terminal Myc epitope-tagged derrière (derrière-Myc) and injected its mRNA into the animal pole of 2-cell-stage embryos. Immunoblotting with anti-Myc antibody detected a processed, mature form in both the whole-embryo extracts and the conditioned media from the cultured embryos (Figure 1A, WT derrière-Myc, lower arrowhead). We then constructed a cleavage-deficient mutant of derrière (Cm-derrière-Myc) according to the design of the cleavage-deficient mutant of BMP (Hawley et al., 1995). As expected, Cm-derrière was not processed to a mature form (Figure 1A, Cm-derrière-Myc). Moreover, co-injection of a 10-fold excess of Cm-derrière (non-Myc-tagged) with WT derrière (-Myc) significantly inhibited generation of the mature form (Figure 1A, WT derrière-Myc + Cm-derrière). To test the activity of the secreted, mature form of derrière, we collected conditioned media from defolliculated oocytes, which were injected with the indicated combinations of mRNAs shown in Figure 1B and incubated for 3 days. When tested in animal cap explants, a conditioned medium from WT derrière-injected oocytes induced expression of early and late mesodermal markers, like mature Vg1 (Figure 1B). A conditioned medium from oocytes co-injected with Cm-derrière and WT derrière had a significantly weaker ability to induce these mesodermal markers than that from the WT derrière-injected oocytes. In contrast, the ability of a conditioned medium from BVg1 (a chimeric BMP2-Vg1 protein, which is cleaved to the mature Vg1 protein) (Thomsen and Melton, 1993) injected oocytes to induce marker genes was unaffected by co-injection of Cm-derrière (Figure 1B).
Fig. 1. Detection of a processed, mature form of wild-type derrière and the dominant-negative effect of a cleavage-deficient mutant of derrière (Cm-derrière). (A) Detection of a secerted mature form of derrière in both the whole-embryo extracts (E) and the conditioned media (M) from the wild-type (WT) derrière (WT derrière-Myc, 100 pg) injected embryos, but not in those from the Cm-derrière (a cleavage-deficient mutant of derrière) (Cm-derrière-Myc, 100 pg) injected embryos. Co-injection of Cm-derrière (non-Myc-tagged, 1 ng) with WT derrière (WT derrière-Myc, 100 pg) caused a marked decrease in the mature form. Arrowheads show an immature proprotein (upper) and a mature form (lower) of derrière. (B) A secreted derrière can induce expression of mesodermal marker genes in animal caps, like mature Vg1, and Cm-derrière can inhibit WT derrière specifically. Animal caps were dissected at blastula stage and cultured in the conditioned media obtained from WT derrière-, WT derrière plus Cm-derrière-, BVg1- or BVg1 plus Cm-derrière-injected oocytes until sibling embryos reached stage 11 (early) or stage 26 (late). Expression of Xbra, derrière, EF1α, muscle-actin and XlHbox6 was analyzed by RT–PCR. EF1α served as a loading control. No signal was observed in the absence of reverse transcription (–RT). (C) Expression of Cm-derrière reduced endogenous derrière expression. Expression of endogenous derrière mRNA was analyzed by RT–PCR using the primer pair designed from the 3′-untranslated region of derrière (derrière 3′UTR), and expression of endogenous plus exogenous derrière mRNA was analyzed by RT–PCR using the primer pair designed from the coding region of derrière (derrière). EF1α served as a loading control.
Taken together, these results demonstrate that derrière proprotein is efficiently processed to a functional, secreted form in embryos, and that Cm-derrière functions as a specific dominant-negative construct and thus does not block Vg1. As WT derrière enhanced derrière mRNA expression (Figure 1B), it is possible that derrière expression is regulated by an autofeedback mechanism. To assess this possibility, we injected Cm-derrière mRNA into the marginal zone of four blastomeres at the 4-cell embryo stage and isolated total RNA at early gastrulation. To detect endogenous derrière transcripts, we used a primer pair designed from a 3′ untranslated region of derrière mRNA (Figure 1C, derrière 3′UTR). The RT–PCR analysis showed that expression of Cm-derrière reduced an endogenous derrière expression level (Figure 1C), suggesting the existence of a positive feedback mechanism of derrière expression.
Ectopic expression of derrière on the right side can invert cardiac and visceral left–right orientation
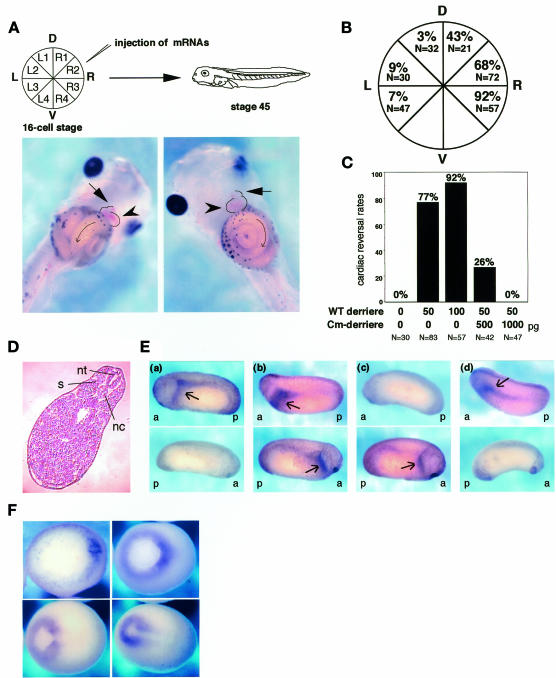
As the prospective mature region of derrière is ∼80% identical to that of Vg1, we examined whether or not derrière also has the ability to affect the development of the left–right asymmetry. We injected derrière mRNA into various vegetal cells of the 16-cell-stage embryos and observed cardiac and visceral left–right orientation of the stage 45 embryos, as illustrated in Figure 2A. In the normal embryo, the ventricle is situated on the left side, with the outflow tract looping to the right side, and gut coils counterclockwise (Figure 2A, left). Because disruption of dorsoanterior or dorsal midline development is known to affect laterality (Danos and Yost, 1996; Lohr et al., 1997; Nascone and Mercola, 1997), we scored only embryos with an apparently undisturbed primary axis, in which notochord, neural tube and somites appeared to be unaffected in injected embryos (Figure 2D). Injection of WT derrière into R3 resulted in the reversal of the left–right orientation of cardiac looping and gut coiling in almost all the injected embryos (Figure 2A, right and 2B). The injection into R2 or R1 also induced inversion of the cardiac and visceral left–right orientation in about half of the population (Figure 2B), and the embryos often showed heterotaxia (not shown). In contrast, injection of WT derrière into L1, L2 or L3 had little or no effect on the left–right asymmetry (Figure 2B). The reversal effect of WT derrière expression in R3 on the left–right asymmetry was dose dependent and rescued completely by co-injection of a 20-fold excess of Cm-derrière (Figure 2C). Thus, Cm-derrière can in fact function dominant negatively. Because derrière is expressed zygotically in vivo (Sun et al., 1999), we examined the effect of expression of derrière shortly after the mid-blastula transition by performing plasmid DNA injections with pCS2 and pCSKA. Both vectors gave essentially the same results. Although injection of 50 pg of WT derrière cDNA into R3 resulted in embryonic lethality in ∼70% (total n = 292) of injected embryos, 41% of the surviving embryos exhibited reversed cardiac looping. As increasing the amount of cDNA caused further increase in embryonic lethality, we could not examine the effect of higher doses of WT derrière in the cDNA injections. Taken together, these results indicate that derrière can invert cardiac and visceral left–right asymmetry, like Vg1.
Fig. 2. Effect of derrière on left–right development and Xnr-1 expression. (A) Experimental scheme (upper) and situs solitus (lower, left panel) and situs inversus (lower, right panel) phenotypes in Xenopus. Cell designations according to Hyatt and Yost (1998)) are shown (upper, left). Embryos were injected at the indicated blastomere with mRNA at the 16-cell stage and were harvested at stage 45 for observation of cardiac and visceral left–right orientation. In situs solitus (left, control), embryos show normal heart orientation, with outflow tract (arrow) to the embryo’s right and ventricle (arrowhead) to the left, and normal gut orientation with counterclockwise coiling. In situs inversus (right, derrière-injected into R3), embryos show reversed heart orientation, with outflow tract (arrow) to the embryo’s left and ventricle (arrowhead) to the right, and reversed gut orientation with clockwise coiling. (B) Cardiac left–right reversal by injection of WT derrière. Embryos were injected with 100 pg of WT derrière mRNA in the indicated region. The percentages shown here indicate the cardiac reversal rates. (C) Cm-derrière can rescue the inversion of cardiac orientation induced by injection of WT derrière into R3. The indicated doses of mRNAs were injected into R3 at the 16-cell stage and the embryos were observed at stage 45 for cardiac and visceral left–right orientation. The percentages shown here indicate the cardiac reversal rates. (D) Transverse section of an embryo injected with WT derrière mRNA into R3. nt, neural tuge; nc, notochord; s, somite. (E) Altered Xnr-1 expression patterns by injection of derrière. Embryos were collected at stages 22–24 and processed for whole-mount in situ hybridization analysis of Xnr-1 expression. Upper panels, lateral view with anterior to the left (a, anterior; p, posterior); bottom panels, lateral view with anterior to the right. Embryos injected with 50 pg of WT derrière mRNA into L3, R2 and R3 exhibited normal left expression of Xnr-1 (a), bilateral expression of Xnr-1 (b) and right expression of Xnr-1 (c), respectively. Xnr-1 expression in an uninjected embryo is shown in (d). Arrows show Xnr-1 staining. See the text for details. (F) Whole-mount in situ hybridization of derrière. All embryos are viewed from the vegetal surface with dorsal to the right. Upper left panel, stage 10.5; upper right panel, stage 11; bottom left panel, stage 12; bottom right panel, stage 13. Expression of derrière is symmetrical at any stages shown.
Expression of Cm-derrière on the left side partially randomizes cardiac left–right orientation
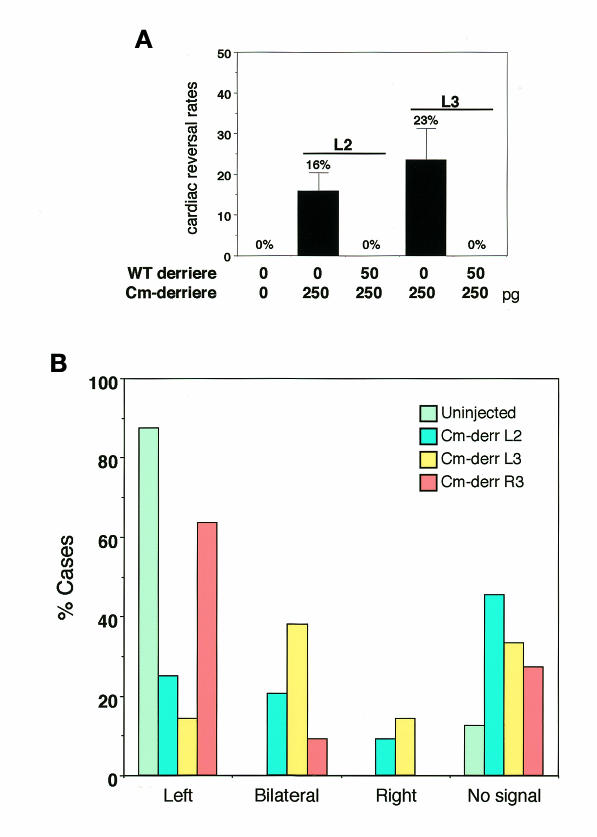
If derrière normally regulates left–right asymmetry, the above result implies that it should function in the left side of the embryo, like the suggested role of Vg1, and inhibition of the function of derrière in the left side should lead to randomization of left–right asymmetry. To test this possibility, we injected dominant-negative derrière (Cm-derrière) RNA into the left side of 16-cell-stage embryos. Although injection of Cm-derrière at 4-cell stage led to posterior truncation (not shown; Sun et al., 1999), embryos injected with Cm-derrière into one of the vegetal cells at 16-cell stage could develop normally. The Cm-derrière injection (250 pg) into L2 and L3 altered the orientation of cardiac looping in 16 and 23% of the injected embryos, respectively (Figure 3A). Although these reversal rates are somewhat lower than the rate of complete randomization (50%), they are much higher than those of the injection into R2 (6%, n = 34) or R3 (6%, n = 33). Moreover, co-injection of WT derrière into L2 or L3 rescued completely the disturbed left–right orientation induced by injection of Cm-derrière and led to normal left–right development (Figure 3A), indicating that the effect of expression of Cm-derrière is specific.
Fig. 3. Expression of Cm-derrière disturbs left–right asymmetry. (A) Embryos were injected with 250 pg of Cm-derrière mRNA or 250 pg of Cm-derrière mRNA plus 50 pg of WT derrière mRNA in L2 or L3 at 16-cell stage. Expression of Cm-derrière altered the orientation of cardiac looping (16% L2, SD = 4.6%; N = 120; 23% L3, SD = 7.8%; N = 118), and co-injection of WT derrière rescued completely the alteration of cardiac orientation induced by Cm-derrière (0% L2; N = 35; 0% L3; N = 42). (B) Xnr-1 expression patterns in Cm-derrière-injected embryos. The percentages of cases of indicated Xnr-1 expression in Cm-derrière L2 injected (blue, N = 44), Cm-derrière L3 injected (yellow, N = 42), Cm-derrière R3 injected (orange, N = 22) and uninjected (green, N = 32) embryos are shown.
Ectopic expression of WT- or Cm-derrière alters Xnr-1 expression
It has been shown that the asymmetric nodal expression in the left LPM is highly conserved in vertebrates and is required for left–right development. We thus examined the derrière-injected embryos for the expression of Xnr-1, a Xenopus homolog of nodal. Uninjected embryos showed the left-sided Xnr-1 expression (Figures 2E,d and 3B). When we injected WT derrière into L3 (total n = 27), a majority of embryos (78%) showed normal Xnr-1 expression (completely left-sided expression; Figure 2E,a) and the rest (22%) showed slight expression of Xnr-1 on the right side and much stronger expression on the left side. In contrast, injection of WT derrière into R3 resulted in reversal of Xnr-1 expression (right 75%, Figure 2E,c; bilateral 16%, left 9%; n = 32). Injection of WT derrière into R2 resulted in a complex Xnr-1 expression pattern (total n = 54): bilateral expression (30%, Figure 2E,b), right-sided expression (30%), left-sided expression (26%) and no detectable expression (14%). Altered Xnr-1 expression patterns by injection of derrière are correlated with the rates of cardiac and visceral reversal in the derrière-injected embryos.
Next, we examined the effect of Cm-derrière on Xnr-1 expression. While injection of Cm-derrière into R3 resulted in normal Xnr-1 expression (left 64%, right 0%, bilateral 9%, not detected 27%; n = 22; Figure 3B), injection of Cm-derrière into L2 (total n = 44) or L3 (total n = 42) resulted in marked increases of bilateral (L2, 21%; L3, 38%; Figure 3B) and right-sided (L2, 9%; L3, 14%; Figure 3B) Xnr-1 expression. Thus, ectopic expression of Cm-derrière on the left side can partially randomize Xnr-1 expression.
Taken together, these results suggest that derrière is involved in the molecular pathway developing the left–right asymmetry upstream of Xnr-1, probably functioning in the left side of the embryo, although the result that expression of Cm-derrière cannot induce complete randomization may suggest the existence of another factor that plays the equivalent role. We examined whether derrière exhibits an asymmetric expression pattern at any stages, like other asymmetrically expressed genes. We performed whole-mount in situ hybridization for derrière, but we could not detect its asymmetric expression (Figure 2F). This result does not, however, rule out the possibility of asymmetric expression in a highly localized region of the left side in a specific period. In addition, as recent studies have suggested the existence of the molecular flow producing the left–right gradient of ligands outside the cells in mouse embryos (Nonaka et al., 1998; Okada et al., 1999; Takeda et al., 1999) and also the existence of the asymmetrically localized specific antagonists (Esteban et al., 1999; Yokouchi et al., 1999; Zhu et al., 1999), it is possible that the activity of derrière is regulated by such mechanisms.
Cm-derrière inhibits the inversion of cardiac and visceral orientation induced by BVg1
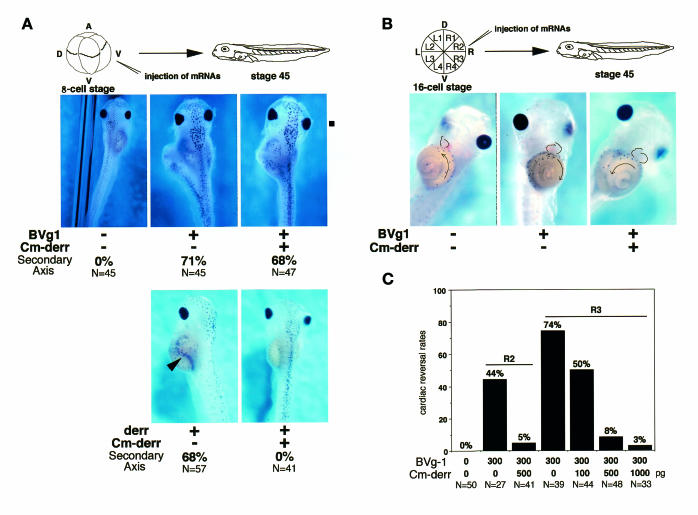
Next we studied a possible interaction between derrière and Vg1. Co-injection of Cm-derrière (500 pg) into the ventral vegetal region of 8-cell embryos, which inhibited the WT derrière (50 pg)-induced formation of a partial secondary axis (Figure 4A), did not affect the ability of BVg1 (300 pg) to induce a secondary axis (Figure 4A), indicating that Cm-derrière acts as a specific inhibitor of the function of derrière and does not inhibit the activity of BVg1 directly. This is also supported by the result of the RT–PCR analysis shown in Figure 1B. In contrast, in left–right development, Cm-derrière rescued the alteration of the left–right orientation induced by BVg1 (Figure 4B). While injection of BVg1 (300 pg) into R2 and R3 altered the orientation of cardiac looping in 44% (n = 36) and 74% (n = 39) of injected embryos, respectively (Figure 4C), co-injection of Cm-derrière (500 pg) and BVg1 (300 pg) resulted in the normal left–right orientation of cardiac looping and visceral coiling (Figure 4B, right panel and 4C), only 5–8% of double-injected embryos showed the inverted phenotype (Figure 4C). The effect of co-injection of Cm-derrière was dose dependent (Figure 4C). These results indicate that Cm-derrière inhibits the ability of BVg1 to invert the left–right axis. In animal cap explants, BVg1 induced expression of derrière transcripts (Figure 1B). It might be possible, therefore, that derrière acts downstream of Vg1 in embryos. Moreover, we examined whether Cm-derrière inhibits the left–right reversal induced by Xnr-1. We injected plasmid pCSKA encoding Xnr-1 into the dorsal right or dorsal left blastomere of 4-cell embryos. Injection of pCSKA/Xnr-1 (50 pg) into the right side altered the orientation of cardiac looping in 68% of injected embryos, and some of them showed heterotaxia (n = 57). In contrast, injection of pCSKA/Xnr-1 (50 pg) into the left side had little effect on the left–right asymmetry, and only 7% of injected embryos showed heterotaxia (n = 54). When we injected Cm-derrière (500 pg) together with pCSKA/Xnr-1 (50 pg) into the right side, 41% of injected embryos (n = 51) showed reversal of cardiac looping. These results showed that Cm-derrière does not block significantly the left–right reversal induced by Xnr-1, whereas it does block almost completely the left–right reversal induced by BVg1 (Figure 4C), and supported our argument that derrière acts upstream of Xnr-1. The slight, inhibitory effect of Cm-derrière on left–right reversal induced by Xnr-1 observed here is consistent with the previous report, which showed that Cm-derrière inhibits the activity of Xnr-1 to some extent (Sun et al., 1999), although the reason is unknown at present.
Fig. 4. Expression of Cm-derrière rescues the inversion of cardiac and visceral orientation induced by BVg1. (A) Effect of Cm-derrière on the ability of BVg1 to induce a secondary axis. Upper panels, embryos were injected with 300 pg of BVg1 mRNA or 300 pg of BVg1 mRNA plus 500 pg of Cm-derrière mRNA in the ventrovegetal blastomere at 8-cell stage and were harvested at stage 45 for morphological analysis. Induction of a partial secondary axis was observed in 71% (upper middle; N = 45) of BVg1-injected embryos and in 68% (upper right; N = 47) of BVg1 and Cm-derrière-co-injected embryos. Thus, Cm-derrière did not inhibit the ability of BVg1 to induce a partial secondary axis. Bottom panels, embryos were injected with 50 pg of derrière mRNA or 50 pg of derrière mRNA plus 500 pg of Cm-derrière mRNA in the ventrovegetal blastomere at 8-cell stage and were harvested at stage 45 for morphological analysis. Induction of a partial secondary axis was observed in 68% (bottom left; N = 57) of derrière-injected embryos, and this effect was completely rescued by co-injection of Cm-derrière (bottom right; N = 41). An arrowhead shows a partial secondary axis. (B and C) Effect of Cm-derrière on the ability of BVg1 to invert the left–right orientation. Embryos were injected with 300 pg of BVg1 mRNA or 300 pg of BVg1 mRNA plus 100, 500 or 1000 pg of Cm-derrière mRNA in R2 or R3 at 16-cell stage and were harvested at stage 45 for observation of cardiac and visceral left–right orientation. Injection of BVg1 mRNA into R2 or R3 resulted in the reversal of cardiac orientation (middle panel, B) in 44% (N = 27) or in 74% (N = 39) of the injected embryos, respectively. Co-injection of BVg1 mRNA and Cm-derrière mRNA into R2 or R3 resulted in the reversal of cardiac orientation (right panel, B). The results are summarized in (C), which indicates that Cm-derrière can rescue the alteration of cardiac orientation induced by expression of BVg1. Cm-derr, Cm-derrière.
It is possible that maternal Vg1 proprotein could be processed to a functional form in the left side of embryos where it could induce derrière transcripts zygotically, although asymmetric expression of the bulk of derrière could not be observed, and these secreted TGF-β family ligands could act cooperatively in establishing left–right asymmetry including the asymmetric nodal (Xnr-1) expression. An alternative possibility is that an endogenous left–right coordinator is derrière itself, and the observed ability of ectopically expressed Vg1 on the right side to invert the left–right axis might result from its ability to induce derrière. The idea that a zygotically expressed gene could function as a left–right coordinator is consistent with the observations that ectopic expression of BVg1 (Hyatt and Yost, 1998) or derrière (this study) after the mid-blastula transition also exhibits the inversion of the left–right axis. In conclusion, our results suggest that the recently identified TGF-β family ligand, derrière, may play an important role in early molecular events initiating and developing left–right asymmetry, although when and how the function of derrière is directed to the left side of the embryo remain to be elucidated.
METHODS
Plasmid construction and microinjection. Using a subtractive hybridization strategy, we isolated derrière as one of activin-inducible genes (H. Hanafusa, N. Masuyama and E. Nishida, in preparation). Full-length cDNA clones were obtained by screening a λZAPII cDNA library made from stage 10.5 embryos. The coding region of derrière or Xnr-1 was amplified by PCR and cloned into the vector CS2 or CSKA. A cleavage-deficient mutant of derrière (Cm-derrière) was constructed according to the strategy of Hawley et al. (1995). The mutagenic oligonucleotide (CAATTGCAAAACTCAAGGAGTCGACGG-GAGTACTCATTCATC) was used to convert the cleavage site RAKR to GVDG. In vitro synthesis of capped mRNA was performed using the Ambion mMESSAGE mMACHINE kit. Templates were as follows: NotI-linearized pCS2derrière and pCS2Cm-derrière; EcoRI-linearized pSP64TBVg1 (Thomsen and Melton, 1993). The RNAs were injected into 2-, 8- or 16-cell-stage embryos. The amounts of injected RNAs and sites of injection are described in the text and figure legends.
Embryo manipulations and immunoblotting. Embryos were in vitro fertilized, dejellied and cultured in 0.1 MBS [1.5 mM HEPES pH 7.4, 8.8 mM NaCl, 0.1 mM KCl, 0.24 mM NaHCO3, 0.082 mM MgSO4, 0.03 mM Ca(NO3)2 and 0.041 mM CaCl2]. In anti-Myc immunoblotting experiments, embryos were injected with WT derrière-Myc mRNA (100 pg), Cm-derrière-Myc mRNA (100 pg), or WT derrière-Myc mRNA (100 pg) and Cm-derrière mRNA (1 ng) at the 2-cell stage and incubated for 12 h. Aliquots of the incubation media from 30 injected embryos and the whole-embryo extracts from five injected embryos were separated by 10% SDS–PAGE and transferred to a PVDF membrane (Millipore). Immunoblotting was performed using the 9E10 anti-Myc antibody (Santa Cruz Biotechnology) and visualized by the ECL western blotting detection system (Amersham).
Oocyte injections and RT–PCR analysis. Oocytes were surgically removed from females, manually defolliculated by digestion with 1.5 mg/ml collagenase in OR2 (5 mM Tris–HCl pH 7.8, 82.5 mM NaCl, 2.5 mM KCl, 1 mM Na2HPO4), injected with 20 ng of in vitro transcribed mRNA and cultured at 19°C in OR2 supplemented with 1 mM MgCl2, 1 mM CaCl2 and 0.5 mg/ml bovine serum albumin (BSA). Conditioned media from oocytes were prepared by incubating oocytes in OR2 with MgCl2, CaCl2 and BSA for 3 days. For RT–PCR analysis, animal cap explants were prepared at stage 8 and cultured in 0.1 MBS supplemented with the oocyte conditioned media until sibling embryos reached stage 11 (early) or stage 26 (late). Total RNA was isolated from six animal cap explants or five whole embryos using TRIzol (Gibco-BRL) according to the manufacturer’s instructions. PCR conditions were: 94°C, 30 s; 58°C, 30 s; 72°C, 30 s; for 25 cycles (EF1α and derrière in Figure 1C for 20 cycles). Primers for Xbra, gsc, EF1α and muscle-actin were as described (Masuyama et al., 1999). The sequences of other primer pairs used were as follows: derrière forward 5′-ATATTATGGACA-ACAGTTCC-3′, reverse 5′-ACTACAAATGATCGATTGCC-3′; derrière 3′UTR forward 5′-GTTTGCTTTGGAGATTGTTC-3′, reverse 5′-TGTTTCATCCAGCAGCTCTG-3′; XlHbox6 forward 5′-GCAATCTGAACCCCTGGACT-3′, reverse 5′-TTAGTGTCG-GGGAAGTTGCC-3′.
In situ hybridization and histological analysis. Injected embryos were fixed at stage 22–24 by MEMFA (0.1 M MOPS pH 7.4, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde) treatment for whole-mount in situ hybridization and histological sectioning. Whole-mount in situ hybridization was performed as described (Masuyama et al., 1999). For histology, fixed embryos were dehydrated through ethanol–xylene series, embedded in paraffin, and 7 µm sections were cut on a rotary microtome. Sections were stained with hematoxylin–eosin (Sigma) according to the manufacturer’s protocol.
Acknowledgments
ACKNOWLEDGEMENTS
We thank K. Kawachi and H. Ellinger-Ziegelbauer for technical comments and helpful discussion, and members of our laboratory for their helpful comments. We are grateful to Dr Douglas A. Melton for providing the BVg1 construct. This work was supported by grants from the Ministry of Education, Science and Culture of Japan (to E.N.).
REFERENCES
- Beddington R.S.P. and Robertson, E.J. (1999) Axis development and early asymmetry in mammals. Cell, 96, 195–209. [DOI] [PubMed] [Google Scholar]
- Brown N.A. and Wolpert, L. (1990) The development of handedness in left/right asymmetry. Development, 109, 1–9. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Vogan, K.J., Tabin, C.J. and Belmonte, J.C.I. (2000) Mechanisms of left–right determination in vertebrates. Cell, 101, 9–21. [DOI] [PubMed] [Google Scholar]
- Danos M.C. and Yost, H.J. (1996) Role of notochord in specification of cardiac left–right orientation in zebrafish and Xenopus. Dev. Biol., 177, 96–103. [DOI] [PubMed] [Google Scholar]
- Esteban C.R., Capdevila, J., Economides, A.N., Pascual, J., Ortiz, A. and Belmonte, J.C.I. (1999) The novel Cer-like protein Caronte mediates the establishment of embryonic left–right asymmetry. Nature, 401, 243–251. [DOI] [PubMed] [Google Scholar]
- Harvey R.P. (1998) Links in the left/right axial pathway. Cell, 94, 273–276. [DOI] [PubMed] [Google Scholar]
- Hawley S.H.B., Wunnenberg-Stapleton, K., Hashimoto, C., Laurent, M.N., Watabe, T., Blumberg, B.W. and Cho, K.W.Y. (1995) Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev., 9, 2923–2935. [DOI] [PubMed] [Google Scholar]
- Hyatt B.A. and Yost, H.J. (1998) The left–right coordinator: the role of Vg1 in organizing left–right axis formation. Cell, 93, 37–46. [DOI] [PubMed] [Google Scholar]
- Hyatt B.A., Lohr, J.L. and Yost, H.J. (1996) Initiation of left–right axis formation by maternal Vg1. Nature, 384, 62–65. [DOI] [PubMed] [Google Scholar]
- Lohr J.L., Danos, M.C. and Yost, H.J. (1997) Left–right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development, 124, 1465–1472. [DOI] [PubMed] [Google Scholar]
- Masuyama N., Hanafusa, H., Kusakabe, M., Shibuya, H. and Nishida, E. (1999) Identification of two Smad4 proteins in Xenopus. J. Biol. Chem., 274, 12163–12170. [DOI] [PubMed] [Google Scholar]
- Meno C., Shimono, A., Saijoh, Y., Yashiro, K., Mochida, K., Ohishi, S., Noji, S., Kondoh, H. and Hamada, H. (1998) lefty-1 is required for left–right determination as a regulator of lefty-2 and nodal. Cell, 94, 287–297. [DOI] [PubMed] [Google Scholar]
- Meno C. et al. (1999) Mouse lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol. Cell, 4, 287–298. [DOI] [PubMed] [Google Scholar]
- Nascone N. and Mercola, M. (1997) Organizer induction determines left–right asymmetry in Xenopus. Dev. Biol., 189, 68–78. [DOI] [PubMed] [Google Scholar]
- Nonaka S., Tanaka, Y., Okada, Y., Takeda, S., Harada, A., Kanai, Y., Kido, M. and Hirokawa, N. (1998) Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell, 95, 829–837. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nonaka, S., Tanaka, Y., Saijyoh, Y., Hamada, H. and Hirokawa, N. (1999) Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell, 4, 459–468. [DOI] [PubMed] [Google Scholar]
- Pagan-Westphal S.M. and Tabin, C.J. (1998) The transfer of left–right positional information during chick embryogenesis. Cell, 93, 25–35. [DOI] [PubMed] [Google Scholar]
- Ramsdell A.F. and Yost, H.J. (1998) Molecular mechanisms of vertebrate left–right development. Trends Genet., 14, 459–465. [DOI] [PubMed] [Google Scholar]
- Sampath K., Cheng, A.M.S., Frisch, A. and Wright, C.V.E. (1997) Functional differences among nodal-related genes in left–right axis determination. Development, 124, 3293–3302. [DOI] [PubMed] [Google Scholar]
- Sun B.I., Bush, S.M., Collins-Racie, L.A., LaVallie, E.R., DiBlasio-Smith, E.A., Wolfman, N.M., McCoy, J.M. and Sive, H.L. (1999) derrière: a TGF-β family member required for posterior development in Xenopus. Development, 126, 1467–1482. [DOI] [PubMed] [Google Scholar]
- Takeda S., Yonekawa, Y., Tanaka, Y., Okada, Y., Nonaka, S. and Hirokawa, N. (1999) Left–right asymmetry and kinesin super family protein KIF3A: new insights in determination of laterality and mesoderm induction by KIF3A–/– mice analysis. J. Cell Biol., 145, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen G.H. and Melton, D.A. (1993) Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell, 74, 433–441. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y., Vogan, K.J., Pearse, R.V., II and Tabin, C.J. (1999) Antagonistic signaling by Caronte, a novel Cerberus-related gene, mediates the establishment of broad domains of left–right asymmetric gene expression. Cell, 98, 573–583. [DOI] [PubMed] [Google Scholar]
- Zhu L., Marvin, M.J., Gardiner, A., Lassar, A.B., Mercola, M., Stern, C.D. and Levin, M. (1999) Cerberus regulates left/right asymmetry of the embryonic head and heart. Curr. Biol., 9, 931–938. [DOI] [PubMed] [Google Scholar]