Abstract
Introduction
Patients undergoing revision total hip surgery (RTHS) have a high prevalence of mild and moderate preoperative anemia, associated with adverse outcomes. The aim of this study was to investigate the association of perioperative allogeneic blood transfusions (ABT) and postoperative complications in preoperatively mild compared to moderate anemic patients undergoing RTHS who did not receive a diagnostic anemia workup and treatment before surgery.
Methods
We included 1,765 patients between 2007 and 2019 at a university hospital. Patients were categorized according to their severity of anemia using the WHO criteria of mild, moderate, and severe anemia in the first Hb level of the case. Patients were grouped as having received no ABT, 1–2 units of ABT, or more than 2 units of ABT. Need for intraoperative ABT was assessed in accordance with institutional standards. Primary endpoint was the compound incidence of postoperative complications. Secondary outcomes included major/minor complications and length of hospital and ICU stay.
Results
Of the 1,765 patients, 31.0% were anemic of any cause before surgery. Transfusion rates were 81% in anemic patients and 41.2% in nonanemic patients. The adjusted risks for compound postoperative complication were significantly higher in patients with moderate anemia (OR 4.88, 95% CI: 1.54–13.15, p = 0.003) but not for patients with mild anemia (OR 1.93, 95% CI: 0.85–3.94, p < 0.090). Perioperative ABT was associated with significantly higher risks for complications in nonanemic patients and showed an increased risk for complications in all anemic patients. In RTHS, perioperative ABT as a treatment for moderate preoperative anemia of any cause was associated with a negative compound effect on postoperative complications, compared to anemia or ABT alone.
Discussion
ABT is associated with adverse outcomes of patients with moderate preoperative anemia before RTHS. For this reason, medical treatment of moderate preoperative anemia may be considered.
Keywords: Total revision hip surgery, Preoperative anemia, Perioperative allogeneic blood transfusion
Introduction
Rising numbers of primary and revision total hip surgeries (RTHS) lead to an increased financial burden on the health care system. Therefore, optimizing care of this patient population is imperative not only at the patient level but also on a health care systems level [1].
Preoperative anemia has been reported as an independent risk factor for perioperative complications and increased length of stay (LOS) with a high prevalence of mild and moderate anemia in RTHS [2]. In primary total hip surgery, preoperative anemia has also been linked to increased use of allogeneic blood transfusion (ABT) [3]. In RTHS, the interactions between preoperative anemia and ABT are less well studied and understood [3]. It remains unclear whether transfusion-associated immunomodulation (TRIM) may provoke immunosuppression and proinflammatory effects or if preoperative moderate to severe anemia by itself, which more likely triggers RBC transfusion, impairs outcome (indication bias) as reported for patients undergoing orthopedic or other types of surgery [4–11]. Furthermore, in high-risk cardiovascular patients undergoing hip fracture surgery, a liberal transfusion strategy did not show any outcome improvement [12]. Hence, the discussion about perioperative anemia and its treatment with ABT in surgery is ongoing [7, 13–16]. We hypothesized that perioperative ABT is associated with adverse outcomes in terms of postoperative complications in patients with moderate compared to mild preoperative anemia of any cause undergoing RTHS.
Materials and Methods
This was a single-center, retrospective cohort study at Charité University Hospital Berlin, Germany, and adheres to the STROBE guidelines. After Institutional Review Board approval (EA1/343/16), data of all patients undergoing RTHS between 2007 and 2019 were consolidated in an anonymized database to investigate the role of perioperative ABT in anemic patients. Given the nature of the study, the need for informed patient consent was waived. Data sources included the hospital information system as well as electronic anesthesia records.
Patient selection was based on OPS – the German version of International Classification of Procedures in Medicine [17] – codes 5-820 (implantation of an endoprosthesis in the hip joint) and 5-821 (revision, change, and removal of an endoprosthesis in the hip joint) and presence of International Classification of Disease (ICD) code T84 indicating related complications, thereby qualifying the procedure as revision surgery. ICD codes were used for classification as aseptic (T84.14) and septic (T84.4-7) revision surgery and for assessing comorbidities (online suppl. Table S1; for all online suppl. material, see https://doi.org/10.1159/000530135). If patients had multiple cases, subsequent cases were excluded. If patients underwent multiple operations in one case (i.e., hospital stay), they were excluded from the study. Patients were categorized as positive or negative for preoperative anemia according to WHO criteria (hemoglobin [Hb] <13 g.dL−1 for men and Hb <12 g.dL−1 for women) and the severity of preoperative anemia stratified according to WHO criteria as mild (Hb in women 11.9–11.0 and 12.9–11.0 g.dL−1 in men), moderate (Hb 10.9–8.0 g.dL−1), and severe (Hb <8.0 g.dL−1) using the first Hb level of a case. Patients were grouped as having received no perioperative ABT, 1–2 units of ABT, or more than 2 units of ABT. Every data of transfusion (type of transfusion, volume, time, bed side test, blood type) was documented in the patient documentation system due to the German Transfusion Law. We extracted the data from the patient documentation systems in collaboration with the Institute of Medical Informatics, Charité – Universitätsmedizin Berlin.
Need for perioperative ABT was assessed in accordance with institutional standards [18] based on national guidelines [19], which were last updated in 2020. Transfusion indications were mainly aggravation of preexisting preoperative anemia and/or acute bleeding with clinical signs of anemic hypoxia. A predonated autologous blood program was not available for our study population. To control for changes in transfusion practices and in intraoperative techniques – e.g., cell salvage and use of antifibrinolytics – during the observation period, patients were divided into an early (2007–2012) and a late (2013–2019) cohort. Operations were performed by a relatively consistent number of surgeons with little change in the surgical team over the entire observation period.
The primary endpoint was the compound incidence of postoperative complications, drawn from ICD-10 codes (online suppl. Table S1) including acute coronary syndrome, acute respiratory failure, acute renal failure, pulmonary embolism, sepsis, cerebrovascular incidence, cardiac arrest, deep vein thrombosis, pneumonia, urinary tract infection, wound infection/dehiscence, and delirium, termed skin failure. Complications were selected based on published data and further categorized as either major or minor complications based upon the American College of Surgeons National Surgical Quality Improvement Program [2, 20]. Secondary endpoints included the incidence of major and minor complications separately, LOS in the hospital, and ICU treatment greater than 24 h. The 24-h cutoff for ICU treatment was chosen to exclude care on the hospital’s postoperative care unit. To differentiate the impact of anemia and transfusion, an interaction analysis was performed (see Statistical Analysis below).
As our study hypothesis ascribes the increased risk of compound complications to ABT in patients with different severities of preoperative anemia, we were aware of the possibility of transfusions being the result of complications. Thus, we conducted a sensitivity analysis for the subgroup of all intraoperatively transfused patients (online suppl. Table S2).
Statistical Analysis
Descriptive statistics were performed using R software [21]. A p value below 0.05 was considered significant. When normal distribution was ruled out by using the Kolmogorov-Smirnov test, results are given as median and interquartile range. Qualitative observations are characterized by frequency and reported with percentage. For a group comparison, the exact Mann-Whitney U test was applied. Multiple logistic regression was applied to characterize the impact of confounders on study endpoints in anemic versus nonanemic patients. All parameters reported in patients’ characteristics were included as explanatory variables.
Multiple logistic regression was applied to further investigate the interaction of anemia and transfusion. First, we added the product of anemia (i.e., negative or positive) and transfusion (i.e., no, 1–2 or >2 units) status as an interaction term in the regression model. When the interaction was statistically significant (p value <0.05), the individual impact of each anemia and transfusion status combination was assessed by a separate regression model that contained the interaction as a factor. For dichotomizing the continuous outcome variable LOS, patients exceeding the median LOS of the entire study population had been classified as positive for this study endpoint.
Results
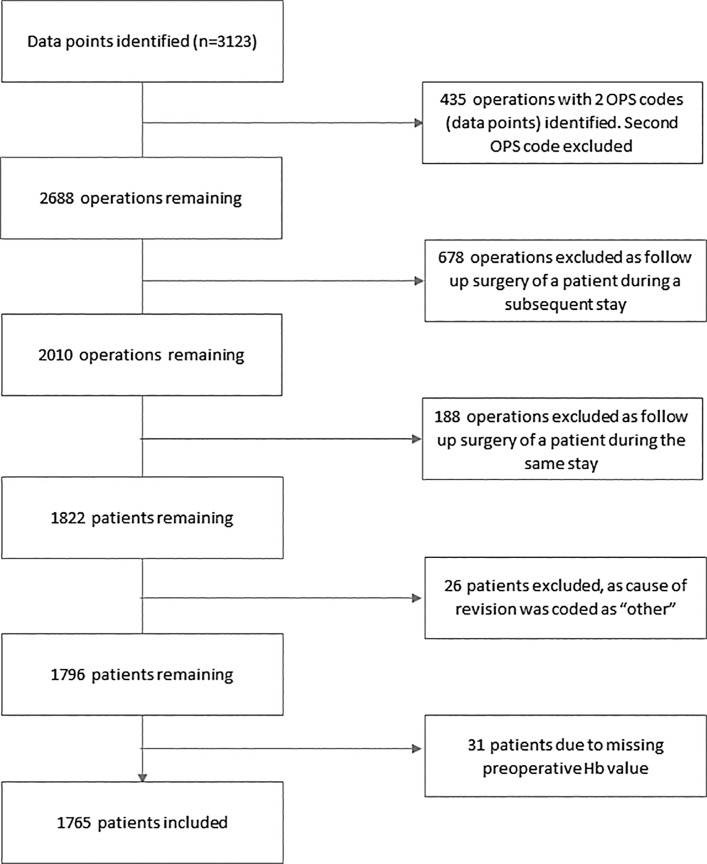
3,123 data points indicating RTHS were identified during the study period. After exclusion of operations as shown in Figure 1, 1,765 patients remained for analysis (shown in Fig. 1).
Fig. 1.
CONSORT diagram of study.
548 (31.0%) of these patients had preoperative anemia. 279 (15.8%), 260 (14.7%), and 9 (0.5) patients had mild, moderate, and severe anemia, respectively. During the study period, no workup of the cause of preoperative anemia or causal anemia treatment was performed before surgery. As the number of severely anemic patients was too small for further analysis, no subgroup analysis of this patient group was done.
Preoperatively anemic patients were significantly older, more likely to be male and to have an ASA grade greater than 2. A significantly higher prevalence of all comorbidities examined that except obesity, COPD and coronary artery disease were observed. Infection of the implanted prosthesis was more often the cause for RTHS in anemic patients (50.0 vs. 19.8%) as opposed to mechanical complications. Preoperatively anemic patients received perioperative ABT at significantly higher rates (81.0 vs. 41.2%, p < 0.001) (Table 1).
Table 1.
Demographics, comorbidities, and transfusion status of study population
| Variables | All patients | Patients without preoperative anemia | Patients with mild anemia | Patients with moderate anemia | Patients with severe anemia | p value |
|---|---|---|---|---|---|---|
| [N = 1,765] | N = 1,217 | N = 279 | N = 260 | N = 9 | ||
| Age, years | 71.0 [61.0–77.0] | 69.0 [59.0–76.0] | 72.0 [64.0–79.0] | 74.5 [66.0–79.0] | 75.0 [41.0–76.0] | <0.001 |
| Sex (female) | 1,097 (62.2%) | 792 (65.1%) | 123 (44.1%) | 176 (67.7%) | 6 (66.7%) | <0.001 |
| ASA grade >2 | 652 (39.3%) | 358 (31.4%) | 132 (50.0%) | 156 (63.2%) | 6 (75.0%) | <0.001 |
| Septic revision | 515 (29.2%) | 241 (19.8%) | 123 (44.1%) | 145 (55.8%) | 6 (66.7%) | <0.001 |
| Diabetes mellitus (IDDM and NIDDM) | 314 (17.8%) | 174 (14.3%) | 64 (22.9%) | 75 (28.8%) | 1 (11.1%) | <0.001 |
| Obesity (BMI >30) | 207 (11.7%) | 132 (10.8%) | 31 (11.1%) | 42 (16.2%) | 2 (22.2%) | 0.062 |
| Hypertension | 1,085 (61.5%) | 713 (58.6%) | 178 (63.8%) | 190 (73.1%) | 4 (44.4%) | <0.001 |
| Heart failure | 173 (9.8%) | 90 (7.4%) | 46 (16.5%) | 36 (13.8%) | 1 (11.1%) | <0.001 |
| COPD | 76 (4.3%) | 44 (3.6%) | 20 (7.2%) | 12 (4.6%) | 0 (0.0%) | 0.078 |
| Coronary artery disease | 206 (11.7%) | 131 (10.8%) | 44 (15.8%) | 29 (11.2%) | 2 (22.2%) | 0.070 |
| Chronic kidney disease | 221 (12.5%) | 101 (8.3%) | 59 (21.1%) | 59 (22.7%) | 2 (22.2%) | <0.001 |
| Patients receiving ABT | 946 (53.6%) | 502 (41.2%) | 197 (70.6%) | 239 (91.9%) | 8 (88.9%) | <0.001 |
| ABT, units | 1.00 [0.00–3.00] | 0.00 [0.00–2.00] | 2.00 [0.00–4.00] | 4.00 [2.00–6.00] | 5.00 [4.00–6.00] | <0.001 |
Values are number (proportion) or median (IQR [range]).
ASA, American Society of Anesthesiologists; IDDM, insulin-dependent diabetes mellitus; NIDDM, noninsulin dependent diabetes mellitus; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ABT, allogeneic blood transfusion; IQR, interquartile range.
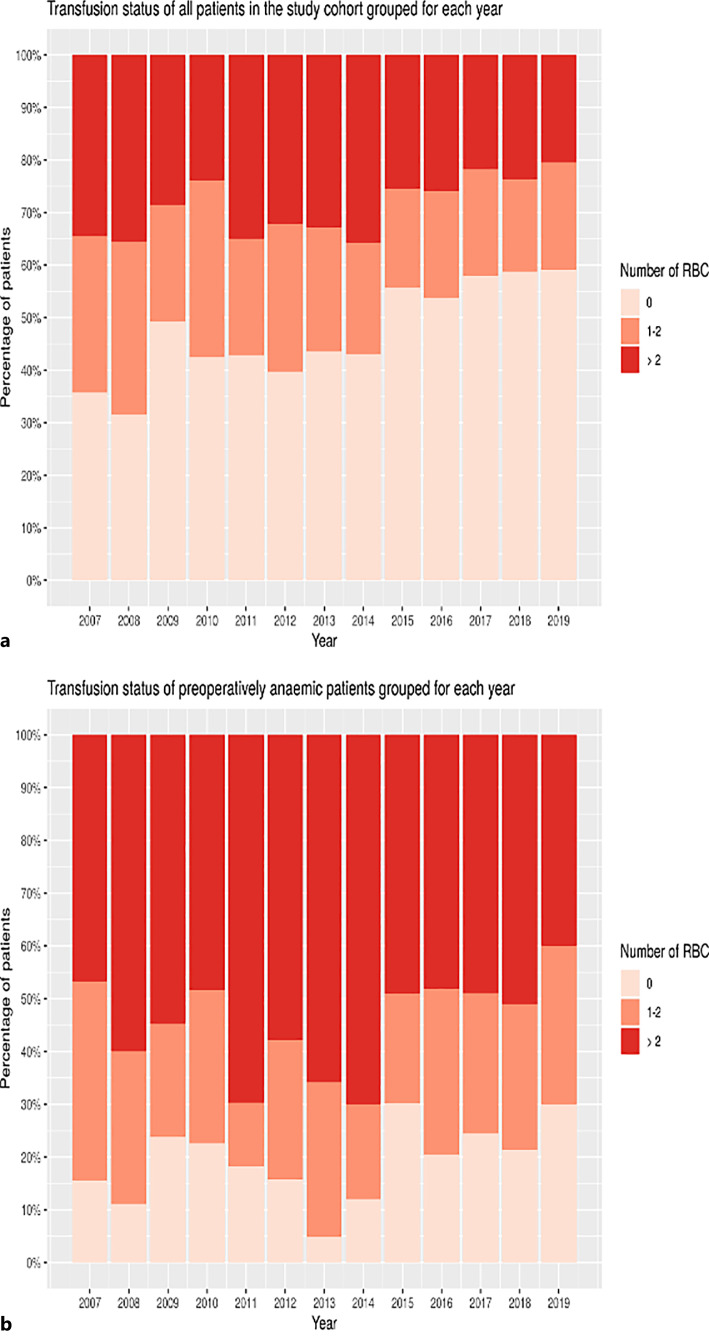
During the study period, a significant decrease of transfusion rates in the overall study population was observed, but not in the anemic group of patients (shown in Fig. 2).
Fig. 2.
a, b Transfusion status in the study cohort grouped for each year. a Transfusion status of all patients in the study cohort grouped for each year. b Transfusion status of preoperative anemic patients in the study cohort grouped for each year. y-axis: proportion of patients; x-axis: year; and figure key: number of red blood cell units (RBC) transfused.
Preoperatively anemic patients suffered from higher rates of compound complications (no anemia 11.3% vs. mild anemia 24.0%, and moderate anemia 43.5%, p < 0.001) as well as major (no anemia 6.0% vs. mild anemia 14.7% and moderate anemia 20.8%, p < 0.001) and minor (no anemia 7.2% vs. mild anemia 19.0% and moderate anemia 36.5%, p < 0.001) complications. Hospital mortality (0.6% for no anemia vs. 1.8% for mild and 3.1% for moderate anemia, p = 0.005) and rates of ICU stays exceeding 24 h (no anemia 4.4% vs. mild anemia 14.3% and moderate anemia 20.8%, p < 0.001) were higher. Mean LOS in hospital was longer (no anemia 11.1 days vs. mild anemia 13.1 days and moderate anemia 16.0 days, p < 0.001) (Table 2).
Table 2.
Postoperative complications of the study population
| Variables | All patients | Patients without preoperative anemia | Patients with mild anemia | Patients with moderate anemia | Patients with severe anemia | p value |
|---|---|---|---|---|---|---|
| [N = 1,765] | [N = 1,217] | [N = 279] | [N = 260] | [N = 9] | ||
| Compound complications | 320 (18.1%) | 137 (11.3%) | 67 (24.0%) | 113 (43.5%) | 3 (33.3%) | <0.001 |
| Major complicationsa | 168 (9.5%) | 73 (6.0%) | 41 (14.7%) | 54 (20.8%) | 0 (0.0%) | <0.001 |
| ACS | 15 (0.9%) | 11 (0.9%) | 2 (0.7%) | 2 (0.8%) | 0 (0.0%) | 1,000 |
| Acute respiratory failure | 115 (6.5%) | 51 (4.2%) | 26 (9.3%) | 38 (14.6%) | 0 (0.0%) | <0.001 |
| Acute kidney failure | 46 (2.6%) | 13 (1.1%) | 10 (3.6%) | 23 (8.9%) | 0 (0.0%) | <0.001 |
| PE | 3 (0.2%) | 2 (0.2%) | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | 0.447 |
| Sepsis | 33 (1.9%) | 13 (1.1%) | 10 (3.6%) | 10 (3.9%) | 0 (0.0%) | 0.003 |
| Cerebrovascular incident | 5 (0.3%) | 2 (0.2%) | 0 (0.0%) | 3 (1.2%) | 0 (0.0%) | 0.073 |
| Cardiac arrest | 12 (0.7%) | 5 (0.4%) | 5 (1.8%) | 2 (0.8%) | 0 (0.0%) | 0.079 |
| Minor complicationsa | 238 (13.5%) | 87 (7.2%) | 53 (19.0%) | 95 (36.5%) | 3 (33.3%) | <0.001 |
| DVT | 7 (0.4%) | 4 (0.3%) | 2 (0.7%) | 1 (0.4%) | 0 (0.0%) | 0.617 |
| Pneumonia | 36 (2.0%) | 12 (1.0%) | 4 (1.4%) | 19 (7.3%) | 1 (11.1%) | <0.001 |
| UTI | 34 (1.9%) | 14 (1.2%) | 8 (2.9%) | 11 (4.2%) | 1 (11.1%) | 0.001 |
| Wound dehiscence/infection | 37 (2.1%) | 9 (0.7%) | 11 (3.9%) | 17 (6.5%) | 0 (0.0%) | <0.001 |
| Delirium | 81 (4.6%) | 26 (2.1%) | 21 (7.5%) | 34 (13.1%) | 0 (0.0%) | <0.001 |
| Pressure ulcer | 115 (6.5%) | 37 (3.0%) | 21 (7.5%) | 55 (21.2%) | 2 (22.2%) | <0.001 |
| Death | 20 (1.1%) | 7 (0.6%) | 5 (1.8%) | 8 (3.1%) | 0 (0.0%) | 0.005 |
| LOS (days) | 12.0 [9.79–15.2] | 11.1 [9.10–14.1] | 13.1 [10.1–17.1] | 16.0 [12.0–24.0] | 16.6 [15.0–22.7] | <0.001 |
| Number of patients on ICU >24 h | 148 (8.4%) | 53 (4.4%) | 40 (14.3%) | 54 (20.8%) | 1 (11.1%) | <0.001 |
Values are number (proportion) or median (IQR [range]).
ACS, acute coronary syndrome; PE, pulmonary embolism; DVT, deep vein thrombosis; UTI, urinary tract infection; ICU, intensive care unit; IQR, interquartile range.
aDefined as one of the following.
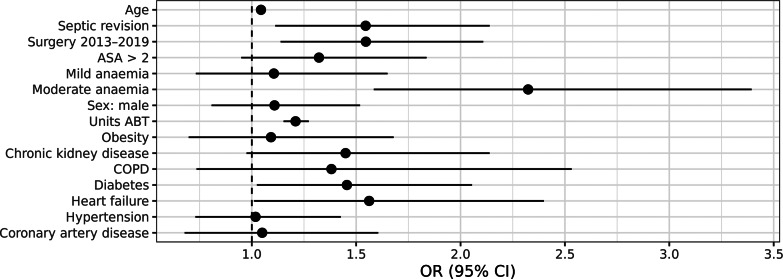
The following confounders were significantly associated with compound complications: surgery between 2013 and 2019 (i.e., second half of study period), age, septic revision, preoperative anemia of moderate grade, ABT units, chronic kidney disease, diabetes, and heart failure (shown in Fig. 3). Furthermore, a significant interaction was found between severity of preoperative anemia and perioperative ABT regarding compound complications:
Fig. 3.
Confounders for compound complications.
Moderate anemia was significantly associated with an increased risk of compound complication (OR 4.88, 95% CI: 1.54–13.15), while mild anemia was not (OR 1.93, 95% CI: 0.85–3.94) (Table 3). In nonanemic patients, a significant dose-dependent increase in complications was associated with ABT for the risk of complications with 1–2 units (OR 1.78, 95% CI: 1.08–2.91), and >2 units (OR 6.60, 95% CI: 4.34–10.13) (Table 3).
Table 3.
Interaction analysis quantifying the association of preoperative anemia and perioperative allogeneic blood transfusion on primary and secondary outcomes
| Compound complications | Major complications | Minor complications | LOS (days) | ICU care >24 h | |
|---|---|---|---|---|---|
| No anemia + 1–2 ABT | 1.78 (1.08–2.91), p = 0.021 | 2.30 (1.12–4.68), p = 0.021 | 1.51 (0.80–2.77), p = 0.188 | 2.44 (1.84–3.24), p < 0.001 | 6.29 (2.31–19.92), p = 0.001 |
| No anemia + >2 ABT | 6.60 (4.34–10.13), p < 0.001 | 9.46 (5.34–17.46), p < 0.001 | 5.45 (3.31–9.09), p < 0.001 | 6.25 (4.48–8.80), p < 0.001 | 27.93 (11.82–92.19), p < 0.001 |
| Mild anemia + No ABT | 1.93 (0.85–3.94), p < 0.090 | 2.67 (0.86–6.96), p = 0.061 | 2.21 (0.87–4.95), p = 0.062 | 1.51 (0.93–2.41), p = 0.088 | 7.28 (1.77–28.07), p = 0.004 |
| Mild anemia + 1–2 ABT | 2.76 (1.34–5.33), p = 0.004 | 3.94 (1.48–9.45), p = 0.003 | 2.27 (0.89–5.09), p = 0.062 | 2.03 (1.27–3.24), p = 0.003 | 11.51 (3.39–40.82), p < 0.001 |
| Mild Anemia + >2 ABT | 10.13 (6.26–16.46), p < 0.001 | 13.53 (7.22–26.10), p < 0.001 | 11.83 (6.96–20.34), p < 0.001 | 9.63 (6.04–15.95), p < 0.001 | 48.97 (20.13–146.62), p < 0.001 |
| Moderate anemia + No ABT | 4.88 (1.54–13.15), p = 0.003 | 0.00a (0.00–0.00) | 7.39 (2.29–20.39), p < 0.001 | 1.77 (0.71–4.24), p = 0.204 | 0.00a (0.00–0.00) |
| Moderate anemia + 1–2 ABT | 6.91 (3.66–12.76), p < 0.001 | 5.23 (1.95–12.68), p < 0.001 | 8.23 (4.10–16.10), p < 0.001 | 3.49 (2.06–6.00), p < 0.001 | 9.79 (2.37–37.99), p = 0.001 |
| Moderate anemia + >2 ABT | 15.81 (10.39–24.41), p < 0.001 | 14.84 (8.42–27.35), p < 0.001 | 16.99 (10.66–27.74), p < 0.001 | 13.09 (8.57–20.71), p < 0.001 | 55.91 (24.01–163.22), p < 0.001 |
Numbers are given as odds ratio with a 95% confidence interval and p-value, with no anemia and no ABT as the reference. ABT numbers are given for units.
ABT, allogeneic blood transfusion.
aNo patient in this category.
Comparing risks along the two axes of severity of preoperative anemia and number of transfusions showed a clear pattern of increased risk of compound complications as primary outcome with either increased severity of anemia or increased number of perioperative ABT. The combination of perioperative ABT with mild or moderate preoperative anemia was associated with a higher risk for the primary and all secondary outcomes in these patients (Table 3).
While mild anemia without ABT was not associated with a significantly elevated risk for any complications, the risk for compound and major complications as well as LOS and ICU care >24 h increased significantly when ABT was given. The highest risk for compound postoperative complications (OR 15.81, 95% CI: 10.39–24.41) and all secondary outcome parameters were observed in patients with moderate preoperative anemia receiving more than 2 units of ABT perioperatively (Table 3).
Discussion
The main finding of this analysis is a negative compound effect of preoperative anemia of any cause and perioperative ABT on the risk of postoperative compound complications in patients undergoing RTHS. ABT is associated with adverse outcomes in terms of postoperative complications in patients with moderate preoperative anemia. While mild anemia by itself was only associated with an increased rate of ICU care >24 h, the combination with perioperative ABT resulted in an increased risk for all outcome parameters. Moderate anemia and perioperative ABT were independently associated with an increased risk in primary and most secondary outcomes. Combining moderate anemia with perioperative ABT showed a negative compound effect on all outcome parameters.
Preoperative anemia and intraoperative ABT have both been reported in joint surgery as independent risk factors for morbidity and mortality [22, 23] and are also strongly associated with one another [3–5, 24]. In revision joint surgery, preoperative anemia has been identified as the most important modifiable independent predictor for both major complications and prolonged hospital stay [2].
The interaction between preoperative anemia and perioperative ABT, however, is less well studied. Most research trying to differentiate the impact of preoperative anemia and intra- and postoperative transfusion has been conducted in cardiac surgery, showing a compound effect of intra- and postoperative transfusions on adverse outcomes in anemic patients [6, 25], although this association is controversially debated in the literature [26, 27]. Similar results have been shown in gynecological surgery with intra- and postoperative ABT increasing the effect of preoperative anemia on mortality and morbidity [8]. A study in orthopedic patients undergoing primary hip, knee, and spine surgery identified perioperative ABT as a significant risk factor for mortality in nonanemic, but not in anemic patients [9]. In contrast to our study, patients in this study were younger, had a lower rate of comorbidities, and were less anemic and revision surgery was not investigated [9]. In contrast, another study in cardiac surgery patients [26] and a systematic review article could not confirm a significant connection between perioperative ABT and elevated mortality [27].This study looks specifically at patients undergoing RTHS who display a high incidence of preoperative anemia and ABT as compared to primary hip arthroplasty surgeries [3, 5, 28]. While the amount of ABT units in our study was in line with the amount of 1–3 red blood cell units in the majority of patients undergoing RTHS in earlier and recent studies [3, 29], a higher rate of postoperative complications in transfused anemic patients has been shown in studies in noncardiac surgery [30]. Our findings align with these results. The significantly higher rate of ICU stays >24 h and the increased LOS in hospital may be attributed to the increased postoperative morbidity as explained by the higher complication rates of most transfused anemic patients in our study.
A prolonged LOS – as shown in our data – was described for preoperatively anemic patients in previous studies involving revision joint [2] and other noncardiac surgery [22, 30, 31]. While these studies did not report results on ICU stay, it is probable that increased rates of postoperative complications in anemic patients would result in a higher rate of ICU admissions.
Perioperative ABT during hospital stay has been reported by Saleh et al. [5] to be associated with prolonged hospital stay in primary hip replacement [5], but in RTHS, only the study by Mahadevan et al. [32] found a significant association of intra- and postoperative ABT with prolonged LOS in hospital. In contrast to our study, the patients in this study were not stratified by the presence of preoperative anemia; thus, the results may be confounded by preoperative anemia. However, our data support their findings in a bigger patient sample and may further indicate that perioperative ABT is not necessarily associated with reduced LOS in hospital in preoperatively anemic patients.
The strongest confounder for compound complication observed in this study was moderate anemia. This is in line with earlier studies [2–4] and, most probably, is the result of a multifactorial genesis including anemia as a marker of a higher comorbidity level, as well as anemia itself, potentially leading to higher intraoperative blood loss [3] and ABT requirements [5, 32]. Therefore, it can be questioned that ABT alone altered postoperative complication. Moderate anemia may represent an indication bias which more likely affects outcome than ABT-related TRIM [10, 11]. Due to missing prospective randomized data investigating TRIM as primary outcome, there is no consistent evidence to account ABT by itself for impaired postoperative outcome in patients with preoperative anemia [33].
Over the 13-year study period, upcoming PBM strategies have shifted clinical practice to a more restrictive approach toward ABT [34]. Our finding of a significant decrease in perioperative ABT reflects this shift. However, we did not find this decrease in the rate of preoperatively anemic patients of any cause and the severity of anemia. Therefore, it might be hypothesized that preoperative treatment of the underlying causes of anemia may reduce the incidence of ABT and the associated potentially harmful effects.
Limitations
We do acknowledge several limitations of our study. The results depend on the hospital’s ICD coding system for comorbidities and complications. The accuracy of these data is dependent on coding accuracy, which is, however, done by dedicated and trained administrative staff following national coding guidelines making systematic errors less likely. We identified underreporting of complications as a potential concern, and contrary to our expectations, we did observe an increase in compound complications in the second half of the study period, which might be attributed to increased patient age resulting in a higher burden of disease. Interestingly, the LOS decreased at the same time which might indicate stricter coding and discharge practices in the second half of the study period.
To control for the possibility of transfusions being the result of prior complications, we performed a subgroup analysis with patients who received intraoperative ABT, which showed similar results to the whole study population (online suppl. Table S2). However, due to the retrospective nature of the study, this association cannot strictly be ruled out. There is also no information on the exact indication for transfusion (e.g., “transfusion threshold”) nor did the database contain intraoperative data (e.g., use of cell salvage), which has been shown to reduce the need for ABT in revision joint surgery [3]. As the focus of the study was the interaction of preoperative anemia and ABT and their impact on outcome of RTHS patients, we did not investigate the association between intraoperative complications and patient outcome nor did we analyze the reasons for prolonged ICU which may have been related to intraoperative complications or preoperative anemia and should be considered in future research. Furthermore, a separate evaluation of patients with revision total hip surgery for mechanical reasons or infections would be important to know and should be considered in future research. Nevertheless, the time from the first surgical procedure to the revision surgery as well as perioperative bleeding complications and volume of blood loss might be interesting as well. Our investigation cannot rule out that there might be a difference in outcome when preop nonanemic patients have bled acutely intraoperatively with consecutive anemia and transfusion compared to preoperative anemic patients with or without intraoperative bleeding or transfusion.
To improve comparability, we adopted an approach similar to the American College of Surgeons National Surgical Quality Improvement Program to stratify major and minor complications. Not all necessary data for certain categories according to the program’s guidelines were available. Our stratification is thus derived from, but not an exact match of, the program’s stratification.
Strengths of our study include stratification according to the severity of anemia, a homogeneous cohort of patients, and inclusion of mechanical and infectious causes for revision, thus reflecting a realistic patient sample with a prevalence of anemia consistent with the previous literature [3].
Conclusions
Moderate preoperative anemia of any cause as well as ABT was independently associated with impaired outcomes after RTHS. A combination of preoperative anemia and ABT showed a negative compound effect. Furthermore, our study shows that overall transfusion rates declined over time, but this was not the case in anemic patients, who continued to be exposed to a high rate of ABT.
Our results support the hypothesis that preoperative treatment of the cause of moderate anemia may improve complication rates and other outcomes after RTHS as stipulated in guidelines [35] and promoted by PBM programs [36, 37]. Nevertheless, future prospective randomized trials of preoperative anemia treatment are required to test this hypothesis.
Statement of Ethics
This study protocol was reviewed and approved by Geschäftsstelle der Charité – Ethikkommission, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany (approval number EA1/343/16). Written informed consent was not required due to the retrospective study design (approved by Geschäftsstelle der Charité – Ethikkommission, Charité – Universitätsmedizin Berlin).
Conflict of Interest Statement
Henning Uden, Franziska Büttner, Michael Krämer, Gerald Vorderwülbecke, Sebastian Hardt, and Jochen Kruppa: None. Christian von Heymann declares to have no financial conflict of interest related to the topic of this manuscript. He also declares that he was mandated from the German Society of Anesthesiology and lntensive Care Medicine (DGAI) to write the German Guideline on Preoperative Anemia (published in April 2018) and that he was part of the writing group of the Patient Blood Management Guideline in cardiac surgery on behalf of the European Society of Cardiothoracic Anaesthesiologists (EACTA) in conjunction with the European Society of Cardiothoracic Surgery (EACTS) (published in September 2017). Outside this work, Christian von Heymann discloses to have received research funding, speaker’s and consultancy honoraria, and travel reimbursements from CSL Behring, Daiichi Sankyo, HICC GbR, Mitsubishi Pharma GmbH, NovoNordisk Pharma GmbH, Shionogi Pharma, and Sobi Pharma. Lutz Kaufner declares to have no financial conflict of interest related to the topic of this manuscript. He also declares that he was mandated from the German Society of Anesthesiology and lntensive Care Medicine (DGAI) to write the German Guideline on Preoperative Anemia (published in April 2018). He discloses to received speaker’s and consultancy honoraria and travel reimbursements from HICC GbR., CSL Behring, and Novo Nordisk outside the submitted work.
Claudia Spies reports grants from Deutsche Forschungs-gemeinschaft/German Research Society, grants from Deutsches Zentrum für Luft- und Raumfahrt e.V. (DLR)/German Aerospace Center, grants from Einstein Stiftung Berlin/Einstein Foundation Berlin, grants from Gemeinsamer Bundesausschuss/Federal Joint Committee (G-BA), grants from Inneruniversitäre Forschungsförderung/Inner University Grants, grants from Projektträger im DLR/Project Management Agency, grants from Stifterverband/Non-Profit Society Promoting Science and Education, grants from European Society of Anaesthesiology and Intensive Care, grants from Baxter Deutschland GmbH, grants from Cytosorbents Europe GmbH, grants from Edwards Lifesciences‚ Germany GmbH, grants from Fresenius Medical Care, grants from Grünenthal GmbH, grants from Masimo Europe Ltd‚ grants from Pfizer Pharma PFE GmbH, personal fees from Georg Thieme Verlag, grants from Dr. F. Köhler Chemie GmbH‚ grants from Sintetica GmbH, grants from Stifterverband für die deutsche Wissenschaft e.V./Philips, grants from Stiftung Charite, grants from AGUETTANT Deutschland GmbH, grants from AbbVie Deutschland GmbH & Co. KG, grants from Amomed Pharma GmbH, grants from InTouch Health, grants from Copra System GmbH, grants from Correvio GmbH, grants from Max-Planck-Gesellschaft zur Förderung der Wissenschaften e.V., grants from Deutsche Gesellschaft für Anästhesiologie & Intensivmedizin (DGAI), grants from Stifterverband für die deutsche Wissenschaft e.V./Metronic, grants from Philips ElectronicsNederland BV, grants from BMG, grants from BMBF, grants from Deutsche Forschungsgemeinschaft/German Research Society, and grants from Drägerwerk AG & Co. KGaA, outside the submitted work. In addition, Dr. Spies has a patent 10 2014 215 211.9 licensed, a patent 10 2018 114 364.8 licensed, a patent 10 2018 110 275.5 licensed, a patent 50 2015 010 534.8 licensed, a patent 50 2015 010 347.7 licensed, and a patent 10 2014 215 212.7 licensed. Felix Balzer reports grants from German Federal Ministry of Education and Research, grants from German Federal Ministry of Health, grants from Berlin Institute of Health, personal fees from Elsevier Publishing, grants from Hans Böckler Foundation, other from Robert Koch Institute, grants from Einstein Foundation, and grants from Berlin University Alliance, outside the submitted work.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Henning Uden and Christian von Heymann: This author helped design and perform the study; and draft, read, and approve the manuscript. Franziska Büttner and Claudia Spies: This author helped draft, read, and approve the manuscript. Michael Krämer and Lutz Kaufner: This author helped design the database; and read and approve the manuscript. Gerald Vorderwülbecke and Jochen Kruppa: This author helped perform the statistical analysis; and draft, read, and approve the manuscript. Felix Balzer: This author helped design the study; and draft, read, and approve the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Patel A, Pavlou G, Mújica-Mota RE, Toms AD. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Joint J. 2015;97-B(8):1076–81. 10.1302/0301-620X.97B8.35170. [DOI] [PubMed] [Google Scholar]
- 2. Liodakis E, Bergeron SG, Zukor DJ, Huk OL, Epure LM, Antoniou J. Perioperative complications and length of stay after revision total hip and knee arthroplasties: an analysis of the NSQIP database. J Arthroplasty. 2015;30(11):1868–71. 10.1016/j.arth.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 3. Kasivisvanathan R, Ramesh V, Rao Baikady R, Nadaraja S. Preoperative anaemia is associated with increased allogeneic pack red cell transfusion in revision hip and knee joint arthroplasty: a retrospective analysis of 5387 patients over a 10-year period at a single high volume centre. Transfus Med. 2016;26(4):271–7. 10.1111/tme.12322. [DOI] [PubMed] [Google Scholar]
- 4. Walsh TS, Palmer J, Watson D, Biggin K, Seretny M, Davidson H, et al. Multicentre cohort study of red blood cell use for revision hip arthroplasty and factors associated with greater risk of allogeneic blood transfusion. Br J Anaesth. 2012;108(1):63–71. 10.1093/bja/aer326. [DOI] [PubMed] [Google Scholar]
- 5. Saleh A, Small T, Chandran Pillai AL, Schiltz NK, Klika AK, Barsoum WK. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J Bone Joint Surg Am. 2014;96(18):e155. 10.2106/JBJS.M.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Heymann C, Kaufner L, Sander M, Spies C, Schmidt K, Gombotz H, et al. Does the severity of preoperative anemia or blood transfusion have a stronger impact on long-term survival after cardiac surgery? J Thorac Cardiovasc Surg. 2016;152(5):1412–20. 10.1016/j.jtcvs.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 7. Gu WJ, Gu XP, Wu XD, Chen H, Kwong JSW, Zhou LY, et al. Restrictive versus liberal strategy for red blood-cell transfusion: a systematic review and meta-analysis in orthopaedic patients. J Bone Joint Surg Am. 2018;100(8):686–95. 10.2106/JBJS.17.00375. [DOI] [PubMed] [Google Scholar]
- 8. Richards T, Musallam KM, Nassif J, Ghazeeri G, Seoud M, Gurusamy KS, et al. Impact of preoperative anaemia and blood transfusion on postoperative outcomes in gynaecological surgery. PLoS One. 2015;10(7):e0130861. 10.1371/journal.pone.0130861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smilowitz NR, Oberweis BS, Nukala S, Rosenberg A, Zhao S, Xu J, et al. Association between anemia, bleeding, and transfusion with long-term mortality following noncardiac surgery. Am J Med. 2016;129(3):315–23.e2. 10.1016/j.amjmed.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wissenschaftliche Erläuterungen zur Stellungnahme Transfusionsassoziierte Immunmodulation (TRIM) des Arbeitskreises Blut vom 13 Bundesgesundheitsbl 2020. 63 Bundesgesundheitsbl. 1022–4. [DOI] [PMC free article] [PubMed]
- 11.Wissenschaftliche Erläuterungen zur Stellungnahme Transfusionsassoziierte Immunmodulation (TRIM) des Arbeitskreises Blut vom 13. Februar 2020. Bundesgesundheitsbl 63. 1025–53. [DOI] [PMC free article] [PubMed]
- 12. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–62. 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunskill SJ, Millette SL, Shokoohi A, Pulford EC, Doree C, Murphy MF, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev. 2015;21(4):CD009699. 10.1002/14651858.CD009699.pub2,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378(9800):1396–407. 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 15. Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst L, et al. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis. BMJ. 2016;352:i1351. 10.1136/bmj.i1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng S, Machina M, Beattie WS. Influence of anaemia and red blood cell transfusion on mortality in high cardiac risk patients undergoing major non-cardiac surgery: a retrospective cohort study. Br J Anaesth. 2017;118(6):843–51. 10.1093/bja/aex090. [DOI] [PubMed] [Google Scholar]
- 17.Deutschen Institut für Medizinische Dokumentation und Information: The German procedure classification. Available from: https://www.dimdi.de/dynamic/en/classifications/ops/index.html (accessed November 2, 2021).
- 18. Kox WJ, Spies CD. Check-up Anästhesiologie, Anästhesie–Intensivmedizin–Schmerztherapie–Notfallmedizin Standards. Springer. 2005. [Google Scholar]
- 19. Querschnitts-Leitlinien zur Therapie mit Blutkomponenten und Plasmaderivaten. https://www.bundesaerztekammer.de/themen/medizin-und-ethik/wissenschaftlicher-beirat/stellungnahmen-richtlinien-jahresberichte/haemotherapie-transfusionsmedizin/querschnitts-leitlinien-baek-zur-therapie-mit-blutkomponenten-und-plasmaderivaten-gesamtnovelle-2020 (accessed February 17, 2023). [Google Scholar]
- 20. Lu M, Sing DC, Kuo AC, Hansen EN. Preoperative anemia independently predicts 30-day complications after aseptic and septic revision total joint arthroplasty. J Arthroplasty. 2017;32(9S):S197–201. 10.1016/j.arth.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 21. R Development Core Team . R: a language and environment for statistical computing. Vienna, Austria: the R Foundation for Statistical Computing; 2011. Available from: http://www.R-project.org/ (accessed November 2, 2021). [Google Scholar]
- 22. Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113(2):482–95. 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen AB, Mehnert F, Overgaard S, Johnsen SP. Allogeneic blood transfusion and prognosis following total hip replacement: a population-based follow up study. BMC Musculoskelet Disord. 2009;10:167. 10.1186/1471-2474-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carling MS, Jeppsson A, Eriksson BI, Brisby H. Transfusions and blood loss in total hip and knee arthroplasty: a prospective observational study. J Orthop Surg Res. 2015;10:48. Published 2015 Mar 28. 10.1186/s13018-015-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Engoren M, Schwann TA, Habib RH, Neill SN, Vance JL, Likosky DS. The independent effects of anemia and transfusion on mortality after coronary artery bypass. Ann Thorac Surg. 2014;97(2):514–20. 10.1016/j.athoracsur.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 26. Padmanabhan H, Brookes MJ, Luckraz H. Association between anemia and blood transfusion with long-term mortality after cardiac surgery. Ann Thorac Surg. 2020 Aug;110(2):749–50. 10.1016/j.athoracsur.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 27. Padmanabhan H, Siau K, Curtis J, Ng A, Menon S, Luckraz H, et al. Preoperative anemia and outcomes in cardiovascular surgery: systematic review and meta-analysis. Ann Thorac Surg. 2019 Dec;108(6):1840–8. 10.1016/j.athoracsur.2019.04.108. [DOI] [PubMed] [Google Scholar]
- 28. Saleh E, McClelland DB, Hay A, Semple D, Walsh TS. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth. 2007;99(6):801–8. 10.1093/bja/aem299. [DOI] [PubMed] [Google Scholar]
- 29. Surgenor DM, Wallace EL, Churchill WH, Hao SH, Chapman RH, Poss R. Red cell transfusions in total knee and total hip replacement surgery. Transfusion. 1991 Jul–Aug;31(6):531–7. 10.1046/j.1537-2995.1991.31691306252.x. [DOI] [PubMed] [Google Scholar]
- 30. Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283–92. 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 31. Bulte CSE, Boer C, Hemmes SNT, Serpa Neto A, Binnekade JM, Hedenstierna G, et al. The effects of preoperative moderate to severe anaemia on length of hospital stay: a propensity score-matched analysis in non-cardiac surgery patients. Eur J Anaesthesiol. 2021;38(6):571–81. 10.1097/EJA.0000000000001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahadevan D, Challand C, Keenan J. Revision total hip replacement: predictors of blood loss, transfusion requirements, and length of hospitalisation. J Orthop Traumatol. 2010;11(3):159–65. 10.1007/s10195-010-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54(10 Pt 2):2646–57. 10.1111/trf.12687. [DOI] [PubMed] [Google Scholar]
- 35. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) –Ständige Kommission Leitlinien . Präoperative Anämie: diagnostik und Therapie der Präoperativen Anämie (S3 Leitlinie) Auflage. 2018. Available from: https://www.awmf.org/uploads/tx_szleitlinien/001-024Ll_S3_Praeoperative-Anaemie_2018-04.pdf (accessed February 17, 2020). [Google Scholar]
- 36. Meybohm P, Froessler B, Goodnough LT, Klein AA, Muñoz M, Murphy MF, et al. “Simplified international recommendations for the implementation of patient blood management” (SIR4PBM). Perioper Med. 2017;6:5. Published 2017 Mar 17. 10.1186/s13741-017-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–52. 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.