Abstract
The spindle assembly checkpoint detects defects in spindle structure or in the alignment of the chromosomes on the metaphase plate and delays the onset of anaphase until defects are corrected. Thus far, the evidence regarding the presence of a spindle checkpoint during meiosis in male Drosophila has been indirect and contradictory. On the one hand, chromosomes without pairing partners do not prevent meiosis progression. On the other hand, some conserved components of the spindle checkpoint machinery are expressed in these cells and behave as their homologue proteins do in systems with an active spindle checkpoint. To establish whether the spindle checkpoint is active in Drosophila spermatocytes we have followed meiosis progression by time-lapse microscopy under conditions where the checkpoint is likely to be activated. We have found that the presence of a relatively high number of misaligned chromosomes or a severe disruption of the meiotic spindle results in a significant delay in the time of entry into anaphase. These observations provide the first direct evidence substantiating the activity of a meiotic spindle checkpoint in male Drosophila.
INTRODUCTION
The aim of cell division is to ensure the correct partitioning of the genetic material between the resulting daughters. To optimize this process, segregation is preceded by the alignment of the chromosomes in the metaphase plate, midway between the two spindle poles. Chromosome alignment at the metaphase plate is achieved by a complex process governed by the equilibrium between the pulling and pushing forces exerted by the microtubules on each kinetochore pair. In many systems, a back-up mechanism known as the spindle checkpoint inhibits entry into anaphase when it detects failures of chromosome alignment (Gorbsky, 1995; Rudner and Murray, 1996; Wells, 1996; Nicklas, 1997). Several lines of evidence argue against the presence of a spindle checkpoint in male Drosophila meiosis. The first comes from the behaviour of compound chromosomes that are mandatory univalents during the first meiotic division because they do not have a homologue to pair with (Ashburner, 1989). These univalent chromosomes can rarely attain the bi-oriented conformation required for stabilization at the metaphase plate. The fact that these chromosomes are transmitted to the offspring shows that chromosome misalignment does not prevent Drosophila male meiosis.
A similar conclusion can be drawn from the analysis of mutants like mei-S332 and ord that result in precocious separation of sister chromatids (Goldstein, 1980; Lin and Church, 1982; Church and Lin, 1988; Kerrebrock et al., 1992). Like univalents in the first meiotic division, these prematurely separated chromatids do not inhibit meiosis II progression. These observations suggest that an efficient spindle checkpoint is not active in Drosophila spermatocytes. Nevertheless, some conserved components of the checkpoint machinery, like Bub1 and Bub3, have recently been shown to be expressed in meiotic spermatocytes (Basu et al., 1998, 1999). Moreover, their subcellular localization is consistent with them playing an active role during meiosis: their association with the kinetochores is very tight before metaphase, becomes weaker as the bivalents align at the metaphase plate, and is lost as the cell enters anaphase (Basu et al., 1998, 1999). The same behaviour is observed in the mitotic cells of the Drosophila ganglia where the spindle checkpoint is known to be active. Moreover, the localization of Bub1 in meiotic spermatocytes is sensitive to bipolar tension at the kinetochores, further supporting the view that it may be acting as part of a functional spindle checkpoint (Basu et al., 1999). Additional evidence for the presence of a tension-sensing mechanism in Drosophila male meiosis is provided by the behaviour of Zw10 and Rod. Zw10 is redistributed from the kinetochores to the spindle microtubules when the chromosomes achieve bipolar orientation, but remains attached to the kinetochores of univalent chromosomes suggesting that redistribution is a response to tension (Williams et al., 1996). The Rod protein behaves identically to Zw10 in this regard (Scaërou et al., 1999).
A simple hypothesis to reconcile these seemingly contradictory observations is that a spindle checkpoint does exist in Drosophila meiotic spermatocytes, but that it operates with significantly reduced efficiency. One prediction of this viewpoint (Basu et al., 1999) is that conditions that should enable the checkpoint would delay anaphase onset, but would not completely block meiosis. To test this hypothesis we have followed meiosis by time-lapse microscopy of cultured spermatocytes under conditions that are expected to trigger the spindle checkpoint (Rudner and Murray, 1996).
RESULTS
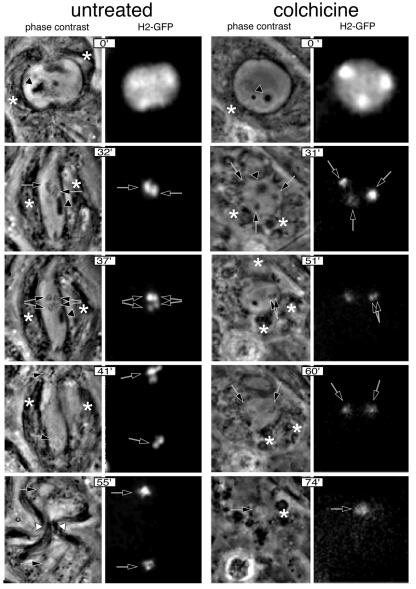
The first experimental condition studied to determine the presence of a checkpoint in Drosophila spermatocytes was colchicine treatment. Colchicine is a potent inhibitor of microtubule polymerization, one of the most effective ways to activate the checkpoint (Rieder and Palazzo, 1992). The results of these experiments are summarized in Figure 1. Five time points showing some of the main landmarks of meiosis I in Drosophila spermatocytes are shown. In the control spermatocyte, these include late prophase (Church and Lin, 1985) (0 min), stabilized bivalents at the metaphase plate (32 min), the pole-ward movement of the chromosomes during anaphase (37 min), chromosome decondensation (41 min), nuclear formation and cytokinesis (55 min). In the colchicine-treated spermatocyte, the chromosomes fail to cluster in a plate, homologous chromosomes do not segregate at any significant distance and there is no hint of cytokinesis, as expected in the absence of a functional meiotic spindle. Nevertheless, chromosome condensation proceeds normally (31 min), homologous chromosomes separate from one another (51 min), the chromosomes decondense (60 min) and nuclear formation occurs (74 min) in the colchicine-treated cell. Thus, the main landmarks of meiosis I progression, with the obvious exception of those that require a functional meiotic spindle, can be observed in the colchicine-treated spermatocyte. From this observation we conclude that the absence of a proper spindle does not arrest meiosis in these cells. Nevertheless, meiosis progression takes significantly longer in colchicine-treated spermatocytes. A closer look at the timing of the landmarks reveals that this increase in the duration of meiosis is mainly accounted for by a delay in the time of entry into anaphase.
Fig. 1. Meiosis I progression in control and colchicine-treated wild-type males observed by time-lapse phase-contrast and fluorescence microscopy. Meiosis was followed from early prometaphase to the end of meiosis I at a rate of 20 frames/min using a combination of phase-contrast and fluorescence microscopy. The chromosomes were labelled with a His2–GFP fusion protein. Only five significant time points are shown in this figure. Control: time 0′ corresponds to late prophase. At this stage, the two centrosomes have migrated to opposite poles and organize large asters. The incipient meiotic spindle is outlined by associated phase-dark parafusorial membranes (asterisks) that contain numerous mitochondria (Rieder et al., 1994). A non-chromosomal phase-dark nuclear structure, which remains present throughout meiosis (McKee et al., 1998) can also be seen (arrowhead). At time 32′, the spermatocyte contains a fully formed, elongated spindle. Two bivalents can be seen stabilized at the metaphase plate in this focal plane (arrows). At time 37′, anaphase has just started. Two pairs of homologous chromosomes can be seen segregating from each other (double arrows). At time 41′, the chromosomes have reached the poles and are decondensing. Chromosome decondensation is first apparent midway through anaphase. At time 55′, the two daughter nuclei are formed (arrows) and the cytokinesis furrow begins to pinch the central spindle (white arrowheads). The parafusorial membranes are tightly associated with the spindle at this stage. Colchicine: time point 0′ corresponds to an early stage of chromatin condensation very similar to that shown by the control cell. The parafusorial membranes are not organized. At time point 31′, chromosome condensation has proceeded, but the chromosomes do not congress at a metaphase plate. At time point 51′ the bivalent that is within the focal plane can be seen to split into two homologous chromosomes (double arrow). Despite their separation, the two homologues do not segregate from each other to any noticeable distance. At time 60′, the chromosomes begin to decondense (arrows). At time point 74′, the nuclear envelope forms around the unsegregated chromosomes. Several clusters of parafusorial membranes can be seen scattered within this cell (asterisk). The lack of organization of these membranes throughout meiosis is fully consistent with a general failure in microtubule polymerization and provides an excellent internal control for the effect of colchicine in these cells.
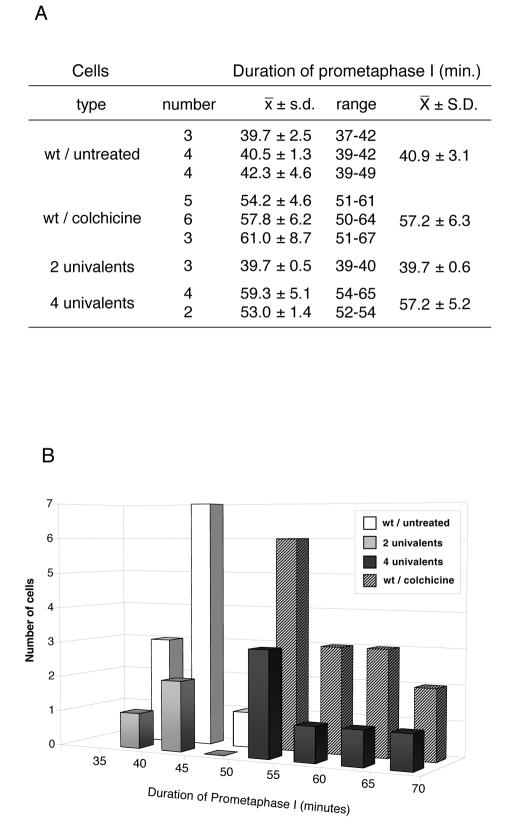
To quantify these differences further, we measured the timing of meiosis in 15 colchicine-treated spermatocytes and 12 control spermatocytes followed by time-lapse microscopy. We found that the average time and standard deviation of prometaphase length is 40.9 min ± 3.0 in control cells and 57.2 min ± 6.3 in colchicine-treated spermatocytes (Figure 2). Therefore, entry into anaphase is delayed for an average of 16 min. Consistent with this observation, we found that the onset of chromosome decondensation is also delayed by colchicine treatment (67.6 min ± 5.8 versus 46.0 min ± 4.6). Thus, in the absence of a proper meiotic spindle, entry into anaphase is arrested for up to 40% of the normal duration of prometaphase. After this time, the arrest is overridden and meiosis can proceed even if the conditions that triggered the delay have not changed. These observations suggest that a spindle checkpoint, defined as a mechanism that delays the onset of anaphase when chromosomes are not properly aligned on the spindle, operates in Drosophila spermatocytes.
Fig. 2. Duration of prometaphase I under conditions that may trigger a spindle checkpoint. (A) Duration of prometaphase I was estimated as the interval spanning between the beginning of chromosome condensation and the onset of anaphase I. Four different conditions including wild-type untreated cells, wild-type cells treated with colchicine, and cells containing two [C(3L);C(3R)] or four [C(2L);C(2R);C(3L);C(3R)] univalent chromosomes were studied. Each line shows the number of cells followed in each single culture, together with the average (x), standard deviation (s.d.) and range of prometaphase length. The cumulative average of all the cultures from the same experiment and the corresponding standard deviation are also shown (X ± S.D.). Colchicine treatment of wild-type spermatocytes, as well as the presence of four univalents result in a delay of around 16 min, with respect to the control, while the presence of two univalents has no effect. (B) Graphic representation of the data presented in (A) showing the frequency distribution of the duration of prometaphase in the four experimental conditions studied.
Total failure of spindle assembly is a very extreme condition which may not be encountered often in nature. In many systems, the spindle checkpoint is able to detect more subtle defects which are more likely to occur under normal conditions. For instance, in some species the spindle assembly checkpoint is so refined that it can be activated by a single unattached kinetochore (Gorbsky, 1995; Nicklas et al., 1995; Wells, 1996). To determine the sensitivity of the spindle checkpoint of Drosophila spermatocytes we followed meiosis in cells that contained misaligned chromosomes, but did not have any spindle defects. We used two combinations of misaligned chromosomes. The first included four compound chromosomes, each made of two copies of the left or right arm of the second or third chromosome (Ashburner, 1989). The second combination carried only two compounds derived from the third chromosome. These compound chromosomes are mandatory univalents that oscillate between the two poles and seldom reach a stable position at the metaphase plate during the first meiotic division (see accompanying video clips at EMBO Reports Online). The results from these experiments are summarized in Figure 3. As expected, all the stages of meiosis progression can be observed in spermatocytes carrying either two or four univalents. Nevertheless, meiosis is significantly delayed in the spermatocyte carrying four compounds. This can easily be appreciated in Figure 3 where the X and Y chromosomes of the four-compound bearing spermatocyte are still paired 55 min after the start of prometaphase (black arrow). To quantify these differences further, we followed meiosis by time-lapse microscopy in three or four cells carrying two and four compound chromosomes, respectively. The average time and standard deviation of prometaphase length in these cells was 39.6 min ± 0.5 in spermatocytes that carry two compound chromosomes and 57.1 min ± 5.1 in cells carrying four compounds (Figure 2). Thus, as far as the duration of meiosis I is concerned, the two-compound combination studied has no effect, while the combination of four compound chromosomes results in a significant delay in the time of entry into anaphase. These observations provide further support to our conclusion that a spindle checkpoint operates in Drosophila spermatocytes.
Fig. 3. Meiosis I progression in the presence of two [C(3L);C(3R)] and four [C(2L);C(2R);C(3L);C(3R)] univalent chromosomes observed by time-lapse phase-contrast microscopy. Meiosis was followed from late prometaphase to the end of meiosis I at a rate of 20 frames/min. Only four significant time points are shown in this figure. Two univalents: time point 0′ corresponds to early prometaphase. One univalent (white arrow) and one bivalent (black arrow) can be observed at this focal plane. At time 17′, the bivalents (black arrow) are stabilized at the metaphase plate while the two univalents (white arrows) oscillate between the two poles. At time 34′, anaphase has started. Two pairs of segregating homologues (double arrows) and one univalent located near one pole (white arrow) can be observed. At time point 41′, the chromosomes have reached the poles and are fully decondensed (arrowheads). The chromosomes were also followed by fluorescence microscopy to detect the His2–GFP fusion (not shown). Four univalents: time point 0′ corresponds to the beginning of prometaphase. One univalent (white arrow) can be observed at this focal plane. At time points 21′, 28′ and 55′, the four univalents (white arrows), and the major bivalent, the X/Y pair (black arrow), remain condensed. The X and Y chromosomes do not segregate during this period. Anaphase onset in this cell started at time point 59′ (not shown), a delay of >20 min over the cell that carries two univalents.
DISCUSSION
We have shown that the presence of a relatively high number of misaligned chromosomes or a severe disruption of the meiotic spindle in Drosophila spermatocytes results in a significant delay in the time of entry into anaphase I. These observations provide the first direct evidence substantiating the activity of a meiotic spindle checkpoint in male Drosophila. To date, the only spindle checkpoint component of Drosophila that has been characterized in some detail is bub1 (Basu et al., 1999). There is only one mutant allele of this gene, produced by the insertion of a P-element into the 5′-untranslated leader of the bub1 RNA, 48 bp upstream of the initiator ATG. Molecular and genetic analysis suggests that this insertion results in a severe loss-of-function of the bub1 gene. Homozygous bub1 larvae present a variety of mitotic defects including chromatin bridges and lagging chromatids during anaphase, chromosome fragmentation and aneuploidy. Moreover, bub1 mutant cells cannot maintain sister chromatid cohesion when the spindle is disrupted. Unfortunately, bub1 homozygous testes are very small and contain a reduced number of meiotic figures. Due to these limitations, we have, so far, been unable to record meiosis progression in bub1 mutant spermatocytes. The few meiotic figures that have been observed displayed severe spindle abnormalities during metaphase and anaphase, and multiple nuclei of variable volume in telophase (Basu et al., 1999).
Colchicine treatment or the presence of four univalent chromosomes delays anaphase I onset in Drosophila spermatocytes by an average of 16 min. This represents a 40% increase in the normal duration of prometaphase. This is a relatively moderate delay, but falls within the range of what has been observed in other cell types. The stringency of the spindle checkpoint is rather variable among different cell lines (Kung et al., 1990; Rieder and Palazzo, 1992). For instance, HeLa S3 cells, one of the most extreme cases, remain arrested in mitosis with elevated levels of cyclinB/p34cdc2 for days until they eventually die. Likewise, mantid spermatocytes that contain a free X chromosome are blocked in meiosis I and do not form sperm (see reference quoted in Nicklas, 1997). Nevertheless, many animal cells, including cells from newts, sea urchins, rodents and humans, are only transiently arrested and can even go through one or more cell cycles in the presence of colchicine or similar drugs (Rieder and Palazzo, 1992). The extent of this transient arrest ranges from several-fold to just a fraction of the normal duration of prometaphase (Kung et al., 1990).
It is difficult to estimate what the actual contribution of the delay observed in Drosophila spermatocytes may be in terms of preventing errors in chromosome segregation. On the one hand, such delay is not sufficient to prevent the differentiation of aneuploid spermatids when compound chromosomes are present. On the other hand, it may facilitate the correct orientation of misaligned chromosomes under normal conditions, thus contributing to reduce the incidence of chromosome missegregation in wild-type flies. In the case of mitotic cells, it has been proposed that a transient arrest could have beneficial consequences because it would allow a chance for survival, as opposed to the certain death faced by a permanently arrested cell (Rudner and Murray, 1996). This reasoning may not apply to germ line cells where loosing a fraction of the sperm may be more advantageous than generating aneuploid offspring (Nicklas, 1997).
Due to the possible contribution of unknown variations in the genetic background of the two stocks that were analysed, we cannot draw any general quantitative conclusions regarding the sensitivity of the monitoring system that we have observed. Nevertheless, it seems clear that, under the conditions used in our assay, the monitoring mechanism is able to respond to the presence of four univalents, but it is not able to detect two. As with the length of the delay, the sensitivity of the spindle checkpoint monitoring mechanism is very variable among different cell types. In some systems, the presence of a single kinetochore that is not attached to the spindle is able to activate the checkpoint (Gorbsky, 1995; Wells, 1996; Nicklas, 1997). In contrast, sea urchin zygotes, that stay almost 3-fold longer in mitosis when the spindle is depolymerized by colcemid treatment, initiate anaphase at the same time as the controls when 50% of the chromosomes (approximately 20) are unattached or mono-oriented (Sluder et al., 1994).
It has been proposed (McKee, 1998) that Drosophila spermatocytes may have a spindle checkpoint that, when triggered, results in a general disabling of the spermatids that result from affected spermatocytes (McKee, 1998; McKee et al., 1998). According to this hypothesis, this checkpoint would be sensitive to the same type of meiotic errors that the checkpoint in grasshopper and mantid spermatocytes detect, and thus would guard against the transmission of aneuploid gametes. However, instead of triggering a ‘wait anaphase’ response it would trigger a pathway leading to disability of the resulting spermatids. Although this hypothesis accounts well for the observations regarding the sex chromosomes’ meiotic drive it does not explain the behaviour of translocations between autosomes (McKee, 1998), nor does it account for the seemingly normal viability of spermatids carrying compound arm chromosomes.
In conclusion, our observations suggests that a spindle checkpoint, defined as a mechanism that delays anaphase when chromosomes are not properly aligned on the spindle, operates in Drosophila spermatocytes. This checkpoint does not necessarily require a total breakdown of the meiotic spindle to become active. It can also sense the presence of misaligned chromosomes, although a minimum number of these seem to be required. These observations provide a functional assay to assess the role of the Drosophila proteins that are homologues of known checkpoint components in other organisms.
METHODS
Fly stocks. Control and colchicine-treated spermatocytes were w1118; P[His2AvDGFP, w+]. P[His2AvDGFP] is a transposable element inserted in the third chromosome that expresses a fusion between His2A and green fluorescent protein (GFP) (Clarkson and Saint, 1999). The His2–GFP fusion protein localizes to the chromosomes through male meiosis. To study the effect of univalents on the checkpoint, we examined meiotic timing in spermatocytes of flies with the genotype w; C(3L), P[His2AvDGFP]; C(3R), es, which carry two compound chromosomes (and thus two univalents) and flies with the genotype y2; C(2L),dp; C(2R),px; C(3L),h; C(3R), which carry four compound chromosomes (i.e. four univalents). The C(3L), P[His2AvDGFP] chromosome was derived from the third chromosome of the stock used as the source of control flies by standard genetic procedures (Ashburner, 1989).
Spermatocyte culture, microscopy and timing. Spermatocytes were cultured as described by Church and Lin (1985) with minor modifications. Cultures were kept at 24°C ± 1 with a Bioptechs objective-temperature controller. Fluorescence and phase-contrast observations were made with a Leica DM IRB/E microscope equipped with a 63×/1.32 objective. Phase-contrast allows the identification of the major cytological landmarks of meiosis progression in these cells, including spindle position and shape which are revealed by the phase-dark parafusorial membranes that decorate the spindle throughout meiosis (Tates, 1971). Fluorescence microscopy was used to unequivocally identify the chromosomes that were labelled with a His2–GFP fusion protein. Time-lapse images were captured with a Cohu camera at a rate of 20 frames/min. Time measurements were taken from the time at which the first signs of chromosome condensation could be observed. The onset of chromosome condensation is fairly conspicuous and is characterized by the appearance of phase-dark chromatin structures and the shrinkage of the nucleus. It also correlates with the first stages of clustering of the fluorescence signal produced by the GFP–His2 fusion protein. Colchicine treatment was carried out by feeding young male flies with a colchicine solution as described by Theurkauf et al. (1993).
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Tony Hyman, Juan Mata, Salud Llamazares, Pilar Arana and Claudio Sunkel for their comments and suggestions and to Bob Saint for the flies expressing the His2–GFP fusion protein. E.R. is supported by a Marie Curie postdoctoral research fellowship. Work in our laboratory is partially supported by an EU TMR grant
REFERENCES
- Ashburner M. (1989) Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Basu J., Logarinho, E., Herrmann, S., Bousbaa, H., Li, Z., Chan, G.T.K., Yen, T.J., Sunkel, C.E. and Goldberg, M.L. (1998) Localization of the Drosophila checkpoint control protein Bub3 to the kinetochore requires Bub1 but not Zw10 or Rod. Chromosoma, 107, 376–385. [DOI] [PubMed] [Google Scholar]
- Basu J., Bousbaa, H., Logarinho, E., Li, Z., Williams, B.C., Lopes, C., Sunkel, C.E. and Goldberg, M.L. (1999) Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol., 146, 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church K. and Lin, H.-P.P. (1985) Kinetochore microtubules and chromosome movement during prometaphase in Drosophila melanogaster spermatocytes studied in life and with the electron microscope. Chromosoma, 92, 273–282. [DOI] [PubMed] [Google Scholar]
- Church K. and Lin, H.-P.P. (1988) Drosophila: A model for the study of aneuplopidy. In Aneuploidy, part B. Alan R. Liss, Inc., pp. 227–255.
- Clarkson M. and Saint, R.A. (1999) His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol., 18, 457–462. [DOI] [PubMed] [Google Scholar]
- Goldstein L.S.B. (1980) Mechanism of chromosome orientation revealed by two meiotic mutants in Drosophila melanogaster. Chromosoma, 78, 79–111. [DOI] [PubMed] [Google Scholar]
- Gorbsky G.J. (1995) Kinetochores, microtubules and the metaphase checkpoint. Trends Cell Biol., 5, 143–149. [DOI] [PubMed] [Google Scholar]
- Kerrebrock A.W., Miyazaki, W.Y., Birnby, D. and Orr-Weaver, T.L. (1992) The Drosophila mei-S332 gene promotes sister chromatid cohesion in meiosis following kinetochore differentiation. Genetics, 130, 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung A.L., Sherwood, S.W. and Schimke, R.T. (1990) Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc. Natl Acad. Sci. USA, 87, 9553–9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-P.P. and Church, K. (1982) Meiosis in Drosophila melanogaster. III. The effect of orientation disruptor (ord) on gonial mitotic and meiotic divisions in males. Genetics, 102, 751–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee B.D. (1998) Pairing sites and the role of chromosome pairing in meiosis and spermatogenesis in male Drosophila. Curr. Top. Dev. Biol., 37, 77–115. [DOI] [PubMed] [Google Scholar]
- McKee B.D., Wilhelm, K., Merrill, C. and Ren, X. (1998) Male sterility and meiotic drive associated with sex chromosome rearrangements in Drosophila. Role of X-Y pairing. Genetics, 149, 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki W.Y. and Orr-Weaver, T.L. (1992) Sister-chromatid misbehavior in Drosophila ord mutants. Genetics, 132, 1047–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R.B. (1997) How cells get the right chromosomes. Science, 275, 632–637. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Ward, S.C. and Gorbsky, G.J. (1995) Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol., 130, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L. and Palazzo, R.E. (1992) Colcemid and the mitotic cycle. J. Cell Sci., 102, 387–392. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Schultz, A., Cole, R. and Sluder, G. (1994) Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol., 127, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner A.D. and Murray, A.W. (1996). The spindle assembly checkpoint. Curr. Opin. Cell Biol. 8, 773–780. [DOI] [PubMed] [Google Scholar]
- Scaërou F., Aguilera, I., Saunders, R., Kane, N., Blottiere, L. and Karess, R. (1999) The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J. Cell Sci., 112, 3757–3768. [DOI] [PubMed] [Google Scholar]
- Sluder G., Miller, F.J., Thompson, E.A. and Wolf, D.E. (1994) Feedback control of the metaphase-anaphase transition in sea urchin zygotes: role of maloriented chromosomes. J. Cell Biol., 126, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tates A.D. (1971) Cytodifferentiation during spermatogenesis in Drosophila melanogaster: an electron microscopy study. S-Gravenhage: Drukkerij, J.H. Pasmans.
- Theurkauf W.E., Alberts, B.M., Jan, Y.N. and Jongens, T.A. (1993). A central role for microtubules in the differentiation of Drosophila oocytes. Development, 118, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Wells W.A.E. (1996). The spindle assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol., 6, 228–234. [DOI] [PubMed] [Google Scholar]
- Williams B.C., Gatti, M. and Goldberg, M.L. (1996) Bipolar spindle attachments affect redistributions of ZW10, a Drosophila centromere/kinetochore component required for accurate chromosome segregation. J. Cell Biol., 134, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]