Abstract
Aortic dissection is a life-threatening condition for which diagnosis mainly relies on imaging examinations, while reliable biomarkers to detect or monitor are still under investigation. Recent advances in technologies provide an unprecedented opportunity to yield the identification of clinically valuable biomarkers, including proteins, ribonucleic acids (RNAs), and deoxyribonucleic acids (DNAs), for early detection of pathological changes in susceptible patients, rapid diagnosis at the bedside after onset, and a superior therapeutic regimen primarily within the concept of personalized and tailored endovascular therapy for aortic dissection.
Keywords: Aortic dissection, Biomarker, Protein, Cell-free DNA, miRNA, Inflammation, Lipid-metabolism
Introduction
Aortic dissection (AD) is caused by a proximal tear in the intimal layer of the aorta or bleeding of vasa vasorum within the aortic wall, which contributes to and perpetuates the separation of the layers of the aortic wall. The intimal flap extends in both antegrade and retrograde directions and progresses to affect side-branch arteries. Normal blood flowing into the false lumen causes symptoms of ischemia and complications such as internal organ malperfusion, aortic valve insufficiency, heart failure, cardiac tamponade, and death.[1] The mortality of acute AD increases by 1%–3% per hour before the intervention or medical treatment and reaches up to 21% for 1 day and 74% for 7 days.[2] AD is likely to be misdiagnosed or overdiagnosed because of its rarity and concomitant comorbidities that often mask the primary symptoms.
Currently, the diagnosis of AD relies on imaging examinations, such as computed tomography angiography (CTA), which provides an in-depth understanding of the anatomical structure of the aortic wall with the advantages of being easily available, having a shorter scanning time, and being able to evaluate the whole aorta and branch vessels.[3] However, due to the large radiation dose of enhanced computed tomography (CT), contrast agent-induced nephropathy, and failure to provide an aortic function or dynamic evaluation, imaging can be applied only under certain circumstances. Moreover, patients with an increased genetic risk of AD may not present aortic dilatation, which restricts imaging examination as a means of a screening method for AD in the general population, making it more necessary to develop valuable early stage biomarkers for AD.
The holy grail of biomarkers is to reliably screen high-risk patients, rapidly identify or exclude this disease, and accurately assess disease prognosis during follow-up in a cost-effective and resource-efficient manner, and even brings the entire field of AD management into the mainstream of chronic disease management.[4] Broadly speaking, candidate biomarkers include proteins, ribonucleic acids (RNAs), and deoxyribonucleic acids (DNAs), the majority of which are still under preclinical investigation. This article aims to summarize these candidate biomarkers, elucidate their roles in AD diagnosis, progression prediction, and prognostic evaluation, and anticipate their transformation into clinical practice.
Protein as a Biomarker of Diagnosis and Prognosis in AD
Over the past few years, biomarker-selecting methods have changed from those based on the understanding of pathogenesis to a now common alternative method through a series of proteomics screening techniques, including gel electrophoresis, mass spectrometry, and multiple antibody array techniques. In the wake of advanced proteomics technologies providing unexampled opportunities to accelerate the discovery of biomarkers at the molecular level, cytokines, enzymes, and cytoskeletal proteins are found to actively participate in the pathophysiological condition at the early stage or acute phase of AD, hence, are proposed as reliable biomarkers for diagnosis and prognosis [Table 1].
Table 1.
Summary of the sensitivities and specificities of biomarkers alone or in combination in AD at the cut-off values with the respective time windows.
| Biomarkers | Concentration | Time (h) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| D-dimer for diagnosis | 500 ng/mL | 24 | 97 | 59 |
| 1600 ng/mL | 6 | 100 | NR | |
| D-dimer for in-hospital mortality | 5.92 μg/mL | NR | 87.19 | 64.70 |
| IL-6 | 18.36 pg/mL | NR | 87.4 | 70.8 |
| IL-10 | 20 ng/L | NR | 55 | 98 |
| CRP for in-hospital death | 14.30 mg/L | NR | 87.10 | 53.85 |
| CP | 36.82 mg/dL | NR | 90.6 | 92.9 |
| sST2 | 34.6 ng/mL | NR | 99.1 | 84.9 |
| 40.0 ng/mL | NR | 87.7 | 91.3 | |
| Combination of ANGPTL8, hs-CRP, and D-dimer | NR | NR | 98.46 | 79.49 |
| sLOX-1 | 150 pg/mL | NR | 89.5 | 94.3 |
| smMHC | 2.5 ng/mL | 3/12/24 | 90.9/90/85 | 98/97/97 |
| 10 ng/mL | NR | NR | 100 | |
| Calponin | ||||
| Acidic calponin | 2.3 ng/mL | 6/24 | 50/58 | 87/72 |
| Basic calponin | 159 ng/ml | 6/24 | 63/50 | 73/66 |
| Polycystin | 357.33 pg/mL | 85.7 | 75.6 | |
| Calcium-binding protein S100 | 1.10 ng/mL | NR | 84.4 | 85.5 |
| IMA | 79.35 U/mL | 24 | 80.6 | 84.8 |
| MMP8 | 3.6 ng/mL | NR | 100.0 | 9.5 |
| MMP9 | 20 ng/mL | NR | 96.2 | 16.2 |
| sELAF | NR | NR | 64.0 | 98.8 |
| 97.07ng/mL | NR | 82.86 | NR | |
| sELAF for open or partially open pseudolumen | 285.4 ng/mL | NR | 88.9 | 99.8 |
| TNC for in-hospital death | >103.4 ng/mL | NR | 83.87 | 83.33 |
| Combination of TNC and D-dimer | NR | NR | 90.30 | 86.6 |
| ACAN | 14.3 ng/mL | NR | 81 | 97 |
| miR-15a | NR | NR | 75.7 | 100 |
| miR-23a | NR | NR | 91.9 | 85.7 |
| let7b | NR | NR | 79.4 | 92.9 |
| US33-5p | NR | NR | 73.5 | 85.7 |
| 4-miRNA panel | NR | Within 48 h | 93.33 | 86.67 |
| miR-25 | 1.353 | after onset | 86.67 | 93.33 |
| miR-29a | 1.354 | NR | 93.33 | 93.33 |
| miR-155 | 1.457 | NR | 73.33 | 86.67 |
| miR-26b | 0.500 | NR | 73.33 | 86.67 |
| circMARK3 | 1.497 | NR | 90.0 | 86.7 |
| circMARK3 and miR-1273-3p | 0.4807 | NR | 93.3 | 86.7 |
| DNA methylation pattern | NR | NR | 86 | 75 |
ACAN: Aggrecan; AD: Aortic dissection; ANGPTL8: Angiopoietin Like 8; CP: Ceruloplasmin; CRP: C-reactive protein; DNA: Deoxyribonucleic acid; FC: Fold change; hs-CRP: High sensitive C-reactive protein; IL: Interleukin; IMA: Ischemia-modified albumin; LDL: Low-density lipoprotein; miRNA: MicroRNA; MMP: Matrix metalloproteinase; NR: Not reported; RNA: Ribonucleic acid; sELAF: Soluble elastin fragments; sLOX-1: Soluble form of lectin-like oxLDL receptor 1; smMHC: Smooth muscle myosin heavy chain; sST2: Soluble suppression of tumorigenesis-2; TNC: Tenascin-C.
D-dimer
D-dimer, originally found as a fibrin degradation product, is elevated in the blood during active fibrosis in clinically common diseases such as deep vein thrombosis, myocardial infarction, cerebral infarction, pulmonary embolism, and malignant tumors. It was not until 2009, however, that proof for this hypothesis was provided in the prospective multicenter International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) study, which demonstrated that the cut-off level of 500 ng/mL, which was widely used to rule out pulmonary embolism, can also accurately exclude AD during the first 24 h after onset with a sensitivity of 97%, specificity of 59%, negative and positive likelihood ratios of 0.06 and 2.58, respectively [Table 1].[5] Acute AD was considered to be excluded with a sensitivity of 100%, when D-dimer levels were <100 ng/mL.[6] In addition, elevated D-dimer levels >1600 ng/mL would rule in AD within the first 6 h after onset.[5] The aortic dissection detection risk score (ADD-RS) was historically proposed as a primary screening tool with a high sensitivity of 95.7%,[7] while D-dimer was further introduced as a secondary marker for patients with low ADD-RS. A study from Italy verified that negative D-dimer levels, which was <500 ng/mL, had a sensitivity of 100% to rule out AD patients with the ADD-RS of 0.[8]
In addition, D-dimer presented a sensitivity of 93.5%, and a specificity of 63.2% for the detection of patients with the acute aortic syndrome (AAS), with a negative predictive value (NPV) of 98.9%.[9] The diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes (ADvISED) study showed a similar result: a positive D-dimer result had an overall sensitivity of 96.7% and a specificity of 64% to diagnose AAS. The combination of intermediate and low ADD-RS and D-dimer levels <500 ng/mL showed a failure rate of 3.3%, whereas D-dimer was not discriminatory in patients with a higher ADD-RS.[10]
Nevertheless, owing to the short biological half-life (<8 h), it has limited power to detect subacute or chronic AD, which might prevent it from being widely used in the clinic.
Recent studies have confirmed that increased D-dimer levels following endovascular repair in the thoracic aorta were associated with less overall survival and more severe complications.[11] The sensitivity and specificity for prediction of in-hospital mortality were 87.19% and 64.70%, respectively, when D-dimer was over 5.92 μg/mL [Table 1].[12] The average D-dimer level at 9 μg/mL from before to after intervention treatment during hospitalization was an independent risk factor for in-hospital death.[13] Although not statistically significant, the IRAD-Bio study also found postoperative false lumen patency in type A dissections to be associated with higher level of D-dimer.[5] In conclusion, the significance of D-dimer as a prognostic marker for AD appears in the near future.
Inflammatory markers
The initial focus on the comprehension of what roles inflammatory responses and factors play in cardiovascular diseases arose from the observation and experiment that many facets of phenotypes could be simulated by the known biological effects of proinflammatory cytokines. Increasing evidence indicates that the innate immune response pathway consisting of the inflammasome NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3), interleukin (IL)-1 to IL-6, the C-reactive protein (CRP), and fibrinogen derived from liver was involved in cardiovascular disorders and thus that specific inflammatory cytokines might have a profound role in their early diagnosis.
IL-6
IL-6 was a crucial cytokine of innate immune responses that participates in a series of physiological or pathophysiological conditions related to immune cell proliferation, differentiation, and regulation. Circulating IL-6 levels in patients with acute AD were considerably increased than those in normal controls.[14,15,16,17] Post-implantation syndrome (PIS), which occurred in 15.8% of patients after endovascular treatment, was associated with higher peak value of IL-6 than non-PIS patients.[18] Furthermore, the IL-6 level in plasma of the non-survival AD group was higher than that of the survival group. The cut-off value of the IL-6 level for the prediction was 18.36 pg/mL, with a sensitivity and a specificity of 87.4% and 70.8%, respectively [Table 1].[19] A similar trend was reported in which the IL-6 level and tumor necrosis factor α (TNF-α) level in plasma were significantly elevated in AD. The time intervals of the levels of IL-6 and TNF-α increasing to peak were shorter than those of CRP.[20] The dynamically changed IL-6 levels in AD climbed steadily after onset and peaked 1–2 days, followed by a gradual slide to normal range during the next 2 months.[14] The relatively wide time window improves its diagnostic and prognostic accuracy in clinical applications.
IL-10
IL-10 concentrations were confirmed six to seven times higher in AD plasmas than all other diagnoses, such as thoracic aortic aneurysm (TAA), acute myocardial infarction (AMI), or pulmonary embolism (PE), collectively. IL-10 individually presented a superior performance for AD diagnosis with a sensitivity of 55.0% and a specificity of 98.0%, with the cut-off value of 20 ng/L [Table 1], enabling itself to be a potential biomarker to AD and accurately discriminate suspected AD, AMI, and PE.[21]
CRP
CRP, stimulated by several cytokines in the acute phase of inflammation, was recommended as the first choice of inflammation marker in clinical diagnosis and treatment. Studies have pointed toward a clear association between plasma CRP levels at hospital admission and long-term adverse events in patients with AD. The short-term mortality significantly increased as the CRP value exceeded 6.3 mg/L.[22] When CRP exceeded 14.30 mg/L, the sensitivity and specificity for forecasting in-hospital mortality were 87.10% and 53.85%, respectively.[12] In addition, the CRP level over 15 mg/L was a vital risk factor for poor prognosis [Table 1].[23]
Ceruloplasmin (CP)
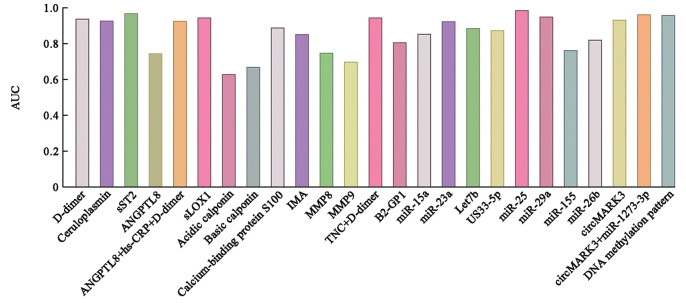
CP, a multicopper oxidase family member, is mainly synthesized and largely released in acute phase reactions.[24] A previous study pointed out that though CRP was regarded as the gold standard, serum CP might be helpful in detecting and monitoring chronic inflammation, and elevated CP levels are associated with an increased incidence of abdominal aorta aneurysm,[25] heart failure,[26] atrial fibrillation,[27] as well as cardiovascular risk in chronic dialysis.[28] Serum CP evidently increased and was positively related to CRP or platelet levels. The area under the curve (AUC) for the diagnosis of AD was 0.929 at the cut-off value of 36.82 mg/dL, with a sensitivity of 90.6% and a specificity of 92.9% [Table 1 and Figure 1]. Furthermore, serum CP was increased in cases with thrombosed false lumen than those with patent false lumen cases, suggesting that higher CP exposed patients to raised risk of thrombosed false lumen. Therefore, CP can act as a candidate biomarker for the diagnosis of AD and the risk factor for thrombosed false lumen.[29]
Figure 1.
The AUC of biomarkers for the diagnosis of AD. AD: Aortic dissection; ANGPTL 8: Angiopoietin Like 8; AUC: Area under the curve; GP1: glycoprotein1; hs-CRP: High sensitive C-reactive protein; IMA: Ischemia-modified albumin; LDL: Low-density lipoprotein; miR: MicroRNA; MMP: Matrix metalloproteinase; RNA: Ribonucleic acid; sLOX-1: Soluble form of lectin-like oxLDL receptor 1; sST2: Soluble suppression of tumorigenesis-2; TNC: Tenascin-C.
Soluble suppression of tumorigenesis-2 (sST2)
sST2 was primarily involved in inflammatory processes and T-cell-mediated immune responses. As a soluble cytokine receptor with a large molecular weight, sST2 was quite stable in the circulation and was considered a more precise biomarker of inflammation. Wang et al[30] corroborated that sST2 was a more valuable biomarker than D-dimer to exclude the diagnosis of AD. sST2 showed increased levels in acute AD than either in myocardial infarction or in pulmonary embolism. sST2 at a cut-off level of 34.6 ng/mL yielded a sensitivity of 99.1% and a specificity of 84.9% [Table 1], as well as positive predictive value (PPV) and NPV of 68.7% and 99.7%, respectively, with the area under curve (AUC) of 0.97 for sST2 [Figure 1]. It was also reported that sST2 showed the highest accuracy of 90.1% with a sensitivity of 87.7% and a specificity of 91.3%, and a positive predictive value of 77.1% and a positive likelihood ratio of 10.1% at a cutoff level of 40 ng/mL, which might also support a role in positive prediction [Figure 1].The level of sST2 peaked at approximately 24 h after symptom onset.
Adipokine adiponectin
There was growing evidence that the perivascular adipose tissue surrounding the vasculature played a role in modulating inflammation and vascular tone via the regional release of adipokines.[31] The pleiotropic adipokine adiponectin, such as angiopoietin like 8 (ANGPTL8) and ANGPTL2, down-regulated the expression of inflammatory cytokines in abdominal aortic aneurysm.[32,33,34] Increasing ANGPTL8 levels in the blood resulted in a higher odds ratio as an independent risk factor for AD and were positively correlated with hs-CRP and aortic diameter. The AUC for ANGPTL8 was 0.746, while the AUC for the combination of ANGPTL8, D-dimer, and hs-CRP was 0.927, with a sensitivity of 98.46% and a specificity of 79.49% [Table 1 and Figure 1].[32]
Lipid metabolism-related markers
Low-density lipoprotein (LDL)
LDL played an active role in promoting foam cell formation, and inducing proliferation, migration, and phenotypic switching of vascular smooth muscle cell in the initiation and progression of atherosclerosis.[35] A soluble form of lectin-like oxLDL receptor 1 (sLOX-1) was generated by being cleaved at the membrane-proximal extracellular domain from LOX-1, the major oxLDL receptor located in the endothelial cells. A majority of studies had confirmed the performance of sLOX-1 in terms of determining the severity of stable coronary artery disease and acute coronary syndrome.[36,37,38,39] sLOX-1 levels was considerably higher in the AD and acute coronary syndrome (ACS) patients than in control and that in AD group were even substantially higher compared with the ACS group. The AUC for sLOX-1 at optimal threshold of 150 pg/mL to differentiate AD from ACS was 0.946 [Figure 1], with a sensitivity of 89.5% and a specificity of 94.3% [Table 1], while that of cardiac troponin T (cTnT) was merely 0.580.[40] Further multicenter studies are required to identify the diagnostic accuracy for sLOX-1 and in combination with D-dimer in large sample sizes.
Lysophosphatidylcholine (LPC)
LPC was a fundamental cell signaling molecule that is produced through the phospholipase A2 acting on phosphatidylcholine. Sphingomyelins (SM) and ceramides were interrelated through the"sphingomyelinase-ceramide pathway", and the latter were products of SM hydrolysis under the action of the enzyme sphingomyelinase.[41] The sphingomyelinase-ceramide pathway generally contributes to pro-inflammatory, pro-oxidative, and pro-apoptotic processes, such as promoting vascular smooth muscle cell calcification,[42,43] leading to atherosclerosis, aging, and other cardiovascular events.[44,45,46] SMs, typically SM C16:0 and SM C24:1 predominated in the normal blood.[47] A general increase in the amount of total SM levels in AD was uncovered through the metabolic profiles of ascending thoracic aortic wall tissue.[48] Meanwhile, LPCs and sphingolipids, including sphingosine, phytosphingosine, SM, and ceramide, were identified to differ between the AD groups and the control.[49] LPC levels were remarkably diminished in both the Stanford type A and type B AD, while sphingolipids were only reduced in the Stanford type A AD. The identification of potential biomarkers for the diagnosis of AD and discrimination between type A and type B AD might be facilitated by combining these two families of metabolites.
Homocysteine (Hcy)
Hcy was an intermediate product during the process of the physiologically biosynthesizing the amino acids cysteine and methionine. A clear association between total Hcy and the presence of coronary and peripheral vascular disease had been pointed out in recent studies.[50] Hcy, as an independent risk factor for atherosclerosis,[51] mediates the formation of cardiovascular disease by different mechanisms, including promoting vascular smooth muscle cells (VSMCs) proliferation, triggering endothelial dysfunction and oxidative stress, inducing synthesis and deterioration of collagen, and launching an inflammatory response.[52] A recent study prospectively confirmed the efficacy of Hcy levels in predicting adverse cardiovascular disease events.[53] Patients with aortic dilatation or AD were characterized by higher levels of serum Hcy than those with mild cardiovascular manifestations. Total Hcy was evidently higher in Marfan's syndrome patients who have severe cardiovascular manifestations vs. patients with mild manifestations.[54]
Smooth muscle cell markers
Smooth muscle myosin heavy chain (smMHC), a specific smooth muscle protein, was released from impaired aortic medial smooth muscle cell during the initial and progression of AD and reached a concentration 20 times higher followed by a rapid decline to the normal range. The smMHC value in serum, first reported in AD, greatly increased within 24 h of onset and exceeded 7 ng/mL until 24 h after onset, which was 5–10 times higher than controls, following a substantial decrease to a normal value after 24 h.[55]
Another study showed that the sensitivity and the specificity of the smMHC at a cut-off level of 2.5 ng/mL within the first 12 h was 90% and 97%, respectively [Table 1].[56] While within 3 h after onset, the elevated levels of circulating smMHC showed a sensitivity of 90.9% and a specificity of 98%. Levels of smMHC exceeding 10 ng/mL showed 100% specificity for AD. Furthermore, smMHC level was significantly increased in patients with proximal lesions than in distal lesions, which interestedly showed decreased levels (<2.5 ng/mL). Chances were that smMHC was lower in patients with distal lesions, probably because less smooth muscle exists in the abdominal aorta than in the thoracic aorta. The receiver operating characteristic (ROC) curve confirmed its superior sensitivity for proximal lesions than for distal lesions within 3–6 h after onset and thereafter,[57] presenting a potential value in locating the lesions.
Creatine kinase-BB isozyme
Creatine kinase-BB isozyme, which also reflected aortic smooth muscle damage, was found to be 7- or 8-fold higher in AD patients than in normal controls. It peaked at 6 h after onset with a longer time window than that of smMHC.[58]
Calponin
Calponin, troponin-like protein of smooth muscle, analogous to cardiac troponins present in myocardial ischemia or necrosis had caught the eyes of researchers. Patients with AD have acid and basic calponin levels elevated in both proximal and distal aortic diseases. The acidic calponin at the cut-off point of 2.3 ng/mL and basic calponin with optimum values of 159 ng/mL showed a sensitivity of 50% and specificity of 87%, and a sensitivity of 63% and specificity of 73% during the first 6 h, respectively. Calponin presented a relatively long time, with a sensitivity of 58% and specificity of 72% for acidic calponin, a sensitivity of 50% and specificity of 66% for basic calponin in the initial 24 h, and an acceptable NPV but a disappointing PPV [Figure 1 and Table 1].[5] A meta-analysis involving four studies pointed to a strong relationship between elevated troponin levels, which was present in 26.8% of patients with AD, and a high risk of short-term death during hospitalization.[59] Collectively, calponin exhibited promising potential in the diagnosis of AD and is presently being pursued with hopes of its clinical application and bedside practice.[3]
Polycystin 1 (PC1)
PC1, predominately expressed in both endothelial cells and smooth muscle cells, casts a fundamental role in maintaining the structural stability and functional integrity of the vessel wall.[60,61,62,63] Serum PC1 was increased in patients with AD than in other diseases or healthy subjects, with a sensitivity of 85.7% and a specificity of 75.6%, at the cut-off value of 357.33 pg/mL [Table 1].[64]
Cardiac markers
N-terminal pro-brain natriuretic peptide (NT-proBNP)
NT-proBNP was a well-established diagnostic and prognostic biomarker in patients with heart failure. Although NT-proBNP showed no superiority in the early diagnosis of acute AD in the emergency setting,[65] higher levels of NT-proBNP also were independently associated with in-hospital mortality. NT-proBNP levels >647 pg/mL indicated the occurrence of postoperative heart failure in patients with AD.[66] In addition, a higher sensitivity in predicting in-hospital death was achieved with the combination of NT-proBNP levels and aortic diameters.[67]
Calcium-binding protein S100
Calcium-binding protein S100 had various biological functions and involves in cell proliferation and differentiation, protein phosphorylation, and transcription factor regulation via the regulation of intracellular calcium ions.[68] The calcium-binding protein S100A1, one of the S100 protein families, was reported as a pivotal regulator of myocardial systolic and diastolic functions.[69] S100A1 was regarded as an early diagnostic marker of ischemic coronary artery disease.[70] The concentration of S100A1 was further elevated in patients with AD, typically in AD complicated by aortic regurgitation, pericardial effusion, or in-hospital death. When the plasma concentration of S100A1 was 1.10 ng/mL, the sensitivity for the diagnosis of AD was 84.4%, and the specificity was 85.5% [Table 1].[71]
Ischemia-modified albumin (IMA)
IMA was an extensively investigated diagnostic marker of the early stage of myocardial ischemia in patients.[72] However, IMA lacked diagnostic specificity, resulting in a high proportion of false positives.[73,74] Studies had found elevated IMA in a wide range of diseases, including acute coronary syndrome,[75] chronic liver and kidney diseases,[76] malaria,[77] and preeclampsia.[78] IMA tended to be positively correlated to time from symptom onset at baseline.[79] Patients with poor prognosis exhibited higher levels of IMA within 24 h from the onset, indicating that IMA was an independent risk factor for in-hospital mortality with the best threshold of 79.35 U/mL. The AUC at this IMA level was 0.854, while the sensitivity and specificity to anticipate in-hospital death were 80.6% and 84.8%, respectively [Figure 1 and Table 1].[80]
Extracellular matrix markers
The vessel wall extracellular matrix (ECM) was a dynamic structure, which was instrumental in regulating vascular function in healthy and pathophysiological conditions.[81] The vascular ECM was composed of structural proteins, such as collagens and elastin, and non-structural proteins, including glycoproteins, proteoglycans, growth factors, and proteases. The collagen and elastin remained to be the most principal ECM proteins in the arterial wall, though the composition of ECM varies along the aortic wall layers and longitudinal directions. The balance of structural proteins regulated by proteolytic enzymes matrix metalloproteinases (MMPs) and their inhibitors tissue inhibitors of metalloproteinases (TIMPs) has been a hotspot in the field of aneurysms.[82] Recently, a growing number of studies have focused on the role of non-structural proteins, especially proteoglycans.[83,84,85]
Matrix metalloproteinases (MMPs)
MMPs mainly involved in the aortic remodeling have been extensively studied in the past several years. MMP-2 and TIMP-2 were reported to be significantly lower in the AD group.[85] Whereas the expression of MMP-1 and MMP-9 was increased in patients with aneurysm and AD compared with healthy controls, and higher MMP-2 and MMP-9 expressions were typically recorded in AD than in aneurysm.[86] Increased MMP-9 levels were also observed in the subacute phase of medically treated type B AD.[84] Plasma levels of MMP-8 and MMP-9 were closely related, and both MMP-8 and MMP-9 showed a stronger association with D-dimer. For MMP-8, the AUC for patients with AD was 0.75 vs. all controls, 0.75 vs. aortic aneurysm, 0.67 vs. inflammatory disease, and 0.82 vs. acute coronary syndrome. Plasma MMP-8 had a sensitivity of 100.0% and a specificity of 9.5% at a cut-off of 3.6 ng/mL. For MMP-9, the AUC for patients with AD was 0.70 vs. all controls, 0.77 vs. aortic aneurysm, 0.69 vs. inflammatory disease, and 0.73 vs. acute coronary syndrome. Plasma MMP-9 had a sensitivity of 96.2% and a specificity of 16.2% at a cut-off of 20.0 ng/mL [Table 1]. Furthermore, a combination of MMP-8 and D-dimer increases the AUC of the ROC curve in predicting acute AD, representing a promising marker to accurately rule out AD.[87]
Soluble elastin fragments (sELAF)
sELAF, degraded by proteolytic enzymes, were released into the plasma when a great number of elastic fibers in the vascular wall rupture. Its dynamic change with confounding factors had made it difficult for clinical application in the present.[3] Elastin showed an average diagnostic performance with a sensitivity of 64.0%, a specificity of 98.8%, PPV of 94.1%, and NPV of 98.1% [Table 1]. Another study implied that sELAF had a better diagnostic sensitivity of 82.86% at a cut-off value of 97.07 ng/mL.[64] The elevated plasma sELAF level increased as early as 0.7 h after onset and was sustained for 72 h, which might be considered an effective diagnostic marker. The increased sELAF at the cut-off value of 285.4 ng/mL can effectively predict an open or a partially open false lumen of AD patients with a sensitivity of 88.9% and a specificity of 99.8% [Table 1].[88] A combination of smMHC and sELAF showed high sensitivity and specificity in the diagnosis of AD, but these tests were regarded as not practical in clinical emergency scenarios.[89]
Tenascin-C (TNC)
TNC was an ECM glycoprotein that can be synthesized by a broad set of cell types, exhibiting a main function of de-adhesion and cell proliferation promotion in the response to inflammatory mediators and mechanical stress. Elevated TNC level was identified in aortic aneurysm, pulmonary arterial hypertension, and restenosis patients.[90] TNC levels were positively correlated with the maximal aortic diameter and the degree of histological damage to the aortic wall.[91] A recent study showed that serum TNC levels were increased in patients with AD, and TNC levels were correlated with the peak hs-CRP and D-dimer levels on the seventh day after onset.[92] TNC levels reached the top point at 12–24 h and diminished to normal in the next 24 h. The serum TNC level was significantly higher in non-survivors than survivors, showing the potential of being an independent risk factor in predicting in-hospital death among patients with acute AD. ROC analysis showed that the AUC of TNC was comparable to that of D-dimer and superior to that of CRP in predicting in-hospital death at the cut-off point TNC >103.4 ng/mL, with a sensitivity of 83.87% and specificity of 83.33% [Table 1]. The AUC of TNC combined with D-dimer reached up to 0.946, with a sensitivity of 90.30% and a specificity of 86.60% [Figure 1 and Table 1],[12] indicating that TNC can enhance the ability of D-dimer to evaluate the short-term prognosis of acute AD.
Aggrecan (ACAN)
ACAN, a major proteoglycan, was significantly increased at a 4- to 5-fold higher concentration compared to the controls and maintained elevated levels for 72 h after onset without major fluctuations. The optimum discrimination limit of 14.3 ng/mL resulted in a specificity of more than 97% and a sensitivity of 81%, as well as a PPV and NPV of 72.7% and 98%, respectively [Table 1].[93]
Vinculin
Vinculin was a crucial cytoskeletal protein located at focal adhesions involved in mediating the mechanochemical pathway of the cytoskeleton in cell component and ECM.[94,95] It contributed to abnormal proliferation, adhesion, and vascular smooth muscle cells switching from contractile to the synthetic phenotype. Synthetic VSMCs could secrete excessive MMPs, resulting in an imbalanced state of MMPs and TIMPs and elastin proteolysis in the outer layer of the aortic wall.[96] Vinculin increased significantly among the differential proteins in AD (15.8 ng/mL) than in AMI patients (8.6 ng/mL) than healthy volunteers (5.3 ng/mL). The concentration of vinculin increased swiftly in the early stage after onset (often <12 h) and then remained at a high level for 48 h in patients with AD, indicating a satisfactory time window.[97]
Other proteins
TGF-β
TGF-β was a signaling molecule that binds to fibrillin-1, an ECM protein encoded by the FBN1 gene. The deficiency of fibrillin-1 can affect the overactivation of TGF-β, giving rise to an abnormal TGF-β bioavailability in Marfan patients.[98] The TGF-β level was elevated at 24.5 ng/mL, which was approximately 5-fold higher, in non-Marfan patients within 24 h of symptom onset. Approximately 2-fold elevations were observed in type A AD (28.5 ng/mL) compared with type B AD (14.4 ng/mL).[99]
Osteoprotegerin (OPG) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
An elevated OPG/TRAIL ratio was identified as the best predictor of overall, 30-day, and post-30-day mortality, with a hazard ratio of 2.32. Two cut-off OPG/TRAIL ratio values can categorize the mortality of patients into low-risk (OPG/TRAIL ratio <4) and high-risk (OPG/TRAIL ratio >33).[100]
Beta-2 glycoprotein1 (β2-GP1)
β2-GP1 mainly participated in the immune system through direct interaction with membrane toll-like receptors, promoting activation of endothelial cells and expression of proinflammatory cytokines.[101] The AUC of B2-GP1 for diagnosing AD was 0.81 [Figure 1], indicating that B2-GP1 might be a potential biomarker.[102]
Uric acid
Uric acid was an end-product of purine nucleotide degradation. It was closely related to many conditions, such as hypertension, dyslipidemia, obesity, and impaired glucose metabolism. Uric acid levels were higher in patients with AD than in controls.[103,104,105,106] Moreover, hyperuricemia was reported to be independently associated with the risk of AD.[107,108] In addition, a meta-analysis of seven case–control studies confirmed no significant difference between type A and type B AD.[109]
RNA as a Biomarker of Diagnosis and Prognosis in AD
MicroRNA (miRNA)
miRNA was an endogenous non-coding RNA molecule that orchestrates gene expression at the transcriptional and posttranscriptional levels via targeting mRNA for cleavage or translational repression.[110] In recent years, a plethora of evidence suggested that miRNAs, which were strikingly stable in plasma,[111] played crucial roles in the pathologic processes of cardiovascular diseases.[112,113]
miR-29
The miR-29 family, comprising miR-29a, miR-29b, and miR29c, are essential regulators of ECM homeostasis, with targets in elastin,[114] collagens, and MMPs.[115] miR-29a expression was up-regulated in patients with bicuspid aortic valve-thoracic aneurysm, a disease that progressively enlarges and predisposes tissue to acute AD in the aortic concavity,[116,117,118] while a decline was shown in the convexity with demonstrated regional differences,[117] suggesting that miR-29a might play a role in adapting the thoracic aorta to the environment of hemodynamic stress created by the bicuspid aortic valve. Nevertheless, miR-29a was declined in aortic tissue obtained during open chest surgery both before[119] and after dissection.[120]
miR-30
miR-30 family members were involved in the development of multiple organs and tissues, including the heart, blood vessels, intestinal tract, and malignant tumor. Up-regulated expression of miR-30 promotes the occurrence and progression of AD, which possibly aims at LOX, suggesting a potential role of miR-30a as a strong regulator of LOX in aortic VSMCs.[121]
miR-143/145
miR-143/145 were two of the best-characterized miRNAs in cardiovascular conditions, which collaboratively played a crucial role in VSMC differentiation and phenotype switching.[122,123,124] Contractile VSMC populations in the aorta were maintained, with miR-145 promoting contractile gene expression and miR-143 inhibiting synthetic gene expression,[123] therefore, contributing to structural modifications of the aorta.[120] Recently, Jing et al[89] verified that circulating miRNAs were novel potential biomarkers for the diagnosis of AD.
miRNA panel
Four miRNAs (miR-15a, miR-23a, let7b, and US33-5p) were significantly increased in the AD group compared with the control group. ROC analysis for miR-15a exhibited a sensitivity of 75.7% and specificity of 100%, for miR-23a presented a sensitivity of 91.9% and specificity of 85.7%, for let7b showed a sensitivity of 79.4% and specificity of 92.9%, and for US33-5p revealed a sensitivity of 73.5% and specificity of 85.7%. The AUCs for four miRNAs were 0.855, 0.925, 0.887, and 0.815, respectively.
Another four miRNAs, including miR-25, miR-29a, miR-155, and miR-26b, may also serve as potential biomarkers for diagnosing AD patients. When compared with healthy controls, patients with AD had significantly higher expression of miR-25, miR-29a, and miR-155, while miR-26b was markedly diminished. ROC analysis for miR-25 exhibited a sensitivity of 86.67% and specificity of 93.33%, for miR-29a presented a sensitivity of 93.33% and specificity of 93.33%, for miR-155 showed a sensitivity of 73.33% and specificity of 86.67%, and for miR-26b revealed a sensitivity of 73.33% and specificity of 86.67% [Table 1].The 4-miRNA panel showed a sensitivity of 96%, a specificity of 100%, and an AUC of 0.995, with an optimal cut-off value of 46.50%. In the following single-blind trial, the 4-miRNA panel reliably exhibited a sensitivity of 93.33%, specificity of 86.67%, and AUC of 0.973 [Figure 1].[125]
The differential expression profile of miRNAs yielded a series of upregulated expressed circulating miRNAs, incorporating miR-4313, miR933, miR-1281, and miR-123831, whose accuracy is currently under clinical verification in a large sample of AD patients and controls.[126]
circMARK3
The preliminary landscape of circRNA expression profiles suggested that circMARK3, the upstream regulatory molecule of tyrosineprotein kinase Fgr, was differentially expressed in the occurrence and development of AD. The ROC of serum circMARK3 as a biomarker for AD presented a sensitivity of 90.0%, specificity of 86.7% [Table 1], and AUC of 0.934 at a cut-off value of 1.497 [Figure 1]. The AUC of the combination of circMARK3 and miR-1273-3p was 0.9644, with the cutoff value of 0.4807 and the corresponding sensitivity of 93.3% and specificity of 86.7%, respectively [Figure 1 and Table 1].[127]
Recent studies had highlighted the potential application of non-coding RNA-regulated processes in the pathogenesis of AD. However, comparison of the conclusions among different studies presented a low consistency of differentially regulated miRNAs. There were possible reasons. First, a circulating miRNA profile may differ depending on different confounding factors such as population, sex, presence of comorbidities, medication history, the nature of the sample, and collection methods.[128,129,130] Such confounding factors should be clarified and controlled during study design and statistical analysis to minimize potential bias. Second, it can be difficult to determine whether miRNA up- or down-regulation has a causal effect in terms of AD development or, oppositely, results from a compensatory mechanism to regulate. While animal models that allow detailed mechanistic approaches would provide some insight.
DNA as a Biomarker of Diagnosis and Prognosis in AD
Cell-free DNA (cfDNA) from plasma heralds a revolution in the battle against cancer.[131] It was rapidly emerging as a significant and minimally invasive adjunct to standard tumor biopsies and, in some diseases, even a potential alternative approach.[132] Each cfDNA fragment harbors molecular markers of its cellular origin, such as DNA methylation status.[133]
Although based on a minority of patient samples, recent studies had suggested a high degree of consistency among DNA alterations detected in between arterial walls and plasma samples from the same patient, in which researchers see the potential of blood test to distinguish AD from other diseases. Methylation of the CpG site significantly increased in the promoter of MMP2 in the AD group.[134] The DNA methylation landscape supported that AD was associated with an inflammatory vascular remodeling process and a dedifferentiated smooth muscle cell phenotype.[135] Moreover, a different methylation profile in AD patients who underwent surgery and healthy controls presented numerous differentially methylated regions (DMRs) enriched in the areas of vasculature and heart development. A prediction model was built based on the maximal 50 differentially methylated regions (DMRs) with methylation variance for cfDNA from plasma to evaluate the DNA methylation pattern as a biomarker in AD diagnosis. A high sensitivity of 86% and specificity of 75% [Table 1] were achieved with the AUC of 0.96 [Figure 1].[136] The results suggested the potential of cfDNA leveraging informative methylation patterns to be a non-invasive biomarker for screening heritable thoracic aortic disease, predicting disease before the symptoms fully manifest, indicating the clinical characteristics of subtype classification, and then accordingly guiding the timing of intervention, treatment and prognosis management for individual aortopathy.
These results shed light on the value of genetic diagnosis in hereditary connective tissue diseases. The link between genetic variants and various phenotypes might enhance our ability to stratify individuals based on their genetic profile. Although the time-consuming genetic test might not be suitable for the detection of AD in the acute phase,[67] they were very valuable in predicting patients with high risk and were conducive to determining the etiology of AD and differentially diagnosing hereditary diseases associated with AD, which contributed to AD prevention in future generations.
Conclusion
Biomarkers play an important role in the diagnosis and prognosis of multiple diseases. Recent scientific innovations and cutting-edge technologies have provided unprecedented opportunities to detect key factors involved in the pathogenesis and progression of AD, and an increasing number of biomarkers with potential clinical translational value have been identified. There are no ideal biomarkers available for clinical diagnosis or prognostic assessment of AD currently, and there is an urgent need for multicenter, large-sample randomized clinical trials to provide evidence for clinical practice with the aim of monitoring pathological changes in genetically susceptible patients in the pre-disease stage, rapidly confirming or ruling out disease at early onset, predicting disease progression, assessing disease prognosis, and providing personalized treatment strategies for endovascular treatment of AD.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81970412) and the Science and Technology Innovation Plan of the Shanghai Science and Technology Commission (No. 18441902400).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhao YF, Fu WG, Wang LX. Biomarkers in aortic dissection: Diagnostic and prognostic value from clinical research. Chin Med J 2024;137:257–269. doi: 10.1097/CM9.0000000000002719
References
- 1.Nienaber CA Clough RE Sakalihasan N Suzuki T Gibbs R Mussa F, et al. Aortic dissection. Nat Rev Dis Primers 2016;2: 16053. doi: 10.1038/nrdp.2016.53. [DOI] [PubMed] [Google Scholar]
- 2.Hirst AE, Jr., Johns VJ, Jr., Kime SW, Jr. Dissecting aneurysm of the aorta: A review of 505 cases. Medicine (Baltimore) 1958;37: 217–279. doi: 10.1097/00005792-195809000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T Lyon A Saggar R Heaney LM Aizawa K Cittadini A, et al. Editor's choice-Biomarkers of acute cardiovascular and pulmonary diseases. Eur Heart J Acute Cardiovasc Care 2016;5: 416–433. doi: 10.1177/2048872616652309. [DOI] [PubMed] [Google Scholar]
- 4.Dalman RL, Wanhainen A, Mani K, Modarai B. Top 10 candidate aortic disease trials. J Intern Med 2020;288: 23–37. doi: 10.1111/joim.13042. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T Distante A Zizza A Trimarchi S Villani M Salerno Uriarte JA, et al. Diagnosis of acute aortic dissection by D-dimer: The international registry of acute aortic dissection substudy on biomarkers (IRAD-Bio) experience. Circulation 2009;119: 2702–2707. doi: 10.1161/CIRCULATIONAHA.108.833004. [DOI] [PubMed] [Google Scholar]
- 6.Sodeck G Domanovits H Schillinger M Ehrlich MP Endler G Herkner H, et al. D-dimer in ruling out acute aortic dissection: A systematic review and prospective cohort study. Eur Heart J 2007;28: 3067–3075. doi: 10.1093/eurheartj/ehm484. [DOI] [PubMed] [Google Scholar]
- 7.Rogers AM Hermann LK Booher AM Nienaber CA Williams DM Kazerooni EA, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation results from the international registry of acute aortic dissection. Circulation 2011;123: 2213–2218. doi: 10.1161/circulationaha.110.988568. [DOI] [PubMed] [Google Scholar]
- 8.Nazerian P Morello F Vanni S Bono A Castelli M Forno D, et al. Combined use of aortic dissection detection risk score and D-dimer in the diagnostic workup of suspected acute aortic dissection. Int J Cardiol 2014;175: 78–82. doi: 10.1016/j.ijcard.2014.04.257. [DOI] [PubMed] [Google Scholar]
- 9.Gorla R Erbel R Kahlert P Tsagakis K Jakob H Mahabadi AA, et al. Accuracy of a diagnostic strategy combining aortic dissection detection risk score and D-dimer levels in patients with suspected acute aortic syndrome. Eur Heart J Acute Cardiovasc Care 2017;6: 371–378. doi: 10.1177/2048872615594497. [DOI] [PubMed] [Google Scholar]
- 10.Nazerian P Mueller C Soeiro AM Leidel BA Salvadeo SAT Giachino F, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes: The ADvISED prospective multicenter study. Circulation 2018;137: 250–258. doi: 10.1161/CIRCULATIONAHA.117.029457. [DOI] [PubMed] [Google Scholar]
- 11.Iyano K, Kawada T, Aiba M, Takaba T. Correlation of hemostatic molecular markers and morphology of the residual false lumen in chronic aortic dissection. Ann Thorac Cardiovasc Surg 2004;10: 106–112. [PubMed] [Google Scholar]
- 12.Guo T, Zhou X, Zhu A, Peng W, Zhong Y, Chai X. The role of serum tenascin-C in predicting in-hospital death in acute aortic dissection. Int Heart J 2019;60: 919–923. doi: 10.1536/ihj.18-462. [DOI] [PubMed] [Google Scholar]
- 13.Gorla R Erbel R Kahlert P Tsagakis K Jakob H Mahabadi AA, et al. Diagnostic role and prognostic implications of D-dimer in different classes of acute aortic syndromes. Eur Heart J Acute Cardiovasc Care 2017;6: 379–388. doi: 10.1177/2048872615594500. [DOI] [PubMed] [Google Scholar]
- 14.Zhong MH, Jun GU, Zhang EY. Clinical significances of plasma interleukin-6, C-reaction protein and tumor necrosis factor-alpha in patients with aortic dissection (in Chinese). Sichuan Da Xue Xue Bao Yi Xue Ban 2015;46: 234–237. doi: 10.13464/j.scuxbyxb.2015.02.015. [PubMed] [Google Scholar]
- 15.Gu J Hu J Qian H Shi Y Zhang E Guo Y, et al. Intestinal barrier dysfunction: A novel therapeutic target for inflammatory response in acute Stanford type A aortic dissection. J Cardiovasc Pharmacol Ther 2016;21: 64–69. doi: 10.1177/1074248415581176. [DOI] [PubMed] [Google Scholar]
- 16.Wu HJ Zhang W Shu YW Fan H Li H Zeng QT, et al. Comparison of plasma pro-inflammatory cytokine expressions in patients with different types of acute aortic dissection (in Chinese). Chin Crit Care Med 2014;26: 10. doi: 10.3760/cma.J.isn.2095-4352.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Yang L. Practical significance of D-dimer in the diagnosis of acute aortic dissection Stanford type A (in Chinese). Chin J Esth Med 2012;21: 1. doi: 10.3969/j.issn.1008-6455.2012.z1.071. [Google Scholar]
- 18.Gorla R Erbel R Kahlert P Tsagakis K Jakob H Mahabadi AA, et al. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur J Cardiothorac Surg 2016;49: 1239–1247. doi: 10.1093/ejcts/ezv355. [DOI] [PubMed] [Google Scholar]
- 19.Qu H, Zhu J, Li X, Yang Y. Predictive value of interleukin-6 for mortality in patients with acute aortic dissection (in Chinese). Chin J Clinicians 2015;9: 17. doi: 10.3877/cma.j.issn.1674-0785.2015.17.008. [Google Scholar]
- 20.Gu J Hu J Zhang HW Xiao ZH Fang Z Qian H, et al. Time-dependent changes of plasma inflammatory biomarkers in type A aortic dissection patients without optimal medical management. J Cardiothorac Surg 2015;10: 1–7. doi: 10.1186/s13019-014-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrer A Schoenrath F Torzewski M Schmid J Franke UFW Göbel N, et al. Novel blood biomarkers for a diagnostic workup of acute aortic dissection. Diagnostics (Basel) 2021;11: 615. doi: 10.3390/diagnostics11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawada Y Onoda K Imanaka-Yoshida K Maruyama J Yamamoto K Yoshida T, et al. Tenascin-C synthesized in both donor grafts and recipients accelerates artery graft stenosis. Cardiovasc Res 2007;74: 366–376. doi: 10.1016/j.cardiores.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Sakakura K Kubo N Ako J Wada H Fujiwara N Funayama H, et al. Peak C-reactive protein level predicts long-term outcomes in type B acute aortic dissection. Hypertension 2010;55: 422–429. doi: 10.1161/HYPERTENSIONAHA.109.143131. [DOI] [PubMed] [Google Scholar]
- 24.Gitlin JD. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J Biol Chem 1988;263: 6281–6287. doi: 10.1016/S0021-9258(18)68783-6. [PubMed] [Google Scholar]
- 25.Powell JT, Muller BR, Greenhalgh RM. Acute phase proteins in patients with abdominal aortic aneurysms. J Cardiovasc Surg (Torino) 1987;28: 528–530. [PubMed] [Google Scholar]
- 26.Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, Simic D, Radovanovic S, Simic T. Novel biomarkers of heart failure. Adv Clin Chem 2017;79: 93–152. doi: 10.1016/bs.acc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Arenas de Larriva AP Norby FL Chen LY Soliman EZ Hoogeveen RC Arking DE, et al. Circulating ceruloplasmin, ceruloplasmin-associated genes, and the incidence of atrial fibrillation in the atherosclerosis risk in communities study. Int J Cardiol 2017;241: 223–228. doi: 10.1016/j.ijcard.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panichi V Taccola D Rizza GM Consani C Migliori M Filippi C, et al. Ceruloplasmin and acute phase protein levels are associated with cardiovascular disease in chronic dialysis patients. J Nephrol 2004;17: 715–720. [PubMed] [Google Scholar]
- 29.Ma C Zhao H Shi F Li M Liu X Ji C, et al. Serum ceruloplasmin is the candidate predictive biomarker for acute aortic dissection and is related to thrombosed false lumen: A propensity score-matched observational case-control study. Biol Trace Elem Res 2021;199: 895–911. doi: 10.1007/s12011-020-02219-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y Tan X Gao H Yuan H Hu R Jia L, et al. Magnitude of soluble ST2 as a novel biomarker for acute aortic dissection. Circulation 2018;137: 259–269. doi: 10.1161/CIRCULATIONAHA.117.030469. [DOI] [PubMed] [Google Scholar]
- 31.Boa BCS, Yudkin JS, van Hinsbergh VWM, Bouskela E, Eringa EC. Exercise effects on perivascular adipose tissue: Endocrine and paracrine determinants of vascular function. Br J Pharmacol 2017;174: 3466–3481. doi: 10.1111/bph.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y Jiao X Li L Hu C Zhang X Pan L, et al. Increased circulating angiopoietin-like protein 8 levels are associated with thoracic aortic dissection and higher inflammatory conditions. Cardiovasc Drugs Ther 2020;34: 65–77. doi: 10.1007/s10557-019-06924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tazume H Miyata K Tian Z Endo M Horiguchi H Takahashi O, et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2012;32: 1400–1409. doi: 10.1161/ATVBAHA112.247866. [DOI] [PubMed] [Google Scholar]
- 34.Wågsäter D Vorkapic E van Stijn CM Kim J Lusis AJ Eriksson P, et al. Elevated adiponectin levels suppress perivascular and aortic inflammation and prevent angii-induced advanced abdominal aortic aneurysms. Sci Rep 2016;6: 31414. doi: 10.1038/srep31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann A, Brunssen C, Wolk S, Reeps C, Morawietz H. Soluble LOX-1: A novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J Am Heart Assoc 2020;9: e013803. doi: 10.1161/JAHA.119.013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashida K Kume N Murase T Minami M Nakagawa D Inada T, et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: A novel marker for early diagnosis. Circulation 2005;112: 812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- 37.Pirillo A, Catapano AL. Soluble lectin-like oxidized low density lipoprotein receptor-1 as a biochemical marker for atherosclerosis-related diseases. Dis Markers 2013;35: 413–418. doi: 10.1155/2013/716325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kume N, Mitsuoka H, Hayashida K, Tanaka M, Kominami G, Kita T. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome – Comparison with other biomarkers. J Cardiol 2010;56: 159–165. doi: 10.1016/j.jjcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhao ZW, Zhu XL, Luo YK, Lin CG, Chen LL. Circulating soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are associated with angiographic coronary lesion complexity in patients with coronary artery disease. Clin Cardiol 2011;34: 172–177. doi: 10.1002/clc.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi N Hata N Kume N Yokoyama S Takano M Shinada T, et al. Detection of acute aortic dissection by extremely high soluble lectin-like oxidized LDL receptor-1 (sLOX-1) and low troponin T levels in blood. Int J Cardiol 2013;165: 557–559. doi: 10.1016/j.ijcard.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Otahal A, Fuchs R, Alallaf FA, Blaas D. Release of vesicular stomatitis virus spike protein G-pseudotyped lentivirus from the host cell is impaired upon low-density lipoprotein receptor overexpression. J Virol 2015;89: 11723–11726. doi: 10.1128/JVI.01869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Augé N Escargueil-Blanc I Lajoie-Mazenc I Suc I Andrieu-Abadie N Pieraggi MT, et al. Potential role for ceramide in mitogen-activated protein kinase activation and proliferation of vascular smooth muscle cells induced by oxidized low density lipoprotein. J Biol Chem 1998;273: 12893–12900. doi: 10.1074/jbc.273.21.12893. [DOI] [PubMed] [Google Scholar]
- 43.Augé N Maupas-Schwalm F Elbaz M Thiers JC Waysbort A Itohara S, et al. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation 2004;110: 571–578. doi: 10.1161/01.CIR.0000136995.83451.1D. [DOI] [PubMed] [Google Scholar]
- 44.Edsfeldt A Dunér P Ståhlman M Mollet IG Asciutto G Grufman H, et al. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol 2016;36: 1132–1140. doi: 10.1161/ATVBAHA.116.305675. [DOI] [PubMed] [Google Scholar]
- 45.Bao JX, Su YT, Cheng YP, Zhang HJ, Xie XP, Chang YM. Vascular sphingolipids in physiological and pathological adaptation. Front Biosci (Landmark Ed) 2016;21: 1168–1186. doi: 10.2741/4448. [DOI] [PubMed] [Google Scholar]
- 46.Chaurasia B, Summers SA. Ceramides – Lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 2015;26: 538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Hammad SM Pierce JS Soodavar F Smith KJ Gadban MA Rembiesa B, et al. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J Lipid Res 2010;51: 3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blaas I Heinz K Würtinger P Türkcan A Tepeköylü C Grimm M, et al. Vein graft thrombi, a niche for smooth muscle cell colonization – A hypothesis to explain the asymmetry of intimal hyperplasia. J Thromb Haemost 2016;14: 1095–1104. doi: 10.1111/jth.13295. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X Wang R Zhang T Liu F Zhang W Wang G, et al. Identification of lysophosphatidylcholines and sphingolipids as potential biomarkers for acute aortic dissection via serum metabolomics. Eur J Vasc Endovasc Surg 2019;57: 434–441. doi: 10.1016/j.ejvs.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Okura T Miyoshi KI Irita J Enomoto D Nagao T Kukida M, et al. Hyperhomocysteinemia is one of the risk factors associated with cerebrovascular stiffness in hypertensive patients, especially elderly males. Sci Rep 2014;4: 5663. doi: 10.1038/srep05663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang X Liu J Zhao J Mao J Zhang X Feng L, et al. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014;236: 73–81. doi: 10.1016/j.atherosclerosis.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Unhee L, Cassano PA. Homocysteine and blood pressure in the third national health and nutrition examination survey, 1988–1994. Am J Epidemiol 2002;156: 1105–1113. doi: 10.1093/aje/kwf157. [DOI] [PubMed] [Google Scholar]
- 53.Veeranna V Zalawadiya SK Niraj A Pradhan J Ference B Burack RC, et al. Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 2011;58: 1025–1033. doi: 10.1016/j.jacc.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 54.Giusti B Porciani MC Brunelli T Evangelisti L Fedi S Gensini GF, et al. Phenotypic variability of cardiovascular manifestations in Marfan syndrome. Possible role of hyperhomocysteinemia and C677T MTHFR gene polymorphism. Eur Heart J 2003;24: 2038–2045. doi: 10.1016/j.ehj.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Katoh H Suzuki T Hiroi Y Ohtaki E Suzuki S Yazaki Y, et al. Diagnosis of aortic dissection by immunoassay for circulating smooth muscle myosin. Lancet 1995;345: 191–192. doi: 10.1016/s0140-6736(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T Katoh H Watanabe M Kurabayashi M Hiramori K Hori S, et al. Novel biochemical diagnostic method for aortic dissection. Results of a prospective study using an immunoassay of smooth muscle myosin heavy chain. Circulation 1996;15: 1244–1249. doi: 10.1161/01.cir.93.6.1244. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T Katoh H Tsuchio Y Hasegawa A Kurabayashi M Ohira A, et al. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection. The smooth muscle myosin heavy chain study. An Intern Med 2000;133: 537–541. doi: 10.7326/0003-4819-133-7-200010030-00013. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki T, Katoh H, Kurabayashi M, Yazaki Y, Nagai R. Biochemical diagnosis of aortic dissection by raised concentrations of creatine kinase BB-isozyme. Lancet 1997;350: 784–785. doi: 10.1016/S0140-6736(05)62569-X. [DOI] [PubMed] [Google Scholar]
- 59.Vrsalovic M. Prognostic effect of cardiac troponin elevation in acute aortic dissection: A meta-analysis. Int J Cardiol 2016;214: 277–278. doi: 10.1016/j.ijcard.2016.03.230. [DOI] [PubMed] [Google Scholar]
- 60.Lantinga-van Leeuwen IS Dauwerse JG Baelde HJ Leonhard WN van de Wal A Ward CJ, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet 2004;13: 3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 61.Hassane S Claij N Lantinga-van Leeuwen IS Van Munsteren JC Van Lent N Hanemaaijer R, et al. Pathogenic sequence for dissecting aneurysm formation in a hypomorphic polycystic kidney disease 1 mouse model. Arterioscler Thromb Vasc Biol 2007;27: 2177–2183. doi: 10.1161/ATVBAHA.107.149252. [DOI] [PubMed] [Google Scholar]
- 62.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A 2001;98: 12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng J, Ge S, Zhang L, Che H, Liang C. Aortic dissection is associated with reduced polycystin-1 expression, an abnormality that leads to increased ERK phosphorylation in vascular smooth muscle cells. Eur J Histochem 2016;60: 2711. doi: 10.4081/ejh.2016.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng W Peng Z Chai X Zhu Q Yang G Zhao Q, et al. Potential biomarkers for early diagnosis of acute aortic dissection. Heart Lung 2015;44: 205–208. doi: 10.1016/j.hrtlng.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Mir MA. Aortic dissection – In pursuit of a serum marker. Am J Emerg Med 2008;26: 942–945. doi: 10.1016/j.ajem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Sodeck G Domanovits H Schillinger M Janata K Thalmann M Ehrlich MP, et al. Pre-operative N-terminal pro-brain natriuretic peptide predicts outcome in type A aortic dissection. J Am Coll Cardiol 2008;51: 1092–1097. doi: 10.1016/j.jacc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Wen D, Zhou XL, Li JJ, Hui RT. Biomarkers in aortic dissection. Clin Chim Acta 2011;412: 688–695. doi: 10.1016/j.cca.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 68.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J 2006;396: 201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fargnoli AS, Katz MG, Williams RD, Kendle AP, Steuerwald N, Bridges CR. Liquid jet delivery method featuring S100A1 gene therapy in the rodent model following acute myocardial infarction. Gene Ther 2016;23: 151–157. doi: 10.1038/gt.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Han C, Zhang P, Zang W, Guo R. Association between serum S100A1 level and Global Registry of Acute Coronary Events score in patients with non-ST-segment elevation acute coronary syndrome. J Int Med Res 2018;46: 2670–2678. doi: 10.1177/0300060518769524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han C, Liu Q, Li Y, Zang W, Zhou J. S100A1 as a potential biomarker for the diagnosis of patients with acute aortic dissection. J Int Med Res 2021;49: 1–9. doi: 10.1177/03000605211004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montagnana M Lippi G Tessitore N Salvagno GL Targher G Gelati M, et al. Effect of hemodialysis on traditional and innovative cardiac markers. J Clin Lab Anal 2008;22: 59–65. doi: 10.1002/jcla.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christenson RH Duh SH Sanhai WR Wu AH Holtman V Painter P, et al. Characteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: A multicenter study. Clin Chem 2001;47: 464–470. doi: 10.1093/clinchem/47.3.464. [PubMed] [Google Scholar]
- 74.Bhagavan NV Lai EM Rios PA Yang J Ortega-Lopez AM Shinoda H, et al. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem 2003;49: 581–585. doi: 10.1373/49.4.581. [DOI] [PubMed] [Google Scholar]
- 75.Gurumurthy P, Borra SK, Yeruva RK, Victor D, Babu S, Cherian KM. Estimation of ischemia modified albumin (IMA) levels in patients with acute coronary syndrome. Indian J Clin Biochem 2014;29: 367–371. doi: 10.1007/s12291-013-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J Zhao Y Xu C Hong Y Lu H Wu J, et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Sci Rep 2014;4: 5832. doi: 10.1038/srep05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh K, Muddeshwar MG, Lokhande M, Ghosh K. Albumin cobalt binding or ischaemia modified albumin: A test of great prognostic value in malaria. Mediterr J Hematol Infect Dis 2017;9: e2017041. doi: 10.4084/MJHID.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vyakaranam S, Bhongir AV, Patlolla D, Chintapally R. Maternal serum ischemia modified albumin as a marker for hypertensive disorders of pregnancy: A pilot study. Int J Reprod Contracept Obstet Gynecol 2015;4: 611–616. doi: 10.18203/2320-1770.ijrcog20150061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sbarouni E, Georgiadou P, Marathias A, Panagiotakos D, Geroulanos S, Voudris V. Ischemia-modified albumin in acute aortic dissection. J Clin Lab Anal 2010;24: 399–402. doi: 10.1002/jcla.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang G, Zhou Y, He H, Pan X, Chai X. Ischemia-modified albumin, a novel predictive marker of in-hospital mortality in acute aortic dissection patients. Front Physiol 2019;10: 1253. doi: 10.3389/fphys.2019.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duca L Blaise S Romier B Laffargue M Gayral S El Btaouri H, et al. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc Res 2016;110: 298–308. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 82.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1995;15: 1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 83.Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol 2018;71-72: 225–239. doi: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sangiorgi G Trimarchi S Mauriello A Righini P Bossone E Suzuki T, et al. Plasma levels of metalloproteinases-9 and -2 in the acute and subacute phases of type A and type B aortic dissection. J Cardiovasc Med (Hagerstown) 2006;7: 307–315. doi: 10.2459/01.JCM.0000223251.26988.c5. [DOI] [PubMed] [Google Scholar]
- 85.Manabe T, Imoto K, Uchida K, Doi C, Takanashi Y. Decreased tissue inhibitor of metalloproteinase-2/matrix metalloproteinase ratio in the acute phase of aortic dissection. Surg Today 2004;34: 220–225. doi: 10.1007/s00595-003-2683-3. [DOI] [PubMed] [Google Scholar]
- 86.Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg 2004;78: 2106–2110; discussion 2110-1. doi: 10.1016/j.athoracsur.2004.05.088. [DOI] [PubMed] [Google Scholar]
- 87.Giachino F Loiacono M Lucchiari M Manzo M Battista S Saglio E, et al. Rule out of acute aortic dissection with plasma matrix metalloproteinase 8 in the emergency department. Crit Care 2013;17: R33. doi: 10.1186/cc12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shinohara T Suzuki K Okada M Shiigai M Shimizu M Maehara T, et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol 2003;23: 1839–1844. doi: 10.1161/01.ATV.0000085016.02363.80. [DOI] [PubMed] [Google Scholar]
- 89.Dong J Bao J Feng R Zhao Z Lu Q Wang G, et al. Circulating microRNAs: A novel potential biomarker for diagnosing acute aortic dissection. Sci Rep 2017;7: 12784. doi: 10.1038/s41598-017-13104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Imanaka-Yoshida K, Yoshida T, Miyagawa-Tomita S. Tenascin-C in development and disease of blood vessels. Anat Rec (Hoboken) 2014;297: 1747–1757. doi: 10.1002/ar.22985. [DOI] [PubMed] [Google Scholar]
- 91.Kimura T Shiraishi K Furusho A Ito S Hirakata S Nishida N, et al. Tenascin C protects aorta from acute dissection in mice. Sci Rep 2014;4: 4051. doi: 10.1038/srep04051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nozato T Sato A Hirose S Hikita H Takahashi A Endo H, et al. Preliminary study of serum tenascin-C levels as a diagnostic or prognostic biomarker of type B acute aortic dissection. Int J Cardiol 2013;168: 4267–4269. doi: 10.1016/j.ijcard.2013.04.211. [DOI] [PubMed] [Google Scholar]
- 93.König KC Lahm H Dreßen M Doppler SA Eichhorn S Beck N, et al. Aggrecan: A new biomarker for acute type A aortic dissection. Sci Rep 2021;11: 10371. doi: 10.1038/s41598-021-89653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Izard T, Brown DT. Mechanisms and functions of vinculin interactions with phospholipids at cell adhesion sites. J Biol Chem 2016;291: 2548–2555. doi: 10.1074/jbc.r115.686493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bays JL, DeMali KA. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol Life Sci 2017;74: 2999–3009. doi: 10.1007/s00018-017-2511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurihara T Shimizu-Hirota R Shimoda M Adachi T Shimizu H Weiss SJ, et al. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation 2012;126: 3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 97.Wang HQ Yang H Tang Q Gong YC Fu YH Wan F, et al. Identification of vinculin as a potential diagnostic biomarker for acute aortic dissection using label-free proteomics. Biomed Res Int 2020;2020: 7806409. doi: 10.1155/2020/7806409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pepe G, Giusti B, Sticchi E, Abbate R, Gensini GF, Nistri S. Marfan syndrome: Current perspectives. Appl Clin Genet 2016;9: 55–65. doi: 10.2147/TACG.S96233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzuki T Trimarchi S Sawaki D Grassi V Costa E Rampoldi V, et al. Circulating transforming growth factor-beta levels in acute aortic dissection. J Am Coll Cardiol 2011;58: 775. doi: 10.1016/j.jacc.2010.01.079. [DOI] [PubMed] [Google Scholar]
- 100.Lu J Li P Ma K Li Y Yuan H Zhu J, et al. OPG/TRAIL ratio as a predictive biomarker of mortality in patients with type A acute aortic dissection. Nat Commun 2021;12: 3401. doi: 10.1038/s41467-021-23787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bai A. β2-glycoprotein I and its antibodies involve in the pathogenesis of the antiphospholipid syndrome. Immunol Lett 2017;186: 15–19. doi: 10.1016/j.imlet.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Cheng N Wang H Zhang W Wang H Jin X Ma X, et al. Comparative proteomic investigation of plasma reveals novel potential biomarker groups for acute aortic dissection. Dis Markers 2020;2020: 4785068. doi: 10.1155/2020/4785068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Liu SQ, Duan WX, Yu SQ, Yi DH. Risk factors of aortic dissection disease in Chinese population (in Chinese). Chin J Evidence Based Cardiovasc Med 2014;6: 411–415. doi: 10.3969/j.1674-4055.2014.04.10. [Google Scholar]
- 104.Wang Y, Cadres DO, Hospital XC. Correlations of D-two dimer, blood uric acid and aortic dissection (in Chinese). South China J Cardiovasc Dis 2014;20: 347–349. doi: 10.3969/j.issn.1007-9688.2014.03.023. [Google Scholar]
- 105.Song W, Yan WJ, Li HJ, Wang AP, Feng W, Du B. Correlation between peripheral blood biochemical markers and aortic dissection (in Chinese). Chin Med 2016;11: 195–198. doi: 10.3760/cma.j.issn.1673-4777.2016.02.014. [Google Scholar]
- 106.Tang Y. Relation research between serum uric acid level and aortic dissection (in Chinese). Chin J Cardio Res 2012;10(12): 925–927. doi: 10.3969/j.issn.1672-5301.2012.12.013. [Google Scholar]
- 107.Zhang L, Zhou J, Jing Z. Serum uric acid might be associated with aortic dissection in Chinese men. Int J Cardiol 2016;203: 420–421. doi: 10.1016/j.ijcard.2015.10.185. [DOI] [PubMed] [Google Scholar]
- 108.Takeuchi T Adachi H Ohuchida M Nakamura T Satoh A Jacobs DR Jr., et al. A case-control study found that low albumin and smoking were associated with aortic dissection. J Clin Epidemiol 2004;57: 386–391. doi: 10.1016/j.jclinepi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 109.Li X Jiang S He J Li N Fan Y Zhao X, et al. Uric acid in aortic dissection: A meta-analysis. Clin Chim Acta 2018;484: 253–257. doi: 10.1016/j.cca.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116: 281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 111.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res 2011;39: 7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol 2013;75: 69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang W, Yu Q, Wang Q, Cao F. Roles of miRNA in cardiovascular development and dysfunction. Curr Med Chem 2013;20: 3613–3622. doi: 10.2174/0929867311320290007. [DOI] [PubMed] [Google Scholar]
- 114.van Rooij E Sutherland LB Thatcher JE DiMaio JM Naseem RH Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 2008;105: 13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y Taylor NE Lu L Usa K Cowley AW Jr. Ferreri NR, et al. Renal medullary microRNAs in dahl salt-sensitive rats miR-29b regulates several collagens and related genes. Hypertension 2010;55: 974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ikonomidis JS Ivey CR Wheeler JB Akerman AW Rice A Patel RK, et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J Thorac Cardiovasc Surg 2013;145: 1326–1333. doi: 10.1016/j.jtcvs.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Albinsson S Della Corte A Alajbegovic A Krawczyk KK Bancone C Galderisi U, et al. Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microRNA signatures in mildly dilated ascending aorta. Heart Vessels 2017;32: 750–767. doi: 10.1007/s00380-016-0942-7. [DOI] [PubMed] [Google Scholar]
- 118.Goliopoulou A Oikonomou E Antonopoulos A Koumallos N Gazouli M, et al. Expression of tissue microRNAs in ascending aortic aneurysms and dissections. Angiology 2022;74: 88–94. doi: 10.1177/00033197221098295. [DOI] [PubMed] [Google Scholar]
- 119.Jones JA Stroud RE O'Quinn EC Black LE Barth JL Elefteriades JA, et al. Selective microRNA suppression in human thoracic aneurysms: Relationship of miR-29a to aortic size and proteolytic induction. Circ Cardiovasc Genet 2011;4: 605–613. doi: 10.1161/CIRCGENETICS.111.960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao M Zou S Weng J Hou L Yang L Zhao Z, et al. A microRNA profile comparison between thoracic aortic dissection and normal thoracic aorta indicates the potential role of microRNAs in contributing to thoracic aortic dissection pathogenesis. J Vasc Surg 2011;53: 1341–1349.e3. doi: 10.1016/j.jvs.2010.11.113. [DOI] [PubMed] [Google Scholar]
- 121.Yu Y Shi E Gu T Tang R Gao S Wang Y, et al. Overexpression of microRNA-30a contributes to the development of aortic dissection by targeting lysyl oxidase. J Thorac Cardiovasc Surg 2017;154: 1862–1869. doi: 10.1016/j.jtcvs.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 122.Boettger T Beetz N Kostin S Schneider J Krüger M Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 2009;119: 2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cordes KR Sheehy NT White MP Berry EC Morton SU Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009;460: 705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L. The miR-143/145 cluster is a novel transcriptional target of jagged-1/notch signaling in vascular smooth muscle cells. J Biol Chem 2011;286: 28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu Z Wang Q Pan J Sheng X Hou D Chong H, et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci Rep 2017;7: 13659. doi: 10.1038/s41598-017-13696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang XJ Huang B Yang YM Zhang L Su WJ Tian L, et al. Differential expression of microRNAs in aortic tissue and plasma in patients with acute aortic dissection. J Geriatr Cardiol 2015;12: 655–661. doi: 10.11909/j.issn.1671-5411.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tian C Tang X Zhu X Zhou Q Guo Y Zhao R, et al. Expression profiles of circRNAs and the potential diagnostic value of serum circMARK3 in human acute Stanford type A aortic dissection. PLoS One 2019;14: e0219013. doi: 10.1371/journal.pone.0219013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang K Yuan Y Cho JH Mcclarty S Baxter D Galas DJ, et al. Comparing the microRNA spectrum between serum and plasma. PLoS One 2012;7: e41561. doi: 10.1371/journal.pone.0041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Witwer KW. Circulating microRNA biomarker studies: Pitfalls and potential solutions. Clin Chem 2015;61: 56–63. doi: 10.1373/clinchem.2014.221341. [DOI] [PubMed] [Google Scholar]
- 130.Schwarzenbach H, da Silva AM, Calin G, Pantel K. Data normalization strategies for microRNA quantification. Clin Chem 2015;61: 1333–1342. doi: 10.1373/clinchem.2015.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang M Forbes ME Bitting RL O'Neill SS Chou PC Topaloglu U, et al. Incorporating blood-based liquid biopsy information into cancer staging: Time for a TNMB system? Ann Oncol 2018;29: 311–323. doi: 10.1093/annonc/mdx766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med 2018;379: 1754–1765. doi: 10.1056/NEJMra1706174. [DOI] [PubMed] [Google Scholar]
- 133.Lo YMD, Han DSC, Jiang P, Chiu RWK. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021;372: eaaw3616. doi: 10.1126/science.aaw3616. [DOI] [PubMed] [Google Scholar]
- 134.Li N Lin H Zhou H Zheng D Xu G Shi H, et al. Efficient detection of differentially methylated regions in the genome of patients with thoracic aortic dissection and association with MMP2 hypermethylation. Exp Ther Med 2020;20: 1073–1081. doi: 10.3892/etm.2020.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pan S Lai H Shen Y Breeze C Beck S Hong T, et al. DNA methylome analysis reveals distinct epigenetic patterns of ascending aortic dissection and bicuspid aortic valve. Cardiovas Res 2017;113: 692–704. doi: 10.1093/cvr/cvx050. [DOI] [PubMed] [Google Scholar]
- 136.Liu P Zhang J Du D Zhang D Jin Z Qiu W, et al. Altered DNA methylation pattern reveals epigenetic regulation of Hox genes in thoracic aortic dissection and serves as a biomarker in disease diagnosis. Clin Epigenetics 2021;13: 124. doi: 10.1186/s13148-021-01110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]