The need for an enzyme to catalyze the slow conversion between carbon dioxide and bicarbonate enabled physiologists to predict the existence of carbonic anhydrase almost 70 years ago. Shortly thereafter the enzyme was purified from erythrocytes (Meldrum and Roughton, 1933; Stadie and O’Brien, 1933). The enzyme, now called α-carbonic anhydrase (α-CA), was found to contain a zinc ion (Keilin and Mann, 1940).
A large number of α-CA isoenzymes, primarily from mammalian species, have subsequently been identified and characterized, and several of their molecular structures have been elucidated, starting with that of human α-CAII (Liljas et al., 1972). When carbonic anhydrase from plants and certain bacteria was characterized and sequenced, it turned out to be oligomeric and its amino acid sequence had no similarity to the previously studied enzymes (Hewett-Emmett and Tashian, 1996). This form is called β-carbonic anhydrase (β-CA). Subsequently, a carbonic anhydrase from archaea was identified (γ-carbonic anhydrase, γ-CA). In this case, the amino acid sequence was again strikingly different (Alber and Ferry, 1994). In spite of the differences in sequence, however, all forms of carbonic anhydrase have an essential zinc ion in the active site. It became evident that only a comparison of the molecular structures might clarify the relationships between the different enzymes.
Light was shed on this problem by recent papers in The Journal of Biochemistry and The EMBO Journal, which reported the structure of β-CA from plant and algae, respectively (Kimber and Pai, 2000; Mitsuhashi et al., 2000), following the earlier elucidation of the structure of γ-CA (Kisker et al., 1996). These publications illustrate that all of the carbonic anhydrases are completely different from one another at the level of their tertiary and quaternary structures, but that the active sites show essential features of remarkable similarity.
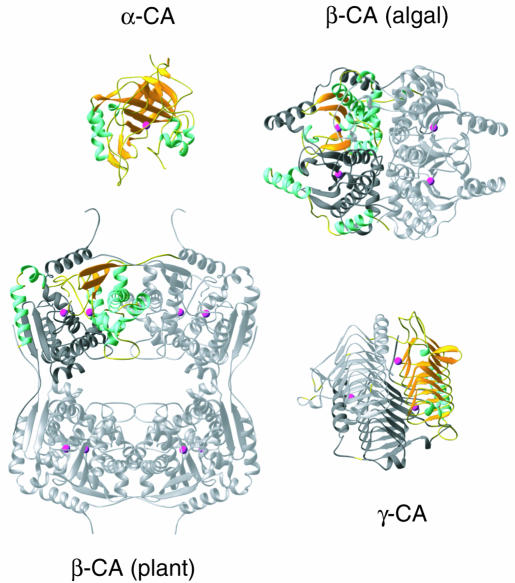
The differences between the various carbonic anhydrases are illustrated in Figure 1. Notably, γ-CA has a primitive β-helix structure (shown in yellow) in which a short repeated amino acid sequence produces the main part of the fold, whereas the α- and β-CAs have folds that are constructed by different arrangements of α-helices and β-strands. Differences in quaternary structure exist even within the class of β-CA (Figure 1). Thus, in the two β-CAs for which structures have been elucidated, the plant enzyme forms an unusual octamer structure, which is not based on 4-fold but on repeated 2-fold symmetry, while the algal polypeptide has an internal repeat, forming a covalently connected dimer (colorful monomer plus dark gray monomer) that in turn dimerizes with a second molecule (light gray).
Fig. 1. The tertiary structures of the three forms of carbonic anhydrase. From top to bottom: α-CA monomer; algal β-CA dimer with internal repeat [the unique (monomer) fold is highlighted]; pea β-CA octamer; γ-CA trimer. For the oligomeric forms of the enzyme, one monomer is highlighted with the β-strands in yellow and the α-helices in blue. The other monomers are in light gray. The zinc ions are indicated by red spheres.
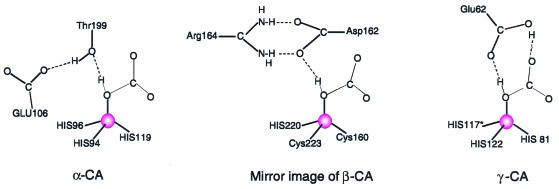
While the active site (identified by the zinc ion in red) of α-CA lies in a crevice within the monomer, those of β-CA and γ-CA are situated in grooves between the subunits of the oligomers. Not only are the zinc ion ligands different, but the essential residues of the active sites differ as well (Figure 2). The superposition of the active sites is not straightforward, but α-CA is best compared to the mirror image of β-CA, as pointed out by Kimber and Pai (2000). A further difference between the β-CAs is that the plant enzyme has an acetate ion bound at the zinc in a way reminiscent of a substrate, while the acetate is replaced by the active site aspartate in the algal enzyme.
Fig. 2. Schematic representations of the active sites of the three forms of carbonic anhydrase with a bound bicarbonate ion (α-CA, Liljas et al., 1994; β-CA, Protein Data Bank entry 1QRL). The location of HCO3 in the active site of γ-CA is tentative and made in analogy to the situation in the other two forms. The proximity of an obligate hydrogen bond acceptor close to the free ligand position at the zinc ion (red sphere) seems in all cases to dictate the ion’s orientation. Thus, the catalytic mechanism is likely to be the same.
In spite of the differences highlighted above, these enzymes catalyze the same reaction, in which the zinc ion activates a water molecule that reacts with carbon dioxide, or destabilizes bicarbonate in the reverse reaction. Even though the residues that have essential functions are different, the catalytic mechanisms are probably very similar, since in all cases, an obligate hydrogen bond acceptor is within hydrogen bonding distance of the available ligand position at the zinc ion, and the OH of bicarbonate would necessarily be the metal ligand (Figure 2). Carbonic anhydrases provide an excellent example of convergent evolution. It is of significant interest to clarify how the same function has evolved three times completely independently, to generate enzymes that appear to be different superficially but that actually have great underlying similarities. The species distribution for the three classes of protein does not reveal a pattern that could explain how they are related. What was the driving force that created three versions of this wheel?
References
- Alber B.E. and Ferry, J.G. (1994) A carbonic anhydrase from the archaeon Methanosarcina thermophila. Proc. Natl Acad. Sci. USA, 91, 6909–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett-Emmett D. and Tashian, R.E. (1996) Functional diversity, conservation, and convergence in the evolution of the α-, β-, and γ-carbonic anhydrase gene families. Mol. Phylogenet. Evol., 5, 50–77. [DOI] [PubMed] [Google Scholar]
- Keilin D. and Mann, T. (1940) Carbonic anhydrase, purification and nature of the enzyme. Biochem. J., 34, 1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber M.S. and Pai, E.F. (2000) The active site architecture of Pisum sativumβ-carbonic anhydrase is a mirror image of that of α-carbonic anhydrases. EMBO J., 19, 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisker C., Schindelin, H., Alber, B.E., Ferry, J.G. and Rees, D.C. (1996) A left-hand β-helix revealed by the crystal structure of a carbonic anhydrase from the archaeon Methanosarcina thermophila. EMBO J., 15, 2323–2330. [PMC free article] [PubMed] [Google Scholar]
- Liljas A. et al. (1972) Crystal structure of human carbonic anhydrase C. Nature New Biol., 235, 131–137. [DOI] [PubMed] [Google Scholar]
- Liljas A., Håkansson, K., Jonsson, B.H. and Xue, Y. (1994) Inhibition and catalysis of carbonic anhydrase. Recent crystallographic analyses. Eur. J. Biochem., 219, 1–10. [DOI] [PubMed] [Google Scholar]
- Meldrum N.U. and Roughton, F.J.W. (1933) Carbonic anhydrase. Its preparation and properties. J. Physiol., 80, 113–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Mizushima, T., Yamashita, E., Yamamoto, M., Kumasaka, T., Moriyama, H., Ueki, T., Miyachi, S. and Tsukihara, T. (2000) X-ray structure of β-carbonic anhydrase from the red alga, Porphyridium purpureum, reveals a novel catalytic site for CO2 hydration. J. Biol. Chem., 275, 5521–5526. [DOI] [PubMed] [Google Scholar]
- Stadie W.C. and O’Brien, H. (1933) The catalysis of the hydration of carbon dioxide and the dehydration of carbonic acid by an enzyme isolated from red blood cells. J. Biol. Chem., 103, 521–529. [Google Scholar]