Abstract
The Cdc25 A phosphatase is required for the G1–S transition of the cell cycle and is overexpressed in human cancers. We found that it is ubiquitylated and rapidly degraded by the proteasome and that its levels increase from G1 until mitosis. By treating cells with the DNA synthesis inhibitor hydroxyurea, Cdc25 A rapidly decreased in abundance, and this was accompanied by an increase in Cdk2 phosphotyrosine content and a decrease in Cdk2 kinase activity. Cdc25 A overexpression altered the ability of cells to arrest in the presence of hydroxyurea, and caused them to undergo premature chromosome condensation. Cdc25 A overexpression could render tumor cells less sensitive to DNA replication checkpoints, thereby contributing to their genomic instability.
INTRODUCTION
Cdc25 phosphatases are essential regulators of cell cycle transitions. In lower eukaryotes, Cdc25 is an activator of the G2–M transition by virtue of its ability to dephosphorylate the Cdc2 protein kinase (see Draetta and Eckstein, 1997 for review). In mammalian cells, three Cdc25-related proteins have been identified (Sadhu et al., 1990; Galaktionov and Beach, 1991a; Nagata et al., 1991) that allow growth of Schizosaccharomyces pombe lacking Cdc25. While Cdc25 B and C appear to regulate progression from G2 to M phase (Millar et al., 1991; Gabrielli et al., 1996; Lammer et al., 1998; Karlsson et al., 1999), Cdc25 A is required for S phase entry (Hoffmann et al., 1994; Jinno et al., 1994; Vigo et al., 1999), and its overexpression leads to an acceleration of S phase entry (Blomberg and Hoffmann, 1999; Sexl et al., 1999). In addition, Cdc25 A is a transcriptional target of c-Myc (Galaktionov et al., 1996) and of E2F (Vigo et al., 1999) and can cooperate with activated Ras in inducing mouse cell transformation and in vivo tumor growth (Galaktionov et al., 1995). Cdc25 A overexpression can also induce growth of cells expressing a mutated CSF1-R in semi-solid medium (Sexl et al., 1999). Furthermore, a subset of aggressive human cancers shows increased expression of Cdc25 A or B (Galaktionov et al., 1995; Gasparotto et al., 1997; Kudo et al., 1997; Dixon et al., 1998; Hernandez et al., 1998; Wu et al., 1998).
Cdc25s are able to dephosphorylate both threonine and tyrosine residues in cyclin-dependent kinases (see Draetta and Eckstein 1996, for review). Given its role in activating the G1–S transition, it has been suggested that Cdc25 A dephosphorylates and activates Cdk2. Cdk2 is phosphorylated on tyrosine in intact cells (Gu et al., 1992) and can both physically interact with and be a substrate of Cdc25 A (Hoffmann et al., 1994; Blomberg and Hoffmann, 1999). Tyrosine phosphorylation of Cdk4 has also been detected (Terada et al., 1995; Iavarone and Massague, 1997), making this protein a candidate Cdc25 A substrate. In addition, Cdc25 A can dephosphorylate the Cut transcriptional repressor (Coqueret et al., 1998).
We have investigated the regulation of Cdc25 A in the cell cycle. We present evidence of its degradation through the ubiquitin pathway and demonstrate that its downregulation is a key element of the DNA replication checkpoint. Overexpression of the protein will indeed interfere with a cell cycle block induced by hydroxyurea (HU) and will precipitate cells into an abortive mitosis.
RESULTS AND DISCUSSION
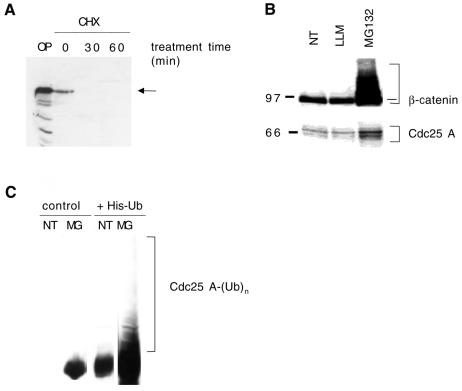
By treating growing HeLa cells with the protein synthesis inhibitor cycloheximide, we found that Cdc25 A rapidly decreased as early as 30 min after treatment (Figure 1A). Since previous studies have shown that the single S. pombe Cdc25 protein is ubiquitylated and degraded by the proteasome (Nefsky and Beach, 1996), and that in mammalian cells Cdc25 B is also degraded by the proteasome (Baldin et al., 1997), we asked whether the ubiquitin–proteasome pathway was also involved in Cdc25 A processing. For this purpose, cells were treated with the specific proteasome inhibitor MG132, or with LLM, a calpain inhibitor (Figure 1B). β-catenin was used as a positive control, since it is rapidly degraded through the ubiquitin–proteasome pathway (see Maniatis, 1999, for review). The Cdc25 A and β-catenin levels increased in lysates from cells treated with MG132, while no accumulation of either was seen in those made from cells treated with LLM (Figure 1B).
Fig. 1. (A) Cell lysates (50 µg) were treated with 10 µg/ml cycloheximide (CHX) and immunoblotted with Cdc25 A antibodies. OP, overexpressed Cdc25 A. (B) Lysates (50 µg) from cells treated for 8 h with 5 µM LLM or MG132 were immunoblotted for β-catenin (top) or Cdc25 A (bottom). (C) Cells (10-cm plates) were transfected with a plasmid (8 µg) encoding wild-type Cdc25 A and 2 µg of either an empty vector or a vector encoding His6-ubiquitin. Twenty-four hours after removal of the DNA precipitate, cells were left untreated (NT) or treated for 8 h with 5 µM MG132 (MG). Ubiquitylated forms were recovered on nickel agarose, and total eluates immunoblotted with Cdc25 A antibodies. Ubiquitylated species are indicated.
To determine the involvement of ubiquitylation in Cdc25 A degradation, we transfected cells with a vector encoding Cdc25 A along with either a control vector or a vector encoding a histidine-tagged ubiquitin (His6-ubiquitin). Ubiquitylated species were isolated as described by Treier et al. (1994). Transfected cells were left untreated or treated with MG132 for 8 h and then lysed in guanidium–HCl and incubated with nickel-agarose. Bound proteins were analyzed by immunoblotting with Cdc25 A antibodies. Figure 1C shows the presence of slower migrating bands in the samples cotransfected with Cdc25 A and His6-ubiquitin and treated with the proteasome inhibitor. Even if Cdc25 A was found to interact directly, like other proteins do, with nickel-agarose, these high Mr bands were not detectable in the absence of proteasome inhibitor treatment or in samples not transfected with His6-ubiquitin. We conclude therefore that Cdc25 A is ubiquitylated and rapidly degraded through the proteasome.
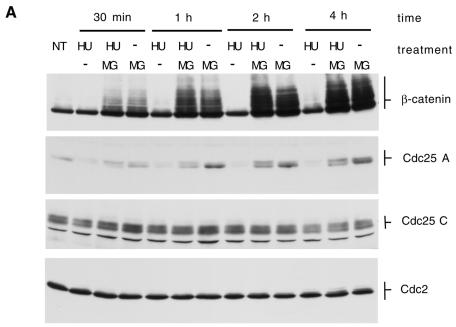
Consistent with a role in S phase entry (Hoffmann et al., 1994; Jinno et al., 1994), Cdc25 A accumulates and its activity becomes detectable in late G1. We attempted to study the regulation of Cdc25 A in conditions where S phase is inhibited, by using HU, a known ribonucleotide reductase inhibitor (Krakoff et al., 1968; Yabro, 1992). Treatment with HU for as little as 30 min induced a dramatic decrease in Cdc25 A protein levels. Concomitant treatment with HU and MG132 abolished the HU effect, resulting in an accumulation of the protein over time (Figure 2A). These effects occurred concomitantly with a complete inhibition of DNA synthesis (measured by BrdU incorporation, not shown, see also below), suggesting that a decrease in Cdc25 A protein level after HU treatment is a primary effect of DNA synthesis inhibition. Upon longer electrophoretic runs the Cdc25 A band resolved into multiple bands, which accumulated differently upon treatment with MG132 and HU compared with MG132 alone, and might represent phosphorylation events. Contrary to what was observed for Cdc25 A, treatment with HU and/or MG132 did not produce changes in the Cdc25 C or Cdc2 protein levels. Furthermore, the β-catenin levels were not altered by HU treatment, despite the fact that both Cdc25 A and β-catenin accumulated rapidly after MG132 addition. Treatment with HU induced a marked decrease in Cdc25 A protein levels in any of a number of cell lines tested (not shown), proving that this is a general phenomenon rather than being cell-line specific.
Fig. 2. (A) HeLa cells were treated with 2.5 mM HU, HU plus 5 µM MG132 (HU/MG) or MG132 and lysates (50 µg) analyzed by immunoblotting with the indicated antibodies. NT, untreated cells. (B) HeLa cells (15-cm plates) were transfected with a plasmid encoding Cdc25 A (6 µg) with or without an N-terminal FLAG tag (left and right panel respectively). EV, cells transfected with empty vector. DNA precipitates were left on cells for 15 h. Cells were then split into three plates. After 12 h, the medium was changed and cells were treated with HU or HU plus MG132 for an additional 12 h. Thirty micrograms of lysate were loaded per lane, and blots probed with Cdc25 or FLAG antibodies. Asterisks indicate non-specific bands recognized by the FLAG antibody. (C) Cells were treated as in Figure 1C. Twenty-four hours after removal of the DNA precipitate, cells were either left untreated (NT) or treated for 8 h with 2.5 mM HU or HU plus 5 µM MG132 (MG). (D) Cdc25 A immunoprecipitations were performed on lysates (1 mg) from cells untreated (NT) or treated for 2 h with HU (2.5 mM).
Our results are compatible with the possibility that HU induces an acceleration of Cdc25 A degradation via the proteasome pathway. To support this point further, either a wild-type or a FLAG epitope-tagged Cdc25 A cDNA was inserted into a cytomegalovirus (CMV) promoter expression vector. The construct was transfected in cells that were then treated with either HU or HU plus the MG132 proteasome inhibitor. Treatment with HU reduced the levels of the CMV-expressed proteins, similarly to what was seen for endogenous Cdc25 A (Figure 2B). This demonstrates that the observed decrease in Cdc25A levels was not caused by a post-translational modification masking the epitope recognized by the antiserum, nor by specific inhibition of Cdc25A transcription or translation.
We next asked whether Cdc25 A was also ubiquitylated in cells subjected to HU treatment. We transfected cells with Cdc25 A and either an empty vector or a vector encoding His6-ubiquitin. After treatment with HU or HU and MG132 (Figure 2C) we lysed cells in denaturing conditions and purified ubiquitylated species on nickel-agarose as described above. High Mr bands reactive with Cdc25 A antibodies and compatible with ubiquitylated bands were detected in samples treated with both HU and MG132. No such bands were detected in control lanes, in the absence of His6-ubiquitin or of MG132 addition, suggesting that the treatment stimulates the degradation of Cdc25 A through the ubiquitin–proteasome pathway.
This degradation signal appears to depend specifically on inhibition of DNA synthesis, since upon release from the DNA replication block, Cdc25 A did return to its normal levels within a time frame that is not compatible with recovery from DNA damage (not shown). We also obtained similar results using DNA synthesis inhibitors like aphidicolin and Raltitrex® (not shown), which function through distinct mechanisms, and in addition, we did not observe significant induction of Cdc25 A degradation upon treating G1 or M phase cells with HU (not shown).
Our data suggest that, similarly to what is shown for cyclin D1 (Diehl et al., 1998), Cdc25 A is a short-lived protein whose degradation is regulated by phosphorylation, and that further reduction in the protein half-life can occur in response to signaling events. Whether or not this involves, as is the case for cyclin D1, a change in the subcellular localization of the protein from the nucleus to the cytosol is not clear at present. Phosphorylation of Cdc25 A by a checkpoint kinase (Chk1, Chk2) could induce, as for Cdc25 C, its binding to the 14-3-3 proteins and subsequent shuttling (see Kumagai and Dunphy, 1999; Lopez-Girona et al., 1999 and references therein). Thus far though, we have been unable to detect an interaction between Cdc25 A and 14-3-3 proteins, either in vitro or in intact cells.
Since a previous study (Hoffmann et al., 1994) had shown that the Cdc25 A phosphatase activity increased in HU synchronized cells, and in this study we show that Cdc25 A protein levels dramatically decreased after HU treatment, we set out to investigate this discrepancy. Control cells or cells treated with HU were used as a source of Cdc25 A and activation of the Cdc2–cyclin B complex was used as a measure of Cdc25 A activity. Cdc25 A was immunoprecipitated from each lysate and incubated with inactive Cdc2–cyclin B complexes (Cans et al., 1999). Figure 2D shows that cells treated with HU contained a very low amount of Cdc25 A activity compared with untreated cells, consistent with the observed decrease in total protein levels (not shown, see e.g. Figure 1A). The activity measured by Hoffmann et al. (1994) could be ascribed to the low residual amount of Cdc25 A in the treated cells compared with early G1 cells.
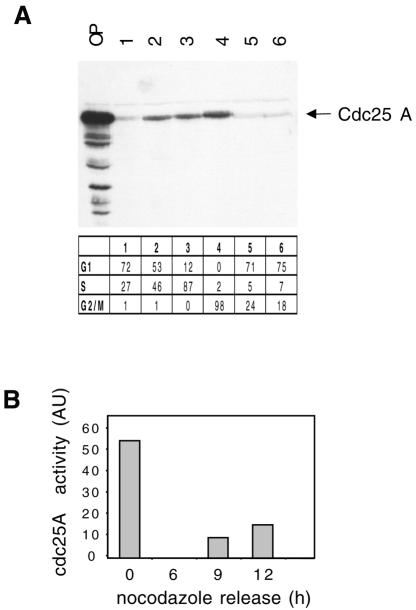
We found that the Cdc25 A protein levels continued to increase as cells progressed past the G1–S transition. Synchronized populations of HeLa cells enriched in various stages of the cell cycle were obtained either by thymidine block and release or by nocodazole block and release. Cdc25 A was almost undetectable in G1 cells, very low in thymidine-treated cells, and then started to accumulate during S phase and until mitosis (Figure 3A). Identical results were obtained by centrifugal elutriation (not shown), suggesting that the observed variations were not due to the chemical synchronization used. We also analyzed the Cdc25 A phosphatase activity during the cell cycle. Cells were blocked in metaphase by nocodazole addition and then released into fresh medium for varying times. Cdc25A was immunoprecipitated and assayed for phosphatase activity. Figure 3B shows that the activity was detected at high levels in mitotic cells, and then decreased sharply as cells entered G1, to resume again as they progressed into S phase. Taken together, our data show that Cdc25 A protein levels increase from G1 to mitosis and that its activity parallels its protein abundance.
Fig. 3. (A) Cells were synchronized at the G1–S border by a thymidine block for 15 h (lane 1) and subsequent release into complete medium for 3 h (lane 2) or 6 h (lane 3). Cells in M phase were obtained by nocodazole addition for 15 h (50 ng/ml); round mitotic cells were collected (lane 4) and plated in complete medium for 3 and 5 h (lanes 5 and 6). Fifty micrograms of total lysate from each sample were analyzed by immunoblotting with Cdc25 A antibodies. OP, overexpressed Cdc25 A. Cell cycle position was verified by FACS analysis. (B) HeLa cells were synchronized in mitosis with nocodazole and then released into complete medium for 0, 6, 9 and 12 h. Cell cycle position was verified by FACS analysis (Figure 4D). Cdc25 A phosphatase activity was measured as activation of Cdc2–cycB.
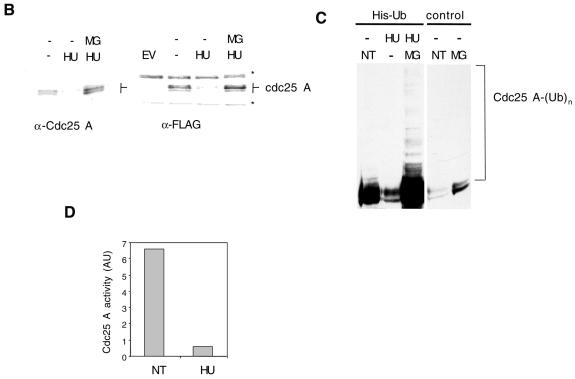
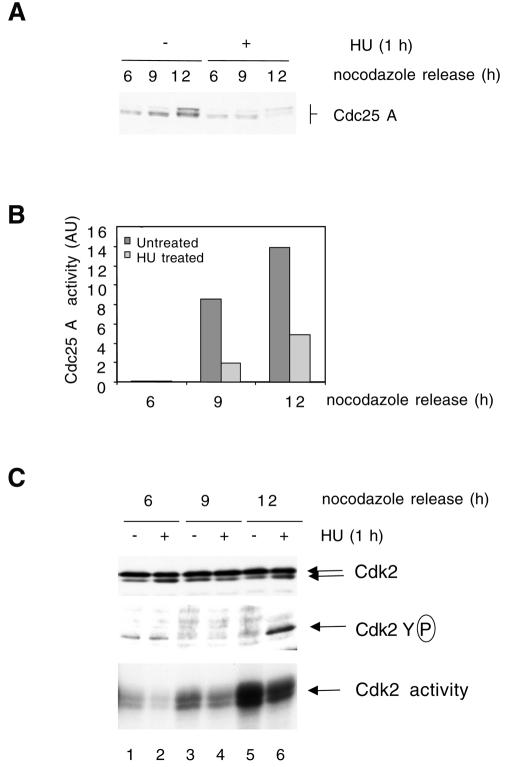
To understand better the relationships between S phase inhibition and Cdc25 A degradation, we studied the effects of adding HU to a synchronized cell population. Mitotic cells were generated by treatment with nocodazole, and then released. Samples were taken at 6, 9 and 12 h after release, with or without addition of HU for the last hour, and also incubated with bromodeoxyuridine (BrdU) prior to collection. The Cdc25 A levels increased with time with the appearance of slowly migrating species. Treatment with HU for 1 h inhibited Cdc25 A accumulation (Figure 4A). Measurement of Cdc2–cyclin B activation by Cdc25 A shows that Cdc25 A activity increased concomitantly with its protein level and that treatment with HU resulted in a substantial reduction in activity (Figure 4B).
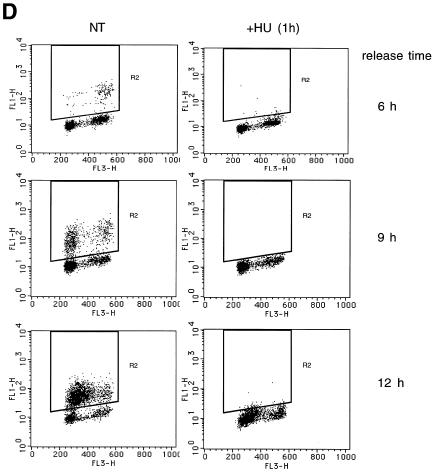
Fig. 4. Cells were blocked in metaphase with nocodazole. Attached cells were collected 6, 9 and 12 h after replating. HU (2.5 mM) was added for the last hour and BrdU was added for the last 15 min. (A) Immunoblotting with Cdc25 A antibodies (50 µg lysate). (B) Cdc25 A immunoprecipitations (1 mg lysate) for phosphatase activity measurements. Background activity of Cdc2–cycB in the absence of Cdc25 A was subtracted from totals shown. (C) Immunoblotting with Cdk2 antibodies (50 µg) (top panel). Cdk2 immunoprecipitation and immunoblotting with anti-pTyr antibodies (middle panel) or H1 kinase assay (bottom panel). (D) Flow cytometry analysis.
Cdk2 can be a substrate of Cdc25 A (Blomberg and Hoffmann, 1999). The observed changes in Cdc25 A activity correlated also with measurements of Cdk2 activity (Figure 4C). HU treatment of S phase cells resulted in the appearance of tyrosine phosphorylation of Cdk2 and this strongly correlated with loss of Cdc25 A activity. Cdk2 was found to be largely inactive in G1, its activity started to increase in early S phase and it was maximal in S phase. Both in early and late S phase HU addition efficiently inhibited Cdk2 activity. By flow cytometry, cells were mostly in G1 by 6 h after nocodazole release, they started to enter S phase 9 h after release and were mostly in S phase 12 h after release. BrdU incorporation was completely inhibited by 1 h HU treatment at all times analyzed (Figure 4D). The small population of G2 cells found to be present in all samples is likely to be due to cells that did not return into cycle after release. Our results suggest that Cdc25 A degradation in response to HU treatment strongly correlates with Cdk2 inactivation. Cdc25 A could therefore be a target of a DNA replication checkpoint.
Cdc25 A is needed for the G1–S transition, and its degradation in cells where DNA synthesis cannot take place is paralleled by a decrease in Cdk2 activity. Downregulation of Cdk2 appears to be a direct consequence of Cdc25 A downregulation, since Cdk2–cyclin A and/or cyclin E are Cdc25 A substrates. Since Cdc25 A was shown to be a rate-limiting factor for Cdk2 activation during G1–S progression (Blomberg and Hoffmann, 1999; Sexl et al., 1999), its decrease upon DNA synthesis inhibition would alter the balance between Cdk2 phosphorylation and dephosphorylation to the advantage of the inactivating kinase. It is also clear that the establishment of cell populations overexpressing Cdc25 A will not automatically result in a reduction of Cdk2 tyrosine dephosphorylation (Sexl et al., 1999). However, Cdc25 A-overexpressing cells will enter S phase prematurely once released from growth arrest, suggesting the existence of a critical Cdc25 A substrate(s) other than Cdk2.
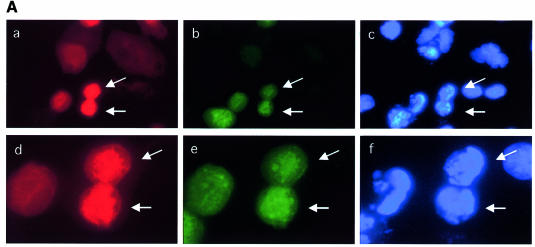
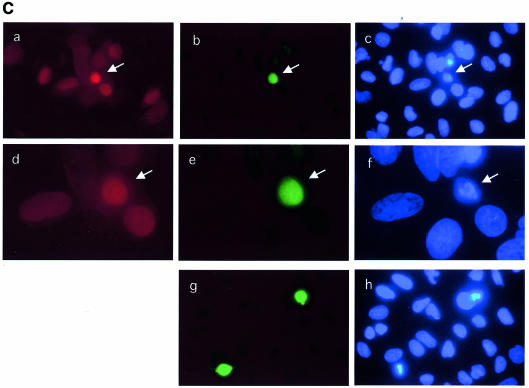
If Cdc25 A degradation in response to HU is required for cell cycle arrest, it should be possible to interfere with such a block by overexpressing Cdc25 A. To gain more precise information on this point, U2OS cells were microinjected with an expression vector encoding Cdc25 A along with immunoglobulin, used to detect the injected cells. Cells were then incubated for 6 h in the absence or presence of HU, and 15 min prior to sample collection BrdU was added. Figure 5A shows that the Cdc25 A-overexpressing cells showed hyper-condensed and fragmented nuclei at high frequency with a morphology of cells undergoing premature mitosis. Remarkably, some of such nuclei stained positively for BrdU, suggesting that these cells were actively in S phase. Upon addition of HU, the Cdc25 A-injected cells did not incorporate BrdU, although a small proportion of them were weakly positive for BrdU staining. To quantify the amount of nuclear aberrations induced by Cdc25 overexpression, microinjections were performed using the wild-type Cdc25 A expression vector and an empty vector as a control, in the presence or the absence of HU. At least 200 injected cells were counted for each experiment, and the average was calculated from three independent experiments (not shown). We found that 25–30% of the cells overexpressing Cdc25 A displayed an altered nuclear morphology, independent from the addition of HU.
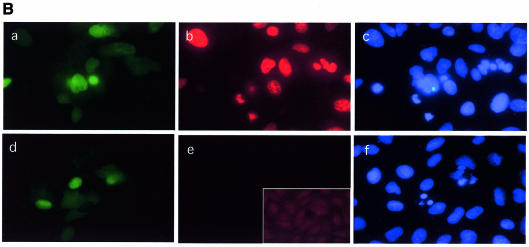
Fig. 5. (A) Growing cells were microinjected with a plasmid encoding wild-type Cdc25 A (100 ng/µl) plus immunoglobulin to identify injected cells and incubated for 6 h in the absence (a, b and c) or the presence (d, e and f) of HU. Fifteen minutes prior to sample collection, BrdU was added to the culture medium. Coverslips were fixed in 4% PAF, permeabilized with Triton X-100, treated with HCl to denature the DNA and stained for BrdU (b and e). Microinjected cells were identified by immunoglobulin staining (a and d). Nuclei were counterstained with Hoechst 33352 (c and f). (B and C) Cells were microinjected and incubated for 6 h in the absence (B) or the presence (C) of HU. Cells were stained with the MPM2 antibody (b, e and g in each part) and counterstained for immunoglobulin (a and d in each part) to identify injected cells. Nuclei were stained with Hoechst 33352 (c, f and h in each part). Magnification is 40× for (a–c) and (g–h), and 60× for (d–e).
Cdc25 A-induced cell death did not appear to be apoptotic (as defined by lack of TUNEL staining) but rather displayed the characteristics of premature mitosis. This was not surprising since we show here that Cdc25 A levels and activity increase during the cell cycle, reaching a maximum during mitosis. It is possible that Cdc25 A overexpression upsets the balance of Cdk–cyclin activation, thus driving premature mitosis. To test this possibility, cell microinjection was performed, in the absence (Figure 5B) or presence (Figure 5C) of HU, and coverslips were fixed after 6 h. Cells were stained with MPM2, a monoclonal antibody that recognizes mitosis-specific phosphoepitopes. Some of the microinjected cells displaying abnormally condensed chromatin were positive for MPM2 staining. Specificity of MPM2 staining was checked by staining non-injected cells. Similar results were obtained upon microinjection of HU-presynchronized cells. A parallel experiment showed that condensed nuclei were weakly positive for TUNEL staining (not shown), probably due to the generation of free DNA ends following chromosome fragmentation. This was far less intense than the stain caused by apoptotic stimuli. From these observations we conclude that Cdc25 A overexpression can induce mitotic events independent of completion of DNA synthesis. We believe that Cdc25 A degradation is a necessary event for growth arrest, since its overexpression causes a failure of cells to arrest properly in the cycle. Cdc25 A overexpression causes cells to overcome the effect of DNA replication inhibitors and make them enter an abortive mitosis.
Cdc25 B and C are needed for G2–M progression, and Cdc25 A for G1–S progression. It has been proposed that Cdc25 B initiates cytoplasmic mitosis by activating centrosome nucleation, prior to induction of nuclear mitosis, which requires the nuclear activity of Cdc25 C. Cdc25 B activity starts to accumulate in late S and G2, whereas Cdc25 C activity increases later, at G2–M. Our data also suggest that Cdc25 A has a role in the initiation of mitosis, consistent with the sustained increase in protein abundance and activity until mitosis, and with previously published data showing that microinjection of Cdc25 A antibodies can interfere with mitosis (Galaktionov and Beach, 1991b). Cdc25 A could act as the major nuclear phosphatase during interphase, and an increase in its levels would serve to counteract the activity of the Wee1-family kinases towards Cdk2–cyclin A–E and perhaps Cdk4–cyclin D, as well as to contribute to the activation of the nuclear pool of Cdc2–cyclin B (Heald et al., 1993). The balance between the activities of Wee1 and Cdc25 A would control DNA replication and prevent unscheduled mitosis.
METHODS
Cell culture conditions, drug treatments, conditions for cell lysis, immunoprecipitaion and immunoblotting were as previously described (Pagano et al., 1992; Naviglio et al., 1998). Monoclonal antibodies to Cdc25 A, Cdc2 and cyclin B and polyclonal antibodies to Cdc25 C and Cdk2 were from Santa Cruz. Polyclonal antibodies to β-catenin were from Transduction laboratories, anti-FLAG antibody from Kodak, and anti-MPM2 from Upstate Biotech. For in vivo ubiquitylation, cells were harvested and processed as described in Treier et al. (1994). Kinase assays were performed as described in Pagano et al. (1992). Cdc25 A phosphatase activity was measured as activation of cyclin B1–Cdc2 (Cans et al., 1999). Inactive cyclin B1–Cdc2 was immunoprecipitated with anti-cyclin B1 antibodies using 200 µg of lysate per reaction. Cdc25 was immunoprecipitated in parallel from 1 mg of cell extracts. Beads were then mixed, incubated in 50 µl of phosphatase buffer (20 mM Tris–Cl pH 8.3, 150 mM NaCl, 2 mM EDTA, 0.1% Triton X-100, 5 mM dithiothreitol) at 30°C for 1 h, and then assayed for kinase activity. Cell microinjection, immunofluorescence and flow cytometry conditions were performed as described (Naviglio et al., 1998).
NOTE ADDED IN PROOF
During the preparation of this manuscript, a similar manuscript was published in Science, 288, 1425–1429 by Mailand et al. (2000).
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by grants from AIRC-FIRC, Telethon, CNR and MinSan.
REFERENCES
- Baldin V., Cans, C., Knibiehler, M. and Ducommun, B. (1997) Phosphorylation of human CDC25B phosphatase by CDK1–cyclin A triggers its proteasome-dependent degradation. J. Biol. Chem., 272, 32731–32734. [DOI] [PubMed] [Google Scholar]
- Blomberg I. and Hoffmann, I. (1999) Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol., 19, 6183–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cans C., Sert, V., De Rycke, J., Baldin, V. and Ducommun, B. (1999) Use of CDC2 from etoposide-treated cells as substrate to assay CDC25 phosphatase activity. Anticancer Res., 19, 1241–1244. [PubMed] [Google Scholar]
- Chaturvedi P. et al.(1999) Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene, 18, 4047–4054. [DOI] [PubMed] [Google Scholar]
- Coqueret O., Berube, G. and Nepveu, A. (1998) The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J., 17, 4680–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J.A., Cheng, M., Roussel, M.F. and Sherr, C.J. (1998) Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev., 12, 3499–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D., Moyana, T. and King, M.J. (1998) Elevated expression of the cdc25A protein phosphatase in colon cancer. Exp. Cell Res., 240, 236–243. [DOI] [PubMed] [Google Scholar]
- Draetta G. and Eckstein, J. (1997) Cdc25 protein phosphatases in cell proliferation. Biochim. Biophys. Acta, 1332, M53–M63. [DOI] [PubMed] [Google Scholar]
- Gabrielli B.G., De Souza, C.P., Tonks, I.D., Clark, J.M., Hayward, N.K. and Ellem, K.A. (1996) Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J. Cell Sci., 109, 1081–1093. [DOI] [PubMed] [Google Scholar]
- Galaktionov K. and Beach, D. (1991) Specific activation of cdc25 tyrosine phosphatase by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell, 67, 1181–1194. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Lee, A.K., Eckstein, J., Draetta, G., Meckler, J., Loda, M. and Beach, D. (1995) CDC25 phosphatases as potential human oncogenes. Science, 269, 1575–1577. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Chen, X. and Beach, D. (1996) Cdc25 cell-cycle phosphatase as a target of c-myc. Nature, 382, 511–517. [DOI] [PubMed] [Google Scholar]
- Gasparotto D., Maestro, R., Piccinin, S., Vukosavljevic, T., Barzan, L., Sulfaro, S. and Boiocchi, M. (1997) Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res., 57, 2366–2368. [PubMed] [Google Scholar]
- Gu Y., Rosenblatt, J. and Morgan, D. (1992) Cell cycle regulation of Cdk2 activity by phosphorylation of thr160 and tyr15. EMBO J., 11, 3995–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., McLoughlin, M. and McKeon, F. (1993) Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell, 74, 463–474. [DOI] [PubMed] [Google Scholar]
- Hernandez S. et al. (1998) cdc25 cell cycle-activating phosphatases and c-myc expression in human non-Hodgkin’s lymphomas. Cancer Res., 58, 1762–1767. [PubMed] [Google Scholar]
- Hoffmann I., Draetta, G. and Karsenti, E. (1994) Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1–S transition. EMBO J., 13, 4302–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A. and Massague, J. (1997) Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-β in cells lacking the CDK inhibitor p15. Nature, 387, 417–422. [DOI] [PubMed] [Google Scholar]
- Jinno S., Suto, K., Nagata, A., Igarashi, M., Kanaoka, Y., Nojima, H. and Okayama, H. (1994) Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J., 13, 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C., Katich, S., Hagting, A., Hoffmann, I. and Pines, J. (1999) Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J. Cell Biol., 146, 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakoff I.H., Brown, N.C. and Reichard, P. (1968) Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res., 28. [PubMed] [Google Scholar]
- Kudo Y., Yasui, W., Ue, T., Yamamoto, S., Yokozaki, H., Nikai, H. and Tahara, E. (1997) Overexpression of cyclin-dependent kinase-activating CDC25B phosphatase in human gastric carcinomas. Jpn J. Cancer Res., 88, 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy, W.G. (1999) Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev., 13, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer C., Wagerer, S., Saffrich, R., Mertens, D., Ansorge, W. and Hoffmann, I. (1998) The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J. Cell Sci., 111, 2445–2453. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A., Furnari, B., Mondesert, O. and Russell, P. (1999) Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature, 397, 172–175. [DOI] [PubMed] [Google Scholar]
- Maniatis T. (1999) A ubiquitin ligase complex essential for the NF-κB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev., 13, 505–510. [DOI] [PubMed] [Google Scholar]
- Millar J.B., Blevitt, J., Gerace, L., Sadhu, K., Featherstone, C. and Russell, P. (1991) p55CDC25 is a nuclear protein required for the initiation of mitosis in human cells. Proc. Natl Acad. Sci. USA, 88, 10500–10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata A., Igarashi, M., Jinno, S., Suto, K. and Okayama, H. (1991) An additional homolog of the fission yeast cdc25 gene occurs in humans and is highly expressed in some cancer cells. New Biol., 3, 959–967. [PubMed] [Google Scholar]
- Naviglio S., Matteucci, C., Matoskova, B., Nagase, T., Nomura, N., Di Fiore, P.P. and Draetta, G.F. (1998) UBPY, a growth regulated human ubiquitin isopeptidase. EMBO J., 17, 3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefsky B. and Beach, D. (1996) Pub1 acts as an E6-AP-like protein ubiquitin ligase in the degradation of cdc25. EMBO J., 15, 1301–1312. [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Pepperkok, R., Verde, F., Ansorge, W. and Draetta, G. (1992) Cyclin A is required at two points in the human cell cycle. EMBO J., 11, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich A.G. and Hartwell, L.H. (1995) A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell, 82, 841–847. [DOI] [PubMed] [Google Scholar]
- Sadhu K., Reed, S.I., Richardson, H. and Russell, P. (1990) Human homolog of fission yeast cdc25 is predominantly expressed in G1. Proc. Natl Acad. Sci. USA, 87, 5139–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santocanale C. and Diffley, J.F. (1998) A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature, 395, 615–618. [DOI] [PubMed] [Google Scholar]
- Sexl V., Diehl, J.A., Sherr, C.J., Ashmun, R., Beach, D. and Roussel, M.F. (1999) A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene, 18, 573–582. [DOI] [PubMed] [Google Scholar]
- Shirahige K., Hori, Y., Shiraishi, K., Yamashita, M., Takahashi, K., Obuse, C., Tsurimoto, T. and Yoshikawa, H. (1998) Regulation of DNA-replication origins during cell-cycle progression. Nature, 395, 618–621. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tatsuka, M., Jinno, S. and Okayama, H. (1995) Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature, 376, 358–362. [DOI] [PubMed] [Google Scholar]
- Treier M., Staszewski, L. and Bohmann, D. (1994) Ubiquitin-dependent c-Jun degradation in vivo is mediated by the ∂ domain. Cell, 78, 787–798. [DOI] [PubMed] [Google Scholar]
- Vigo E., Muller, H., Prosperini, E., Hateboer, G., Cartwright, P., Moroni, M.C. and Helin, K. (1999) CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol., 19, 6379–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Fan, Y.H., Kemp, B.L., Walsh, G. and Mao, L. (1998) Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res., 58, 4082–4085. [PubMed] [Google Scholar]
- Yabro J.W. (1992) Mechanism of action of hydroxyurea. Semin. Oncol., 19, 1–10. [PubMed] [Google Scholar]